Abstract
Background
Systematic screening for active pulmonary tuberculosis (TB) is recommended for high-risk populations however the lack of an accurate, simple, and low-cost screening test that can be used in high burden areas is a major obstacle to its implementation. We evaluated whether C-reactive protein (CRP) possesses the necessary test characteristics to screen individuals for active pulmonary TB.
Methods
We performed a systematic review and meta-analysis of studies evaluating the diagnostic accuracy of CRP (cut-point of 10 mg/L) for pulmonary TB. We searched four databases for eligible articles published before January 31, 2015 and extracted data for individual studies. We synthesized data separately for outpatient and inpatient studies and generated pooled summary estimates (95% CI) for sensitivity and specificity using random-effects meta-analysis. We performed pre-specified subgroup analyses to determine pooled summary estimates of CRP for diagnosis-seeking vs. screening populations and for patients with and without HIV infection.
Findings
We identified nine unique studies enrolling 1723 patients from the outpatient and inpatient setting. In the outpatient setting, CRP had high sensitivity (93%, 95% CI: 85–97) and moderate specificity (62%, 95% CI: 42–79) for active pulmonary TB. CRP was just as sensitive and specific for active pulmonary TB among patients with confirmed HIV-infection. Among hospitalized patients, specificity of CRP was poor (21%, 95% CI: 6–52).
Interpretation
CRP shows considerable promise as a tool to facilitate systematic screening for active TB, even among PLHIV. CRP-based TB screening should now be studied in other high-risk groups to determine the full impact of this simple and low-cost test.
INTRODUCTION
Despite substantial investments in global tuberculosis (TB) control, TB incidence remains high with over 9 million TB cases in 2013 alone.1 High-risk groups such as people living with HIV (PLHIV) shoulder a disproportionately heavy disease burden.1 The World Health Organization (WHO) now recommends systematic screening of high-risk groups,2 but the lack of an accurate yet simple screening tool is a key barrier. A good screening test would rule-out TB in the majority of patients without disease (sensitivity ≥ 90%) and limit referrals for more costly confirmatory testing (e.g., Xpert and/or culture) to patients with a high likelihood of having TB (specificity ≥ 70%).3 A test with these characteristics that is also low-cost and can be performed by front-line health workers has been ranked among the highest priority needs for TB diagnostics.3
Current TB screening tools endorsed by the WHO are inadequate and include symptom assessment (cough ≥ 2 weeks for people without HIV or any of four symptoms suggestive of TB for people living with HIV) and chest radiography (CXR).2 A symptom-based approach to TB screening requires a priori knowledge of the patient’s HIV status to be sufficiently sensitive and has poor specificity for active TB, particularly among key high-risk groups such as people living with HIV (specificity range: 5–61%).4–11 Although CXR is sufficiently sensitive and has higher specificity,11,12 it requires costly infrastructure and trained interpreters, both of which are often absent in lower-level health centers where most patients with symptoms suggestive of TB first present for care. To facilitate scale-up of systematic screening of high-risk groups, there is an urgent need to identify an accurate and practical screening tool.
C-reactive protein (CRP) is an acute phase reactant whose levels rise in response to IL-6 mediated pyogenic infections such as active TB. Prior studies have consistently shown CRP to have high sensitivity for TB13–22 and that TB-associated elevations in CRP levels are independent of HIV status.14 In addition, CRP can be measured using a low-cost (< 2 USD per test), point-of-care (POC) assay. To determine whether CRP is an adequate screening test, we performed a systematic review to assess the accuracy of CRP for identifying pulmonary TB.
METHODS
Search strategy and selection criteria
We performed an online search, with assistance from a professional research librarian, to identify all studies that measured blood CRP levels from patients undergoing screening or evaluation for active TB. We searched PubMed, Embase, the Cochrane Library, and Web of Science for relevant studies published on or before January 31, 2014; we updated our search to identify additional studies published between February 1, 2014 and January 31, 2015 (Table 1, Online Supplement). To minimize the impact of potential publication bias, we also performed an online search of all relevant abstracts presented after 2004 at The Union World Lung Health Conference.
Table 1.
Characteristics of included studies.
| First author, year | Country | Study population | Total patients, N (% HIV+) | Active TB, n (%) | CRP test/manufacturer, assay type | Reference standard | Industry involvementa |
|---|---|---|---|---|---|---|---|
| Outpatient pulmonary TB | |||||||
|
| |||||||
| TB screening | |||||||
|
| |||||||
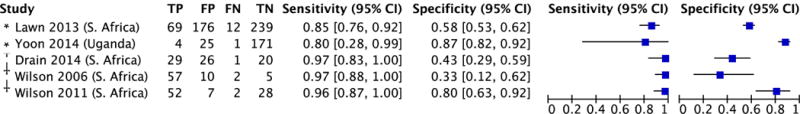
| Lawn, 2013 | South Africa | Outpatients initiating ART | 496 (100) | 81 (16) | Quantikine/R&D Systems, ELISA | MGIT | Kit donation |
| Yoon, 2014 | Uganda | Outpatients initiating ART | 201 (100) | 5 (2.5) | iCHROMA/Boditech, Sandwich immunoassayb | Standardized clinical criteria | Kit donation |
| TB diagnosis | |||||||
| Drain, 2014 | South Africa | Outpatients with TB symptoms and negative sputum AFB smears | 76 (100) | 30 (39.5) | NycoCard/Axis-Shield, Sandwich immunoassayb | LJ and MGIT | Kit donation |
| Wilson, 2006 | South Africa | Outpatients with TB symptoms and negative sputum AFB smears or unable to produce sputum | 74 (100) | 59 (80) | Synchron CX7/Beckman Coulter, Immunoturbidometry | LJ and MGIT | Funding |
| Wilson, 2011 | South Africa | Outpatients with TB symptoms and negative sputum AFB smears or unable to produce sputum | 204 (44) | 116 (57) | Olympus AU640/Olympus, Immunoturbidometry Dimension RXL/Dade-Behring, Immunoturbidometryc |
MGIT | Funding |
|
| |||||||
| Inpatient pulmonary TB | |||||||
|
| |||||||
| Bandyopadhyay, 2011 | India | Inpatients with FUOd | 52 (23) | 9 (17) | Not provided/Abbott, Immunoturbidometry | Sputum and/or BAL culturee | Unclear |
| Cho, 2012 | South Korea | Inpatients with TB symptoms | 73 (3) | 40 (55) | Not provided, Immunoturbidometry | Sputum and/or BAL culturee or positive NAAT | Unclear |
| Lee,2011 | South Korea | Inpatients with TB symptoms | 272 (0) | 82 (30) | CRPL3/Roche Diagnostics, Immunoturbidometry | Sputum and/or BAL culturee | None |
| Sage, 2010 | United Kingdom | Inpatients with TB symptoms | 247 (100) | 28 (11) | Not provided | Sputum and/or BAL culturee | Unclear |
| Wilson, 2006 | South Africa | Inpatients with TB symptoms and negative sputum AFB smears or unable to produce sputum | 28 (100) | 26 (93) | Synchron CX7/Beckman Coulter, Immunoturbidometry | LJ and MGIT | Funding |
Abbreviations: TB (tuberculosis); CRP (C-reactive protein); FUO (fever of unknown origin); BAL (bronchoalveolar lavage); NAAT (nucleic acid amplification test); AFB (acid-fast bacilli); LJ (Löwenstein-Jensen); MGIT (mycobacteria growth indicator tube); ART (antiretroviral therapy).
Industry involvement was determined after reviewing the author’s conflict of interest statement and included kit donation (free and/or discounted test materials) and funding. If a conflict of interest statement was not available with the published article, industry involvement was described as ‘unclear.’
CRP measured using a point-of-care assay.
Study utilized 2 CRP assays during the study period; study participants had CRP levels measured with one assay only.
FUO defined as: 1) T ≥ 38.3°C on several occasions, 2) fever lasting ≥ 3 weeks, 3) failure to reach a diagnosis despite 3 outpatient visits or 3 days in the hospital without elucidation of a cause or 1 week of ambulatory investigation.
Solid and/or liquid culture not specified.
We included studies that measured serum, plasma or whole blood CRP levels in children and adults undergoing pulmonary TB evaluation for symptoms suggestive of active pulmonary TB or high-risk (e.g., HIV-infected) individuals being screened for active pulmonary TB. We excluded: 1) non-English language studies; 2) animal studies; 3) studies presented in only conference abstracts, reviews, letters to the editor, case-series/reports, and case-control studies; 4) studies with an inadequate microbiologic or clinical gold standard method for diagnosing pulmonary TB (see Reference Standard below); 5) studies evaluating only extra-pulmonary TB as the target condition; 6) studies that measured CRP using a non-quantitative assay; 7) studies recruiting only patients with comorbid conditions that are themselves associated with elevated CRP levels (e.g., inflammatory bowel disease); and 8) studies with < 5 active pulmonary TB cases.
Three reviewers (CY, LHC, SMP) independently screened the citations for relevance and reviewed full-text articles using the pre-specified eligibility criteria. The reviewers resolved disagreements about study selection and data extraction by consensus. We extracted study data using a standardized form, including the number of true positives (TP), false positives (FP), false negatives (FN), and true negatives (TN).
Index tests
Eligible studies used quantitative lab-based and/or point-of-care (POC) assays to measure CRP levels and utilized a variety of CRP cut-points. To standardize assessment of diagnostic accuracy, we selected a well-established CRP cut-point of 10 mg/L; large-scale epidemiological studies have found CRP levels ≥ 10 mg/L to be clinically significant because such levels are suggestive of ongoing pyogenic infection and/or another pathologic systemic inflammatory process.23,24 We contacted study authors via email to obtain additional information for studies that did not present sufficient data to allow us to extract or calculate the number of TP, FP, FN, and TN using a CRP cutoff of ≥ 10 mg/L. We excluded studies if the authors did not provide the necessary information.
Reference standard
We included studies that used as a reference standard sputum mycobacterial culture (Löwenstein-Jensen [LJ] and/or mycobacteria growth indicator tube [MGIT]) results. If microbiological testing was not performed in all patients, we included studies that used standardized clinical criteria including longitudinal follow-up of at least one year to exclude active pulmonary TB; our rationale for including studies with this clinical criteria is that patients with undiagnosed active TB are likely to manifest symptoms over a 12 month period and then identified during longitudinal follow-up.
Assessment of Study Quality
We assessed the quality of each included study using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool, a validated tool for diagnostic accuracy studies.25 Because of growing concerns about conflicts of interest in diagnostic studies and guidelines, we also reported whether private industries had any involvement with the design or conduct of the study including donation of test materials, monetary support and participation in data analysis.
Statistical Analysis
We used descriptive statistics to present key study characteristics. We calculated individual study estimates of sensitivity, specificity, and their 95% confidence intervals (CI). We adopted the following pre-specified approach to account for expected heterogeneity. Because test accuracy depends largely on the spectrum of clinical disease severity in a study population, we synthesized data separately for outpatient and inpatient studies. We also performed subgroup analyses to determine sensitivity and specificity estimates of CRP for diagnosis-seeking vs. screening populations and for patients with and without confirmed HIV infection. For all analyses, we assessed heterogeneity visually using forest plots and statistically using the chi-squared and I2 tests. We then calculated pooled sensitivity and specificity estimates of CRP for pulmonary TB using random effects modeling (hierarchical summary receiver operating characteristic [HSROC] models), which provides more conservative estimates than fixed effects modeling when heterogeneity is a concern.26,27 We calculated pooled estimates when ≥ four studies, each with ≥ 10 patients, were available in any subgroup and reported individual study estimates when < four studies were available. We performed secondary analyses that excluded studies that 1) did not use sputum mycobacterial culture as the reference standard for pulmonary TB and 2) had industry-involvement. We performed all analyses using Stata version 13 (StataCorp, College Station, TX), and generated forest plots using Review Manager 5 (The Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen).
RESULTS
Search results
Our initial search identified 1198 citations published before on or before January 31, 2014, including 16 abstracts presented at The Union World Lung Health Conference (Figure 1). After removing 361 duplicate citations, we excluded 726 citations based on title and/or abstract alone. We reviewed 111 full-text articles, of which 97 were excluded for the reasons listed in Figure 1. Of the remaining 14 articles, 5 were excluded because we were not able to obtain sufficient data from the authors to calculate diagnostic accuracy of CRP for TB using a cut-point of 10 mg/L or in reference to our pre-defined gold standard (Table 2, Online Supplement). We updated our literature search to include citations published between February 1, 2014 and January 31, 2015 and identified no additional studies eligible for this systematic review and meta-analysis. One study evaluated two different CRP assays (POC sandwich vs. lab-based immunoturbidometric assay) within the same study population and found no differences in their diagnostic accuracy for pulmonary TB.28 To avoid underestimating heterogeneity, we included accuracy data obtained from only the POC assay.28 None of the included studies reported enrolling pediatric patients.
Figure 1. Flow diagram of studies.
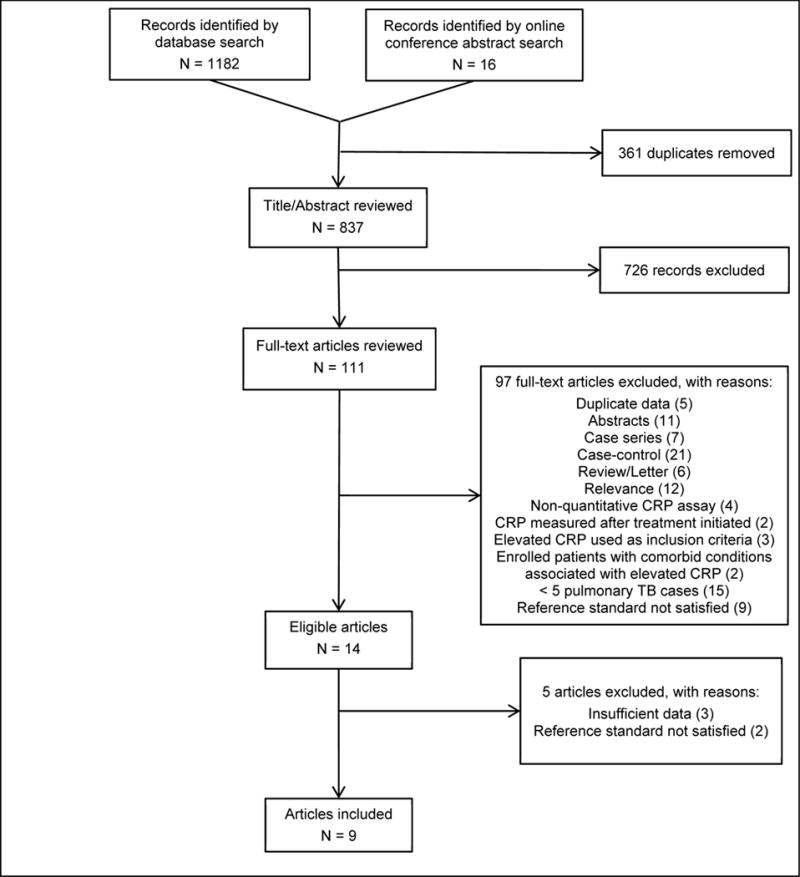
Abbreviations: CRP (C-reactive protein); TB (tuberculosis).
Legend: An updated search performed on October 20, 2015 to identify additional studies published between February 1, 2014 and January 31, 2015 yielded no additional eligible studies
Outpatients
Study quality
Five studies enrolled patients from the outpatient setting, including one study that enrolled patients from both the outpatient and inpatient setting.17 Figures 2A and 2B describe the summary and individual study risks of bias and applicability concerns. Most studies were designed to evaluate the diagnostic accuracy of CRP for pulmonary TB.8,9,22,28 All studies selected patients either consecutively or by random sample.8,9,17,22,28 Two studies enrolled a representative spectrum of patients8,9 while three studies restricted enrollment to patients with a high probability of pulmonary TB (e.g., symptomatic patients with suspected smear-negative pulmonary TB).17,22,28 All studies reported the time elapsed between blood collection for CRP testing and specimen collection for pulmonary TB evaluation.8,9,17,22,28 Most studies reported that pulmonary TB status was assessed without knowledge of CRP results.8,9,22,28 All but one study used culture as the reference standard for pulmonary TB.9 All studies acknowledged industry involvement8,9,17,22,28 including two studies that reported receiving donated POC CRP assays from the manufacturer.9,28
Figure 2. Outpatient pulmonary tuberculosis study quality using the Quality Assessment of Diagnostic Studies (QUADAS-2) tool.
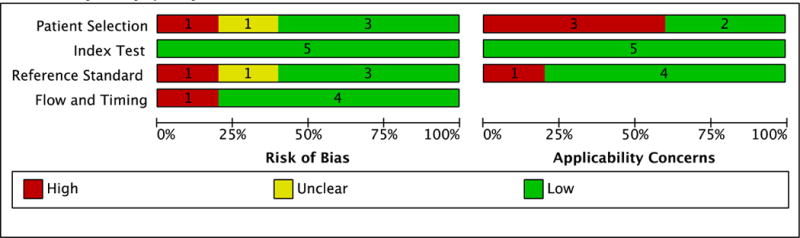
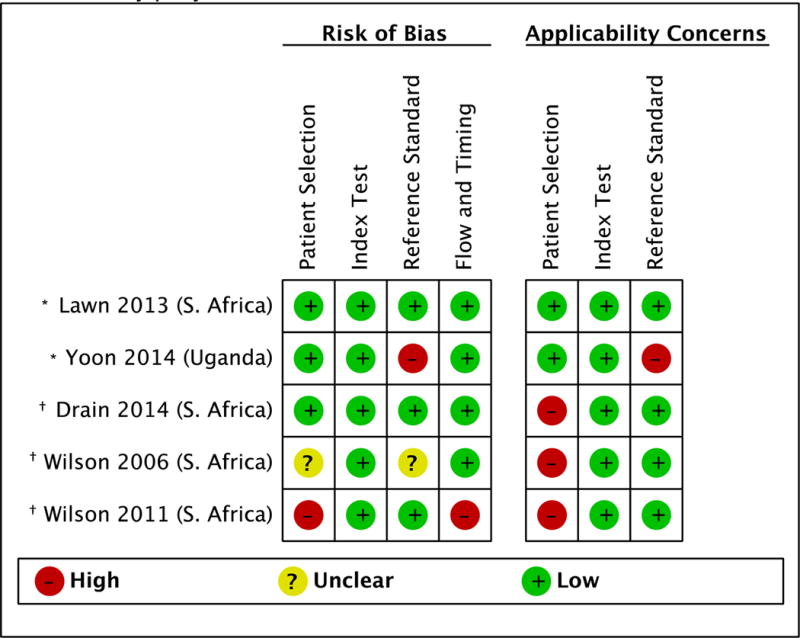
A. Summary study quality.
B. Individual study quality.
*C-reactive protein evaluated as a screening test for pulmonary tuberculosis among people living with HIV
†C-reactive protein evaluated as a diagnostic test for pulmonary tuberculosis among people with smear-negative sputa
Study characteristics
Table 1 describes the characteristics of the five studies enrolling 1051 patients from the outpatient setting, of whom 291 (28%) had pulmonary TB. All studies were conducted in countries with high TB/HIV burden and included patients infected with HIV (936 HIV-infected patients),8,9,17,22,28 including four studies that restricted enrollment to HIV-infected individuals.8,9,17,28 Two studies (N=697) evaluated CRP as a screening test for pulmonary TB,8,9 enrolling a high-risk population for whom TB screening is recommended: patients with advanced HIV/AIDS (CD4 cell count ≤ 200 cells/μL) initiating antiretroviral therapy (ART). The remaining three studies evaluated CRP as a diagnostic test for pulmonary TB among patients self-reporting symptoms suggestive of pulmonary TB.17,22,28 The proportion of pulmonary TB cases in the included studies was lowest (2.5%) for screening studies9 and highest (80%) for diagnostic studies.17
Sensitivity and specificity
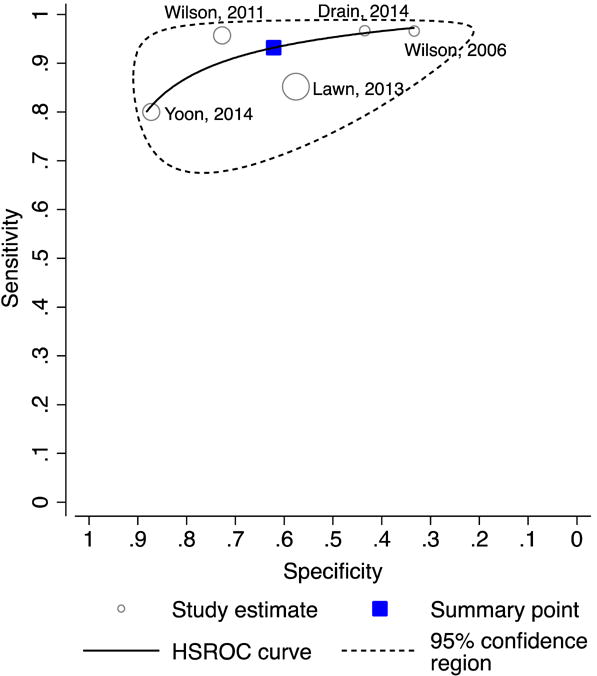
There was significant heterogeneity in specificity (range 33–87%; I2=96%, p<0.0001) but not sensitivity (range 80–97%; I2=39%, p=0.16) estimates across studies (Figure 3A). The pooled sensitivity of CRP was 93% (95% CI: 85–97) and pooled specificity was 62% (95% CI: 42–79; Figure 3B).
Figure 3. Diagnostic accuracy of CRP for pulmonary tuberculosis among outpatients.
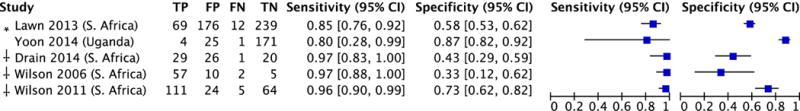
A. Forest plot.
B. Hierarchical summary receiver operating characteristics (HSROC) plot.
Abbreviations: CRP (C-reactive protein)
*CRP evaluated as a screening test for tuberculosis
†CRP evaluated as a diagnostic test for symptomatic patients undergoing tuberculosis evaluation
Pooled sensitivity 93% (95% CI: 85–97); test for heterogeneity I2 = 39%, p=0.16
Pooled specificity 62% (95% CI: 42–79); test for heterogeneity I2 = 96%, p<0.0001
Subgroup analyses
Sensitivity ranged from 80–85% and specificity from 58–87% in the two studies that evaluated CRP in the context of TB screening.8,9 As expected, sensitivity was higher (range 96–97%) and specificity lower (range 33–73%) in the three studies (N=354) that enrolled patients self-reporting symptoms suggestive of pulmonary TB.17,22,28 Among outpatients with confirmed HIV infection (N=936; Figures 4A and 4B), pooled sensitivity (93%, 95% CI: 85–97; I2=40, p=0.16) and pooled specificity (64%, 95% CI: 42–81; I2=96%, p<0.0001) estimates were nearly identical to the overall estimates. Sensitivity was 100% (95% CI: 63–100%) and specificity was 67% (95% CI: 22–96%) among 115 HIV-negative outpatients enrolled in one study.22
Figure 4. Diagnostic accuracy of CRP for pulmonary tuberculosis among ambulatory patients with confirmed HIV infection.
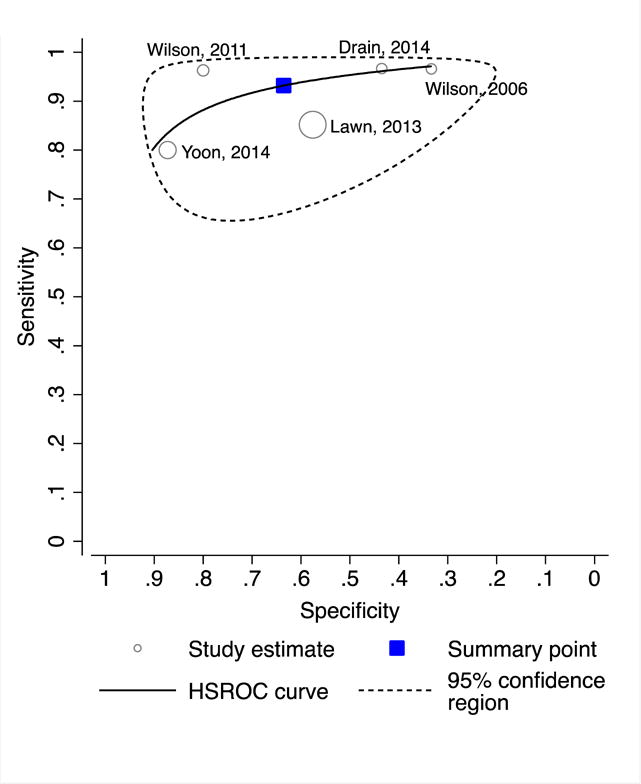
A. Forest plot
B. Hierarchical summary receiver operating characteristics (HSROC) plot.
Abbreviations: CRP (C-reactive protein)
*CRP evaluated as a screening test for tuberculosis
†CRP evaluated as a diagnostic test for symptomatic patients undergoing tuberculosis evaluation
Pooled sensitivity 93% (95% CI: 85–97); test for heterogeneity I2 = 40%, p=0.16
Pooled specificity 64% (95% CI: 42–81); test for heterogeneity I2 = 96%, p<0.0001
Inpatients
Study quality
Supplementary Figures 1A and 1B (Online Supplement) describe the summary and individual risks of bias and applicability concerns of the five studies that enrolled hospitalized patients.17,21,29–31 Unlike most outpatient studies, none of the inpatient studies were designed to evaluate the diagnostic accuracy of CRP for pulmonary TB;17,21,29–31 in general, these studies were judged to have higher and/or unclear risk of bias and greater concerns for applicability for all domains. Only two studies reported enrolling a consecutive or random sample of patients,17,21 while the remaining studies did not provide sufficient information to determine the patient selection method.29–31 Most studies did not enroll a representative spectrum of patients.17,29–31 Although all studies used culture as the reference standard for TB,17,21,29–31 none of the studies explicitly stated that researchers assessing TB status were blinded to CRP results and only one study reported the time elapsed between blood collection for CRP testing and specimen collection for TB evaluation.17 Industry involvement was unknown for three studies29–31 and acknowledged by one study.17
Study characteristics
The five studies enrolled 672 hospitalized patients. Two studies were conducted in countries with high TB/HIV burden17,29 and the remaining three studies were conducted in countries with low to intermediate TB burden (Table 1).21,30,31 Overall, 185 patients (28%) had pulmonary TB, and prevalence ranged from 11 to 93%. Four studies included patients infected with HIV (N=287),17,21,29,31 including two studies that restricted enrollment to HIV-infected individuals.17,21 All studies enrolled hospitalized patients who self-reported symptoms suggestive of TB:17,21,29–31 two studies enrolled patients with pulmonary infiltrates on chest imaging,30,31 one study enrolled HIV-infected patients with respiratory symptoms,21 one study enrolled HIV-infected patients with suspected smear-negative pulmonary TB,17 and one study enrolled patients with fever of unknown origin (FUO).29
Sensitivity and specificity
There was significant heterogeneity in sensitivity (range 56–96%; I2=80%, p=0.001) and specificity (range 0–67%; I2=95%, p<0.0001) estimates across studies (Figure 2, Online Supplement). The pooled sensitivity of CRP was 78% (95% CI: 58–90) and pooled specificity was 21% (95% CI: 6–52).
Subgroup analyses
No study evaluated CRP as a screening tool for pulmonary TB among hospitalized patients. Three studies each enrolled ≥ 10 patients with confirmed HIV-infection (N=287); study estimates of sensitivity were high (range 89–100%) while specificity estimates were low (range 0–40%; Figure 3A, Online Supplement).17,21,29 Three studies enrolled 384 HIV-negative patients; individual study estimates of sensitivity (range 43–82%) and specificity (range 0–76%) exhibited substantial variability (Figure 3B, Online Supplement).29–31
DISCUSSION
Systematic screening for TB among high-risk groups is a key aspect of the WHO’s TB elimination strategy.32 However, current tools for systematic screening have inadequate test characteristics in key high-risk groups (e.g., symptom-based screening)4–11 or have high cost and infrastructure requirements (e.g., CXR).11,34,35 In this systematic review, we found that CRP – which can be measured using an inexpensive, point-of-care assay – has similar sensitivity and better specificity than what has been reported for symptom-based screening. Point-of-care CRP testing should therefore be evaluated as a tool to improve the efficiency and lower the cost of systematic screening programs relative to current options.
The WHO’s target product profile for a TB screening test recommends that sensitivity be at least 90% and specificity at least 70%.3 A recent modeling study concluded that no ideal TB screening algorithm exists that meets these criteria across all populations and settings.36 Several systematic reviews have shown that symptom-based screening is more sensitive (range: 84–90%) in patients with HIV infection compared to those without, limiting its general applicability.4,11 In addition, specificity is poor (range: 5–61%) among people living with HIV,4–11 particularly in sub-Saharan Africa. CXR either alone or following symptom screening has better and more consistent performance characteristics,11,34,35 but is not routinely available and requires trained personnel to interpret results. Digital imaging and computer-aided diagnosis have shown promise and may overcome some of these barriers, but start-up costs are substantial. Thus, to implement WHO systematic screening recommendations, there is an urgent need for a low-cost, simple and accurate screening test.
The findings from this systematic review suggest that CRP has strong potential to facilitate systematic screening of high-risk groups. In the outpatient context – where most systematic screening would take place – we found that the pooled sensitivity of CRP was 93% (95% CI: 85–97). Moreover, results were consistent across studies and similar for patients with and without HIV-infection. CRP can therefore be expected to meet or exceed the minimum sensitivity threshold recommended for a TB screening test in most contexts. Pooled specificity was 62% (95% CI: 42–79) – slightly lower than the recommended threshold for a TB screening test. However, three of five studies evaluated CRP in the context of TB diagnosis rather than TB screening. Patients self-presenting with TB symptoms are expected to have a higher prevalence of pyogenic infections or other systemic inflammatory conditions mimicking TB than populations for whom systematic screening is recommended (e.g., people living with HIV presenting for routine HIV/AIDS care, prisoners, and household contacts of patients with active TB), lowering the specificity of CRP for active TB. Indeed, among inpatient studies – where the prevalence of diseases mimicking TB is high – we found that the pooled specificity of CRP was only 21% (95% CI: 6–52). At the other extreme, healthy outpatient populations – such as ones used to establish CRP cut-points – have few individuals with CRP levels ≥ 10mg/L because the prevalence of pyogenic infections and other systemic inflammatory conditions is low. When viewed within this context, CRP specificity in populations targeted for systematic screening is likely to be higher than the pooled value of 62% that we report here.
Our systematic review has several strengths. First, we synthesized data separately for outpatient and inpatient studies and performed pre-specified subgroup analyses to evaluate the accuracy of CRP for patients self-presenting with TB symptoms vs. patients undergoing provider-initiated TB screening and for patients with and without HIV. These analyses allowed us to address additional questions important for identifying the appropriate clinical context for CRP testing in the work-up of TB. We found sensitivity of CRP for pulmonary TB to be similar among patients with and without HIV infection, in both the inpatient and outpatient settings. Second, we standardized assessment of diagnostic accuracy of CRP by using a well-established cut-point point (10 mg/L) for all studies.23,24 Third, most studies included in our systematic review enrolled high-risk patients (the target population for systematic TB screening) and/or patients living in TB endemic countries (settings most in need of and therefore most likely to use a simple and low-cost screening test for TB). Lastly, our systematic review utilized a standardized protocol as recommended by the Cochrane Collaboration’s Diagnostic Test Accuracy Working Group37 and reported our findings in accordance with the PRISMA guidelines for systematic reviews and meta-analyses.38
Our systematic review has several potential limitations. First, we identified only two studies that evaluated CRP as a TB screening test,8,9 one of which did not use a microbiological reference standard.9 Of note, excluding this study did not meaningfully change pooled sensitivity or specificity estimates for outpatient studies. More studies that enroll populations targeted for systematic screening and that include a culture-based reference standard are clearly needed, and our synthesis of data from the outpatient and inpatient context provides the justification needed to move forward with such studies. Second, over one-third of eligible studies were excluded because we were unable to obtain data from authors to calculate diagnostic accuracy using a CRP cut-point of 10 mg/L. However, none of these studies evaluated CRP in the context of TB screening and only two enrolled an outpatient population (Table 2, Online Supplement). Last, as with all systematic reviews, there is a possibility of publication bias. However, many of the included studies had “negative” findings (i.e., poor sensitivity and/or specificity).
In summary, CRP shows considerable promise as a tool to facilitate systematic screening of high-risk populations. It has similar sensitivity and higher specificity than symptom-based screening, and is simpler and less resource intensive to implement than CXR. However, to support policy recommendations, high quality studies that enroll populations targeted for systematic screening are needed. In addition to diagnostic accuracy, such studies should also assess the impact of POC CRP-based TB screening on the timing of TB diagnosis and treatment, proportion of patients who initiate preventive therapy if eligible, and health system- and patient-related costs relative to current TB screening options.
Supplementary Material
Acknowledgments
Funding: National Institutes of Health, Presidential Emergency Plan for AIDS Relief, Nina Ireland Program for Lung Health
References
- 1.World Health Organization. Global Tuberculosis Report. Geneva, Switzerland: WHO; 2015. Available from: http://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.World Health Organization. Systematic screening for active tuberculosis: principles and recommendations. Geneva, Switzerland: WHO; 2013. Available from: http://www.who.int/tb/tbscreening/en/. [PubMed] [Google Scholar]
- 3.World Health Organization. High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. Geneva, Switzerland: WHO; 2014. Available from: http://www.who.int/tb/publications/tpp_report/en/. [Google Scholar]
- 4.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim L, Heilig CM, McCarthy KD, et al. Symptom screen for identification of highly infectious tuberculosis in people living with HIV in Southeast Asia. J Acquir Immune Defic Syndr. 2012;60(5):519–524. doi: 10.1097/QAI.0b013e318256b3db. [DOI] [PubMed] [Google Scholar]
- 6.Hanifa Y, Fielding KL, Charalambous S, et al. Tuberculosis among adults starting antiretroviral therapy in South Africa: the need for routine case finding. Int J Tuberc Lung Dis. 2012;16(9):1252–1259. doi: 10.5588/ijtld.11.0733. [DOI] [PubMed] [Google Scholar]
- 7.Kufa T, Mngomezulu V, Charalambous S, et al. Undiagnosed tuberculosis among HIV clinic attendees: association with antiretroviral therapy and implications for intensified case finding, isoniazid preventive therapy, and infection control. J Acquir Immune Defic Syndr. 2012;60:e22–e28. doi: 10.1097/QAI.0b013e318251ae0b. [DOI] [PubMed] [Google Scholar]
- 8.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic and prognostic value of serum C-reactive protein for screening for HIV-associated tuberculosis. Int J Tuberc Lung Dis. 2013;17:636–643. doi: 10.5588/ijtld.12.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon C, Davis JL, Huang L, et al. Point-of-care C-reactive protein testing to facilitate implementation of isoniazid preventive therapy for people living with HIV. J Acquir Immune Defic Syndr. 2014;65(5):551–556. doi: 10.1097/QAI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad Khan F, Verkuijl S, Parrish A, et al. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS. 2014;28(10):1463–1472. doi: 10.1097/QAD.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van’t Hoog AH, Langendam MW, Mitchell E, et al. A Systematic Review of the Sensitivity and Specificity of Symptom- and Chest-Radiography Screening for Active Pulmonary Tuberculosis in HIV-Negative Persons and Persons with Unknown HIV Status, Report to WHO. 2013 doi: 10.1002/14651858.CD010890.pub2. Available from: http://www.who.int/tb/Review2Accuracyofscreeningtests.pdf. [DOI] [PMC free article] [PubMed]
- 12.Agizew T, Bachhuber MA, Nyirenda S, et al. Association of chest radiographic abnormalities with tuberculosis disease in asymptomatic HIV-infected adults. Int J Tuberc Lung Dis. 2010;14(3):324–331. [PubMed] [Google Scholar]
- 13.Lawn SD, Obeng J, Acheampong JW, Griffin GE. Resolution of the acute-phase response in West African patients receiving treatment for pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4(4):340–344. [PubMed] [Google Scholar]
- 14.Lawn SD, Wiktor S, Coulibaly D, Ackah AN, Lal RB. Serum C-reactive protein and detection of tuberculosis in persons co-infected with the human immunodeficiency virus. Trans R Soc Trop Med Hyg. 2001;95(1):41–42. doi: 10.1016/s0035-9203(01)90328-1. [DOI] [PubMed] [Google Scholar]
- 15.Polzin A, Pletz M, Erbes R, et al. Procalcitonin as a diagnostic tool in lower respiratory tract infections and tuberculosis. Eur Respir J. 2003;21:939–943. doi: 10.1183/09031936.03.00055103. [DOI] [PubMed] [Google Scholar]
- 16.Schleicher GK, Herbert V, Brink A, et al. Procalcitonin and C-reactive protein levels in HIV-positive subjects with tuberculosis and pneumonia. Eur Respir J. 2005;25(4):688–692. doi: 10.1183/09031936.05.00067604. [DOI] [PubMed] [Google Scholar]
- 17.Wilson D, Nachega J, Morroni C, Chaisson R, Maartens G. Diagnosing smear-negative tuberculosis using case definitions and treatment response in HIV-infected adults. Int J Tuberc Lung Dis. 2006;10(1):31–38. [PubMed] [Google Scholar]
- 18.Choi CM, Kang CI, Jeung WK, Kim DH, Lee CH, Yim JJ. Role of the C-reactive protein for the diagnosis of TB among military personnel in South Korea. Int J Tuberc Lung Dis. 2007;11(2):233–236. [PubMed] [Google Scholar]
- 19.Breen RA, Leonard O, Perrin FM, et al. How good are systemic symptoms and blood inflammatory markers at detecting individuals with tuberculosis? Int J Tuberc Lung Dis. 2008;12(1):44–49. [PubMed] [Google Scholar]
- 20.Kang YA, Kwon SY, Yoon HI, Lee JH, Lee CT. Role of C-reactive protein and procalcitonin in differentiation of tuberculosis from bacterial community acquired pneumonia. Korean J Intern Med. 2009;24(4):337–342. doi: 10.3904/kjim.2009.24.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sage EK, Noursadeghi M, Evans HE, et al. Prognostic value of C-reactive protein in HIV-infected patients with Pneumocystis jirovecii pneumonia. Int J STD AIDS. 2010;21(4):288–292. doi: 10.1258/ijsa.2010.009551. [DOI] [PubMed] [Google Scholar]
- 22.Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear negative tuberculosis in an ambulatory high HIV prevalence population. PLoS One. 2011;6(1):e15248. doi: 10.1371/journal.pone.0015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claus DR, Osmand AP, Gewurz H. Radioimmunoassay of human C-reactive protein and levels in normal sera. J Lab Clin Med. 1976;87:120–128. [PubMed] [Google Scholar]
- 24.Shine B, de Beer FC, Pepys MB. Solid phase radioimmunoassays for human C-reactive protein. Clin Chim Acta. 1981;117:13–23. doi: 10.1016/0009-8981(81)90005-x. [DOI] [PubMed] [Google Scholar]
- 25.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics. 2007;8:239–251. doi: 10.1093/biostatistics/kxl004. [DOI] [PubMed] [Google Scholar]
- 27.Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149:8898897. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drain P, Mayeza L, Bartman P, et al. Diagnostic accuracy and clinical role of rapid C-reactive protein testing in HIV-infected individuals with presumed tuberculosis in South Africa. Int J Tuberc Lung Dis. 2014;18(1):20–26. doi: 10.5588/ijtld.13.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandyopadhyay D, Bandyopadhyay R, Paul R, Roy D. Etiological study of Fever of unknown origin in patients admitted to medicine ward of a teaching hospital of eastern India. J Glob Infect Dis. 2011;3(4):329–333. doi: 10.4103/0974-777X.91052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YJ, Lee J, Kim YY, et al. Performance of whole-blood interferon-gamma release assay in patients admitted to the emergency department with pulmonary infiltrates. BMC Infectious Disease. 2011;11:107. doi: 10.1186/1471-2334-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho K, Cho E, Kwon S, et al. Factors associated with indeterminate and false negative results of quantiferon-TB gold in-tube test in active tuberculosis. Tuberc Respir Dis. 2012;72:416–425. doi: 10.4046/trd.2012.72.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. The End TB Strategy, global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva, Switzerland: WHO; 2015. Available from: http://www.who.int/tb/post2015_strategy/en/. [Google Scholar]
- 33.Shah NS, Anh MH, Thuy TT, et al. Population-based chest X-ray screening for pulmonary tuberculosis in people living with HIV/AIDS, An Giang, Vietnam. Int J Tuberc Lung Dis. 2008;12(4):404–410. [PubMed] [Google Scholar]
- 34.Theron G, Pooran A, Peter J, et al. Do adjunct tuberculosis tests, when combined with Xpert MTB/RIF, improve accuracy and thecost of diagnosis in a resource-poor setting? Eur Respir J. 2012;40(1):161–168. doi: 10.1183/09031936.00145511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samandari T, Bishai D, Luteijn M, et al. Costs and consequences of additional chest x-ray in a tuberculosis prevention program in Botswana. Am J Respir Crit Care Med. 2011;183(8):1103–1111. doi: 10.1164/rccm.201004-0620OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van’t Hoog AH, Onozaki I, Lonnroth K. Choosing algorithms for TB screening: a modelling study to compare yield, predictive value and diagnostic burden. BMC Infect Dis. 2014;14:532. doi: 10.1186/1471-2334-14-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011 Available from: www.cochrane-handbook.org.
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS MED. 2009;6(6):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


