Abstract
Background
Recently it has been shown in animal studies that deep brain stimulation (DBS) of auditory structures was able to reduce tinnitus-like behavior. However, the question arises whether hearing might be impaired when interfering in auditory-related network loops with DBS.
Methods
The auditory brainstem response (ABR) was measured in rats during high frequency stimulation (HFS) and low frequency stimulation (LFS) in the central nucleus of the inferior colliculus (CIC, n = 5) or dentate cerebellar nucleus (DCBN, n = 5). Besides hearing thresholds using ABR, relative measures of latency and amplitude can be extracted from the ABR. In this study ABR thresholds, interpeak latencies (I–III, III–V, I–V) and V/I amplitude ratio were measured during off-stimulation state and during LFS and HFS.
Results
In both the CIC and the CNBN groups, no significant differences were observed for all outcome measures.
Discussion
DBS in both the CIC and the CNBN did not have adverse effects on hearing measurements. These findings suggest that DBS does not hamper physiological processing in the auditory circuitry.
Keywords: Deep brain stimulation, Tinnitus, Dentate cerebellar nucleus, Hearing loss, Auditory brainstem response, Inferior colliculus
Introduction
Deep brain stimulation (DBS) in auditory structures has been performed in animal studies as a treatment for tinnitus (Luo et al., 2012; Smit et al., 2016). The rationale behind this treatment is to interfere with the pathological neuronal activity in the central nervous system and interrupt the network loop that is essential for the persistence of tinnitus (Smit et al., 2015).
The fundamental knowledge of the effect of deep DBS in auditory structures on hearing is essential before applying this treatment in a clinical setting (Smit et al., 2015). It has been shown in rats, using the sound-induced pre-pulse inhibition test with click stimuli, that during high frequency stimulation (HFS) of the external nucleus of the inferior colliculus (IC) hearing thresholds did not change (Smit et al., 2016). As far as we know, a more detailed hearing assessment during DBS in auditory structures has not been assessed thus far.
To assess hearing thresholds in more detail, the auditory brainstem response (ABR) was measured in this study. The ABR assesses changes in neural integrity and is commonly used in laboratory animal studies to estimate hearing (Rosahl et al., 2000; Turner et al., 2006). In humans, ABRs are used in daily practice to assess possible hearing loss of a retrocochlear origin (Stockard & Rossiter, 1977).
Two structures were targeted in this study, the central nucleus of the IC (CIC) and the dentate cerebellar nucleus (DCBN). The CIC is the principal auditory part of the IC and has a well-defined tonotopy (Aitkin & Moore, 1975; De Martino et al., 2013). In animal models of tinnitus, the IC shows tonotopic reorganization, increased spontaneous firing rate, increased bursting activity and increased neural synchrony (Bauer et al., 2008; Chen & Jastreboff, 1995; Robertson et al., 2013; Wang, Ding & Salvi, 2002). A recent study showed that HFS of the external nucleus of the IC in rats decreased tinnitus-like behavior (Smit et al., 2016). The cerebellum is a structure that is not involved in the auditory pathways but is associated with tinnitus (Brozoski, Ciobanu & Bauer, 2007; Osaki et al., 2005; Sedley et al., 2012; Shulman & Strashun, 1999). It was demonstrated that ablation of the paraflocculus completely diminished tinnitus in rats (Bauer et al., 2013). The majority of fibers in the cerebellum, including the paraflocculus, originate from the deep cerebellar nuclei, especially the DCBN, which is the largest (Gayer & Faull, 1988; Gould, 1979). Therefore, the CIC and the DCBN could be considered as respectively an auditory and a non-auditory potential DBS target for the treatment of tinnitus.
DBS can be performed with low frequency stimulation (LFS), which mainly has an excitatory effect, and as HFS, which generally is described as a global inhibitory effect similar as ablation (Benabid et al., 1998; Breit, Schulz & Benabid, 2004; Dostrovsky & Lozano, 2002). Following ablation of IC in animals models, decreased amplitude and latency of peak V have been found (Achor & Starr, 1980; Buchwald & Huang, 1975; Durrant et al., 1994; Kaga, Shinoda & Suzuki, 1997). Peak V is the last of the five peaks of the ABR and represents neural activity of the IC. Because of a high variability in amplitude among subjects, the V/I amplitude ratio is a more consistent measure than the absolute value (Musiek et al., 1984; Musiek, Reeves & Baran, 1985). The relative measures of the latencies are the interpeak latencies (I–III, III–V, I–V), which represent the central transmission latency best (Eggermont & Don, 1986; Picton et al., 1977; Squires, Chu & Starr, 1978). There is little evidence that stimulation of cerebellar structures has influence on the ABR (Crispino & Bullock, 1984).
We hypothesized that for CIC stimulation, the V/I amplitude ratio of the ABR would be lower and the I–V or III–V interpeak latencies would be prolonged during HFS and not during LFS of the CIC. Our hypothesis was that stimulating a non-auditory structure such as the DCBN would not have any influence on the ABR.
Methods
Animals
Male rats (Sprague Dawley, 250–300 g; Charles River, Amsterdam, The Netherlands) were housed individually under conditions of constant room temperature and humidity with a reversed 12u/12u light/dark cycle and had free access to water and food. The Animal Experiments Committee of the Maastricht University approved the experiments (approval reference number 2012–069).
Surgical procedure
Subcutaneous electrodes were implanted for ABR recordings and during the same surgery DBS electrodes were implanted in the brain (Fig. 1). Animals were anesthetized by intraperitoneal administration of ketamine (90 mg/kg) and xylazine (10 mg/kg). The head of the rats was immobilized in a stereotactic apparatus (Stoelting Co, Wood Dale, IL, USA) with mouth and blunt ear-bars. Permanent Teflon-coated stainless steel electrodes were subcutaneously implanted. One wire electrode was subcutaneously tunneled to the mastoid and a second wire electrode was attached to a screw on the vertex. Based on coordinates from a stereotactic atlas (Paxinos & Watson, 2007), bilateral electrodes (Technomed, Beek, The Netherlands) were inserted in the CIC (bregma −8.8, depth 4.5, interspace 3.8) or in the DCBN (bregma −11.5, depth 6.5, interspace 6.8). The postoperative recovery time was one week.
Figure 1. Surgery of implantation of ABR and DBS electrodes.
(A) After exposing the skull, the vertex electrode is attached with a screw in the skull and the mastoid electrode is subcutaneously tunneled to the mastoid and also fixated with a screw. Three boreholes are made for anchoring screws to later fixate the structure with dental cement. (B) Boreholes for the DBS electrodes are drilled at coordinates calculated from the bregma level. Calculation of the boreholes and placement of the DBS electrodes are performed within a stereotactic frame. (C) All electrodes are in place and the construct is fixated with dental cement. ABR, auditory brainstem response; DBS, deep brain stimulation.
Deep brain stimulation
DBS was performed with bipolar, concentric electrodes using monophasic rectangular pulses. The electrical stimulus pulses were created by an A310 acupulser and an A360 stimulus isolator (World Precision Instruments, Berlin, Germany). During DBS, stimuli were given with a frequency of 100 Hz (HFS) and 10 Hz (LFS) with an amplitude of 100 µA and a pulse width of 60 µs. Electrodes are gold-plated with platinum–iridium inner wire (negative contact) and stainless steel outer part (positive contact). The inner and outer electrodes are insulated except for a 75 µm exposed tip (Tan et al., 2010).
Rats were divided in two groups, one group received implantation of electrodes in the CIC (n = 5) and the other group in the DCBN (n = 5). In the off-stimulation state, designated as the control situation, no electrical stimulation was given. During stimulation-off state, LFS and HFS, ABRs were recorded in separate sessions.
Auditory brainstem response
ABR measurements were performed in a random manner of the three situations (off-stimulation, LFS, HFS) with a one week interval. Stimulation was turned on approximately 5 min before ABR recordings. HFS consisted of monophasic rectangular pulses, with a frequency of 100 Hz, amplitude of 100 µA per electrode and a pulse width of 60 µs (A310 Acupulser; World Precision Instruments, Berlin, Germany). Similar settings were used in a study which showed tinnitus reduction during HFS in rats (Smit et al., 2016). LFS consisted of the same parameters with a frequency of 10 Hz.
To achieve anesthesia during ABR recordings, intraperitoneal administration of ketamine (90 mg/kg) and xylazine (10 mg/kg) was used, which is preferred over isoflurane when assessing hearing thresholds in rats (Ruebhausen, Brozoski & Bauer, 2012).
During the ABR procedure, animals were placed into a sound-attenuating chamber. Cables were plugged into the socket of the head of the animal and connected to the recording device (Powerlab 8/35 connected to a Dual Bio Amp amplifier (ADInstruments, Castle Hill, Australia)). An electrode connected to the left hind paw served as the ground.
Custom-made auditory stimuli (10, 16, 24 and 32 kHz) were created with Matlab 2011a (Mathworks, Natick, MA, USA) and consisted of 5 ms bursts with a cos2 rise and fall filter and were played at a rate of 20 per second at decreasing intensities from 90 to 0 dB peSPL with steps of 10 dB. To prevent synchronous occurrence of stimulation artifacts with the ABRs, one in 10 stimuli had an interval of 55 ms instead of 45 ms. To gain an approximately similar amount of data after filtering of stimulation artifacts, 500 auditory stimuli were given per intensity in the off-stimulation state, 700 during LFS and 1,000 during HFS. Sounds were calibrated with a Bruel & Kjaer 2231 decibel meter with a 4191 microphone (range 2–40 kHz), which was placed at the location of the rat’s right ear. Sound intensities are reported as the peak equivalent sound pressure level (peSPL).
Auditory stimuli were processed with an external soundcard with a sample rate of 192 kHz (Creative E-MU 0204), amplified with Ultrasonic power amplifier (Avisoft Bioacoustics, Berlin) and played with an Ultrasonic Dynamic Speaker Vifa (Avisoft Bioacoustics, Berlin, Germany) to the right ear. To standardize sound presentation between recording sessions it was monitored that in every session the same position of the rat and the same distance between the loudspeaker and the ear was used (2 cm). The contralateral ear was plugged with modeling clay.
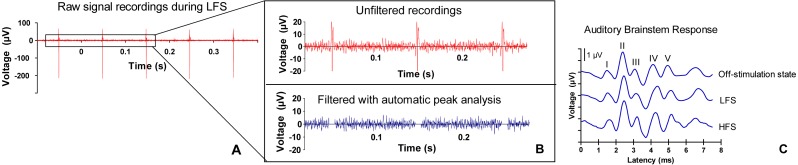
Auditory stimuli were digitally triggered. The recordings were done in Labchart Pro 7 (ADInstruments, Castle Hill, Australia) at a sample frequency of 20 kHz and raw data were imported into Matlab. With a customized script, the signal was amplified 100,000 times and band-pass filtered (300–3,000 Hz). Evoked responses were averaged and data which contained DBS artifacts were automatically removed based on a peak-detection analysis. Using a customized Matlab script, peaks were automatically detected if the signal was above a manual depicted maximal baseline value. Before and after the maximal value of the peak of the artifact 2.5 ms of data were converted in Not-a-Number (NaN). The ABR and DBS stimuli were not phase-locked so per epoch a different part was converted in NaN. All epochs were averaged to calculate the mean ABR signal (Fig. 2B).
Figure 2. ABR signal processing.
(A) Example of a raw signal that was measured during low frequency stimulation (LFS). (B) Stimulation artifacts are filtered with automatic peak detection analysis. (C) Example of an auditory brainstem response (ABR) (burst frequency 10 kHz) during off-stimulation state, during LFS and during high frequency stimulation (HFS) in the central nucleus of the inferior colliculus. The five ABR peaks are numbered I–V. Morphology and latency of ABR peaks in the current study were consistent with other animal studies (Backoff & Caspary, 1994; Dehmel, Eisinger & Shore, 2012; Zheng et al., 2012). The first peak arises approximately 1.5 ms after stimulus onset. Although there is overlap, the first peak represents neural activity of the cochlear nerve. The second peak is considered to be mainly generated by cochlear nuclear cells, the third peak by the contralateral superior olivary complex cells and the fourth peak by the lateral lemniscus. The fifth peak originates from the inferior colliculus (Biacabe et al, 2001; Chen & Chen, 1991; Simpson et al., 1985).
Two independent blinded observers visually identified ABR thresholds and peaks. In case of disagreement, a third observer was sought and the concordant data were accepted. The auditory threshold was defined as the lowest decibel level (peSPL) of the stimulus, which produced a distinctive ABR.
For latency analysis, the five positive peaks were determined at 90 dB peSPL and numbered I–V based on the recordings of vertex upward deflections (for an example see Fig. 2C). Latencies of peaks were measured from stimulus onset. Interpeak latency was defined as the time between respective peaks.
The amplitude was expressed as the peak-to-peak amplitude ratio of peak V subtracted by peak I.
Electrode localization
Animals were deeply anesthetized with pentobarbital (75 mg/kg) and perfused transcardially with Tyrode’s buffer (0.1 M) and fixative containing 4% paraformaldehyde, 15% picric acid and 0.05% glutaraldehyde in 0.1 M phosphate buffer (pH 7.6). After post-fixation for 12 h, the brains were cut to coronal sections using a vibrotome. To assess the electrode localization, the sections containing the target area and the electrode trajectory were stained with hematoxylin-eosin (Merck, Darmstadt, Germany). Definition of anatomic structures was based on the stereotactical atlas (Paxinos & Watson, 2007).
Statistical analysis
Dependent data were analyzed using the Wilcoxon signed-rank Test for two groups and a Friedman test for multiple groups. Since multiple comparisons were made when comparing the stimulation-off state with LFS and HFS, modified p-values (alpha = 0.05) are given as corrected by means of the Holm-Bonferonni sequential correction (Holm, 1979). Data are presented as mean ± standard error of the mean (SEM). All data were analyzed with SPSS (Version 20, IBM, Somers, NY, USA).
Results
Electrode localization
Histological evaluation showed that all electrodes were implanted correctly in the target structures (Fig. 3A and Fig. 3B).
Figure 3. Histology.
Representative examples of electrode positions (white lines) in the CIC (A) and DCBN (B). All electrodes were implanted bilaterally. ECIC, external nucleus of the inferior colliculus; CIC, central nucleus of inferior colliculus; DCIC, dorsal cortex of inferior colliculus; DCBN, dentate cerebellar nucleus; icp, inferior cerebellar peduncle; ICBN, interposed cerebellar nucleus; scp, superior cerebellar peduncle. Scale bar: 500 µm.
Hearing thresholds
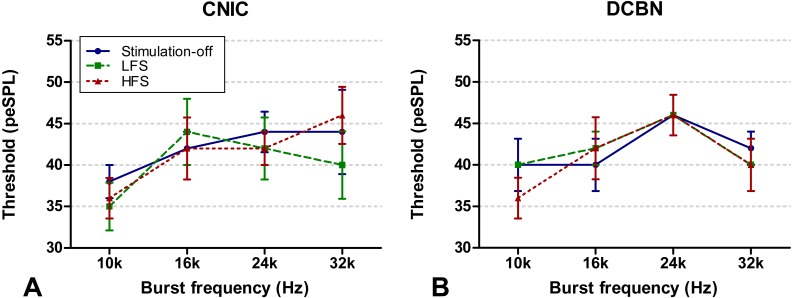
Hearing response thresholds were determined as the minimal intensity stimulus at which an ABR was evident. Thresholds of different stimulus frequencies (10, 16, 24 and 32 kHz) are depicted in Fig. 4A for the CIC group and in Fig. 4B for the DCBN group. In one rat two thresholds (10 Hz LFS and 32 Hz LFS) were not possible to determine. In both groups, no statistically significant differences were found during HFS and LFS compared to off-stimulation.
Figure 4. ABR thresholds.
ABR thresholds of the CIC (A) and DCBN group (B) measured during the DBS-off state (blue, circles, solid line), LFS (green, squares, striped line) and HFS (red, triangles, dotted line). There was no statistically significant difference. The vertical lines indicate the standard error of the mean. ABR, auditory brainstem response; CIC, central nucleus of the inferior colliculus; DBS, deep brain stimulation; DCBN, dentate cerebellar nucleus; LFS, low frequency stimulation; HFS, high frequency stimulation.
Latencies and amplitudes
From all ABRs, 5 distinctive peaks could be determined at 90 dB peSPL (Fig. 1). In Table 1 the mean interpeak latencies (I–III, III–V and I–V) are shown for different burst frequencies (10, 16, 24 and 32 kHz). In both the CIC and the DCBN group, no statistically significant differences were found for high and low frequency DBS compared to no stimulation (Table 1).
Table 1. Interpeak latencies (IL) and V/I amplitude ratio (AR) for 10 k, 16 k, 24 k and 32 k burst sounds.
Mean values with standard deviation are given. Adjusted Holm-Bonferroni pvalues are used.
| Frequency (Hz) | Peaks | CIC group | DCBN group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stim-off | LFS | p | HFS | p | Stim-off | LFS | p | HFS | p | ||
| 10 k | IL I–III | 1.504 (.170) | 1.524 (.125) | >.99 | 1.470 (.285) | >.99 | 1.520 (.157) | 1.590 (.162) | 0.20 | 1.530 (.136) | >.99 |
| IL III–V | 1.908 (.213) | 1.925 (.128) | >.99 | 1.745 (.285) | >.99 | 1.842 (.028) | 1.842 (.116) | >.99 | 1.953 (.109) | .32 | |
| IL I–V | 3.411 (.312) | 3.449 (.143) | >.99 | 3.24 (.291) | >.99 | 3.362 (.153) | 3.433 (.245) | >.99 | 3.514 (.130) | .22 | |
| AR V/I | 1.542 (1.739) | 1.238 (.832) | >.99 | 1.174 (.517) | >.99 | 1.349 (.946) | 1.1310 (.398) | >.99 | 1.326 (.287) | >.99 | |
| 16 k | IL I–III | 1.560 (.160) | 1.540 (.131) | >.99 | 1.490 (.145) | 0.25 | 1.560 (.147) | 1.540 (.084) | >.99 | 1.561 (.062) | >.99 |
| IL III–V | 1.822 (.257) | 1.943 (.091) | >.99 | 1.862 (.113) | >.99 | 1.863 (.113) | 1.933 (.166) | .85 | 1.9630 (.050) | >.99 | |
| IL I–V | 3.383 (.395) | 3.483 (.218) | >.99 | 3.353 (.248) | >.99 | 3.423 (.252) | 3.474 (.214) | >.99 | 3.5236 (.094) | >.99 | |
| AR V/I | .979 (.410) | .9067 (.320) | .69 | 1.310 (.410) | >.99 | 1.178 (.913) | 1.278 (1.470) | >.99 | 1.078 (.289) | >.99 | |
| 24 k | IL I–III | 1.550 (.157) | 1.550 (.130) | >.99 | 1.570 (.232) | >.99 | 1.560 (.113) | 1.520 (.090) | >.99 | 1.621 (.120) | >.99 |
| IL III–V | 1.853 (.186) | 2.004 (.135) | 0.25 | 1.974 (.180) | >.99 | 1.712 (.202) | 1.893 (.293) | .25 | 1.750 (.200) | >.99 | |
| IL I–V | 3.403 (.329) | 3.554 (.243) | 0.26 | 3.544 (.309) | >.99 | 3.272 (.250) | 3.413 (.279) | >.99 | 3.371 (.147) | .51 | |
| AR V/I | .957 (.242) | .916 (.594) | >.99 | 1.11 (.595) | >.99 | .536 (.265) | .445 (.305) | .50 | .834 (.470) | .50 | |
| 32 k | IL I–III | 1.550 (.109) | 1.478 (.093) | >.99 | 1.428 (.261) | >.99 | 1.570 (.097) | 1.520 (.066) | .23 | 1.570 (.157) | >.99 |
| IL III–V | 1.883 (.318) | 2.010 (.376) | >.99 | 1.850 (.240) | >.99 | 1.822 (.065) | 1.903 (.272) | >.99 | 1.862 (.366) | >.99 | |
| IL I–V | 3.433 (.400) | 3.488 (.372) | >.99 | 3.278 (.416) | >.99 | 3.393 (.145) | 3.423 (.283) | >.99 | 3.433 (.362) | >.99 | |
| AR V/I | .491 (.437) | .505 (.278) | .69 | .617 (.523) | .28 | .404 (.296) | .551 (.209) | .50 | .870 (.961) | .45 | |
Notes.
Abbreviations
- CIC
- central nucleus of interior colliculus
- DCBN
- dentate cerebellar nucleus
- stim-off
- stimulation-off state
- LFS
- deep brain stimulation at 10 Hz
- HFS
- deep brain stimulation at 100 Hz
The V/I amplitude ratio was calculated at all burst frequencies. In both groups, there was no statistical significant difference when comparing no stimulation with HFS and LFS. Tables A1–A4 in Appendix shows the absolute latencies and interpeak latencies.
When looking at the latency and amplitude data, a relation between ABR latencies and amplitudes, with frequencies of burst tones was noticed. For further analysis, we grouped the off-stimulation data of the CIC and DCBN group since only baseline measurements were analyzed. The latency, e.g., of peak I, differed between burst frequencies (X2(3) = 20.12, p < 0.01). The raw data (see Appendix) show a shorter latency with increasing frequencies of burst tones. The V/I amplitude ratio does not differ amongst frequencies (X2(3) = 4.92, p = .178). Amplitudes of peak I did not differ between frequencies (X2(3) = 3.240, p = .355), but the amplitude of peak V was different between frequencies (X2(3) = 17.160, p < 0.01). Also peak V amplitude decreases with increasing burst frequency.
Discussion
We successfully measured ABRs during stimulation-off state, LFS and HFS. Our results showed that LFS as well as HFS in the CIC and DCBN do not influence ABR thresholds, interpeak latencies and amplitude ratios in rats.
ABR thresholds
The finding that ABR thresholds were not influenced by LFS and HFS suggests that hearing in these frequencies is not impaired by DBS. Nonetheless, several caveats must be taken into account when interpreting ABR thresholds. Although common frequencies were tested (10, 16, 24 and 32 kHz) in these studies, hearing loss can occur in other specific frequency bands. In rats, hearing thresholds based on ABRs tend to be at least 10–20 dB higher than those determined behaviorally (Borg, 1982; Heffner et al., 1994). The thresholds in the current study (ranging from 36 to 46 dB peSPL) are thus an overestimation of the actual hearing level. To get the most reproducible ABR data in various measurements, we implanted ABR electrodes. In contrast to the commonly used subcutaneous electrodes, these implanted electrodes always measure from exactly the same anatomical position (Buchwald et al., 1981; Hall, 1990; McGee, Ozdamar & Kraus, 1983). To our knowledge, no other studies determined ABR thresholds during HFS and LFS of the CIC or DCBN. Likewise, determination of thresholds in ablation studies, whose results are thought to be similar to HFS, have not been performed.
ABR latency
In addition to thresholds, the latency and amplitude can be extracted from the five ABR peaks. Interpeak latencies are generally accepted as measures of conduction time of the central auditory pathway (Eggermont & Don, 1986; Picton et al., 1977; Squires, Chu & Starr, 1978). The interpeak latency of waves I–III, III–V and I–V reflect the time to traverse in the caudal, rostral and the whole brainstem, respectively. A prolonged interpeak latency reflects a lesion in central auditory processing (Burkhard, Eggermont & Don, 2007; Hood, 1998). Occasionally, a decreased latency of peak V was noted in ablation studies of the IC. This decrease of peak V latency was only an acute effect (Achor & Starr, 1980).
In this study, no statistically significant differences were found between the interpeak latencies at baseline compared to low and high frequency DBS. This can be interpreted as no functional relevant lesion at the IC is induced by DBS. However, many studies found no differences in latencies when ablating the IC, but found a difference in amplitude (Achor & Starr, 1980; Buchwald & Huang, 1975; Caird & Klinke, 1987). Therefore, we also performed analysis of the ABR amplitude.
ABR amplitude
Synchronously activated neurons contribute to the amplitude of the waveform (Burkhard, Eggermont & Don, 2007). The IC has a central role in the auditory pathway (Aitkin & Moore, 1975; De Martino et al., 2013). Previous studies have shown that lesioning of the IC resulted in a decrease of the amplitude of peak V (Achor & Starr, 1980; Buchwald & Huang, 1975; Caird & Klinke, 1987). In most studies a large part or the whole IC was ablated. One study only found an abolished peak V when ablation of the lateroventral part of the IC, in contrast to ablating the central nucleus (Funai & Funasaka, 1983). In humans, absence of the IC also resulted in abolished peak V peaks (Durrant et al., 1994). It is assumed that electrode implantation does not influence the amplitude of the evoked potentials, since only minimal tissue damage is seen along the electrode trajectory (Tan et al., 2010).
Although the precise role of the cerebellum and its associated nuclei in hearing is not known, it might have a modulatory effect on hearing. The cerebellum receives direct connections from the cochlear nucleus (Huang, Liu & Huang, 1982) and indirect connections from the IC (Aitkin & Boyd, 1978; Huffman & Henson, 1990). Furthermore, auditory stimuli as well as stimulation of the auditory cortex elicited responses from auditory cells in the paraflocculus (Azizi, Burne & Woodward, 1985).
One study assessed the ABR during cerebellar stimulation. High frequency stimulation (400 Hz) of the cerebellar surface resulted in a difference of the IV/I amplitude ratio, where peak IV represented in this particular study the IC. The IV/I amplitude ratio increased in case of a short electrical-sound stimulus interval (<10 ms), and decreased with larger intervals (>10 ms). In this particular study, peak IV represented the IC (Crispino & Bullock, 1984). In our study, the electrical and sound stimuli were played in an asynchronous manner and therefore various interval times are achieved. This could explain why we did not found any difference in the amplitude ratio. As far as we know, no ABRs were recorded in a cerebellar ablation study.
General ABR findings
It is a well-known phenomenon that high frequency tones show shorter latency peaks than lower frequency sounds, because high frequency sounds stimulate the more basal portions of the basilar membrane (Alvarado et al., 2012). This is also seen in our data. We also found that the peak V amplitude ratio decreased with increasing frequency of the tone given. As far as we know this is a new finding, which has not been reported earlier.
Mechanism of DBS in the auditory system
Our results show that latencies were not prolonged and amplitudes were not decreased during DBS, indicating that DBS in the CIC and DCBN probably does not have an overall inhibitory effect on physiological central auditory processing up to the IC (peak V). This finding is supported by one of the main working mechanisms of DBS. Namely that DBS with frequencies above 100 Hz disrupts abnormal information flow in a network (Chiken & Nambu, 2014), without influencing the normal neurophysiological activity.
HFS is also often referred to as having an inhibitory effect and thus mimicking the effect of a lesion (Benabid et al., 1998; Dostrovsky & Lozano, 2002). The pathological neural network loop related to tinnitus is interrupted by performing HFS within this loop (Smit et al., 2016). This hypothesis is supported by the disruption theory; DBS can dissociate the input and output in a stimulation nucleus and thereby disrupting abnormal information flow such as increased burst activity. Physiological information can still be normally processed through different nuclei (Chiken & Nambu, 2014). It can be hypothesized that this is the same when DBS is applied in the auditory pathway and physiological auditory information processing remains intact.
Future studies
In the current study animal did not receive noise trauma for induction of tinnitus. We hypothesize that if DBS does not result in hearing loss in the normal hearing, this will also not be the case when there is hearing loss in association with tinnitus. The current stimulation parameters can be used for tinnitus treatment; in a recent study that showed a decrease of tinnitus during IC stimulation (Smit et al., 2016), the same stimulation parameters were used as in the current study. In our study no pre-operative assessment of the ABR was performed.
Conclusions
In conclusion, HFS and LFS in the CIC and DCBN did not result in increased ABR thresholds and changes in interpeak latencies. Based on these observations, no evidence for changes in information processing in the auditory circuit were found during low and high frequency DBS in the CIC and DCBN. These findings suggest that DBS in the auditory pathways can be performed without hampering physiological processing of auditory information.
Supplemental Information
Appendix: Absolute values of latencies and amplitudes
Table A1. Absolute values of latencies and amplitudes from the five peaks of the auditory brainstem response of 10 kHz auditory stimuli.
Mean values with standard deviation are given.
| Peak | Wave | CIC group | DCBN group | ||||
|---|---|---|---|---|---|---|---|
| Stim-off | LFS | HFS | Stim-off | LFS | HFS | ||
| Latencies | I | 1.639 (.073) | 1.599 (.110) | 1.618 (.080) | 1.540 (.165) | 1.490 (.076) | 1.570 (.090) |
| II | 2.460 (.092) | 2.479 (.121) | 2.392 (.079) | 2.386 (.162) | 2.325 (.066) | 2.356 (.083) | |
| III | 3.143 (.181) | 3.122 (.208) | 3.088 (.144) | 3.060 (.280) | 3.080 (.109) | 3.101 (.084) | |
| IV | 4.288 (.281) | 4.167 (.191) | 4.122 (.238) | 4.077 (.260) | 4.118 (.165) | 4.148 (.098) | |
| V | 5.050 (.275) | 5.047 (.180) | 4.765 (.291) | 4.903 (.270) | 4.923 (.206) | 4.983 (.015) | |
| Amplitudes | I | .007 (.018) | .028 (.030) | .037 (.042) | .030 (.016) | .030 (.011) | .041 (.047) |
| II | .221 (.018) | .218 (.042) | .252 (.054) | .159 (.012) | .181 (.009) | .185 (.048) | |
| III | .042 (.018) | .074 (.037) | .076 (.038) | .078 (.031) | .097 (.066) | .117 (.069) | |
| IV | .070 (.017) | .086 (.042) | .117 (.035) | .061 (.055) | .087 (.041) | .103 (.053) | |
| V | .030 (.059) | .054 (.049) | .063 (.043) | .077 (.028) | .069 (.023) | .093 (.039) | |
Notes.
Abbreviations
- CIC
- central nucleus of interior colliculus
- DCBN
- dentate cerebellar nucleus
- stim-off
- stimulation-off state
- LFS
- deep brain stimulation at 10 Hz
- HFS
- deep brain stimulation at 100 Hz
Table A2. Absolute values of latencies and amplitudes from the five peaks of the auditory brainstem response of 16 kHz auditory stimuli.
Mean values with standard deviation are given.
| Peak | Wave | CIC group | DCBN group | ||||
|---|---|---|---|---|---|---|---|
| Stim-off | LFS | HFS | Stim-off | LFS | HFS | ||
| Latencies | I | 1.400 (.153) | 1.419 (.042) | 1.530 (.076) | 1.399 (.042) | 1.389 (.027) | 1.470 (.066) |
| II | 2.245 (.153) | 2.275 (.055) | 2.325 (.075) | 2.215 (.128) | 2.285 (.084) | 2.275 (.065) | |
| III | 2.960 (.292) | 2.960 (.125) | 3.020 (.155) | 2.96 (.140) | 2.929 (.075) | 3.030 (.090) | |
| IV | 3.956 (.367) | 4.067 (.190) | 4.057 (.230) | 3.977 (.118) | 4.027 (.155) | 4.098 (.145) | |
| V | 4.782 (.534) | 4.903 (.206) | 4.883 (.249) | 4.822 (.240) | 4.863 (.213) | 4.993 (.120) | |
| Amplitudes | I | .042 (.040) | .039 (.031) | .037 (.027) | .036 (.018) | .025 (.016) | .058 (.046) |
| II | .182 (.028) | .162 (.030) | .189 (.043) | .139 (.052) | .119 (.018) | .178 (.023) | |
| III | .079 (.048) | .080 (.048) | .085 (.029) | .088 (.047) | .079 (.040) | .118 (.052) | |
| IV | .050 (.019) | .057 (.031) | .089 (.029) | .047 (.015) | .072 (.030) | .113 (.035) | |
| V | .067 (.047) | .050 (.028) | .077 (.029) | .080 (.089) | .034 (.023) | .086 (.040) | |
Notes.
Abbreviations
- CIC
- central nucleus of interior colliculus
- DCBN
- dentate cerebellar nucleus
- stim-off
- stimulation-off state
- LFS
- deep brain stimulation at 10 Hz
- HFS
- deep brain stimulation at 100 Hz
Table A3. Absolute values of latencies and amplitudes from the five peaks of the auditory brainstem response of 24 kHz auditory stimuli.
Mean values with standard deviation are given.
| Peak | Wave | CIC group | DCBN group | ||||
|---|---|---|---|---|---|---|---|
| Stim-off | LFS | HFS | Stim-off | LFS | HFS | ||
| Latencies | I | 1.379 (.126) | 1.379 (.027) | 1.480 (.131) | 1.299 (.083) | 1.399 (.075) | 1.419 (.066) |
| II | 2.235 (.200) | 2.215 (.071) | 2.293 (.111) | 2.134 (.116) | 2.225 (.083) | 2.305 (.066) | |
| III | 2.929 (.234) | 2.929 (.114) | 3.050 (.131) | 2.859 (.140) | 2.919 (.094) | 3.040 (.121) | |
| IV | 3.896 (.326) | 3.987 (.186) | 4.097 (.272) | 3.906 (.170) | 4.007 (.116) | 4.108 (.166) | |
| V | 4.782 (.391) | 4.933 (.228) | 5.023 (.262) | 4.570 (.215) | 4.812 (.275) | 4.790 (.129) | |
| Amplitudes | I | .026 (.025) | .023 (.038) | .033 (.027) | .032 (.011) | .028 (.014) | .046 (.045) |
| II | .116 (.020) | .094 (.020) | .110 (.028) | .090 (.016) | .088 (.015) | .117 (.029) | |
| III | .080 (.062) | .072 (.062) | .074 (.031) | .070 (.033) | .055 (.034) | .085 (.072) | |
| IV | .037 (.033) | .192 (.301) | .083 (.054) | .053 (.024) | .054 (.009) | .077 (.041) | |
| V | .034 (.036) | .033 (.045) | .043 (.028) | .022 (.014) | .013 (.013) | .050 (.039) | |
Notes.
Abbreviations
- CIC
- central nucleus of interior colliculus
- DCBN
- dentate cerebellar nucleus
- stim-off
- stimulation-off state
- LFS
- deep brain stimulation at 10 Hz
- HFS
- deep brain stimulation at 100 Hz
Table A4. Absolute values of latencies and amplitudes from the five peaks of the auditory brainstem response of 32 kHz auditory stimuli.
Mean values with standard deviation are given.
| Peak | Wave | CIC group | DCBN group | ||||
|---|---|---|---|---|---|---|---|
| Stim-off | LFS | HFS | Stim-off | LFS | HFS | ||
| Latencies | I | 1.369 (.826) | 1.408 (.051) | 1.497 (.188) | 1.289 (.104) | 1.268 (.042) | 1.409 (.155) |
| II | 2.235 (.145) | 2.222 (.106) | 2.272 (.122) | 2.104 (.180) | 1.980 (.444) | 2.325 (.153) | |
| III | 2.919 (.155) | 2.885 (.124) | 2.926 (.139) | 2.859 (1.80) | 2.789 (.098) | 2.980 (.199) | |
| IV | 3.946 (.210) | 3.912 (.130) | 3.982 (.207) | 3.866 (.232) | 4.097 (.614) | 4.027 (.216) | |
| V | 4.802 (.413) | 4.896 (.371) | 4.776 (.304) | 4.681 (.222) | 4.691 (.271) | 4.842 (.277) | |
| Amplitudes | I | .017 (.011) | .024 (.032) | .052 (.124) | .035 (.017) | .023 (.013) | .043 (.041) |
| II | .097 (.019) | .156 (.150) | .167 (.225) | .078 (.021) | .091 (.030) | .092 (.030) | |
| III | .062 (.015) | .093 (.091) | .134 (.145) | .058 (.031) | .056 (.063) | .058 (.040) | |
| IV | .057 (.048) | .129 (.141) | .190 (.268) | .034 (.026) | .048 (.028) | .066 (.042) | |
| V | .021 (.022) | .032 (.054) | .039 (.090) | .015 (.146) | .013 (.025) | 0.042 (.043) | |
Notes.
Abbreviations
- CIC
- central nucleus of interior colliculus
- DCBN
- dentate cerebellar nucleus
- stim-off
- stimulation-off state
- LFS
- deep brain stimulation at 10 Hz
- HFS
- deep brain stimulation at 100 Hz
Funding Statement
This study was supported by the Heinsius Houbolt Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Jasper V. Smit conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Ali Jahanshahi and Marcus L.F. Janssen conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Robert J. Stokroos and Yasin Temel conceived and designed the experiments, wrote the paper, reviewed drafts of the paper.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Animal Experiments Committee of Maastricht University approved the experiments.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as a Data S1.
References
- Achor & Starr (1980).Achor LJ, Starr A. Auditory brain stem responses in the cat. II. Effects of lesions. Electroencephalography and Clinical Neurophysiology. 1980;48:174–190. doi: 10.1016/0013-4694(80)90302-8. [DOI] [PubMed] [Google Scholar]
- Aitkin & Boyd (1978).Aitkin LM, Boyd J. Acoustic input to the lateral pontine nuclei. Hearing Research. 1978;1:67–77. doi: 10.1016/0378-5955(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Aitkin & Moore (1975).Aitkin LM, Moore DR. Inferior colliculus. II. Development of tuning characteristics and tonotopic organization in central nucleus of the neonatal cat. Journal of Neurophysiology. 1975;38:1208–1216. doi: 10.1152/jn.1975.38.5.1208. [DOI] [PubMed] [Google Scholar]
- Alvarado et al. (2012).Alvarado JC, Fuentes-Santamaria V, Jareno-Flores T, Blanco JL, Juiz JM. Normal variations in the morphology of auditory brainstem response (ABR) waveforms: a study in Wistar rats. Neuroscience Research. 2012;73:302–311. doi: 10.1016/j.neures.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Azizi, Burne & Woodward (1985).Azizi SA, Burne RA, Woodward DJ. The auditory corticopontocerebellar projection in the rat: inputs to the paraflocculus and midvermis. An anatomical and physiological study. Experimental Brain Research. 1985;59:36–49. doi: 10.1007/BF00237663. [DOI] [PubMed] [Google Scholar]
- Backoff & Caspary (1994).Backoff PM, Caspary DM. Age-related changes in auditory brainstem responses in Fischer 344 rats: effects of rate and intensity. Hear Research. 1994;73:163–172. doi: 10.1016/0378-5955(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Bauer et al. (2013).Bauer CA, Kurt W, Sybert LT, Brozoski TJ. The cerebellum as a novel tinnitus generator. Hearing Research. 2013;295:130–139. doi: 10.1016/j.heares.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer et al. (2008).Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. Journal of Neuroscience Research. 2008;86:2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid et al. (1998).Benabid AL, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollak P. Long-term electrical inhibition of deep brain targets in movement disorders. Movement Disorders. 1998;13(Suppl 3):119–125. doi: 10.1002/mds.870131321. [DOI] [PubMed] [Google Scholar]
- Biacabe et al (2001).Biacabe B, Chevallier JM, Avan P, Bonfils P. Functional anatomy of auditory brainstem nuclei: application to the anatomical basis of brainstem auditory evoked potentials. Auris Nasus Larynx. 2001;28:85–94. doi: 10.1016/s0385-8146(00)00080-8. [DOI] [PubMed] [Google Scholar]
- Borg (1982).Borg E. Auditory thresholds in rats of different age and strain. A behavioral and electrophysiological study. Hearing Research. 1982;8:101–115. doi: 10.1016/0378-5955(82)90069-7. [DOI] [PubMed] [Google Scholar]
- Breit, Schulz & Benabid (2004).Breit S, Schulz JB, Benabid AL. Deep brain stimulation. Cell and Tissue Research. 2004;318:275–288. doi: 10.1007/s00441-004-0936-0. [DOI] [PubMed] [Google Scholar]
- Brozoski, Ciobanu & Bauer (2007).Brozoski TJ, Ciobanu L, Bauer CA. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI) Hearing Research. 2007;228:168–179. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Buchwald et al. (1981).Buchwald JS, Hinman C, Norman RJ, Huang CM, Brown KA. Middle- and long-latency auditory evoked responses recorded from the vertex of normal and chronically lesioned cats. Brain Research. 1981;205:91–109. doi: 10.1016/0006-8993(81)90722-8. [DOI] [PubMed] [Google Scholar]
- Buchwald & Huang (1975).Buchwald JS, Huang C. Far-field acoustic response: origins in the cat. Science. 1975;189:382–384. doi: 10.1126/science.1145206. [DOI] [PubMed] [Google Scholar]
- Burkhard, Eggermont & Don (2007).Burkhard RF, Eggermont JJ, Don M. Lippincott/Williams & Wilkins; Philadelphia/Baltimore: 2007. Auditory evoked potentials: basic principles and clinical application. [Google Scholar]
- Caird & Klinke (1987).Caird DM, Klinke R. The effect of inferior colliculus lesions on auditory evoked potentials. Electroencephalography and Clinical Neurophysiology. 1987;68:237–240. doi: 10.1016/0168-5597(87)90034-7. [DOI] [PubMed] [Google Scholar]
- Chen & Chen (1991).Chen TJ, Chen SS. Generator study of brainstem auditory evoked potentials by a radiofrequency lesion method in rats. Experimental Brain Research. 1991;85:537–542. doi: 10.1007/BF00231737. [DOI] [PubMed] [Google Scholar]
- Chen & Jastreboff (1995).Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hearing Research. 1995;82:158–178. doi: 10.1016/0378-5955(94)00174-O. [DOI] [PubMed] [Google Scholar]
- Chiken & Nambu (2014).Chiken S, Nambu A. Disrupting neuronal transmission: mechanism of DBS? Frontiers in Systems Neuroscience. 2014;8:33. doi: 10.3389/fnsys.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispino & Bullock (1984).Crispino L, Bullock TH. Cerebellum mediates modality-specific modulation of sensory responses of midbrain and forebrain in rat. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:2917–2920. doi: 10.1073/pnas.81.9.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel, Eisinger & Shore (2012).Dehmel S, Eisinger D, Shore SE. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Frontiers in Systems Neuroscience. 2012;6:42. doi: 10.3389/fnsys.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino et al. (2013).De Martino F, Moerel M, Van de Moortele PF, Ugurbil K, Goebel R, Yacoub E, Formisano E. Spatial organization of frequency preference and selectivity in the human inferior colliculus. Nature Communications. 2013;4:1386. doi: 10.1038/ncomms2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostrovsky & Lozano (2002).Dostrovsky JO, Lozano AM. Mechanisms of deep brain stimulation. Movement Disorders. 2002;17(Suppl 3):S63–S68. doi: 10.1002/mds.10143. [DOI] [PubMed] [Google Scholar]
- Durrant et al. (1994).Durrant JD, Martin WH, Hirsch B, Schwegler J. 3CLT ABR analyses in a human subject with unilateral extirpation of the inferior colliculus. Hearing Research. 1994;72:99–107. doi: 10.1016/0378-5955(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Eggermont & Don (1986).Eggermont JJ, Don M. Mechanisms of central conduction time prolongation in brain-stem auditory evoked potentials. Archives of Neurology. 1986;43:116–120. doi: 10.1001/archneur.1986.00520020010007. [DOI] [PubMed] [Google Scholar]
- Funai & Funasaka (1983).Funai H, Funasaka S. Experimental study on the effect of inferior colliculus lesions upon auditory brain stem response. Audiology. 1983;22:9–19. doi: 10.3109/00206098309072766. [DOI] [PubMed] [Google Scholar]
- Gayer & Faull (1988).Gayer NS, Faull RL. Connections of the paraflocculus of the cerebellum with the superior colliculus in the rat brain. Brain Research. 1988;449:253–270. doi: 10.1016/0006-8993(88)91042-6. [DOI] [PubMed] [Google Scholar]
- Gould (1979).Gould BB. The organization of afferents to the cerebellar cortex in the cat: projections from the deep cerebellar nuclei. Journal of Comparative Neurology. 1979;184:27–42. doi: 10.1002/cne.901840103. [DOI] [PubMed] [Google Scholar]
- Hall (1990).Hall RD. Estimation of surviving spiral ganglion cells in the deaf rat using the electrically evoked auditory brainstem response. Hearing Research. 1990;49:155–168. doi: 10.1016/0378-5955(90)90102-U. [DOI] [PubMed] [Google Scholar]
- Heffner et al. (1994).Heffner HE, Heffner RS, Contos C, Ott T. Audiogram of the hooded Norway rat. Hearing Research. 1994;73:244–247. doi: 10.1016/0378-5955(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Holm (1979).Holm S. A simple sequential rejective method procedure. ScandInavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Hood (1998).Hood LJ. Clinical applications of the Auditory Brainstem response. Singular Publishing Group; San Diego: 1998. [Google Scholar]
- Huang, Liu & Huang (1982).Huang CM, Liu G, Huang R. Projections from the cochlear nucleus to the cerebellum. Brain Research. 1982;244:1–8. doi: 10.1016/0006-8993(82)90897-6. [DOI] [PubMed] [Google Scholar]
- Huffman & Henson (1990).Huffman RF, Henson Jr OW. The descending auditory pathway and acousticomotor systems: connections with the inferior colliculus. Brain Research. Brain Research Reviews. 1990;15:295–323. doi: 10.1016/0165-0173(90)90005-9. [DOI] [PubMed] [Google Scholar]
- Kaga, Shinoda & Suzuki (1997).Kaga K, Shinoda Y, Suzuki JI. Origin of auditory brainstem responses in cats: whole brainstem mapping, and a lesion and HRP study of the inferior colliculus. Acta Oto-Laryngologica. 1997;117:197–201. doi: 10.3109/00016489709117768. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2012).Luo H, Zhang X, Nation J, Pace E, Lepczyk L, Zhang J. Tinnitus suppression by electrical stimulation of the rat dorsal cochlear nucleus. Neuroscience Letters. 2012;522:16–20. doi: 10.1016/j.neulet.2012.05.072. [DOI] [PubMed] [Google Scholar]
- McGee, Ozdamar & Kraus (1983).McGee TJ, Ozdamar O, Kraus N. Auditory middle latency responses in the guinea pig. American Journal of Otolaryngology. 1983;4:116–122. doi: 10.1016/S0196-0709(83)80013-1. [DOI] [PubMed] [Google Scholar]
- Musiek et al. (1984).Musiek FE, Kibbe K, Rackliffe L, Weider DJ. The auditory brain stem response I–V amplitude ratio in normal, cochlear, and retrocochlear ears. Ear and Hearing. 1984;5:52–55. doi: 10.1097/00003446-198401000-00011. [DOI] [PubMed] [Google Scholar]
- Musiek, Reeves & Baran (1985).Musiek FE, Reeves AG, Baran JA. Release from central auditory competition in the split-brain patient. Neurology. 1985;35:983–987. doi: 10.1212/WNL.35.7.983. [DOI] [PubMed] [Google Scholar]
- Osaki et al. (2005).Osaki Y, Nishimura H, Takasawa M, Imaizumi M, Kawashima T, Iwaki T, Oku N, Hashikawa K, Doi K, Nishimura T, Hatazawa J, Kubo T. Neural mechanism of residual inhibition of tinnitus in cochlear implant users. NeuroReport. 2005;16:1625–1628. doi: 10.1097/01.wnr.0000183899.85277.08. [DOI] [PubMed] [Google Scholar]
- Paxinos & Watson (2007).Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; London: 2007. [DOI] [PubMed] [Google Scholar]
- Picton et al. (1977).Picton TW, Woods DL, Barobeau-Brain JBA, Healeu TMG. Evoked potential audiometry. Journal of Otolaryngology. 1977;6:90–119. [PubMed] [Google Scholar]
- Robertson et al. (2013).Robertson D, Bester C, Vogler D, Mulders WH. Spontaneous hyperactivity in the auditory midbrain: relationship to afferent input. Hearing Research. 2013;295:124–129. doi: 10.1016/j.heares.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Rosahl et al. (2000).Rosahl SK, Tatagiba M, Gharabaghi A, Matthies C, Samii M. Acoustic evoked response following transection of the eighth nerve in the rat. Acta Neurochir. 2000;142:1037–1045. doi: 10.1007/s007010070060. [DOI] [PubMed] [Google Scholar]
- Ruebhausen, Brozoski & Bauer (2012).Ruebhausen MR, Brozoski TJ, Bauer CA. A comparison of the effects of isoflurane and ketamine anesthesia on auditory brainstem response (ABR) thresholds in rats. Hearing Research. 2012;287:25–29. doi: 10.1016/j.heares.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Sedley et al. (2012).Sedley W, Teki S, Kumar S, Barnes GR, Bamiou DE, Griffiths TD. Single-subject oscillatory gamma responses in tinnitus. Brain. 2012;135:3089–3100. doi: 10.1093/brain/aws220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman & Strashun (1999).Shulman A, Strashun A. Descending auditory system/cerebellum/tinnitus. International Tinnitus Journal. 1999;5:92–106. [PubMed] [Google Scholar]
- Simpson et al. (1985).Simpson GV, Knight RT, Brailowsky S, Prospero-Garcia O, Scabini D. Altered peripheral and brainstem auditory function in aged rats. Brain Research. 1985;348:28–35. doi: 10.1016/0006-8993(85)90355-5. [DOI] [PubMed] [Google Scholar]
- Smit et al. (2015).Smit JV, Janssen ML, Schulze H, Jahanshahi A, Van Overbeeke JJ, Temel Y, Stokroos RJ. Deep brain stimulation in tinnitus: current and future perspectives. Brain Research. 2015;1608:51–65. doi: 10.1016/j.brainres.2015.02.050. [DOI] [PubMed] [Google Scholar]
- Smit et al. (2016).Smit JV, Janssen ML, Van Zwieten G, Jahanshahi A, Temel Y, Stokroos RJ. Deep brain stimulation of the inferior colliculus in the rodent suppresses tinnitus. Brain Research. 2016;1650:118–124. doi: 10.1016/j.brainres.2016.08.046. [DOI] [PubMed] [Google Scholar]
- Squires, Chu & Starr (1978).Squires KC, Chu NS, Starr A. Auditory brain stem potentials with alcohol. Electroencephalography and Clinical Neurophysiology. 1978;45:577–584. doi: 10.1016/0013-4694(78)90158-X. [DOI] [PubMed] [Google Scholar]
- Stockard & Rossiter (1977).Stockard JJ, Rossiter VS. Clinical and pathologic correlates of brain stem auditory response abnormalities. Neurology. 1977;27:316–325. doi: 10.1212/WNL.27.4.316. [DOI] [PubMed] [Google Scholar]
- Tan et al. (2010).Tan S, Vlamings R, Lim L, Sesia T, Janssen ML, Steinbusch HW, Visser-Vandewalle V, Temel Y. Experimental deep brain stimulation in animal models. Neurosurgery. 2010;67:1073–1079. doi: 10.1227/NEU.0b013e3181ee3580. [DOI] [PubMed] [Google Scholar]
- Turner et al. (2006).Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behavioral Neuroscience. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Wang, Ding & Salvi (2002).Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hearing Research. 2002;168:238–249. doi: 10.1016/S0378-5955(02)00360-X. [DOI] [PubMed] [Google Scholar]
- Zheng et al. (2012).Zheng Y, Vagal S, McNamara E, Darlington CL, Smith PF. A dose-response analysis of the effects of L-baclofen on chronic tinnitus caused by acoustic trauma in rats. Neuropharmacology. 2012;62:940–946. doi: 10.1016/j.neuropharm.2011.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as a Data S1.