Abstract
Nosocomial infections caused by vancomycin-resistant Enterococcus have become a major problem. Bacteriophage therapy is proposed as a potential alternative therapy. Bacteriophages are viruses that infect bacteria and are ubiquitous in nature. Lytic bacteriophage was isolated from sewage water that infects VREF, the causative agent of endocarditis, bacteraemia, and urinary tract infections (UTIs). The phage produced clear plaques with unique clear morphology and well-defined boundaries. TEM results of phage revealed it to be 108 ± 0.2 nm long and 90 ± 0.5 nm wide. The characterization of bacteriophage revealed that infection process of phage was calcium and magnesium dependent and phage titers were highest under optimum conditions for VREF, with an optimal temperature range of 37–50°C. The maximum growth was observed at 37°C, hence having 100% viability. The latent period for phage was small with a burst size of 512 viral particles per bacterial cell. The phage was tested against various clinical strains and results proved it to be host specific. It can be used as a potential therapeutic agent for VREF infections. The phage efficiently eradicated VREF inoculated in cattle compost, poultry compost, and a soil sample which makes it a potential agent for clearing compost and soil sample.
1. Introduction
Enterococcus faecalis is a facultative anaerobe, nonmotile, Gram-positive bacterium found in soil, water, food, plants, and sewage [1]. Enterococci inhabit oral cavity, intestines, and female genital tract of human and are recognized as opportunistic pathogens. Enterococci not only are the causative agents of UTIs, bacteraemia, and endocarditis but also infect the bloodstream, intra-abdominal and pelvic regions, surgical sites, and central nervous system [2]. The occurrence of vancomycin-resistant enterococci (VRE) has reached endemic magnitude in various medical centers due to antibiotic resistance [3]. This resistance can be intrinsic, as against most beta-lactams and aminoglycosides [4], or acquired due to either mutations in the genome or addition of resistance encoding genes, as against tetracycline, macrolides, chloramphenicol, and glycopeptides [5]. VRE pose a serious medical threat leaving no treatment option for some infections on one hand and transfer of such resistance traits to other opportunistic pathogens on the other [3, 6].
Bacteriophages are emerging as potential biocontrol agents that specifically target some bacteria in both animals and humans [7]. Today bacteriophages are considered as potential option to be used as antimicrobial agents due to increasing failure of antibiotics. Phages were used for the first time in 1919 as therapeutic agents [8]. Phage therapy is therapeutic use of bacteriophage to destroy pathogenic bacteria [9]. Phage therapy has been proven to be superior to conventional chemotherapy in certain cases [10, 11]. In recent years increasing focus on phage control has been observed as diseases caused by multiple drug-resistant bacteria, including VRE, are becoming more prevalent [12].
Effective tools must be adopted to ensure VRE-free animal and agriculture environment. Avoparcin, growth promoter and homologue of vancomycin, is extensively used in animal husbandry and agricultural industry [13]. Nontherapeutic use of antibiotics in agriculture leads to antibiotic resistance in gut bacteria, such as Enterococci. Not only can this resistance infect people, but these resistance genes can be transferred to other organisms (World Health Organization 2003). Animal production is in close relation to human society due to the use of animal products and recycling resources. The risk of VRE contamination in human population can easily be increased with incidence of its contamination in animal husbandry. As animal waste compost is widely used in farming, it should be ensured that compost is VRE-free to avoid its contamination in agriculture land [12].
Among 36 phages previously isolated for Enterococcus [14], only few against VRE have been reported [9, 12, 15, 16]. The objective of this study was to isolate and characterize bacteriophage against vancomycin-resistant Enterococcus faecalis as a potential agent for VRE control. The aims of the study included the bacterial reduction in natural as well as inoculated compost and soil.
2. Materials and Methods
2.1. Bacterial Identification
After overnight incubation of bacterial strain, established microbiological methods (colony morphology, Gram staining, and biochemical test) were used for identification of bacterial strain [1]. Analytical profile index (API) test kit [11], a standardized identification system for Enterococcaceae family, was used for biochemical tests. Kirby Bauer method was used to check the antimicrobial susceptibility of the strain. For 16S rRNA sequencing, DNA was manually isolated using methods described by Carozzi et al., 1991. The 16S rRNA gene was amplified by using polymerase chain reaction (PCR) with universal 16S rRNA primers (RS-1, 5′-AAACTCAAATGAATTGACGG-3′; RS-3, 5′-ACGGGCGGTGTGTAC-3′) as described earlier [8]. Reaction was carried out by initial denaturation at 95°C for 5 minutes and 35 cycles of amplification (95°C: 45 seconds, 51°C: 45 seconds, and 72°C: 1 minute) with a final elongation time of 5 minutes at 72°C. The amplified product was electrophoresed on 1% agarose gel and eluted from gel using Invitrogen gel extraction kit (Carlsbad, USA). Sequencing was carried out by Molecular Biology Products (Karachi, Pakistan). The sequence homology was checked by subjecting the 16S rRNA sequence to BLAST analysis (https://www.ncbi.nlm.nih.gov/BLAST).
2.2. Phage Enrichment and Amplification
Bacteriophages specific to VREF were enriched with methods described by Stenholm et al. with some modifications [17, 18]. Sewage water sample was centrifuged at 1400 rpm for 15 minutes to remove algal cell and debris. Sewage water sample was incubated with isolated host bacterial strain to enrich the phage population.
The above prepared sample concentrates (5 mL) were added to 30 mL OD600 VREF grown overnight at 37°C. Enrichment cultures were incubated overnight at 37°C with shaking at 150 rpm. Enrichment culture was incubated overnight and 1 mL was transferred to eppendorf tubes and one drop of 1% chloroform was added to disrupt bacterial cells and release phages. The culture was centrifuged at 14000 rpm for 10 minutes to pellet down bacterial cell debris. The supernatant was filtered using 0.45 μm and 0.20 μm syringe filters sequentially (Minisart filters, Germany) and transferred to a new tube. Plaque assay on LB agar plate (tryptone 10 g/L, yeast extract 5 g/L, NaCl 10 g/L, and agar 15 g/L) with top agar (LB media having 0.75% agar) was carried out for the detection of phage. Autoclaved test tubes with 100 μL of sewage sample along with 100 μL of the VREF culture were incubated for 5–10 minutes at room temperature. About 2-3 mL of top agar was added to the test tubes and the tubes were poured to appropriate plates. The plates were then allowed to solidify after which they were incubated at 37°C overnight and examined for plaques. SM buffer [100 mM NaCl, 8 mM MgSO4, and 50 mM Tris HCl (pH 7.5)] and 0.002% (w/v) gelatin at 4°C with the addition of 7% dimethyl sulfur oxide (DMSO) were used to store the purified phages at –80°C.
2.3. Calcium and Magnesium Ions Effect on Adsorption Rate of Phage
A culture of VREF (100 mL), incubated at 37°C for 16 h, was taken and divided into four flasks of 25 mL each. Among two sets of flasks, one was inoculated with 500 μL (10 × 107 pfu/ml) phage only (control), while the second flask was inoculated with both 500 μl phage and 250 μl CaCl2 (10 mM). In the other set, one flask was inoculated with 500 μL (10 × 107 pfu/ml) phage only (control), while the second flask was inoculated with both 500 μl phage and 250 μl MgCl2 (10 mM). Samples were taken at different time intervals of 0, 10, 20, and 30 minutes to measure the number of free phages in control and Ca and Mg added solution. The number of free phages was determined using the double layer agar assay [18].
2.4. One-Step Growth, Latent Period, and Phage Burst Size
The method previously described for determining the latent period and burst size by Jamal et al. (2015) was followed [18]. VREF culture (50 mL) was incubated to mid exponential phase O.D600 of 0.4–0.6 and the cells were harvested by centrifugation. Pellet was resuspended in 0.5 mL LB broth media and mixed with 0.5 mL phage having 10 × 107 pfu. Phage was allowed to adsorb to bacteria for 1 minute and mixture was centrifuged at 13000 rpm for 30 seconds to remove free phages. The pellet was resuspended in 100 mL fresh media and culture was incubated at 37°C continuously. Samples from the incubated flask were taken at 3-minute intervals and the phage titer was determined by double layer agar assay.
2.5. Transmission Electron Microscopy of SRG1 Phage Morphology
The purified SRG1 phage particles were transferred to the surface of a formvar carbon film (on a 200-mesh copper grid), negatively stained by addition of 2% uranyl acetate, and blotted immediately with filter paper; the grid was then air-dried for viewing by transmission electron microscopy (Hitachi, H-7000) at 100 kV. The guidelines provided by international committee on taxonomy of viruses (ICTV) were used for classification of the SRG1 phage based on the electron microscopy results.
2.6. Thermal and pH Stability
The pH stability for phage was carried out as previously described by Capra et al. [19, 20]. Under pH values of 1, 3, 5, 7, 9, and 11, 500 μL of phages was treated and incubated at 37°C for 1 hour. After incubation, each treated sample was tested against the host in double layer agar assay to check the viability of phages.
Thermal stability test for the phage was performed according to the method described by Capra et al. [19, 20]. Phage filtrates were taken in eppendorf tubes and treated under different temperatures for 1 hour. After incubation, plaque assay was performed for each treated sample.
2.7. Host Range Determination
The host specificity of the phage was assessed on a range of clinical pathogenic bacterial strains including Pseudomonas aeruginosa, Methicillin resistant Staphylococcus aureus (MRSA), Citrobacter sp., Acinetobacter sp., E. coli, E. faecalis, and E. faecium. To test the susceptibility of bacterial isolates, drop-on-lawn technique was used [21]. After overnight incubation at 37°C, plates were checked for any plaque formation against an uninfected negative control.
2.8. Bacterial Reduction Assay
Two flasks containing 100 mL LB broth media were inoculated with VREF and incubated at 37°C in shaking incubator. When O.D600 was in the range of 0.4–0.6, one flask was inoculated with 1 mL of phage filtrate. The other flask was taken as control having no phages. The flasks were then incubated at 37°C and the O.D600 readings were taken at interval of 2 hours for 24 hours using spectrophotometer [18].
2.9. Application of Bacteriophage to Compost and Soil
Compost is organic matter that has been decomposed and recycled as a fertilizer and is a key ingredient in organic farming. In LB media, 7 ml of bacterial culture was grown and CFU (colony forming unit) was calculated (3.5 × 109). The poultry and cattle compost samples along with soil sample were measured and dried at 70°C for 4 hrs. 1.5 mL of bacterial culture was taken in an eppendorf and was then centrifuged at 13,000 rpm for 10 minutes. Supernatant was removed and pellet was washed twice with double distilled water. E. faecalis along with double distilled water was enumerated in LB media. 1 mL of diluted E. faecalis (3.2 × 107) was added to 0.5 g of both compost and soil samples. The compost and soil sample were added to each flask and 500 μl of phage filtrate was added. The mixture was vortexed and incubated at 37°C for 2 days. Soil and compost were diluted with 5 mL of sterilized dH2O and vortexed for 2 minutes. Serial dilutions were made and bacterial colonies were enumerated on LB agar plates [12].
3. Results
3.1. Bacterial Identification
Morphological characterization showed off white/beige colored round pinpoint colonies with entire margins. Enterococcus was confirmed to be Gram-positive cocci by using Gram staining technique. The strain was confirmed to be VREF which belongs to Enterococcaceae family by biochemical test API 20E. PCR yielded an expected size amplicon (470 bp) that was subjected to DNA sequencing from both orientations (Figure 1). The resulting sequence was deposited to database and aligned to search for homologous sequences. In Basic Local Alignment Search Tool (BLAST) analysis, it showed high nucleotide sequence identity of 100% to Enterococcus faecalis. The sequence was submitted to NCBI and accession number was obtained as Enterococcus faecalis KF448071.
Figure 1.
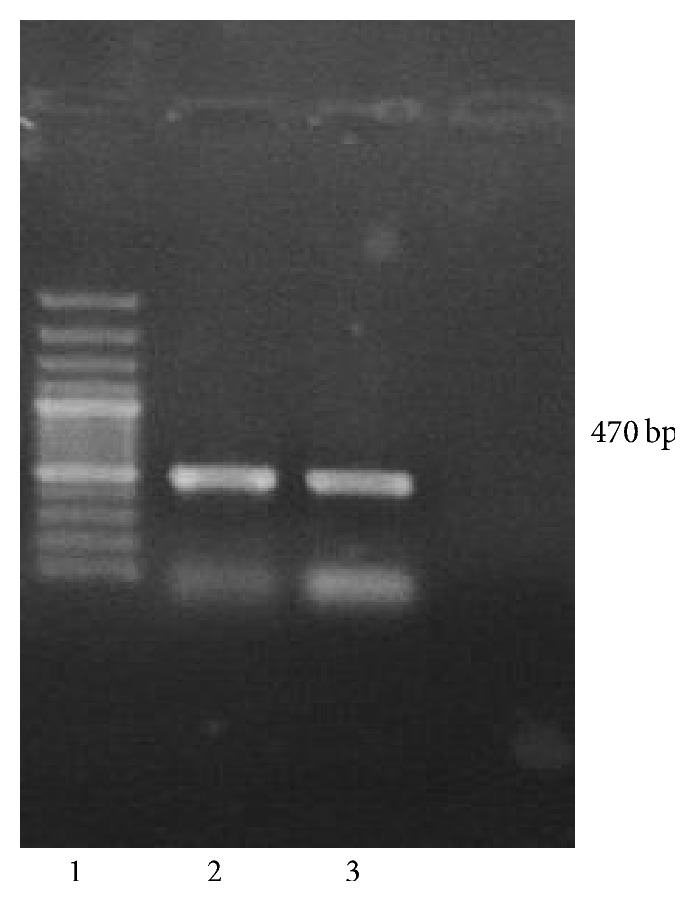
PCR amplification of Enterococcus faecalis 16S rRNA gene. Lane 2 and lane 3 show bands at approximately 470 bp. Lane 1 shows 1 kb ladder.
The Kirby Bauer method showed that the strain was resistant to antibiotics including vancomycin, erythromycin, teicoplanin, and ciprofloxacin.
3.2. Bacteriophage Isolation
Phage SRG1 was isolated for VREF from water sample collected from sewage in Islamabad, using the double layer agar assay technique after enrichment of the phage. Plaques were obtained after incubation of plates at 37°C overnight having plaque size ranging from 0.2 to 0.5 mm in diameter and unique clear morphology with well-defined boundaries (Figure 2).
Figure 2.
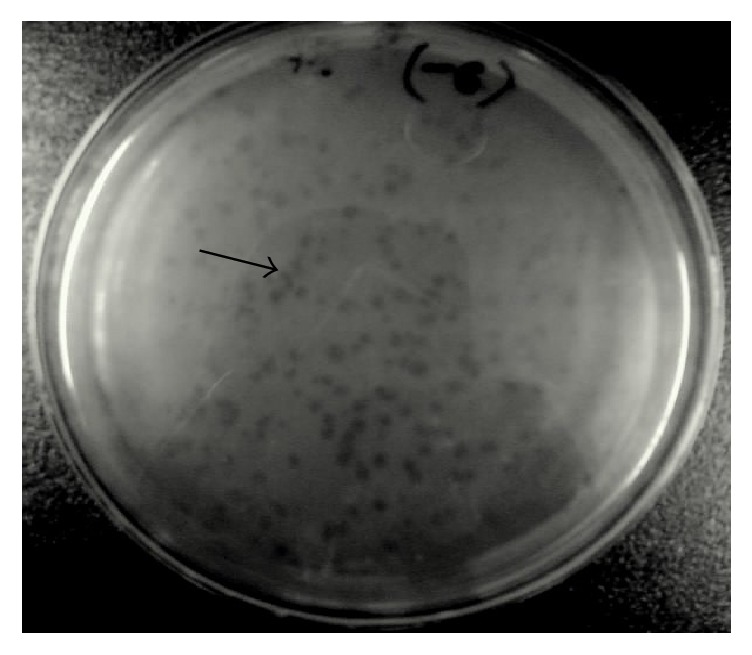
Double layer agar assay, arrows showing pinpoint plaques of phage. Calculated pfu was 10 × 107 with plaque size of 0.2–0.5 mm in diameter.
3.3. Analysis of Calcium and Magnesium Ions Effect on Phage Adsorption Rate
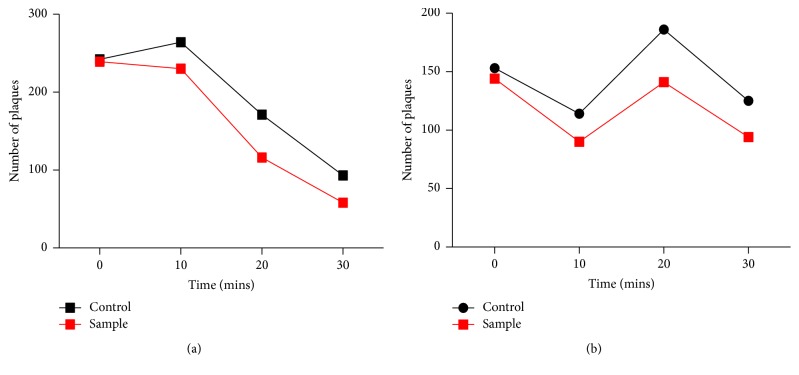
The effect of calcium and magnesium ions on adsorption of phage was analyzed by adding (10 mM) CaCl2 and MgCl2 to the phage and VREF. The number of free phages left in the solution at different time intervals of 0, 10, 20, and 30 minutes was detected using the plaque assay. Statistical analysis showed significant difference between control and the calcium ion treated group (Figures 3(a) and 3(b)). The ions might have enhanced phage adsorption.
Figure 3.
(a) Effect of calcium ions on adsorption rate of phage and VREF. (b) Effect of magnesium ions on adsorption rate of phage and VREF. Divalent metal ions had positive effect on the binding of phage to bacterial cell surface receptors as evident by decreased number of free phages in comparison to controls.
The percentile of free phages was found by the following formula:
| (1) |
where No is the PFU/ml of phages at T = 0 min while N is PFU/ml at T = 10, 20, and 30 mins.
3.4. Latent Period and Phage Burst Size
One-step growth experiment was performed for determination of the latent time period and burst size of phage. The graph obtained shows the lag, log, and the stationary phase (Figure 4). The latent time was determined to be 21 minutes. The burst size of the phage was 512 virions. Determination of burst size was based on the ratio of mean yield of phage that infected the bacterial cells to the mean phage particles liberated.
Figure 4.
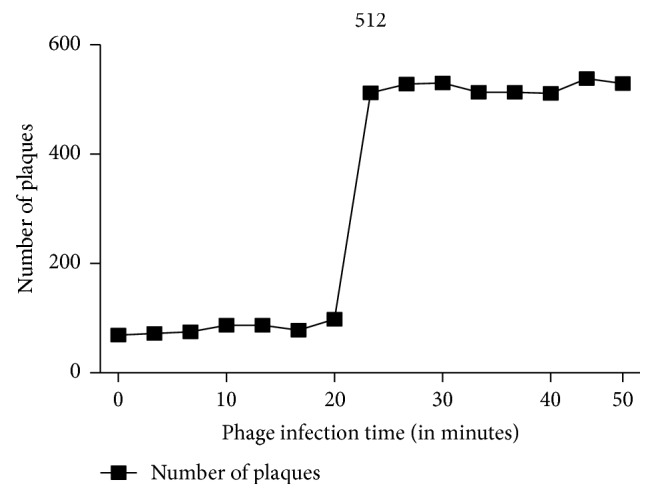
One-step growth experiment showing latent phase (21 mins) with burst size (amount of infectious virus produced, per infected cell) of 521 phages and plateau and of the phage SRG1.
3.5. Transmission Electron Microscopy of SRG1 Phage Morphology
The phage morphology was observed by transmission electron microscopy. The phage head was 108 ± 0.2 nm long and 90 ± 0.5 nm wide with icosahedral sides and a long tail of about 107 ± 03 nm and width 29 nm. The phage was assigned to family Myoviridae on the basis of its morphology (Figure 5).
Figure 5.
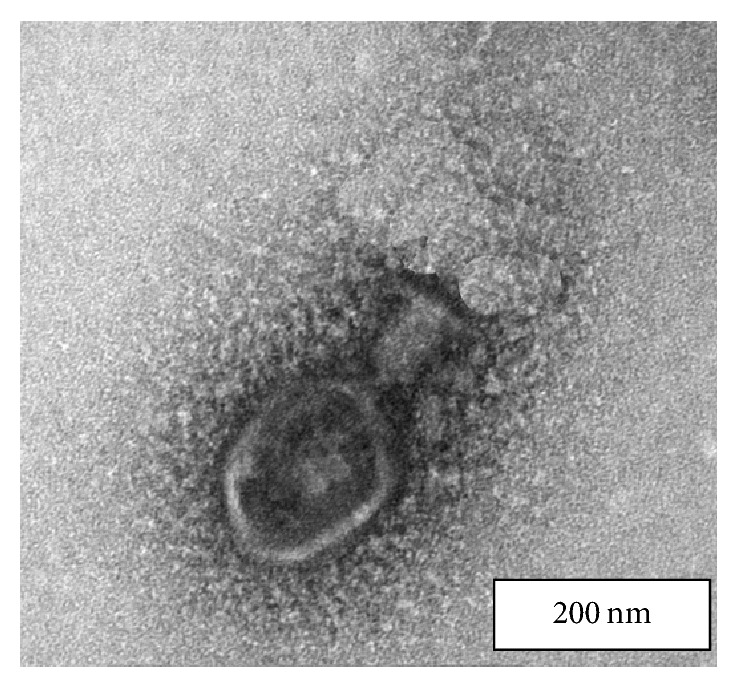
Transmission electron micrographs of the purified SRG1 phage; scale bars, 200 nm.
3.6. pH and Thermal Stability
Phage stability was checked in a range of pH in order to determine optimum pH for phage. At pH 7, incubation for one hour showed no apparent reduction. Number of phages reduced at extreme pH 3.0 and pH 5.0 whereas no active phage was found at pH 1. The number of phages increases with increasing pH. (Figure 6(a)).
Figure 6.
(a) pH stability test showed maximum infectivity at extreme basic pH. (b) Heat stability test showed that 37°C is optimum temperature for phage SRG1.
The potential of phage to resist heat was checked by heat stability test. At 37°C, phage showed 100% activity and found to be stable till 37–65°C. No phages could be observed at 70°C (Figure 6(b)).
3.7. Host Range Determination
Clinical strains of eight pathogens including Pseudomonas aeruginosa, Methicillin resistant Staphylococcus aureus (MRSA), Citrobacter sp., Acinetobacter sp., E. coli, E. faecalis, and E. faecium were used to determine the host range of phage by means of drop-on-lawn technique [21]. The plates were incubated overnight at 37°C. The phage showed slight activity against E. faecalis but overall it had a narrow host range (Table 1).
Table 1.
Determination of host range of phage by using spot and plaque assay.
| Sr number | Hospital strain number | Bacterial strain | Bacteriophage effect | |
|---|---|---|---|---|
| Spot assay | Plaque assay | |||
| (1) | 2934 | Citrobacter sp. | (—) | (—) |
| (2) | 2938 | Staphylococcus aureus | (—) | (—) |
| (3) | 3568 | Pseudomonas aeruginosa Z | (—) | (—) |
| (4) | 2895 | Staphylococcus aureus | (—) | (—) |
| (5) | 2966 | Acinetobacter sp. | (—) | (—) |
| (6) | 3168 | E. coli | (—) | (—) |
| (7) | 2908/22 | E. coli | (—) | (—) |
| (8) | 2953/5 | E. coli | (—) | (—) |
| (9) | U-806 | E. faecalis | Activity | (—) |
| (10) | TH-785 | E. faecalis | (—) | (—) |
| (11) | B-215 | E. faecalis | (—) | (—) |
| (12) | FT-490 | E. faecalis | (—) | (—) |
| (13) | H-707 | E. faecalis | (—) | (—) |
| (14) | U-439 | E. faecalis | (—) | (—) |
| (15) | B-216 | E. faecalis | Activity | (—) |
| (16) | FT-167 | E. faecalis | (—) | (—) |
| (17) | U-170 | E. faecalis | Activity | (—) |
| (18) | U-790 | E. faecium | (—) | (—) |
| (19) | FT-607 | E. faecium | (—) | (—) |
| (20) | FT-549 | E. faecium | (—) | (—) |
3.8. Bacterial Reduction Assay
Infection of VREF was observed with phage for 24 hrs. The turbidity of VREF broth decreased remarkably due to phage infection as compared to the control (Figure 7). The comparison of O.D600 for phage infected and control culture revealed remarkable difference. However, after 11 hours of incubation increase in O.D600 was observed which might be attributed to phage resistant bacterial cells or the cell debris (Figure 7).
Figure 7.
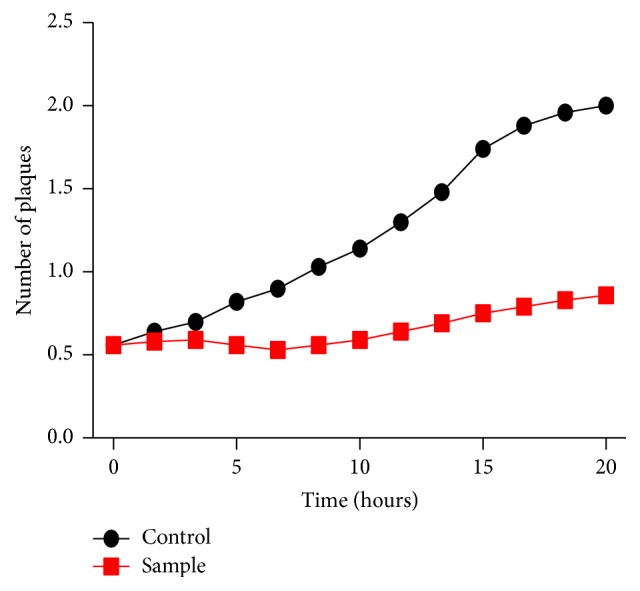
Lysis of VREF in broth culture by phage SRG1 over the time period of 20 hrs.
3.9. Bacterial Reduction in Compost
Three types of cattle compost, 1 poultry compost, and soil samples were inoculated by the E. faecalis. Bacteriophage was added and the bacterial reduction was observed after 24 and 48 hours and compared with controls without phage. The compost and soil samples were measured and dried at 70°C for 4 hrs. About 1 mL of diluted VREF broth was added to 0.5 g of both compost and soil samples. 500 μl of phage filtrate was added to each flask and after incubation CFU was calculated (Table 2).
Table 2.
The reduction of E. faecalis by phage in different samples; the phage titer pfu/ml 10 × 107 was used along with cfu/ml of original culture 3.5 × 109 and cfu/ml of diluted culture 1.6 × 108.
| Sample | CFU after 24 hrs, control | CFU after 48 hrs, control | CFU after 24 hrs, sample | CFU after 48 hrs, sample |
|---|---|---|---|---|
| Compost A (Plantfert) | 1.95 × 108 | 2.20 × 108 | 0 | 0 |
| Compost B (few) | 2.04 × 108 | 2.25 × 108 | 0 | 0 |
| Compost C (Plantafor, Germany) | 1.74 × 108 | 1.86 × 108 | 0 | 0 |
| Compost P | 1.8 × 108 | 2.16 × 108 | 7.0 × 107 | 2.4 × 107 |
| Soil sample | 1.88 × 108 | 2.11 × 108 | 9.0 × 107 | 1.10 × 107 |
4. Discussions
Enterococcus species are known to be opportunistic organisms causing fatal and difficult-to-treat infections [1]. The emergence of resistance in Enterococci against antibiotics of aminoglycoside, β lactams, and glycopeptides (vancomycin and teicoplanin) is a major problem for medical community. Vancomycin and teicoplanin are the agents of choice for infections caused by enterococci [4] and emergence of VRE poses a serious threat because of increased mortality and morbidity [22]. The prevalence of VRE has increased dramatically and six VRE outbreaks have been reported in literature to date, worldwide [23–28].
Bacteriophage therapy can serve as alternate for VRE infections as contemporary medicine has failed to cope with this serious problem. In countries like Russia, Poland, and Eastern Europe bacteriophage therapy has been used for enteric and systemic treatments [8]. Institute of Bacteriophage, Microbiology and Virology, Georgia, is a center for phage therapy and conducts research programs to use phage as a priority for bacterial infections [8].
In this study, we have isolated lytic phage named SRG1 specific for clinical isolate of vancomycin-resistant Enterococcus faecalis (VREF) from sewage water [17]. Other groups have also reported isolation of phages against VRE [9, 12, 15] from sewage, compost, and water channel. Temperature, pH, metal ions CaCl2 and MgCl2, and the physiological stage of host are among the factors that affect in vitro activity of the phage [29, 30]. According to previous reports metal ions may be involved in the adsorption, attachment, and penetration of phage in the host cell [31, 32]. Ca and Mg ions have been suggested to stabilize the weak interaction of phage with receptors by increasing the phage population on host surface that increases the alteration of cell surface receptors in insertion of phage genome into the host cell [33, 34]. Phage SRG1 showed more infectivity with a 10 mM calcium chloride and magnesium chloride concentration.
In this study, phage showed increased activity at pH range of 7–11. The reason may be adaptation of phage to slightly basic pH of sewage water from which it has been isolated. In our study, the phage showed excellent stability to alkaline pH and its infectivity reduced at acidic pH. The optimum temperature for phage was observed to be 37°C and this can be explained as Enterococcus being part of normal flora of humans. Adsorption rate of phage as well as the metabolic activities of host was elevated by increase in temperature [32].
One-step growth experiments exhibited all stages of phage life cycle. Latent time period of the phage was found to be 21 minutes and studies report optimum latent time attributes to high phage fitness [35]. The gradual rise can be attributed to cell debris as well as the phage resistant cells that have developed immunity against the phage [36].
Host range of SRG 1 was checked on a range of clinical pathogenic bacterial strains and the results showed that the phage was highly host specific. The infectivity of phage to some isolates of E. faecalis was observed with spot assay; however, in plaque assay no phage activity was observed. The reason for infectivity in spot assay might be the cytolysin produced by the bacteria.
Application of phage 107 pfu/ml to three different cattle composts and one poultry raw compost having 108 cfu/ml showed complete eradication of not only experimentally inoculated bacteria but also naturally occurring E. faecalis. 50% bacterial reduction was observed in experimentally inoculated soil sample. In a study by Otawa, the phage vrep-5 led to the decrease of VRE abundance up to >3 log10 [12]. In consideration of today's need for organic recycling of animal waste, troubles with pathogens and bacterial drug resistance need to be resolved by inexpensive and effective methods. Enormous amounts of animal waste are emitted daily, and as yet, we have no efficient way of killing specific pathogens in compost. Our study provides a considerable way of killing these pathogens present in compost and soil by means of phage particles.
5. Conclusions
Bacteriophage isolation against VRE is a major step for the development of strategies to control VRE infections. Phage characterization shows that it belongs to family Myoviridae which not only efficiently lyses VRE but also has outstanding thermal and pH stability that makes it a potential candidate for antibacterial therapy. However, the phage is highly host specific and is not effective against other clinical isolates included in our study which suggests that more virulent phages are yet to be discovered in the future. The phage completely eradicated experimentally inoculated VRE in compost and soil samples which proves it to be a potential agent for treatment processes.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Fisher K., Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009;155(6):1749–1757. doi: 10.1099/mic.0.026385-0. [DOI] [PubMed] [Google Scholar]
- 2.Murray B. E., Weinstock G. M. Enterococci: New aspects of an old organism. Proceedings of the Association of American Physicians. 1999;111(4):328–334. doi: 10.1046/j.1525-1381.1999.99241.x. [DOI] [PubMed] [Google Scholar]
- 3.Tucci V., Haran M. A., Isenberg H. D. Epidemiology and control of vancomycin-resistant enterococci in an adult and children's hospital. American Journal of Infection Control. 1997;25(5):371–376. doi: 10.1016/S0196-6553(97)90080-8. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor L., Randhawa V. S., Deb M. Antimicrobial resistance of enterococcal blood isolates at a pediatric care hospital in India. Japanese Journal of Infectious Diseases. 2005;58(2):101–103. [PubMed] [Google Scholar]
- 5.Kayser F. H. Safety aspects of enterococci from the medical point of view. International Journal of Food Microbiology. 2003;88(2-3):255–262. doi: 10.1016/S0168-1605(03)00188-0. [DOI] [PubMed] [Google Scholar]
- 6.Mayhall C. G. The epidemiology and control of VRE: still struggling to come of age. Infection Control and Hospital Epidemiology. 1999;20(10):650–652. doi: 10.1086/501559. [DOI] [PubMed] [Google Scholar]
- 7.Leverentz B., Conway W. S., Camp M. J., et al. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Applied and Environmental Microbiology. 2003;69(8):4519–4526. doi: 10.1128/AEM.69.8.4519-4526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haq I. U., Chaudhry W. N., Akhtar M. N., Andleeb S., Qadri I. Bacteriophages and their implications on future biotechnology: a review. Virology journal. 2012;9:p. 9. doi: 10.1186/1743-422X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchiyama J., Rashel M., Maeda Y., et al. Isolation and characterization of a novel Enterococcus faecalis bacteriophage phiEF24C as a therapeutic candidate. Federation of European Microbiological Societies. 2008;278(2):200–206. doi: 10.1111/j.1574-6968.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 10.Marza J. A. S., Soothill J. S., Boydell P., Collyns T. A. Multiplication of therapeutically administered bacteriophages in Pseudomonas aeruginosa infected patients. Burns. 2006;32(5):644–646. doi: 10.1016/j.burns.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Fox J. L. The business of developing antibacterials. Nature Biotechnology. 2006;24(12):1521–1528. doi: 10.1038/nbt1206-1521. [DOI] [PubMed] [Google Scholar]
- 12.Otawa K., Hirakata Y., Kaku M., Nakai Y. Bacteriophage control of vancomycin-resistant enterococci in cattle compost. Journal of Applied Microbiology. 2012;113(3):499–507. doi: 10.1111/j.1365-2672.2012.05361.x. [DOI] [PubMed] [Google Scholar]
- 13.Klare I., Heier H., Claus H., Reissbrodt R., Witte W. vanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiology Letters. 1995;125(2-3):165–171. doi: 10.1016/0378-1097(94)00493-B. [DOI] [PubMed] [Google Scholar]
- 14.Fard R. M. N., Barton M. D., Heuzenroeder M. W. Novel bacteriophages in enterococcus spp. Current Microbiology. 2010;60(6):400–406. doi: 10.1007/s00284-009-9555-z. [DOI] [PubMed] [Google Scholar]
- 15.Biswas B., Adhya S., Washart P., et al. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infection and Immunity. 2002;70(1):204–210. doi: 10.1128/IAI.70.1.204-210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchiyama J., Rashel M., Takemura I., Wakiguchi H., Matsuzaki S. In silico and in vivo evaluation of bacteriophage φEF24C, a candidate for treatment of Enterococcus faecalis infections. Applied and Environmental Microbiology. 2008;74(13):4149–4163. doi: 10.1128/AEM.02371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenholm A. R., Dalsgaard I., Middelboe M. Isolation and characterization of bacteriophages infecting the fish pathogen Flavobacterium psychrophilum. Applied and Environmental Microbiology. 2008;74(13):4070–4078. doi: 10.1128/AEM.00428-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamal M., Hussain T., Das C. R., Andleeb S. Isolation and characterization of a Myoviridae MJ1 bacteriophage against multi-drug resistant Escherichia coli 3. Jundishapur Journal of Microbiology. 2015;8(11) doi: 10.5812/jjm.25917.e25917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capra M. L., Quiberoni A., Reinheimer J. Phages of Lactobacillus casei/paracasei: Response to environmental factors and interaction with collection and commercial strains. Journal of Applied Microbiology. 2006;100(2):334–342. doi: 10.1111/j.1365-2672.2005.02767.x. [DOI] [PubMed] [Google Scholar]
- 20.Capra M. L., Quiberoni A., Reinheimer J. A. Thermal and chemical resistance of Lactobacillus casei and Lactobacillus paracasei bacteriophages. Letters in Applied Microbiology. 2004;38(6):499–504. doi: 10.1111/j.1472-765X.2004.01525.x. [DOI] [PubMed] [Google Scholar]
- 21.Zimmer M., Scherer S., Loessner M. J. Genomic analysis of Clostridium perfringens bacteriophage φ3626, which integrates into guaA and possibly affects sporulation. Journal of Bacteriology. 2002;184(16):4359–4368. doi: 10.1128/JB.184.16.4359-4368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayakawa K., Marchaim D., Martin E. T., et al. Comparison of the clinical characteristics and outcomes associated with vancomycin-resistant Enterococcus faecalis and vancomycin-resistant E. faecium bacteremia. Antimicrobial Agents and Chemotherapy. 2012;56(5):2452–2458. doi: 10.1128/AAC.06299-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uttley A. H. C., Collins C. H., Fitch L. E., et al. High-level vancomycin-resistant enterococci causing hospital infections. Epidemiology and Infection. 1989;103(1):173–181. doi: 10.1017/S0950268800030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karanfil L. V., Murphy M., Josephson A., et al. A cluster of vancomycin-resistant enterococcusfaecium in an intensive care unit. Infection Control & Hospital Epidemiology. 1992;13(4):195–200. doi: 10.1086/646509. [DOI] [PubMed] [Google Scholar]
- 25.Livornese L. L., Jr., Dias S., Samel C., et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Annals of Internal Medicine. 1992;117(2):112–116. doi: 10.7326/0003-4819-117-2-112. [DOI] [PubMed] [Google Scholar]
- 26.Rubin L. G., Isenberg H. D. Vancomycin-Resistant enterococcus faecium in hospitalized children. Infection Control & Hospital Epidemiology. 1992;13(12):700–705. doi: 10.1086/648342. [DOI] [PubMed] [Google Scholar]
- 27.Boyle J. F., Soumakis S. A., Rendo A. Epidemiologic analysis and genotypic characterization of a nosocomial outbreak of vancomycin-resistant enterococci. Journal of Clinical Microbiology. 1993;31(5):1280–1285. doi: 10.1128/jcm.31.5.1280-1285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handwerger S., Raucher B., Altarac D., et al. Nosocomial outbreak due to enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clinical Infectious Diseases. 1993;16(6):750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 29.De Paepe M., Taddei F. Viruses' life history: towards a mechanistic basis of a trade-off between survival and reproduction among phages. PLoS Biology. 2006;4(7):p. e193. doi: 10.1371/journal.pbio.0040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poranen M. M., Ravantti J. J., Grahn A. M., Gupta R., Auvinen P., Bamford D. H. Global changes in cellular gene expression during bacteriophage PRD1 infection. Journal of Virology. 2006;80(16):8081–8088. doi: 10.1128/JVI.00065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luria S. E., Steiner D. L. The role of calcium in the penetration of bacteriophage T5 into its host. Journal of Bacteriology. 1954;67(6):635–639. doi: 10.1128/jb.67.6.635-639.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moldovan R., Chapman-McQuiston E., Wu X. L. On kinetics of phage adsorption. Biophysical Journal. 2007;93(1):303–315. doi: 10.1529/biophysj.106.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russel M., Whirlow H., Sun T.-P., Webster R. E. Low-frequency infection of F- bacteria by transducing particles of filamentous bacteriophages. Journal of Bacteriology. 1988;170(11):5312–5316. doi: 10.1128/jb.170.11.5312-5316.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe K., Takesue S. The requirement for calcium in infection with Lactobacillus phage. Journal of General Virology. 1972;17(1):19–30. doi: 10.1099/0022-1317-17-1-19. [DOI] [PubMed] [Google Scholar]
- 35.Wang I.-N. Lysis timing and bacteriophage fitness. Genetics. 2006;172(1):17–26. doi: 10.1534/genetics.105.045922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhry W. N., Haq I. U., Andleeb S., Qadri I. Characterization of a virulent bacteriophage LK1 specific for Citrobacter freundii isolated from sewage water. Journal of Basic Microbiology. 2014;54(6):531–541. doi: 10.1002/jobm.201200710. [DOI] [PubMed] [Google Scholar]