Abstract
Almost all polymerase II transcripts undergo alternative pre-mRNA splicing. Here, we review the functions of alternative splicing events that have been experimentally determined. The overall function of alternative splicing is to increase the diversity of mRNAs expressed from the genome. Alternative splicing changes proteins encoded by mRNAs, which has profound functional effects. Experimental analysis of these protein isoforms showed that alternative splicing regulates binding between proteins, between proteins and nucleic acids as well as between proteins and membranes. Alternative splicing regulates the localization of proteins, their enzymatic properties and their interaction with ligands. In most cases, changes caused by individual splicing isoforms are small. However, cells typically coordinate numerous changes in ‘splicing programs’, which can have strong effects on cell proliferation, cell survival and properties of the nervous system. Due to its widespread usage and molecular versatility, alternative splicing emerges as a central element in gene regulation that interferes with almost every biological function analyzed.
Keywords: Alternative Splicing, pre-mRNA, gene function
1.0 Introduction
The comparison of mRNA with genomic sequences in the late 1970s showed that prior to the export into the cytosol, viral sequences are removed from the pre-mRNA and the remaining sequences are joined together (Berget et al., 1977; Chow et al., 1977). It was quickly found that almost all mammalian polymerase II transcripts undergo this process, called pre-mRNA splicing. Due to splicing, only a small fraction of sequences from the primary transcripts are joined together and exported as exons into the cytosol, forming the mature mRNA. The majority of intervening sequences (introns) remain in the nucleus where they are subsequently degraded (reviewed in (Sharp, 2005)). It is now clear that the vast majority of pre-mRNAs contain exons that can be alternatively included into the mature mRNA or removed from it, which is called alternative splicing.
Frequently transcripts contain several alternative exons and their usage can be combined, largely increasing the diversity of the mRNA expressed from the genome and giving alternative splicing a central role in forming complex organisms. Alternative splicing patterns constantly change under physiological conditions, allowing an organism to respond to changes in the environment by determining which part of the genome it expresses. Most of the changes in alternative splicing are studied in artificial experimental systems, but alternative exon usage changes in real life scenarios. The stress of exams on medical students causes a change in alternative pre-mRNA splicing of the phosphatidylinositol 3-kinase-related protein kinase (SMG-1). This change may have later effects on nonsense-mediated RNA decay and the p53 pathway (Kurokawa et al., 2010).
Alternative splicing can play a role even before life and after death. The importance of alternative splicing before fertilization is illustrated by Nitric Oxide Synthase 1 where splicing isoforms are involved in controlling the erectile function (Hurt et al., 2006). The role after death is shown by the poor meat quality of turkeys that underwent transport and heat stress prior to slaughtering. This stress changes the splicing patterns of ryanodine receptors, ultimaltey leading to an increase of water content in the meat and lowering the quality (Strasburg and Chiang, 2009).
A change in alternative splicing can lead to human diseases, as summarized in section 3.1.4. However, changes in alternative splicing can be exploited for useful purposes, as they could be part of a pest control approach that generates male flies with a splicing defect eliminating female offspring (Fu et al., 2007).
Most of the splicing isoforms are only known through sequence comparison. The realization of alternative splicing's importance resulted in more functional studies of alternative exons reviewed here. This paper extends an earlier review published about seven years ago (Stamm et al., 2005) and shows impressive progress in the field. Functions of alternative exons published prior to 2005 are not covered here (but can be found in (Stamm et al., 2005)). They are summarized in Supplemental Figure 1.
Despite more than 20,000 publications dealing with alternative splicing, we still do not know the function of most alternative exons. However, key features emerge: most changes caused by alternative splicing are subtle and often hard to detect, changes in alternative splicing of different genes are controlled by ‘splicing programs’ centered around 50-300 regulatory proteins that work in combination. These splicing programs controlling different genes can have drastic physiological effects.
We will first give a brief summary of alternative splicing regulation and then describe functions of alternative exons that were experimentally determined during the past seven years.
2.0 Mechanism of alternative splicing
An alternative exon can only exert a function on the protein level after it is recognized by the splicing machinery and included in the mRNA. We therefore briefly summarize the mechanism of alternative pre-mRNA splicing. Previous work resulted in an explosion of mechanistical insights into alternative splicing regulation, which has been covered in excellent reviews (Matlin et al., 2005; Li et al., 2007b; Graveley, 2009; Wahl et al., 2009). Readers interested in a more basic introduction to alternative splicing and RNA are referred to specialized books covering the subject (Elliott and Ladomery, 2011; Stamm et al., 2012).
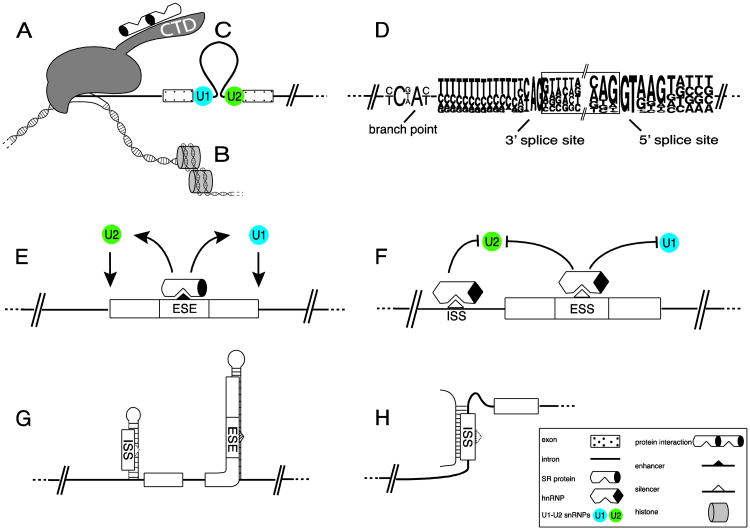
Human exons are between 50 and 300 nt in length, averaging about 137 nt (Berget, 1995). Thus they represent short sequences of the pre-mRNA that are surrounded by larger introns, which have an average length of 3,400 nt (Deutsch and Long, 1999). A large macromolecular complex, the spliceosome, recognizes exons and removes the intervening sequences (introns) while the pre-mRNA is synthesized by RNA polymerase II in the nucleus. The spliceosome is composed of at least 170 proteins and five snRNAs (small nuclear RNAs) (Behzadnia et al., 2007). Exons are defined by three major sequence elements: the 5′ splice site, the 3′ splice site and the branch point (Figure 1). The spliceosome recognizes these elements and assembles in a stepwise manner on the nascent pre-mRNA. First, the U1 snRNP binds to the 5′ splice site, then splicing factor 1 binds to the branch point, which facilitates binding of the U2AF factor on the 3′ splice site. This E (early) complex is committed to the splicing reaction by the substitution of SF1 by U2 snRNP, resulting in the prespliceosomal A complex. Through the exchange and recruitment of more factors, the A complex is transformed into the splicosomal B complex that removes the intron and joins the exons in a transesterification reaction.
Figure 1. Control elements of alternative exons.
The usage of alternative exons is controlled by the combination of multiple factors. A: RNA polymerase II assembles splicing factors on its CTD, which can predetermine the splicing outcome of a nascent pre-mRNA. This example shows SR-proteins, but other factors assemble as well. B: Exon boundaries correlate with DNA nucleosome occupancy and alternative exon usage is influenced by chromatin marks. C: Large intron regions can loop out to bring exons together. D: An exon is defined by three crucial elements: the branch point, the 3′ splice site and the 5′ splice site, which follow consensus sequences indicated. E: Exons need additional factors to be recognized. These factors bind to exonic or intronic sequences. In this example, two SR-proteins bind to exonic enhancers (ESE: exonic splicing enhancer) and help stabilize binding of U2 and U1 snRNP. F: Exon usage can be repressed by exonic splicing silencers (ESS) and intronic splicing silencers (ISS) that prevent U1 or U2 snRNP binding. G: RNA secondary structures can mask exonic or intronic splicing enhancer and silencer, as proteins binding to these RNA elements typically recognize single stranded RNA. H: small RNAs can bind to splicing regulatory elements located on different mRNAs.
2.1 Common features of alternative exons determined by genome-wide analysis
Alternative spliced exons share the sequence features of constitutively used exons, but, in general, these sequences deviate more from the consensus sequences (i.e. are weaker), which implies a lowered affinity to the spliceosome resulting in reduced recognition. The branch point of constitutive exons is typically within 40 nt of the 3′ splice site, but this distance can be much larger in alternative exons, up to 400 nt. In these cases, there are no AG nucleotides between the branch point and the 3′ splice site, called the AG dinucleotide exclusion zone (Gooding et al., 2006). The higher phylogenetic conservation of branch points preceeding alternative exons reflects a difference between constitutive and alternative exons, which indicates that the cis-elements marking alternative exons are so weak they cannot tolerate more perturbations (Kol et al., 2005).
Similarly, splice sites and exonic enhancers are generally weaker in alternative exons (Wang et al., 2005b), an evolutionarily conserved feature (Garg and Green, 2007). This conservation indicates it is an evolutionary advantage for an exon to be used alternatively.
An important finding of genome-wide analysis was the discovery of evolutionary conserved intronic regions, which suggests deep intronic regions contribute to splicing regulation (Sugnet et al., 2006; Yeo et al., 2007), (Sironi et al., 2005; Venables, 2007; Voelker and Berglund, 2007). These motifs are typically not discovered by in vitro methods that can analyze only shorter sequences and could explain the mechanism of action of disease-causing deep intronic sequences. (Kashima et al., 2007; Spena et al., 2007; Davis et al., 2009).
2.2 Proteins regulating alternative splicing
The 5′ splice site, the 3′ splice site and the branch point sequences follow only loose consensus sequences. Therefore, additional factors are required for exon recognition, which are brought into play by RNA sequence elements that can be either exonic or intronic. These short sequences bind to proteins that stabilize the binding of U1, U2 or SF1 to form the A complex. Likewise, binding of proteins to splicing silencers that block A complex formation inhibit exon recognition. The proteotypical exon enhancing proteins are SR-proteins and contain a domain rich in arginine and serine, called RS-domain. In contrast, most hnRNPs bind to splicing silencers and inhibit exon recognition. As a general rule, SR-proteins and SR-domain containing proteins promote exon inclusion and hnRNPs antagonize exon inclusion. However, there are numerous exceptions to this rule, for example the GTPase Rac1, where a critical exon is regulated by the completion of two SR-proteins, SRp20 and ASF/SF2 (Goncalves et al., 2009) and the myosin phosphatase targeting subunit-1 (MYPT1), which is regulated by antagonism between the two hnRNPs PTB and TIA-1 (Shukla et al., 2005). Furthermore, SF2/ASF and htra2-beta, two SR-proteins cause exon skipping of several exons regulated by ceramide (Sumanasekera et al., 2012).
The active concentration of splicing regulatory proteins can be influenced by the cell through different expression levels or through sequestration in cellular organelles, which resulted in the concept that alternative splicing is regulated by the ratios of antagonistic splicing factors. PTB (polypyrimidine tract binding protein) is an hnRNP and a well-studied example of the regulation by antagonistic proteins. For example, PTB competes with RBM4 for a CU-rich regulatory element in alpha-tropomyosin (Lin and Tarn, 2005), with TIA1 for a U-rich cis-element in myosin phosphatase (Shukla et al., 2005) and in FAS (Izquierdo et al., 2005) with U2AF for regulation in the src gene (Sharma et al., 2005) and with SRp30c for regulation of the hnRNPA1 gene (Paradis et al., 2007). Antagonism can also occur among SR-proteins, for example between SRp20 and SF2/ASF in the rac1b gene (Goncalves et al., 2009). Splicing regulatory proteins cannot only antagonize each other, but can also enhance their action on an exon, where they act like coactivators. This is exemplified by Fox-3 and PSF that together promote usage of a neuron-specific exon in the myosin heavy chain (Kim et al., 2011).
The importance of the concentration of splicing factors is further highlighted by the fact that most splicing factors autoregulate their expression levels, often by generating inactive variants as seen in FOX proteins (Damianov and Black, 2010), SF2/ASF (Sun et al., 2010) and tra2-beta1 (Stoilov et al., 2004). As the splicing factors work within protein, RNA complexes, their action depends on the binding site's sequence context.
Global analysis of drosophila cells shows that different splicing factors control a variable number of pre-mRNAs and that many alternative exons are regulated by multiple trans-acting factors, suggesting the relative ratios of these factors control exon usage (Blanchette et al., 2005).
2.3 Emerging role of RNA in alternative splicing reaction
Despite the large progress that has been made in determining the regulation of model exons, our mechanistical understanding is not complete. The comparison between exonic splicing regulatory sequences and alternative splicing usage in human and chimpanzee shows that changes in cis-elements are not associated with alternative exon variations between the two species (Irimia et al., 2009), suggesting the existence of other regulatory factors.
The current models of splice site selection outlined in Figure 1 propose proteins as the main regulators. However, there are less than 50 proteins known that regulate alternative splicing and less than 300 proteins known that bind RNA (Chen and Manley, 2009; Graveley, 2009), which contrasts with the more than 2,500 identified transcription factors (Babu et al., 2004). RNA itself could be involved in this regulation and there is emerging evidence of a larger role of non-coding RNA in exon selection. These RNA elements can be located on the pre-mRNA and regulate the binding of U1 snRNP by stabilizing the interaction of U1 with competing 5′ splice sites (Yu et al., 2008). The RNAs can also be generated from other transcripts and then regulate pre-mRNAs (reviewed in (Khanna and Stamm, 2010)).
miRNAs are a well-studied group of small RNAs and were shown to influence alternative splicing. For example, miR-124 acts on the splicing regulator PTB (Makeyev et al., 2007) and influences PTB-dependent splicing events indirectly. miR-10a/10b, miR-28 and miR-505 target SF2/ASF (Verduci et al., 2010; Meseguer et al., 2011) and influence SF2/ASF dependent exons. Similarly, miR-23a/b regulates the expression of CUGBP and ETR-3-like proteins that control the majority of alternative splicing in the developing muscle (Kalsotra et al., 2010).
Longer RNAs that regulate alternative splicing include MALAT1 that regulates the phosphorylation of SR-proteins (Tripathi et al., 2010), as well as fragments of snoRNAs that regulate numerous alternative exons (Kishore et al., 2010).
RNA elements within a pre-mRNA influence splice site selection, often by influencing the secondary structure (reviewed by (Buratti and Baralle, 2004)). Most proteins regulating alternative splicing bind to single stranded RNA and changing the RNA structure can “mask” such binding sites by forming double-stranded RNA structures (Hiller et al., 2007b). The effect of secondary structure is well studied for the DSCAM pre-mRNA, where the structure of the pre-mRNA regulates alternative exon usage. In the DSCAM pre-mRNA, one alternative exon is chosen from 48 alternative exons by the formation of a double-stranded RNA structure between a conserved sequence in the pre-mRNA and the alternative exon (Graveley, 2005). The 5′ splice site of tau exon 10 is possibly determined by its sequestration in a secondary structure (Varani et al., 1999) that is possibly opened by the RNA helicase p68/DDX5 (Kar et al., 2011). However, there is experimental evidence that regulatory proteins contribute to exon 10 regulation by binding near the proposed secondary structure, which is currently investigated (Wang et al., 2011).
Finally, metabolites can change pre-mRNA structures. Three Neurospora crassa genes contain a thiamine aptamer in introns located at the 5′ end of genes involved in thiamine metabolism. Two of these introns have alternative 5′ splice sites. The thiamine aptamer sterically blocks one of the alternative 5′ splice sites in the absence of thiamine pyrophosphate. Upon binding of thiamine phosphate, the aptamer changes its conformation, making the alternative 5′ splice site accessible to the splicing machinery. Thus, a metabolite can change alternative splicing by changing the secondary structure of a pre-mRNA (Cheah et al., 2007).
Since not all short non-coding RNAs have been identified, it is likely that RNAs play a larger role in splice site selection than previously thought. A new cloning method provided evidence for widespread expression of dsRNAs in mouse brain, suggesting that pre-mRNA regulation by antisense RNAs is much more common than previously anticipated (Shen et al., 2011). tRNAs are another emerging regulation by RNA where the initiator tRNA, devoid of a loaded methionine, can regulate alternative splicing events that depend on the AUG start codon for their usage (Kamhi et al., 2010).
2.4 Alternative splicing is integrated with other mechanisms of gene expression
Alternative exon usage is coordinated with other events of gene expression, most notably transcription. Two models have been suggested for the mechanism: the recruitment model and the kinetic model. The recruitment model assumes splicing factors assemble at the CTD (carboxy terminal domain) of RNA polymerase II and are released onto the nascent pre-mRNA during transcription. As these factors influence splice sites in a concentration dependent manner, the pre-loading of the CTD influences alternative exon usage (Das et al., 2006; Das et al., 2007b). The kinetic model postulates that protein complexes need time to assemble on an exon, which leads to its recognition. Everything that slows down a polymerase would give more time for the recruitment of the regulatory complexes and would favor alternative exon usage, as these exons usually depend more strongly on auxiliary factors (reviewed in (Kornblihtt, 2006; Kornblihtt, 2007)). The kinetic model attained considerable attention as genome-wide studies showed that exons associate with different histone modifications than introns (Kolasinska-Zwierz et al., 2009). DNA that encodes constitutive exons shows a higher degree of nucleosome occupancy than alternative exons or introns (Tilgner et al., 2009; Chen et al., 2010). Furthermore, exons associate with distinct histone modifications that are at least partially evolutionarily conserved (Andersson et al., 2009; Kolasinska-Zwierz et al., 2009; Huff et al., 2010). These histone marks could contribute to the regulation of alternative splicing (Luco et al., 2010). (Reviewed in (Kornblihtt et al., 2009; Schor et al., 2010)).
Histone modifications are not the only epigenetic modifications associated with alternative splicing. For example, the methylation pattern of honeybees correlates with splice sites and, most interestingly, is regulated by the different intake of royal jelly in workers and queens during development (Lyko et al., 2010). Therefore, it becomes clear that epigenetic modifications correlate with alternative splicing and influence the process mechanistically. This should be kept in mind when analyzing the function of alternative exons, as the exon usage could simply reflect an overall change in chromatin structure that changes alternative splicing as a ‘side effect’.
2.5 Global analysis indicates that alternative exons are jointly regulated in networks of exons
The development of new technologies, especially genome-wide splicing arrays, deep-sequencing and CLIP (Cross link and immunoprecipitation) analysis (Moore and Silver, 2008; Liu and Elliott, 2010), and the emergence of high-throughput sequencing (Sultan et al., 2008) provided new insights into the global regulation of alternative exons and their function. For example, it was found that 76% of all genes are expressed during brain development, with a striking coordination of alternatively spliced exons that implies they are functionally relevant (Johnson et al., 2009). Similar coordinated changes in expression of alternative splicing were observed in a cell model of myogenic differentiation (Bland et al., 2010): during differentiation of a primary model of human erythrocytes (Yamamoto et al., 2009), during epithlial mesenchymal transition (Warzecha et al., 2010), during chemically induced cell death that results in a coordinated change of splicing of apoptotic factors (Moore et al., 2010) and after stimulation with insulin (Hartmann et al., 2009). The analysis of these model systems showed that numerous alternative exons changed to achieve a biological outcome simultaneously. Some of the exons changed under different experimental conditions could just be co-regulated, without a direct contribution to the phenotype.
Similar to functional networks of alternative exons, there are networks of exons regulated by a common factor. These exons could be identified by CLIP analysis that shows the in vivo binding profiles between a protein factor and RNAs, especially when coupled with microarray analysis that shows functional changes. In the CLIP method, an RNA binding protein is cross-linked in situ to the pre-mRNA, the complexes are immunoprecipitated, the RNA isolated and cloned or subjected to direct sequencing in HITS/CLIP. CLIP was pioneered for NOVA (Ule et al., 2003; Licatalosi et al., 2008; Ruggiu et al., 2009) and has since been applied to numerous other factors, including TIA-1 (Wang et al., 2010), hnRNPC (Konig et al., 2010), PTB (Xue et al., 2009; Llorian et al., 2010), SF2/ASF (Sanford et al., 2009) and FOX2 (Yeo et al., 2009). Functionally, the role of given splicing factors on alternative splicing have been determined by array analysis after knockdown of a splicing factor by siRNA. These experiments identified new target genes of splicing factors, as for SAM68 (Chawla et al., 2009).
The CLIP method allows detailed evolutionary comparisons. For example, the targets of the mammalian NOVA-1 and its drosphila ortholog Pasilla have been compared. Interestingly, NOVA-1 and Pasilla regulate a completely different set of genes in both organisms, but recognize exons with similarly arranged regulatory sequences. This indicates that sequences regulated by a splicing factor evolve rapidly, whereas the regulatory principle is highly conserved (Brooks et al., 2011).
Almost all genome-wide studies of alternative splicing are based on assays that detect changes in mRNA expression. As protein expression from RNA is regulated by a wide variety of mechanisms, such as miRNA action, nonsense-mediated decay and translational efficiency, it is important to test whether the proteome reflects the changes in alternative splicing. The emerging proteomic studies indicate that indeed the proteome reflects the diversity seen on the RNA level (Tress et al., 2008; Severing et al., 2011).
2.6 Outlook
Despite the large body of work accumulated in the past 10 years, more amazing features of the splicing process are being discovered. Trans-splicing, the connection of two different mRNAs, has been long known in trypanosomes, but there is evidence it can also occur in humans and drosphila (Horiuchi and Aigaki, 2006; Al-Balool et al., 2011). Related to trans-splicing, a rearrangement of exon order different from their genomic order has been observed (Dixon et al., 2005). Pre-mRNA splicing is strictly a nuclear event, but can occur in the cytosol of platelets, which are devoid of a nucleus (Denis et al., 2005). Finally, there is evidence that splicing can bypass the 3′ end formation signals and connect the RNA of two neighboring genes, which could create fusion transcripts from adjacent genes (Frith et al., 2007).
Most of the mechanistical insight into alternative splicing derived from biochemical experiments and transfection studies, where the behavior of millons of molecules is averaged. These studies are now being refined by single molecule analysis. By visualizing the binding of PTB on its target pre-mRNA, evidence was found that PTB stabilizes RNA loops that flank an exon (Cherny et al., 2010), which questions earlier studies that proposed PTB creates one larger loop containing the regulated exon (Wagner and Garcia-Blanco, 2001).
Most of the functional studies of splicing isoforms are performed in cell models that are easy to manipulate. To obtain a more physiological picuture, an increasing amount of mouse models has been generated (reviewed in (Moroy and Heyd, 2007)). These studies sometimes gave surprising results, for example, they generated a mouse line that showed an increased sensitivity to dioxin (Pohjanvirta, 2009). Similarly, mice expressing only selected mu-receptors responded to heroin, but not to the chemically related morphine, which reflects the preference of most human addicts (Pan et al., 2009). More challenging insights can be expected when functional studies move from cells to whole organisms in the future.
Tremendous progress has been made in determining the mechanisms of splice site selection and in determining the genome-wide regulation of alternative exons during the past years. The data suggest that most alternatively spliced isoforms are part of a program that changes the isoform repertoire of multiple genes. These coordinated changes have to be taken into account when analyzing the function of a given exon. It is possible that a functional change is not caused by a single exon, but by the coordinated change of multiple, coregulated alternative exons. The coregulated exons may not be apparent in an experiment. Now we can at least start to predict the regulation of exons based on their sequence elements and can determine exons that are coregulated by a splicing factor experimentally (Das et al., 2007a). The field can therefore start to analyze the physiological functions of coordinated changes in alternative splicing.
3.0 Function of alternative splicing
3.1 General principles
The overall function of alternative splicing is to increase the diversity of the mRNA expressed from the genome. Due to the combinatorial control mechanisms that regulate alternative exon recognition, splicing programs coordinate the generation of mRNA isoforms from multiple genes. Evolution can select some of these isoforms to fulfill defined functions. Other isoforms could simply represent co-regulated exons without any direct function. It is also possible that a specific isoform shows only a functional effect when expressed with other isoforms generated by a coordinated change in splicing. The magnitude of alternative splicing regulation ranges from subtle modifications of protein functions, for example in ion channels to making binary on/off switches, observed in apoptosis genes.
3.1.1 Genome-wide overview of functions
Genome-wide studies indicate protein-parts encoded by alternative exons are predominantly located in coiled regions on the outside of the protein. Therefore, alternative exons do not change the general structure of the protein, but mainly influence local structures on the protein surface (Wang et al., 2005d). Alternative exons are often located in unstructured protein regions, allowing the introduction of protein domains without disrupting the overall protein structure (Romero et al., 2006). Together, this indicates the major function of alternative splicing is to modify, but not to radically change the function of a protein. Evolutionary analysis supports this notion, as human-mouse comparison shows that short alternative exons preserving the reading frame are conserved between human and mouse, indicating that small, incremental changes are caused by alternative splicing (Zhang et al., 2007a).
3.1.2 Alternative splicing as a part of evolution
Alternative splicing is an evolutionarily old process and likely appeared in the common ancestor of eukaryotes (Irimia et al., 2007). The function of individual exons can be determined by evolutionary comparison (reviewed in (Keren et al., 2010)). All comparisons rely on the hypothesis that functionally important alternative exons will be conserved in evolution. In contrast, non-functional exons will be eliminated by purifying selection. A frequently used property of alternative exons is the symmetry of an exon. An exon is symmetric when it can be divided by three, which indicates it keeps the reading frame. In general, conserved alternative exons in reading frames are symmetrical, whereas exons in non-protein coding regions and species-specific exons show a higher fraction of non-symmetrical exons (Magen and Ast, 2005; Xing and Lee, 2005).
The amount of alternative splicing increases from invertebrates to vertebrates (Kim et al., 2007a), suggesting the generation of new alternative exons could be a driving force in evolution. A characteristic of primates is the occurrence of Alu elements that comprise about 10% of the human genome (Hasler et al., 2007). These Alu insertions can evolve into exons, which contribute to generating a more complex transcriptome in primates (Lev-Maor et al., 2003; Dagan et al., 2004).
Mouse-human comparison showed that about 11% of cassette exons are alternatively used in one species, but constitutively used in the other species. This demonstrates that alternative splicing contributes to species-specific differences (Claverie-Martin et al., 2005; Lei and Vorechovsky, 2005; Pan et al., 2005; Mola et al., 2007). Within one species, alternatively spliced exons are conserved mostly in the nervous system. In contrast, testis and cancer cell lines show the least amount of conserved alternative splicing. For testis, this could suggest a deliberate increase of variation in expression to allow for evolutionary selection. The diverse expression of alternative exons in cancer cells could be caused by a general breakdown of surveillance mechanism, but could also allow the selection of cancer cells likely to grow in an organism (Kan et al., 2005).
3.1.3 Splicing in individuals
Individuals belonging to the same species show differences in alternative splicing. When 250 exons from 22 human individuals were compared, 6 out of 70 alternative exons showed consistent differences among individuals (Hull et al., 2007). There are now numerous examples, where a certain haplotype contributes to differences in alternative splicing patterns (Hull et al., 2007; Douglas et al., 2009; Iwata et al., 2009) (reviewed in (Graveley, 2008)). These studies suggest that differences in alternative splicing patterns contribute to differences in gene expression and phenotypes among individuals. In the future it will be interesting to see how single nucleotide polymorphisms contribute to alternative splicing and differences among individuals.
3.1.4 Splicing in disease
Given the widespread functions of alternative splicing, it is not surprising that aberrant regulation of alternative splicing leads to human disease. This connection to human health is being increasingly recognized and has been covered in numerous reviews (Wang and Cooper, 2007; Kim et al., 2008a; Cooper et al., 2009; Tazi et al., 2009; Barta and Schumperli, 2010; Dhir and Buratti, 2010; Hallegger et al., 2010; Raponi and Baralle, 2010; Tazi et al., 2010; Ward and Cooper, 2010). In most cases, changes in alternative splicing are caused by point mutations, which have been collected in databases (Bechtel et al., 2008b; Bechtel et al., 2008a).
Changes of alternative splicing in cancer cells are well-studied, as a deregulation of alternative splicing is a hallmark of cancer (Klinck et al., 2008; Venables et al., 2009; Misquitta-Ali et al., 2011) (reviewed in (Kim et al., 2008b; David and Manley, 2010)). Several protein isoforms generated by alternative splicing are crucial for cancer progression and are the subject of experimental therapeutic intervention. For example, the RON tyrosine kinase gene can generate a constitutively active kinase due to the skipping of an alternative exon (Collesi et al., 1996). The exon, controlled by the splicing factor SF2/ASF, determines the epithelial to mesenchymal transition, which is the invasiveness of the cancer cells (Ghigna et al., 2005). It is now investigated whether manipulating this splicing event changes tumor progression.
Most tumors rely on anerobic glycolysis for their energy requirements, which acidifies the surrounding media (Gatenby et al., 2006). Therefore tumors have a lower requirement of oxygen and a different expression profile than glycolytic enzymes. One of these enzymes is pyruvate kinase, which is upregulated in some cancer cells (Christofk et al., 2008). Currently it is controversial whether a splice variant or the total pyruvate kinase is upregulated (Bluemlein et al., 2011), but functional studies showed a reversal from the embryonic to the adult splicing forms of pyruvate kinase slows cancer growth in mouse models (Christofk et al., 2008). The deregulation of pyruvate kinase splicing could indicate a cancer-specific program, as the trans-acting factors regulating the splicing event are under the control of c-myc (David et al., 2010) (Clower et al., 2010).
The lower pH of the tumor's microenvironment influences alternative splicing by changing the intracellular localization of numerous splicing factors. Most splicing factors are nuclear under steady state conditions, but they shuttle between the nucleus and the cytosol, typically driven by phosphorylation (Stamm, 2008). For example, under hypoxia (Daoud et al., 2002) and chemically induced acidic conditions (Hirschfeld et al., 2009), the splicing factor tra2-beta1 accumulates in the cytosol and is depleted in the nucleus. As a result, the splicing of its target genes is altered. Important for cancer, the matricellular protein Cyr61 (cysteine rich 61) that promotes metastasis changes its splicing pattern to an isoform supporting metastasis (Hirschfeld et al., 2009). Therefore, changes in the tumor microenvironment could contribute to the deregulation of alternative splicing in cancer.
Lists of alternative exons deregulated in different cancer cells have been compiled in the literature (see for example (Xu and Lee, 2003; Klinck et al., 2008; Venables et al., 2009)).
Several neurological diseases are characterized by changes in alternative splicing. One of the best-investigated diseases is Myotonic Dystrophy type 1 (DM1). There, a CTG expansion in the 3′ untranslated region of the DMPK gene causes a sequestration of two splicing regulatory proteins: CUGBP1 and MBNL1. As a result, a network of alternative splicing events is changed, which causes abnormatilies in heart development and skeletal muscle (reviewed (Ranum and Cooper, 2006; Poulos et al., 2011)).
3.2 Overall changes of cellular properties
The influence of splice variants on global cellular processes has been studied using transfection assays that either overexpress or remove a splicing isoform. The readout of these experiments is typically cell proliferation, which can have a significant physiological relevance, for example in cancer. However, the detailed molecular mechanisms for the observed effect remain unclear.
Well-studied examples include apoptosis, where alternative splicing can act like an on/off switch for several genes encoding pro-apoptotic or anti-apoptotic enzymes. For example, alternative inclusion of parts of the active center of caspase 3 generates pro- and anti-apoptotic forms (Vegran et al., 2005). However, the overall contribution of alternative splicing to apoptosis is more complex, as splice variants can increase (Mosley and Keri, 2006) or inhibit apoptosis (Lee et al., 2010a). The role of splicing isoforms is not absolute, but depends on the cell type where the isoforms are expressed (Caldas et al., 2007) (Figure 2, Table 1).
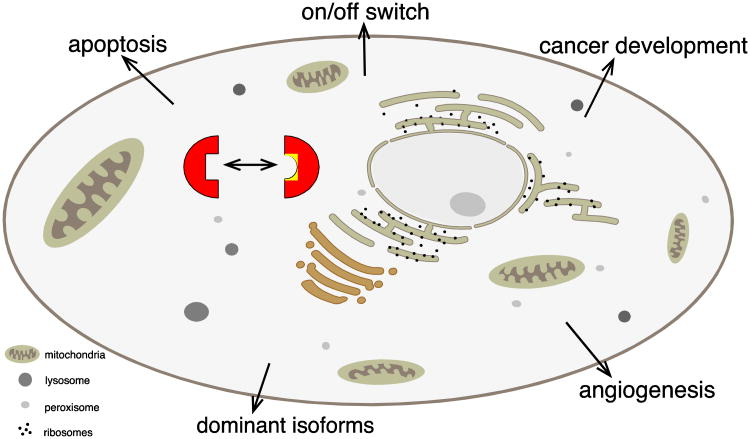
Figure 2. Overall changes in cellular properties due to alternative splicing.
A eukaryotic cell is schematically depicted. Alternative splicing changes protein isoforms (red half squares) by introducing new protein sequences (yellow) that are encoded by alternative exons. Changes in global cellular processes are arranged similar to Table 1 that lists specific examples.
Table 1. Cellular processes changed by alternative splicing.
| A. Apoptosis | ||
|---|---|---|
| Name | Function | Reference |
| Birc2 (baculoviral IAP repeat containing 2) Mus musculus | Exon in the CARD (Caspase activation and recruitment domain) domain increases anti-apoptotic activity | (Mosley and Keri, 2006) |
| BIRC5 (baculoviral IAP repeat containing 5) | Alternative splicing generates two isoforms, which interact with the wild-type and regulate proliferation and apoptosis | (Caldas et al., 2005) |
| BIRC5 (baculoviral IAP repeat containing 5) | BIRC5 exon 2B inclusion causes loss of anti-apoptotic potential | (Vegran et al., 2005) |
| CASP10 (Caspase 10, apoptosis-related cysteine peptidase) | Truncated protein generated by new exon inclusion enhances NF-kappaB activity | (Wang et al., 2007) |
| CASP3 (caspase 3, apoptosis-related cysteine peptidase) | Deletion of CASP3 exon 6 in the catalytic site generates a protein with anti-apoptotic function | (Vegran et al., 2005) |
| CASP3 (caspase 3, apoptosis-related cysteine peptidase) | Exon skipping rises a short isoform and increases chemoresistance | (Vegran et al., 2006) |
| CASP6 (Caspase 6, apoptosis-related cysteine peptidase) | Alternative spliced isoform inhibits the wild-type protein activation | (Lee et al., 2010a) |
| CFLAR (CASP8 and FADD-like apoptosis regulator) | The CFLAR longer form lacking a cysteine residue is inactive and inhibits apoptosis | (Sharp et al., 2005) |
| CMTM8 (CKLF-like MARVEL transmembrane domain containing 8) | Deletion of exon 2 induces apoptosis | (Li et al., 2007a) |
| DAPK1 (death-associated protein kinase 1) | Alternative splicing in the kinase domain generates a new isoform, which induces wild-type isoform destabilization | (Lin et al., 2009a) |
| HYAL1 (hyaluronoglucosaminidase 1) | Exon skipping induces cell cycle arrest and apoptosis | (Lokeshwar et al., 2006) |
| KLF6 (Kruppel-like factor 6) | Different 5′ site use causes cell apoptosis and reduces cell proliferation | (Hanoun et al., 2010) |
| MADD (MAP-kinase activating death domain) | Exon 13 and 16 inclusion generates a pro-apoptotic form inhibiting cell proliferation | (Mulherkar et al., 2006; Mulherkar et al., 2007) |
| OLR1 (oxidized low density lipoprotein (lectin-like) receptor 1) | Exon skipping has anti-apoptotic effect | (Mango et al., 2005) |
| RIPK2 (receptor-interacting serine-threonine kinase 2) | Exon 2 skipping leads to a form lacking kinase domain and caspase activity | (Krieg et al., 2009) |
| TP73 (tumor protein p73) | Alternative splicing isoforms in C-terminus region, alpha and beta, have anti- and pro-apoptotic function, respectively | (Nyman et al., 2005) |
| B. Competition between isoform functions (switch function) | ||
| Name | Function | Reference |
| GHRH (growth hormone releasing hormone) | Intron retention rises a truncated protein that is a dominant negative | (McElvaine and Mayo, 2006) |
| Hrh3 (histamine receptor H3) Mus musculus | Alternative splicing creates different C-terminus proteins that act as a dominant negative | (Bakker et al., 2006) |
| NPPB (Natriuretic peptide B) | Exon-skipped isoform attenuates expression and secretion of wild-type isoform | (Torrado et al., 2010) |
| PPARG (peroxisome proliferator-activated receptor gamma) | Exon inclusion gives rise a dominant negative form | (Kim et al., 2006) |
| SLC6A2 (solute carrier family 6 (neurotransmitter transporter, noradrenalin), member 2) | Short isoform has dominant negative effect on long isoform | (Sogawa et al., 2010) |
| SLC6A3 (solute carrier family 6 (neurotransmitter transporter, dopamine), member 3) | Short isoform has dominant negative effect on long isoform | (Sogawa et al., 2010) |
| Vipr2 (vasoactive intestinal peptide receptor 2) Mus musculus | Alternatively spliced isoform is a competitive interactant of wild-type | (Huang et al., 2006b) |
| C. Cancer/ Cell proliferation | ||
| Name | Function | Reference |
| AR (androgen receptor) | Exon gives rise to a constitutive ligand-independent form and improves therapy-resistant prostate cancer | (Dehm et al., 2008) |
| CCND1 (Cyclin D1) | Alternative splicing generates a protein lacking exon 5 and enhances cell invasiveness | (Kim et al., 2009) |
| CD83 (CD83 molecule) | Alternative splicing of two exons in transmembrane and cytosolic domains inhibit T cell proliferation | (Dudziak et al., 2005) |
| CD99 (CD99 molecule) | Deletion in cytoplasmatic domain generates a truncated protein and enhances tumor malignancy | (Scotlandi et al., 2007) |
| CD99 (CD99 molecule) | Alternative splicing generates a short intracytoplasmatic domain and promotes breast cancer | (Byun et al., 2006) |
| Cyld (cylindromatosis (turban tumor syndrome)) Mus musculus | Splice variant causes an increase of B cell survival | (Hovelmeyer et al., 2007) |
| DNMT3B (DNA (cytosine-5-)-methyltransferase 3 beta) | Exon 5 deletion creates a new isoform expressed in cancer cells and contributes to the genomic DNA methylation defects | (Gopalakrishnan et al., 2009) |
| ERBB4 (v-erb-a erythroblastic leukemia viral oncogene homolog 4 (avian) | Exon skipping in phosphotidylinositol-3 kinase binding site enhances proliferation | (Muraoka-Cook et al., 2009) |
| ESR2 (estrogen receptor 2 (ER beta) | Exon 6 is anti-proliferative | (Treeck et al., 2007) |
| ESR2 estrogen receptor 2 (ER beta) | Exon inclusion has an anti-tumor effect | (Treeck et al., 2008) |
| GLI1 (GLI family zinc finger 1) | Exon 3 and part of exon 4 are protective against transforming process | (Lo et al., 2009) |
| H2AFY (H2A histone family, member Y) | Alternative splicing generates two isoforms, one of which blocks cell growth | (Novikov et al., 2011) |
| KLF6 (Kruppel-like factor 6) | Alternatively spliced isoform enhances cell proliferation | (Narla et al., 2005) |
| MET (met proto-oncogene (hepatocyte growth factor receptor) | Exon 14 exclusion in the juxtamembrane domain enhances oncogenic activity | (Lee et al., 2006) |
| MITF (microphthalmia-associated transcription factor) | Alternative exon encoding six amino acids reduces DNA synthesis and influences proliferation | (Bismuth et al., 2005) |
| MST1R (macrophage stimulating 1 receptor (c-met-related tyrosine kinase) | Exon skipping enhances cancer proliferation | (Xu et al., 2005) |
| P2RX7 (purinergic receptor P2X, ligand-gated ion channel, 7) | Splicing variant generates a protein lacking ligand binding and promotes cervical cancer proliferation | (Feng et al., 2006b) |
| PPARG (peroxisome proliferator-activated receptor gamma) | Alternative splicing generates a protein lacking ligand binding domain that promotes tumorigenesis | (Sabatino et al., 2005) |
| PRLR (Prolactin receptor) | Short isoform downregulates long isoform and prevents growth of breast cancer cells | (Tan and Walker, 2010) |
| PUF60 (poly-U binding splicing factor 60KDa) | Exon 2 contributes to c-myc repression and cancer progression | (Matsushita et al., 2006) |
| RUNX1 (runt-related transcription factor 1) RUNX1T1 (runt-related transcription factor 1; translocated to, 1 (cyclin D-related) | Exon induces leukemogenesis | (Yan et al., 2006) |
| TNFSF13B (tumor necrosis factor (ligand) superfamily, member 13b) | Exon 3 has proliferative activity | (Gavin et al., 2005) |
| VCAN (versican) | Alternative splice isoforms contain CS (chondroitin sulfate) beta or alpha domain and enhance or inhibit cell proliferation, respectively | (Sheng et al., 2005) |
| VEGFA (vascular endothelial growth factor A) | Alternative splicing gives rise to a shorter isoform that induces cell proliferation | (Herve et al., 2005) |
| D. Angiogenesis | ||
| Name | Function | Reference |
| BIRC5 (baculoviral IAP repeat containing 5) | Exon 3 modulates angiogenesis | (Caldas et al., 2007) |
| F3 (coagulation factor III (thromboplastin, tissue factor)) | Splicing variant codes for a different C-terminus protein, which enhances angiogenesis | (van den Berg et al., 2009) |
| VASH1 (vasohibin 1) | Alternative splicing isoform generates a shorter protein with anti-angiogenic effect | (Kern et al., 2008) |
| E. Dominant effect on cellular functions | ||
| Name | Function | Reference |
| CHEK2 (checkpoint kinase 2) | Heterodimerization of splice variants decrease wild-type activity | (Berge et al., 2010) |
| EMR2 (egf-like module containing, mucin-like, hormone receptor-like 2) | An 11-amino acid deletion in exon 12 inhibits wild-type protein | (Davies et al., 2007) |
| Gipr (gastric inhibitory polypeptide receptor) Mus musculus | Intron retention generates a truncated protein with a dominant negative effect | (Harada et al., 2008a) |
| OLR1 (oxidized low density lipoprotein (lectin-like) receptor 1) | Splicing isoform with different C-terminus interacts with the full-length ORL1 and inhibits its binding activity | (Biocca et al., 2008) |
| Robo3 (roundabout, axon guidance receptor, homolog 3 (Drosophila) Mus musculus | Two isoforms, generated by differential intron retention, have opposite functions in regulating commissural axon guidance | (Chen et al., 2008) |
| SIAH1 (seven in absentia homolog 1 (Drosophila) | A partial deletion of exon 2 generates a truncated protein and inhibits the full-length isoform | (Mei et al., 2007) |
| F. Nervous system | ||
| Name | Function | Reference |
| Birc5 (baculoviral IAP repeat-containing protein 5) Mus musculus | Alternative splicing isoforms are expressed differently after mouse sciatic nerve injury | (Amiri et al., 2009) |
| CadN (cadherin-N) Drosophila melanogaster | Exon is involved in photoreceptor neuron development | (Nern et al., 2005) |
| DNM3 (dynamin 3) | Exon leads to aberrant synaptogenesis | (Gray et al., 2005) |
| fru (fruitless) Drosophila melanogaster | Different transcripts lead to sex-specific aggressiveness and dominance | (Vrontou et al., 2006) |
| Lrp8 (low density lipoprotein receptor-related protein 8, apolipoprotein e receptor) Mus musculus | Exon has neuron protective function | (Beffert et al., 2006) |
| Myh10 (myosin, heavy chain 10, non-muscle) Mus musculus | Two alternative splice isoforms regulate mouse brain development | (Ma et al., 2006) |
| Ncam1 (neural cell adhesion molecule 1) Mus musculus | The Ncam-Vase (variable alternative splice exon) isoform is responsible for the changing of structural plasticity of hippocampus and poor learning performance | (Qin et al., 2005) |
| NLGN1 (neuroligin 1) | Splice variant generates a shorter protein, which induces rapid presynaptic differentiation | (Lee et al., 2010b) |
| Pcdh1 (protocadherin 1) Mus musculus | Alternative splicing isoform levels regulate learning and memory functions in the brain | (Fukuda et al., 2008) |
| G. Miscellaneous | ||
| Name | Function | Reference |
| CCKBR (cholecystokinin B receptor) | Intron 4 retention encodes an additional 69 a.a. within the third intracellular loop domain, resulting in plasma membrane dissociation and rapid resensitization | (Chao et al., 2005) |
| CXCR3 (chemokine (C-X-C motif) receptor 3) | Splice variants activate different signaling pathways | (Datta et al., 2006) |
| Dnm2 (dynamin 2) Mus musculus | Different splicing variants of DNM2 display different effectiveness at rescuing p75 export from the TGN | (Liu et al., 2008) |
| FGF8 (fibroblast growth factor 8 (androgen-induced) | An alternative 3′ splice site generates a longer protein and induces mesoderm formation | (Fletcher et al., 2006) |
| KCNMA1 (potassium large conductance calcium-activated channel, subfamily M, alpha member 1) | Exon insert is responsible for sensitivity of hypoxia | (McCartney et al., 2005) |
| MAD2L1 (Mad2, mitotic arrest deficient-like 1) | Expression of inefficient splice isoform result in decreased expression of efficient isoform | (Yin et al., 2006) |
| MAPKAP1 (mitogen-activated protein kinase associated protein 1) | Different splicing isoforms regulated the TORC2 (mammalian target of rapamycin complex 2) signaling | (Frias et al., 2006) |
| Mhc (Myosin heavy chain) Drosophila melanogaster | Alternative splicing of exon 15 correlates with different muscle physiological properties | (Suggs et al., 2007) |
| Mhc (Myosin heavy chain) Drosophila melanogaster | Exon 7 plays an important role in establishing fiber speed and flight performance | (Swank et al., 2006) |
| MYH11 (myosin, heavy chain 11, smooth muscle) | Exon encoding seven amino acids regulates contractive properties of myosin heavy chain | (Low et al., 2006) |
| MYO1B (myosin 1B) | Alternative splicing of the LCBD (light-chain binding domain) results in proteins with increased range of force sensitivities | (Laakso et al., 2010) |
| Nos1 (nitric oxide synthase 1 (neuronal) Mus musculus | Alternatively spliced form controls erectile activity | (Hurt et al., 2006) |
| POU5F1 (POU class 5 homeobox 1) | Alternative splicing N-terminus isoform is crucial for totipotent cells | (Cauffman et al., 2006) |
| PYCARD (PYD and CARD domain containing) | Exon 2 skipping in PGR (proline and glycine-rich) domain may affect the structure of the protein and its activation | (Matsushita et al., 2009a) |
| Slc12a1 (solute carrier family 12 (sodium/potassium/chloride transporters), member 1) Mus musculus | Exon controls part of macula densa signaling | (Oppermann et al., 2006) |
| SNAP25 (synaptosomal-associated protein, 25kDa) | Isoforms regulate exocytotic burst and membrane secretion | (Nagy et al., 2005) |
| TBXA2R (thromboxane A2 receptor) | Two C-terminus spliced isoforms could heterodimerize and auto-regulate the gene expression | (Sasaki et al., 2006) |
| TPM1 (tropomyosin 1 (alpha) | Three alternatively spliced exons alter the folding of tropomyosin | (Kremneva et al., 2006) |
| TREX2 (three prime repair exonuclease 2) | No differences between two spliced isoforms | (Chen et al., 2007) |
The role of intronic SNPs (single nucleotide polymorphism) in modifying alternative splicing is an emerging area of research. Its potential importance is illustrated by a deep intronic SNP in the oxidized low-density lipoprotein (lectin-like) receptor 1 gene. The SNP is associated with exon 5 skipping that correlates with acute coronary syndromes, where the new shorter variant called LOXIN protects cells from apoptosis. Although it is not fully clear how the SNP influences splice site selection mechanistically, the example shows that inherited variations of alternative splicing contribute to complex human diseases (Mango et al., 2005).
Alternative splicing isoforms have been implicated in almost all aspects of cancer development: cell proliferation, cell invasiveness, methylation defects and chemotherapy resistance. It is often difficult to discern whether a particular splice site selection causes the observed effect or is merely the result of the cancerous transformation. In addition to functional examples in Table 1B, extensive isoform profiling of cancer cells has been performed ((Xu and Lee, 2003; Klinck et al., 2008; Venables et al., 2009)). Related to the role in cancer development, the role of isoforms in angiogenesis that controls blood supplies to tumors has been documented.
Similar to molecularly well-characterized examples, different mechanisms are used to modify cellular processes, for example destabilization of the protein (Lin et al., 2009a), addition of inserts in functional domains (Vegran et al., 2005; Mosley and Keri, 2006; Krieg et al., 2009) or activation of signaling pathways (Datta et al., 2006).
Finally, it should be pointed out that not all splicing isoforms have a measurable effect, for example there was no observable difference between TREX2 isoforms (Chen et al., 2007). As such negative results are difficult to publish, they are likely under reported.
3.3 Change in transcription factors
Transcription is regulated by the formation of protein complexes on the promoter sequences of DNA. As these protein complexes are assembled by combining several weak interactions to form one high-affinity complex, they offer multiple regulation points for alternative splicing variants that generally have moderate influence on binding to DNA or other proteins in the complex (Figure 3).
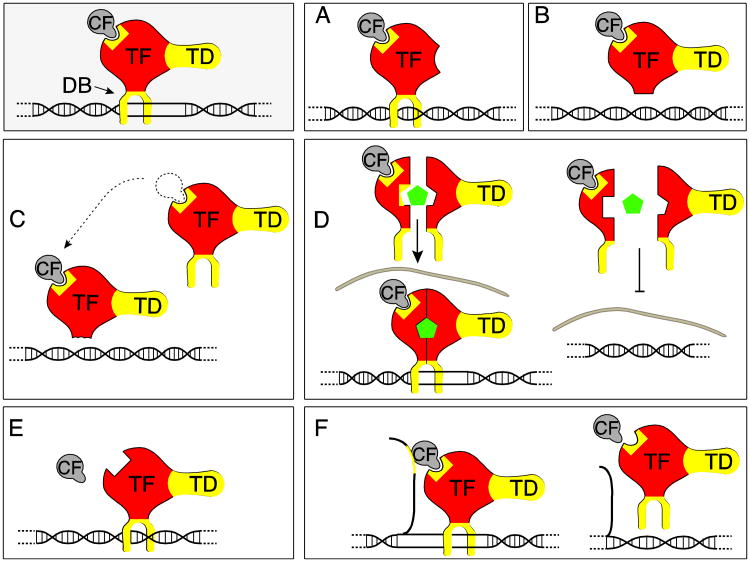
Figure 3. Changes in transcription factors.
The gray box schematically depicts the general structure of a transcription factor complex. TD: transactivation domain; CF: Co-factor; TF: core transcription factor, DB: DNA binding domain. Components of transcription factors that undergo alternative splicing with well-studied effects are indicated in yellow. The numbering (A-F) of the Figure refers to Table 2 that lists specific examples.
A. Change in transactivation domain
B. Loss in DNA binding
C. Generation of dominant negative isoforms, here a factor that lost its DNA binding domain, but can still compete for cofactors (indicated by arrow).
D. Alternative splicing of nuclear recpetors. In this example, the ligand binding domain is subject to alternative splicing (yellow area) that interferes with ligand binding (green pentagon), which regulates translocation of the dimerized receptor complex into the nucleus (indicated by gray nuclear membrane).
E. The binding of transcriptional cofactors is regulated by alternative splicing.
F. ncRNAs can direct transcription factors to DNA, which is regulated by alternative splicing of the ncRNAs.
Changes in alternative splicing alter the structure of the transactivation domain, which influences the activation of RNA polymerase II in either a negative or positive way (Table 2, A). Skipping of alternative exons that encode the DNA binding domain leads to transcription factors that have lost their ability to bind to promoters. These isoforms can either be inactive (Figure 3, B) or act in a dominant negative way. This is usually achieved by replacing the transcription factor isoform with a DNA binding domain from the transcription factor complex (Figure 3, C). Nuclear receptors are particularly well-studied. They typically form after multimerization of cytosolic receptors initiated by ligand binding that leads to their translocation into the nucleus. The protein interaction in the multimerization domains is subject to regulation by alternative splicing and usually leads to inactive variants. However, typical for alternative splicing regulation, alternative exons do not just inactivate a factor, but can also modulate its activity (Goodson et al., 2005). Since such effects are subtler, they are likely under reported (Figure 3, D).
Table 2. Transcription factors.
| A. Change in Transactivation domain | ||
|---|---|---|
| Name | Function | Reference |
| GLI2 (GLI family zinc finger 2) | Premature stop codons in repressor domain results in higher transactivation activity | (Speek et al., 2006) |
|
Hsf (heat shock factor) Drosophila melanogaster |
Heat/cold stress changes transactivation and dimerization domains, resulting in different transcriptional activity | (Fujikake et al., 2005) |
| MEF2C (myocyte enhancer factor 2C) | Skipping of a domain increases the transactivation properties | (Zhu et al., 2005) |
| MYB (v-myb myeloblastosis viral oncogene homolog) | Modulation of transcriptional activities through at least six alternative exons, different splicing regulation in lymphoma and during hematopoetic differentiation | (O'Rourke and Ness, 2008) |
| NFKBIZ (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, zeta) | Skipping of an exon in the central part of the transactivation domain generates isoforms not able to transactivate | (Motoyama et al., 2005) |
| NR1I2 (nuclear receptor subfamily 1, group I, member 2) | Skipping of 37 nt in the ligand binding domain generates dominant negative isoform that no longer transactivates | (Lin et al., 2009b) |
| POU1F1 (POU class 1 homeobox 1) Ovis aries | Insertion in transactivation domain reduces transactivation | (Bastos et al., 2006) |
| Prpf19 (pre-mRNA-processing factor 19) Mus musculus | Exon inclusion generates an isoform that represses a transcription activator | (Urano et al., 2006) |
| RBM14 (RNA binding motif protein 14) | Modulates transactivation activity through different C terminus | (Iwasaki et al., 2005) |
| Sim2 (single-minded homolog 2 (Drosophila)) Mus musculus | Change in a repressive domain leads to transactivation | (Metz et al., 2006) |
| THRB (thyroid hormone receptor, beta) | Increased binding to a transcriptional co-activator after thyroid hormone binding | (Wan et al., 2005) |
| ZFPM2 (zinc finger protein, multitype 2) | Regulation of a repression motif in a tissue specific way, variant can no longer perform cofactor-mediated transactivation | (Dale et al., 2007) |
| ZNF318 (zinc-finger protein 318) | Usage of zinc-finger motif changes a repressor to a transactivator | (Tao et al., 2006) |
| B. DNA-binding | ||
| Name | Function | Reference |
| Dmrt1 (doublesex and mab-3 related transcription factor 1) Mus musculus | DM domain (zinc finger-like DNA-binding domain), variant acts dominant negative | (Lu et al., 2007) |
| ESRRG (estrogen-related receptor gamma) | Loss of a part of the DNA-binding domain and transactivation properties | (Kojo et al., 2006) |
|
FOXP2 (forkhead box P2) |
Loss of DNA-binding domain leads to dominant negative form due to sequestration | (Vernes and Fisher, 2009) |
| hth (Homothorax) Drosophila melanogaster | Change in DNA-binding domain | (Noro et al., 2006) |
|
PAX3 (paired box 3) PAX7 (paired box 7) |
Isoforms have different affinity to DNA, resulting in different transcriptional activity | (Du et al., 2005) |
| PAX6 (paired box 6) | Change in DNA-binding domains | (Azuma et al., 2005) |
| REL (v-rel reticuloendotheliosis viral oncogene homolog (avian) | Exon 9 encodes for an inhibitory domain, its deletion increases DNA-binding activity | (Leeman et al., 2008) |
|
SMAD2 (SMAD family member 2) |
Change in DNA-binding activity | (Dunn et al., 2005) |
| Taf1 (TATA-binding protein-associated factor 1) Drosophila melanogaster | Change in DNA-binding domain | (Metcalf and Wassarman, 2006) |
|
TCF7l2 (transcription factor 7-like 2 (T-cell specific, HMG-box)) |
Change in C clamp DNA recognition motif creates isoforms with different transactivation domains | (Weise et al., 2010) |
| TFCP2 (transcription factor CP2) | Change in DNA-binding domain | (Kang et al., 2005) |
| THRA (thyroid hormone receptor, alpha) | Deletion of hinge domain abolishes DNA-binding and causes sequestration of active full-length form in the cytosol | (Casas et al., 2006) |
| ZGPAT (zinc finger, CCCH-type with G patch domain) | Loss of Zn-finger domain abolishes DNA-binding and creates dominant negative form | (Yu et al., 2010) |
| C. Loss of regulation: Dominant negative forms/constitutively active | ||
| Name | Function | Reference |
| FOXO4 (forkhead box O4) | Dominant negative activity due to N-terminal changes | (Lee et al., 2008) |
| NR1I3 (nuclear receptor subfamily 1, group I, member 3) Sus scrofa | Dominant negative effect due to altered ligand binding domain | (Gray et al., 2009) |
| POU1F1 (POU class 1 homeobox 1) | Insertion into transactivation domain generates dominant negative isoform | (Jonsen et al., 2009) |
| PTCH1 (patched 1) | Alternative exon usage inserts premature stop codon. Factors truncated due to premature stop codon and act as dominant negative | (Uchikawa et al., 2006) |
| SREBPF1 (sterol regulatory element binding transcription factor 1) | Premature stop codon abolishes transmembrane domain and generates constitutively active isoform | (Harada et al., 2008b) |
| TP53 (tumor protein p53) | Dominant negative form due to partial of core domain | (Garcia-Alai et al., 2008) |
| D. Change in nuclear receptors/ligand binding | ||
| Name | Function | Reference |
| carm1 (coactivator-associated arginine methyltransferase 1) Xenopus laevis | Alternative usage exon 14 generates isoforms with opposite effects on ligand-mediated transcription | (Matsuda et al., 2007) |
|
NCOR2 (nuclear receptor corepressor 2) |
Alternative splicing generates two isoforms with different affinities for nuclear receptors | (Goodson et al., 2005) |
|
NR1H3 (nuclear receptor subfamily 1, group H, member 3) |
Exon encodes part of the ligand binding domain and its skipping abolishes receptor activity | (Chen et al., 2005b) |
|
Nr1i2 (nuclear receptor subfamily 1, group I, member 2) Mus musculus |
Loss of 41 aa adjacent to ligand binding pocket represses function of full-length form | (Matic et al., 2010) |
| THRB (thyroid hormone receptor, beta) | Stop codon in ligand binding domain creates inactive receptor/transcription factor | (Tagami et al., 2010) |
| E. Change in intracellular localization | ||
| Name | Function | Reference |
|
ESRRB (estrogen-related receptor beta) |
Change in F-domain that alters nuclear localization and ligand binding | (Zhou et al., 2006) |
| ESRRB (estrogen-related receptor beta) | Alternative sequence at the C-terminus influence intra-nuclear mobility and activation of reporter genes | (Bombail et al., 2010) |
| Gtf2i (general transcription factor II-I) Mus musculus | On/off switch after change in localization due to cell stimulation | (Hakre et al., 2006) |
| NOSTRIN (nitric oxide synthase trafficker) | Truncated isoform has nuclear rather then cytoplasmic localization and negatively regulates transcription of its own gene | (Wiesenthal et al., 2009) |
| F. Different interaction with proteins of transcriptional machinery | ||
| Name | Function | Reference |
| BRD8 (bromodomain containing 8) | Splicing regulates protein interaction motif that determines binding to different receptors | (Hosoya et al., 2008) |
| CARM1 (coactivator-associated arginine methyltransferase 1) | Isoform of the transcriptional co-activator CARM1 with different C-terminus associates with U1C and affects 5′ splice site selection | (Ohkura et al., 2005) |
| IKBKG (inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase gamma) | Change in coiled-coil domain needed for protein-protein interaction results in ligand response change | (Hai et al., 2006) |
|
POU1F1 (POU class 1 homeobox 1) |
Homodimerization of splice variants decreases their efficiency as transcriptional activators | (Sporici et al., 2005) |
| TAF4B (TAF4b RNA polymerase II, TATA box binding protein (TBP)-associated factor, 105kDa) | Loss of entire TFIID-associating domain through generation of a stop codon | (Wu et al., 2005) |
| TBX5 (T-box 5) | Premature stop codon generates different C-terminus with altered ability to bind to co-factors | (Georges et al., 2008) |
| thraa (thyroid hormone receptor, alpha) Danio rerio | Alternative C-terminus (F-domain) modulates interaction with co-activator | (Takayama et al., 2008) |
| G. NcRNA | ||
| Name | Function | Reference |
| DLX6-AS1 (DLX6 antisense RNA 1 (non-protein coding)) | Alternative splice form of non-coding RNA activates by binding to dlx-2 | (Feng et al., 2006a) |
| NR1H2 (nuclear receptor subfamily 1, group H, member 2) | Intronic sequence inserted into pre-mRNA generates RNA co-activator of receptor produced from the same gene, variant acts as RNA, not protein | (Hashimoto et al., 2009a) |
| H. Miscellaneous | ||
| Name | Function | Reference |
| CREM (cAMP responsive element modulator) | CREM isoforms encode either transcriptional activators or repressors. Deregulation of activator isoforms can lead to male infertility | (Blocher et al., 2005) |
| GPR56 (G protein-coupled receptor 56) | Splice variants of the receptor activate transcription factors | (Kim et al., 2010a) |
| Myod1 (myogenic differentiation 1) Danio rerio | Alternative exon present in chromatin modifying domain | (Fernandes et al., 2007) |
| NCOA1 (nuclear receptor coactivator 1) | Different alternative splicing in C-terminus region leads to different receptor- and ligand-specific effects | (Meijer et al., 2005) |
| ncor2 (nuclear receptor corepressor 2) Xenopus laevis | Loss of CoRNR box changes co-repressor activity | (Malartre et al., 2006) |
| NR4A2 (nuclear receptor subfamily 4, group A, member 2) | The frame shift generates new C-terminus that reduces transcriptional activity | (Michelhaugh et al., 2005) |
| PPARGC1A (peroxisome proliferator-activated receptor gamma, co-activator 1 alpha) | Protein is a co-activator of a nuclear receptor. In absence of the ligand, the interaction with the receptor is lower for the alternatively spliced, truncated form | (Zhang et al., 2009) |
| SRA1 (steroid receptor RNA activator 1) | Alternative splicing variants SRC-1a co-activates transcription from single GREs, whereas SRC-1e co-activates the transcription with multiple response elements containing promoters | (Meijer et al., 2005) |
| SRF (serum response factor (c-fos serum response element-binding transcription factor)) | Isoform auto-represses by binding to its own promoter | (Zhang et al., 2007b) |
| TNIP1 (TNFAIP3 interacting protein 1) | Changes transcriptional activation | (Shiote et al., 2006) |
Intracellular and intranuclear localization can be regulated by alternative splicing, which leads to inactive transcription factors when accumulated in the cytosol. The FOXP2 variants show a drastic example of such a regulation that also includes sequestration. The loss of the DNA binding domain results in the cytoplasmic localization of FOXP2 variants. Since these variants still contain a dimerization domain, they can form complexes in the cytosol, which downregulates the transcriptional activity of the full-length form (Vernes and Fisher, 2009).
The effect of alternative splicing is not limited to protein isoforms, but extends to ncRNA (non-coding RNA). ncRNAs bind to transcription factors where different ncRNA isoforms generated by alternative splicing can modulate the activity of a transcription factor (Feng et al., 2006a; Hashimoto et al., 2009a) (Figure 3, E).
Most of the studies were performed by transfection analysis. It is important to keep in mind that alternative splicing frequently occurs in a cell-type or tissue-specific way, as illustrated by the MYB gene. The MYB gene modulates transcriptional activities through at least six alternative exons, which are used differentially in lymphomas and during hematopoetic differentiation. This example shows how cell-type specific splice site selection can contribute to cell-type specific transcriptional programs (O'Rourke and Ness, 2008). Alternative splicing of transcription factor isoforms contributes to regulatable on/off switches that can be triggered by extracellular signals. For example, the TFII-I gene in resting cells generates two isoforms, the cytosolic delta isoform and the nuclear beta isoform. The beta isoform blocks activation of the c-fos gene. Upon growth factor stimulation, the beta isoform moves from the nucleus to the cytosol and the delta isoform from the cytosol to the nucleus. In the nucleus, the delta isoformactivates c-fos (Hakre et al., 2006). This example also illustrates that changes in transcriptional activity are only visible after cellular stimulation.
3.4 Change in localization of proteins
Alternative splicing can change the intracellular localization of proteins by altering localization signals, sequences for post-translational modification or interaction sites with other proteins (Figure 4). These changes can result in all or nothing effects, for example when an exon introduces a nuclear localization site (Miyake et al., 2005). More often these changes are gradual, where alternative exons shift the relative distribution of protein isoforms among cellular compartments, such as in the ING4 variants (Unoki et al., 2006). Proteins often acquire new functions in different compartments. For example, the lipin gene generates a nuclear and a cytoplasmatic variant due to inclusion of an alternative exon. The nuclear form acts as a transcription factor, whereas the cytosolic form has phosphatidate (PA) phosphatase activity (Han and Carman, 2010; Zhang et al., 2012) (reviewed in (Csaki and Reue, 2010)). In this gene, the alternative splicing of the crucial exon is regulated by the splicing factor tra2-beta1, which is downregulated in obese subjects (Pihlajamaki et al., 2011). This suggests a deregulation of a splicing factor can control fat metabolism by sending a key regulatory protein into different cellular compartments.
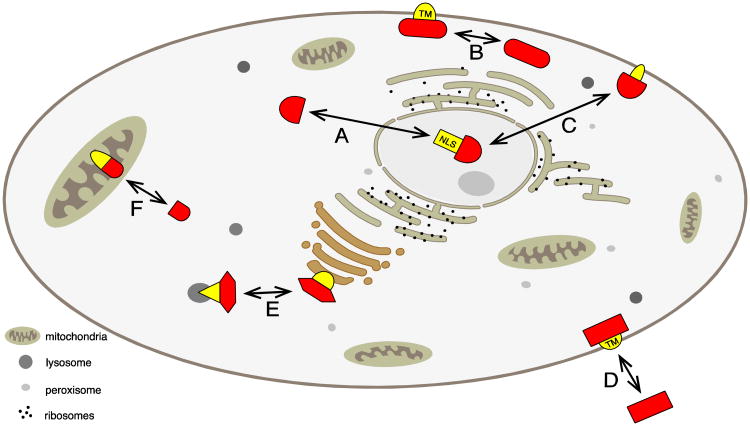
Figure 4. Changes in intracellular localization due to alternative splicing.
A eukaryotic cell is schematically shown. Proteins that undergo alternative splicing are shown in red, parts encoded by alternative exons are shown in yellow. The Figure is arranged similar to Table 3, in clockwise arrangement.
A. Change between cytosol and nucleus, typically by altering a nuclear localization signal (NLS).
B. Change between plasma membrane and cytosol, typically by changing a transmembrane region (TM).
C. Change between nucleus and membrane associated forms.
D. Generation of soluble, secreted forms, typically by changing a transmembrane region (TM).
E. Localization between different internal membranes.
F. Localization in the mitochondria, typically by regulating a mitochondrial-targeting signal (MTS).
Most of the studies investigating the intracellular localization of splice variants analyze used tagged cDNA, mostly GFP, myc and Flag tags. In contrast to pre-mRNAs, these cDNA constructs do not undergo nonsense-mediated decay. It is therefore crucial to test that the endogenous mRNAs are actually expressed as a protein.
A special case of localization is the association of proteins with membranes (Table 3, C-E). By deleting membrane-binding domains, alternative splicing can control membrane association of proteins. The soluble proteins can either accumulate in the cytosol or be secreted, depending on their membrane topology. This mostly results in a loss of function of the normally membrane-bound protein. However, the soluble proteins can have dominant effects, as shown in the MET oncogene, where a soluble, secreted form inhibited the signaling of the membrane-bound form (Tiran et al., 2008). Finally, the localization among different membrane compartments is regulated by alternative splicing, which leads to the accumulation of the variants in different cellular organelles (Table 3, D).
Table 3. Change in protein localization due to alternative splicing.
| A. Change between nucleus and cytosol | ||
|---|---|---|
| Name | Function | Reference |
| ATN1 (atrophin 1) | Different splice site acceptor causes two variants: the form with a glutamine residue is nuclear and the GLN-excluded is cytosolic | (Tadokoro et al., 2005) |
| Atxn7 (ataxin 7) Mus musculus | Alternative exon 12b increases cytosolic localization | (Strom et al., 2005) |
| BSX (brain-specific homeobox) | Alternatively spliced variant moves from the nucleus to the cytoplasm | (Chu and Ohtoshi, 2007) |
| CAMK2A (calcium/calmodulin-dependent protein kinase II alpha) | Exon encodes an NLS and locates the protein into the nucleus | (O'Leary et al., 2006) |
| CCND1 (Cyclin D1) | Exon 4 deletion causes nuclear retention | (Leveque et al., 2007) |
| FOXP2 (forkhead box P2) | Inclusion of an alternative exon generates proteins that leave the nucleus and aggregate in the cytosol | (Vernes et al., 2006) |
| GLI1 (GLI family zinc finger 1) | Full-length form is nuclear, N-terminal alternative splicing leads to cytosolic accumulation | (Shimokawa et al., 2008) |
| HNRNPA2B1 (heterogeneous nuclear ribonucleoprotein A2/B1) | The inclusion of exon 2 and exon 9 generates a predominantly nuclear form, skipping of the exons generates cytosolic forms | (Han et al., 2010) |
| ILF3 (interleukin enhancer binding factor 3, 90kDa) | Different C-termini isoforms: the long form is nuclear and the short form is cytoplasmatic | (Parrott et al., 2005) |
| ING4 (inhibitor of growth family, member 4) | Isoform V1 is predominantly nuclear, isoform V2 and V4 accumulate more in the cytosol, but are also nuclear | (Unoki et al., 2006) |
| Lpin1 (lipin 1) Mus musculus | Exon 7 skipping isoform moves from the nucleus to the cytoplasm | (Peterfy et al., 2005) |
| Mlf (Myelodysplasia/myeloid leukemia factor) Drosophila melanogaster | Splicing creates two variants with different C-termini. One is nuclear, one is both nuclear and cytosolic | (Martin-Lanneree et al., 2006) |
| Orc1 (origin recognition complex, subunit 1) Mus musculus | The short variant lacking part of exon 5 is retained in the cytosol. The full-length form is nuclear | (Miyake et al., 2005) |
| RBFOX1 (RNA binding protein, fox-1 homolog (C. elegans) 1) | Skipping of exon creates a nuclear isoform | (Lee et al., 2009) |
| SEPHS1 (selenophosphate synthetase 1) | One variant is localized at both the plasma and the nuclear membrane and the others are in the cytoplasm | (Kim et al., 2010b) |
| Smox (spermine oxidase) Mus musculus | Exon 6a encodes a nuclear signal domain; skipping causes a cytosolic localization | (Bianchi et al., 2005) |
| TRIM21 (tripartite motif containing 21) | Exon 4 skipping leads to isoform that is present in both the nucleus and the cytosol | (Wada et al., 2006) |
| U2AF1L4 (U2 small nuclear RNA auxiliary factor 1-like 4) | Variant lacking a nuclear localization signal is localized in the cytoplasm. The full-length isoform displays nucleo-cytoplasmic shuttling | (Heyd et al., 2008) |
| UBP1 (upstream binding protein 1 (LBP-1a)) | Alternative exon contains a NLS (nuclear localization signal) that localizes isoform into the nucleus. The isoform with the skipped exon is cytosolic | (Sato et al., 2005) |
| ZNF268 (zinc finger protein 268) | Gene regulated by multiple cassette exons. One splice variant lacking two cassette exons is predominantly nuclear, whereas other splice variants are mainly cytosolic | (Shao et al., 2006) |
| ZNF415 (zinc finger protein 415) | Splicing events create five forms. Only the form lacking KRAB (Krüppel-associated box) A box is nuclear | (Cheng et al., 2006) |
| B. Change in membrane association | ||
| Name | Function | Reference |
| Aqp4 (aquaporin 4) Rattus norvegicus | Lack of exon 2 causes intracellular localization, protein does not transport water. Exon 2 inclusion generates a membrane bound form that transports water | (Moe et al., 2008) |
| CA9 (carbonic anhydrase IX) | Exon skipping generates a truncated protein that accumulates in the cytosol and competes with the full-length protein for the regulation of the extracellular pH | (Malentacchi et al., 2009) |
| CADM1 (cell adhesion molecule 1) | Generation of a shorter soluble form (sCADM1) | (Hagiyama et al., 2009) |
| CD40 (CD40 molecule, TNF receptor superfamily member 5) | Skipping of exon 5 and/or exon 6 generates a soluble isoform that can have antagonistic effects | (Eshel et al., 2008) |
| CD55 (CD55 molecule, decay accelerating factor for complement (Cromer blood group) | Intron 7 retention generates a membrane-bound variant | (Osuka et al., 2006) |
| Crhr2 (corticotropin releasing hormone receptor 2) Mus musculus | The full-length protein is membrane-bound. Exon 6 skipping generates a soluble form | (Chen et al., 2005a) |
| CSF2RA (colony stimulating factor 2 receptor, alpha, low-affinity (granulocyte-macrophage) | Exon in juxtamembrane domain increases solubility of protein and decreases cell surface localization | (Pelley et al., 2007) |
| ECE1 (endothelin converting enzyme 1) | Exon 3 encodes a transmembrane domain. Exon skipping generates a cytosolic variant | (Meidan et al., 2005) |
| FLT1 (fms-related tyrosine kinase 1 (vascular endothelial growth factor/vascular permeability factor receptor) | Alternative splicing gives rise to two proteins: the shortest is soluble, the longest is transmembrane | (Thomas et al., 2007) |
| FRMD3 (FERM domain containing 3) | Exon 5 skipping creates a soluble isoform | (Seo et al., 2009b) |
| HPN (hepsin) | Exon encodes a transmembrane domain and causes cytosolic retention when skipped | (Li et al., 2005) |
| HRAS (v-Ha-ras Harvey rat sarcoma viral oncogene homolog) | Different C-termini variants, one variant is nuclear and cytoplasmic, the second variant is associated with the CTaSma membrane | (Jeong et al., 2006) |
| HTR7 (5-hydroxytryptamine (serotonin) receptor 7, adenylate cyclase-coupled) | C-terminal isoforms have different internalization behavior upon agonist presence | (Guthrie et al., 2005) |
| IL13RA2 (interleukin 13 receptor, alpha 2) | Exon 10 encodes a transmembrane domain, its skipping creates a soluble form | (Tabata et al., 2006) |
| IL17RD (interleukin 17 receptor D) | Alternative splicing generates two N-termini isoforms; the longer form is localized in the membrane, the shorter in the cytoplasm | (Rong et al., 2007) |
| IL6R (interleukin 6 receptor) | Exon skipping creates a cytoplasmic form of the receptor | (Chalaris et al., 2011) |
| LST1 (leukocyte specific transcript 1) | Exon 3 produces a membrane-bound form when present and a soluble form when absent | (Mulcahy et al., 2006) |
| MET (met proto-oncogene (hepatocyte growth factor receptor) | The splice variant lacks the transmembrane domain and acts as a dominant negative form | (Tiran et al., 2008) |
| OSMR (oncostatin M receptor) | The full-length form is membrane bound. A splice variant lacking part of exon 8 is soluble | (Diveu et al., 2006) |
| PROCR (protein C receptor, endothelial) | Loss of transmembrane domain creates a soluble receptor isoform that remains cytosolic | (Molina et al., 2008) |
| RGS5 (regulator of G-protein signaling 5) | Skipping of exon 2 and 3 generates an exclusively cytosolic form. The full-length form is also membrane bound | (Liang et al., 2005) |
| SLC6A2 (solute carrier family 6 (neurotransmitter transporter, noradrenalin), member 2) | Exon 15 causes expression at the cell surface, when exon is skipped the protein moves in the intracellular compartment | (Sogawa et al., 2007) |
| SYT8 (synaptotagmin VIII) | Exon 6 encodes a transmembrane domain and produces a cytosolic variant when skipped | (Monterrat et al., 2006) |
| TMEFF2 (transmembrane protein with EGF-like and two follistatin-like domains 2) | The full-length form is a membrane protein. The truncated variant is cytosolic | (Quayle and Sadar, 2006) |
| USH2A (Usher syndrome 2A (autosomal recessive, mild)) | Long isoforms encode transmembrane regions and are associated with the membrane, whereas shorter isoforms are cytosolic and the exon usage is cell-type specific | (Adato et al., 2005) |
| C. Change between nucleus and membrane | ||
| Name | Function | Reference |
| DLG1 (discs, large homolog 1 (Drosophila) | Exon insertion 2 (I2) variant localizes in the nucleus, exon insertion 3 (I3) variant localizes to the membrane | (Roberts et al., 2007) |
| ERBB4 (v-erb-a erythroblastic leukemia viral oncogene homolog 4 (avian) | Exon inclusion generates a protein that less efficiently translocates from the plasma membrane to the nucleus | (Sundvall et al., 2007) |
| STX1B (syntaxin 1B) | Loss of a C-terminal transmembrane region creates a nuclear isoform | (Pereira et al., 2008) |
| D. Creation of secreted forms | ||
| Name | Function | Reference |
| CD209 (CD209 molecule) | Exon 3 encodes a transmembrane domain, its skipping leads to a form that is cytoplasmatic rather than secreted | (Martinez et al., 2005) |
| CFHR4 (complement factor H-related 4) | The splice variant is a truncated protein and is secreted | (Jozsi et al., 2005) |
| CXCL16 (chemokine (C-X-C motif) ligand 16) | Variant lacking transmembrane and cytoplasmic domains is a secreted protein | (van der Voort et al., 2010) |
| EPOR (erythropoietin receptor) | Intron retention generates a shorter protein isoform that circulates in the blood and inhibits the function of full-length erythropoietin receptor located on the erythroblast's membrane | (Khankin et al., 2010) |
| ERBB3 (v-erb-b2 erythroblastic leukemia viral oncogene homolog 3 (avian)) | Variants lacking TM domain are secreted proteins that still promote growth | (Lin et al., 2008) |
| FGFR3 (fibroblast growth factor receptor 3) | A variant lacking exon 8 to 10 is secreted | (Tomlinson et al., 2005) |
| FLT1 (fms-related tyrosine kinase 1 (vascular endothelial growth factor/vascular permeability factor receptor)) | Secreted form functions as an inhibitor for full-length FLT1 receptor | (Thomas et al., 2010) |
| Il15 (interleukin 15) Mus musculus | A shorter variant lacking hydrophobic domain is not secreted and retained in the cytosol | (Nishimura et al., 2005) |
| IL27RA (interleukin 27 receptor, alpha) | Soluble isoform only encodes the extracellular region of the full-length receptor, but can functionally substitute the full-length receptor | (Hashimoto et al., 2009b) |
| LILRB1 (leukocyte immunoglobulin-like receptor, subfamily B (with TM and ITIM domains), member 1) | Soluble isoforms lacking transmembrane and cytoplasmic domains function as negative dominant regulators to full-length receptors | (Jones et al., 2009) |
| Nell2 (NEL-like 2 (chicken) Rattus norvegicus | Exon 3 encodes part of the secretion signal and its skipping leads to a variant that is not secreted | (Hwang et al., 2007) |
| Rxfp1 (relaxin/insulin-like family peptide receptor 1) Mus musculus | Exon 4 inclusion generates a truncated isoform that is secreted; the full-length form is membrane bound | (Scott et al., 2005) |
| SEL1L (sel-1 suppressor of lin-12-like (C. elegans)) | Exon skipping creates isoforms that are no longer in the endoplasmatic reticulum, but are secreted | (Cattaneo et al., 2009) |
| TNFRSF1B (tumor necrosis factor receptor superfamily, member 1B) | Skipping of two alternative exons leads to a soluble TNFRS1B receptor that is associated with greater insulin sensitivity | (Fernandez-Real et al., 2006) |
| E. Different internal membranes | ||
| Name | Function | Reference |
| CLDN10 (claudin 10) | Among the six variants, exon 4 inclusion generates isoforms localized in the cytoplasmic membrane, while exon 4 excluded isoforms are retained in the endoplasmic reticulum | (Gunzel et al., 2009) |
| ENOX2 (ecto-NOX disulfide-thiol exchanger 2) | The full-length variant is retained in internal membranes, the exon 4 skipped form into the plasma membrane | (Tang et al., 2007) |
| GDNF (glial cell derived neurotrophic factor) | Shorter transcript lacking the pro-domain region is localized mostly in vesicles, while the full-length transcripts primarily in the Golgi complex | (Lonka-Nevalaita et al., 2010) |
| GLG1 (golgi glycoprotein 1) | Splicing produces two C-terminal variants localizing either at the Golgi apparatus or the cell surface | (Ahn et al., 2005) |
| Grik3 (glutamate receptor, ionotropic, kainate 3) Mus musculus | Splicing generates two C-termini, one is expressed at the plasma membrane and one is retained in the endoplasmic reticulum | (Jaskolski et al., 2005) |
| LHCGR (luteinizing hormone/choriogonadotropin receptor) | The splicing variant lacking exon 9 does not localize at the cell surface and is degraded in the lysosome | (Minegishi et al., 2007) |
| Lhcgr (luteinizing hormone/choriogonadotropin receptor) Rattus norvegicus | The full-length is a transmembrane receptor; Exon 11 skipping generates an isoform retained in the endoplasmic reticulum | (Apaja et al., 2006) |
| MOG (myelin oligodendrocyte glycoprotein) | Exon 7 and 8 target proteins to cell surface, exon 10 inclusion variant is in the endoplasmic reticulum | (Boyle et al., 2007) |
| ninaD (neither inactivation nor afterpotential D) Drosophila melanogaster | The long form is localized at the cell surface, exon skipping generates forms found in the intracellular membrane | (Voolstra et al., 2006) |
| NPSR1 (neuropeptide S receptor 1) | Truncated isoforms caused by exon skipping are found in intracellular membranes, the full-length form is at the cell surface | (Vendelin et al., 2005) |
| Ntsr2 (neurotensin receptor 2) Rattus norvegicus | The full-length form is at the cell surface; a variant lacking two transmembrane domains is in intracellular membranes | (Perron et al., 2005) |
| SLC18A1 (solute carrier family 18 (vesicular monoamine), member 1) | The full-length form localizes to the membrane of secretory vesicles. Exon skipping generates a variant localized in the endoplasmic reticulum | (Essand et al., 2005) |
| SLC22A5 (solute carrier family 22 (organic cation/carnitine transporter), member 5) | Intron retention in the first extracellular loop causes retention of the protein in the endoplasmic reticulum | (Maekawa et al., 2007) |
| SLC30A2 (solute carrier family 30 (zinc transporter), member 2) | The shorter isoform is localized in the cytoplasma membrane and the longer isoform is primarily localized in the endosomal compartment | (Lopez and Kelleher, 2009) |
| SLC30A5 solute carrier family 30 (zinc transporter), member 5 | The short isoform is localized in the plasma membrane, the long isoform in the Golgi system | (Jackson et al., 2007) |
| F. Mitochondria and other cell parts | ||
| Name | Function | Reference |
| CCKBR (cholecystokinin B receptor) | Insertion in the third intracellular loop mainly causes intracellular localization of the receptor and decreases its amount on the plasma membrane | (Chao et al., 2005) |
| CS (citrate synthase) | Exon encodes mitochondrial localization signal | (Cheng et al., 2009) |
| CYP24A1 (cytochrome P450, family 24, subfamily A, polypeptide 1) | Alternative splicing produces a truncated protein that lacks the mitochondrial signal domain and is inactive | (Ren et al., 2005) |
| MOBP (myelin-associated oligodendrocyte basic protein) | Skipping of exon 3 leads to a mitochondrial form; the full-length variant is nuclear. In a different splicing event, the long isoform is targeted to the nucleus, the short isoform generated through an alternative 5′ splice site locates to the mitochondria | (Montague et al., 2005) |
| NUDT1 (nudix (nucleoside diphosphate linked moiety X)-type motif 1) | SNP in exon 2 reduces mitochondrial translocation efficiency | (Sakai et al., 2006) |
| NUDT6 (nudix (nucleoside diphosphate linked moiety X)-type motif 6) | Isoforms containing the mitochondrial targeting sequence are mitochondiral, others are nuclear and cytosolic | (Zhang et al., 2008) |
| PIF1 (PIF1 5′-to-3′ DNA helicase homolog (S. cerevisiae) | Exon in the C-terminus region directs protein from the nucleus into the mitochondria | (Futami et al., 2007) |
| SIRT3 (sirtuin 3) | The long isoform with mitochondrial localization sequence is predominantly located in mitochondria, while the shorter isoform lacking that sequence has lower mitochondrial expression | (Bao et al., 2010) |
| XPNPEP3 (X-prolyl aminopeptidase (aminopeptidase P) 3, putative) | Exon 3 encodes a stop codon and produces a cytosolic variant. The exon 3 skipped form is mitochondrial | (Ersahin et al., 2005) |
| G. Miscellaneous | ||
| Name | Function | Reference |
| CD6 (CD6 molecule) | Exon 5 skipping in the third SRCR (scavenger receptor cysteine-rich) domain causes a failure to localize in the immunological synapse | (Castro et al., 2007) |
| CRHR1 (corticotropin releasing hormone receptor 1) | CRHR1 splicing isoforms change localization and activity of the main isoform CRHR1α | (Zmijewski and Slominski, 2009) |
| CRHR2 (corticotropin releasing hormone receptor 2) | Variant lacking a transmembrane region is subject to degradation | (Evans and Seasholtz, 2009) |
| CTNS (cystinosin, lysosomal cystine transporter) | Full-length isoform is in lysosomes. Exon skipping deletes lysosomal targeting signal and creates an isoform localized in the plasma membrane, in lysosomes and in other cytosolic structures | (Taranta et al., 2008) |
| ERBB4 (v-erb-a erythroblastic leukemia viral oncogene homolog 4 (avian)) | Cytoplasmic domain differs in the four variants. Depending on exon usage, some variants are in endosomal vesicles and subject to endocytosis | (Sundvall et al., 2008) |
| FMNL1 (formin-like 1) | Intron retention interrupts C-terminal diaphanous auto-regulatory domain. This isoform is localized on cell membrane | (Han et al., 2009) |
| ING4 (inhibitor of growth family, member 4) | Variant containing a NoLS (nucleolar localization signal) localized in the tandem splice sites translocate from nucleus to nucleolus | (Tsai et al., 2008) |
| MAPK3 (mitogen-activated protein kinase 3) | Alternatively spliced isoform lacks cytosolic retention domain and localizes to the Golgi apparatus | (Shaul and Seger, 2006) |
| NOXO1 (NADPH oxidase organizer 1) | Different splice site in exon 3 gives rise to four proteins; two are within the intracellular membrane, one in the plasma membrane and the last in the nucleus | (Ueyama et al., 2007) |
| RXFP1 (relaxin/insulin-like family peptide receptor 1) | Alternative splicing produces different isoforms. One is localized at the cell surface, one is cytoplasmatic and the third is secreted | (Muda et al., 2005) |
| SLC1A2 (solute carrier family 1 (glial high affinity glutamate transporter), member 2) | Exon 9 skipped isoform lacks endoplasmic reticulum translocation motif and is not functional | (Lauriat et al., 2007) |
| SLC35A2 (solute carrier family 35 (UDP-galactose transporter), member A2) | One form is in the Golgi system and a second form in the endoplasmic reticulum | (Kabuss et al., 2005) |
| svr (silver) Drosophila melanogasgter | Alternative splicing generates different isoforms; the short variants are secreted; the long form with exon 8 is at the cell surface; the longest is found in the Golgi complex | (Kalinina et al., 2006) |
| TGME49_000320 (hypoxanthine-xanthine-guanine phosphoribosyltransferase) Toxoplasma gondii | Skipping generates a short cytosolic variant whereas the full-length protein is in the inner membrane complex | (Chaudhary et al., 2005) |
| WHSC1 (Wolf-Hirschhorn syndrome candidate 1) | Variants localized in the nucleus but not in the nucleoli | (Keats et al., 2005) |
A change in localization due to splice site selection can have severe physiological consequences. For example, unusual intron retention creates a soluble, secreted form of the erythropoietin receptor that is released into the blood where it competes with the full-length receptor, which contributes to erythropoietin resistance in end-stage kidney diseases (Khankin et al., 2010).
3.5 Change in enzymatic properties
Alternative splicing can change enzymatic properties (Figure 5). The best studied class of enzymes affected by alternative splicing are kinases, which are frequently inactivated by inclusion or deletion of alternative protein parts in their active center. In most cases, a change in usage of the alternative exon completely abolishes the activity (Table 4, A). However, in some cases the activity is only modulated, typically reduced (Ghosh et al., 2006) (Dolzhanskaya et al., 2006). Frequently, described enzymatic inactive forms are generated by premature stop codons. Care should be taken in analyzing these isoforms to ensure the shortened variants are actually expressed as mRNAs as they could be dregraded (Schneider et al., 2005), typically by NMD (nonsense mediated decay). Other mechanisms that influence the activity are the control of enzyme secretion or the control of substrate binding (Lira et al., 2008).
Figure 5. Change of enzymatic activity due to alternative splicing.
The alternative exon is shown as a red circle, parts encoded by alternative exons are shown in yellow. A: The enzyme converts a substrate (circle) into a product (star). B: Alternative splicing influences the substrate binding, which prevents substrate formation (striped star). C: Alternative splicing changes the catalytic center (short triangle), which prevents substrate formation (examples in Table 4A).
Table 4. Change of enzymatic properties due to alternative splicing.
| A. Loss of enzymatic activity | ||
|---|---|---|
| Name | Function | Reference |
| Acsbg1 (acyl-CoA synthetase bubblegum family member 1) Rattus norvegicus | The alternative exon 8 is essential for the fatty acyl-CoA synthetase activity of GR-LACS | (Li et al., 2006) |
| AGRN (agrin) | An eight amino acid insert at the B-side is necessary for the enzymatic activity | (Scotton et al., 2006) |
| CES2 (carboxylesterase 2) | Alternative splicing in exon 10 results in an enzymatically inactive protein | (Schiel et al., 2007) |
| Ctsm (Cathepsin M) Mus musculus and Rattus norvegicus | Aberrant splicing of exon 7 leads to catalytically inactive isoforms | (Bode et al., 2005) |
| Entpd2 (ectonucleoside triphosphate diphosphohydrolase 2) Rattus norvegicus | Different in cellular distribution, catalytic properties and regulation by protein kinases | (Wang et al., 2005a) |
| FMR1 (fragile × mental retardation 1) | Reduction in methylation activity | (Dolzhanskaya et al., 2006) |
| Grk6 (G-protein-coupled receptor kinase 6) Mus musculus | C-terminus is required for the catalytic activity and contains three auto-regulatory elements | (Vatter et al., 2005) |
| hpse (heparanase) Xenopus laevis | Skipping of cassette exon generates catalytic inactive form | (Bertolesi et al., 2008) |
| IDE(insulin-degrading enzyme) | Isoform with an exon 15b has inefficient proteolytic activity | (Farris et al., 2005) |
| IRAK1 (interleukin-1 (IL-1) receptor-associated kinase 1) | Alternative exon necessary for kinase activity | (Rao et al., 2005) |
| LPIN1 (lipin 1) | Alpha isoform has the highest turnover number, followed by the beta and gamma isoforms | (Han and Carman, 2010) |
| mhc (Myosin heavy chain) Drosophila melanogaster | Alternatively spliced exon 7 domain modulates myosin ATPase activity | (Miller et al., 2005) |
| MTHFD1L (methylenetetrahydrofolate dehydrogenase (NADP+ dependent) 1-like) | Short isoform lacking exon 8 is soluble and enzymatically inactive | (Prasannan and Appling, 2009) |
| NOS3 (nitric oxide synthase 3 (endothelial cell) | Novel 3'splice sites within intron 13 given three splice variants of NOS3 resulting truncated proteins lacking enzyme activity | (Lorenz et al., 2007) |
| PIP5K1A (phosphatidylinositol-4-phosphate 5-kinase, type I, alpha) | Different kinase activity that regulates exocytosis | (Wang et al., 2005c) |
| PIP5K1B (phosphatidylinositol-4-phosphate 5-kinase, type I, beta) | Beta isoform has lower kinase activity and does not prime exocytosis | (Wang et al., 2005c) |
| PTGS1 (prostaglandin-endoperoxide synthase 1 (prostaglandin G/H synthase and cyclooxygenase)) | Isoform lacking the last 111 nucleotides of exon 9 cannot be detected as protein. The recombinant protein is catalytically inactive | (Schneider et al., 2005) |
| SAT1 (spermidine/spermine N1-acetyltransferase 1) | Hypoxia and iron-deficiency promote inclusion of 110 nucleotides of intron 3 leading to a truncated isoform lacking catalytic activity | (Kim et al., 2005a) |
| TAZ (tafazzin) | The isoform lacking exon 5 has transacylase activity | (Xu et al., 2009) |
| TMLHE (trimethyllysine hydroxylase, epsilon) | TMLHE-b isoform lacking the last 90 amino acids at the C-terminal end is inactive | (Monfregola et al., 2005) |
| TPH2 (tryptophan hydroxylase 2) | TPH2B variant has a higher activity than TPH2B in which part of intron 3 is retained | (Grohmann et al., 2010) |
| Trhde (thyrotropin-releasing hormone degrading enzyme) Rattus norvegicus | Alternatively spliced isoform lacking part of the C-terminal domain is enzymatically inactive | (Chavez-Gutierrez et al., 2005) |
| Tssk6 (testis-specific serine kinase 5) Mus musculus | Insertion of 10 amino acid residues in region VIb results in inactive splice variants | (Wei et al., 2007) |
| B. Generation of constitutively active variants | ||
| Name | Function | Reference |
| Mst1r (macrophage stimulating 1 receptor (c-met-related tyrosine kinase) Mus musculus | Exon deletion is responsible for constitutive kinase activation | (Wei et al., 2005) |
| Pak3 (p21 protein (Cdc42/Rac)-activated kinase 3) Mus musculus | Alternative exons are inserted into the catalytic domain and make the kinase constitutively active | (Kreis et al., 2008) |
| C. Dominant negative effects | ||
| Name | Function | Reference |
| ENTPD3 (Nucleoside triphosphate diphosphohydrolase 3 (NTPDase3)) | Alternative exon of NTPDase3 (nucleoside triphosphate diphosphohydrolase 3) is necessary for activity, the presence of the inactive variant downregulates the activity of the active variant | (Crawford et al., 2007) |
| UGT1A (UDP glucuronosyltransferase 1 family, polypeptide A complex locus) | The inactive isoform inhibits the UGT1A-mediated glucuronidation from acting as a dominant-negative repressor | (Bellemare et al., 2010) |
| D. Miscellaneous | ||
| ACSL5 (acyl-CoA synthetase long-chain family member 5) | Splice variant lacking exon 20 is inactive at highly alkaline pH | (Gassler et al., 2007) |
| ACSL6 (acyl-CoA synthetase long-chain family member 6) | Specific fatty acid gate-domain residues are essential for activity. Isoform lacking the domain is inactive, isoforms containing different motifs have different activity | (Soupene et al., 2010) |
| CETP (cholesteryl ester transfer protein, plasma) | Alternative exons create less efficiently secreted variants | (Lira et al., 2008) |
| FYN (FYN oncogene related to SRC, FGR, YES) | Inclusion of either exon 7A or 7B changes the SH3-dependent interaction and tyrosine phosphorylation of Sam68. It affects the auto-inhibition of FYN | (Brignatz et al., 2009) |
| PRKDC (protein kinase, DNA-activated, catalytic polypeptide) | Splice variant II and III are conserved and expressed predominantly in non-dividing cells | (Convery et al., 2005) |
| PTGER3 (prostaglandin E receptor 3 (subtype EP3)) | Prostaglandin-E2 mediated phosphorylation of ERK1/2 by PTGER3 isoform II and III involves activation of Galpha(i)/PI3K/PKC/Src and EGFR-dependent pathway, ERK ½ phosphorylation by isoform Ia is minimal and involves activation of Galpha(i)/Src and EGFR-dependent pathway | (Israel and Regan, 2009) |
| RAC1 (ras-related C3 botulinum toxin substrate 1 (rho family, small GTP binding protein Rac1)) | Exon encodes a 19-a.a. insertion that leads to decreased GTPase activity and reduced affinity to GDP | (Orlichenko et al.) |
| TNFRSF1B (tumor necrosis factor receptor superfamily, member 1B) | Skipping of two alternative exons leads to a soluble TNFRS1B receptor associated with greater insulin sensitivity | (Fernandez-Real et al., 2006) |
Kinases can be regulated by autoinhibition. A removal of this domain generates constitutively active enzymes (Table 4, B).
In few instances, alternative isoforms have a dominant negative effect on the active isoform, which is achieved by the formation of heterodimers (Table 4, C). Finally, alternative splicing can change the substrate specificity of enzymes, for example in the cytosolic phospholipase A2 beta (Ghosh et al., 2006).
3.6 Alternative exons that encode protein domains
Alternative exons frequently encode part of protein domains. These exons are usually identified by database comparison and their function is deduced from the general function of the domain that is deleted by exon skipping. Table 5 lists examples of identified domains where a change of binding to a defined interaction partner was not determined. Tables 6 and 2B list changes in binding domains that alter the interaction with DNA, other proteins and defined ligands. The role of the domain is illustrated in Figure 6.
Table 5. Alternative exons that encode protein domains.
| Name | Function | Reference |
|---|---|---|
| AAK1 (AP2 associated kinase 1) | Variant encodes clathrin-binding domain | (Henderson and Conner, 2007) |
| CD55 (CD55 molecule, decay accelerating factor for complement (Cromer blood group)) | Alternative splicing generates either a protein with a GPI anchor or with a transmembrane domain | (Mizuno et al., 2007) |
| Cd80 (CD80 molecule) Mus musculus | Exon encodes for IgV (immunoglobulin-like domains) and acts as a negative regulator | (Bugeon et al., 2006) |
| dgk-1 (DiacylGlycerol Kinase) Caenorhabditis elegans | Alternative splicing generates two new forms with the kinase domain and part of the PH (pleckstrin homology) domain | (Jose and Koelle, 2005) |
| DYRK4 (dual-specificity tyrosine-(Y)-phosphorylation regulated kinase 4) | Exon encodes nuclear localization domain | (Papadopoulos et al., 2011) |
| ERBB2IP (erbb2 interacting protein) | Variant lacks the SID (SMAD-interacting domain) and loses its ability to inhibit TGFβ (transforming growth factor beta) | (Dai et al., 2007) |
| FGFR1 (fibroblast growth factor receptor 1) | Exon encodes one immunoglobulin domain that increases cellular proliferation in high density cultures | (Zhang et al., 2006) |
| matn1 (matrilin 1, cartilage matrix protein) Danio rerio | Exon encodes a threonine/serine domain | (Ko et al., 2005) |
| MUC1 (mucin 1, cell surface associated) | Exons encode mucin domain | (Levitin et al., 2005) |
| NOD2 (nucleotide-binding oligomerization domain containing 2) | Alternative splicing produces a new variant that lacks a LRR (Leucine-rich repeat) domain | (Leung et al., 2007) |
| RPGRIP1 (retinitis pigmentosa GTPase regulator interacting protein 1) | Alternative stop codon deletes C2 and RID (Rho GTPase inactivation domain) | (Lu and Ferreira, 2005) |
| Syne2 (spectrin repeat containing, nuclear envelope 2) Mus musculus | Exon codes for an actin-binding domain. Its skipping causes thickening of epidermis | (Luke et al., 2008) |
| TNFRSF13B (tumor necrosis factor receptor superfamily, member 13B) | Exon encodes a CRD (cysteine-rich domain) | (Hymowitz et al., 2005) |
Table 6. Protein binding regulated by alternative splicing.
| A. Binding being regulated by individual domains | ||
|---|---|---|
| Name | Function | Reference |
| ADAM15 (ADAM metallopeptidase domain 15) | Exons encode different putative SH3 domain binding sites, causing selective binding to SH3-containing proteins | (Kleino et al., 2009) |
| CACNA1D (calcium channel, voltage-dependent, L type, alpha 1D subunit) | Exon encodes a SH3 and a class I PDZ binding domain that binds Shank proteins | (Olson et al., 2005) |
| CADPS2 (Ca++-dependent secretion activator 2) | Exon 3 encodes a dynactin 1-binding domain and affects axonal CADPS2 protein distribution | (Sadakata et al., 2007) |
| Col4a3bp (collagen, type IV, alpha 3 (Goodpasture antigen) binding protein) Danio rerio | Splicing isoform produces a protein missing a serine-rich domain. The splicing isoform overexpression doesn't rescue the knockdown phenotype | (Granero-Molto et al., 2008) |
| FMR1 (fragile × mental retardation 1) | Exon 15 encodes C-terminal G-R rich domain that can reduce methylation by PRMTs | (Dolzhanskaya et al., 2006) |
| FRMD3 (FERM domain containing 3) | Alternative exon 5, encoding a peptide in the FERM domain of protein 4.1R, is necessary for plasma membrane targeting | (Seo et al., 2009a) |
| Hph (HIF prolyl hydroxylase) Drosophila melanogaster | Alternative splicing gives rise to an isoform missing a MYND-type (myeloid, Nervy and DEAF-1) zinc finger domain, which is upregulated in adult stages | (Acevedo et al., 2010) |
| ITSN1 (intersectin 1 (SH3 domain protein)) | Exon 20 encodes five a.a. in the first SH3A (SH3 domain) and changes the binding properties of the SH3 domain | (Tsyba et al., 2008) |
| LDB2 (LIM domain binding 2) | Exon encodes LID (lim interaction domain) and regulates protein interactions | (Tran et al., 2006) |
| MAPT (microtubule-associated protein tau) | Exon includes microtuble binding site, its skipping weakens binding | (Levy et al., 2005) |
| MAPT (microtubule-associated protein tau) | Alternative exon weakens the binding of the SH3 domain of fyn | (Bhaskar et al., 2005) |
| MYO1B (myosin IB) | Exon encodes IQ motifs that regulate interaction with calmodulin | (Lin et al., 2005) |
| Pde1a (phosphodiesterase 1A, calmodulin-dependent) Mus musculus | The new variant lacks the first calmodulin-binding domain, but not the second | (Vasta et al., 2005) |
| POU3F2 (POU class 3 homeobox 2) | Exon encodes beta domain that blocks the interaction between Pit-1 and CBP | (Sporici et al., 2005) |
| PPARG (peroxisome proliferator-activated receptor gamma) | Deletion of the ligand binding domain | (Sabatino et al., 2005) |
| SH2D2A (SH2 domain containing 2A) | Exon encodes four tyrosines that interact with a SH2 domain of Lck | (Granum et al., 2006) |
| TNFRSF13B (tumor necrosis factor receptor superfamily, member 13B) | Alternative splicing controls cystein rich domain, which controls affinity to APRIL and BAFF ligands | (Hymowitz et al., 2005) |
| B. Binding to individual proteins | ||
| Name | Function | Reference |
| ABI1 (abl-interactor 1) | Splicing isoforms of Abi1 have different relative affinities for activated Rac1 in Wave 2 complex, thus regulating macropinocytosis | (Dubielecka et al.) |
| AMOT (angiomotin) | Exons encode a N-terminal extension, which mediates the interaction between AMOT and membrane associated guanylate kinase MAGI-1 | (Bratt et al., 2005) |
| BIN1 (bridging integrator 1) | The tumor-specific Bin 1 with exon 12A inclusion inhibits Bin 1 binding to c-Myc | (Pineda-Lucena et al., 2005) |
| BRAF (v-raf murine sarcoma viral oncogene homolog B1) | The presence of an alternative exon 8b increases the binding of the B-Raf N terminus to a MEK kinase | (Hmitou et al., 2007) |
| CXCL12 (chemokine (C-X-C motif) ligand 12) | The splicing variant CXCL12 gamma displays higher binding affinity to heparane sulfate expressed at the cell surface | (Laguri et al., 2007) |
| DSG1 (desmoglein 1) | Retained intron encodes a specific peptide sequence that binds to Drbeta1*0102 | (Mouquet et al., 2006) |
| ID1 (inhibitor of DNA binding 1, dominant negative helix-loop-helix protein) | Different affinity to CASK (calcium/calmodulin-dependent serine protein kinase (MAGUK family)) | (Qi et al., 2005) |
| KIF1B (kinesin family member 1B) | Splicing variants show different affinity for microtubles and have different motor activities | (Matsushita et al., 2009b) |
| MAPT (microtubule-associated protein tau) | Tau isoforms 6D/6P, due to exon 6 alternative splicing, remain soluble and inhibit polymerization of hTau40 | (Lapointe et al., 2009) |
| MYO5A (myosin VA (heavy chain 12, myoxin)) | Exon B constitutes an essential part of the DYNLL2 binding site. A brain-specific isoform in which exon B is skipped can no longer interact with DYNLL2 | (Wagner et al., 2006) |
| MYO5A (myosin VA (heavy chain 12, myoxin)) | Altenative exon B that encodes three residues is essential for DLC2 binding | (Hodi et al., 2006) |
| NRXN1 (neurexin 1) | Only the neurexins lacking an insert in exon can bind to LRRTM2 as ligands | (Ko et al., 2009) |
| OCRL (oculocerebrorenal syndrome of Lowe) | Splicing alters the clathrin binding properties | (Choudhury et al., 2009) |
| PLCB1 (phospholipase C, beta 1 (phosphoinositide-specific)) | Alternative splicing variant PLCbeta1b, but not PLCbeta1a, associates with Galphaq and inhibits PLC in responses to alpha1-adrenergic receptor activation | (Grubb et al., 2008) |
| PNRC1 (proline-rich nuclear receptor coactivator 1) | Alternative 3′ splice sites of exon 1 creates splicing variant RNRC1c with an increased affinity to NRs (nuclear receptors) and PNRC1f with no binding ability to NRs | (Wang et al., 2008) |
| RGS8 (regulator of G-protein signaling 8) | The shorter isoform of RGS8 has lower binding strength to mACjRs | (Itoh et al., 2006) |
| VCAM1 (vascular cell adhesion molecule 1) | Exons encode the Ig-like domain 4 of VCAM1. The 6D isoform, lacking domain four, has a higher affinity to to integrin alpha4beta1 | (Woodside et al., 2006) |
| C. Binding to ligands | ||
| Name | Function | Reference |
| ADCYAP1R1 (adenylate cyclase activating polypeptide 1 (pituitary) receptor type I) | Alternative splicing generates PAC1 isoforms with diversity in ligand specificity, binding affinity and downstream signaling | (Ushiyama et al.) |
| EDNRA (endothelin receptor type A) | Splicing changes affinity to receptor antagonist BQ123 | (Chester et al., 2007) |
| EPM2A (epilepsy, progressive myoclonus type 2A, Lafora disease (laforin)) | Different binding ability to glycogen and affinity to Malin | (Dubey and Ganesh, 2008) |
| H2AFY(H2A histone family, member Y) | Alternative splicing regulates the interaction between mH2A and NAD metabolites OAADPR and ADPR | (Kustatscher et al., 2005) |
| HRH3 (histamine receptor H3) | Splicing variants have different affinity and potency for H3R agonists | (Bongers et al., 2007) |
| IGF1R (insulin-like growth factor 1 receptor) | Splicing variants of hybrid IR (insulin receptor) do not change the binding properties to insulin and IGF-I | (Slaaby et al., 2006) |
| INSR (insulin receptor) | Exon 11 modulates the time course of IRS-1 activation | (Deutsch et al., 2008b) |
| LEPR (leptin receptor) Gallus gallus | Exon 9 encodes leptin binding domain and modulates leptin action | (Liu and Sharp, 2007) |
| NTSR2 (neurotensin receptor 2) | Alternative exon introduces a stop codon that abolishes two of the transmembrane domains and the ligand binding | (Perron et al., 2005) |
| OPRM1 (opioid receptor, mu 1) | Variants loose binding to [(3)H]diprenorphine | (Choi et al., 2006) |
Figure 6. Regulation of protein-protein and protein-ligand interaction by alternative splicing.
Examples are given in Table 6, A-B.
A. Alternative splicing of the domain-binding pocket regulates protein interactions.
B. Alternative splicing of domains that bind to binding pockets regulates protein interactions.
C. Alternative splicing changes binding of a ligand (green pentagon) to a protein (red).
3.7 Change in binding to other proteins
As most alternative exons are localized on the protein surface, alternative splicing can regulate the binding to other proteins. Exons can encode complete interaction domains or part of binding domains, which changes the interaction with other proteins (Table 6, A; Figure 6A, B). In most cases, the binding affinity is modulated but the binding is not abolished completely. Several cases have been reported where a change in alternative splicing outside a known protein domain changes the interaction with other proteins, summarized in Table 6, B.
Similar to a change in protein binding is the influence of alternative splicing on binding of low molecular weight ligands or hormones (Figure 6C). The classical example is the insulin receptor, where skipping of the alternative exon 11 generates a receptor that shows the highest affinity for IGF-II (insulin-like growth factor II) (reviewed in (Belfiore et al., 2009)). This system is more complicated in vivo as the insulin receptor and the IGF-IR (insulin-like growth factor I receptor) can form heterodimers. There is no difference among the splice variants in these heterodimers (Slaaby et al., 2006), demonstrating the ability of alternative exons to ‘fine-tune’ a biological response.
Another interaction regulated by alternative splicing is the binding of proteins to DNA, which can be controlled by alternative exons in the DNA binding domain, summarized in Table 2, B. In addition, binding to membranes is influenced by alternative splicing and changes the localization of the proteins, summarized in Table 3.
3.8 Change in channel proteins
In general, the effects evoked by alternative splicing are relatively small. Electrophysiological techniques can analyze channel proteins and determine the properties of a few or even a single molecule. Alternative splicing can change every aspect of ion channels: gating times, gating voltages, ion sensitivity and inhibition by other substances (Table 7, Figure 7). Similar to other events generated by alternative splicing, some variants can exhibit dominant effects, for example variants of the glutamate transporter SLC1A3 (Vallejo-Illarramendi et al., 2005).
Table 7. Change of channel protein properties.
| Name | Function | Reference |
|---|---|---|
| ANO1 (anoctamin 1, calcium activated chloride channel) | TMEM 16A splicing variants exhibit differences in voltage dependence and Ca++ sensitivity | (Ferrera et al., 2009) |
| CACNA1A (calcium channel, voltage-dependent, P/Q type, alpha 1A subunit) | Mutations of FHM-1 exhibit differential effects on voltage-dependent properties, on recovery from inactivation and on accumulation of inactivation during tonic and burst firing of Cav2.1 splice variants | (Adams et al., 2009) |
| CACNA1B (calcium channel, voltage-dependent, N type, alpha 1B subunit) | Change in the channel opening time and expression density | (Dabertrand et al., 2006) |
| CACNA1B (calcium channel, voltage-dependent, N type, alpha 1B subunit) | Exon 37a acts as a molecular switch that tailors the channels toward specific roles in pain | (Altier et al., 2007) |
| CACNA1C (calcium channel, voltage-dependent, L type, alpha 1C subunit) | Changes in the kinetics and voltage-dependence of inactivation, recovery from inactivation and rundown of the Ca++ current | (Tiwari et al., 2006) |
| CACNA1C (calcium channel, voltage-dependent, L type, alpha 1C subunit) | Exon 33 encodes part of the IVS3-S4 linker region of the Cav1.2 calcium channel. Cav1.2SM, lacking exon 33, displays hyperpolarized shifts for steady-state inactivation and is more sensitive to nifedipine inhibition | (Liao et al., 2007) |
| CACNA1D (calcium channel, voltage-dependent, L type, alpha 1D subunit) | The longer isoform of the Ca(V) 1.4 channel retunes channel affinity to apoCaM | (Liu et al.) |
| CACNA1H (calcium channel, voltage-dependent, T type, alpha 1H subunit) | Change in gating behavior, sensitivity to neuromodulation and interaction with extracellular matrix | (Zhong et al., 2006) |
| CACNA1H (calcium channel, voltage-dependent, T type, alpha 1H subunit) | Exon 25, encoding part of the III-IV linker of Cav3.2, influences the voltage-dependence and kinetics of both activation and inactivation | (Cooper et al., 2008) |
| CACNB1 (calcium channel, voltage-dependent, beta 1 subunit) | The isoform of Cavbeta1, lacking exons that encode the alpha-binding pocket, can modulate the gating behavior of L-type Ca++ channels | (Roszelle et al., 2008) |
| CACNB4 (calcium channel, voltage-dependent, beta 4 subunit) | Alternative splicing regulates cell-specific expression | (Vendel et al., 2006) |
| GABRB2 (gamma-aminobutyric acid (GABA) A receptor, beta 2) | Change in hyperpolarization | (Zhao et al., 2006) |
| GRIA1 (glutamate receptor, ionotropic, AMPA 1) GRIA2 (glutamate receptor, ionotropic, AMPA 2) GRIA3 (glutamate receptor, ionotropic, AMPA 3) GRIA4 (glutamate receptor, ionotropic, AMPA 4) | Change in resensitation kinetics | (Schlesinger et al., 2005) |
| GRIA2 (glutamate receptor, ionotropic, AMPA 2) | Flip/flop splicing alters GluR2 assembly and endoplasmic reticulum secretion kinetics | (Deutsch et al., 2008c) |
| GRIN1 (glutamate receptor, ionotropic, N-methyl D-aspartate 1) | Change in inhibition by ethanol | (Jin and Woodward, 2006) |
| KCNMA1 (potassium large conductance calcium-activated channel, subfamily M, alpha member 1) | Skipping of exon that encodes an acidic cluster-like motif results in a dominant negative subunit that supresses cell surface expression of BK channels | (Chen et al.) |
| KCNQ4 (potassium voltage-gated channel, KQT-like subfamily, member 4) | Exons encode different calmodulin binding domains of KCNQ4 channel, thus the channels can be modulated differentially by calmodulin | (Xu et al., 2007) |
| Oprm1 (opioid receptor, mu 1) Mus musculus | Exon 4 encodes the MOR1-derived recycling sequence. OPRM1 splicing variants lacking exon 4 recycle inefficiently after ligand-induced entocytosis | (Tanowitz et al., 2008) |
| PDK2 (pyruvate dehydrogenase kinase, isozyme 2) | The isoform with exon 7 skipping no longer interacts with polycyctin-1 | (Cooper et al., 2010) |
| RYR1 (ryanodine receptor 1 (skeletal)) | The ASI(+) splicing variant contributes to an inhibitory module in RYR1 that influences skeletal muscle EC coupling | (Kimura et al., 2009) |
| RYR2 (ryanodine receptor 2 (cardiac)) | Splicing isoforms modulate intracellular Ca++ fluxes following caffeine exposure | (Deutsch et al., 2008a) |
| SLC1A3 (solute carrier family 1 (glial high affinity glutamate transporter), member 3) | Loss of glutamate uptake | (Vallejo-Illarramendi et al., 2005) |
| SLC8A2 (solute carrier family 8 (sodium/calcium exchanger), member 2) | The BD splicing variant of NCX, with a differnt Ca++binding site, displays no Ca++ binding | (Breukels and Vuister) |
Figure 7. Change of ion channels due to alternative splicing.
The ion-channel is shown in red and the channel opening indicated by yellow half circles. The triangle indicates a ligand-binding site; the ligand is shown as a hexgon. A: Channel in its open conformation. Dots indicate the flow of ions. B: Alternative splicing changes the pore, which in this case leads to a larger flux. C: A change in the ligand-binding site prevents channel opening.
3.9 Influence on mRNA function
Alternative splicing not only occurs in the coding regions of pre-mRNA, but also in the untranslated regions, summarized in Table 8. An emerging theme is the regulation of mRNA stability through miRNAs, which can be affected by alternative splicing of the miRNA-binding sites, frequently located in the UTR, as has been seen in the SLC11A2 gene (Andolfo et al., 2010).
Table 8. Changes in RNA stability and function.
| Name | Function | Reference |
|---|---|---|
| CD247 (CD247 molecule) | When the retained intron in the 3′ UTR is spliced out, the mRNA is less stable and the protein is less efficiently translated. The splicing out is seen more in patients with Systemic Lupus Erythematosus | (Chowdhury et al., 2005) |
| GLRX (glutaredoxin (thioltransferase)) | The alternative 3′ end of the last exons changes stability of the mRNA when assayed with a luciferase reporter construct | (Park and Levine, 2005) |
| SLC11A2 (solute carrier family 11 (proton-coupled divalent metal ion transporters), member 2) | The 3′UTR splicing variant of DMT1 lacking an IRE binds to Let-7D on its 3′UTR | (Andolfo et al., 2010) |
| SLC40A1 (solute carrier family 40 (iron-regulated transporter), member 1) | Alternative splicing changes the UTR and creates/abolishes an iron response element through cassette exons and alternative 3′ splice sites and changes translation | (Cianetti et al., 2005) |
3.10 Other functions of alternative exons
Alternative exons were found to change the stability of proteins by different mechanisms, that includes a change in the C-terminus making proteins more susceptible to degradation, as seen for the migration and invasion inhibitory protein MIIP (Song et al., 2005) and the iodotyrosine deiodinase (Gnidehou et al., 2006) (Table 9, A).
Table 9. Other functions.
| A. Influence on protein stability | ||
|---|---|---|
| Name | Function | Reference |
| ATXN3 (ataxin 3) | Splicing regulates the aggregation and degradation of protein isoforms | (Harris etal.) |
| IYD (iodotyrosine deiodinase) | Splicing regulates protein stability | (Gnidehou et al., 2006) |
| MAPT (microtubule-associated protein tau) | Tau isoforms 6D/6P, due to exon 6 alternative splicing, remain soluble and inhibit polymerization of hTau40 | (Lapointe et al., 2009) |
| MIIP (migration and invasion inhibitory protein) | Exon 7 skipped isoform has a different C-terminus and is degraded | (Song et al., 2005) |
| B. Alternative splicing of splicing factors | ||
| Name | Function | Reference |
| TIA1 (TIA1 cytotoxic granule-associated RNA binding protein) | Skipping of exon 5 generates a shorter isoform and increases splicing stimulatory activity | (Izquierdo and Valcarcel, 2007) |
| WT1 (Wilms tumor 1) | Insertion of a small exon abolishes the effect of WT1 on alternative splicing | (Markus etal., 2006) |
3.11 General conclusion
These examples demonstrate that alternative splicing influences almost all aspects of protein functions, making it a central element in gene expression. Given the widespread usage of alternative splicing that affects almost every gene, the experimentally verified examples here are only the tip of the iceberg. It is likely that genome-wide approaches will unveil new functions and new regulatory modules in the future.
4.0 Databases for work on alternative splicing
The experimental analysis of splicing problems has been greatly facilitated by user-friendly, Web-based analysis tools and databases, summarized in Table 10. Databases for alternative splicing were mostly generated computationally from different species (Table 10, A), but have also been generated from protein databases (Table 10, B). Importantly, numerous specialized databases listed in Table 10, B, help in analyzing alternative splicing functions and are useful to put a splicing event into a bigger picture.
Table 10. Bioinformatic resources for alternative splicing.
| A. General Databases | ||||
|---|---|---|---|---|
| Databases | Notes | Species | URL | Reference |
| AceView | Computationally generated from cDNA alignment onto genome (annotations include alternative splicing variants) | Human, Mouse, Rat, Arabidopsis, C. Elegans | http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/ | (Thierry-Mieg and Thierry-Mieg, 2006) |
| AS-ALPS | Computationally generated (predict protein structural changes due to alternative splicing) | Human, Mouse, Fly, C. Elegans, Arabidopsis, O. Sativa, Swiss-Prot | http://as-alps.nagahama-i-bio.ac.jp or http://genomenetwork.nig.ac.jp/as-alps/ | (Shionyu et al., 2009) |
| ASAP II | Alternative splicing annotation and intergenome comparison for 15 species (human, mouse, rat, western clawed frog, chicken, cow, dog, C. elegans, ciona, zebrafish, fruit fly, fugu, yellow fever mosquito, honeybee and African malaria mosquito) | 15 Species | http://bioinfo.mbi.ucla.edu/ASAP2/ | (Kim et al., 2007b) |
| ASMD | Computationally generated alternative splicing information using mutation data | Human | http://mco321125.meduohio.edu/∼jbechtel/asmd/1.1/ | (Bechtel et al., 2008a) |
| ASPicDB | Computationally generated (splice-site detection and full-length transcript modeling) | Human, Arabidopsis | http://www.caspur.it/ASPicDB | (Castrignano et al., 2006) (Castrignano et al., 2008) (Martelli et al., 2011) |
| AVATAR | Computationally generated by mapping ESTs and mRNAs to the whole human genome | Human | http://avatar.iecs.fcu.edu.tw/ | (Hsu et al., 2005) |
| FAST DB | Computationally generated from EST and mRNA | Human And Mouse | http://www.fast-db.com/ | (de la Grange et al., 2007) (Lerivray et al., 2006) |
| H-DBAS | Human-transcriptome database for alternative splicing (based on H-invitational full-length cDNAs) | Human And Mouse | http://h-invitational.jp/h-dbas/ | (Takeda et al., 2007) (Takeda et al., 2010) |
| SCN1A infobase | Manually generated from literature and updated with computational calculations | Human | http://www.scn1a.info/ | (Lossin, 2009) |
| Splice Browser | Computationally generated using high-throughput RNA sequencing and microarray profiling (identified novel splicing events including developmental regulated AS) | C. Elegans | http://splicebrowse.ccbr.utoronto.ca/ | (Ramani et al., 2011) |
| B. Specialized Databases | ||||
| Databases | Notes | Species | URL | Reference |
| ProSAS | Protein structure based analysis of alternative splicing events | Human, Mouse, Rat, Chimpanzee | http://www2.bio.ifi.lmu.de/ProSAS/ | (Birzele et al., 2008) |
| RegRNA | Interactive Web server for identifying sequence and structure homologs of regulatory RNAs (motifs gathered from literature and databases) | Human, Mouse, Rat | http://regrna.mbc.nctu.edu.tw/ | (Huang et al., 2006a) |
| snoRNP DB | Computationally generated from NCBI (a collection of 8994 snoRNA sequences and 589 snoRNA-associated protein sequences, both confirmed and putative) | Many species from Bacteria, Archaea and Eukayotes | http://evolveathome.com/snoRNA/snoRNA.php | (Ellis et al., 2010) |
| SplicInfo | Collection of occurrences of exon skipping, 5′-alternative splicing, 3′-alternative splicing and intron retention; prediction tool for secondary structure; identify regulatory motifs | Human | http://spliceinfo.mbc.nctu.edu.tw/ | (Huang et al., 2005a) |
| ssSNPTargetD B | Genome-wide splice site SNPs database | Human and Mouse | http://variome.kobic.re.kr/ssSNPTarget/ | (Yang et al., 2009) |
| TassDB | Tandem Splice Site DataBase (2 different versions: TassDB1 and TassDB2) | Human, Mouse, Rat, Dog, Chicken, Zebrafish, Drosophila, C. Elegans | http://www.tassdb.info/ | (Hiller et al., 2007) (Sinha et al., 2010) |
| UMD | Universal Mutation Databases with tools to calculate consensus values for potential splice sites and splicing enhancers | Human | http://www.umd.be/ | (Beroud et al., 2005) |
| UTRdb | 5′ and 3′ UTR variants and UTR Site motifs including ones generated from alternative splicing | Human, Mouse and other species | http://utrdb.ba.itb.cnr.it/ | (Grillo et al., 2010) |
| C. Software tools | ||||
| Software Tools | Notes | Species | URL | Reference |
| Free Web-based Tools | ||||
| ASePCR | RE-PCR emulator compatible with RefSeq, Ensembl, ECgene, AceView and GenBank (allows search for alternative splicing patterns of orthologous genes of different species) | Human, Mouse, Rat, Chicken | http://s47.rna.kr/ASePCR/ | (Kim et al., 2005b) |
| ASTALAVISTA | Improved visualization tool for complex alternative splicing events (can be used for whole transcriptome data from GENCODE, REFSEQ, ENSEMBL or user submission) | Many Species from Bacteria, Archaea and Eukaryotes | http://genome.crg.es/astalavista/ | (Foissac and Sammeth, 2007) |
| AUGUSTUS | Tool for eukaryotic gene prediction including alternative splicing using a Generalized Hidden Markov Model (can be locally installed) | Human, Fly, Arabidopsis, C. Cinereus (Fungus) | http://bioinf.uni-greifswald.de/augustus/ | (Stanke et al., 2006c) (Stanke et al., 2006a) (Stanke et al., 2006b) |
| Sfmap | Webserver for prediction of splicing factor binding sites in genomic data | Human (Other Eukaryotes accepted but not considered for evolutionary conservation) | http://sfmap.technion.ac.il/ | (Paz et al., 2010) |
| Skippy | Splicing assessment tool; scores human exon variants for exon-skipping events; identifies potential splice-affecting genome variants | Human | http://research.nhgri.nih.gov/skippy/index.shtml | (Woolfe et al., 2010) |
| SpliceCenter | Computational/analysis tools for RT-PCR primer/probe sets, RNAi effectors, microarrays and protein-targeting technologies | Human | http://projects.insilico.us/SpliceCenter/SpliceOverview.jsp | (Ryan et al., 2008) |
| SpliceMiner | Web interface for querying Evidence Viewer Database (filters splice variants from NCBI) and for mapping probes for splice variants; used for microarray analysis | Human, Mouse, Rat, Fruit Fly, C. Elegans, Cow, Zebrafish, Asian Rice, Arabidopsis | http://projects.insilico.us/SpliceMiner/intro.jsp | (Kahn et al., 2007) |
| Open Source Programs (Local Installation) | ||||
| ExAlt | Predict alternatively spliced exons using a non-expression based statistical method | Drosophila | http://www.cbcb.umd.edu/software/exalt/ | (Allen and Salzberg, 2006) |
| MARS | Extension to Twinscan (uses pairwise informant sequences from mouse, rat, dog, opossum, chicken and frog genome to predict alternatively spliced transcripts de novo) | Human, Mouse, Rat, Dog, Opossum, Chicken, From | http://www.ebi.ac.uk/∼flicek/MARS/ | (Flicek and Brent, 2006) |
| SNPSplicer | Utility for screening SNPs for effects on alternative splicing | Human | http://www.ikmb.uni-kiel.de/snpsplicer/ | (ElSharawy et al., 2006) |
| Splign | Computationally generated (improved method for co-aligning EST to genome) | Human | http://www.ncbi.nlm.nih.gov/sutils/splign/splign.cgi | (Kapustin et al., 2008) |
| Proprietary Software | ||||
| AspAlt | Alternative splicing and alternative transcription annotations and provide inter-database comparisons among Ensembl, RefSeq and AceView (Genome International) | 46 Eukaryotes | http://www.genome.com/products-1/integrated-genomics-resources/products-integrated-genomics-resources-igr-aspalt | (Bhasi et al., 2009) |
| EuSplice | Analysis tool for alternative splicing events with data mining and graphics capabilities for inter-genomic comparison (Genome International) | Eukaryotes | http://www.genome.com/products-1/integrated-genomics-resources/eusplice | (Bhasi et al., 2007) |
Finally, software tools are available to analyze splicing events, which are listed in Table 10, C. With a few exceptions listed in Table 10, all resources are available for free on the Web.
Supplementary Material
Highlights.
Almost every human pre-mRNA undergoes alternative splicing
The overall function of alternative splicing is to increase the diversity of mRNAs expressed from the genome
Changes caused by individual splicing isoforms are typically small
Splicing changes are coordinated, generating significant biological effects through combination
Alternative splicing interferes with almost every biological function analyzed
Acknowledgments
This work is supported by NIH RO1 GM083187 and an Endowment of the Universty of Kentucky. We thank Eva R. McEnrue for editorial help.
Abbreviations
- CLIP
Cross link and immunoprecipitation
- CTD
carboxy terminal domain
- Cyr61
cysteine rich 61
- ESE
exonic sequence enhancer
- HITS-CLIP
High-throughput sequencing of RNA isolated by crosslinking immunoprecipitation
- hnRNP
heterogenous ribonuclear proteins
- IR
insulin receptor
- ISS
inhibitory splicing sequence
- ncRNA
non-coding RNA
- NLS
nuclear localization signal
- NMD
nonsense mediated decay
- NoLS
nucleolar localization signal
- NOVA
neuro-oncological ventral antigen 1
- NR
nuclear receptors
- PTB
polypyrimidine tract binding protein
- SNP
single nucleotide polymorphism
- SR
serine arginine rich
- U2AF
U2 auxillary factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo JM, Centanin L, Dekanty A, Wappner P. Oxygen sensing in Drosophila: multiple isoforms of the prolyl hydroxylase fatiga have different capacity to regulate HIFalpha/Sima. PLoS One. 2010;5:e12390. doi: 10.1371/journal.pone.0012390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PJ, Garcia E, David LS, Mulatz KJ, Spacey SD, Snutch TP. Ca(V)2.1 P/Q-type calcium channel alternative splicing affects the functional impact of familial hemiplegic migraine mutations: implications for calcium channelopathies. Channels (Austin) 2009;3:110–21. doi: 10.4161/chan.3.2.7932. [DOI] [PubMed] [Google Scholar]
- Adato A, Lefevre G, Delprat B, Michel V, Michalski N, Chardenoux S, Weil D, El-Amraoui A, Petit C. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Human molecular genetics. 2005;14:3921–32. doi: 10.1093/hmg/ddi416. [DOI] [PubMed] [Google Scholar]
- Ahn J, Febbraio M, Silverstein RL. A novel isoform of human Golgi complex-localized glycoprotein-1 (also known as E-selectin ligand-1, MG-160 and cysteine-rich fibroblast growth factor receptor) targets differential subcellular localization. J Cell Sci. 2005;118:1725–31. doi: 10.1242/jcs.02310. [DOI] [PubMed] [Google Scholar]
- Al-Balool HH, Weber D, Liu Y, Wade M, Guleria K, Nam PL, Clayton J, Rowe W, Coxhead J, Irving J, Elliott DJ, Hall AG, Santibanez-Koref M, Jackson MS. Post-transcriptional exon shuffling events in humans can be evolutionarily conserved and abundant. Genome research. 2011;21:1788–99. doi: 10.1101/gr.116442.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JE, Salzberg SL. A phylogenetic generalized hidden Markov model for predicting alternatively spliced exons. Algorithms Mol Biol. 2006;1:14. doi: 10.1186/1748-7188-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altier C, Dale CS, Kisilevsky AE, Chapman K, Castiglioni AJ, Matthews EA, Evans RM, Dickenson AH, Lipscombe D, Vergnolle N, Zamponi GW. Differential role of N-type calcium channel splice isoforms in pain. J Neurosci. 2007;27:6363–73. doi: 10.1523/JNEUROSCI.0307-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri S, Movahedin M, Mowla SJ, Hajebrahimi Z, Tavallaei M. Differential gene expression and alternative splicing of survivin following mouse sciatic nerve injury. Spinal Cord. 2009;47:739–44. doi: 10.1038/sc.2009.26. [DOI] [PubMed] [Google Scholar]
- Andersson R, Enroth S, Rada-Iglesias A, Wadelius C, Komorowski J. Nucleosomes are well positioned in exons and carry characteristic histone modifications. Genome research. 2009;19:1732–41. doi: 10.1101/gr.092353.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolfo I, De Falco L, Asci R, Russo R, Colucci S, Gorrese M, Zollo M, Iolascon A. Regulation of divalent metal transporter 1 (DMT1) non-IRE isoform by the microRNA Let-7d in erythroid cells. Haematologica. 2010;95:1244–52. doi: 10.3324/haematol.2009.020685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apaja PM, Tuusa JT, Pietila EM, Rajaniemi HJ, Petaja-Repo UE. Luteinizing hormone receptor ectodomain splice variant misroutes the full-length receptor into a subcompartment of the endoplasmic reticulum. Mol Biol Cell. 2006;17:2243–55. doi: 10.1091/mbc.E05-09-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma N, Tadokoro K, Asaka A, Yamada M, Yamaguchi Y, Handa H, Matsushima S, Watanabe T, Kohsaka S, Kida Y, Shiraishi T, Ogura T, Shimamura K, Nakafuku M. The Pax6 isoform bearing an alternative spliced exon promotes the development of the neural retinal structure. Human molecular genetics. 2005;14:735–45. doi: 10.1093/hmg/ddi069. [DOI] [PubMed] [Google Scholar]
- Babu MM, Luscombe NM, Aravind L, Gerstein M, Teichmann SA. Structure and evolution of transcriptional regulatory networks. Current opinion in structural biology. 2004;14:283–91. doi: 10.1016/j.sbi.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Bakker RA, Lozada AF, van Marle A, Shenton FC, Drutel G, Karlstedt K, Hoffmann M, Lintunen M, Yamamoto Y, van Rijn RM, Chazot PL, Panula P, Leurs R. Discovery of naturally occurring splice variants of the rat histamine H3 receptor that act as dominant-negative isoforms. Mol Pharmacol. 2006;69:1194–206. doi: 10.1124/mol.105.019299. [DOI] [PubMed] [Google Scholar]
- Bao J, Lu Z, Joseph JJ, Carabenciov D, Dimond CC, Pang L, Samsel L, McCoy JP, Jr, Leclerc J, Nguyen P, Gius D, Sack MN. Characterization of the murine SIRT3 mitochondrial localization sequence and comparison of mitochondrial enrichment and deacetylase activity of long and short SIRT3 isoforms. Journal of cellular biochemistry. 2010;110:238–47. doi: 10.1002/jcb.22531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta A, Schumperli D. Editorial on alternative splicing and disease. RNA biology. 2010;7:388–9. doi: 10.4161/rna.7.4.12818. [DOI] [PubMed] [Google Scholar]
- Bastos E, Avila S, Cravador A, Renaville R, Guedes-Pinto H, Castrillo JL. Identification and characterization of four splicing variants of ovine POU1F1 gene. Gene. 2006;382:12–9. doi: 10.1016/j.gene.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Bechtel JM, Rajesh P, Ilikchyan I, Deng Y, Mishra PK, Wang Q, Wu X, Afonin KA, Grose WE, Wang Y, Khuder S, Fedorov A. The Alternative Splicing Mutation Database: a hub for investigations of alternative splicing using mutational evidence. BMC research notes. 2008a;1:3. doi: 10.1186/1756-0500-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel JM, Rajesh P, Ilikchyan I, Deng Y, Mishra PK, Wang Q, Wu X, Afonin KA, Grose WE, Wang Y, Khuder S, Fedorov A. Calculation of splicing potential from the Alternative Splicing Mutation Database. BMC research notes. 2008b;1:4. doi: 10.1186/1756-0500-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Nematollah Farsian F, Masiulis I, Hammer RE, Yoon SO, Giehl KM, Herz J. ApoE receptor 2 controls neuronal survival in the adult brain. Curr Biol. 2006;16:2446–52. doi: 10.1016/j.cub.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Behzadnia N, Golas MM, Hartmuth K, Sander B, Kastner B, Deckert J, Dube P, Will CL, Urlaub H, Stark H, Luhrmann R. Composition and three-dimensional EM structure of double affinity-purified, human prespliceosomal A complexes. Embo J. 2007;26:1737–48. doi: 10.1038/sj.emboj.7601631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocrine reviews. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- Bellemare J, Rouleau M, Harvey M, Guillemette C. Modulation of the human glucuronosyltransferase UGT1A pathway by splice isoform polypeptides is mediated through protein-protein interactions. The Journal of biological chemistry. 2010;285:3600–7. doi: 10.1074/jbc.M109.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge EO, Staalesen V, Straume AH, Lillehaug JR, Lonning PE. Chk2 splice variants express a dominant-negative effect on the wild-type Chk2 kinase activity. Biochim Biophys Acta. 2010;1803:386–95. doi: 10.1016/j.bbamcr.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Berget SM. Exon Recognition in vertebrate splicing. J Biol Chem. 1995;270:2411–2414. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- Berget SM, Moore C, Sharp PA. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977;74:3171–5. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beroud C, Hamroun D, Collod-Beroud G, Boileau C, Soussi T, Claustres M. UMD (Universal Mutation Database): 2005 update. Hum Mutat. 2005;26:184–91. doi: 10.1002/humu.20210. [DOI] [PubMed] [Google Scholar]
- Bertolesi GE, Michaiel G, McFarlane S. Two heparanase splicing variants with distinct properties are necessary in early Xenopus development. The Journal of biological chemistry. 2008;283:16004–16. doi: 10.1074/jbc.M708525200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasi A, Pandey RV, Utharasamy SP, Senapathy P. EuSplice: a unified resource for the analysis of splice signals and alternative splicing in eukaryotic genes. Bioinformatics. 2007;23:1815–23. doi: 10.1093/bioinformatics/btm084. [DOI] [PubMed] [Google Scholar]
- Bhasi A, Philip P, Sreedharan VT, Senapathy P. AspAlt: A tool for inter-database, inter-genomic and user-specific comparative analysis of alternative transcription and alternative splicing in 46 eukaryotes. Genomics. 2009;94:48–54. doi: 10.1016/j.ygeno.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Bhaskar K, Yen SH, Lee G. Disease-related modifications in tau affect the interaction between Fyn and Tau. The Journal of biological chemistry. 2005;280:35119–25. doi: 10.1074/jbc.M505895200. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Amendola R, Federico R, Polticelli F, Mariottini P. Two short protein domains are responsible for the nuclear localization of the mouse spermine oxidase mu isoform. FEBS J. 2005;272:3052–9. doi: 10.1111/j.1742-4658.2005.04718.x. [DOI] [PubMed] [Google Scholar]
- Biocca S, Filesi I, Mango R, Maggiore L, Baldini F, Vecchione L, Viola A, Citro G, Federici G, Romeo F, Novelli G. The splice variant LOXIN inhibits LOX-1 receptor function through hetero-oligomerization. J Mol Cell Cardiol. 2008;44:561–70. doi: 10.1016/j.yjmcc.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Birzele F, Kuffner R, Meier F, Oefinger F, Potthast C, Zimmer R. ProSAS: a database for analyzing alternative splicing in the context of protein structures. Nucleic Acids Res. 2008;36:D63–8. doi: 10.1093/nar/gkm793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismuth K, Maric D, Arnheiter H. MITF and cell proliferation: the role of alternative splice forms. Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society. 2005;18:349–59. doi: 10.1111/j.1600-0749.2005.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette M, Green RE, Brenner SE, Rio DC. Global analysis of positive and negative pre-mRNA splicing regulators in Drosophila. Genes & development. 2005;19:1306–14. doi: 10.1101/gad.1314205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland CS, Wang ET, Vu A, David MP, Castle JC, Johnson JM, Burge CB, Cooper TA. Global regulation of alternative splicing during myogenic differentiation. Nucleic acids research. 2010;38:7651–64. doi: 10.1093/nar/gkq614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocher S, Fink L, Bohle RM, Bergmann M, Steger K. CREM activator and repressor isoform expression in human male germ cells. International journal of andrology. 2005;28:215–23. doi: 10.1111/j.1365-2605.2005.00532.x. [DOI] [PubMed] [Google Scholar]
- Bluemlein K, Gruning NM, Feichtinger RG, Lehrach H, Kofler B, Ralser M. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget. 2011;2:393–400. doi: 10.18632/oncotarget.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode S, Peters C, Deussing JM. Placental cathepsin M is alternatively spliced and exclusively expressed in the spongiotrophoblast layer. Biochimica et biophysica acta. 2005;1731:160–7. doi: 10.1016/j.bbaexp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Bombail V, Collins F, Brown P, Saunders PT. Modulation of ER alpha transcriptional activity by the orphan nuclear receptor ERR beta and evidence for differential effects of long- and short-form splice variants. Molecular and cellular endocrinology. 2010;314:53–61. doi: 10.1016/j.mce.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Bongers G, Krueger KM, Miller TR, Baranowski JL, Estvander BR, Witte DG, Strakhova MI, van Meer P, Bakker RA, Cowart MD, Hancock AA, Esbenshade TA, Leurs R. An 80-amino acid deletion in the third intracellular loop of a naturally occurring human histamine H3 isoform confers pharmacological differences and constitutive activity. J Pharmacol Exp Ther. 2007;323:888–98. doi: 10.1124/jpet.107.127639. [DOI] [PubMed] [Google Scholar]
- Boyle LH, Traherne JA, Plotnek G, Ward R, Trowsdale J. Splice variation in the cytoplasmic domains of myelin oligodendrocyte glycoprotein affects its cellular localisation and transport. J Neurochem. 2007;102:1853–62. doi: 10.1111/j.1471-4159.2007.04687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt A, Birot O, Sinha I, Veitonmaki N, Aase K, Ernkvist M, Holmgren L. Angiomotin regulates endothelial cell-cell junctions and cell motility. J Biol Chem. 2005;280:34859–69. doi: 10.1074/jbc.M503915200. [DOI] [PubMed] [Google Scholar]
- Breukels V, Vuister GW. Binding of calcium is sensed structurally and dynamically throughout the second calcium-binding domain of the sodium/calcium exchanger. Proteins. 78:1813–24. doi: 10.1002/prot.22695. [DOI] [PubMed] [Google Scholar]
- Brignatz C, Paronetto MP, Opi S, Cappellari M, Audebert S, Feuillet V, Bismuth G, Roche S, Arold ST, Sette C, Collette Y. Alternative splicing modulates autoinhibition and SH3 accessibility in the Src kinase Fyn. Molecular and cellular biology. 2009;29:6438–48. doi: 10.1128/MCB.00398-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AN, Yang L, Duff MO, Hansen KD, Park JW, Dudoit S, Brenner SE, Graveley BR. Conservation of an RNA regulatory map between Drosophila and mammals. Genome research. 2011;21:193–202. doi: 10.1101/gr.108662.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugeon L, Wong KK, Rankin AM, Hargreaves RE, Dallman MJ. A negative regulatory role in mouse cardiac transplantation for a splice variant of CD80. Transplantation. 2006;82:1334–41. doi: 10.1097/01.tp.0000239343.01775.54. [DOI] [PubMed] [Google Scholar]
- Buratti E, Baralle FE. Influence of RNA secondary structure on the pre-mRNA splicing process. Mol Cell Biol. 2004;24:10505–14. doi: 10.1128/MCB.24.24.10505-10514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun HJ, Hong IK, Kim E, Jin YJ, Jeoung DI, Hahn JH, Kim YM, Park SH, Lee H. A splice variant of CD99 increases motility and MMP-9 expression of human breast cancer cells through the AKT-, ERK-, and JNK-dependent AP-1 activation signaling pathways. J Biol Chem. 2006;281:34833–47. doi: 10.1074/jbc.M605483200. [DOI] [PubMed] [Google Scholar]
- Caldas H, Fangusaro JR, Boue DR, Holloway MP, Altura RA. Dissecting the role of endothelial SURVIVIN DeltaEx3 in angiogenesis. Blood. 2007;109:1479–89. doi: 10.1182/blood-2006-02-003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldas H, Jiang Y, Holloway MP, Fangusaro J, Mahotka C, Conway EM, Altura RA. Survivin splice variants regulate the balance between proliferation and cell death. Oncogene. 2005;24:1994–2007. doi: 10.1038/sj.onc.1208350. [DOI] [PubMed] [Google Scholar]
- Casas F, Busson M, Grandemange S, Seyer P, Carazo A, Pessemesse L, Wrutniak-Cabello C, Cabello G. Characterization of a novel thyroid hormone receptor alpha variant involved in the regulation of myoblast differentiation. Molecular endocrinology. 2006;20:749–63. doi: 10.1210/me.2005-0074. [DOI] [PubMed] [Google Scholar]
- Castrignano T, D'Antonio M, Anselmo A, Carrabino D, D'Onorio De Meo A, D'Erchia AM, Licciulli F, Mangiulli M, Mignone F, Pavesi G, Picardi E, Riva A, Rizzi R, Bonizzoni P, Pesole G. ASPicDB: a database resource for alternative splicing analysis. Bioinformatics. 2008;24:1300–4. doi: 10.1093/bioinformatics/btn113. [DOI] [PubMed] [Google Scholar]
- Castrignano T, Rizzi R, Talamo IG, De Meo PD, Anselmo A, Bonizzoni P, Pesole G. ASPIC: a web resource for alternative splicing prediction and transcript isoforms characterization. Nucleic Acids Res. 2006;34:W440–3. doi: 10.1093/nar/gkl324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro MA, Oliveira MI, Nunes RJ, Fabre S, Barbosa R, Peixoto A, Brown MH, Parnes JR, Bismuth G, Moreira A, Rocha B, Carmo AM. Extracellular isoforms of CD6 generated by alternative splicing regulate targeting of CD6 to the immunological synapse. J Immunol. 2007;178:4351–61. doi: 10.4049/jimmunol.178.7.4351. [DOI] [PubMed] [Google Scholar]
- Cattaneo M, Lotti LV, Martino S, Cardano M, Orlandi R, Mariani-Costantini R, Biunno I. Functional characterization of two secreted SEL1L isoforms capable of exporting unassembled substrate. The Journal of biological chemistry. 2009;284:11405–15. doi: 10.1074/jbc.M805408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman G, Liebaers I, Van Steirteghem A, Van de Velde H. POU5F1 isoforms show different expression patterns in human embryonic stem cells and preimplantation embryos. Stem Cells. 2006;24:2685–91. doi: 10.1634/stemcells.2005-0611. [DOI] [PubMed] [Google Scholar]
- Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J. The soluble Interleukin 6 receptor: generation and role in inflammation and cancer. European journal of cell biology. 2011;90:484–94. doi: 10.1016/j.ejcb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Chao C, Ives KL, Goluszko E, Kolokoltsov AA, Davey RA, Townsend CM, Jr, Hellmich MR. SRC regulates constitutive internalization and rapid resensitization of a cholecystokinin 2 receptor splice variant. The Journal of biological chemistry. 2005;280:33368–73. doi: 10.1074/jbc.M506337200. [DOI] [PubMed] [Google Scholar]
- Chaudhary K, Donald RG, Nishi M, Carter D, Ullman B, Roos DS. Differential localization of alternatively spliced hypoxanthine-xanthine-guanine phosphoribosyltransferase isoforms in Toxoplasma gondii. J Biol Chem. 2005;280:22053–9. doi: 10.1074/jbc.M503178200. [DOI] [PubMed] [Google Scholar]
- Chavez-Gutierrez L, Bourdais J, Aranda G, Vargas MA, Matta-Camacho E, Ducancel F, Segovia L, Joseph-Bravo P, Charli JL. A truncated isoform of pyroglutamyl aminopeptidase II produced by exon extension has dominant-negative activity. Journal of neurochemistry. 2005;92:807–17. doi: 10.1111/j.1471-4159.2004.02916.x. [DOI] [PubMed] [Google Scholar]
- Chawla G, Lin CH, Han A, Shiue L, Ares M, Jr, Black DL. Sam68 regulates a set of alternatively spliced exons during neurogenesis. Molecular and cellular biology. 2009;29:201–13. doi: 10.1128/MCB.01349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah MT, Wachter A, Sudarsan N, Breaker RR. Control of alternative RNA splicing and gene expression by eukaryotic riboswitches. Nature. 2007;447:497–500. doi: 10.1038/nature05769. [DOI] [PubMed] [Google Scholar]
- Chen AM, Perrin MH, Digruccio MR, Vaughan JM, Brar BK, Arias CM, Lewis KA, Rivier JE, Sawchenko PE, Vale WW. A soluble mouse brain splice variant of type 2alpha corticotropin-releasing factor (CRF) receptor binds ligands and modulates their activity. Proc Natl Acad Sci U S A. 2005a;102:2620–5. doi: 10.1073/pnas.0409583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Jeffries O, Rowe IC, Liang Z, Knaus HG, Ruth P, Shipston MJ. Membrane trafficking of large conductance calcium-activated potassium channels is regulated by alternative splicing of a transplantable, acidic trafficking motif in the RCK1-RCK2 linker. J Biol Chem. 285:23265–75. doi: 10.1074/jbc.M110.139758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Beaven S, Tontonoz P. Identification and characterization of two alternatively spliced transcript variants of human liver × receptor alpha. Journal of lipid research. 2005b;46:2570–9. doi: 10.1194/jlr.M500157-JLR200. [DOI] [PubMed] [Google Scholar]
- Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol. 2009;10:741–54. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Ma SM, Dumitrache LC, Hasty P. Biochemical and cellular characteristics of the 3′ -> 5′ exonuclease TREX2. Nucleic Acids Res. 2007;35:2682–94. doi: 10.1093/nar/gkm151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Luo L, Zhang L. The organization of nucleosomes around splice sites. Nucleic acids research. 2010;38:2788–98. doi: 10.1093/nar/gkq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron. 2008;58:325–32. doi: 10.1016/j.neuron.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Cheng TL, Liao CC, Tsai WH, Lin CC, Yeh CW, Teng CF, Chang WT. Identification and characterization of the mitochondrial targeting sequence and mechanism in human citrate synthase. Journal of cellular biochemistry. 2009;107:1002–15. doi: 10.1002/jcb.22200. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Wang Y, Li Y, Deng Y, Hu J, Mo X, Li N, Li Y, Luo N, Yuan W, Xiao J, Zhu C, Wu X, Liu M. A novel human gene ZNF415 with five isoforms inhibits AP-1- and p53-mediated transcriptional activity. Biochem Biophys Res Commun. 2006;351:33–9. doi: 10.1016/j.bbrc.2006.09.161. [DOI] [PubMed] [Google Scholar]
- Cherny D, Gooding C, Eperon GE, Coelho MB, Bagshaw CR, Smith CW, Eperon IC. Stoichiometry of a regulatory splicing complex revealed by single-molecule analyses. The EMBO journal. 2010;29:2161–72. doi: 10.1038/emboj.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester AH, Azam R, Felkin LE, George R, Brand N. Correlation between vascular responsivensss and expression of novel transcripts of the ETA-receptor in human vascular tissue. Vascular pharmacology. 2007;46:181–7. doi: 10.1016/j.vph.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Choi HS, Kim CS, Hwang CK, Song KY, Wang W, Qiu Y, Law PY, Wei LN, Loh HH. The opioid ligand binding of human mu-opioid receptor is modulated by novel splice variants of the receptor. Biochemical and biophysical research communications. 2006;343:1132–40. doi: 10.1016/j.bbrc.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Choudhury R, Noakes CJ, McKenzie E, Kox C, Lowe M. Differential clathrin binding and subcellular localization of OCRL1 splice isoforms. J Biol Chem. 2009;284:9965–73. doi: 10.1074/jbc.M807442200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow LT, Gelinas RE, Broker TR, Roberts RJ. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell. 1977;12:1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- Chowdhury B, Tsokos CG, Krishnan S, Robertson J, Fisher CU, Warke RG, Warke VG, Nambiar MP, Tsokos GC. Decreased stability and translation of T cell receptor zeta mRNA with an alternatively spliced 3′-untranslated region contribute to zeta chain down-regulation in patients with systemic lupus erythematosus. The Journal of biological chemistry. 2005;280:18959–66. doi: 10.1074/jbc.M501048200. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Chu HY, Ohtoshi A. Cloning and functional analysis of hypothalamic homeobox gene Bsx1a and its isoform, Bsx1b. Mol Cell Biol. 2007;27:3743–9. doi: 10.1128/MCB.01561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianetti L, Segnalini P, Calzolari A, Morsilli O, Felicetti F, Ramoni C, Gabbianelli M, Testa U, Sposi NM. Expression of alternative transcripts of ferroportin-1 during human erythroid differentiation. Haematologica. 2005;90:1595–606. [PubMed] [Google Scholar]
- Claverie-Martin F, Flores C, Anton-Gamero M, Gonzalez-Acosta H, Garcia-Nieto V. The Alu insertion in the CLCN5 gene of a patient with Dent's disease leads to exon 11 skipping. Journal of human genetics. 2005;50:370–4. doi: 10.1007/s10038-005-0265-5. [DOI] [PubMed] [Google Scholar]
- Clower CV, Chatterjee D, Wang Z, Cantley LC, Vander Heiden MG, Krainer AR. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1894–9. doi: 10.1073/pnas.0914845107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collesi C, Santoro MM, Gaudino G, Comoglio PM. A splicing variant of the RON transcript induces constitutive tyrosine kinase activity and an invasive phenotype. Mol Cell Biol. 1996;16:5518–26. doi: 10.1128/mcb.16.10.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convery E, Shin EK, Ding Q, Wang W, Douglas P, Davis LS, Nickoloff JA, Lees-Miller SP, Meek K. Inhibition of homologous recombination by variants of the catalytic subunit of the DNA-dependent protein kinase (DNA-PKcs) Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1345–50. doi: 10.1073/pnas.0406466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BT, Roszelle BN, Long TC, Deutsch S, Manning KB. The 12 cc Penn State pulsatile pediatric ventricular assist device: fluid dynamics associated with valve selection. Journal of biomechanical engineering. 2008;130:041019. doi: 10.1115/1.2939342. [DOI] [PubMed] [Google Scholar]
- Cooper BT, Roszelle BN, Long TC, Deutsch S, Manning KB. The influence of operational protocol on the fluid dynamics in the 12 cc Penn state pulsatile pediatric ventricular assist device: the effect of end-diastolic delay. Artificial organs. 2010;34:E122–33. doi: 10.1111/j.1525-1594.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–93. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford PA, Gaddie KJ, Smith TM, Kirley TL. Characterization of analternative splice variant of human nucleoside triphosphate diphosphohydrolase 3 (NTPDase3): a possible modulator of nucleotidase activity and purinergic signaling. Archives of biochemistry and biophysics. 2007;457:7–15. doi: 10.1016/j.abb.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csaki LS, Reue K. Lipins: multifunctional lipid metabolism proteins. Annual review of nutrition. 2010;30:257–72. doi: 10.1146/annurev.nutr.012809.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabertrand F, Morel JL, Sorrentino V, Mironneau J, Mironneau C, Macrez N. Modulation of calcium signalling by dominant negative splice variant of ryanodine receptor subtype 3 in native smooth muscle cells. Cell Calcium. 2006;40:11–21. doi: 10.1016/j.ceca.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Dagan T, Sorek R, Sharon E, Ast G, Graur D. AluGene: a database of Alu elements incorporated within protein-coding genes. Nucleic Acids Res. 2004;32 Database issue:D489–92. doi: 10.1093/nar/gkh132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai F, Chang C, Lin X, Dai P, Mei L, Feng XH. Erbin inhibits transforming growth factor beta signaling through a novel Smad-interacting domain. Mol Cell Biol. 2007;27:6183–94. doi: 10.1128/MCB.00132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RM, Remo BF, Svensson EC. An alternative transcript of the FOG-2 gene encodes a FOG-2 isoform lacking the FOG repression motif. Biochemical and biophysical research communications. 2007;357:683–7. doi: 10.1016/j.bbrc.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damianov A, Black DL. Autoregulation of Fox protein expression to produce dominant negative splicing factors. Rna. 2010;16:405–16. doi: 10.1261/rna.1838210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud R, Mies G, Smialowska A, Oláh L, Hossmann K, Stamm S. Ischemia induces a translocation of the splicing factor tra2-beta1 and changes alternative splicing patterns in the brain. J Neurosci. 2002;22:5889–5899. doi: 10.1523/JNEUROSCI.22-14-05889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D, Clark TA, Schweitzer A, Yamamoto M, Marr H, Arribere J, Minovitsky S, Poliakov A, Dubchak I, Blume JE, Conboy JG. A correlation with exon expression approach to identify cis-regulatory elements for tissue-specific alternative splicing. Nucleic acids research. 2007a;35:4845–57. doi: 10.1093/nar/gkm485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Dufu K, Romney B, Feldt M, Elenko M, Reed R. Functional coupling of RNAP II transcription to spliceosome assembly. Genes & development. 2006;20:1100–9. doi: 10.1101/gad.1397406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Yu J, Zhang Z, Gygi MP, Krainer AR, Gygi SP, Reed R. SR proteins function in coupling RNAP II transcription to pre-mRNA splicing. Molecular cell. 2007b;26:867–81. doi: 10.1016/j.molcel.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Datta D, Flaxenburg JA, Laxmanan S, Geehan C, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer research. 2006;66:9509–18. doi: 10.1158/0008-5472.CAN-05-4345. [DOI] [PubMed] [Google Scholar]
- David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–8. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes & development. 2010;24:2343–64. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JQ, Chang GW, Yona S, Gordon S, Stacey M, Lin HH. The role of receptor oligomerization in modulating the expression and function of leukocyte adhesion-G protein-coupled receptors. J Biol Chem. 2007;282:27343–53. doi: 10.1074/jbc.M704096200. [DOI] [PubMed] [Google Scholar]
- Davis RL, Homer VM, George PM, Brennan SO. A deep intronic mutation in FGB creates a consensus exonic splicing enhancer motif that results in afibrinogenemia caused by aberrant mRNA splicing, which can be corrected in vitro with antisense oligonucleotide treatment. Human mutation. 2009;30:221–7. doi: 10.1002/humu.20839. [DOI] [PubMed] [Google Scholar]
- de la Grange P, Dutertre M, Correa M, Auboeuf D. A new advance in alternative splicing databases: from catalogue to detailed analysis of regulation of expression and function of human alternative splicing variants. BMC Bioinformatics. 2007;8:180. doi: 10.1186/1471-2105-8-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer research. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–91. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch M, Long M. Intron-exon structures of eukaryotic model organisms. Nucleic Acids Res. 1999;27:3219–28. doi: 10.1093/nar/27.15.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Long KD, Gaskins BL, Burket JA, Mastropaolo J. Sodium butyrate, an epigenetic interventional strategy, attenuates a stress-induced alteration of MK-801's pharmacologic action. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2008a;18:565–8. doi: 10.1016/j.euroneuro.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Long KD, Gaskins BL, Mastropaolo J. Guanosine possesses specific modulatory effects on NMDA receptor-mediated neurotransmission in intact mice. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2008b;18:299–302. doi: 10.1016/j.euroneuro.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Mastropaolo J, Long KD, Gaskins BL. Epigenetic therapeutic strategies for the treatment of neuropsychiatric disorders: ready for prime time? Clinical neuropharmacology. 2008c;31:104–19. doi: 10.1097/WNF.0b013e318067e255. [DOI] [PubMed] [Google Scholar]
- Dhir A, Buratti E. Alternative splicing: role of pseudoexons in human disease and potential therapeutic strategies. The FEBS journal. 2010;277:841–55. doi: 10.1111/j.1742-4658.2009.07520.x. [DOI] [PubMed] [Google Scholar]
- Diveu C, Venereau E, Froger J, Ravon E, Grimaud L, Rousseau F, Chevalier S, Gascan H. Molecular and functional characterization of a soluble form of oncostatin M/interleukin-31 shared receptor. J Biol Chem. 2006;281:36673–82. doi: 10.1074/jbc.M607005200. [DOI] [PubMed] [Google Scholar]
- Dixon RJ, Eperon IC, Hall L, Samani NJ. A genome-wide survey demonstrates widespread non-linear mRNA in expressed sequences from multiple species. Nucleic acids research. 2005;33:5904–13. doi: 10.1093/nar/gki893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzhanskaya N, Merz G, Denman RB. Alternative splicing modulates protein arginine methyltransferase-dependent methylation of fragile × syndrome mental retardation protein. Biochemistry. 2006;45:10385–93. doi: 10.1021/bi0525019. [DOI] [PubMed] [Google Scholar]
- Douglas KB, Windels DC, Zhao J, Gadeliya AV, Wu H, Kaufman KM, Harley JB, Merrill J, Kimberly RP, Alarcon GS, Brown EE, Edberg JC, Ramsey-Goldman R, Petri M, Reveille JD, Vila LM, Gaffney PM, James JA, Moser KL, Alarcon-Riquelme ME, Vyse TJ, Gilkeson GS, Jacob CO, Ziegler JT, Langefeld CD, Ulgiati D, Tsao BP, Boackle SA. Complement receptor 2 polymorphisms associated with systemic lupus erythematosus modulate alternative splicing. Genes and immunity. 2009;10:457–69. doi: 10.1038/gene.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Lawrence EJ, Strzelecki D, Rajput P, Xia SJ, Gottesman DM, Barr FG. Co-expression of alternatively spliced forms of PAX3, PAX7, PAX3-FKHR and PAX7-FKHR with distinct DNA binding and transactivation properties in rhabdomyosarcoma. International journal of cancer Journal international du cancer. 2005;115:85–92. doi: 10.1002/ijc.20844. [DOI] [PubMed] [Google Scholar]
- Dubey D, Ganesh S. Modulation of functional properties of laforin phosphatase by alternative splicing reveals a novel mechanism for the EPM2A gene in Lafora progressive myoclonus epilepsy. Hum Mol Genet. 2008;17:3010–20. doi: 10.1093/hmg/ddn199. [DOI] [PubMed] [Google Scholar]
- Dubielecka PM, Cui P, Xiong X, Hossain S, Heck S, Angelov L, Kotula L. Differential regulation of macropinocytosis by Abi1/Hssh3bp1 isoforms. PLoS One. 5:e10430. doi: 10.1371/journal.pone.0010430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudziak D, Nimmerjahn F, Bornkamm GW, Laux G. Alternative splicing generates putative soluble CD83 proteins that inhibit T cell proliferation. J Immunol. 2005;174:6672–6. doi: 10.4049/jimmunol.174.11.6672. [DOI] [PubMed] [Google Scholar]
- Dunn NR, Koonce CH, Anderson DC, Islam A, Bikoff EK, Robertson EJ. Mice exclusively expressing the short isoform of Smad2 develop normally and are viable and fertile. Genes & development. 2005;19:152–63. doi: 10.1101/gad.1243205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D, Ladomery M. Molecular Biology of RNA. Oxford University Press; 2011. [Google Scholar]
- Ellis JC, Brown DD, Brown JW. The small nucleolar ribonucleoprotein (snoRNP) database. RNA. 2010;16:664–6. doi: 10.1261/rna.1871310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSharawy A, Manaster C, Teuber M, Rosenstiel P, Kwiatkowski R, Huse K, Platzer M, Becker A, Nurnberg P, Schreiber S, Hampe J. SNPSplicer: systematic analysis of SNP-dependent splicing in genotyped cDNAs. Hum Mutat. 2006;27:1129–34. doi: 10.1002/humu.20377. [DOI] [PubMed] [Google Scholar]
- Ersahin C, Szpaderska AM, Orawski AT, Simmons WH. Aminopeptidase P isozyme expression in human tissues and peripheral blood mononuclear cell fractions. Arch Biochem Biophys. 2005;435:303–10. doi: 10.1016/j.abb.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Eshel D, Toporik A, Efrati T, Nakav S, Chen A, Douvdevani A. Characterization of natural human antagonistic soluble CD40 isoforms produced through alternative splicing. Molecular immunology. 2008;46:250–7. doi: 10.1016/j.molimm.2008.08.280. [DOI] [PubMed] [Google Scholar]
- Essand M, Vikman S, Grawe J, Gedda L, Hellberg C, Oberg K, Totterman TH, Giandomenico V. Identification and characterization of a novel splicing variant of vesicular monoamine transporter 1. J Mol Endocrinol. 2005;35:489–501. doi: 10.1677/jme.1.01875. [DOI] [PubMed] [Google Scholar]
- Evans RT, Seasholtz AF. Soluble corticotropin-releasing hormone receptor 2alpha splice variant is efficiently translated but not trafficked for secretion. Endocrinology. 2009;150:4191–202. doi: 10.1210/en.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris W, Leissring MA, Hemming ML, Chang AY, Selkoe DJ. Alternative splicing of human insulin-degrading enzyme yields a novel isoform with a decreased ability to degrade insulin and amyloid beta-protein. Biochemistry. 2005;44:6513–25. doi: 10.1021/bi0476578. [DOI] [PubMed] [Google Scholar]
- Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes & development. 2006a;20:1470–84. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YH, Li X, Wang L, Zhou L, Gorodeski GI. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem. 2006b;281:17228–37. doi: 10.1074/jbc.M602999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes JM, Kinghorn JR, Johnston IA. Differential regulation of multiple alternatively spliced transcripts of MyoD. Gene. 2007;391:178–85. doi: 10.1016/j.gene.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Straczkowski M, Lainez B, Chacon MR, Kowalska I, Lopez-Bermejo A, Garcia-Espana A, Nikolajuk A, Kinalska I, Ricart W. An alternative spliced variant of circulating soluble tumor necrosis factor-alpha receptor-2 is paradoxically associated with insulin action. European journal of endocrinology / European Federation of Endocrine Societies. 2006;154:723–30. doi: 10.1530/eje.1.02145. [DOI] [PubMed] [Google Scholar]
- Ferrera L, Caputo A, Ubby I, Bussani E, Zegarra-Moran O, Ravazzolo R, Pagani F, Galietta LJ. Regulation of TMEM16A chloride channel properties by alternative splicing. J Biol Chem. 2009;284:33360–8. doi: 10.1074/jbc.M109.046607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher RB, Baker JC, Harland RM. FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development. 2006;133:1703–14. doi: 10.1242/dev.02342. [DOI] [PubMed] [Google Scholar]
- Flicek P, Brent MR. Using several pair-wise informant sequences for de novo prediction of alternatively spliced transcripts. Genome Biol. 2006;7 Suppl 1:S8 1–9. doi: 10.1186/gb-2006-7-s1-s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissac S, Sammeth M. ASTALAVISTA: dynamic and flexible analysis of alternative splicing events in custom gene datasets. Nucleic Acids Res. 2007;35:W297–9. doi: 10.1093/nar/gkm311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–70. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Frith MC, Carninci P, Kai C, Kawai J, Bailey TL, Hayashizaki Y, Mattick JS. Splicing bypasses 3′ end formation signals to allow complex gene architectures. Gene. 2007;403:188–93. doi: 10.1016/j.gene.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Fu G, Condon KC, Epton MJ, Gong P, Jin L, Condon GC, Morrison NI, Dafa'alla TH, Alphey L. Female-specific insect lethality engineered using alternative splicing. Nature biotechnology. 2007;25:353–7. doi: 10.1038/nbt1283. [DOI] [PubMed] [Google Scholar]
- Fujikake N, Nagai Y, Popiel HA, Kano H, Yamaguchi M, Toda T. Alternative splicing regulates the transcriptional activity of Drosophila heat shock transcription factor in response to heat/cold stress. FEBS letters. 2005;579:3842–8. doi: 10.1016/j.febslet.2005.05.074. [DOI] [PubMed] [Google Scholar]
- Fukuda E, Hamada S, Hasegawa S, Katori S, Sanbo M, Miyakawa T, Yamamoto T, Yamamoto H, Hirabayashi T, Yagi T. Down-regulation of protocadherin-alpha A isoforms in mice changes contextual fear conditioning and spatial working memory. Eur J Neurosci. 2008;28:1362–76. doi: 10.1111/j.1460-9568.2008.06428.x. [DOI] [PubMed] [Google Scholar]
- Futami K, Shimamoto A, Furuichi Y. Mitochondrial and nuclear localization of human Pif1 helicase. Biol Pharm Bull. 2007;30:1685–92. doi: 10.1248/bpb.30.1685. [DOI] [PubMed] [Google Scholar]
- Garcia-Alai MM, Tidow H, Natan E, Townsley FM, Veprintsev DB, Fersht AR. The novel p53 isoform “delta p53” is a misfolded protein and does not bind the p21 promoter site. Protein science : a publication of the Protein Society. 2008;17:1671–8. doi: 10.1110/ps.036996.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg K, Green P. Differing patterns of selection in alternative and constitutive splice sites. Genome research. 2007;17:1015–22. doi: 10.1101/gr.6347907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassler N, Roth W, Funke B, Schneider A, Herzog F, Tischendorf JJ, Grund K, Penzel R, Bravo IG, Mariadason J, Ehemann V, Sykora J, Haas TL, Walczak H, Ganten T, Zentgraf H, Erb P, Alonso A, Autschbach F, Schirmacher P, Knuchel R, Kopitz J. Regulation of enterocyte apoptosis by acyl-CoA synthetase 5 splicing. Gastroenterology. 2007;133:587–98. doi: 10.1053/j.gastro.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B, Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer research. 2006;66:5216–23. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- Gavin AL, Duong B, Skog P, Ait-Azzouzene D, Greaves DR, Scott ML, Nemazee D. deltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J Immunol. 2005;175:319–28. doi: 10.4049/jimmunol.175.1.319. [DOI] [PubMed] [Google Scholar]
- Georges R, Nemer G, Morin M, Lefebvre C, Nemer M. Distinct expression and function of alternatively spliced Tbx5 isoforms in cell growth and differentiation. Molecular and cellular biology. 2008;28:4052–67. doi: 10.1128/MCB.02100-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigna C, Giordano S, Shen H, Benvenuto F, Castiglioni F, Comoglio PM, Green MR, Riva S, Biamonti G. Cell motility is controlled by SF2/ASF through alternative splicing of the Ron protooncogene. Mol Cell. 2005;20:881–90. doi: 10.1016/j.molcel.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Ghosh M, Loper R, Gelb MH, Leslie CC. Identification of the expressed form of human cytosolic phospholipase A2beta (cPLA2beta): cPLA2beta3 is a novel variant localized to mitochondria and early endosomes. The Journal of biological chemistry. 2006;281:16615–24. doi: 10.1074/jbc.M601770200. [DOI] [PubMed] [Google Scholar]
- Gnidehou S, Lacroix L, Sezan A, Ohayon R, Noel-Hudson MS, Morand S, Francon J, Courtin F, Virion A, Dupuy C. Cloning and characterization of a novel isoform of iodotyrosine dehalogenase 1 (DEHAL1) DEHAL1C from human thyroid: comparisons with DEHAL1 and DEHAL1B. Thyroid : official journal of the American Thyroid Association. 2006;16:715–24. doi: 10.1089/thy.2006.16.715. [DOI] [PubMed] [Google Scholar]
- Goncalves V, Matos P, Jordan P. Antagonistic SR proteins regulate alternative splicing of tumor-related Rac1b downstream of the PI3-kinase and Wnt pathways. Human molecular genetics. 2009;18:3696–707. doi: 10.1093/hmg/ddp317. [DOI] [PubMed] [Google Scholar]
- Gooding C, Clark F, Wollerton MC, Grellscheid SN, Groom H, Smith CW. A class of human exons with predicted distant branch points revealed by analysis of AG dinucleotide exclusion zones. Genome biology. 2006;7:R1. doi: 10.1186/gb-2006-7-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson ML, Jonas BA, Privalsky ML. Alternative mRNA splicing of SMRT creates functional diversity by generating corepressor isoforms with different affinities for different nuclear receptors. The Journal of biological chemistry. 2005;280:7493–503. doi: 10.1074/jbc.M411514200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan S, Van Emburgh BO, Shan J, Su Z, Fields CR, Vieweg J, Hamazaki T, Schwartz PH, Terada N, Robertson KD. A novel DNMT3B splice variant expressed in tumor and pluripotent cells modulates genomic DNA methylation patterns and displays altered DNA binding. Molecular cancer research : MCR. 2009;7:1622–34. doi: 10.1158/1541-7786.MCR-09-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granero-Molto F, Sarmah S, O'Rear L, Spagnoli A, Abrahamson D, Saus J, Hudson BG, Knapik EW. Goodpasture antigen-binding protein and its spliced variant, ceramide transfer protein, have different functions in the modulation of apoptosis during zebrafish development. J Biol Chem. 2008;283:20495–504. doi: 10.1074/jbc.M801806200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum S, Sundvold-Gjerstad V, Dai KZ, Kolltveit KM, Hildebrand K, Huitfeldt HS, Lea T, Spurkland A. Structure function analysis of SH2D2A isoforms expressed in T cells reveals a crucial role for the proline rich region encoded by SH2D2A exon 7. BMC immunology. 2006;7:15. doi: 10.1186/1471-2172-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley BR. Mutually exclusive splicing of the insect Dscam pre-mRNA directed by competing intronic RNA secondary structures. Cell. 2005;123:65–73. doi: 10.1016/j.cell.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley BR. The haplo-spliceo-transcriptome: common variations in alternative splicing in the human population. Trends in genetics : TIG. 2008;24:5–7. doi: 10.1016/j.tig.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveley BR. Alternative splicing: regulation without regulators. Nature structural & molecular biology. 2009;16:13–5. doi: 10.1038/nsmb0109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Peacock JN, Squires EJ. Characterization of the porcine constitutive androstane receptor (CAR) and its splice variants. Xenobiotica; the fate of foreign compounds in biological systems. 2009;39:915–30. doi: 10.3109/00498250903330348. [DOI] [PubMed] [Google Scholar]
- Gray NW, Kruchten AE, Chen J, McNiven MA. A dynamin-3 spliced variant modulates the actin/cortactin-dependent morphogenesis of dendritic spines. J Cell Sci. 2005;118:1279–90. doi: 10.1242/jcs.01711. [DOI] [PubMed] [Google Scholar]
- Grillo G, Turi A, Licciulli F, Mignone F, Liuni S, Banfi S, Gennarino VA, Horner DS, Pavesi G, Picardi E, Pesole G. UTRdb and UTRsite (RELEASE 2010): a collection of sequences and regulatory motifs of the untranslated regions of eukaryotic mRNAs. Nucleic Acids Res. 2010;38:D75–80. doi: 10.1093/nar/gkp902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann M, Hammer P, Walther M, Paulmann N, Buttner A, Eisenmenger W, Baghai TC, Schule C, Rupprecht R, Bader M, Bondy B, Zill P, Priller J, Walther DJ. Alternative splicing and extensive RNA editing of human TPH2 transcripts. PLoS ONE. 2010;5:e8956. doi: 10.1371/journal.pone.0008956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb DR, Vasilevski O, Huynh H, Woodcock EA. The extreme C-terminal region of phospholipase Cbeta1 determines subcellular localization and function; the “b” splice variant mediates alpha1-adrenergic receptor responses in cardiomyocytes. FASEB J. 2008;22:2768–74. doi: 10.1096/fj.07-102558. [DOI] [PubMed] [Google Scholar]
- Gunzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Muller D. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. Journal of cell science. 2009;122:1507–17. doi: 10.1242/jcs.040113. [DOI] [PubMed] [Google Scholar]
- Guthrie CR, Murray AT, Franklin AA, Hamblin MW. Differential agonist-mediated internalization of the human 5-hydroxytryptamine 7 receptor isoforms. J Pharmacol Exp Ther. 2005;313:1003–10. doi: 10.1124/jpet.104.081919. [DOI] [PubMed] [Google Scholar]
- Hagiyama M, Ichiyanagi N, Kimura KB, Murakami Y, Ito A. Expression of a soluble isoform of cell adhesion molecule 1 in the brain and its involvement in directional neurite outgrowth. The American journal of pathology. 2009;174:2278–89. doi: 10.2353/ajpath.2009.080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T, Yeung ML, Wood TG, Wei Y, Yamaoka S, Gatalica Z, Jeang KT, Brasier AR. An alternative splice product of IkappaB kinase (IKKgamma), IKKgamma-delta, differentially mediates cytokine and human T-cell leukemia virus type 1 tax-induced NF-kappaB activation. Journal of virology. 2006;80:4227–41. doi: 10.1128/JVI.80.9.4227-4241.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakre S, Tussie-Luna MI, Ashworth T, Novina CD, Settleman J, Sharp PA, Roy AL. Opposing functions of TFII-I spliced isoforms in growth factor-induced gene expression. Molecular cell. 2006;24:301–8. doi: 10.1016/j.molcel.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Hallegger M, Llorian M, Smith CW. Alternative splicing: global insights. The FEBS journal. 2010;277:856–66. doi: 10.1111/j.1742-4658.2009.07521.x. [DOI] [PubMed] [Google Scholar]
- Han GS, Carman GM. Characterization of the human LPIN1-encoded phosphatidate phosphatase isoforms. The Journal of biological chemistry. 2010;285:14628–38. doi: 10.1074/jbc.M110.117747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SP, Friend LR, Carson JH, Korza G, Barbarese E, Maggipinto M, Hatfield JT, Rothnagel JA, Smith R. Differential subcellular distributions and trafficking functions of hnRNP A2/B1 spliceoforms. Traffic. 2010;11:886–98. doi: 10.1111/j.1600-0854.2010.01072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Eppinger E, Schuster IG, Weigand LU, Liang X, Kremmer E, Peschel C, Krackhardt AM. Formin-like 1 (FMNL1) is regulated by N-terminal myristoylation and induces polarized membrane blebbing. The Journal of biological chemistry. 2009;284:33409–17. doi: 10.1074/jbc.M109.060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoun N, Bureau C, Diab T, Gayet O, Dusetti N, Selves J, Vinel JP, Buscail L, Cordelier P, Torrisani J. The SV2 variant of KLF6 is down-regulated in hepatocellular carcinoma and displays anti-proliferative and pro-apoptotic functions. Journal of hepatology. 2010;53:880–8. doi: 10.1016/j.jhep.2010.04.038. [DOI] [PubMed] [Google Scholar]
- Harada N, Yamada Y, Tsukiyama K, Yamada C, Nakamura Y, Mukai E, Hamasaki A, Liu X, Toyoda K, Seino Y, Inagaki N. A novel GIP receptor splice variant influences GIP sensitivity of pancreatic beta-cells in obese mice. Am J Physiol Endocrinol Metab. 2008a;294:E61–8. doi: 10.1152/ajpendo.00358.2007. [DOI] [PubMed] [Google Scholar]
- Harada N, Yonemoto H, Yoshida M, Yamamoto H, Yin Y, Miyamoto A, Hattori A, Wu Q, Nakagawa T, Nakano M, Teshigawara K, Mawatari K, Hosaka T, Takahashi A, Nakaya Y. Alternative splicing produces a constitutively active form of human SREBP-1. Biochemical and biophysical research communications. 2008b;368:820–6. doi: 10.1016/j.bbrc.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Harris GM, Dodelzon K, Gong L, Gonzalez-Alegre P, Paulson HL. Splice isoforms of the polyglutamine disease protein ataxin-3 exhibit similar enzymatic yet different aggregation properties. PLoS One. 5:e13695. doi: 10.1371/journal.pone.0013695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann B, Castelo R, Blanchette M, Boue S, Rio DC, Valcarcel J. Global analysis of alternative splicing regulation by insulin and wingless signaling in Drosophila cells. Genome biology. 2009;10:R11. doi: 10.1186/gb-2009-10-1-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Ishida E, Matsumoto S, Shibusawa N, Okada S, Monden T, Satoh T, Yamada M, Mori M. A liver × receptor (LXR)-beta alternative splicing variant (LXRBSV) acts as an RNA co-activator of LXR-beta. Biochemical and biophysical research communications. 2009a;390:1260–5. doi: 10.1016/j.bbrc.2009.10.132. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Kurita M, Matsuoka M. Identification of soluble WSX-1 not as a dominant-negative but as an alternative functional subunit of a receptor for an anti-Alzheimer's disease rescue factor Humanin. Biochemical and biophysical research communications. 2009b;389:95–9. doi: 10.1016/j.bbrc.2009.08.095. [DOI] [PubMed] [Google Scholar]
- Hasler J, Samuelsson T, Strub K. Useful ‘junk’: Alu RNAs in the human transcriptome. Cell Mol Life Sci. 2007;64:1793–800. doi: 10.1007/s00018-007-7084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DM, Conner SD. A novel AAK1 splice variant functions at multiple steps of the endocytic pathway. Mol Biol Cell. 2007;18:2698–706. doi: 10.1091/mbc.E06-09-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve MA, Buteau-Lozano H, Mourah S, Calvo F, Perrot-Applanat M. VEGF189 stimulates endothelial cells proliferation and migration in vitro and up-regulates the expression of Flk-1/KDR mRNA. Experimental cell research. 2005;309:24–31. doi: 10.1016/j.yexcr.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Heyd F, Carmo-Fonseca M, Moroy T. Differential isoform expression and interaction with the P32 regulatory protein controls the subcellular localization of the splicing factor U2AF26. The Journal of biological chemistry. 2008;283:19636–45. doi: 10.1074/jbc.M801014200. [DOI] [PubMed] [Google Scholar]
- Hiller M, Nikolajewa S, Huse K, Szafranski K, Rosenstiel P, Schuster S, Backofen R, Platzer M. TassDB: a database of alternative tandem splice sites. Nucleic Acids Res. 2007a;35:D188–92. doi: 10.1093/nar/gkl762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller M, Zhang Z, Backofen R, Stamm S. Pre-mRNA secondary structures influence exon recognition. PLOS Genetics. 2007b;3:e204. doi: 10.1371/journal.pgen.0030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld M, zur Hausen A, Bettendorf H, Jager M, Stickeler E. Alternative splicing of Cyr61 is regulated by hypoxia and significantly changed in breast cancer. Cancer research. 2009;69:2082–90. doi: 10.1158/0008-5472.CAN-08-1997. [DOI] [PubMed] [Google Scholar]
- Hmitou I, Druillennec S, Valluet A, Peyssonnaux C, Eychene A. Differential regulation of B-raf isoforms by phosphorylation and autoinhibitory mechanisms. Molecular and cellular biology. 2007;27:31–43. doi: 10.1128/MCB.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi Z, Nemeth AL, Radnai L, Hetenyi C, Schlett K, Bodor A, Perczel A, Nyitray L. Alternatively spliced exon B of myosin Va is essential for binding the tail-associated light chain shared by dynein. Biochemistry. 2006;45:12582–95. doi: 10.1021/bi060991e. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Aigaki T. Alternative trans-splicing: a novel mode of pre-mRNA processing. Biology of the cell / under the auspices of the European Cell Biology Organization. 2006;98:135–40. doi: 10.1042/BC20050002. [DOI] [PubMed] [Google Scholar]
- Hosoya T, Monden T, Fukabori Y, Hashimoto K, Satoh T, Kasai K, Yamada M, Mori M. A novel splice variant of the nuclear coactivator p120 functions strongly for androgen receptor: characteristic expression in prostate disease. Endocrine journal. 2008;55:657–65. doi: 10.1507/endocrj.k07e-133. [DOI] [PubMed] [Google Scholar]
- Hovelmeyer N, Wunderlich FT, Massoumi R, Jakobsen CG, Song J, Worns MA, Merkwirth C, Kovalenko A, Aumailley M, Strand D, Bruning JC, Galle PR, Wallach D, Fassler R, Waisman A. Regulation of B cell homeostasis and activation by the tumor suppressor gene CYLD. J Exp Med. 2007;204:2615–27. doi: 10.1084/jem.20070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FR, Chang HY, Lin YL, Tsai YT, Peng HL, Chen YT, Cheng CY, Shih MY, Liu CH, Chen CF. AVATAR: a database for genome- wide alternative splicing event detection using large scale ESTs and mRNAs. Bioinformation. 2005;1:16–8. doi: 10.6026/97320630001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HD, Horng JT, Lin FM, Chang YC, Huang CC. SpliceInfo: an information repository for mRNA alternative splicing in human genome. Nucleic Acids Res. 2005;33:D80–5. doi: 10.1093/nar/gki129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Chien CH, Jen KH, Huang HD. RegRNA: an integrated web server for identifying regulatory RNA motifs and elements. Nucleic Acids Res. 2006a;34:W429–34. doi: 10.1093/nar/gkl333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MC, Miller AL, Wang W, Kong Y, Paul S, Goetzl EJ. Differential signaling of T cell generation of IL-4 by wild-type and short-deletion variant of type 2 G protein-coupled receptor for vasoactive intestinal peptide (VPAC2) J Immunol. 2006b;176:6640–6. doi: 10.4049/jimmunol.176.11.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff JT, Plocik AM, Guthrie C, Yamamoto KR. Reciprocal intronic and exonic histone modification regions in humans. Nature structural & molecular biology. 2010;17:1495–9. doi: 10.1038/nsmb.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull J, Campino S, Rowlands K, Chan MS, Copley RR, Taylor MS, Rockett K, Elvidge G, Keating B, Knight J, Kwiatkowski D. Identification of common genetic variation that modulates alternative splicing. PLOS Genetics. 2007;3:e99. doi: 10.1371/journal.pgen.0030099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt KJ, Sezen SF, Champion HC, Crone JK, Palese MA, Huang PL, Sawa A, Luo X, Musicki B, Snyder SH, Burnett AL. Alternatively spliced neuronal nitric oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2006;103:3440–3. doi: 10.1073/pnas.0511326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang EM, Kim DG, Lee BJ, Choi J, Kim E, Park N, Kang D, Han J, Choi WS, Hong SG, Park JY. Alternative splicing generates a novel non-secretable cytosolic isoform of NELL2. Biochem Biophys Res Commun. 2007;353:805–11. doi: 10.1016/j.bbrc.2006.12.115. [DOI] [PubMed] [Google Scholar]
- Hymowitz SG, Patel DR, Wallweber HJ, Runyon S, Yan M, Yin J, Shriver SK, Gordon NC, Pan B, Skelton NJ, Kelley RF, Starovasnik MA. Structures of APRIL-receptor complexes: like BCMA, TACI employs only a single cysteine-rich domain for high affinity ligand binding. The Journal of biological chemistry. 2005;280:7218–27. doi: 10.1074/jbc.M411714200. [DOI] [PubMed] [Google Scholar]
- Irimia M, Rukov JL, Penny D, Roy SW. Functional and evolutionary analysis of alternatively spliced genes is consistent with an early eukaryotic origin of alternative splicing. BMC evolutionary biology. 2007;7:188. doi: 10.1186/1471-2148-7-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia M, Rukov JL, Roy SW. Evolution of alternative splicing regulation: changes in predicted exonic splicing regulators are not associated with changes in alternative splicing levels in primates. PLoS ONE. 2009;4:e5800. doi: 10.1371/journal.pone.0005800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel DD, Regan JW. EP(3) prostanoid receptor isoforms utilize distinct mechanisms to regulate ERK 1/2 activation. Biochimica et biophysica acta. 2009;1791:238–45. doi: 10.1016/j.bbalip.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagatomo K, Kubo Y, Saitoh O. Alternative splicing of RGS8 gene changes the binding property to the M1 muscarinic receptor to confer receptor type-specific Gq regulation. J Neurochem. 2006;99:1505–16. doi: 10.1111/j.1471-4159.2006.04220.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Koibuchi N, Chin WW. Synovial sarcoma translocation (SYT) encodes a nuclear receptor coactivator. Endocrinology. 2005;146:3892–9. doi: 10.1210/en.2004-1513. [DOI] [PubMed] [Google Scholar]
- Iwata H, Kitano T, Umetsu K, Yuasa I, Yamazaki K, Kemkes-Matthes B, Ichinose A. Distinct C-terminus of the B subunit of factor XIII in a population-associated major phenotype: the first case of complete allele-specific alternative splicing products in the coagulation and fibrinolytic systems. Journal of thrombosis and haemostasis : JTH. 2009;7:1084–91. doi: 10.1111/j.1538-7836.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo JM, Majos N, Bonnal S, Martinez C, Castelo R, Guigo R, Bilbao D, Valcarcel J. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Molecular cell. 2005;19:475–84. doi: 10.1016/j.molcel.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Izquierdo JM, Valcarcel J. Fas-activated serine/threonine kinase (FAST K) synergizes with TIA-1/TIAR proteins to regulate Fas alternative splicing. J Biol Chem. 2007;282:1539–43. doi: 10.1074/jbc.C600198200. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Helston RM, McKay JA, O'Neill ED, Mathers JC, Ford D. Splice variants of the human zinc transporter ZnT5 (SLC30A5) are differentially localized and regulated by zinc through transcription and mRNA stability. J Biol Chem. 2007;282:10423–31. doi: 10.1074/jbc.M610535200. [DOI] [PubMed] [Google Scholar]
- Jaskolski F, Normand E, Mulle C, Coussen F. Differential trafficking of GluR7 kainate receptor subunit splice variants. J Biol Chem. 2005;280:22968–76. doi: 10.1074/jbc.M413166200. [DOI] [PubMed] [Google Scholar]
- Jeong MH, Bae J, Kim WH, Yoo SM, Kim JW, Song PI, Choi KH. p19ras interacts with and activates p73 by involving the MDM2 protein. J Biol Chem. 2006;281:8707–15. doi: 10.1074/jbc.M513853200. [DOI] [PubMed] [Google Scholar]
- Jin C, Woodward JJ. Effects of 8 different NR1 splice variants on the ethanol inhibition of recombinant NMDA receptors. Alcoholism, clinical and experimental research. 2006;30:673–9. doi: 10.1111/j.1530-0277.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Sestan N. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DC, Roghanian A, Brown DP, Chang C, Allen RL, Trowsdale J, Young NT. Alternative mRNA splicing creates transcripts encoding soluble proteins from most LILR genes. European journal of immunology. 2009;39:3195–206. doi: 10.1002/eji.200839080. [DOI] [PubMed] [Google Scholar]
- Jonsen MD, Duval DL, Gutierrez-Hartmann A. The 26-amino acid beta-motif of the Pit-1beta transcription factor is a dominant and independent repressor domain. Molecular endocrinology. 2009;23:1371–84. doi: 10.1210/me.2008-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose AM, Koelle MR. Domains, amino acid residues, and new isoforms of Caenorhabditis elegans diacylglycerol kinase 1 (DGK-1) important for terminating diacylglycerol signaling in vivo. J Biol Chem. 2005;280:2730–6. doi: 10.1074/jbc.M409460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozsi M, Richter H, Loschmann I, Skerka C, Buck F, Beisiegel U, Erdei A, Zipfel PF. FHR-4A: a new factor H-related protein is encoded by the human FHR-4 gene. European journal of human genetics : EJHG. 2005;13:321–9. doi: 10.1038/sj.ejhg.5201324. [DOI] [PubMed] [Google Scholar]
- Kabuss R, Ashikov A, Oelmann S, Gerardy-Schahn R, Bakker H. Endoplasmic reticulum retention of the large splice variant of the UDP-galactose transporter is caused by a dilysine motif. Glycobiology. 2005;15:905–11. doi: 10.1093/glycob/cwi085. [DOI] [PubMed] [Google Scholar]
- Kahn AB, Ryan MC, Liu H, Zeeberg BR, Jamison DC, Weinstein JN. SpliceMiner: a high-throughput database implementation of the NCBI Evidence Viewer for microarray splice variant analysis. BMC Bioinformatics. 2007;8:75. doi: 10.1186/1471-2105-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina E, Fontenele-Neto JD, Fricker LD. Drosophila S2 cells produce multiple forms of carboxypeptidase D with different intracellular distributions. J Cell Biochem. 2006;99:770–83. doi: 10.1002/jcb.20972. [DOI] [PubMed] [Google Scholar]
- Kalsotra A, Wang K, Li PF, Cooper TA. MicroRNAs coordinate an alternative splicing network during mouse postnatal heart development. Genes & development. 2010;24:653–8. doi: 10.1101/gad.1894310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamhi E, Raitskin O, Sperling R, Sperling J. A potential role for initiator-tRNA in pre-mRNA splicing regulation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11319–24. doi: 10.1073/pnas.0911561107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Z, Garrett-Engele PW, Johnson JM, Castle JC. Evolutionarily conserved and diverged alternative splicing events show different expression and functional profiles. Nucleic acids research. 2005;33:5659–66. doi: 10.1093/nar/gki834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HC, Chae JH, Lee YH, Park MA, Shin JH, Kim SH, Ye SK, Cho YS, Fiering S, Kim CG. Erythroid cell-specific alpha-globin gene regulation by the CP2 transcription factor family. Molecular and cellular biology. 2005;25:6005–20. doi: 10.1128/MCB.25.14.6005-6020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapustin Y, Souvorov A, Tatusova T, Lipman D. Splign: algorithms for computing spliced alignments with identification of paralogs. Biol Direct. 2008;3:20. doi: 10.1186/1745-6150-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar A, Fushimi K, Zhou X, Ray P, Shi C, Chen X, Liu Z, Chen S, Wu JY. RNA helicase p68 (DDX5) regulates tau exon 10 splicing by modulating a stem-loop structure at the 5′ splice site. Mol Cell Biol. 2011;31:1812–21. doi: 10.1128/MCB.01149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima T, Rao N, Manley JL. An intronic element contributes to splicing repression in spinal muscular atrophy. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3426–31. doi: 10.1073/pnas.0700343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats JJ, Maxwell CA, Taylor BJ, Hendzel MJ, Chesi M, Bergsagel PL, Larratt LM, Mant MJ, Reiman T, Belch AR, Pilarski LM. Overexpression of transcripts originating from the MMSET locus characterizes all t(4;14)(p16;q32)-positive multiple myeloma patients. Blood. 2005;105:4060–9. doi: 10.1182/blood-2004-09-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren H, Lev-Maor G, Ast G. Alternative splicing and evolution: diversification, exon definition and function. Nature reviews Genetics. 2010;11:345–55. doi: 10.1038/nrg2776. [DOI] [PubMed] [Google Scholar]
- Kern J, Bauer M, Rychli K, Wojta J, Ritsch A, Gastl G, Gunsilius E, Untergasser G. Alternative splicing of vasohibin-1 generates an inhibitor of endothelial cell proliferation, migration, and capillary tube formation. Arterioscler Thromb Vasc Biol. 2008;28:478–84. doi: 10.1161/ATVBAHA.107.160432. [DOI] [PubMed] [Google Scholar]
- Khankin EV, Mutter WP, Tamez H, Yuan HT, Karumanchi SA, Thadhani R. Soluble erythropoietin receptor contributes to erythropoietin resistance in end-stage renal disease. PloS one. 2010;5:e9246. doi: 10.1371/journal.pone.0009246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Stamm S. Regulation of alternative splicing by short non-coding nuclear RNAs. RNA Biol. 2010;19:480–5. doi: 10.4161/rna.7.4.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Nishi K, Isono T, Okuyama Y, Tambe Y, Okada Y, Inoue H. Cyclin D1b variant promotes cell invasiveness independent of binding to CDK4 in human bladder cancer cells. Molecular carcinogenesis. 2009;48:953–64. doi: 10.1002/mc.20547. [DOI] [PubMed] [Google Scholar]
- Kim E, Goren A, Ast G. Alternative splicing and disease. RNA Biol. 2008a;5:17–9. doi: 10.4161/rna.5.1.5944. [DOI] [PubMed] [Google Scholar]
- Kim E, Goren A, Ast G. Insights into the connection between cancer and alternative splicing. Trends in genetics : TIG. 2008b;24:7–10. doi: 10.1016/j.tig.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Kim E, Magen A, Ast G. Different levels of alternative splicing among eukaryotes. Nucleic acids research. 2007a;35:125–31. doi: 10.1093/nar/gkl924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Woo IS, Kang ES, Eun SY, Kim HJ, Lee JH, Chang KC, Kim JH, Seo HG. Identification of a truncated alternative splicing variant of human PPARgamma1 that exhibits dominant negative activity. Biochem Biophys Res Commun. 2006;347:698–706. doi: 10.1016/j.bbrc.2006.06.147. [DOI] [PubMed] [Google Scholar]
- Kim JE, Han JM, Park CR, Shin KJ, Ahn C, Seong JY, Hwang JI. Splicing variants of the orphan G-protein-coupled receptor GPR56 regulate the activity of transcription factors associated with tumorigenesis. Journal of cancer research and clinical oncology. 2010a;136:47–53. doi: 10.1007/s00432-009-0635-z. [DOI] [PubMed] [Google Scholar]
- Kim JY, Lee KH, Shim MS, Shin H, Xu XM, Carlson BA, Hatfield DL, Lee BJ. Human selenophosphate synthetase 1 has five splice variants with unique interactions, subcellular localizations and expression patterns. Biochemical and biophysical research communications. 2010b;397:53–8. doi: 10.1016/j.bbrc.2010.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Ryu JH, Park JW, Kim MS, Chun YS. Induction of a SSAT isoform in response to hypoxia or iron deficiency and its protective effects on cell death. Biochemical and biophysical research communications. 2005a;331:78–85. doi: 10.1016/j.bbrc.2005.03.121. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim YC, Adelstein RS, Kawamoto S. Fox-3 and PSF interact to activate neural cell-specific alternative splicing. Nucleic acids research. 2011;39:3064–78. doi: 10.1093/nar/gkq1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Alekseyenko AV, Roy M, Lee C. The ASAP II database: analysis and comparative genomics of alternative splicing in 15 animal species. Nucleic Acids Res. 2007b;35:D93–8. doi: 10.1093/nar/gkl884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Lim D, Lee S, Kim H. ASePCR: alternative splicing electronic RT-PCR in multiple tissues and organs. Nucleic Acids Res. 2005b;33:W681–5. doi: 10.1093/nar/gki407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Lueck JD, Harvey PJ, Pace SM, Ikemoto N, Casarotto MG, Dirksen RT, Dulhunty AF. Alternative splicing of RyR1 alters the efficacy of skeletal EC coupling. Cell Calcium. 2009;45:264–74. doi: 10.1016/j.ceca.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore S, Khanna A, Zhang Z, Hui J, Balwierz PJ, Stefan M, Beach C, Nicholls RD, Zavolan M, Stamm S. The snoRNA MBII-52 (SNORD 115) is processed into smaller RNAs and regulates alternative splicing. Human molecular genetics. 2010;19:1153–64. doi: 10.1093/hmg/ddp585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleino I, Ortiz RM, Yritys M, Huovila AP, Saksela K. Alternative splicing of ADAM15 regulates its interactions with cellular SH3 proteins. J Cell Biochem. 2009;108:877–85. doi: 10.1002/jcb.22317. [DOI] [PubMed] [Google Scholar]
- Klinck R, Bramard A, Inkel L, Dufresne-Martin G, Gervais-Bird J, Madden R, Paquet ER, Koh C, Venables JP, Prinos P, Jilaveanu-Pelmus M, Wellinger R, Rancourt C, Chabot B, Abou Elela S. Multiple alternative splicing markers for ovarian cancer. Cancer research. 2008;68:657–63. doi: 10.1158/0008-5472.CAN-07-2580. [DOI] [PubMed] [Google Scholar]
- Ko J, Fuccillo MV, Malenka RC, Sudhof TC. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009;64:791–8. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YP, Kobbe B, Paulsson M, Wagener R. Zebrafish (Danio rerio) matrilins: shared and divergent characteristics with their mammalian counterparts. Biochem J. 2005;386:367–79. doi: 10.1042/BJ20041486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo H, Tajima K, Fukagawa M, Isogai T, Nishimura S. A novel estrogen receptor-related protein gamma splice variant lacking a DNA binding domain exon modulates transcriptional activity of a moderate range of nuclear receptors. The Journal of steroid biochemistry and molecular biology. 2006;98:181–92. doi: 10.1016/j.jsbmb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Kol G, Lev-Maor G, Ast G. Human-mouse comparative analysis reveals that branch-site plasticity contributes to splicing regulation. Human molecular genetics. 2005;14:1559–68. doi: 10.1093/hmg/ddi164. [DOI] [PubMed] [Google Scholar]
- Kolasinska-Zwierz P, Down T, Latorre I, Liu T, Liu XS, Ahringer J. Differential chromatin marking of introns and expressed exons by H3K36me3. Nature genetics. 2009;41:376–81. doi: 10.1038/ng.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig J, Zarnack K, Rot G, Curk T, Kayikci M, Zupan B, Turner DJ, Luscombe NM, Ule J. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nature structural & molecular biology. 2010;17:909–15. doi: 10.1038/nsmb.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt AR. Chromatin, transcript elongation and alternative splicing. Nat Struct Mol Biol. 2006;13:5–7. doi: 10.1038/nsmb0106-5. [DOI] [PubMed] [Google Scholar]
- Kornblihtt AR. Coupling transcription and alternative splicing. Adv Exp Med Biol. 2007;623:175–89. doi: 10.1007/978-0-387-77374-2_11. [DOI] [PubMed] [Google Scholar]
- Kornblihtt AR, Schor IE, Allo M, Blencowe BJ. When chromatin meets splicing. Nature structural & molecular biology. 2009;16:902–3. doi: 10.1038/nsmb0909-902. [DOI] [PubMed] [Google Scholar]
- Kreis P, Rousseau V, Thevenot E, Combeau G, Barnier JV. The four mammalian splice variants encoded by the p21-activated kinase 3 gene have different biological properties. Journal of neurochemistry. 2008;106:1184–97. doi: 10.1111/j.1471-4159.2008.05474.x. [DOI] [PubMed] [Google Scholar]
- Kremneva E, Nikolaeva O, Maytum R, Arutyunyan AM, Kleimenov SY, Geeves MA, Levitsky DI. Thermal unfolding of smooth muscle and nonmuscle tropomyosin alpha-homodimers with alternatively spliced exons. FEBS J. 2006;273:588–600. doi: 10.1111/j.1742-4658.2005.05092.x. [DOI] [PubMed] [Google Scholar]
- Krieg A, Le Negrate G, Reed JC. RIP2-beta: a novel alternative mRNA splice variant of the receptor interacting protein kinase RIP2. Mol Immunol. 2009;46:1163–70. doi: 10.1016/j.molimm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Kuwano Y, Tominaga K, Kawai T, Katsuura S, Yamagishi N, Satake Y, Kajita K, Tanahashi T, Rokutan K. Brief naturalistic stress induces an alternative splice variant of SMG-1 lacking exon 63 in peripheral leukocytes. Neuroscience letters. 2010;484:128–32. doi: 10.1016/j.neulet.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Kustatscher G, Hothorn M, Pugieux C, Scheffzek K, Ladurner AG. Splicing regulates NAD metabolite binding to histone macroH2A. Nature structural & molecular biology. 2005;12:624–5. doi: 10.1038/nsmb956. [DOI] [PubMed] [Google Scholar]
- Laakso JM, Lewis JH, Shuman H, Ostap EM. Control of myosin-I force sensing by alternative splicing. Proc Natl Acad Sci U S A. 2010;107:698–702. doi: 10.1073/pnas.0911426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguri C, Sadir R, Rueda P, Baleux F, Gans P, Arenzana-Seisdedos F, Lortat-Jacob H. The novel CXCL12gamma isoform encodes an unstructured cationic domain which regulates bioactivity and interaction with both glycosaminoglycans and CXCR4. PLoS One. 2007;2:e1110. doi: 10.1371/journal.pone.0001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe NE, Horowitz PM, Guillozet-Bongaarts AL, Silva A, Andreadis A, Binder LI. Tau 6D and 6P isoforms inhibit polymerization of full-length tau in vitro. Biochemistry. 2009;48:12290–7. doi: 10.1021/bi901304u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauriat TL, Richler E, McInnes LA. A quantitative regional expression profile of EAAT2 known and novel splice variants reopens the question of aberrant EAAT2 splicing in disease. Neurochem Int. 2007;50:271–80. doi: 10.1016/j.neuint.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Lee AW, Champagne N, Wang X, Su XD, Goodyer C, Leblanc AC. Alternatively spliced caspase-6B isoform inhibits the activation of caspase-6A. J Biol Chem. 2010a;285:31974–84. doi: 10.1074/jbc.M110.152744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Kim JM, Lee MK, Jameson JL. Splice variants of the forkhead box protein AFX exhibit dominant negative activity and inhibit AFXalpha-mediated tumor cell apoptosis. PLoS ONE. 2008;3:e2743. doi: 10.1371/journal.pone.0002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Dean C, Isacoff E. Alternative splicing of neuroligin regulates the rate of presynaptic differentiation. J Neurosci. 2010b;30:11435–46. doi: 10.1523/JNEUROSCI.2946-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Tang ZZ, Black DL. An inducible change in Fox-1/A2BP1 splicing modulates the alternative splicing of downstream neuronal target exons. Genes & development. 2009;23:2284–93. doi: 10.1101/gad.1837009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Gao CF, Lee CC, Kim MD, Vande Woude GF. An alternatively spliced form of Met receptor is tumorigenic. Exp Mol Med. 2006;38:565–73. doi: 10.1038/emm.2006.66. [DOI] [PubMed] [Google Scholar]
- Leeman JR, Weniger MA, Barth TF, Gilmore TD. Deletion analysis and alternative splicing define a transactivation inhibitory domain in human oncoprotein REL. Oncogene. 2008;27:6770–81. doi: 10.1038/onc.2008.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Vorechovsky I. Identification of splicing silencers and enhancers in sense Alus: a role for pseudoacceptors in splice site repression. Molecular and cellular biology. 2005;25:6912–20. doi: 10.1128/MCB.25.16.6912-6920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerivray H, Mereau A, Osborne HB. Our favourite alternative splice site. Biol Cell. 2006;98:317–21. doi: 10.1042/BC20050084. [DOI] [PubMed] [Google Scholar]
- Leung E, Hong J, Fraser A, Krissansen GW. Splicing of NOD2 (CARD15) RNA transcripts. Mol Immunol. 2007;44:284–94. doi: 10.1016/j.molimm.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Lev-Maor G, Sorek R, Shomron N, Ast G. The birth of an alternatively spliced exon: 3′ splice-site selection in Alu exons. Science. 2003;300:1288–91. doi: 10.1126/science.1082588. [DOI] [PubMed] [Google Scholar]
- Leveque C, Marsaud V, Renoir JM, Sola B. Alternative cyclin D1 forms a and b have different biological functions in the cell cycle of B lymphocytes. Exp Cell Res. 2007;313:2719–29. doi: 10.1016/j.yexcr.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Levitin F, Baruch A, Weiss M, Stiegman K, Hartmann ML, Yoeli-Lerner M, Ziv R, Zrihan-Licht S, Shina S, Gat A, Lifschitz B, Simha M, Stadler Y, Cholostoy A, Gil B, Greaves D, Keydar I, Zaretsky J, Smorodinsky N, Wreschner DH. A novel protein derived from the MUC1 gene by alternative splicing and frameshifting. J Biol Chem. 2005;280:10655–63. doi: 10.1074/jbc.M406943200. [DOI] [PubMed] [Google Scholar]
- Levy SF, Leboeuf AC, Massie MR, Jordan MA, Wilson L, Feinstein SC. Three- and four-repeat tau regulate the dynamic instability of two distinct microtubule subpopulations in qualitatively different manners. Implications for neurodegeneration. The Journal of biological chemistry. 2005;280:13520–8. doi: 10.1074/jbc.M413490200. [DOI] [PubMed] [Google Scholar]
- Li D, Jin C, Yin C, Zhang Y, Pang B, Tian L, Han W, Ma D, Wang Y. An alternative splice form of CMTM8 induces apoptosis. Int J Biochem Cell Biol. 2007a;39:2107–19. doi: 10.1016/j.biocel.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Li J, Sheng Y, Tang PZ, Tsai-Morris CH, Dufau ML. Tissue-cell- and species-specific expression of gonadotropin-regulated long chain acyl-CoA synthetase (GR-LACS) in gonads, adrenal and brain. Identification of novel forms in the brain. J Steroid Biochem Mol Biol. 2006;98:207–17. doi: 10.1016/j.jsbmb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Li Q, Lee JA, Black DL. Neuronal regulation of alternative pre-mRNA splicing. Nature reviews Neuroscience. 2007b;8:819–31. doi: 10.1038/nrn2237. [DOI] [PubMed] [Google Scholar]
- Li Y, Yu Z, Zhao X, Shen SH. Identification and characterization of hepsin/-TM, a non-transmembrane hepsin isoform. Biochim Biophys Acta. 2005;1681:157–65. doi: 10.1016/j.bbaexp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Liang Y, Li C, Guzman VM, Chang WW, Evinger AJ, 3rd, Sao D, Woodward DF. Identification of a novel alternative splicing variant of RGS5 mRNA in human ocular tissues. FEBS J. 2005;272:791–9. doi: 10.1111/j.1742-4658.2004.04516.x. [DOI] [PubMed] [Google Scholar]
- Liao P, Yu D, Li G, Yong TF, Soon JL, Chua YL, Soong TW. A smooth muscle Cav1.2 calcium channel splice variant underlies hyperpolarized window current and enhanced state-dependent inhibition by nifedipine. J Biol Chem. 2007;282:35133–42. doi: 10.1074/jbc.M705478200. [DOI] [PubMed] [Google Scholar]
- Licatalosi DD, Mele A, Fak JJ, Ule J, Kayikci M, Chi SW, Clark TA, Schweitzer AC, Blume JE, Wang X, Darnell JC, Darnell RB. HITS-CLIP yields genome-wide insights into brain alternative RNA processing. Nature. 2008;456:464–9. doi: 10.1038/nature07488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Tarn WY. Exon selection in alpha-tropomyosin mRNA is regulated by the antagonistic action of RBM4 and PTB. Molecular and cellular biology. 2005;25:10111–21. doi: 10.1128/MCB.25.22.10111-10121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Cheng CJ, Lee YC, Ye X, Tsai WW, Kim J, Pasqualini R, Arap W, Navone NM, Tu SM, Hu M, Yu-Lee LY, Logothetis CJ. A 45-kDa ErbB3 secreted by prostate cancer cells promotes bone formation. Oncogene. 2008;27:5195–203. doi: 10.1038/onc.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Tang N, Ostap EM. Biochemical and motile properties of Myo1b splice isoforms. The Journal of biological chemistry. 2005;280:41562–7. doi: 10.1074/jbc.M508653200. [DOI] [PubMed] [Google Scholar]
- Lin Y, Stevens C, Harrison B, Pathuri S, Amin E, Hupp TR. The alternative splice variant of DAPK-1, s-DAPK-1, induces proteasome-independent DAPK-1 destabilization. Mol Cell Biochem. 2009a;328:101–7. doi: 10.1007/s11010-009-0079-4. [DOI] [PubMed] [Google Scholar]
- Lin YS, Yasuda K, Assem M, Cline C, Barber J, Li CW, Kholodovych V, Ai N, Chen JD, Welsh WJ, Ekins S, Schuetz EG. The major human pregnane × receptor (PXR) splice variant, PXR.2, exhibits significantly diminished ligand-activated transcriptional regulation. Drug metabolism and disposition: the biological fate of chemicals. 2009b;37:1295–304. doi: 10.1124/dmd.108.025213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira ME, Loomis AK, Paciga SA, Lloyd DB, Thompson JF. Expression of CETP and of splice variants induces the same level of ER stress despite secretion efficiency differences. Journal of lipid research. 2008;49:1955–62. doi: 10.1194/jlr.M800078-JLR200. [DOI] [PubMed] [Google Scholar]
- Liu X, Sharp PJ. Deletions in mRNA encoding the chicken leptin receptor gene binding domain. Comp Biochem Physiol B Biochem Mol Biol. 2007;146:250–5. doi: 10.1016/j.cbpb.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Liu X, Yang PS, Yang W, Yue DT. Enzyme-inhibitor-like tuning of Ca(2+) channel connectivity with calmodulin. Nature. 463:968–72. doi: 10.1038/nature08766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Elliott DJ. Coupling genetics and post-genomic approaches to decipher the cellular splicing code at a systems-wide level. Biochemical Society transactions. 2010;38:237–41. doi: 10.1042/BST0380237. [DOI] [PubMed] [Google Scholar]
- Liu YW, Surka MC, Schroeter T, Lukiyanchuk V, Schmid SL. Isoform and splice-variant specific functions of dynamin-2 revealed by analysis of conditional knock-out cells. Mol Biol Cell. 2008;19:5347–59. doi: 10.1091/mbc.E08-08-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorian M, Schwartz S, Clark TA, Hollander D, Tan LY, Spellman R, Gordon A, Schweitzer AC, de la Grange P, Ast G, Smith CW. Position-dependent alternative splicing activity revealed by global profiling of alternative splicing events regulated by PTB. Nature structural & molecular biology. 2010;17:1114–23. doi: 10.1038/nsmb.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HW, Zhu H, Cao X, Aldrich A, Ali-Osman F. A novel splice variant of GLI1 that promotes glioblastoma cell migration and invasion. Cancer Res. 2009;69:6790–8. doi: 10.1158/0008-5472.CAN-09-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokeshwar VB, Estrella V, Lopez L, Kramer M, Gomez P, Soloway MS, Lokeshwar BL. HYAL1-v1, an alternatively spliced variant of HYAL1 hyaluronidase: a negative regulator of bladder cancer. Cancer Res. 2006;66:11219–27. doi: 10.1158/0008-5472.CAN-06-1121. [DOI] [PubMed] [Google Scholar]
- Lonka-Nevalaita L, Lume M, Leppanen S, Jokitalo E, Peranen J, Saarma M. Characterization of the intracellular localization, processing, and secretion of two glial cell line-derived neurotrophic factor splice isoforms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:11403–13. doi: 10.1523/JNEUROSCI.5888-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez V, Kelleher SL. Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc. The Biochemical journal. 2009;422:43–52. doi: 10.1042/BJ20081189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M, Hewing B, Hui J, Zepp A, Baumann G, Bindereif A, Stangl V, Stangl K. Alternative splicing in intron 13 of the human eNOS gene: a potential mechanism for regulating eNOS activity. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:1556–64. doi: 10.1096/fj.06-7434com. [DOI] [PubMed] [Google Scholar]
- Lossin C. A catalog of SCN1A variants. Brain Dev. 2009;31:114–30. doi: 10.1016/j.braindev.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Low R, Leguillette R, Lauzon AM. (+)Insert smooth muscle myosin heavy chain (SM-B): from single molecule to human. The international journal of biochemistry & cell biology. 2006;38:1862–74. doi: 10.1016/j.biocel.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Lu H, Huang X, Zhang L, Guo Y, Cheng H, Zhou R. Multiple alternative splicing of mouse Dmrt1 during gonadal differentiation. Biochem Biophys Res Commun. 2007;352:630–4. doi: 10.1016/j.bbrc.2006.11.066. [DOI] [PubMed] [Google Scholar]
- Lu X, Ferreira PA. Identification of novel murine- and human-specific RPGRIP1 splice variants with distinct expression profiles and subcellular localization. Investigative ophthalmology & visual science. 2005;46:1882–90. doi: 10.1167/iovs.04-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luco RF, Pan Q, Tominaga K, Blencowe BJ, Pereira-Smith OM, Misteli T. Regulation of alternative splicing by histone modifications. Science. 2010;327:996–1000. doi: 10.1126/science.1184208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke Y, Zaim H, Karakesisoglou I, Jaeger VM, Sellin L, Lu W, Schneider M, Neumann S, Beijer A, Munck M, Padmakumar VC, Gloy J, Walz G, Noegel AA. Nesprin-2 Giant (NUANCE) maintains nuclear envelope architecture and composition in skin. J Cell Sci. 2008;121:1887–98. doi: 10.1242/jcs.019075. [DOI] [PubMed] [Google Scholar]
- Lyko F, Foret S, Kucharski R, Wolf S, Falckenhayn C, Maleszka R. The honey bee epigenomes: differential methylation of brain DNA in queens and workers. PLoS biology. 2010;8:e1000506. doi: 10.1371/journal.pbio.1000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Kawamoto S, Uribe J, Adelstein RS. Function of the neuron-specific alternatively spliced isoforms of nonmuscle myosin II-B during mouse brain development. Mol Biol Cell. 2006;17:2138–49. doi: 10.1091/mbc.E05-10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa S, Mori D, Nishiya T, Takikawa O, Horinouchi T, Nishimoto A, Kajita E, Miwa S. OCTN2VT, a splice variant of OCTN2, does not transport carnitine because of the retention in the endoplasmic reticulum caused by insertion of 24 amino acids in the first extracellular loop of OCTN2. Biochim Biophys Acta. 2007;1773:1000–6. doi: 10.1016/j.bbamcr.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Magen A, Ast G. The importance of being divisible by three in alternative splicing. Nucleic acids research. 2005;33:5574–82. doi: 10.1093/nar/gki858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Molecular cell. 2007;27:435–48. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malartre M, Short S, Sharpe C. Xenopus embryos lacking specific isoforms of the corepressor SMRT develop abnormal heads. Developmental biology. 2006;292:333–43. doi: 10.1016/j.ydbio.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Malentacchi F, Simi L, Nannelli C, Andreani M, Janni A, Pastorekova S, Orlando C. Alternative splicing variants of carbonic anhydrase IX in human non-small cell lung cancer. Lung cancer. 2009;64:271–6. doi: 10.1016/j.lungcan.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Mango R, Biocca S, del Vecchio F, Clementi F, Sangiuolo F, Amati F, Filareto A, Grelli S, Spitalieri P, Filesi I, Favalli C, Lauro R, Mehta JL, Romeo F, Novelli G. In vivo and in vitro studies support that a new splicing isoform of OLR1 gene is protective against acute myocardial infarction. Circ Res. 2005;97:152–8. doi: 10.1161/01.RES.0000174563.62625.8e. [DOI] [PubMed] [Google Scholar]
- Markus MA, Heinrich B, Raitskin O, Adams DJ, Mangs H, Goy C, Ladomery M, Sperling R, Stamm S, Morris BJ. WT1 interacts with the splicing protein RBM4 and regulates its ability to modulate alternative splicing in vivo. Exp Cell Res. 2006;312:3379–88. doi: 10.1016/j.yexcr.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Martelli PL, D'Antonio M, Bonizzoni P, Castrignano T, D'Erchia AM, D'Onorio De Meo P, Fariselli P, Finelli M, Licciulli F, Mangiulli M, Mignone F, Pavesi G, Picardi E, Rizzi R, Rossi I, Valletti A, Zauli A, Zambelli F, Casadio R, Pesole G. ASPicDB: a database of annotated transcript and protein variants generated by alternative splicing. Nucleic Acids Res. 2011;39:D80–5. doi: 10.1093/nar/gkq1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Lanneree S, Lasbleiz C, Sanial M, Fouix S, Besse F, Tricoire H, Plessis A. Characterization of the Drosophila myeloid leukemia factor. Genes Cells. 2006;11:1317–35. doi: 10.1111/j.1365-2443.2006.01023.x. [DOI] [PubMed] [Google Scholar]
- Martinez O, Brackenridge S, El-Idrissi Mel A, Prabhakar BS. DC-SIGN, but not sDC-SIGN, can modulate IL-2 production from PMA- and anti- CD3-stimulated primary human CD4 T cells. Int Immunol. 2005;17:769–78. doi: 10.1093/intimm/dxh258. [DOI] [PubMed] [Google Scholar]
- Matic M, Corradin AP, Tsoli M, Clarke SJ, Polly P, Robertson GR. The alternatively spliced murine pregnane × receptor isoform, mPXR(delta171-211) exhibits a repressive action. The international journal of biochemistry & cell biology. 2010;42:672–82. doi: 10.1016/j.biocel.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Matlin AJ, Clark F, Smith CW. Understanding alternative splicing: towards a cellular code. Nature reviews Molecular cell biology. 2005;6:386–98. doi: 10.1038/nrm1645. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Paul BD, Choi CY, Shi YB. Contrasting effects of twoalternative splicing forms of coactivator-associated arginine methyltransferase 1 on thyroid hormone receptor-mediated transcription in Xenopus laevis. Molecular endocrinology. 2007;21:1082–94. doi: 10.1210/me.2006-0448. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Takeoka M, Sagara J, Itano N, Kurose Y, Nakamura A, Taniguchi S. A splice variant of ASC regulates IL-1beta release and aggregates differently from intact ASC. Mediators Inflamm. 2009a;2009:287387. doi: 10.1155/2009/287387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Tomonaga T, Shimada H, Shioya A, Higashi M, Matsubara H, Harigaya K, Nomura F, Libutti D, Levens D, Ochiai TAn. Essential Role of Alternative Splicing of c-myc Suppressor FUSE-Binding Protein–Interacting Repressor in Carcinogenesis. Cancer Res. 2006;66:1409–1417. doi: 10.1158/0008-5472.CAN-04-4459. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Yamamoto R, Mitsui K, Kanazawa H. Altered motor activity of alternative splice variants of the mammalian kinesin-3 protein KIF1B. Traffic. 2009b;10:1647–54. doi: 10.1111/j.1600-0854.2009.00975.x. [DOI] [PubMed] [Google Scholar]
- McCartney CE, McClafferty H, Huibant JM, Rowan EG, Shipston MJ, Rowe IC. A cysteine-rich motif confers hypoxia sensitivity to mammalian large conductance voltage- and Ca-activated K (BK) channel alpha-subunits. Proc Natl Acad Sci U S A. 2005;102:17870–6. doi: 10.1073/pnas.0505270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElvaine AT, Mayo KE. A dominant-negative human growth hormone-releasing hormone (GHRH) receptor splice variant inhibits GHRH binding. Endocrinology. 2006;147:1884–94. doi: 10.1210/en.2005-1488. [DOI] [PubMed] [Google Scholar]
- Mei Y, Xie C, Xie W, Wu Z, Wu M. Siah-1S, a novel splice variant of Siah-1 (seven in absentia homolog), counteracts Siah-1-mediated downregulation of beta-catenin. Oncogene. 2007;26:6319–31. doi: 10.1038/sj.onc.1210449. [DOI] [PubMed] [Google Scholar]
- Meidan R, Klipper E, Gilboa T, Muller L, Levy N. Endothelin-converting enzyme-1, abundance of isoforms a-d and identification of a novel alternatively spliced variant lacking a transmembrane domain. J Biol Chem. 2005;280:40867–74. doi: 10.1074/jbc.M505679200. [DOI] [PubMed] [Google Scholar]
- Meijer OC, Kalkhoven E, van der Laan S, Steenbergen PJ, Houtman SH, Dijkmans TF, Pearce D, de Kloet ER. Steroid receptor coactivator-1 splice variants differentially affect corticosteroid receptor signaling. Endocrinology. 2005;146:1438–48. doi: 10.1210/en.2004-0411. [DOI] [PubMed] [Google Scholar]
- Meseguer S, Mudduluru G, Escamilla JM, Allgayer H, Barettino D. MicroRNAs-10a and -10b contribute to retinoic acid-induced differentiation of neuroblastoma cells and target the alternative splicing regulatory factor SFRS1 (SF2/ASF) The Journal of biological chemistry. 2011;286:4150–64. doi: 10.1074/jbc.M110.167817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf CE, Wassarman DA. DNA binding properties of TAF1 isoforms with two AT-hooks. The Journal of biological chemistry. 2006;281:30015–23. doi: 10.1074/jbc.M606289200. [DOI] [PubMed] [Google Scholar]
- Metz RP, Kwak HI, Gustafson T, Laffin B, Porter WW. Differential transcriptional regulation by mouse single-minded 2s. The Journal of biological chemistry. 2006;281:10839–48. doi: 10.1074/jbc.M508858200. [DOI] [PubMed] [Google Scholar]
- Michelhaugh SK, Vaitkevicius H, Wang J, Bouhamdan M, Krieg AR, Walker JL, Mendiratta V, Bannon MJ. Dopamine neurons express multiple isoforms of the nuclear receptor nurr1 with diminished transcriptional activity. Journal of neurochemistry. 2005;95:1342–50. doi: 10.1111/j.1471-4159.2005.03458.x. [DOI] [PubMed] [Google Scholar]
- Miller BM, Zhang S, Suggs JA, Swank DM, Littlefield KP, Knowles AF, Bernstein SI. An alternative domain near the nucleotide-binding site of Drosophila muscle myosin affects ATPase kinetics. Journal of molecular biology. 2005;353:14–25. doi: 10.1016/j.jmb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Minegishi T, Nakamura K, Yamashita S, Omori Y. The effect of splice variant of the human luteinizing hormone (LH) receptor on the expression of gonadotropin receptor. Mol Cell Endocrinol. 2007;260-262:117–25. doi: 10.1016/j.mce.2005.11.051. [DOI] [PubMed] [Google Scholar]
- Misquitta-Ali CM, Cheng E, O'Hanlon D, Liu N, McGlade CJ, Tsao MS, Blencowe BJ. Global profiling and molecular characterization of alternative splicing events misregulated in lung cancer. Molecular and cellular biology. 2011;31:138–50. doi: 10.1128/MCB.00709-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake Y, Mizuno T, Yanagi K, Hanaoka F. Novel splicing variant of mouse Orc1 is deficient in nuclear translocation and resistant for proteasome-mediated degradation. The Journal of biological chemistry. 2005;280:12643–52. doi: 10.1074/jbc.M413280200. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Donev RM, Harris CL, Morgan B. CD55 in rat male reproductive tissue: differential expression in testis and expression of a unique truncated isoform on spermatozoa. Molecular immunology. 2007;44:1613–22. doi: 10.1016/j.molimm.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Moe SE, Sorbo JG, Sogaard R, Zeuthen T, Petter Ottersen O, Holen T. New isoforms of rat Aquaporin-4. Genomics. 2008;91:367–77. doi: 10.1016/j.ygeno.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Mola G, Vela E, Fernandez-Figueras MT, Isamat M, Munoz-Marmol AM. Exonization of Alu-generated splice variants in the survivin gene of human and non-human primates. Journal of molecular biology. 2007;366:1055–63. doi: 10.1016/j.jmb.2006.11.089. [DOI] [PubMed] [Google Scholar]
- Molina E, Hermida J, Lopez-Sagaseta J, Puy C, Montes R. The functional properties of a truncated form of endothelial cell protein C receptor generated by alternative splicing. Haematologica. 2008;93:878–84. doi: 10.3324/haematol.12376. [DOI] [PubMed] [Google Scholar]
- Monfregola J, Cevenini A, Terracciano A, van Vlies N, Arbucci S, Wanders RJ, D'Urso M, Vaz FM, Ursini MV. Functional analysis of TMLH variants and definition of domains required for catalytic activity and mitochondrial targeting. Journal of cellular physiology. 2005;204:839–47. doi: 10.1002/jcp.20332. [DOI] [PubMed] [Google Scholar]
- Montague P, McCallion AS, Barrie JE, Edgar JM, McLaughlin M, Davies RW, Griffiths IR. Characterization of the murine splice variant Mobp155: developmental CNS expression pattern and subcellular localization of epitope-tagged protein. Glia. 2005;50:80–5. doi: 10.1002/glia.20155. [DOI] [PubMed] [Google Scholar]
- Monterrat C, Boal F, Grise F, Hemar A, Lang J. Synaptotagmin 8 is expressed both as a calcium-insensitive soluble and membrane protein in neurons, neuroendocrine and endocrine cells. Biochim Biophys Acta. 2006;1763:73–81. doi: 10.1016/j.bbamcr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Silver PA. Global analysis of mRNA splicing. Rna. 2008;14:197–203. doi: 10.1261/rna.868008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Wang Q, Kennedy CJ, Silver PA. An alternative splicing network links cell-cycle control to apoptosis. Cell. 2010;142:625–36. doi: 10.1016/j.cell.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroy T, Heyd F. The impact of alternative splicing in vivo: mouse models show the way. Rna. 2007;13:1155–71. doi: 10.1261/rna.554607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley JD, Keri RA. Splice variants of mIAP1 have an enhanced ability to inhibit apoptosis. Biochem Biophys Res Commun. 2006;348:1174–83. doi: 10.1016/j.bbrc.2006.07.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama M, Yamazaki S, Eto-Kimura A, Takeshige K, Muta T. Positive and negative regulation of nuclear factor-kappaB-mediated transcription by IkappaB-zeta, an inducible nuclear protein. The Journal of biological chemistry. 2005;280:7444–51. doi: 10.1074/jbc.M412738200. [DOI] [PubMed] [Google Scholar]
- Mouquet H, Farci S, Joly P, Maillere B, Leblond J, Drouot L, Leprince J, Tonon MC, Loiseau P, Charron D, Tron F, Gilbert D. A truncated alternative spliced isoform of human desmoglein 1 contains a specific T cell epitope binding to the pemphigus foliaceus-associated HLA class II DRbeta1*0102 molecule. J Immunol. 2006;177:6517–26. doi: 10.4049/jimmunol.177.9.6517. [DOI] [PubMed] [Google Scholar]
- Muda M, He C, Martini PG, Ferraro T, Layfield S, Taylor D, Chevrier C, Schweickhardt R, Kelton C, Ryan PL, Bathgate RA. Splice variants of the relaxin and INSL3 receptors reveal unanticipated molecular complexity. Mol Hum Reprod. 2005;11:591–600. doi: 10.1093/molehr/gah205. [DOI] [PubMed] [Google Scholar]
- Mulcahy H, O'Rourke KP, Adams C, Molloy MG, O'Gara F. LST1 and NCR3 expression in autoimmune inflammation and in response to IFN-gamma, LPS and microbial infection. Immunogenetics. 2006;57:893–903. doi: 10.1007/s00251-005-0057-2. [DOI] [PubMed] [Google Scholar]
- Mulherkar N, Prasad KV, Prabhakar BS. MADD/DENN splice variant of the IG20 gene is a negative regulator of caspase-8 activation. Knockdown enhances TRAIL-induced apoptosis of cancer cells. The Journal of biological chemistry. 2007;282:11715–21. doi: 10.1074/jbc.M701085200. [DOI] [PubMed] [Google Scholar]
- Mulherkar N, Ramaswamy M, Mordi DC, Prabhakar BS. MADD/DENN splice variant of the IG20 gene is necessary and sufficient for cancer cell survival. Oncogene. 2006;25:6252–61. doi: 10.1038/sj.onc.1209650. [DOI] [PubMed] [Google Scholar]
- Muraoka-Cook RS, Sandahl MA, Strunk KE, Miraglia LC, Husted C, Hunter DM, Elenius K, Chodosh LA, Earp HS., 3rd ErbB4 splice variants Cyt1 and Cyt2 differ by 16 amino acids and exert opposing effects on the mammary epithelium in vivo. Molecular and cellular biology. 2009;29:4935–48. doi: 10.1128/MCB.01705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Milosevic I, Fasshauer D, Muller EM, de Groot BL, Lang T, Wilson MC, Sorensen JB. Alternative splicing of SNAP-25 regulates secretion through nonconservative substitutions in the SNARE domain. Molecular biology of the cell. 2005;16:5675–85. doi: 10.1091/mbc.E05-07-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla G, DiFeo A, Yao S, Banno A, Hod E, Reeves HL, Qiao RF, Camacho-Vanegas O, Levine A, Kirschenbaum A, Chan AM, Friedman SL, Martignetti JA. Targeted inhibition of the KLF6 splice variant, KLF6 SV1, suppresses prostate cancer cell growth and spread. Cancer Res. 2005;65:5761–8. doi: 10.1158/0008-5472.CAN-05-0217. [DOI] [PubMed] [Google Scholar]
- Nern A, Nguyen LV, Herman T, Prakash S, Clandinin TR, Zipursky SL. An isoform-specific allele of Drosophila N-cadherin disrupts a late step of R7 targeting. Proc Natl Acad Sci U S A. 2005;102:12944–9. doi: 10.1073/pnas.0502888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Fujimoto A, Tamura N, Yajima T, Wajjwalku W, Yoshikai Y. A novel autoregulatory mechanism for transcriptional activation of the IL-15 gene by a nonsecretable isoform of IL-15 generated by alternative splicing. FASEB J. 2005;19:19–28. doi: 10.1096/fj.04-2633com. [DOI] [PubMed] [Google Scholar]
- Noro B, Culi J, McKay DJ, Zhang W, Mann RS. Distinct functions of homeodomain-containing and homeodomain-less isoforms encoded by homothorax. Genes & development. 2006;20:1636–50. doi: 10.1101/gad.1412606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikov L, Park JW, Chen H, Klerman H, Jalloh AS, Gamble MJ. QKI-mediated alternative splicing of the histone variant MacroH2A1 regulates cancer cell proliferation. Molecular and cellular biology. 2011;31:4244–55. doi: 10.1128/MCB.05244-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman U, Sobczak-Pluta A, Vlachos P, Perlmann T, Zhivotovsky B, Joseph B. Full-length p73alpha represses drug-induced apoptosis in small cell lung carcinoma cells. J Biol Chem. 2005;280:34159–69. doi: 10.1074/jbc.M500394200. [DOI] [PubMed] [Google Scholar]
- O'Leary H, Sui X, Lin PJ, Volpe P, Bayer KU. Nuclear targeting of the CaMKII anchoring protein alphaKAP is regulated by alternative splicing and protein kinases. Brain Res. 2006;1086:17–26. doi: 10.1016/j.brainres.2006.02.120. [DOI] [PubMed] [Google Scholar]
- O'Rourke JP, Ness SA. Alternative RNA splicing produces multiple forms of c-Myb with unique transcriptional activities. Molecular and cellular biology. 2008;28:2091–101. doi: 10.1128/MCB.01870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkura N, Takahashi M, Yaguchi H, Nagamura Y, Tsukada T. Coactivator-associated arginine methyltransferase 1, CARM1, affects pre-mRNA splicing in an isoform-specific manner. The Journal of biological chemistry. 2005;280:28927–35. doi: 10.1074/jbc.M502173200. [DOI] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:1050–62. doi: 10.1523/JNEUROSCI.3327-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann M, Mizel D, Huang G, Li C, Deng C, Theilig F, Bachmann S, Briggs J, Schnermann J, Castrop H. Macula densa control of renin secretion and preglomerular resistance in mice with selective deletion of the B isoform of the Na, K,2Cl co-transporter. J Am Soc Nephrol. 2006;17:2143–52. doi: 10.1681/ASN.2006040384. [DOI] [PubMed] [Google Scholar]
- Orlichenko L, Geyer R, Yanagisawa M, Khauv D, Radisky ES, Anastasiadis PZ, Radisky DC. The 19-amino acid insertion in the tumor-associated splice isoform Rac1b confers specific binding to p120 catenin. J Biol Chem. 285:19153–61. doi: 10.1074/jbc.M109.099382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuka F, Endo Y, Higuchi M, Suzuki H, Shio Y, Fujiu K, Kanno R, Oishi A, Terashima M, Fujita T, Gotoh M. Molecular cloning and characterization of novel splicing variants of human decay-accelerating factor. Genomics. 2006;88:316–22. doi: 10.1016/j.ygeno.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Pan Q, Bakowski MA, Morris Q, Zhang W, Frey BJ, Hughes TR, Blencowe BJ. Alternative splicing of conserved exons is frequently species-specific in human and mouse. Trends in genetics : TIG. 2005;21:73–7. doi: 10.1016/j.tig.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Pan YX, Xu J, Xu M, Rossi GC, Matulonis JE, Pasternak GW. Involvement of exon 11-associated variants of the mu opioid receptor MOR-1 in heroin, but not morphine, actions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4917–22. doi: 10.1073/pnas.0811586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos C, Arato K, Lilienthal E, Zerweck J, Schutkowski M, Chatain N, Muller-Newen G, Becker W, de la Luna S. Splice variants of the dual specificity tyrosine phosphorylation-regulated kinase 4 (DYRK4) differ in their subcellular localization and catalytic activity. The Journal of biological chemistry. 2011;286:5494–505. doi: 10.1074/jbc.M110.157909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis C, Cloutier P, Shkreta L, Toutant J, Klarskov K, Chabot B. hnRNP I/PTB can antagonize the splicing repressor activity of SRp30c. Rna. 2007;13:1287–300. doi: 10.1261/rna.403607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JB, Levine M. Cloning, sequencing, and characterization of alternatively spliced glutaredoxin 1 cDNA and its genomic gene: chromosomal localization, mrna stability, and origin of pseudogenes. The Journal of biological chemistry. 2005;280:10427–34. doi: 10.1074/jbc.M412450200. [DOI] [PubMed] [Google Scholar]
- Parrott AM, Walsh MR, Reichman TW, Mathews MB. RNA binding and phosphorylation determine the intracellular distribution of nuclear factors 90 and 110. J Mol Biol. 2005;348:281–93. doi: 10.1016/j.jmb.2005.02.047. [DOI] [PubMed] [Google Scholar]
- Paz I, Akerman M, Dror I, Kosti I, Mandel-Gutfreund Y. SFmap: a web server for motif analysis and prediction of splicing factor binding sites. Nucleic Acids Res. 2010;38:W281–W285. doi: 10.1093/nar/gkq444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelley JL, Nicholls CD, Beattie TL, Brown CB. Discovery and characterization of a novel splice variant of the GM-CSF receptor alpha subunit. Exp Hematol. 2007;35:1483–94. doi: 10.1016/j.exphem.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Pereira S, Massacrier A, Roll P, Verine A, Etienne-Grimaldi MC, Poitelon Y, Robaglia-Schlupp A, Jamali S, Roeckel-Trevisiol N, Royer B, Pontarotti P, Leveque C, Seagar M, Levy N, Cau P, Szepetowski P. Nuclear localization of a novel human syntaxin 1B isoform. Gene. 2008;423:160–71. doi: 10.1016/j.gene.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Perron A, Sarret P, Gendron L, Stroh T, Beaudet A. Identification and functional characterization of a 5-transmembrane domain variant isoform of the NTS2 neurotensin receptor in rat central nervous system. The Journal of biological chemistry. 2005;280:10219–27. doi: 10.1074/jbc.M410557200. [DOI] [PubMed] [Google Scholar]
- Peterfy M, Phan J, Reue K. Alternatively spliced lipin isoforms exhibit distinct expression pattern, subcellular localization, and role in adipogenesis. J Biol Chem. 2005;280:32883–9. doi: 10.1074/jbc.M503885200. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki J, Lerin C, Itkonen P, Boes T, Floss T, Schroeder J, Dearie F, Crunkhorn S, Burak F, Jimenez-Chillaron JC, Kuulasmaa T, Miettinen P, Park PJ, Nasser I, Zhao Z, Zhang Z, Xu Y, Wurst W, Ren H, Morris AJ, Stamm S, Goldfine AB, Laakso M, Patti ME. Expression of the splicing factor gene SFRS10 is reduced in human obesity and contributes to enhanced lipogenesis. Cell metabolism. 2011;14:208–18. doi: 10.1016/j.cmet.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-Lucena A, Ho CS, Mao DY, Sheng Y, Laister RC, Muhandiram R, Lu Y, Seet BT, Katz S, Szyperski T, Penn LZ, Arrowsmith CH. A structure-based model of the c-Myc/Bin1 protein interaction shows alternative splicing of Bin1 and c-Myc phosphorylation are key binding determinants. J Mol Biol. 2005;351:182–94. doi: 10.1016/j.jmb.2005.05.046. [DOI] [PubMed] [Google Scholar]
- Pohjanvirta R. Transgenic mouse lines expressing rat AH receptor variants--a new animal model for research on AH receptor function and dioxin toxicity mechanisms. Toxicology and applied pharmacology. 2009;236:166–82. doi: 10.1016/j.taap.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Poulos MG, Batra R, Charizanis K, Swanson MS. Developments in RNA splicing and disease. Cold Spring Harbor perspectives in biology. 2011;3:a000778. doi: 10.1101/cshperspect.a000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasannan P, Appling DR. Human mitochondrial C1-tetrahydrofolate synthase: submitochondrial localization of the full-length enzyme and characterization of a short isoform. Archives of biochemistry and biophysics. 2009;481:86–93. doi: 10.1016/j.abb.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Su Y, Sun R, Zhang F, Luo X, Yang Z. CASK inhibits ECV304 cell growth and interacts with Id1. Biochemical and biophysical research communications. 2005;328:517–21. doi: 10.1016/j.bbrc.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Qin S, Zheng F, Chen GH, Fang H, Wang XM, Zhou JN. Variable alternative spliced exon (VASE)-containing and VASE-lacking neural cell adhesion molecule in the dorsal and ventral hippocampus of SAMP8 mice. J Neurosci Res. 2005;80:838–44. doi: 10.1002/jnr.20527. [DOI] [PubMed] [Google Scholar]
- Quayle SN, Sadar MD. A truncated isoform of TMEFF2 encodes a secreted protein in prostate cancer cells. Genomics. 2006;87:633–7. doi: 10.1016/j.ygeno.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Ramani AK, Calarco JA, Pan Q, Mavandadi S, Wang Y, Nelson AC, Lee LJ, Morris Q, Blencowe BJ, Zhen M, Fraser AG. Genome-wide analysis of alternative splicing in Caenorhabditis elegans. Genome Res. 2011;21:342–8. doi: 10.1101/gr.114645.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranum LP, Cooper TA. RNA-Mediated Neuromuscular Disorders. Annu Rev Neurosci. 2006;29:259–77. doi: 10.1146/annurev.neuro.29.051605.113014. [DOI] [PubMed] [Google Scholar]
- Rao N, Nguyen S, Ngo K, Fung-Leung WP. A novel splice variant of interleukin-1 receptor (IL-1R)-associated kinase 1 plays a negative regulatory role in Toll/IL-1R-induced inflammatory signaling. Molecular and cellular biology. 2005;25:6521–32. doi: 10.1128/MCB.25.15.6521-6532.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raponi M, Baralle D. Alternative splicing: good and bad effects of translationally silent substitutions. The FEBS journal. 2010;277:836–40. doi: 10.1111/j.1742-4658.2009.07519.x. [DOI] [PubMed] [Google Scholar]
- Ren S, Nguyen L, Wu S, Encinas C, Adams JS, Hewison M. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J Biol Chem. 2005;280:20604–11. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- Roberts S, Calautti E, Vanderweil S, Nguyen HO, Foley A, Baden HP, Viel A. Changes in localization of human discs large (hDlg) during keratinocyte differentiation are [corrected] associated with expression of alternatively spliced hDlg variants. Exp Cell Res. 2007;313:2521–30. doi: 10.1016/j.yexcr.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Romero PR, Zaidi S, Fang YY, Uversky VN, Radivojac P, Oldfield CJ, Cortese MS, Sickmeier M, LeGall T, Obradovic Z, Dunker AK. Alternative splicing in concert with protein intrinsic disorder enables increased functional diversity in multicellular organisms. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8390–5. doi: 10.1073/pnas.0507916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Z, Ren Y, Cheng L, Li Z, Li Y, Sun Y, Li H, Xiong S, Chang Z. Sef-S, an alternative splice isoform of sef gene, inhibits NIH3T3 cell proliferation via a mitogen-activated protein kinases p42 and p44 (ERK1/2)-independent mechanism. Cellular signalling. 2007;19:93–102. doi: 10.1016/j.cellsig.2006.05.033. [DOI] [PubMed] [Google Scholar]
- Roszelle BN, Cooper BT, Long TC, Deutsch S, Manning KB. The 12 cc Penn State pulsatile pediatric ventricular assist device: flow field observations at a reduced beat rate with application to weaning. ASAIO journal. 2008;54:325–31. doi: 10.1097/MAT.0b013e3181695cfe. [DOI] [PubMed] [Google Scholar]
- Ruggiu M, Herbst R, Kim N, Jevsek M, Fak JJ, Mann MA, Fischbach G, Burden SJ, Darnell RB. Rescuing Z+ agrin splicing in Nova null mice restores synapse formation and unmasks a physiologic defect in motor neuron firing. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3513–8. doi: 10.1073/pnas.0813112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MC, Zeeberg BR, Caplen NJ, Cleland JA, Kahn AB, Liu H, Weinstein JN. SpliceCenter: a suite of web-based bioinformatic applications for evaluating the impact of alternative splicing on RT-PCR, RNAi, microarray, and peptide-based studies. BMC Bioinformatics. 2008;9:313. doi: 10.1186/1471-2105-9-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino L, Casamassimi A, Peluso G, Barone MV, Capaccio D, Migliore C, Bonelli P, Pedicini A, Febbraro A, Ciccodicola A, Colantuoni V. A novel peroxisome proliferator-activated receptor gamma isoform with dominant negative activity generated by alternative splicing. The Journal of biological chemistry. 2005;280:26517–25. doi: 10.1074/jbc.M502716200. [DOI] [PubMed] [Google Scholar]
- Sadakata T, Washida M, Iwayama Y, Shoji S, Sato Y, Ohkura T, Katoh-Semba R, Nakajima M, Sekine Y, Tanaka M, Nakamura K, Iwata Y, Tsuchiya KJ, Mori N, Detera-Wadleigh SD, Ichikawa H, Itohara S, Yoshikawa T, Furuichi T. Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients. J Clin Invest. 2007;117:931–43. doi: 10.1172/JCI29031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y, Oda H, Yoshimura D, Furuichi M, Kang D, Iwai S, Hara T, Nakabeppu Y. The GT to GC single nucleotide polymorphism at the beginning of an alternative exon 2C of human MTH1 gene confers an amino terminal extension that functions as a mitochondrial targeting signal. J Mol Med (Berl) 2006;84:660–70. doi: 10.1007/s00109-006-0053-5. [DOI] [PubMed] [Google Scholar]
- Sanford JR, Wang X, Mort M, Vanduyn N, Cooper DN, Mooney SD, Edenberg HJ, Liu Y. Splicing factor SFRS1 recognizes a functionally diverse landscape of RNA transcripts. Genome research. 2009;19:381–94. doi: 10.1101/gr.082503.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Miyosawa K, Ohkubo S, Nakahata N. Physiological significance of thromboxane A(2) receptor dimerization. J Pharmacol Sci. 2006;100:263–70. doi: 10.1254/jphs.fp0050839. [DOI] [PubMed] [Google Scholar]
- Sato F, Yasumoto K, Kimura K, Numayama-Tsuruta K, Sogawa K. Heterodimerization with LBP-1b is necessary for nuclear localization of LBP-1a and LBP-1c. Genes to cells : devoted to molecular & cellular mechanisms. 2005;10:861–70. doi: 10.1111/j.1365-2443.2005.00884.x. [DOI] [PubMed] [Google Scholar]
- Schiel MA, Green SL, Davis WI, Sanghani PC, Bosron WF, Sanghani SP. Expression and characterization of a human carboxylesterase 2 splice variant. The Journal of pharmacology and experimental therapeutics. 2007;323:94–101. doi: 10.1124/jpet.107.127027. [DOI] [PubMed] [Google Scholar]
- Schlesinger F, Tammena D, Krampfl K, Bufler J. Desensitization and resensitization are independently regulated in human recombinant GluR subunit coassemblies. Synapse. 2005;55:176–82. doi: 10.1002/syn.20110. [DOI] [PubMed] [Google Scholar]
- Schneider C, Boeglin WE, Brash AR. Human cyclo-oxygenase-1 and an alternative splice variant: contrasts in expression of mRNA, protein and catalytic activities. The Biochemical journal. 2005;385:57–64. doi: 10.1042/BJ20041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor IE, Allo M, Kornblihtt AR. Intragenic chromatin modifications: A new layer in alternative splicing regulation. Epigenetics : official journal of the DNA Methylation Society. 2010;5 doi: 10.4161/epi.5.3.11316. [DOI] [PubMed] [Google Scholar]
- Scotlandi K, Zuntini M, Manara MC, Sciandra M, Rocchi A, Benini S, Nicoletti G, Bernard G, Nanni P, Lollini PL, Bernard A, Picci P. CD99 isoforms dictate opposite functions in tumour malignancy and metastases by activating or repressing c-Src kinase activity. Oncogene. 2007;26:6604–18. doi: 10.1038/sj.onc.1210481. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Tregear GW, Bathgate RA. LGR7-truncate is a splice variant of the relaxin receptor LGR7 and is a relaxin antagonist in vitro. Ann N Y Acad Sci. 2005;1041:22–6. doi: 10.1196/annals.1282.005. [DOI] [PubMed] [Google Scholar]
- Scotton P, Bleckmann D, Stebler M, Sciandra F, Brancaccio A, Meier T, Stetefeld J, Ruegg MA. Activation of muscle-specific receptor tyrosine kinase and binding to dystroglycan are regulated by alternative mRNA splicing of agrin. The Journal of biological chemistry. 2006;281:36835–45. doi: 10.1074/jbc.M607887200. [DOI] [PubMed] [Google Scholar]
- Seo PS, Jeong JJ, Zeng L, Takoudis CG, Quinn BJ, Khan AA, Hanada T, Chishti AH. Alternatively spliced exon 5 of the FERM domain of protein 4.1R encodes a novel binding site for erythrocyte p55 and is critical for membrane targeting in epithelial cells. Biochim Biophys Acta. 2009a;1793:281–9. doi: 10.1016/j.bbamcr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo PS, Jeong JJ, Zeng L, Takoudis CG, Quinn BJ, Khan AA, Hanada T, Chishti AH. Alternatively spliced exon 5 of the FERM domain of protein 4.1R encodes a novel binding site for erythrocyte p55 and is critical for membrane targeting in epithelial cells. Biochimica et biophysica acta. 2009b;1793:281–9. doi: 10.1016/j.bbamcr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severing EI, van Dijk AD, van Ham RC. Assessing the contribution of alternative splicing to proteome diversity in Arabidopsis thaliana using proteomics data. BMC plant biology. 2011;11:82. doi: 10.1186/1471-2229-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Zhu C, Zhao Z, Guo M, Qiu H, Liu H, Wang D, Xue L, Gao L, Sun C, Li W. KRAB-containing zinc finger gene ZNF268 encodes multiple alternatively spliced isoforms that contain transcription regulatory domains. International journal of molecular medicine. 2006;18:457–63. [PubMed] [Google Scholar]
- Sharma S, Falick AM, Black DL. Polypyrimidine tract binding protein blocks the 5′ splice site-dependent assembly of U2AF and the prespliceosomal E complex. Molecular cell. 2005;19:485–96. doi: 10.1016/j.molcel.2005.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DA, Lawrence DA, Ashkenazi A. Selective knockdown of the long variant of cellular FLICE inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis. J Biol Chem. 2005;280:19401–9. doi: 10.1074/jbc.M413962200. [DOI] [PubMed] [Google Scholar]
- Sharp PA. The discovery of split genes and RNA splicing. Trends in biochemical sciences. 2005;30:279–81. doi: 10.1016/j.tibs.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Shaul YD, Seger R. ERK1c regulates Golgi fragmentation during mitosis. J Cell Biol. 2006;172:885–97. doi: 10.1083/jcb.200509063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Eyras E, Wu J, Khanna A, Josiah S, Rederstorff M, Zhang MQ, Stamm S. Direct cloning of double-stranded RNAs from RNase protection analysis reveals processing patterns of C/D box snoRNAs and provides evidence for widespread antisense transcript expression. Nucleic Acids Res. 2011;39:9720–9730. doi: 10.1093/nar/gkr684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W, Wang G, Wang Y, Liang J, Wen J, Zheng PS, Wu Y, Lee V, Slingerland J, Dumont D, Yang BB. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol Biol Cell. 2005;16:1330–40. doi: 10.1091/mbc.E04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa T, Tostar U, Lauth M, Palaniswamy R, Kasper M, Toftgard R, Zaphiropoulos PG. Novel human glioma-associated oncogene 1 (GLI1) splice variants reveal distinct mechanisms in the terminal transduction of the hedgehog signal. The Journal of biological chemistry. 2008;283:14345–54. doi: 10.1074/jbc.M800299200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionyu M, Yamaguchi A, Shinoda K, Takahashi K, Go M. AS-ALPS: a database for analyzing the effects of alternative splicing on protein structure, interaction and network in human and mouse. Nucleic Acids Res. 2009;37:D305–9. doi: 10.1093/nar/gkn869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiote Y, Ouchida M, Jitsumori Y, Ogama Y, Matsuo Y, Ishimaru F, Tanimoto M, Shimizu K. Multiple splicing variants of Naf1/ABIN-1 transcripts and their alterations in hematopoietic tumors. International journal of molecular medicine. 2006;18:917–23. [PubMed] [Google Scholar]
- Shukla S, Del Gatto-Konczak F, Breathnach R, Fisher SA. Competition of PTB with TIA proteins for binding to a U-rich cis-element determines tissue-specific splicing of the myosin phosphatase targeting subunit 1. Rna. 2005;11:1725–36. doi: 10.1261/rna.7176605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lenser T, Jahn N, Gausmann U, Friedel S, Szafranski K, Huse K, Rosenstiel P, Hampe J, Schuster S, Hiller M, Backofen R, Platzer M. TassDB2 - A comprehensive database of subtle alternative splicing events. BMC bioinformatics. 2010;11:216. doi: 10.1186/1471-2105-11-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi M, Menozzi G, Comi GP, Cagliani R, Bresolin N, Pozzoli U. Analysis of intronic conserved elements indicates that functional complexity might represent a major source of negative selection on non-coding sequences. Human molecular genetics. 2005;14:2533–46. doi: 10.1093/hmg/ddi257. [DOI] [PubMed] [Google Scholar]
- Slaaby R, Schaffer L, Lautrup-Larsen I, Andersen AS, Shaw AC, Mathiasen IS, Brandt J. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. The Journal of biological chemistry. 2006;281:25869–74. doi: 10.1074/jbc.M605189200. [DOI] [PubMed] [Google Scholar]
- Sogawa C, Kumagai K, Sogawa N, Morita K, Dohi T, Kitayama S. C-terminal region regulates the functional expression of human noradrenaline transporter splice variants. Biochem J. 2007;401:185–95. doi: 10.1042/BJ20060495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa C, Mitsuhata C, Kumagai-Morioka K, Sogawa N, Ohyama K, Morita K, Kozai K, Dohi T, Kitayama S. Expression and function of variants of human catecholamine transporters lacking the fifth transmembrane region encoded by exon 6. PLoS One. 2010;5:e11945. doi: 10.1371/journal.pone.0011945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SW, Fuller GN, Zheng H, Zhang W. Inactivation of the invasion inhibitory gene IIp45 by alternative splicing in gliomas. Cancer Res. 2005;65:3562–7. doi: 10.1158/0008-5472.CAN-04-3392. [DOI] [PubMed] [Google Scholar]
- Soupene E, Dinh NP, Siliakus M, Kuypers FA. Activity of the acyl-CoA synthetase ACSL6 isoforms: role of the fatty acid Gate-domains. BMC biochemistry. 2010;11:18. doi: 10.1186/1471-2091-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speek M, Njunkova O, Pata I, Valdre E, Kogerman P. A potential role of alternative splicing in the regulation of the transcriptional activity of human GLI2 in gonadal tissues. BMC molecular biology. 2006;7:13. doi: 10.1186/1471-2199-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spena S, Asselta R, Plate M, Castaman G, Duga S, Tenchini ML. Pseudo-exon activation caused by a deep-intronic mutation in the fibrinogen gamma-chain gene as a novel mechanism for congenital afibrinogenaemia. British journal of haematology. 2007;139:128–32. doi: 10.1111/j.1365-2141.2007.06758.x. [DOI] [PubMed] [Google Scholar]
- Sporici RA, Hodskins JS, Locasto DM, Meszaros LB, Ferry AL, Weidner AM, Rinehart CA, Bailey JC, Mains IM, Diamond SE. Repression of the prolactin promoter: a functional consequence of the heterodimerization between Pit-1 and Pit-1 beta. Journal of molecular endocrinology. 2005;35:317–31. doi: 10.1677/jme.1.01678. [DOI] [PubMed] [Google Scholar]
- Stamm S. Regulation of alternative splicing by reversible phosphorylation. J Biol Chem. 2008;283:1223–7. doi: 10.1074/jbc.R700034200. [DOI] [PubMed] [Google Scholar]
- Stamm S, Ben-Ari S, Rafalska I, Tang Y, Zhang Z, Toiber D, Thanaraj TA, Soreq H. Function of alternative splicing. Gene. 2005;344C:1–20. doi: 10.1016/j.gene.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Stamm S, Smith CWJ, R L. Alternative Splicing: Theory and Protocols. Wiley; 2012. [Google Scholar]
- Stanke M, Keller O, Gunduz I, Hayes A, Waack S, Morgenstern B. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 2006a;34:W435–9. doi: 10.1093/nar/gkl200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M, Schoffmann O, Morgenstern B, Waack S. Gene prediction in eukaryotes with a generalized hidden Markov model that uses hints from external sources. BMC Bioinformatics. 2006b;7:62. doi: 10.1186/1471-2105-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M, Tzvetkova A, Morgenstern B. AUGUSTUS at EGASP: using EST, protein and genomic alignments for improved gene prediction in the human genome. Genome Biol. 2006c;7 Suppl 1:S11, 1–8. doi: 10.1186/gb-2006-7-s1-s11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoilov P, Daoud R, Nayler O, Stamm S. Human tra2-beta1 autoregulates its protein concentration by influencing alternative splicing of its pre-mRNA. Hum Mol Genet. 2004;13:509–524. doi: 10.1093/hmg/ddh051. [DOI] [PubMed] [Google Scholar]
- Strasburg GM, Chiang W. Pale, soft, exudative turkey--The role of ryanodine receptor variation in meat quality. Poultry science. 2009;88:1497–505. doi: 10.3382/ps.2009-00181. [DOI] [PubMed] [Google Scholar]
- Strom AL, Forsgren L, Holmberg M. Identification and characterization of Spinocerebellar Ataxia Type 7 (SCA7) isoform SCA7b in mice. Biochimica et biophysica acta. 2005;1731:149–53. doi: 10.1016/j.bbaexp.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Suggs JA, Cammarato A, Kronert WA, Nikkhoy M, Dambacher CM, Megighian A, Bernstein SI. Alternative S2 hinge regions of the myosin rod differentially affect muscle function, myofibril dimensions and myosin tail length. J Mol Biol. 2007;367:1312–29. doi: 10.1016/j.jmb.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugnet CW, Srinivasan K, Clark TA, O'Brien G, Cline MS, Wang H, Williams A, Kulp D, Blume JE, Haussler D, Ares M., Jr Unusual intron conservation near tissue-regulated exons found by splicing microarrays. PLoS computational biology. 2006;2:e4. doi: 10.1371/journal.pcbi.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan M, Schulz MH, Richard H, Magen A, Klingenhoff A, Scherf M, Seifert M, Borodina T, Soldatov A, Parkhomchuk D, Schmidt D, O'Keeffe S, Haas S, Vingron M, Lehrach H, Yaspo ML. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science. 2008;321:956–60. doi: 10.1126/science.1160342. [DOI] [PubMed] [Google Scholar]
- Sumanasekera C, Kelemen O, Beullens M, Aubol BE, Adams JA, Sunkara M, Morris A, Bollen M, Andreadis A, Stamm S. C6 Pyridinium Ceramide Influences Alternative pre-mRNA Splicing By Inhibiting Protein Phosphatase-1. Nucleic Acids Res. 2012 doi: 10.1093/nar/gkr1289. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Zhang Z, Sinha R, Karni R, Krainer AR. SF2/ASF autoregulation involves multiple layers of post-transcriptional and translational control. Nature structural & molecular biology. 2010;17:306–12. doi: 10.1038/nsmb.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundvall M, Korhonen A, Paatero I, Gaudio E, Melino G, Croce CM, Aqeilan RI, Elenius K. Isoform-specific monoubiquitination, endocytosis, and degradation of alternatively spliced ErbB4 isoforms. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4162–7. doi: 10.1073/pnas.0708333105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundvall M, Peri L, Maatta JA, Tvorogov D, Paatero I, Savisalo M, Silvennoinen O, Yarden Y, Elenius K. Differential nuclear localization and kinase activity of alternative ErbB4 intracellular domains. Oncogene. 2007;26:6905–14. doi: 10.1038/sj.onc.1210501. [DOI] [PubMed] [Google Scholar]
- Swank DM, Braddock J, Brown W, Lesage H, Bernstein SI, Maughan DW. An alternative domain near the ATP binding pocket of Drosophila myosin affects muscle fiber kinetics. Biophysical journal. 2006;90:2427–35. doi: 10.1529/biophysj.105.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata Y, Chen W, Warrier MR, Gibson AM, Daines MO, Hershey GK. Allergy-driven alternative splicing of IL-13 receptor alpha2 yields distinct membrane and soluble forms. J Immunol. 2006;177:7905–12. doi: 10.4049/jimmunol.177.11.7905. [DOI] [PubMed] [Google Scholar]
- Tadokoro K, Yamazaki-Inoue M, Tachibana M, Fujishiro M, Nagao K, Toyoda M, Ozaki M, Ono M, Miki N, Miyashita T, Yamada M. Frequent occurrence of protein isoforms with or without a single amino acid residue by subtle alternative splicing: the case of Gln in DRPLA affects subcellular localization of the products. J Hum Genet. 2005;50:382–94. doi: 10.1007/s10038-005-0261-9. [DOI] [PubMed] [Google Scholar]
- Tagami T, Yamamoto H, Moriyama K, Sawai K, Usui T, Shimatsu A, Naruse M. Identification of a novel human thyroid hormone receptor beta isoform as a transcriptional modulator. Biochemical and biophysical research communications. 2010;396:983–8. doi: 10.1016/j.bbrc.2010.05.038. [DOI] [PubMed] [Google Scholar]
- Takayama S, Hostick U, Haendel M, Eisen J, Darimont BAn. F-domain introduced by alternative splicing regulates activity of the zebrafish thyroid hormone receptor alpha. General and comparative endocrinology. 2008;155:176–89. doi: 10.1016/j.ygcen.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda J, Suzuki Y, Nakao M, Kuroda T, Sugano S, Gojobori T, Imanishi T. H-DBAS: alternative splicing database of completely sequenced and manually annotated full-length cDNAs based on H-Invitational. Nucleic Acids Res. 2007;35:D104–9. doi: 10.1093/nar/gkl854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda J, Suzuki Y, Sakate R, Sato Y, Gojobori T, Imanishi T, Sugano S. H-DBAS: human-transcriptome database for alternative splicing: update 2010. Nucleic Acids Res. 2010;38:D86–90. doi: 10.1093/nar/gkp984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D, Walker AM. Short form 1b human prolactin receptor down-regulates expression of the long form. J Mol Endocrinol. 2010;44:187–94. doi: 10.1677/JME-09-0101. [DOI] [PubMed] [Google Scholar]
- Tang X, Tian Z, Chueh PJ, Chen S, Morre DM, Morre DJ. Alternative splicing as the basis for specific localization of tNOX, a unique hydroquinone (NADH) oxidase, to the cancer cell surface. Biochemistry. 2007;46:12337–46. doi: 10.1021/bi700973k. [DOI] [PubMed] [Google Scholar]
- Tanowitz M, Hislop JN, von Zastrow M. Alternative splicing determines the post-endocytic sorting fate of G-protein-coupled receptors. J Biol Chem. 2008;283:35614–21. doi: 10.1074/jbc.M806588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao RH, Kawate H, Ohnaka K, Ishizuka M, Hagiwara H, Takayanagi R. Opposite effects of alternative TZF spliced variants on androgen receptor. Biochemical and biophysical research communications. 2006;341:515–21. doi: 10.1016/j.bbrc.2005.12.213. [DOI] [PubMed] [Google Scholar]
- Taranta A, Petrini S, Palma A, Mannucci L, Wilmer MJ, De Luca V, Diomedi-Camassei F, Corallini S, Bellomo F, van den Heuvel LP, Levtchenko EN, Emma F. Identification and subcellular localization of a new cystinosin isoform. American journal of physiology Renal physiology. 2008;294:F1101–8. doi: 10.1152/ajprenal.00413.2007. [DOI] [PubMed] [Google Scholar]
- Tazi J, Bakkour N, Marchand V, Ayadi L, Aboufirassi A, Branlant C. Alternative splicing: regulation of HIV-1 multiplication as a target for therapeutic action. The FEBS journal. 2010;277:867–76. doi: 10.1111/j.1742-4658.2009.07522.x. [DOI] [PubMed] [Google Scholar]
- Tazi J, Bakkour N, Stamm S. Alternative splicing and disease. Biochim Biophys Acta. 2009;1792:14–26. doi: 10.1016/j.bbadis.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006;7 Suppl 1:S12, 1–14. doi: 10.1186/gb-2006-7-s1-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CP, Andrews JI, Liu KZ. Intronic polyadenylation signal sequences and alternate splicing generate human soluble Flt1 variants and regulate the abundance of soluble Flt1 in the placenta. FASEB J. 2007;21:3885–95. doi: 10.1096/fj.07-8809com. [DOI] [PubMed] [Google Scholar]
- Thomas CP, Raikwar NS, Kelley EA, Liu KZ. Alternate processing of Flt1 transcripts is directed by conserved cis-elements within an intronic region of FLT1 that reciprocally regulates splicing and polyadenylation. Nucleic acids research. 2010;38:5130–40. doi: 10.1093/nar/gkq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilgner H, Nikolaou C, Althammer S, Sammeth M, Beato M, Valcarcel J, Guigo R. Nucleosome positioning as a determinant of exon recognition. Nature structural & molecular biology. 2009;16:996–1001. doi: 10.1038/nsmb.1658. [DOI] [PubMed] [Google Scholar]
- Tiran Z, Oren A, Hermesh C, Rotman G, Levine Z, Amitai H, Handelsman T, Beiman M, Chen A, Landesman-Milo D, Dassa L, Peres Y, Koifman C, Glezer S, Vidal-Finkelstein R, Bahat K, Pergam T, Israel C, Horev J, Tsarfaty I, Ayalon-Soffer M. A novel recombinant soluble splice variant of Met is a potent antagonist of the hepatocyte growth factor/scatter factor-Met pathway. Clin Cancer Res. 2008;14:4612–21. doi: 10.1158/1078-0432.CCR-08-0108. [DOI] [PubMed] [Google Scholar]
- Tiwari S, Zhang Y, Heller J, Abernethy DR, Soldatov NM. Atherosclerosis-related molecular alteration of the human CaV1.2 calcium channel alpha1C subunit. Proc Natl Acad Sci U S A. 2006;103:17024–9. doi: 10.1073/pnas.0606539103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson DC, L'Hote CG, Kennedy W, Pitt E, Knowles MA. Alternative splicing of fibroblast growth factor receptor 3 produces a secreted isoform that inhibits fibroblast growth factor-induced proliferation and is repressed in urothelial carcinoma cell lines. Cancer Res. 2005;65:10441–9. doi: 10.1158/0008-5472.CAN-05-1718. [DOI] [PubMed] [Google Scholar]
- Torrado M, Iglesias R, Centeno A, Lopez E, Mikhailov AT. Exon-skipping brain natriuretic peptide variant is overexpressed in failing myocardium and attenuates brain natriuretic peptide production in vitro. Exp Biol Med (Maywood) 2010;235:941–51. doi: 10.1258/ebm.2010.010078. [DOI] [PubMed] [Google Scholar]
- Tran YH, Xu Z, Kato A, Mistry AC, Goya Y, Taira M, Brandt SJ, Hirose S. Spliced isoforms of LIM-domain-binding protein (CLIM/NLI/Ldb) lacking the LIM-interaction domain. Journal of biochemistry. 2006;140:105–19. doi: 10.1093/jb/mvj134. [DOI] [PubMed] [Google Scholar]
- Treeck O, Juhasz-Boess I, Lattrich C, Horn F, Goerse R, Ortmann O. Effects of exon-deleted estrogen receptor beta transcript variants on growth, apoptosis and gene expression of human breast cancer cell lines. Breast cancer research and treatment. 2008;110:507–20. doi: 10.1007/s10549-007-9749-7. [DOI] [PubMed] [Google Scholar]
- Treeck O, Pfeiler G, Horn F, Federhofer B, Houlihan H, Vollmer A, Ortmann O. Novel estrogen receptor beta transcript variants identified in human breast cancer cells affect cell growth and apoptosis of COS-1 cells. Molecular and cellular endocrinology. 2007;264:50–60. doi: 10.1016/j.mce.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Tress ML, Bodenmiller B, Aebersold R, Valencia A. Proteomics studies confirm the presence of alternative protein isoforms on a large scale. Genome biology. 2008;9:R162. doi: 10.1186/gb-2008-9-11-r162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, Blencowe BJ, Prasanth SG, Prasanth KV. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Molecular cell. 2010;39:925–38. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KW, Tseng HC, Lin WC. Two wobble-splicing events affect ING4 protein subnuclear localization and degradation. Experimental cell research. 2008;314:3130–41. doi: 10.1016/j.yexcr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Tsyba L, Gryaznova T, Dergai O, Dergai M, Skrypkina I, Kropyvko S, Boldyryev O, Nikolaienko O, Novokhatska O, Rynditch A. Alternative splicing affecting the SH3A domain controls the binding properties of intersectin 1 in neurons. Biochem Biophys Res Commun. 2008;372:929–34. doi: 10.1016/j.bbrc.2008.05.156. [DOI] [PubMed] [Google Scholar]
- Uchikawa H, Toyoda M, Nagao K, Miyauchi H, Nishikawa R, Fujii K, Kohno Y, Yamada M, Miyashita T. Brain- and heart-specific Patched-1 containing exon 12b is a dominant negative isoform and is expressed in medulloblastomas. Biochemical and biophysical research communications. 2006;349:277–83. doi: 10.1016/j.bbrc.2006.08.046. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Lekstrom K, Tsujibe S, Saito N, Leto TL. Subcellular localization and function of alternatively spliced Noxo1 isoforms. Free Radic Biol Med. 2007;42:180–90. doi: 10.1016/j.freeradbiomed.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ule J, Jensen KB, Ruggiu M, Mele A, Ule A, Darnell RB. CLIP identifies Nova-regulated RNA networks in the brain. Science. 2003;302:1212–5. doi: 10.1126/science.1090095. [DOI] [PubMed] [Google Scholar]
- Unoki M, Shen JC, Zheng ZM, Harris CC. Novel splice variants of ING4 and their possible roles in the regulation of cell growth and motility. The Journal of biological chemistry. 2006;281:34677–86. doi: 10.1074/jbc.M606296200. [DOI] [PubMed] [Google Scholar]
- Urano Y, Iiduka M, Sugiyama A, Akiyama H, Uzawa K, Matsumoto G, Kawasaki Y, Tashiro F. Involvement of the mouse Prp19 gene in neuronal/astroglial cell fate decisions. The Journal of biological chemistry. 2006;281:7498–514. doi: 10.1074/jbc.M510881200. [DOI] [PubMed] [Google Scholar]
- Ushiyama M, Ikeda R, Yoshida M, Mori K, Kangawa K, Sugawara H, Inoue K, Yamada K, Miyata A. Alternative splicing of the pituitary adenylate cyclase-activating polypetide (PACAP) receptor contributes to function of PACAP-27. J Mol Neurosci. 42:341–8. doi: 10.1007/s12031-010-9385-2. [DOI] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Domercq M, Matute C. A novel alternative splicing form of excitatory amino acid transporter 1 is a negative regulator of glutamate uptake. Journal of neurochemistry. 2005;95:341–8. doi: 10.1111/j.1471-4159.2005.03370.x. [DOI] [PubMed] [Google Scholar]
- van den Berg YW, van den Hengel LG, Myers HR, Ayachi O, Jordanova E, Ruf W, Spek CA, Reitsma PH, Bogdanov VY, Versteeg HH. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci U S A. 2009;106:19497–502. doi: 10.1073/pnas.0905325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Voort R, Verweij V, de Witte TM, Lasonder E, Adema GJ, Dolstra H. An alternatively spliced CXCL16 isoform expressed by dendritic cells is a secreted chemoattractant for CXCR6+ cells. Journal of leukocyte biology. 2010;87:1029–39. doi: 10.1189/jlb.0709482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani L, Hasegawa M, Spillantini MG, Smith MJ, Murrell JR, Ghetti B, Klug A, Goedert M, Varani G. Structure of tau exon 10 splicingregulatory element RNA and destabilization by mutations of frontotemporal dementia and parkinsonism linked to chromosome 17. Proc Natl Acad Sci USA. 1999;96:8229–8234. doi: 10.1073/pnas.96.14.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta V, Sonnenburg WK, Yan C, Soderling SH, Shimizu-Albergine M, Beavo JA. Identification of a new variant of PDE1A calmodulin-stimulated cyclic nucleotide phosphodiesterase expressed in mouse sperm. Biol Reprod. 2005;73:598–609. doi: 10.1095/biolreprod.104.039180. [DOI] [PubMed] [Google Scholar]
- Vatter P, Stoesser C, Samel I, Gierschik P, Moepps B. The variable C-terminal extension of G-protein-coupled receptor kinase 6 constitutes an accessorial autoregulatory domain. The FEBS journal. 2005;272:6039–51. doi: 10.1111/j.1742-4658.2005.04995.x. [DOI] [PubMed] [Google Scholar]
- Vegran F, Boidot R, Oudin C, Riedinger JM, Bonnetain F, Lizard-Nacol S. Overexpression of caspase-3s splice variant in locally advanced breast carcinoma is associated with poor response to neoadjuvant chemotherapy. Clin Cancer Res. 2006;12:5794–800. doi: 10.1158/1078-0432.CCR-06-0725. [DOI] [PubMed] [Google Scholar]
- Vegran F, Boidot R, Oudin C, Riedinger JM, Lizard-Nacol S. Implication of alternative splice transcripts of caspase-3 and survivin in chemoresistance. Bull Cancer. 2005;92:219–26. [PubMed] [Google Scholar]
- Venables JP. Downstream intronic splicing enhancers. FEBS letters. 2007;581:4127–31. doi: 10.1016/j.febslet.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Venables JP, Klinck R, Koh C, Gervais-Bird J, Bramard A, Inkel L, Durand M, Couture S, Froehlich U, Lapointe E, Lucier JF, Thibault P, Rancourt C, Tremblay K, Prinos P, Chabot B, Elela SA. Cancer-associated regulation of alternative splicing. Nature structural & molecular biology. 2009;16:670–6. doi: 10.1038/nsmb.1608. [DOI] [PubMed] [Google Scholar]
- Vendel AC, Terry MD, Striegel AR, Iverson NM, Leuranguer V, Rithner CD, Lyons BA, Pickard GE, Tobet SA, Horne WA. Alternative splicing of the voltage-gated Ca2+ channel beta4 subunit creates a uniquely folded N-terminal protein binding domain with cell-specific expression in the cerebellar cortex. J Neurosci. 2006;26:2635–44. doi: 10.1523/JNEUROSCI.0067-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendelin J, Pulkkinen V, Rehn M, Pirskanen A, Raisanen-Sokolowski A, Laitinen A, Laitinen LA, Kere J, Laitinen T. Characterization of GPRA, a novel G protein-coupled receptor related to asthma. Am J Respir Cell Mol Biol. 2005;33:262–70. doi: 10.1165/rcmb.2004-0405OC. [DOI] [PubMed] [Google Scholar]
- Verduci L, Simili M, Rizzo M, Mercatanti A, Evangelista M, Mariani L, Rainaldi G, Pitto L. MicroRNA (miRNA)-mediated interaction between leukemia/lymphoma-related factor (LRF) and alternative splicing factor/splicing factor 2 (ASF/SF2) affects mouse embryonic fibroblast senescence and apoptosis. The Journal of biological chemistry. 2010;285:39551–63. doi: 10.1074/jbc.M110.114736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernes SC, Fisher SE. Unravelling neurogenetic networks implicated in developmental language disorders. Biochemical Society transactions. 2009;37:1263–9. doi: 10.1042/BST0371263. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Nicod J, Elahi FM, Coventry JA, Kenny N, Coupe AM, Bird LE, Davies KE, Fisher SE. Functional genetic analysis of mutations implicated in a human speech and language disorder. Human molecular genetics. 2006;15:3154–67. doi: 10.1093/hmg/ddl392. [DOI] [PubMed] [Google Scholar]
- Voelker RB, Berglund JA. A comprehensive computational characterization of conserved mammalian intronic sequences reveals conserved motifs associated with constitutive and alternative splicing. Genome research. 2007;17:1023–33. doi: 10.1101/gr.6017807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, von Lintig J. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry. 2006;45:13429–37. doi: 10.1021/bi060701u. [DOI] [PubMed] [Google Scholar]
- Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469–71. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- Wada K, Tanji K, Kamitani T. Function and subcellular location of Ro52beta. Biochem Biophys Res Commun. 2006;340:872–8. doi: 10.1016/j.bbrc.2005.12.084. [DOI] [PubMed] [Google Scholar]
- Wagner EJ, Garcia-Blanco MA. Polypyrimidine tract binding protein antagonizes exon definition. Molecular and cellular biology. 2001;21:3281–8. doi: 10.1128/MCB.21.10.3281-3288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, Fodor E, Ginsburg A, Hammer JA., 3rd The binding of DYNLL2 to myosin Va requires alternatively spliced exon B and stabilizes a portion of the myosin's coiled-coil domain. Biochemistry. 2006;45:11564–77. doi: 10.1021/bi061142u. [DOI] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Luhrmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–18. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Wan W, Farboud B, Privalsky ML. Pituitary resistance to thyroid hormone syndrome is associated with T3 receptor mutants that selectively impair beta2 isoform function. Molecular endocrinology. 2005;19:1529–42. doi: 10.1210/me.2005-0014. [DOI] [PubMed] [Google Scholar]
- Wang CJ, Vlajkovic SM, Housley GD, Braun N, Zimmermann H, Robson SC, Sevigny J, Soeller C, Thorne PR. C-terminal splicing of NTPDase2 provides distinctive catalytic properties, cellular distribution and enzyme regulation. The Biochemical journal. 2005a;385:729–36. doi: 10.1042/BJ20040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet. 2007;8:749–61. doi: 10.1038/nrg2164. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang P, Sun X, Luo Y, Wang X, Ma D, Wu J. Cloning and characterization of a novel caspase-10 isoform that activates NF-kappa B activity. Biochim Biophys Acta. 2007;1770:1528–37. doi: 10.1016/j.bbagen.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Wang J, Smith PJ, Krainer AR, Zhang MQ. Distribution of SR protein exonic splicing enhancer motifs in human protein-coding genes. Nucleic acids research. 2005b;33:5053–62. doi: 10.1093/nar/gki810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li G, Sugita S. A central kinase domain of type I phosphatidylinositol phosphate kinases is sufficient to prime exocytosis: isoform specificity and its underlying mechanism. The Journal of biological chemistry. 2005c;280:16522–7. doi: 10.1074/jbc.M413263200. [DOI] [PubMed] [Google Scholar]
- Wang P, Yan B, Guo JT, Hicks C, Xu Y. Structural genomics analysis of alternative splicing and application to isoform structure modeling. Proceedings of the National Academy of Sciences of the United States of America. 2005d;102:18920–5. doi: 10.1073/pnas.0506770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Y, Chen B, Zhang Y, Lou G, Chen S, Zhou D. Identification and characterization of PNRC splicing variants. Gene. 2008;423:116–24. doi: 10.1016/j.gene.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang J, Gao L, Stamm S, Andreadis A. An SRp75/hnRNPG complex interacting with hnRNPE2 regulates the 5′ splice site of tau exon 10, whose misregulation causes frontotemporal dementia. Gene. 2011;485:130–8. doi: 10.1016/j.gene.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kayikci M, Briese M, Zarnack K, Luscombe NM, Rot G, Zupan B, Curk T, Ule J. iCLIP predicts the dual splicing effects of TIA-RNA interactions. PLoS biology. 2010;8:e1000530. doi: 10.1371/journal.pbio.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AJ, Cooper TA. The pathobiology of splicing. The Journal of pathology. 2010;220:152–63. doi: 10.1002/path.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warzecha CC, Jiang P, Amirikian K, Dittmar KA, Lu H, Shen S, Guo W, Xing Y, Carstens RP. An ESRP-regulated splicing programme is abrogated during the epithelial-mesenchymal transition. The EMBO journal. 2010;29:3286–300. doi: 10.1038/emboj.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Hao L, Ni S, Liu Q, Xu J, Correll PH. Altered exon usage in the juxtamembrane domain of mouse and human RON regulates receptor activity and signaling specificity. J Biol Chem. 2005;280:40241–51. doi: 10.1074/jbc.M506806200. [DOI] [PubMed] [Google Scholar]
- Wei Y, Fu G, Hu H, Lin G, Yang J, Guo J, Zhu Q, Yu L. Isolation and characterization of mouse testis specific serine/threonine kinase 5 possessing four alternatively spliced variants. Journal of biochemistry and molecular biology. 2007;40:749–56. doi: 10.5483/bmbrep.2007.40.5.749. [DOI] [PubMed] [Google Scholar]
- Weise A, Bruser K, Elfert S, Wallmen B, Wittel Y, Wohrle S, Hecht A. Alternative splicing of Tcf7l2 transcripts generates protein variants with differential promoter-binding and transcriptional activation properties at Wnt/beta-catenin targets. Nucleic acids research. 2010;38:1964–81. doi: 10.1093/nar/gkp1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenthal A, Hoffmeister M, Siddique M, Kovacevic I, Oess S, Muller-Esterl W, Siehoff-Icking A. NOSTRINbeta--a shortened NOSTRIN variant with a role in transcriptional regulation. Traffic. 2009;10:26–34. doi: 10.1111/j.1600-0854.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- Woodside DG, Kram RM, Mitchell JS, Belsom T, Billard MJ, McIntyre BW, Vanderslice P. Contrasting roles for domain 4 of VCAM-1 in the regulation of cell adhesion and soluble VCAM-1 binding to integrin alpha4beta1. J Immunol. 2006;176:5041–9. doi: 10.4049/jimmunol.176.8.5041. [DOI] [PubMed] [Google Scholar]
- Woolfe A, Mullikin JC, Elnitski L. Genomic features defining exonic variants that modulate splicing. Genome Biol. 2010;11:R20. doi: 10.1186/gb-2010-11-2-r20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lu Y, Hu Y, Li R. Cyclic AMP-dependent modification of gonad-selective TAF(II)105 in a human ovarian granulosa cell line. Journal of cellular biochemistry. 2005;96:751–9. doi: 10.1002/jcb.20577. [DOI] [PubMed] [Google Scholar]
- Xing Y, Lee CJ. Protein modularity of alternatively spliced exons is associated with tissue-specific regulation of alternative splicing. PLOS Genetics. 2005;1:e34. doi: 10.1371/journal.pgen.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Lee C. Discovery of novel splice forms and functional analysis of cancer-specific alternative splicing in human expressed sequences. Nucleic Acids Res. 2003;31:5635–43. doi: 10.1093/nar/gkg786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Nie L, Zhang Y, Mo J, Feng W, Wei D, Petrov E, Calisto LE, Kachar B, Beisel KW, Vazquez AE, Yamoah EN. Roles of alternative splicing in the functional properties of inner ear-specific KCNQ4 channels. J Biol Chem. 2007;282:23899–909. doi: 10.1074/jbc.M702108200. [DOI] [PubMed] [Google Scholar]
- Xu XM, Zhou YQ, Wang MH. Mechanisms of cytoplasmic {beta}-catenin accumulation and its involvement in tumorigenic activities mediated by oncogenic splicing variant of the receptor originated from Nantes tyrosine kinase. J Biol Chem. 2005;280:25087–94. doi: 10.1074/jbc.M414699200. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang S, Malhotra A, Edelman-Novemsky I, Ma J, Kruppa A, Cernicica C, Blais S, Neubert TA, Ren M, Schlame M. Characterization of tafazzin splice variants from humans and fruit flies. The Journal of biological chemistry. 2009;284:29230–9. doi: 10.1074/jbc.M109.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Zhou Y, Wu T, Zhu T, Ji X, Kwon YS, Zhang C, Yeo G, Black DL, Sun H, Fu XD, Zhang Y. Genome-wide analysis of PTB-RNA interactions reveals a strategy used by the general splicing repressor to modulate exon inclusion or skipping. Molecular cell. 2009;36:996–1006. doi: 10.1016/j.molcel.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto ML, Clark TA, Gee SL, Kang JA, Schweitzer AC, Wickrema A, Conboy JG. Alternative pre-mRNA splicing switches modulate gene expression in late erythropoiesis. Blood. 2009;113:3363–70. doi: 10.1182/blood-2008-05-160325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Kanbe E, Peterson LF, Boyapati A, Miao Y, Wang Y, Chen IM, Chen Z, Rowley JD, Willman CL, Zhang DE. A previously unidentified alternatively spliced isoform of t(8;21) transcript promotes leukemogenesis. Nat Med. 2006;12:945–9. doi: 10.1038/nm1443. [DOI] [PubMed] [Google Scholar]
- Yang JO, Kim WY, Bhak J. ssSNPTarget: genome-wide splice-site Single Nucleotide Polymorphism database. Hum Mutat. 2009;30:E1010–20. doi: 10.1002/humu.21128. [DOI] [PubMed] [Google Scholar]
- Yeo GW, Coufal NG, Liang TY, Peng GE, Fu XD, Gage FH. An RNA code for the FOX2 splicing regulator revealed by mapping RNA-protein interactions in stem cells. Nature structural & molecular biology. 2009;16:130–7. doi: 10.1038/nsmb.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo GW, Van Nostrand EL, Liang TY. Discovery and analysis of evolutionarily conserved intronic splicing regulatory elements. PLOS Genetics. 2007;3:e85. doi: 10.1371/journal.pgen.0030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Du Y, Hu W, Qiao T, Ding J, Wu K, Liu Z, Fan D. Mad2beta, an alternative variant of Mad2 reducing mitotic arrest and apoptosis induced by adriamycin in gastric cancer cells. Life sciences. 2006;78:1277–86. doi: 10.1016/j.lfs.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Yu W, Li R, Gui B, Shang Y. sZIP, an alternative splice variant of ZIP, antagonizes transcription repression and growth inhibition by ZIP. The Journal of biological chemistry. 2010;285:14301–7. doi: 10.1074/jbc.M110.107508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Maroney PA, Denker JA, Zhang XH, Dybkov O, Luhrmann R, Jankowsky E, Chasin LA, Nilsen TW. Dynamic regulation of alternative splicing by silencers that modulate 5′ splice site competition. Cell. 2008;135:1224–36. doi: 10.1016/j.cell.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Krainer AR, Zhang MQ. Evolutionary impact of limited splicing fidelity in mammalian genes. Trends in genetics : TIG. 2007a;23:484–8. doi: 10.1016/j.tig.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang P, Greendorfer JS, Jiao J, Kelpke SC, Thompson JA. Alternatively spliced FGFR-1 isoforms differentially modulate endothelial cell activation of c-YES. Arch Biochem Biophys. 2006;450:50–62. doi: 10.1016/j.abb.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Zhang P, Takeuchi K, Csaki LS, Reue K. Lipin-1 Phosphatidic Phosphatase Activity Modulates Phosphatidate Levels to Promote Peroxisome Proliferator-activated Receptor gamma (PPARgamma) Gene Expression during Adipogenesis. The Journal of biological chemistry. 2012;287:3485–94. doi: 10.1074/jbc.M111.296681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SC, MacDonald KA, Baguma-Nibasheka M, Geldenhuys L, Casson AG, Murphy PR. Alternative splicing and differential subcellular localization of the rat FGF antisense gene product. BMC molecular biology. 2008;9:10. doi: 10.1186/1471-2199-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Azhar G, Huang C, Cui C, Zhong Y, Huck S, Wei JY. Alternative splicing and nonsense-mediated mRNA decay regulate gene expression of serum response factor. Gene. 2007b;400:131–9. doi: 10.1016/j.gene.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Huypens P, Adamson AW, Chang JS, Henagan TM, Boudreau A, Lenard NR, Burk D, Klein J, Perwitz N, Shin J, Fasshauer M, Kralli A, Gettys TW. Alternative mRNA splicing produces a novel biologically active short isoform of PGC-1alpha. The Journal of biological chemistry. 2009;284:32813–26. doi: 10.1074/jbc.M109.037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Xu Z, Chen J, Yu Z, Tong KL, Lo WS, Pun FW, Ng SK, Tsang SY, Xue H. Two isoforms of GABA(A) receptor beta2 subunit with different electrophysiological properties: Differential expression and genotypical correlations in schizophrenia. Molecular psychiatry. 2006;11:1092–105. doi: 10.1038/sj.mp.4001899. [DOI] [PubMed] [Google Scholar]
- Zhong X, Liu JR, Kyle JW, Hanck DA, Agnew WS. A profile of alternative RNA splicing and transcript variation of CACNA1H, a human T-channel gene candidate for idiopathic generalized epilepsies. Hum Mol Genet. 2006;15:1497–512. doi: 10.1093/hmg/ddl068. [DOI] [PubMed] [Google Scholar]
- Zhou W, Liu Z, Wu J, Liu JH, Hyder SM, Antoniou E, Lubahn DB. Identification and characterization of two novel splicing isoforms of human estrogen-related receptor beta. The Journal of clinical endocrinology and metabolism. 2006;91:569–79. doi: 10.1210/jc.2004-1957. [DOI] [PubMed] [Google Scholar]
- Zhu B, Ramachandran B, Gulick T. Alternative pre-mRNA splicing governs expression of a conserved acidic transactivation domain in myocyte enhancer factor 2 factors of striated muscle and brain. J Biol Chem. 2005;280:28749–60. doi: 10.1074/jbc.M502491200. [DOI] [PubMed] [Google Scholar]
- Zmijewski MA, Slominski AT. Modulation of corticotropin releasing factor (CRF) signaling through receptor splicing in mouse pituitary cell line AtT-20--emerging role of soluble isoforms. J Physiol Pharmacol. 2009;60 Suppl 4:39–46. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.