Abstract
Store-operated calcium entry (SOCE) is the predominant calcium entry mechanism in most cancer cells. SOCE is mediated by the endoplasmic reticulum calcium sensor STIMs (STIM1 and 2) and plasma membrane channel forming unit Orais (Orai 1–3). In recent years there is increasing evidence indicating that SOCE in cancer cells is dysregulated to promote cancer cell migration, invasion and metastasis. The overexpression of STIM and Orai proteins has been reported to correlate with the metastatic progression of various cancers. The hyperactive SOCE may promote metastatic dissemination and colonization by reorganizing the actin cytoskeleton, degrading the extracellular matrix and remodeling the tumor microenvironment. Here we discuss how these recent progresses provide novel insights to our understanding of tumor metastasis.
Keywords: Store-operated Calcium Entry, SOCE, Calcium signaling, Cancer metastasis, STIM1, Orai1, Review
2. INTRODUCTION
Tumor metastasis is a critical hallmark of cancer that represents the terminal stage of tumor progression. Conceptually, the metastatic cascade can be simplified into the physical translocation of metastatic cells from primary tumor to distant organs and the metastatic expansion of disseminated cancer cells to form macroscopic secondary tumor (1). To disseminate from primary tumor to distant sites, metastatic cancer cells need to invade surrounding tissues, infiltrate the lymph and blood circulation (intravasation), survive in in the transit to distant organs and escape from microvasculature to the distant organ parenchyma. The disseminated cancer cells may remain dormant for an extended period of time before colonizing the distant site and establishing secondary tumors (metastatic expansion) (2). Although metastasis is responsible for about 90% of cancer-related mortalities, the molecular mechanisms underlying metastatic progression remain the most poorly understood aspect of cancer biology.
Ca2+ is a remarkably versatile second messenger that regulates a diverse range of physiological and pathological processes (3). Ca2+ regulates enzymatic activities, protein-protein interactions and the subcellular localization of signaling molecules through calmodulin and other calcium-binding proteins. The cytosolic Ca2+ concentration is tightly controlled by an elaborate Ca2+ signaling system consisting of Ca2+ channels, pumps, exchangers etc. (3, 4). Many extracellular stimuli (e.g. growth factors, chemokines) induce Ca2+ response by promoting Ca2+ release from the internal Ca2+ store and/or Ca2+ influx from the extracellular environment (4). In non-excitable cells (including most cancer cells), store-operated Ca2+ entry (SOCE) is the predominant Ca2+ entry mechanism (5). The store-operated calcium (SOC) channels are activated upon the Ca2+ release from endoplasmic reticulum (ER), which is essential for the cell to replenish the Ca2+ content in the ER after Ca2+ release (5). The concept of SOCE was first proposed by James Putney in 1986 (6), and the Ca2+ release-activated Ca2+ channels (CRAC) were determined in the early 1990s (7, 8); however, the molecular identities of SOC channels were not identified until more recently. In the wake of high throughput RNAi screening technology, the stromal interaction molecule 1 (STIM1) and Orai1 were recently identified to be the ER Ca2+ sensor (9, 10) and the plasma membrane channel pore-forming unit of SOC channels (11–15), respectively. The essential roles of STIM1 and Orai1 in cancer cell migration, invasion and metastasis were first reported in breast cancer in 2009 (16). Since then, there is rapidly accumulating evidence supporting a role for SOC channels in tumor metastasis and progression. It is becoming clear now that the SOCE is frequently activated in a variety of cancers to promote cell motility, invasiveness and metastatic progression. The overexpression of STIM and/or Orai proteins has been correlated with metastatic diseases and shorter survival in several cancers. Genetic or pharmacological blockade of SOC channels is able to inhibit cancer cell proliferation, migration, invasion, and the self-renewal of cancer stem-like cells in vitro, and tumor metastasis and tumorigenesis in various xenograft mouse models.
3. THE STORE-OPERATED CALCIUM CHANNELS
3.1. STIM1 and STIM2
STIM proteins are type 1 single-pass transmembrane proteins predominantly expressed in the ER membrane with N-terminal facing the ER lumen (17). In vertebrates, there are two STIM proteins, STIM1 and STIM2, that ubiquitously express in all cell types (18). STIM1 and STIM2 have distinctly different roles in store-operated Ca2+ signaling (18). STIM1 is a much stronger activator of Orai1 than STIM2 and is mainly responsible for stimulated SOCE. On the other hand, STIM2 is a more sensitive detector for changes in luminal ER Ca2+, and is responsible of maintaining the homeostasis of cytosolic and luminal ER Ca2+ (19). The N-terminal of STIM proteins includes a Ca2+ binding canonical EF-hand motif, non-Ca2+ binding hidden EF-hand and a Sterile α-motif (SAM) mediating STIM protein oligomerization (20, 21). The cytosolic C terminal is composed of two coiled-coil domains(C-C), a CRAC channel activation domain (CAD) or STIM-ORAI activating region (SOAR), Pro/Ser/(P/S)-rich segments, microtubule interacting Ser/Thr-x-Ile-Pro(S/TxIP) sequences and lysine-rich clusters(22, 23). The N-terminal domains of STIMs operate together to form an elegant sensor of luminal ER Ca2+ (18, 20, 21). Upon Ca2+ release from the ER, the canonical EF hand loses Ca2+ binding, which induces conformation changes that lead to STIM oligomerization and translocation to the junctions between ER and plasma membrane (ER-PM junction). The activated STIM proteins adopt an elongated conformation, which allows the lysine-rich motif in the C-terminal to interact with negatively charged phospholipids on the plasma membrane. The conformation changes also release the intra-molecule inhibition of CAD/SOAR domain in the C-terminal and allow the CAD/SOAR to interact with and to activate Orai proteins on the plasma membrane to activate the opening of the channels.
3.2. Orai1, Orai2 and Orai3
The Orai proteins are highly conserved plasma membrane pore forming units of SOC channels (14, 15, 24). There are three closely related Orai isoforms (Orai1-3) in mammals. It is believed that Orai1 is the predominant channel protein responsible for SOCE, although all three isoforms of mammalian Orais are able to conduct highly selective CRAC currents when overexpressed (25). The Orai consist of four transmembrane domains (M1-4), with both N- and C-termini facing the cytosol (26). Although it is well accepted that Orai protein oligomers form the SOC channels with M1 transmembrane domain lining the ion selective pore (15, 24, 26), the stoichiometry of functional channels has been contentious. Earlier data indicate that active Orai channels are tetrameric (27–30). More recent structural and biochemical evidence strongly supports a hexameric model (26, 31). There is also evidence indicating that both the N- and C-termini of Orai1 interacts with the CAD/SOAR domain of STIM proteins (22), although it is still not completely understood how these interactions activate the channel.
4. SOCE In Cancer Metastasis
The hallmarks that distinguish malignant cancer from benign tumor growth are the abilities to invade surrounding tissue and to disseminate distant organ. The hyperactive cell motility and invasiveness are essential for the metastatic dissemination from primary tumor to distant organ. It was reported that blockade of SOCE, by STIM1 and Orai1 shRNAs or by pharmacological inhibitors, remarkably impaired the breast cancer cell mobility and invasiveness in vitro, and abrogated the lung metastasis of breast cancer cells in mouse models(16). Strikingly, stimulation of SOCE by ectopic expression of STIM1 together with Orai1 was able to confer human mammary epithelial cells MCF-10A with ability to invade Matrigel, suggesting that activation of SOCE in non-invasive epithelial cells with invasiveness (16). In the last several years, there is rapidly accumulating evidence supporting the pro-migration and pro-invasion activity of SOCE in a variety of cancers, including breast cancer (32–39), cervical cancer(40–42), hepatocellular carcinoma (43–45), renal carcinoma(46, 47), nasopharyngeal carcinoma (48), glioblastoma (49, 50), colorectal cancer (51–55) and melanoma(56–60). In this section we will discuss the molecular mechanisms by which SOCE promotes cancer cell migration and invasion.
4.1. A brief overview of cancer cell migration and invasion
The motility of most cancer cell is driven by protrusive forces in the front and contractile forces in the back (61). The protrusive forces in lamellipodia of migration cells are generated by a dendritic actin cytoskeleton network (62). The assembly and polymerization of the dendritic F-actin in lamellipodia are catalyzed by actin nucleation complexes consisting of small GTPase Rac, WAVE, N-WASP and Arp2/3 complex (61). The pro-migration and invasion cues in the tumor microenvironment activate the phosphoinositide-3-kinase, which in turn recruits and activates actin nucleation complexes to generate membrane protrusion. In the rear end of the migrating cell, the retraction of the trailing tail and the net movement of nucleus and cell body is dependent on the contractile forces generated by the actomyosin fibers (61). The actomyosin contraction is regulated by small GTPase Rho, Rho-kinase, or by Ca2+/calmodulin-activated myosin-light chain kinase (61, 63). The actin cytoskeleton in migration cell is anchored to the extracellular matrix (ECM) by a type of adhesive protein complex known as focal adhesion. The assembly of nascent focal adhesion in the front and the disassembly of mature focal adhesion in the back coordinate with the actin cytoskeleton dynamics in migrating cells to drive cell motility.
The motility of cancer cells in vivo is often blocked by the ECM barriers. Therefore, gaining invasiveness is a critical step in cancer metastasis (64, 65). Cancer cells are able to invade the surrounding tissue by proteolytic degradation of extracellular matrix, or by squeezing through loosely crosslinked extracellular matrix in the interstitial spaces in a protease-independent manner (66–69). Invadopodia are adhesive membrane protrusions coordinating focalized ECM degradation in malignant tumor cells (70, 71). The actin rich core of invadopodium is regulated by non-receptor tyrosin kinase Src, scaffolding proteins cortactin, Tsk5, Nck and actin nucleating proteins N-WASP and Arp2/3 (71). The matured invadopodia are able to recruit or secret matrix metalloproteases to degrade extracellular matrix (71). Invadopodia have been shown to be critical for metastasis in mouse models and cancer patients (71–73). Although amoeboid cancer cells may pass through reconstituted ECM in a protease-independent manner (68, 69, 74), focalized proteolysis is essential for cancer cells to breach high density ECM in vivo (66, 75).
4.2. SOCE and focal adhesion turnover
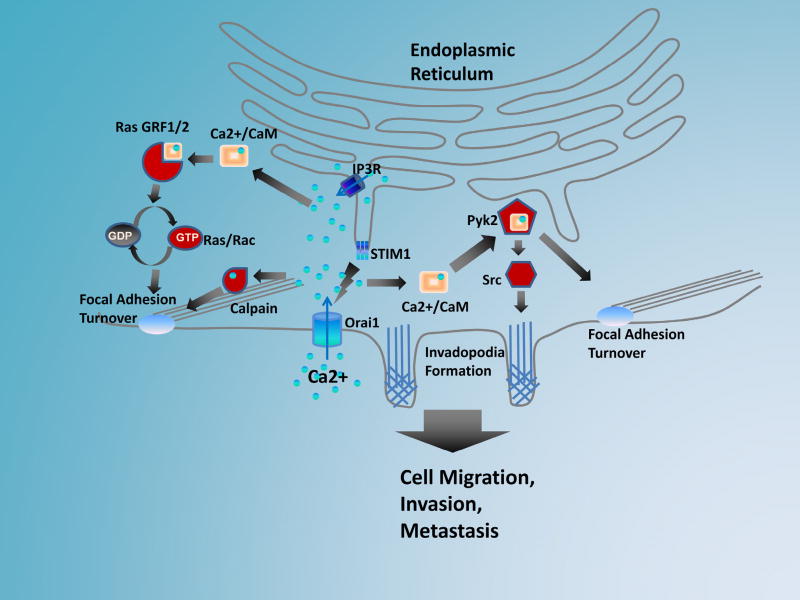
The dynamic turnover of focal adhesions in migrating cells is required for both the leading edge protrusion and trailing tail retraction. The formation of nascent focal complex in the leading edge is essential for the dendritic actin network in the lamellipodia to provide protrusive forces. On the other hand, disassembly of focal adhesion of in the trailing tail is prerequisite for the retraction of trailing tail and the forward movement of the cell body. It is believed that SOCE regulates cancer cell migration at least partially through regulation of focal adhesion turnover (16, 40, 76). Blockade of SOCE in breast and cervical cancer cells lead to large peripheral focal adhesions that have slower assembly and disassembly rates, suggesting impaired focal adhesion turnover in these cells (16, 40). Another interesting observation is that new protrusions in the lamellipodia of migrating cells fail to assemble nascent focal complex and quickly retract (16). Therefore, by regulating focal adhesion assembly and disassembly, SOCE is likely to control both the leading edge protrusion and trailing tail retraction during cancer cell migration. Mechanistically, SOCE regulates focal adhesion turnover through small GTPase, Ras and Rac (Figure 1). Increase in cytosolic Ca2+ activates Ras/Rac, and ectopic expression of constitutively active Ras and Rac is able to rescue focal adhesion turnover and cell migration defects after SOCE inhibition (16, 55). Several Ras and Rac GEFs (e.g. RasGRF1, RasGFR2, RasGRP) are activated by binding to Ca2+ or calmodulin, and thus could potentially mediate the activation of small GTPase downstream of SOCE (Figure 1). SOCE may also control focal adhesion turnover through focal adhesion kinases FAK and Pyk2, since SOCE inhibition also decreases the levels of active FAK and Pyk2 (36, 41, 77). FAK has no Ca2+ or calmodulin binding motif, and therefore FAK is likely to be regulated by SOCE indirectly. Pyk2 has a calmodulin binding IQ motif in its auto-inhibitory IQ motif (78), and could be a direct target mediating SOCE regulation of focal adhesion turnover (Figure 1). Finally, the Ca2+-binding protease calpain may play important role in SOCE-mediated focal adhesion turnover (Figure 1). Calpain contains several Ca2+-binding motifs in its catalytic and regulatory subunits. Ca2+ binding is required for calpain activation (79), and SOCE blockade inhibits its activity in cervical and colon cancer cells (40, 55). The proteolytic processing of some focal adhesion proteins, such as talin, paxillin, FAK, is a rate-limiting step in focal adhesion disassembly (79–82). Interestingly, inhibition of calpain also results in large peripheral focal adhesion and defective focal adhesion disassembly (80), a phenotype that is reminiscent of focal adhesions in SOCE inhibited cancer cells (16).
Figure 1.
Upon Ca2+ depletion from the ER lumen, STIM1 molecules on the ER membrane oligomerize and translocate to the junctions between the ER and plasma membrane. At the ER-PM junction, STIM1 oligomers interact with Orai1 channels to activate SOCE. The hyperactive SOCE in cancer cells promotes metastasis by facilitating focal adhesion turnover and invodopodia formation. The elevation of cytosolic Ca2+ may facilitate focal adhesion turnover through small GTPases Ras/Rac. The binding of Ca2+/calmodulin to IQ-motif containing guanine nucleotide exchanges factors (e.g. RasGRF1 and 2) promotes the GTP binding and Ras/Rac activation. Cytosolic Ca2+ may also facilitate focal adhesion turnover through Ca2+-activated protease calpain, which cleave many focal adhesion proteins. The cytosolic Ca2+ may also activate Pyk2 through Ca2+/calmodulin, which binds to an IQ motif at the FERM domain of Pyk2. The binding of calmodulin to Pyk2 promotes the Pyk2 auto-phosphorylation at tyrosine 402. The phosphorylation of Pyk2 at Y402 provides docking site for the activation of Src kinase, which is able to further promote the assembly of invadopodia and remodeling of extracellular matrix. By regulating focal adhesion turnover and invadopodia formation, the hyperactive SOCE in cancer cells is able to promote cancer cell migration, invasion and metastasis.
In addition to inhibiting focal adhesion disassembly, SOCE blockade also impaired the assembly of nascent focal adhesion in the leading edge of migrating cells (16). The assembly defects could be due to the trapping of focal adhesion structural proteins in mature focal adhesions. Indeed, it has been previously demonstrated that the recycling of the αvβ3 integrin from the trailing-tail to the leading edge is dependent on Ca2+ and calcineurin (83). In addition, Ca2+ may regulate the initiation of focal adhesion assembly through integrin inside-out signaling(84), and the maturation of focal adhesion through myosin-mediated contractility (85, 86).
4.3. The regulation of actomyosin contractility by SOCE
The contractility of the actomyosin network in migrating cell is crucial for the retraction of the trailing tail and the forward movement of the nucleus. Cancer cells are also able to adopt actomyosin contractility-dependent amoeboid cell migration to invade the interstitial extracellular matrix in a protease-independent manner (69). In polarized migrating cells, the Ca2+ concentration is high in the trailing tail and low in the leading edge, forming a front-to-back Ca2+-concentration gradient (87, 88). The high Ca2+ concentration in the trailing tail activates the actomyosin contraction and tail retraction through MLCK (63, 88). Although Ca2+ is generally low in the leading edge, it is rich in transient Ca2+ pulses termed “Ca2+ flicker” (85, 86, 89). These Ca2+ flickers in the front control focal adhesion formation and local contractility through activation of MLCK (85, 86). The local Ca2+ pulse in the leading edge is supported by polarized activation of local Ca2+ release from the ER and local activation of STIM1, which is transported to the front of migrating cells by binding to the microtubule plus ends (85).
4.4. SOCE and cancer cell invasion
Recently, STIM1 and Orai1-mediated SOCE signaling has been shown to be critical for invadopodial assembly and extracellular matrix degradation (57). SOCE promotes invadopodium assembly by activating the non-receptor tyrosine kinase Src (Figure 1), which activate actin-nucleating factors Arp2/3 complexes to promote the assembly of invadopodial precursor (90–93). Consistent with this notion, SOCE blockade inhibits the assembly of invadopodial-precursor, without affecting invadopodial stability (57).
SOCE is critical for not only the assembly initiation, but also the maturation of invadopodia. SOCE blockade with specific shRNA or inhibitors decreases the numbers of invadopodia per cell, as well as the proteolytic activity of remaining invadopodia (57). The defective proteolytic activity of these invadopodia is due to the inhibition of the recycling of MT1-MMP (membrane type 1 matrix metalloprotease) from the endocytic compartment to the plasma membrane (57). MT1-MMP is a matrix metalloprotease with a single transmembrane domain, and one of the predominant protease responsible for cancer invasion (66). The plasma membrane MT1-MMP is constantly endocytosed and trafficked to invadopodia through VAMP7, a v-SNARE that is involved in Ca2+-dependent exocytosis (94, 95). SOCE blockade has no effect on the endocytosis of MT1-MMP, but remarkably slows down the recycling of MT1-MMP from the endocytic compartment to the plasma membrane (57). It is possible that SOCE might regulate
4.5. The regulation of epithelial-to-mesenchymal transition (EMT) by SOCE
The epithelial-to-mesenchymal transition (EMT) is a biological process that allow epithelial cells to adopt many mesenchymal cell phenotypes, such as elevated motility, invasiveness and increased production of extracellular matrix proteins (96). There is emerging data suggesting that SOCE may play a role in the EMT of serval cancers (35, 54, 97, 98). In MCF7 breast cancer cells induction of EMT with transforming growth factor β (TGFβ) increased SOCE and the expression levels of STIM1 and Orai1 (35). The TGFβ-induced EMT was abrogated when STIM1 expression was suppressed with shRNA, suggesting that SOCE is required for TGFβ-mediated EMT (35). The role of SOCE in EMT has also been reported in prostate cancer (99), colon cancer (54) and gastric cancer (97), suggesting that SOCE may regulate cancer metastasis by triggering EMT.
4.6. The role of soce beyond metastatic dissemination
There is now convincing evidence from animal models and human patient specimens suggesting that dysregulation of STIM and Orai proteins promotes the tumor metastasis and progression. Blockade of SOCE with pharmacological inhibitors or RNA interference inhibits the metastasis of breast cancer (16, 39), melanoma (57), colorectal cancer (54), gastric cancer (97) in xenograft models; on the other hand, ectopic expression of STIM1 in SW480 colorectal cancer cells promotes lung metastasis (54). Notably, blockade of SOCE not only inhibits spontaneous metastasis from primary tumor (16, 39), but also reduces metastatic burden from intravenously injected metastatic cancer cells, suggesting that SOCE is critical for disseminated cancer cells to establish secondary tumor.
The metastatic colonization at distant site is an extremely inefficient process. Only a small fraction of disseminated cancer cells are able to successfully establish distant metastases (1). The microenvironment in distant organ is hostile to disseminated cancer cells, and the majority of them undergo apoptosis within 24 hours after extravasation (1). It has been suggested that secreted factors (e.g. VEGF, lysyl oxidase, exosomes) derived from primary tumor are able to remodel the distant sites to facilitate metastatic expansion (100–102). These factors mobilize angiogenesis at distant sites to form pre-metastasis niche, which supports the metastatic colonization of disseminated cancer cells. The STIM1 and Orai1-mediated SOCE promotes the secretion of VEGF and prostaglandins E2 in cervical cancer and colorectal cancer (40, 53), which may contribute to mobilizing endothelial cells at pre-metastasis niche. Furthermore, SOCE could promote metastatic colonization through proteolytic remodeling of the ECM in distant organ, which is essential for cancer cells growth in 3D collagen I matrix (103). The proteolytic remodeling of the ECM is also essential for cancer cells to activate latent growth factors (e. g. TGFβ, VEGF) deposited in the ECM (71). The growth factors released from the ECM may further modify the tumor environment by recruiting endothelial cells, inflammatory immune cells and other stromal cells to create a niche conducive to the survival and expansion of disseminated cancer cells.
5. THE DYSREGULATION OF SOCE IN CANCER
The expression levels of STIM1 and Orai1 have been examined in cervical cancer, glioma, melanoma, hepatocellular carcinoma, non-small cell lung cancer, colorectal cancer, esophageal squamous cell carcinoma, prostate cancer and breast cancer, and these findings have been summarized in two excellent recent reviews (104, 105). The expression levels of STIM1 and/or Orai1 in cervical cancer (40, 42), colorectal cancer (53, 54), gastric cancer (97), non-small cell lung cancer (106) and esophageal cancer (107) are found to be correlated with metastatic progression, poor prognosis and shorter survival. These correlative studies using patient specimens are consistent with findings from cultured cells and xenograft mouse models.
In breast cancer, the highly aggressive basal-like subtype is characterized by an elevated ratio of STIM1/STIM2 ratio (108). Correspondingly, the patients with high STIM1/STIM2 ratio and high STIM1 expression levels have an increased metastatic potential and a decreased survival rate, indicating that STIM proteins are potential regulators of breast cancer progression. The single-nucleotide polymorphisms of ORAI1 gene in breast cancer are strongly associated with lymph node involvement and estrogen receptor status, suggesting the genetic variant of SOCE genes might predispose patients to breast cancer (109). Orai1 isoform is not the only Orai protein that associates with cancer. In estrogen receptor-positive breast cancer cell lines, native SOCE and ICRAC are mediated by STIM1/2 and Orai3, while estrogen receptor-negative breast cancer cells mainly rely on Orai1 (34). The transcription of Orai3 is regulated by estrogen receptor, which may explain the differential expression of Orai3 in different subtypes of breast cancer (34). The overexpression of Orai3 has also been reported in lung adenocarcinoma (110). In a cohort of 200 lung cancer patients, Orai3 protein and mRNA expression was increased in tumor tissues than in matched normal tissues, and Orai3 overexpression independently correlated with overall survival and metastasis free survival (110).
In addition to the canonical regulation of Orai channels by STIM1/2, there are reports of store-independent activation mechanism. It has been reported in luminal breast cancer that SPCA2 is able to activate Orai1 in a STIM1 and ER Ca2+ store-independent manner to promote tumor growth (32). Another example of store-independent activation of Orai1 is the SK3-Orai1 complex in breast cancer and colon cancer models (39, 55). It is reported that SK3 potassium channel forms complex with Orai1 in the plasma membrane lipid rafts to activate Orai1 through plasma membrane hyperpolarization (39). Disruption of lipid rafts with the alklyl-lipid Ohmline interfered with the formation of SK3-Orai1 complex formation and inhibited the breast cancer cell migration (39). There is also evidence that prostate cancer may switch from the canonical SOCE to store-independent arachidonic acid regulated channels (consisting of Orai1/Orai3 heteromer) to support tumor cell proliferation (111). The non-canonical regulation of Orai1 channels is an interesting concept that implicates the complexity of the Ca2+ signaling dysregulation in cancers. However, the biochemistry and electrophysiology of these novel regulatory mechanisms are still not well-defined and future studies in these areas are needed.
Although the overexpression of STIM and Orai proteins has been widely investigated in a variety of cancers, the molecular mechanisms underlying their overexpression are only beginning to be revealed. In glioblastoma and colorectal cancer, the overexpression of STIM2 appeared to be due to amplification of the 4p15 locus, which increases STIM2 gene copy numbers (112, 113). Paradoxically, overexpression of STIM2 promotes cell migration but inhibits cell proliferation (60, 112), implicating complexity of SOCE signaling at different stages of tumor progression. The inflammatory and hypoxic tumor microenvironment may be responsible for overexpression of STIM and Orai in some tumors. The overexpression of STIM1 in hepatocarcinoma is driven by the hypoxic tumor microenvironment and hypoxia-inducible factor-1 (HIF-1) (114). HIF1-α induces the expression of STIM1 by binding to hypoxia response elements in STIM1 promoter (114). In contrast, the expression of STIM2 is not regulated by hypoxia, since its promoter contains no hypoxia response elements (114). Hypoxia, a common feature of the microenvironment of most solid tumors, increases HIF-1α levels by stabilizing the protein. The expression levels of HIF-1α in cancer could also be increased by non-hypoxic regulations (such as mutations of von Hippel-Lindau tumor suppressor) (115). Therefore, it is likely that the hypoxic tumor microenvironment and HIF-1α also contribute to STIM1 overexpression in other solid tumors. In addition to transcriptional regulation, microRNA may regulate STIM1 at the post-transcriptional level. In colorectal cancer, STIM1 was found to be a direct target of microRNA-185, a microRNA that is downregulated in metastatic colorectal cancer cells (54).
6. THE SPATIAL AND TEMPORAL REGULATION OF SOCE IN CANCER CELL
The cytosolic Ca2+ concentration is organized in intricate spatial and temporal patterns. The spatial and temporal coding of is essential for the specificity and versatility of Ca2+ signaling (3, 64, 116). It is estimated that hundreds of proteins encoded by the human genome contain Ca2+ motifs, and many more are indirectly regulated by Ca2+ through calmodulin (116). The spatiotemporal organization of Ca2+ signals allows cells to selectively activate their target proteins at the defined time and subcellular compartments (3). During prolonged stimulation, the cytosolic Ca2+ signals are organized as repeated Ca2+ pulse known as Ca2+ oscillations (3). Each oscillation is coordination between Ca2+ release from the ER and Ca2+ influx from extracellular Ca2+. The oscillating Ca2+ transients provides extended period of Ca2+ stimulation while avoiding toxic side effects associated with Ca2+ overload (117). Such extended Ca2+ signal is essential for many biological processes such as gene transcription and cell migration.
The frequency and amplitude of oscillatory Ca2+ signal may serve as digital signals that control downstream signaling, and STIM1 is believed to be a Ca2+ sensor specialized for coding such digital signaling (118). STIM1 is able to detect the transient decrease in the ER Ca2+ reservoir following each oscillation, and thus activates Orai1 channel to replenish the ER Ca2+ (118). STIM1- and Orai1-mediated SOCE is obligatory for Ca2+ oscillation in human embryonic kidney cells and melanoma cells (57, 119). The assembly of invadopodial precursors in invasive melanoma cells is accompanied by oscillatory Ca2+ signals (57). Depletion of STIM1 and Orai1 decreased oscillation frequency and impaired the assembly of invadopodia, suggesting that oscillatory Ca2+ signal is required for the assembly of invadopodia (57). The hyperactive Ca2+ oscillation has also been reported in esophageal squamous cell carcinoma cells (107). Intriguingly, in esophageal cancer the hyperactive SOCE appeared to be due to Orai1 overexpression alone. shRNA depletion of up to two thirds of STIM1 protein had minimal effect on SOCE in these cells (107).
The spatial organization of Ca2+ channels and Ca2+ effectors into super complexes is another way for the cell to control the robustness and specificity of Ca2+ signaling (3). Under normal physiological condition, the cytosolic Ca2+ from Ca2+ influx/release is quickly sequestered by the ER, mitochondria or pumped out of the cells by plasma membrane Ca2+-ATPase. As a result, diffusion of Ca2+ through opened channels creates local Ca2+ microdomains (120), which allows selective activation of Ca2+ effectors localized to the vicinity of active Ca2+ channels. The local Ca2+ pulses in the front of migrating cells control focal adhesion maturation and lamellipdoium retraction by local activation of myosin contraction (85, 86, 89). It is known that Ca2+ effectors such as calcineurin, myosin and S100 proteins are enriched in proteolytic invadopodia and podosomes (71). The presence of STIM1 and Orai1 in the podosomes of microglia has been recently reported (121). By bringing together SOC channels and Ca2+ effectors, invadopodia may serve as signaling hubs coordinating ECM remodeling and cancer cell invasion.
7. TARGETING SOCE IN METASTATIC CANCER: OPPORTUNITIES AND CHALLENGES
STIM and Orai proteins are frequently overexpressed / activated in metastatic cancer, and the activation of these channels promotes tumor growth and metastatic progression. These exciting new findings suggest that STIM and Orai proteins are promising targets in combating metastatic cancer. Indeed, SOCE blockers SKF96365 and 2-APB have been used to inhibit tumor growth and metastasis in the xenograft mouse models for breast, cervical and esophageal cancers (16, 40, 107). The activity of the store-independent SK3-Orai1 channel could be inhibited by Ohmline, an alkyl-lipid that disrupt the SK3-Orai1 complex by interfering with lipid raft formation. Ohmline treatment inhibited the activity of SK3-Orai1 Ca2+ influx in vitro, and the bone and lung metastasis of MDA-MB-435 breast cancer cells in a mouse model (39). There is also evidence that SOCE is required for chemoresistance in 5-FU treated pancreatic and ovarian cancer cells, suggesting that SOCE blocker could be useful in combination with chemotherapies to treat refractory tumors (122, 123). These studies provide proof-of-principle for targeting STIMs and Orais in cancer treatment.
In addition to inhibit tumor growth and metastasis by directly acting on cancer cells, SOCE inhibitors may also be used to “normalize” the inflammatory tumor microenvironment. Cancers are considered “wounds that do not heal” and chronic inflammation is a common feature of the tumor microenvironment (124). Inflammatory cytokines secreted by tumor cells and infiltrating immune cells promote tumor cell proliferation, migration, invasion and therapy resistance (124). STIM1 promotes COX2 overexpression and prostaglandin production in colorectal cancer cells, implicating a role for SOCE in tumor chronic inflammation (52, 53). SOCE has also been implicating in the production of inflammatory cytokine by endothelial cell, T cell, and mast cells (125–128), while its function in macrophage is more controversial (129, 130). Non-steroidal anti-inflammatory drugs such as Aspirin have shown promising chemopreventive potential in various cancers (131). Notably, many NSAIDs, such as indomethacin, ibuprofen and sulindac, are also able to inhibit SOCE (132). Therefore, these over-the-counter pain killers could potentially be used to target SOCE and the inflammatory pathways downstream of SOCE in cancer. Other Selective SOCE blockers, such as BTP2, Synta-66 (133), might also be used to prevent tumor progression, although their anti-tumor efficacies have yet to be determined.
8. CONCLUSIONS AND FUTURE DIRECTIONS
Recent years have seen significant progress in our understanding of SOCE in cancer progression. There is now convincing evidence indicating that STIM and Orai proteins are overexpressed in solid tumors, and the overexpression of these proteins promotes tumor cell migration, invasion, proliferation, apoptosis resistance and chemoresistance. There is a high interest in developing selective SOCE blockers (133). These inhibitors could potentially be useful in preventing cancer metastasis.
Despite significant progress in this young field, the molecular mechanisms underlying SOCE-mediated cancer metastasis remain poorly understood. Pro-metastasis factors in the tumor microenvironment (e.g. hypoxia, acidic pH, reactive oxygen species, inflammatory cytokines etc) are likely to regulate the expression and activity of STIM and Orai proteins in cancer cells as well as stromal cells. The mechanistic role of SOCE in metastatic cancer cells is now beginning to be understood, while the functions of SOCE in tumor stromal cells remain uncharted territory. Future investigators in these areas will likely bring important insights.
Acknowledgments
This work is supported in part by the NIH (R01 CA175741).
Abbreviation
- SOCE
store operated calcium entry
- ER
endoplasmic reticulum
- CRAC
calcium release-activated calcium channels
- STIM1/2
stromal interaction molecule 1/2
- SAM
sterile α-motif
- CAD
CRAC channel activation domain
- SOAR
STIM-ORAI activating region
- GEF
Guanine nucleotide exchange factor
- FAK
focal adhesion kinase
- Pyk2
protein tyrosine kinase 2 beta
- MLCK
myosin-light chain kinase
- MT1-MMP
membrane type 1 matrix metalloprotease
- VAMP7
Vesicle Associated Membrane Protein 7
- EMT
epithelial-to mesenchymal transition
- TGFβ
transforming growth factor β
- VEGF
vascular endothelial growth factor
- HIF1-α
hypoxia-inducible factor-1
References
- 1.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature reviews. Molecular cell biology. 2003;4(7):517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature reviews. Molecular cell biology. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 5.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiological reviews. 2005;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 6.Putney JW., Jr A model for receptor-regulated calcium entry. Cell calcium. 1986;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 7.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355(6358):353–6. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 8.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993;90(13):6295–9. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15(13):1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169(3):435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 12.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312(5777):1220–3. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci U S A. 2006;103(24):9357–62. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 15.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443(7108):226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15(2):124–34. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 17.Williams RT, Manji SS, Parker NJ, Hancock MS, Van Stekelenburg L, Eid JP, Senior PV, Kazenwadel JS, Shandala T, Saint R, Smith PJ, Dziadek MA. Identification and characterization of the STIM (stromal interaction molecule) gene family: coding for a novel class of transmembrane proteins. Biochem J. 2001;357(Pt 3):673–85. doi: 10.1042/0264-6021:3570673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13(9):549–65. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131(7):1327–39. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng L, Stathopulos PB, Li GY, Ikura M. Biophysical characterization of the EF-hand and SAM domain containing Ca2+ sensory region of STIM1 and STIM2. Biochemical and biophysical research communications. 2008;369(1):240–6. doi: 10.1016/j.bbrc.2007.12.129. [DOI] [PubMed] [Google Scholar]
- 21.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135(1):110–22. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136(5):876–90. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11(3):337–43. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Current biology : CB. 2006;16(20):2073–9. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng X, Wang Y, Zhou Y, Soboloff J, Gill DL. STIM and Orai: dynamic intermembrane coupling to control cellular calcium signals. J Biol Chem. 2009;284(34):22501–5. doi: 10.1074/jbc.R109.018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338(6112):1308–13. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penna A, Demuro A, Yeromin AV, Zhang SL, Safrina O, Parker I, Cahalan MD. The CRAC channel consists of a tetramer formed by Stim-induced dimerization of Orai dimers. Nature. 2008;456(7218):116–20. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mignen O, Thompson JL, Shuttleworth TJ. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J Physiol. 2008;586(2):419–25. doi: 10.1113/jphysiol.2007.147249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruyama Y, Ogura T, Mio K, Kato K, Kaneko T, Kiyonaka S, Mori Y, Sato C. Tetrameric Orai1 is a teardrop-shaped molecule with a long, tapered cytoplasmic domain. J Biol Chem. 2009;284(20):13676–85. doi: 10.1074/jbc.M900812200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson JL, Shuttleworth TJ. How many Orai's does it take to make a CRAC channel? Sci Rep. 2013;3:1961. doi: 10.1038/srep01961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai X, Zhou Y, Nwokonko RM, Loktionova NA, Wang X, Xin P, Trebak M, Wang Y, Gill DL. The Orai1 Store-operated Calcium Channel Functions as a Hexamer. J Biol Chem. 2016 doi: 10.1074/jbc.M116.758813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts-Thomson SJ, Monteith GR, Rao R. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143(1):84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, Smart CE, Brown MA, Kenny PA, Roberts-Thomson SJ, Monteith GR. ORAI1-mediated calcium influx in lactation and in breast cancer. Molecular cancer therapeutics. 2011;10(3):448–60. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- 34.Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3: elective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem. 2010;285(25):19173–83. doi: 10.1074/jbc.M110.102582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu J, Qin K, Zhang Y, Gong J, Li N, Lv D, Xiang R, Tan X. Downregulation of transcription factor Oct4 induces an epithelial-to-mesenchymal transition via enhancement of Ca2+ influx in breast cancer cells. Biochemical and biophysical research communications. 2011;411(4):786–91. doi: 10.1016/j.bbrc.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 36.Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, Trebak M. Orai3 is an estrogen receptor alpha-regulated Ca(2)(+) channel that promotes tumorigenesis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(1):63–75. doi: 10.1096/fj.12-213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B, Yu HH, Ye HL, Luo ZY, Xiao F. Effects of stromal interacting molecule 1 gene silencing by short hairpin RNA on the biological behavior of human gastric cancer cells. Mol Med Rep. 2015;12(2):3047–54. doi: 10.3892/mmr.2015.3778. [DOI] [PubMed] [Google Scholar]
- 38.Hammadi M, Chopin V, Matifat F, Dhennin-Duthille I, Chasseraud M, Sevestre H, Ouadid-Ahidouch H. Human ether a-gogo K(+) channel 1 (hEag1) regulates MDA-MB-231 breast cancer cell migration through Orai1-dependent calcium entry. J Cell Physiol. 2012;227(12):3837–46. doi: 10.1002/jcp.24095. [DOI] [PubMed] [Google Scholar]
- 39.Chantome A, Potier-Cartereau M, Clarysse L, Fromont G, Marionneau-Lambot S, Gueguinou M, Pages JC, Collin C, Oullier T, Girault A, Arbion F, Haelters JP, Jaffres PA, Pinault M, Besson P, Joulin V, Bougnoux P, Vandier C. Pivotal Role of the Lipid Raft SK3-Orai1 Complex in Human Cancer Cell Migration and Bone Metastases. Cancer Res. 2013;73(15):4852–61. doi: 10.1158/0008-5472.CAN-12-4572. [DOI] [PubMed] [Google Scholar]
- 40.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):15225–30. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen YT, Chen YF, Chiu WT, Wang YK, Chang HC, Shen MR. The ER Ca2+ sensor STIM1 regulates actomyosin contractility of migratory cells. J Cell Sci. 2013;126(Pt 5):1260–7. doi: 10.1242/jcs.121129. [DOI] [PubMed] [Google Scholar]
- 42.Chen YT, Chen YF, Chiu WT, Liu KY, Liu YL, Chang JY, Chang HC, Shen MR. Microtubule-associated histone deacetylase 6 supports the calcium store sensor STIM1 in mediating malignant cell behaviors. Cancer Res. 2013;73(14):4500–9. doi: 10.1158/0008-5472.CAN-12-4127. [DOI] [PubMed] [Google Scholar]
- 43.Yang N, Tang Y, Wang F, Zhang H, Xu D, Shen Y, Sun S, Yang G. Blockade of store-operated Ca(2+) entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer Lett. 2013;330(2):163–9. doi: 10.1016/j.canlet.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 44.Yang N, Tang Y, Wang F, Zhang H, Xu D, Shen Y, Sun S, Yang G. Blockade of store-operated Ca(2+) entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer letters. 2013;330(2):163–9. doi: 10.1016/j.canlet.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 45.Wu Z, Qing J, Xia Y, Wang K, Zhang F. Suppression of stromal interaction molecule 1 inhibits SMMC7721 hepatocellular carcinoma cell proliferation by inducing cell cycle arrest. Biotechnol Appl Biochem. 2015;62(1):107–11. doi: 10.1002/bab.1245. [DOI] [PubMed] [Google Scholar]
- 46.Kim JH, Lkhagvadorj S, Lee MR, Hwang KH, Chung HC, Jung JH, Cha SK, Eom M. Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma. Biochem Biophys Res Commun. 2014;448(1):76–82. doi: 10.1016/j.bbrc.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 47.Clarysse L, Gueguinou M, Potier-Cartereau M, Vandecasteele G, Bougnoux P, Chevalier S, Chantome A, Vandier C. cAMP-PKA inhibition of SK3 channel reduced both Ca2+ entry and cancer cell migration by regulation of SK3-Orai1 complex. Pflugers Arch. 2014;466(10):1921–32. doi: 10.1007/s00424-013-1435-5. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Wei J, Kanada M, Yan L, Zhang Z, Watanabe H, Terakawa S. Inhibition of store-operated Ca2+ entry suppresses EGF-induced migration and eliminates extravasation from vasculature in nasopharyngeal carcinoma cell. Cancer Lett. 2013;336(2):390–7. doi: 10.1016/j.canlet.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 49.Motiani RK, Hyzinski-Garcia MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH, Matrougui K, Mongin AA, Trebak M. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch. 2013;465(9):1249–60. doi: 10.1007/s00424-013-1254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu M, Chen L, Zhao P, Zhou H, Zhang C, Yu S, Lin Y, Yang X. Store-operated Ca(2+) entry regulates glioma cell migration and invasion via modulation of Pyk2 phosphorylation. J Exp Clin Cancer Res. 2014;33:98. doi: 10.1186/s13046-014-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong HS, Chang WC. Correlation of clinical features and genetic profiles of stromal interaction molecule 1 (STIM1) in colorectal cancers. Oncotarget. 2015;6(39):42169–82. doi: 10.18632/oncotarget.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang JY, Chen BK, Wang YS, Tsai YT, Chen WC, Chang WC, Hou MF, Wu YC. Involvement of store-operated calcium signaling in EGF-mediated COX-2 gene activation in cancer cells. Cellular signalling. 2012;24(1):162–9. doi: 10.1016/j.cellsig.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Wang JY, Sun J, Huang MY, Wang YS, Hou MF, Sun Y, He H, Krishna N, Chiu SJ, Lin S, Yang S, Chang WC. STIM1 Overexpression Promotes Colorectal Cancer Progression, Cell Motility and COX-2 Expression. Oncogene. 2015;34:4358–67. doi: 10.1038/onc.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Z, Liu X, Feng B, Liu N, Wu Q, Han Y, Nie Y, Wu K, Shi Y, Fan D. STIM1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer. Oncogene. 2015;34(37):4808–20. doi: 10.1038/onc.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gueguinou M, Harnois T, Crottes D, Uguen A, Deliot N, Gambade A, Chantome A, Haelters JP, Jaffres PA, Jourdan ML, Weber G, Soriani O, Bougnoux P, Mignen O, Bourmeyster N, Constantin B, Lecomte T, Vandier C, Potier-Cartereau M. SK3/TRPC1/Orai1 complex regulates SOCE-dependent colon cancer cell migration: a novel opportunity to modulate anti-EGFR mAb action by the alkyl-lipid Ohmline. Oncotarget. 2016;7(24):36168–36184. doi: 10.18632/oncotarget.8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fedida-Metula S, Feldman B, Koshelev V, Levin-Gromiko U, Voronov E, Fishman D. Lipid rafts couple store-operated Ca2+ entry to constitutive activation of PKB/Akt in a Ca2+/calmodulin-, Src- and PP2A-mediated pathway and promote melanoma tumor growth. Carcinogenesis. 2012 doi: 10.1093/carcin/bgs021. [DOI] [PubMed] [Google Scholar]
- 57.Sun J, Lu F, He H, Shen J, Messina J, Mathew R, Wang D, Sarnaik AA, Chang WC, Kim M, Cheng H, Yang S. STIM1- and Orai1-mediated Ca2+ oscillation orchestrates invadopodium formation and melanoma invasion. J Cell Biol. 2014;207(4):535–48. doi: 10.1083/jcb.201407082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umemura M, Baljinnyam E, Feske S, De Lorenzo MS, Xie LH, Feng X, Oda K, Makino A, Fujita T, Yokoyama U, Iwatsubo M, Chen S, Goydos JS, Ishikawa Y, Iwatsubo K. Store-operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS One. 2014;9(2):e89292. doi: 10.1371/journal.pone.0089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hooper R, Zaidi MR, Soboloff J. The heterogeneity of store-operated calcium entry in melanoma. Sci China Life Sci. 2016;59(8):764–9. doi: 10.1007/s11427-016-5087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanisz H, Saul S, Muller CS, Kappl R, Niemeyer BA, Vogt T, Hoth M, Roesch A, Bogeski I. Inverse regulation of melanoma growth and migration by Orai1/STIM2-dependent calcium entry. Pigment Cell Melanoma Res. 2014;27(3):442–53. doi: 10.1111/pcmr.12222. [DOI] [PubMed] [Google Scholar]
- 61.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 62.Ridley AJ. Life at the leading edge. Cell. 2011;145(7):1012–22. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Yang S, Huang XY. Ca2+ Influx through L-type Ca2+ Channels Controls the Trailing Tail Contraction in Growth Factor-induced Fibroblast Cell Migration. J Biol Chem. 2005;280(29):27130–7. doi: 10.1074/jbc.M501625200. [DOI] [PubMed] [Google Scholar]
- 64.Sun J, Lin S, Keeley T, Yang S. Disseminating Melanoma Cells Surf on Calcium Waves. Mol Cell Oncol. 2015;2(4) doi: 10.1080/23723556.2014.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nurnberg A, Kitzing T, Grosse R. Nucleating actin for invasion. Nature reviews. Cancer. 2011;11(3):177–87. doi: 10.1038/nrc3003. [DOI] [PubMed] [Google Scholar]
- 66.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. The Journal of cell biology. 2009;185(1):11–9. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 68.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160(2):267–77. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Madsen CD, Sahai E. Cancer dissemination--lessons from leukocytes. Developmental cell. 2010;19(1):13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 70.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Current opinion in cell biology. 2008;20(2):235–41. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 71.Murphy DA, Courtneidge SA. The 'ins' and 'outs' of podosomes and invadopodia: characteristics, formation and function. Nature reviews. Molecular cell biology. 2011;12(7):413–26. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell. 2011;19(3):372–86. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. Journal of cell science. 2012;125(Pt 3):724–34. doi: 10.1242/jcs.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5(8):711–9. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 75.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nature cell biology. 2007;9(8):893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 76.Yang N, Tang Y, Wang F, Zhang H, Xu D, Shen Y, Sun S, Yang G. Blockade of store-operated Ca(2+) entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer letters. 2012 doi: 10.1016/j.canlet.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 77.Diez-Bello R, Jardin I, Salido GM, Rosado JA. Orai1 and Orai2 mediate store-operated calcium entry that regulates HL60 cell migration and FAK phosphorylation. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbamcr.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 78.Kohno T, Matsuda E, Sasaki H, Sasaki T. Protein-tyrosine kinase CAKbeta/PYK2 is activated by binding Ca2+/calmodulin to FERM F2 alpha2 helix and thus forming its dimer. Biochem J. 2008;410(3):513–23. doi: 10.1042/BJ20070665. [DOI] [PubMed] [Google Scholar]
- 79.Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118(Pt 17):3829–38. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 80.Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nature cell biology. 2004;6(10):977–83. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 81.Bhatt A, Kaverina I, Otey C, Huttenlocher A. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. Journal of cell science. 2002;115(Pt 17):3415–25. doi: 10.1242/jcs.115.17.3415. [DOI] [PubMed] [Google Scholar]
- 82.Carragher NO, Levkau B, Ross R, Raines EW. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125(FAK), paxillin, and talin. J Cell Biol. 1999;147(3):619–30. doi: 10.1083/jcb.147.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377(6544):75–9. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 84.Sjaastad MD, Nelson WJ. Integrin-mediated calcium signaling and regulation of cell adhesion by intracellular calcium. Bioessays. 1997;19(1):47–55. doi: 10.1002/bies.950190109. [DOI] [PubMed] [Google Scholar]
- 85.Tsai FC, Seki A, Yang HW, Hayer A, Carrasco S, Malmersjo S, Meyer T. A polarized Ca2+, diacylglycerol and STIM1 signalling system regulates directed cell migration. Nat Cell Biol. 2014;16(2):133–44. doi: 10.1038/ncb2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsai FC, Meyer T. Ca2+ pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Curr Biol. 2012;22(9):837–42. doi: 10.1016/j.cub.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brundage RA, Fogarty KE, Tuft RA, Fay FS. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science. 1991;254(5032):703–6. doi: 10.1126/science.1948048. [DOI] [PubMed] [Google Scholar]
- 88.Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 1999;400(6742):382–6. doi: 10.1038/22578. [DOI] [PubMed] [Google Scholar]
- 89.Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457(7231):901–5. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seals DF, Azucena EF, Jr, Pass I, Tesfay L, Gordon R, Woodrow M, Resau JH, Courtneidge SA. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7(2):155–65. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Research. 2006;66(6):3034–43. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 92.Yamaguchi H, Yoshida S, Muroi E, Kawamura M, Kouchi Z, Nakamura Y, Sakai R, Fukami K. Phosphatidylinositol 4,5-bisphosphate and PIP5-kinase Ialpha are required for invadopodia formation in human breast cancer cells. Cancer science. 2010;101(7):1632–8. doi: 10.1111/j.1349-7006.2010.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. The Journal of cell biology. 2009;186(4):571–87. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. Journal of cell science. 2009;122(Pt 17):3015–24. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 95.Steffen A, Le Dez G, Poincloux R, Recchi C, Nassoy P, Rottner K, Galli T, Chavrier P. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Current biology : CB. 2008;18(12):926–31. doi: 10.1016/j.cub.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 96.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xia J, Wang H, Huang H, Sun L, Dong S, Huang N, Shi M, Bin J, Liao Y, Liao W. Elevated Orai1 and STIM1 expressions upregulate MACC1 expression to promote tumor cell proliferation, metabolism, migration, and invasion in human gastric cancer. Cancer Lett. 2016;381(1):31–40. doi: 10.1016/j.canlet.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 98.Schaar A, Sukumaran P, Sun Y, Dhasarathy A, Singh BB. TRPC1-STIM1 activation modulates transforming growth factor beta-induced epithelial-to-mesenchymal transition. Oncotarget. 2016 doi: 10.18632/oncotarget.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu Y, Zhang S, Niu H, Ye Y, Hu F, Chen S, Li X, Luo X, Jiang S, Liu Y, Chen Y, Li J, Xiang R, Li N. STIM1 accelerates cell senescence in a remodeled microenvironment but enhances the epithelial-to-mesenchymal transition in prostate cancer. Sci Rep. 2015;5:11754. doi: 10.1038/srep11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114(1):33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 104.Hoth M. CRAC channels, calcium, and cancer in light of the driver and passenger concept. Biochim Biophys Acta. 2016;1863(6 Pt B):1408–17. doi: 10.1016/j.bbamcr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 105.Vashisht A, Trebak M, Motiani RK. STIM and Orai proteins as novel targets for cancer therapy. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol. 2015;309(7):C457–69. doi: 10.1152/ajpcell.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhan ZY, Zhong LX, Feng M, Wang JF, Liu DB, Xiong JP. Over-expression of Orai1 mediates cell proliferation and associates with poor prognosis in human non-small cell lung carcinoma. Int J Clin Exp Pathol. 2015;8(5):5080–8. [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu H, Zhang H, Jin F, Fang M, Huang M, Yang CS, Chen T, Fu L, Pan Z. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget. 2014;5(11):3455–71. doi: 10.18632/oncotarget.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, Smart CE, Brown MA, Kenny PA, Roberts-Thomson SJ, Monteith GR. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther. 2011;10(3):448–60. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- 109.Chang WC, Lee CH, Hirota T, Wang LF, Doi S, Miyatake A, Enomoto T, Tomita K, Sakashita M, Yamada T, Fujieda S, Ebe K, Saeki H, Takeuchi S, Furue M, Chen WC, Chiu YC, Chang WP, Hong CH, Hsi E, Juo SH, Yu HS, Nakamura Y, Tamari M. ORAI1 genetic polymorphisms associated with the susceptibility of atopic dermatitis in Japanese and Taiwanese populations. PLoS One. 2012;7(1):e29387. doi: 10.1371/journal.pone.0029387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Benzerdjeb N, Sevestre H, Ahidouch A, Ouadid-Ahidouch H. Orai3 is a predictive marker of metastasis and survival in resectable lung adenocarcinoma. Oncotarget. 2016 doi: 10.18632/oncotarget.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dubois C, Vanden Abeele F, Lehen'kyi V, Gkika D, Guarmit B, Lepage G, Slomianny C, Borowiec AS, Bidaux G, Benahmed M, Shuba Y, Prevarskaya N. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell. 2014;26(1):19–32. doi: 10.1016/j.ccr.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 112.Aytes A, Mollevi DG, Martinez-Iniesta M, Nadal M, Vidal A, Morales A, Salazar R, Capella G, Villanueva A. Stromal interaction molecule 2 (STIM2) is frequently overexpressed in colorectal tumors and confers a tumor cell growth suppressor phenotype. Molecular carcinogenesis. 2012;51(9):746–53. doi: 10.1002/mc.20843. [DOI] [PubMed] [Google Scholar]
- 113.Ruano Y, Mollejo M, Ribalta T, Fiano C, Camacho FI, Gomez E, de Lope AR, Hernandez-Moneo JL, Martinez P, Melendez B. Identification of novel candidate target genes in amplicons of Glioblastoma multiforme tumors detected by expression and CGH microarray profiling. Mol Cancer. 2006;5:39. doi: 10.1186/1476-4598-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Y, Guo B, Xie Q, Ye D, Zhang D, Zhu Y, Chen H, Zhu B. STIM1 Mediates Hypoxia-Driven Hepatocarcinogenesis via Interaction with HIF-1. Cell Rep. 2015;12(3):388–95. doi: 10.1016/j.celrep.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 115.Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37(3):535–40. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 116.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 117.Dupont G, Combettes L, Bird GS, Putney JW. Calcium oscillations. Cold Spring Harb Perspect Biol. 2011;3(3) doi: 10.1101/cshperspect.a004226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bird GS, Hwang SY, Smyth JT, Fukushima M, Boyles RR, Putney JW., Jr STIM1 is a calcium sensor specialized for digital signaling. Curr Biol. 2009;19(20):1724–9. doi: 10.1016/j.cub.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wedel B, Boyles RR, Putney JW, Jr, Bird GS. Role of the store-operated calcium entry proteins Stim1 and Orai1 in muscarinic cholinergic receptor-stimulated calcium oscillations in human embryonic kidney cells. J Physiol. 2007;579(Pt 3):679–89. doi: 10.1113/jphysiol.2006.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. The Journal of physiology. 2008;586(13):3043–54. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Siddiqui TA, Lively S, Vincent C, Schlichter LC. Regulation of podosome formation, microglial migration and invasion by Ca(2+)-signaling molecules expressed in podosomes. J Neuroinflammation. 2012;9:250. doi: 10.1186/1742-2094-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schmidt S, Liu G, Liu G, Yang W, Honisch S, Pantelakos S, Stournaras C, Honig A, Lang F. Enhanced Orai1 and STIM1 expression as well as store operated Ca2+ entry in therapy resistant ovary carcinoma cells. Oncotarget. 2014;5(13):4799–810. doi: 10.18632/oncotarget.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kondratska K, Kondratskyi A, Yassine M, Lemonnier L, Lepage G, Morabito A, Skryma R, Prevarskaya N. Orai1 and STIM1 mediate SOCE and contribute to apoptotic resistance of pancreatic adenocarcinoma. Biochim Biophys Acta. 2014;1843(10):2263–9. doi: 10.1016/j.bbamcr.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 124.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, Madesh M. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest. 2013;123(2):887–902. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nature immunology. 2008;9(4):432–43. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ma J, McCarl CA, Khalil S, Luthy K, Feske S. T-cell-specific deletion of STIM1 and STIM2 protects mice from EAE by impairing the effector functions of Th1 and Th17 cells. Eur J Immunol. 2010;40(11):3028–42. doi: 10.1002/eji.201040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nature immunology. 2008;9(1):81–8. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 129.Braun A, Gessner JE, Varga-Szabo D, Syed SN, Konrad S, Stegner D, Vogtle T, Schmidt RE, Nieswandt B. STIM1 is essential for Fcgamma receptor activation and autoimmune inflammation. Blood. 2009;113(5):1097–104. doi: 10.1182/blood-2008-05-158477. [DOI] [PubMed] [Google Scholar]
- 130.Vaeth M, Zee I, Concepcion AR, Maus M, Shaw P, Portal-Celhay C, Zahra A, Kozhaya L, Weidinger C, Philips J, Unutmaz D, Feske S. Ca2+ Signaling but Not Store-Operated Ca2+ Entry Is Required for the Function of Macrophages and Dendritic Cells. J Immunol. 2015;195(3):1202–17. doi: 10.4049/jimmunol.1403013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6(2):130–40. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 132.Munoz E, Valero RA, Quintana A, Hoth M, Nunez L, Villalobos C. Nonsteroidal anti-inflammatory drugs inhibit vascular smooth muscle cell proliferation by enabling the Ca2+-dependent inactivation of calcium release-activated calcium/orai channels normally prevented by mitochondria. J Biol Chem. 2011;286(18):16186–96. doi: 10.1074/jbc.M110.198952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Parekh AB. Store-operated CRAC channels: function in health and disease. Nature reviews. Drug discovery. 2010;9(5):399–410. doi: 10.1038/nrd3136. [DOI] [PubMed] [Google Scholar]