Abstract
Increased anxiety and stress are frequently found in children with autism spectrum disorder and are associated with social challenges. Recently, we reported changes in social competence following peer-mediated, theatre-based intervention. The purpose of this study was to examine the impact of the intervention on reducing anxiety and stress. Participants included 30 youth with autism spectrum disorder (8–14years old) randomly assigned to the experimental (17) or waitlist control (13) group. Pretest adjusted, between-group differences were analyzed for state-anxiety, trait-anxiety, play-based cortisol, and diurnal cortisol. Pearson correlations were conducted between anxiety, cortisol, and group play. Significant pretest-adjusted between-group differences at posttest were observed on trait-anxiety (F(1, 27) = 9.16, p=0.005) but not state-anxiety (F(1, 27)=0.03, p=0.86), showing lower trait-anxiety in the experimental group. There were no between-group differences on cortisol. There was a significant negative correlation between group play and trait-anxiety (r=−0.362, p=0.05). Playground cortisol correlated with group play, for the experimental group (r=0.55, p=0.03). The theatre-based, peer-mediated intervention not only contributes to improvement in social competence in youth with autism spectrum disorder but also contributes to reductions in trait-anxiety associated with more social interaction with peers. Results suggest that some degree of physiological arousal is essential for social interaction.
Keywords: anxiety, autism spectrum disorders, competence, cortisol, stress, theatre
Introduction
ASD and Social Functioning
Autism spectrum disorder (ASD) is characterized by impairment in social competence as well as restricted interests and behaviors (American Psychiatric Association, 2013). Social deficits in ASD are resistant to treatment (Eigsti & Shapiro, 2003), present throughout life (Baron-Cohen, 1985; Ballaban-Gil et al., 1996; Billstedt et al., 2007), and appear across multiple domains. Regarding social-cognitive abilities, individuals with ASD have shown impairment in face processing (Lozier et al., 2014) and theory of mind (Baron-Cohen et al., 1985; Castelli et al., 2002). Compared to typically developing (TD) peers, research suggests that individuals with ASD engage in social interactions of decreased quality and quantity (Lord and MaGill-Evans, 1995; Bauminger et al., 2003). Furthermore, numerous correlational studies suggest that difficulty interacting with others may contribute substantially to increased anxiety and stress (Corbett et al., 2010; Lopata et al., 2008; Schupp et al., 2013). Individuals with ASD display elevated anxiety (Bellini, 2006; White et al., 2009), as well as dysregulation of the physiological stress response (Corbett et al., 2008). Moreover, research suggests that within the ASD population, lower levels of social ability are associated with heightened anxiety (Simon and Corbett, 2013).
Stress in ASD
The physiological stress response involves activation of multiple cardiovascular and endocrine systems. One essential stress system is the hypothalamic-pituitary-adrenal (HPA)-axis, which responds significantly to novel situations perceived as threatening (Coplan et al.1996).
Activation of the HPA-axis leads to release of glucocorticoid hormones, including cortisol (O'Connor et al., 2000). Salivary cortisol provides a reliable, non-invasive metric of stress (Kirschbaum and Hellhammer, 1994) and has been used to measure response to social situations (e.g., Corbett et al., 2012; Gunnar et al., 2003).
Compared to TD peers, youth with ASD show greater HPA activity in response to a variety of stressors (Corbett et al., 2008; Spratt et al., 2012). Group differences in HPA response are particularly prominent for stressors of a social nature. For example, elevated salivary cortisol concentration has been observed in ASD during introduction to novel peers (Lopata et al., 2008) and social interaction on a playground (Corbett et al., 2010; Schupp et al., 2013). Familiarity with peers is also influential (Corbett et al., 2014b), and stress may be reduced when interacting with a familiar peer (Lopata et al., 2008). This suggests that social interaction with unfamiliar peers may be a particularly provocative stressor for youth with ASD. Therefore, interventions to reduce stress/anxiety should give particular focus to this social context.
Anxiety in ASD
While the terms stress and anxiety are often used interchangeably, stress in this context refers to a physiological response, while anxiety refers to a mental state of apprehension regarding one’s situation. Among youth with ASD, there is a high rate of co-occurring anxiety disorders (Simonoff et al., 2008; Van Steensel et al., 2011). A majority—as high as 84%—of youth with ASD display levels of anxiety high enough to impair daily function (White et al., 2009). Several research studies suggest correlations between anxiety and specific ASD symptomatology such as repetitive behaviors (Rodgers et al., 2012; Sukhodolsky et al., 2008) or sensory sensitivity (Tsuji et al., 2009; Mazurek et al., 2013): other studies fail to find such correspondence (Renno and Wood, 2013; White et al., 2009). Nonetheless, social situations are undeniably sources of anxiety for many youth with ASD (Bellini, 2006).
Stress and anxiety are often distinguishable, as evidenced by levels that do not correspond when studied together (Lanni et al., 2012; Lopata et al., 2008; Simon and Corbett, 2013)—supporting the approach that the constructs must be assessed separately. Stress can be measured using physiological metrics, but anxiety—as a psychological construct—is often measured through caregiver report or self-report. One widely used scale for measuring anxiety in children is the State Trait-anxiety Inventory for Children (STAI-C; Spielberger, 1983), which consists of two subscales for state and trait anxiety (STAI-C State and STAI-C Trait), distinguished as distinct factors (Dorr, 1981). The STAI-C is useful in measuring anxiety levels among typically developing youth, as well as youth with psychiatric disorders (e.g., Harada et al., 2002; Muris and Meesters, 2004;Spielberger et al., 1999). The STAI-C has also been applied to participants with ASD (Lanni et al., 2012).
Previous work suggests that youth with ASD may have difficulty describing their affective states (i.e., anxiety) at a given time (Bölte et al., 2008; Groden et al., 2006). On the other hand, children between 8 to 12 years with ASD using the STAI-C were ostensibly able to reliably report their persistent (trait) level of anxiety in the context of stressful situations (Simon and Corbett, 2013), and youth with ASD have been shown to reliably report trait irritability (Mikita et al., 2015). This suggests that children with ASD are likely better able to accurately self-report their long-term anxiety than state-dependent anxiety. Thus, STAI-C State may not be a reliable indicator of acute anxiety for the ASD population. The STAI-C Trait, however, appears to be a reliable research metric to utilize for youth with ASD, and maybe valuable in measuring the effectiveness of interventions aimed at reducing anxiety (Simon and Corbett, 2013).
Interventions for Stress/Anxiety
Excessive stress and anxiety have been shown to compromise physical and mental health (Morey et al., 2015). Furthermore, prolonged exposure to stress during childhood and adolescence can lead to permanent morphological changes in brain development, with the potential to negatively affect social behaviors and increase risk for the development of future psychopathology (Giedd, 2004). Members of vulnerable populations, such as children with ASD, can be particularly impacted by the negative effects of persistently or chronically elevated anxiety and stress. Recent clinical research has focused on the development of interventions to help reduce stress and anxiety for individuals with ASD.
Clinical researchers have implemented a variety of interventions, most using elements of Cognitive Behavioral Therapy (CBT)—such as graded exposure, regulation strategies or cognitive restructuring—to diminish symptoms of anxiety in youth with ASD (Danial et al., 2013; Reaven et al., 2012;White et al., 2013). Still, the results of these interventions are variable, and though many include social competency components, interventions aimed at increasing social competence have not been thoroughly explored in relation to anxiety.
Peer-Mediation in Social Competence Intervention
For all children, interaction with peers considerably influences physiological, psychological, and social functioning (Lopata et al, 2008; Schupp et al., 2013). Thus, including peers is a valuable component of social competence intervention (Barry et al., 2003). With proper training, peers can serve to reinforce adaptive social behavior (Banda et al., 2010). The incorporation of peer-mediation leads to greater peer acceptance after intervention (Kasari et al., 2012) and enhanced generalization of skills (Kamps et al., 1992).
Theatre in Social Competence Intervention
Because acting is interactive, it is useful for teaching adaptive social behavior. Important elements of acting and real-world interaction include observing, interpreting, and articulating thoughts and feelings. Skills involved in theatre parallel areas in which youth with ASD display deficits: emotion perception, theory of mind, creative thinking, and reciprocal communication. Practicing theatrical techniques such as improvisation, role-playing, scripted interaction, and performing can help target and improve these deficiencies (Corbett et al., 2014b). There has been a recent surge in research involving the use of theatrical approaches to enhance emotional awareness and social functioning in individuals with and without ASD (Corbett et al., 2011;Corbett et al., 2014a; Corbett et al., 2014b; Goldstein, 2011; Lerner et al. 2011). This promising research shows that theatre-based intervention can lead to enhanced social competence, through increases in social-cognitive functioning, improved social interaction, and possibly changes to neural underpinnings that support these domains (Corbett et al., 2016).
Social Emotional Neuroscience Endocrinology (SENSE) Theatre®
One empirically validated approach is SENSE Theatre®, a peer-mediated, theatre-based intervention for youth with ASD. In SENSE Theatre® participants with ASD are paired with trained TD peers, who serve as models for adaptive social interaction in a novel, supportive social context (Corbett et al., 2016). Specific techniques include role-playing, improvisation, theatre games, video modeling, and character development (Corbett et al., 2014a; Corbett et al., 2014b). Using pre-test, post-test design, participants of SENSE Theatre®have shown significant improvements in face memory, theory of mind, and social interaction (Corbett et al., 2014a; Corbett et al., 2014b). Recently, these findings were extended in a randomized control trial (RCT) in which between group differences were demonstrated in memory for faces, social communication, and group play with peers not affiliated with the treatment (Corbett et al., 2016).
Since social interactions are common stressors for many children with ASD (as discussed above), improving social functioning may be a viable way to lower anxiety and stress. Thus, the primary aim of the current study was to evaluate the impact of SENSE Theatre® on stress and anxiety among children with ASD. The study used a randomized control-trial design, measuring anxiety (self-report) and stress (cortisol) before and after treatment. Participants in the experimental (EXP) group were hypothesized to show greater reduction in anxiety and stress compared to the waitlist-control (WLC) group. Moreover, it was hypothesized that changes would be associated with positive changes in Group Play.
Methods
Participants
Thirty-six youth with ASD were recruited via word-of-mouth and fliers placed at area clinics and autism support organizations. Thirty-three eligible youth (three did not meet criteria for ASD or were determined to be lower functioning demonstrated by an intellectual quotient < 70) were allocated to groups based on simple randomization administered by staff in the Department of Biostatistics not involved in the research. Seventeen youth were randomized into the EXP and 16 to the WLC. Three participants in the WLC did not complete the study. The final sample included 30 youth with ASD between eight-to-14 years.
Inclusion in the study required the participant to have a diagnosis of ASD, which was made based on the Diagnostic and Statistical Manual-5(APA, 2013) and established by: (1) previous diagnosis; (2) current clinical judgment; and (3) corroborated by the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000), administered by research-reliable personnel. Participants were also required to be higher functioning and have an intellectual quotient > 70. An expanded characterization of the sample is found in (Corbett et al., 2016).
The University Institutional Review Board approved the study. Parents and child participants provided written informed consent prior to inclusion in the study. During pre and post-visits, the Peer Interaction Paradigm (described below) was conducted at the Vanderbilt Kennedy Center playground between 2:00–5:00 p.m.
The demographic information for the study sample is presented in Table 1. The mean age for the EXP group was 11.27 (2.51) and WLC was 10.74 (1.89). Out of 30 participants enrolled in the trial, 24 (80%) were male (13 EXP and 11 WLC), which is comparable between the groups. There were 21 total Caucasian (12 EXP, 9 WLC), 1 African-American (1 EXP), 2 Asian (1 EXP, 1 WLC), 4 Latino/Hispanic (1 EXP, 3 WLC), and 2 Multiracial (2 EXP) participants. Nineteen (63%) participants were on psychotropic medication (10 EXP, 9 WLC). Seven were on two or more medications (4 EXP, 3 WLC).
Table 1.
Demographic, diagnostic, and pre-treatment group differences
| Variable | EXP M(SD) |
WLC M(SD) |
df | t | p |
|---|---|---|---|---|---|
| Age | 11.27 (2.51) | 10.74 (1.89) | 28 | −0.66 | 0.52 |
| ADOS (Algorithm Score) | 12.47 (3.96) | 14.42 (5.19) | 27 | 1.11 | 0.28 |
| IQ (WASI) | 106.06 (16.83) | 95.85 (21.19) | 28 | −1.47 | 0.15 |
| STAI-C State | 29.29 (6.54) | 28.08 (4.15) | 28 | −0.59 | 0.56 |
| STAI-C Trait | 39.12 (8.99) | 36.92 (10.05) | 28 | −0.63 | 0.53 |
| PIP Cortisol (Baseline) | −.19 (.18) | .03 (.34) | 28 | 2.36 | 0.03* |
| PIP Cortisol (Beginning of Play) | −.26 (.56) | −.07 (.45) | 28 | 0.99 | 0.33 |
| PIP Cortisol (End of Play) | −.17 (.32) | −.43 (1.1) | 28 | −0.91 | 0.37 |
| PIP Cortisol (Recovery) | −.24 (.27) | −.18 (.37) | 28 | 0.53 | 0.60 |
| CBCL - Affective Problems | 67.67 (6.62) | 64.62 (7.48) | 26 | −1.15 | 0.26 |
| CBCL - Anxiety Problems | 68.73 (6.07) | 65.31 (8.36) | 26 | −1.25 | 0.22 |
| CBCL - Somatic Problems | 56.20 (7.50) | 57.92 (6.91) | 26 | 0.63 | 0.54 |
| CBCL - ADHD Problems | 65.40 (7.50) | 68.38 (6.84) | 26 | 1.09 | 0.28 |
| Group Play | 61.9 (28.44) | 60.8 (38.42) | 27 | −0.09 | 0.93 |
EXP = Experimental Group, WLC = Waitlist Group, ADOS = Autism Diagnostic Observation Schedule, WASI = Wechsler Abbreviated Scale of Intelligence, STAI-C = State Trait-anxiety Inventory for Children, PIP = Peer Interaction Paradigm, CBCL = Child Behavior Checklist.
Intervention
The experimental group received the treatment over 10 weekly four-hour sessions (Corbett et al., 2011). After the follow-up assessment sessions had been completed, the WLC group received the intervention as a 10-session summer camp (Corbett et al., 2014b). At the end of intervention, two public performances were held. The WLC was not assessed after treatment; thus, their treatment data do not appear in this article.
Components of treatment included training typically developing peer actors, SENSE Theatre®sessions, and homework using video models. For an expanded explanation see previous reports (Corbett et al., 2016; Corbett et al., 2014a; Corbett et al., 2014b).
Peer training
Twelve trained peers (M=15.33 years; SD=1.12) were paired with participants with ASD. Training for peers and staff included a comprehensive two-day seminar.
SENSE Theatre®
Sessions were held Saturdays (1:00–5:00 p.m.) at a local secondary school. A schedule of each day was provided in advance and displayed in the theatre. Early sessions included theatrical games, role-playing, and exercises. In subsequent sessions, participants worked on their roles for the play.
Dependent Measures
State-Trait-Anxiety Inventory for Children (STAI-C; Spielberger, 1983)
The STAI-C is a questionnaire consisting of 40 self-report items with a 4-point Likert scale. The scale is based on the original STAI, modified for children, and has been used to measure anxiety in numerous previous intervention studies (e.g., Beidel et al., 2000; Howard and Kendall, 1996; Robb et al., 1995). The inventory measures state (current) and trait (persistent) anxiety. Alpha reliability of the STAI-C is high, ranging from .78–.91 (Muris et al., 2002). Test-retest reliability for STAI-C Trait is higher (.65–.71) than the test-retest reliability for STAI-C State (.31–.41), but this is valid, as the STAI-C State is intended to measure a transient state (Julian, 2011). Furthermore, STAI-C effectively distinguishes between those with and without anxiety disorders (Seligman et al., 2004) and correlates with other measures of anxiety (Spielberger, 1973).
Cortisol
In addition to responding to actual and perceived stressors, cortisol has a diurnal rhythm throughout the day, with peak levels occurring in the morning shortly after waking and lower levels occurring in the evening (Herman & Cullinan, 1997; Sapolsky et al., 2000). Basal levels of cortisol were collected from home by parents to ascertain diurnal baseline over four cycles (two pre- and two post-intervention) using established methods, which included controlled intake of food/liquids (Corbett et al., 2008). Parents were instructed to collect samples via passive drool four times per day (immediately upon waking, 30-min post-waking (CAR), in the afternoon, and evening). To evaluate arousal levels in response to the intervention, participants provided salivary samples at the beginning and end of three days across of the intervention (the first, middle and last session). To examine stress levels during play with novel peers, salivary cortisol was measured at four time-points during each playground visit. Because cortisol detection in saliva has a 20-min lag time, samples were taken to correspond to specific points in time. Specifically, samples were collected at baseline and at 20-minute intervals to reflect the beginning of play, end of play, and 20-minutes post play. Importantly all of these stress samples were collected in the afternoon.
Group Play
Group Play was measured through direct observation using an established playground observation approach. The Peer Interaction Paradigm (PIP) is an ecologically valid semi-structured playground interaction consisting of a 20-minute playground interaction in which the study participant engages in play with two gender- and age-matched confederate peers (Corbett et al., 2010; Schupp et al., 2013). The peers on the playground are research “confederates” and are not part of the theatre intervention. Confederate 1 was a novel peer during the pre-test and returned for the post-test. Confederate 2 was a novel peer during each playground visit. Interactions were recorded and underwent behavioral coding using The Observer XT (Noldus, 2008). Group Play was defined as duration of activity when the participant engaged with the group, using the same types of equipment/toys as peers. For a detailed explanation of the paradigm see previous reports (Corbett et al., 2010; Schupp et al., 2013).
The PIP is a valid measure of the duration and frequency of play during peer interactions with novel typically developing peers (Corbett et al., 2010; Schupp et al., 2013). The primary coder of the PIP was blind to which videos would be checked for reliability and the time period (pre/post). Inter-rater reliability was conducted on a random sample of 20% of the coded videos. For the variable Group Play, reliability was good (k = .85), and similar to previous studies using the PIP (Corbett et al., 2010; Schupp et al., 2013). Group play from the PIP in this investigation was considered a dependent measure and was measured before and after the intervention for the EXP and WLC groups.
Statistical Analysis
Independent sample t-tests were used to determine if there were group differences in diagnostic and pre-test variables. As expected, cortisol levels were skewed and therefore log transformation was conducted using Log10 and transformed values were used in statistical models. Repeated measures analysis of variance was used to test between-group differences in stress cortisol post-intervention, using pre-intervention cortisol values as covariates. Analysis of Covariance (ANCOVA) models were used to test between-group differences in anxiety and diurnal cortisol variables post-intervention using the dependent variable’s pre-intervention value as a covariate. Post-hoc paired-sample t-tests were conducted to assess for changes in trait-anxiety in the two groups. Bootstrapping methods with 95% confidence intervals using the PROCESS application for SPSS were used to test for mediation effects between changes in anxiety and changes in group play (Hayes, 2013). Paired t-tests were used to investigate differences in theatre cortisol across time, in the experimental group. Pearson correlations between dependent variables shown to differentiate groups were also calculated, and post-hoc analyses were conducted to examine group-specific correlations.
Results
Pretest between Group Differences
Pretest between groups differences on diagnostic variables were tested using independent sample t-tests (Table 1). Results showed no significant between-group differences in age, gender, IQ, Group Play, or anxiety.
Treatment Effects on Posttest Anxiety
ANCOVA was used to evaluate the effect of treatment on post-intervention anxiety, adjusted for pre-intervention anxiety. Test of between-subject effects revealed a significant group effect on post-STAI-C Trait, with pre-STAI-C Trait included as a covariate (F(1, 27)=9.16, p=0.005). No group effect was observed for STAI-C State (F (1, 27) =0.03, p=0.86)). Post-hoc paired-sample t-tests were conducted to assess changes in trait anxiety from pre- to post-intervention testing, for the two groups. These results and pre-/post- mean values for trait anxiety are detailed in Table 3. Additionally, mediational analyses were conducted to assess whether changes in group play mediated changes in anxiety. Pre-values for trait-anxiety and group play were included as covariates in the model. Changes in play did not show a significant mediational effect on changes in trait-anxiety (B = −0.32; CI = −3.35 to 2.11). Conversely, the direct effect of the intervention on changes in trait-anxiety remained significant (B = −6.97, CI = −12.62 to −1.31).
Table 3.
Cortisol Levels in Treatment Setting (EXP Group)
| Measure | Mean (SD) |
Measure | Mean (SD) |
df | T | p |
|---|---|---|---|---|---|---|
| Beginning Day 1 | 0.10 (0.34) | End Day 1 | −0.12 (0.38) | 16 | 2.20 | 0.04* |
| Beginning Day 2 | 0.03 (0.36) | End Day 2 | −0.19 (0.25) | 16 | 2.53 | 0.02* |
| Intervention Beginning Day 3 | 0.139 (0.43) | End Day 3 | −0.13 (0.35) | 16 | 1.84 | 0.09 |
| Beginning Day 1 | 0.10 (0.34) | Beginning Day 3 | 0.14 (0.43) | 16 | −0.39 | 0.70 |
| End Day 1 | −0.12 (0.38) | End Day 3 | −0.13 (0.35) | 16 | 0.04 | 0.97 |
Treatment Effects on Posttest Stress
A repeated measures analysis of variance was used to evaluate group effect on post-intervention stress cortisol while adjusting for pre-treatment levels. There was no significant main effect of group on post-intervention playground cortisol (F (3, 21)=0.99, p=0.10). ANCOVA was used to evaluate group effect on post intervention diurnal cortisol and there were no significant differences for immediate waking (F (1, 28)=1.22, p=0.28), CAR (F(1,27)=3.14, p=0.09), or evening (F(1, 28)=2.53 p=0.12) adjusting for pre-treatment home cortisol. Using paired sample t-tests, cortisol levels in the treatment setting across multiple days/time-points were compared for the EXP group. Significant differences in cortisol were found between samples taken at the beginning and end of the first and middle (but not final) days of the intervention, likely reflecting normal diurnal decline (see Table 2). However, there were no significant differences in cortisol levels in the treatment setting across treatment days at corresponding time of day (see Table 2) suggesting no changes in cortisol over treatment.
Table 2.
Changes in Anxiety after Intervention (Separated by Group)
| Measure | Mean (SD) |
Measure | Mean (SD) |
df | T | p |
|---|---|---|---|---|---|---|
| Experimental Group | ||||||
| Pre-STAI-C Trait | 39.12 (8.99) | Post-STAI-C Trait | 34.41 | 16 | 2.10 | 0.05* |
| Waitlist Control Group | ||||||
| Pre-STAI-C Trait | 36.92 (10.05) | Post-STAI-C Trait | 40.62 (10.25) | 12 | −2.37 | .035* |
Correlations
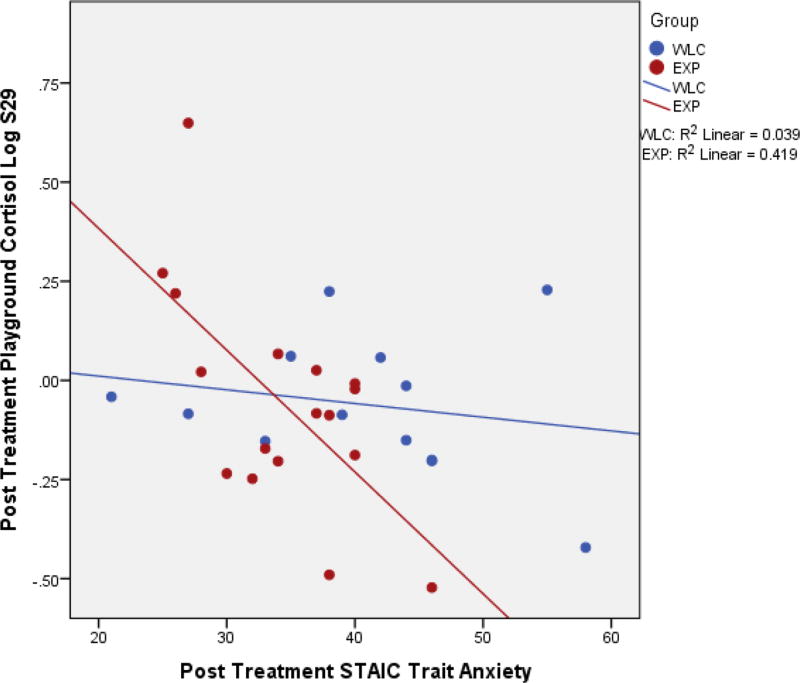
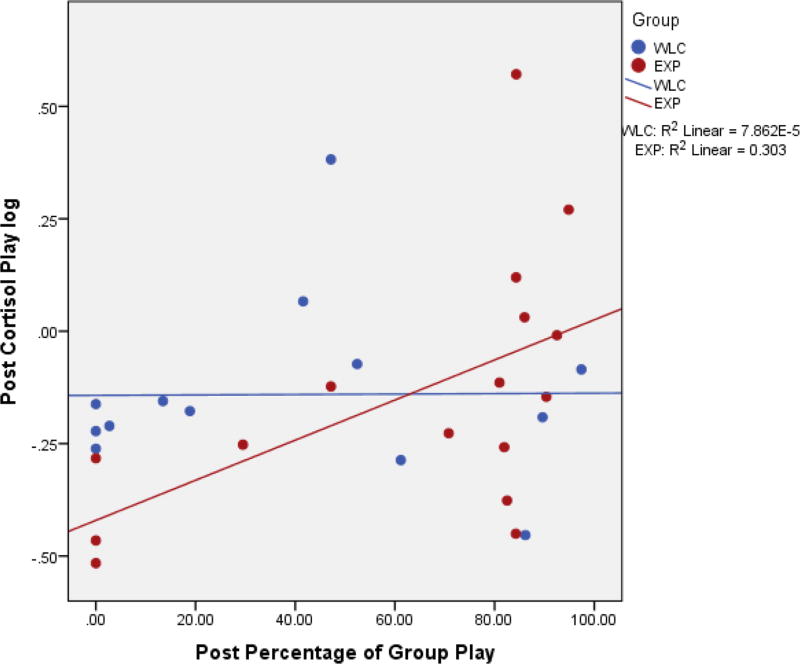
Post-hoc correlation analyses were performed for stress, anxiety, and Group Play, being the only variables shown to differentiate the groups. There were significant negative correlations between post-STAI-C Trait scores and cortisol levels during play (r=−.37, p=0.047) and the recovery period (r=−.390, p=0.03); correlations between cortisol and anxiety (separated by group) are visualized in Figure 1. Furthermore, there was a negative correlation between STAI-C Trait and amount of time spent in Group Play (r=−.362, p=0.05). There were also trend level positive correlations between Group Play and cortisol values for play (r=0.33, p=0.08). Further post-hoc analyses revealed a significant correlation between play cortisol and Group Play, for the experimental group (r=.55, p=0.03); correlations between cortisol and Group Play (separated by group) are visualized in Figure 2.
Figure 1.
Correlation between Post STAI-C Trait and Playground Cortisol.
Figure 2.
Correlation between Post Group Play and Playground Cortisol.
Discussion
Recently, a RCT of a peer-mediated, theatre-based intervention demonstrated that the intervention may be valuable for enhancing social competence in youth with ASD (Corbett et al., 2016). Previous investigations of SENSE Theatre® found decreases in stress/anxiety after intervention (Corbett et al., 2014b). Thus, the current study aimed to investigate whether the RCT resulted in group differences on stress and anxiety. Findings show that there were significant treatment effects in the EXP group resulting in reduction of trait-anxiety. Group differences were not observed for state-anxiety or cortisol. Furthermore, post-intervention stress/anxiety were associated with changes in Group Play.
Consistent with research suggesting reliability of the STAI-C for assessing Trait anxiety in ASD (Simon and Corbett, 2013), the current findings suggest a decrease in trait-anxiety among participants following treatment. This decrease corresponds with previously demonstrated increases in Group Play after intervention (Corbett et al., 2016). Specifically, a negative correlation was shown between social interaction with peers and reported anxiety. However, mediation analysis did not show that changes in group play mediated changes in anxiety. Thus, the findings, while independent, suggest that the intervention modifies both social engagement and anxiety related to interacting with peers. This is consistent with findings that suggest a negative association between social functioning and self-reported trait-anxiety in ASD (Simon and Corbett, 2013). While no decreases in state-anxiety were shown, this could be due to heightened alexithymia in individuals with ASD, which can negatively influence ability to report affective states (Bird and Cook, 2013). Individuals with alexithymia experience heighted physiological arousal associated with emotions such as anxiety, but are unable to reliably report and describe these experiences (Lane et al., 1997). Thus, elevated alexithymia may potentially inhibit individuals with ASD from reporting state-anxiety, even if they are experiencing the associated arousal.
The clinical relevance of the present study is highlighted by comparison with STAI-C Trait scores in populations with anxiety disorders. On pre-intervention measurement, the EXP group reported an average of 39.12 on the STAI-C Trait scale; similarly, the WLC group reported an average of 36.12. These values fall within the general range of STAI-C Trait scores found in youth with diagnosed anxiety disorders (ranging from 30.3–57.8; e.g., Beidel et al., 2000; Hodges, 1990; and Vasey et al., 1995) suggesting clinically relevant distress in the current sample. The improvement after SENSE Theatre®seems relevant as well; notably, while reported trait anxiety decreased significantly in the EXP group post intervention, the WLC group scores increased. Though the range of STAI-C Trait scores in individuals with anxiety disorders is wide, the STAI-C has been shown to be sensitive to treatment gains (Seligman et al., 2004).
While there was an effect of treatment on reducing trait-anxiety, there was no significant effect on cortisol. This suggests that while perception of (trait) anxiety may have been impacted, cortisol reactivity did not change. In the past, salivary cortisol has been used to evaluate whether interventions for youth ASD lead to changes in physiological stress severity (Corbett et al., 2014b; Lopata et al., 2008; Viau et al., 2010). While previous investigations reported reductions in cortisol reactivity following SENSE Theatre® (Corbett et al., 2014b), these studies did not follow a RCT design. Reductions in cortisol may not have been observed due to the significant variability in stress responsivity in individuals with ASD (Corbett et al., 2010). It is also possible the intervention contributes to changes in the performance of social skills; yet, does not result in reductions in the underlying physiological response to social exchange. Thus, despite enhanced arousal, the participants demonstrate more awareness of social stimuli and more motivation to engage with others. It is unclear if the participants must override a higher level of arousal to engage in social encounters (Corbett et al., 2010) or, as postulated below, if the higher cortisol actually facilitates the social exchange. An examination of repeated social exposures over time will help to unravel the extent to which social stress as measured by cortisol is modifiable in children with ASD.
At first, the lack of corresponding results for stress and (state or trait) anxiety in the current study may seem paradoxical. However, this is consistent with previous research showing that these two phenomena do not always directly correspond (Kirschbaum et al., 1995); while changes in cortisol may correspond with acute psychological distress, absolute levels of cortisol do not necessarily correlate with (state or trait) anxiety (Vedhara et al., 2003). Furthermore, individuals with high trait-anxiety have been shown to respond to unpleasant stimuli with blunted cortisol responses, compared to individuals with low trait-anxiety (Hubert and de Jong-Meyer, 1990). So while treatment did not lead to a decrease in stress reactivity, the participants’ ability to cope with stimuli perceived as threatening (i.e., social interaction) was likely improved.
Recent investigation of SENSE Theatre®found a significant effect of intervention on post-treatment Group Play (Corbett et al., 2016); namely, participants in the EXP group engaged in more play with peers. Thus, in order to examine the role of social functioning on participants’ anxiety and stress, correlations between anxiety/stress and Group Play were investigated. Group Play was negatively correlated with anxiety but positively correlated with cortisol, particularly among members of the EXP group. Positive correlations between cortisol and Group Play suggest that increased physiological arousal as measured by salivary cortisol is necessary to facilitate social engagement with peers (Corbett et al., 2014b). It is long established that more demanding tasks require greater arousal (Yerkes & Dodson 1908) and for children with ASD, engaging with peers may be conceived of as challenging (Corbett et al., 2014a).
On the other hand, individuals who engaged in more Group Play endorsed lower anxiety. Consequently, while social interaction may be associated with greater HPA reactivity, participants’ ability to cope with perceived threat of the interaction may be improved following the intervention. The increase in social functioning following participation in the theatre program ostensibly contributed to a reduction in anxiety despite the higher level of arousal that may be necessary for social engagement in children with ASD. These changes in reactivity to social interaction after theatre intervention could be connected to the prominent role of peers.
In SENSE Theatre®, peers serve as models for reciprocal social exchange. It is feasible that practice interacting with peers during the intervention may facilitate positive appraisals of peer interaction during the post-PIP, thereby reducing perceived anxiety. Furthermore, participation in theatre games and social play in the treatment setting may generalize to other settings, leading to higher quality, less distressing interactions on the playground. Peer-mediation has been shown to facilitate positive social effects (Barry et al., 2003;Kamps et al., 1992;Kasari et al., 2012) such as improved memory for faces (Corbett et al., 2016; Corbett et al., 2014b). These improvements likely facilitate more frequent peer interactions, which provoke less anxiety. In summary, participants in the SENSE Theatre® program experienced reduced anxiety, which was correlated with increased engagement with peers. Furthermore, cortisol did not show decrease after treatment, and cortisol correlated positively with play in the EXP group, suggesting that some degree of physiological arousal may be required for youth with ASD to interact with peers.
Limitations
Greater expectancy of improvements in children in the experimental group may have systematically inflated their scores, influencing self-report anxiety. Furthermore, because of decreased ability to report emotional states among many youth with ASD, the STAI-C State results may not be a reliable indicator of state-dependent anxiety. It is also important to acknowledge that despite using cortisol as an index of stress, only self-report measures of anxiety were used. Furthermore, while potentially elevated alexithymia and/or impaired insight among participants may have negatively impacted ability to report state-anxiety, these constructs were not directly assessed. The type of intervention limits the sample size of the cohort under study and thereby may impact the findings. The examination of multiple cohorts in a comprehensive investigation could significantly enhance the size of sample and rigor of the methods. Finally, the inclusion of cortisol as an objective measure is useful; however, it is acknowledged that substantial variability between and within subjects is an important consideration (e.g., Corbett et al., 2010; Schupp et al., 2013). Subsequent studies will address these limitations.
Future Directions
This study’s findings add to the growing body of research investigating the use of theatre as therapy for individuals with ASD (Corbett et al., 2011;Corbett et al., 2014a;Lerner et al., 2011) for impacting social competence as well as stress and anxiety. In future studies, additional physiological and self-report metrics of stress/anxiety maybe incorporated, as well as the inclusion of additional anxiety reduction techniques (e.g., mindfulness and CBT principles).
Summary
The current study extends previous findings, showing that theatre-based therapy for youth with ASD leads to enhanced social competence, and promotes decreased anxiety during social interaction. Findings also suggest that some level of arousal may be adaptive for individuals with ASD when interacting with peers. In conclusion, peer-mediated, theatre-based intervention for youth with ASD shows promise as a treatment to increase multiple domains of social competence while also reducing anxiety.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: 2013. [Google Scholar]
- Ballaban-Gil K, Rapin I, Tuchman R, et al. Longitudinal examination of the behavioral, language, and social changes in a population of adolescents and young adults with autistic disorder. Pediatric Neurology. 1996;15(3):217–223. doi: 10.1016/s0887-8994(96)00219-6. [DOI] [PubMed] [Google Scholar]
- Banda DR, Copple KS, Koul RK, et al. Video modelling interventions to teach spontaneous requesting using AAC devices to individuals with autism:a preliminary investigation. Disability and Rehabilitation. 2010;32(16):1364–72. doi: 10.3109/09638280903551525. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind?”. Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Barry TD, Klinger LG, Lee JM, et al. Examining the effectiveness of an outpatient clinic-based social skills group for high functioning children with autism. Journal of Autism and Developmental Disorders. 2003;33(6):685–701. doi: 10.1023/b:jadd.0000006004.86556.e0. [DOI] [PubMed] [Google Scholar]
- Bauminger N, Shulman C, Agam G. Peer interaction and loneliness in high-functioning children with autism. Journal of Autism and Developmental Disorders. 2003;33(5):489–507. doi: 10.1023/a:1025827427901. [DOI] [PubMed] [Google Scholar]
- Beidel DC, Turner SM, Morris TL. Behavioral treatment of childhood social phobia. Journal of Counseling and Clinical Psychology. 2000;68(6):1072–1080. [PubMed] [Google Scholar]
- Bellini S. The development of social anxiety in adolescents with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities. 2006;21:138–145. [Google Scholar]
- Billstedt E, Gillberg IC, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. Journal of Child Psychology and Psychiatry. 2007;48(11):1102–1110. doi: 10.1111/j.1469-7610.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- Bird G, Cook R. Mixed emotions: The contribution of alexithymia to the emotional symptoms of autism. Translational Psychiatry. 2013;2:e285. doi: 10.1038/tp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölte S, Feineis-Matthews S, Poustka F. Brief report: Emotional processing in high-functioning autism—physiological reactivity and affective report. Journal of Autism and Developmental Disorders. 2008;38(4):776–781. doi: 10.1007/s10803-007-0443-8. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, et al. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proceedings of the National Academy of Science. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Gunther JR, Comins D, et al. Brief report: Theatre as therapy for children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2011;41(4):505–511. doi: 10.1007/s10803-010-1064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Key AP, Qualls L, et al. Improvement in Social Competence Using a Randomized Trial of a Theatre Intervention for Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2016;46(2):658–672. doi: 10.1007/s10803-015-2600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Wegelin JA, et al. Variable cortisol circadian rhythms in children with autism and anticipatory stress. Journal of Psychiatry & Neuroscience. 2008;33(3):227–234. [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Qualls LR, Valencia B, et al. Peer-mediated theatrical engagement for improving reciprocal social interaction in autism spectrum disorder. Frontiers in pediatrics. 2014a;2:110. doi: 10.3389/fped.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Simon D, et al. Elevated cortisol during play is associated with age and social engagement in children with autism. Molecular Autism. 2010;1(1):13. doi: 10.1186/2040-2392-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Swain DM, Coke C, et al. Improvement in Social Deficits in Autism Spectrum Disorders Using a Theatre-Based, Peer-Mediated Intervention. Autism Research. 2014b;7(1):4–16. doi: 10.1002/aur.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Swain DM, Newsom C, Wang L, et al. Biobehavioral profiles of arousal and social motivation in autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2014c;55(8):924–934. doi: 10.1111/jcpp.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial JT, Wood JJ. Cognitive behavioral therapy for children with autism: Review and considerations for future research. Journal of Developmental & Behavioral Pediatrics. 2013;34(9):702–715. doi: 10.1097/DBP.0b013e31829f676c. [DOI] [PubMed] [Google Scholar]
- Eigsti I, Shapiro T. A systems neuroscience approach to autism: biological, cognitive, and clinical perspectives. Mental Retardation and Developmental Disabilities Research Reviews. 2003;9(3):206–216. doi: 10.1002/mrdd.10081. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Science. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Goldstein TR. Correlations among social-cognitive skills in adolescents involved in acting or arts classes. Mind, Brain & Education. 2011;5:97–103. [Google Scholar]
- Groden J, Baron MG, Groden G. Stress and autism: assessment and coping strategies. In: Baron MG, Groden J, Groden G, Lipsitt LP, editors. Stress and coping in autism. New York: Oxford University Press; 2006. pp. 15–51. [Google Scholar]
- Gunnar MR, Sebanc AM, Tout K, Donzella B, van Dulmen MM. Peer rejection, temperament, and cortisol activity in preschoolers. Developmental Psychobiology. 2003;43(4):346–368. doi: 10.1002/dev.10144. [DOI] [PubMed] [Google Scholar]
- Hubert W, de Jong-Meyer R. Psychophysiological response patterns to positive and negative film stimuli. Biological Psychology. 1990;31:73–93. doi: 10.1016/0301-0511(90)90079-c. [DOI] [PubMed] [Google Scholar]
- Julian LJ. Measures of Anxiety. Arthritis Care and Research (Hoboken) 2011;63(11):S467–S472. doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps DM, Leonard BR, Vernon S, et al. Teaching social skills to students with autism to increase peer interactions in an integrated first-grade classroom. Journal of Applied Behavior Analysis. 1992;25(2):281–288. doi: 10.1901/jaba.1992.25-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasari C, Rotheram-Fuller E, Locke J, et al. Making the connection: randomized controlled trial of social skills at school for children with autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2012;53(4):431–439. doi: 10.1111/j.1469-7610.2011.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19(4):313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, et al. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic medicine. 1995;57(1):23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Harada Y, Yamazaki T, Kazuhiko S. Psychosocial problems in attention deficit hyperactive disorder with oppositional defiant disorder. Psychiatry and Clinical Neuroscience. 2002;56(4):365–369. doi: 10.1046/j.1440-1819.2002.01024.x. [DOI] [PubMed] [Google Scholar]
- Hayes AF. SPSS, SAS, and Mplus macros and code. Andrew F. Hayes, Ph. D 2013 [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neuroscience. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hodges K. Depression and anxiety in children: A comparison of self-report questionnaires to clinical interview. Psychological Assessment. 1990;2(4):376–381. [Google Scholar]
- Howard BL, Kendall PC. Cognitive behavioral family therapy for anxiety- disordered children: A multiple baseline evaluation. Cognitive Therapy and Research. 1996;20(5):423–443. [Google Scholar]
- Lane R, Ahern G, Schwartz G, Kaszniak A. Is alexithymia the emotional equivalent of blindsight? Biological Psychiatry. 1997;42:834–844. doi: 10.1016/s0006-3223(97)00050-4. [DOI] [PubMed] [Google Scholar]
- Lanni KE, Schupp CW, Simon D, Corbett BA. Verbal ability, social stress, and anxiety in children with autistic disorder. Autism. 2012;16(2):123–138. doi: 10.1177/1362361311425916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner MD, Mikami AY, Levine K. Socio-dramatic affective-relational intervention for adolescents with asperger syndrome & high functioning autism: pilot study. Autism. 2011;15(1):21–42. doi: 10.1177/1362361309353613. [DOI] [PubMed] [Google Scholar]
- Lopata C, Volker MA, Putnam SK, Thomeer ML, Nida RE. Effect of social familiarity on salivary cortisol and self-reports of social anxiety and stress in children with high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38:1866–1877. doi: 10.1007/s10803-008-0575-5. [DOI] [PubMed] [Google Scholar]
- Lord C, MaGill-Evans J. Peer interactions of autistic children and adolescents. Development and Psychopathology. 1995;7(4):611–626. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, et al. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lozier LM, Vanmeter JW, Marsh AA. Impairment in facial affect recognition associated with autism spectrum disorders:a meta-analysis. Developmental Psychopathology. 2014;26:933–45. doi: 10.1017/S0954579414000479. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Vasa RA, Kalb LG, et al. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2013;41(1):165–176. doi: 10.1007/s10802-012-9668-x. [DOI] [PubMed] [Google Scholar]
- Mikita N, Hollocks MJ, Papadopoulos AS, et al. Irritability in boys with autism spectrum disorders: an investigation of physiological reactivity. Journal of Child Psychology and Psychiatry. 2015;56(10):1118–1126. doi: 10.1111/jcpp.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey JN, Boggero IA, Scott AB, et al. Current directions in stress and human immune function. Current Opinion in Psychology. 2015;5:13–17. doi: 10.1016/j.copsyc.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Meesters C. Children’s somatization symptoms: Correlation with trait anxiety, anxiety sensitivity, and learning experiences. Psychological Reports. 2004;94(3):1269–1275. doi: 10.2466/pr0.94.3c.1269-1275. [DOI] [PubMed] [Google Scholar]
- Muris P, Merckelbach H, Ollendick T, et al. Three traditional and three new childhood anxiety questionnaires: Their reliability and validity in a normal adolescent sample. Behaviour Research and Therapy. 2002;40:753–772. doi: 10.1016/s0005-7967(01)00056-0. [DOI] [PubMed] [Google Scholar]
- Noldus. The Observer XT (Vol. 10.5) Wageningen, The Netherlands: Noldus Information Technology; 2008. [Google Scholar]
- O'Connor TM, O'Halloran DJ, Shanahan F. The stress response and the hypothalamic–pituitary–adrenal axis: from molecule to melancholia. Quarterly Journal of Medicine. 2000;93:323–333. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- Reaven J, Blakeley-Smith A, Culhane-Shelburne K, et al. Group cognitive behavior therapy for children with high-functioning autism spectrum disorders and anxiety: A randomized trial. Journal of Child Psychology and Psychiatry. 2012;53(4):410–419. doi: 10.1111/j.1469-7610.2011.02486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renno P, Wood JJ. Discriminant and convergent validity of the anxiety construct in children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013;43(9):2135–2146. doi: 10.1007/s10803-013-1767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb SL, Nichols RJ, Rutan RL, et al. The Effects of Music Assisted Relaxation on Preoperative Anxiety. Journal of Music Therapy. 1995;32:2–21. [Google Scholar]
- Rodgers J, Glod M, Connolly B, et al. The relationship between anxiety and repetitive behaviours in autism spectrum disorder. Journal of autism and developmental disorders. 2012;42(11):2404–2409. doi: 10.1007/s10803-012-1531-y. [DOI] [PubMed] [Google Scholar]
- Schupp CW, Simon D, Corbett BA. Cortisol responsivity differences in children with autism spectrum disorders during free and cooperative play. Journal of Autism and Developmental Disorders. 2013;43:2405–24. doi: 10.1007/s10803-013-1790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;7:284–301. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Seligman LD, Ollendick TH, Langley AK, et al. The utility of measures of child and adolescent anxiety: A meta-analytic review of the Revised Children's Manifest Anxiety Scale, the State–Trait Anxiety Inventory for Children, and the Child Behavior Checklist. Journal of Clinical Child and Adolescent Psychology. 2004;33(3):557–565. doi: 10.1207/s15374424jccp3303_13. [DOI] [PubMed] [Google Scholar]
- Simon DM, Corbett BA. Examining associations between anxiety and cortisol in high functioning male children with autism. Journal of Neurodevelopmental Disorders. 2013;5(1):32. doi: 10.1186/1866-1955-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, et al. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Edwards CD, Lushene R, et al. STAIC Preliminary Manual. Palo Alto, CA: Consulting Psychologist Press; 1973. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, et al. State-Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- Spielberger CD, Sydeman SJ, Owen AE, et al. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI) In: Marsh ME, editor. The Use of Psychological Testing for Treatment Planning and Outcome Assessment. 2. Mahwah, NJ: Lawrence Erlbaum Associates, Publishers; 1999. pp. 993–1023. [Google Scholar]
- Spratt EG, Nicholas JS, Brady KT, et al. Enhanced cortisol response to stress in children in autism. Journal of Autism and Developmental Disorders. 2012;42(1):75–81. doi: 10.1007/s10803-011-1214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolsky DG, Scahill L, Gadow KD, et al. Parent-rated anxiety symptoms in children with pervasive developmental disorders: Frequency and association with core autism symptoms and cognitive functioning. Journal of Abnormal Child Psychology. 2008;36(1):117–128. doi: 10.1007/s10802-007-9165-9. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Miawaki D, Kawaguchi T, et al. Relationship of hypersensitivity to anxiety and depression in children with high functioning pervasive developmental disorder. Psychiatry and Clinical Neuroscience. 2009;63(2):195–201. doi: 10.1111/j.1440-1819.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- van Steensel FJ, Bögels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: A meta-analysis. Clinical Child and Family Psychology Review. 2011;14(3):302–317. doi: 10.1007/s10567-011-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasey MW, Daleiden EL, Williams LL, et al. Biased attention in childhood anxiety disorders: A preliminary study. Journal of Abnormal Child Psychology. 1995;23(2):267–279. doi: 10.1007/BF01447092. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Miles J, Bennett P, et al. An investigation into the relationship between salivary cortisol, stress, anxiety and depression. Biological psychology. 2003;62(2):89–96. doi: 10.1016/s0301-0511(02)00128-x. [DOI] [PubMed] [Google Scholar]
- Viau R, Arsenault-Lapierre G, Fecteau S, et al. Effect of service dogs on salivary cortisol secretion in autistic children. Psychoneuroendocrinology. 2010;35:1187–1193. doi: 10.1016/j.psyneuen.2010.02.004. [DOI] [PubMed] [Google Scholar]
- White SW, Ollendick T, Albano AM, et al. Randomized controlled trial: Multimodal anxiety and social skill intervention for adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2013;43(2):382–394. doi: 10.1007/s10803-012-1577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Oswald D, Ollendick T, et al. Anxiety in children and adolescents with autism spectrum disorders. Clinical Psychology Review. 2009;29(3):216–229. doi: 10.1016/j.cpr.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerkes R, Dodson J. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18:459–482. [Google Scholar]