Abstract
Introduction
Hepatic artery infusion pump (HAIP) placement is associated with improved outcomes in the treatment of colorectal liver metastases (CRLM). In this study, we examined outcomes following robotic HAIP placement, which were compared with open and laparoscopic placement.
Methods
A retrospective review of HAIP placements by 2 surgeons at a single institution was carried out from a prospectively maintained institutional cancer database. The institutional review board approved this HIPAA-compliant study. All statistical tests were 2-sided and p < 0.05 was considered significant.
Results
There were a total of 53 open HAIP cases, 21 laparoscopic cases, and 24 robotic cases. There were no statistically significant differences between the 3 groups in gender, age, body mass index, aberrant arterial anatomy, disease process being treated, or prior treatment, including chemotherapy, or prior abdominal or hepatic surgical intervention (p > 0.05). Robotic HAIP placement was associated with a significantly lower conversion rate to open operation than laparoscopic pump placement (17 vs. 67%; p = 0.0009). When cases with concomitant resections were excluded, there was a trend towards shorter median length of hospital stay with robotic pump placement compared with open and laparoscopic placement (4 vs. 5 vs. 5 days, respectively; p = 0.09). Complication rates were equivalent among the 3 groups when concomitant resections were excluded.
Conclusion
Robotic HAIP placement is a safe minimally-invasive procedure that is associated with a significantly lower conversion rate to open operation compared with laparoscopic placement and a trend towards shorter hospitalization compared to open.
Introduction
Colorectal cancer is the third most common malignancy in the United States (US), and up to 50% of patients will develop metastatic disease at some point during the course of their disease.1 The liver is the most common site of metastatic disease, and is involved approximately 80% of the time. Multimodal medical and surgical therapies for the treatment of metastatic disease have significantly evolved over the last 2 decades. The treatment of metastatic disease localized to the liver has allowed for the successful addition of highly effective regional hepatic therapies, such as hepatic arterial infusion pump (HAIP) therapy, which have contributed to increasing response and survival rates, including disease cure.2
In a prospective randomized controlled study performed by Kemeny and colleagues, the use of systemic 5-fluorouracil (5-FU) chemotherapy with and without HAIP FUDR therapy was evaluated.3 Two year overall survival (OS) with combined HAIP and systemic therapy was 86%, compared with 72% in the monotherapy arm (p = 0.03). Overall and hepatic progression-free survival (PFS) differences remained statistically significant at 10 years in a subsequent analysis.4 Two additional randomized trials reported an increase in PFS with the use of HAIP.5,6 In a recent phase II trial published by our group, the conversion rate of unresectable CRLM to surgically resectable disease was evaluated using combined HAIP therapy and systemic therapy.7 Forty-nine patients with unresectable disease were included, and conversion to resection, which was the primary outcome, occurred in 47% of patients. These findings established regional HAIP therapy as an option in the treatment of colorectal liver metastases (CRLM). While data for use of HAIP therapy in intrahepatic cholangiocarcinoma (ICC) is less established, early survival data from prospective trials at the Memorial Sloan Kettering Cancer Center (MSKCC) appeared promising.8 A recent retrospective study that evaluated the role of added HAIP therapy in the treatment of unresectable intrahepatic cholangiocarcinoma revealed a statistically significant improvement in OS among 78 patients treated with HAIP and systemic therapy compared to 26 patients who underwent systemic therapy alone (30.8 months vs. 18.4 months; p < 0.001).9
Given the role of minimally-invasive surgery in improving outcomes of some surgical procedures,10–12 surgeons have explored minimally-invasive placement of pumps.13–17 In this report, we provide a descriptive analysis of our series of robotic HAIP placements with a comparison to our open and laparoscopic experience at the MSKCC.
Methods
Before pump placement, all patients underwent multidetector computed-tomography (CT) cross-sectional angiographic imaging in order to thoroughly evaluate disease extent within the liver, to confirm the absence of extrahepatic disease, and to delineate hepatic arterial anatomy. Arterial anatomy was categorized as conventional or variant, with the latter including left and right variance, and further categorized as accessory or replaced. The origin of the GDA was also recorded. Pertinent details related to pump placement at our institution have been previously reported.18,19 Patients underwent pump placement either as an isolated procedure (“HAIP alone”) or in conjunction with another procedure, such as colon or hepatic resection.
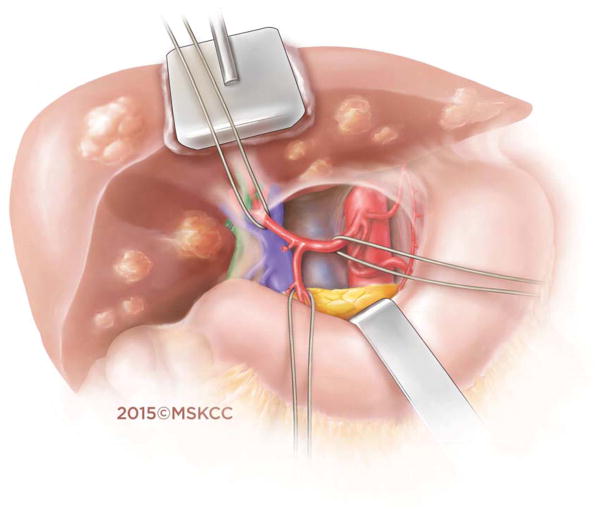
Open surgical placement of the HAIP involved dissection of the hepatic arterial tree, identification of the gastroduodenal artery (GDA), and ligation of all branches in order to prevent extrahepatic perfusion (Figure 1a). Cholecystectomy was routinely carried out in order to prevent the development of chemical cholecystitis following the administration of regional chemotherapy.20 The pump (Codman 3000 series, Raynham, MA) was placed within a subcutaneous pocket (Figure 1b). The distal GDA was ligated and vascular control established by placement of bulldog clamps on the proximal and distal aspects of the common hepatic artery. A distal transverse arteriotomy was then made in the GDA, through which the pump catheter was inserted. The pump catheter was secured in the GDA with silk ties around the catheter beads. Accessory and replaced vessels were ligated. An intraoperative dye test with methylene blue was used to confirm complete hepatic perfusion and rule out extrahepatic perfusion.
Figure 1.
Figure 1a. Hepatic arterial anatomy in preparation for placement of the hepatic arterial infusion pump catheter.
Figure 1b. Precise placement of the hepatic arterial infusion pump catheter into the gastroduodenal artery at the confluence of the common hepatic artery.
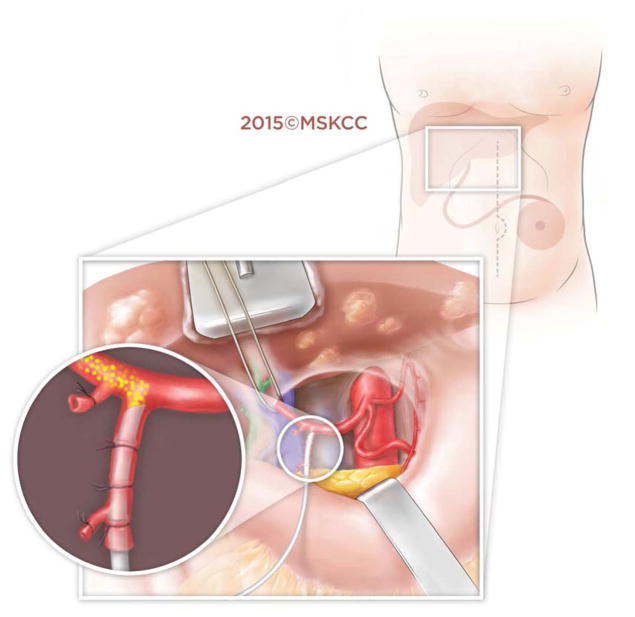
In the robotic approach, we mimicked the open approach and modified it for robotics by marking out the pump pocket prior to incision to ensure no ports were placed in the pocket. We used an unmounted 11-blade to create a precise arteriotomy (Figure 2a). The catheter was secured in similar fashion to the open approach with silk ligatures (Figure 2b). Following pump insertion, perfusion of the liver was similarly confirmed with an injection of methylene blue through the pump (Figure 2c). Port locations are shown in the diagram below (Figure 2d). Finally, injection of long acting local analgesics into the abdominal wall was carried out at the completion of the robotic case.
Figure 2.
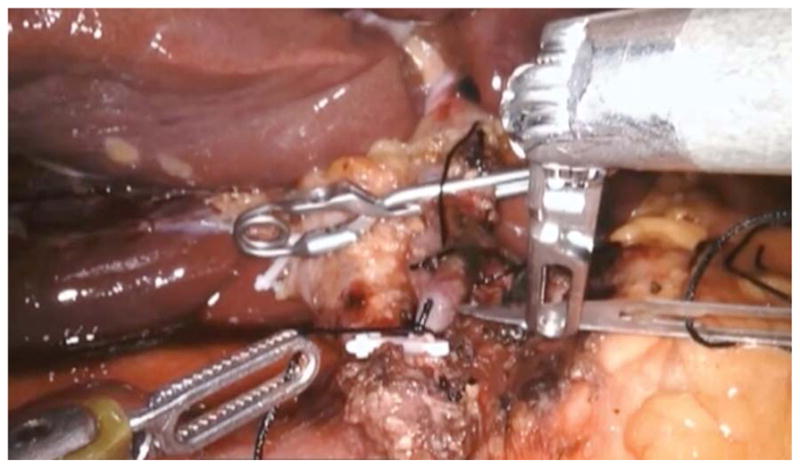
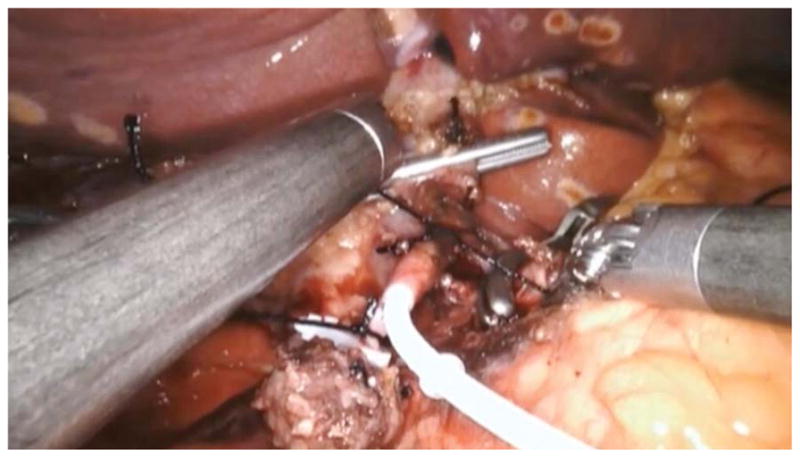
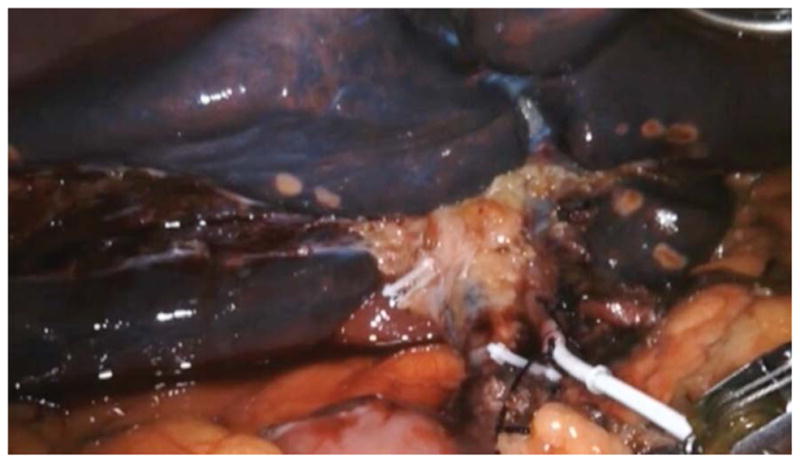
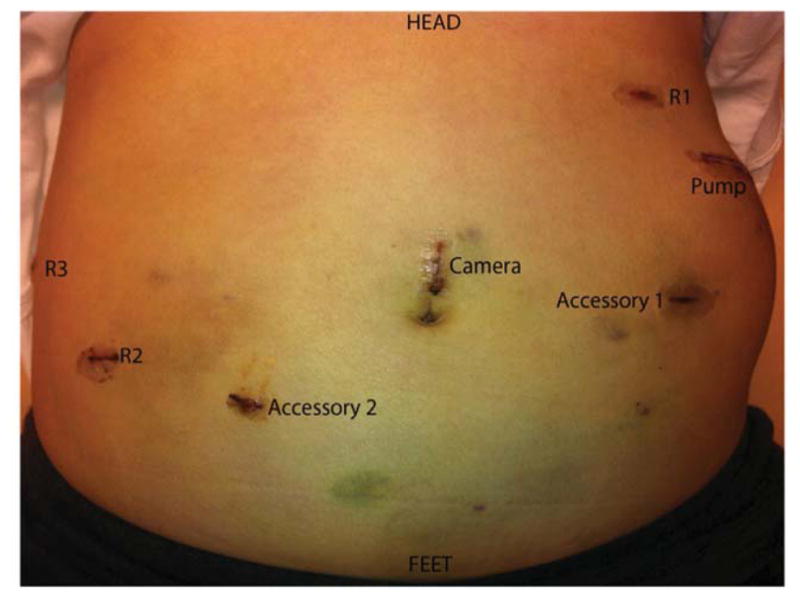
Figure 2a. Still image during robotic hepatic arterial infusion pump placement taken at the point of making an arteriotomy in the gastroduodenal artery.
Figure 2b. Still image during robotic hepatic arterial infusion pump placement taken during securing of the catheter in the in the gastroduodenal artery, which is placed at the confluence of the common hepatic artery.
Figure 2c. Methylene blue injection that is instilled to demonstrate the absence of extrahepatic perfusion in the duodenum, distal stomach, and adjacent lymph nodes, in addition to confirming hepatic cross perfusion.
Figure 2d. Port placement in relation to subcutaneous pocket created for hepatic arterial infusion pump.
Study Design
Approval was obtained from the MSKCC Institutional Review Board. The division’s operative database was queried for patients who underwent HAIP placement by 2 surgeons from July 2003 (1st laparoscopic case) to January 2016. Cases for open and robotic HAIP placement by a single surgeon (TPK) were included and compared with laparoscopic cases previously performed by a single surgeon at the institution (MID). Open cases were carried out from March 2011 to May 2015. Laparoscopic cases were carried out from July 2003 to July 2007. Robotic cases were carried out from April 2011 to January 2016. Patients with all pathological diagnoses were included, including CRLM, hepatocellular carcinoma (HCC), ICC, and metastatic breast disease. There were no cases excluded from analysis.
Data collection was divided into 3 sections. Patient characteristics and demographics were collected and included gender, age, body mass index (BMI), American Society of Anesthesiologists’ (ASA) score, pathologic diagnosis, prior chemotherapy, prior radiation therapy, prior abdominal surgery, prior hepatic surgery, and any aberrant arterial anatomy. Next, intraoperative data were collected, and this included conversion to open HAIP placement for robotic and laparoscopic cases, duration of operation, estimated blood loss (EBL), and the rate of concomitant procedures, including simultaneous hepatic resection, colorectal resection, or any other procedures. Finally, postoperative data were collected, and included length of stay (LOS), the number of patients with nuclear magnetic (NM) perfusion scans as inpatients, number of days to NM perfusion scan, the rate of abnormal NM perfusion scans (requiring angiographic evaluation), and complications. Operative morbidity was recorded and graded in the MSKCC surgical events database, which uses a severity scale that has been previously described.21 Where relevant, cases with simultaneous resections were systematically excluded to allow for analysis of HAIP placement solely.
Data Analysis
The study design was a non-matched observational case series. Descriptive and comparative statistics were performed using GraphPad Prism 6 (2013 GraphPad Software, La Jolla, CA). For 2-column parametric comparisons, a t-test was used. For 2-column non-parametric comparisons, the Mann-Whitney test was used. For 3-column parametric comparisons, a one-way analysis of variance (ANOVA) test was used. For 3-column non-parametric comparison, the Kruskal-Wallis test was used. For comparison of proportions, Chi-squared was used, and Fisher’s exact test was utilized were values were < 5. Finally, for parametric and non-parametric multiple comparisons, the Tukey and Dunn tests were used, respectively. All statistical tests were 2-sided and p < 0.05 was considered statistically significant.
Results
There were a total of 53 open HAIP placements, 21 laparoscopic, and 24 robotic cases. Median follow-up was 20.7 months, 27.8 months, and 12.1 months, respectively. Overall median follow-up was 16.7 months. Pumps were initially placed robotically in 4 patients in 2011. One patient had a hepatic artery dissection 3 months after the pump was placed and a second patient had a GDA bleed from a pseudoaneurysm 9 weeks after following pump placement. While these complications are known to happen after HAIP placement regardless of approach, the program was temporarily halted. It was reinitiated in 2013.
There were no statistically significant differences between the 3 groups in gender, age, BMI, aberrant arterial anatomy rates, disease process being treated, prior chemotherapy or radiation rates, or any prior abdominal or hepatic surgical intervention (Table 1). Median ASA was higher in the open group, which was statistically significant.
Table 1.
Patient demographics
| Parameter | Open | Laparoscopic | Robotic | p-Value |
|---|---|---|---|---|
| All Cases (n = 53) HAIP Alone (n = 22) |
All Cases (n = 21) HAIP Alone (n = 20) |
All Cases (n = 24) HAIP Alone (n = 16) |
||
| Male | 31 | 13 | 13 | 0.87 |
| Mean Age | 57.0 | 54.4 | 58.0 | 0.60 |
| Mean BMI | 28.0 | 26.9 | 29.6 | 0.35 |
| Median ASA Score | 3 | 2 | 2 | < 0.01 |
| CRLM | 42 | 14 | 16 | 0.49 |
| Prior Chemotherapy/Radiation | 38 (71.7) | 12 (57.1) | 16 (66.7) | 0.49 |
| Prior Abdominal Surgery | 31 (58.5) | 17 (81.0) | 14 (58.3) | 0.17 |
| Prior Hepatic Surgery | 6 (11.3) | 0 (0.0) | 1 (4.2) | 0.19 |
| Aberrant Arterial Anatomy | 17 (32.1) | 4 (19.0) | 8 (33.3) | 0.49 |
BMI = body mass index, ASA = American society of anesthesiologists, CRLM = colorectal liver metastases
Robotic HAIP placement was associated with a significantly lower conversion rate to open operation than laparoscopic pump placement (17 vs. 67%; p < 0.01) (Table 2). In the robotic series, there were a total of 4 conversions to open pump placement among 24 patients, of which 2 occurred in cases with concomitant hepatic resection. Three of these conversions occurred among the first 12 patients, as familiarity with the robotic procedure was developing.
Table 2.
Operative data
| Parameter | Open | Laparoscopic | Robotic | p-Value |
|---|---|---|---|---|
| All Cases (n = 53) HAIP Alone (n = 22) |
All Cases (n = 21) HAIP Alone (n = 20) |
All Cases (n = 24) HAIP Alone (n = 16) |
||
| Conversions to Open | -- | 14 (66.7) | 4 (16.7) | < 0.01 |
| Mean OR Time | 221 | 246 | 297 | < 0.01 |
| Mean OR Time HAIP Alone | 154 | 237 | 272 | < 0.01 |
| Mean EBL | 369 | 160 | 170 | < 0.01 |
| Mean EBL HAIP Alone | 200 | 168 | 81 | 0.10 |
| Concomitant Hepatectomy | 24 (45.3) | 0 (0.0) | 4 (16.7) | < 0.01 |
| Concomitant Colectomy | 12 (22.6) | 1 (4.8) | 4 (16.7) | 0.19 |
OR = operating room, HAIP = hepatic arterial infusion pump, EBL = estimated blood loss
In cases including concomitant liver, colorectal, or other resections, mean operating room (OR) time was highest during robotic HAIP placement. Similarly, when cases with concomitant resections were excluded in order to evaluate the effect of HAIP placement alone, mean OR time was statistically significantly highest during robotic placement, followed by laparoscopic placement, and then open placement (272 vs. 237 vs. 154 minutes; p < 0.01). There was no difference between robotic and laparoscopic OR time on multiple comparisons analysis.
With respect to EBL, open HAIP placement was associated with the highest volume of blood loss compared with both open and laparoscopic placement (p < 0.01). However, when patients with concomitant resections were excluded to evaluate EBL associated with HAIP placement alone, there was no difference between the 3 groups. There was, however, a trend towards increased blood loss with open placement and laparoscopic placement compared with robotic placement. There were fewer simultaneous hepatectomies carried out with the minimally-invasive approaches compared with open HAIP placement (p < 0.01). However, there was no difference in the rate of colorectal resections or other procedures between the 3 groups (p = 0.19).
In terms of hospital LOS, patients who underwent minimally-invasive HAIP placement had significantly shorter median LOS compared with patients who underwent open placement (p < 0.01) (Table 3). This was likely a result of simultaneous hepatic resections, which were significantly more frequent in the open group. When patients with concomitant resections were excluded, there was no longer any difference between LOS among the 3 groups. However, there was a trend towards shorter median hospitalization with robotic placement, compared with laparoscopic and open placement (4 vs. 5 vs. 5 days, respectively; p = 0.09).
Table 3.
Postoperative data and complications
| Parameter | Open | Laparoscopic | Robotic | p-Value |
|---|---|---|---|---|
| All Cases (n = 53) HAIP Alone (n = 22) |
All Cases (n = 21) HAIP Alone (n = 20) |
All Cases (n = 24) HAIP Alone (n = 16) |
||
| Median LOS | 6 | 5 | 4 | < 0.01 |
| Median LOS HAIP Alone | 5 | 5 | 4 | 0.09 |
| Patients with Inpatient NM Scan | 51 (96.2) | 21 (100) | 16 (66.7) | < 0.01 |
| Mean Days to NM Scan | 5.3 | 3.3 | 3.9 | < 0.01 |
| Patients with abnormal NM Scan | 2 | 1 | 1 | 0.98 |
| Complications | 40 | 5 | 19 | 0.02 |
| Grades 1–2 | 27 (67.5) | 5 (100) | 15 (78.9) | 0.26 |
| Grades 3–5 | 13 (32.5) | 0 (0.0) | 4 (21.1) | 0.11 |
| Complications HAIP Alone | 13 | 5 | 11 | 0.16 |
| Grades 1–2 | 10 (76.9) | 5 (100) | 9 (81.8) | 0.66 |
| Grades 3–5 | 3 (23.1) | 0 (0.0) | 2 (18.2) | 0.14 |
LOS = length of stay, HAIP = hepatic arterial infusion pump, NM = nuclear medicine
Patients who underwent minimally-invasive procedures were more likely to have their liver NM perfusion scan (to rule out extrahepatic perfusion) sooner than patients who underwent open placement (p < 0.01). There were no differences in the number of abnormal NM perfusion scans (requiring angiographic evaluation) between open, laparoscopic, and robotic pump placement (2 vs. 1 vs. 1, respectively; p = 0.98) (Table 3).
While complication rates appeared to be significantly higher in patients who underwent open and robotic HAIP placement compared with laparoscopic patients (p = 0.02), it is important to note that there were no simultaneous hepatectomies carried out in the laparoscopic group (Table 3). When cases with concomitant resections were excluded from analysis, there were no differences in complications between the 3 groups (p = 0.16).
Finally, when patients who underwent HAIP placement without concomitant procedures were divided equally into 2 time-periods (early vs. late) in order to evaluate the learning curve specifically associated with robotic pump placement, there were 8 patients present in each time-period for evaluation (Table 4). Although there were no statistically significant differences between the 2 groups, there was a trend towards lower median LOS (5.5 vs. 3.5 days; p = 0.37) and lower median OR time (282 vs. 232 minutes; p = 0.30) in the later time-period group compared with the earlier group.
Table 4.
Case series divided into 2 equal time-periods (first half vs. second half)
| HAIP Alone | 1st Half (n = 8) | 2nd Half (n = 8) | p-Value |
|---|---|---|---|
| Median EBL | 75.0 (25–200) | 62.5 (25–150) | 0.48 |
| Median LOS | 5.5 (2–11) | 3.5 (2–12) | 0.37 |
| Median OR Time | 282 (193–408) | 232 (214–355) | 0.30 |
HAIP = hepatic arterial infusion pump, EBL = estimated blood loss, LOS = length of stay, OR = operating room
Discussion
The rationale for HAIP therapy is based on pharmacologic and anatomic principles that permit the administration of high doses of chemotherapeutic agents, which are extracted by the liver during first-pass metabolism, such as floxuridine (FUDR). Regional infusion of FUDR results in high intrahepatic concentrations and minimal systemic effects. Furthermore, liver metastases are perfused almost exclusively by the hepatic artery, rather than the portal vein, which supplies normal hepatocytes, thereby allowing for successful regional infusional therapies that exclusively target malignant cells while sparing normal hepatocytes.22 These concepts have resulted in a unique regional approach and opportunity for the management of malignant liver disease. As interest and use of HAIP therapy has grown in recent years, a barrier to HAIP therapy has been the need for an additional laparotomy and dissection of portal structures necessary for pump placement. Developing a minimally-invasive approach may increase adoption of HAIP therapy by overcoming this barrier.
To our knowledge, this is the largest case series examining the minimally-invasive placement of HAIP in patients undergoing regional hepatic therapy for malignant liver disease. In this report, we provide a description of our initial experience with the robotic procedure. Findings were likely underpowered to detect major statistical significant differences, particularly where early trends were observed. Importantly, however, early data appear to demonstrate safety and feasibility of the robotic procedure. Furthermore, there appears to be a distinct advantage over laparoscopic placement, with a much lower conversion to open rate. We believe the combination of wristed instruments, scaling of movements to reduce tremor, magnified 3-dimensional visualization, and enhanced ergonomics of the robotic platform account for the lower conversion rate in our report. The advantages of the robotic platform in helping surgeons perform minimally-invasive procedures have previously been reported.23
Overall, and particularly where cases with concomitant procedures were excluded in order to evaluate the effects of pump placement alone, the summary of additional robotic findings included a trend towards a lower EBL, a trend towards shorter LOS, and with a similar complication rate and severity profile. While the robotic procedure was associated with a longer OR time, there appeared to be an improvement in OR time, EBL, and hospital LOS as familiarity with the procedure increased. During the development of the robotic program for pump placement at our institution, patients were initially maintained on the traditional pathways in the postoperative setting, until comfort level with the minimally-invasive procedure and outcomes increased. Once the safety profile of the procedure was assured, patients were transitioned to an expedited recovery pathway including minimal narcotics, early feeding, and early discharge, such that patients are now routinely discharged on the second or third postoperative day.
There have been few reports describing minimally-invasive HAIP placement. In a report by Hellen and colleagues, the authors first described robotic placement of the HAIP.16 In their report, the authors defined the novel technique, which was performed similarly to our procedure described above. The exact number of patients who had undergone robotic HAIP placement was not disclosed. Patients were discharged following the procedure on postoperative days 1 or 2, once tolerating a regular diet, and pump therapy was commenced within 1 week of placement. The authors confirmed that the enhanced degrees of freedom, elimination of tremor, the 3-dimensional stereoscopic vision with depth perception, and ergonomic setup all contributed to the success of robotic HAIP placement, while overcoming many of the shortfalls of the laparoscopic approach.
A subsequent case series describing a similar technical procedure and outcomes in 24 patients who underwent robotic HAIP placement was recently published, along with a separate brief technical report.24,25 Although there were no open or laparoscopic groups provided for comparison, the authors provided a description of outcomes that were largely similar to ours. Mean operative time was 282 minutes with a median blood loss of 100ml and a 6-day hospitalization. There was 1 conversion to open HAIP placement. The incidence of grade 3 complications was 13% and pump-related complications occurred in 21% in patients.
In a study by Franklin and colleagues, the authors provided a description of laparoscopic hepatic arterial catheter placement for delivery of regional chemotherapy.15 In their report, there were 20 patients, of whom 12 had undergone prior abdominal surgery. The catheter was placed into the GDA and a subcutaneous “Macroport” was used. There were no open conversions and mean operative time was 186 minutes, with an average EBL of 132cc. Median hospital stay was 3 days, as was median time to tolerance of diet. With no intraoperative complications, the authors concluded that laparoscopic placement of a catheter for delivery of regional hepatic therapies was a safe and feasible option.
The largest laparoscopic HAIP case series was reported by Cheng and colleagues, and included 38 patients with CRLM.13 The authors reported their experience with an emphasis on variant arterial anatomy, which was present in 18 patients (47%). There was only 1 conversion to open (3%) and the authors noted that variant arterial anatomy did not increase pump complications, operative time, or blood loss. The authors confirmed the safety and feasibility of laparoscopic HAIP placement, including in patients with variable arterial anatomy. These included patients with replaced right hepatic arteries that were dissected free from the porta hepatis and ligated to ensure all hepatic arterial inflow was from a vessel supplied with HAIP chemotherapy. In our robotic series, there were no open conversions among 8 patients with variant arterial anatomy, thereby confirming the feasibility of the robotic approach in patients with variant anatomy.
Limitations of our study include the small numbers of cases and limited power to detect significant differences between the groups. Most notably, concomitant procedures, such as hepatectomy, occurred more frequently during open HAIP placement. There were no concomitant hepatectomies performed with laparoscopic placement as the surgeon who performed the procedures did not feel it was feasible or time-efficient to perform both laparoscopic pump placement and hepatectomy. In order to account for the different hepatectomy rates (and other concomitant procedures) between the study arms, separate analyses were performed to exclude cases with concomitant resections in order to provide a more helpful assessment of HAIP placement alone based on surgical approach. However, we also acknowledge the selection bias when comparing robotic cases with open cases performed by a single surgeon, given that a proportion of these open cases would not have been suitable for minimally-invasive surgery due to an added degree of complexity, and potentially accounting for some of the differences seen. Such limitations are due to the retrospective nature of this analysis. At this time, we are no longer performing laparoscopic pump placement, given the high conversion rate associated with this technically challenging procedure. Laparoscopic pump placement is more difficult than robotic placement given that instruments do not reticulate. The movements needed to dissect the GDA, place the catheter in the ideal location, and secure it in place, we believe, are easier with the robotic platform.
In our case series, we provide a report of the safety and feasibility of robotic HAIP placement in patients with malignant liver disease. We believe that robotic HAIP placement is a safe minimally-invasive procedure that is associated with a significantly lower conversion rate to open surgery compared with laparoscopic placement, and a trend towards shorter hospitalization in well selected patients. With longer follow-up and additional patients, it is possible that we will be able to determine if patients have a lower hernia rate, earlier return to work, quicker initiation of chemotherapy, and fewer adhesions when additional hepatic surgery is required. Wristed instruments as well as improved visualization make the robotic platform an easier minimally-invasive approach for HAIP placement than laparoscopic placement. Robotic HAIP placement is worthy of continued analysis and further study.
Acknowledgments
This study was supported in part by NIH/NCI P30 CA0088748 (Cancer Center Support Grant).
Footnotes
The authors have no conflicts of interest, financial or otherwise, to disclose.
This is an original article. This work was presented at the Digestive Disease Week in San Diego, CA on Sunday May 22, 2016
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Qadan M, D’Angelica MI. Complex surgical strategies to improve resectability in borderline-resectable disease. Curr Colorectal Cancer Rep. 2015;11:369–377. doi: 10.1007/s11888-015-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kemeny NE, Chou JF, Boucher TM, et al. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J Surg Oncol. 2016;113:477–484. doi: 10.1002/jso.24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 4.Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med. 2005;352:734–735. doi: 10.1056/NEJM200502173520723. [DOI] [PubMed] [Google Scholar]
- 5.Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. J Clin Oncol. 2002;20:1499–1505. doi: 10.1200/JCO.2002.20.6.1499. [DOI] [PubMed] [Google Scholar]
- 6.Lygidakis NJ, Sgourakis G, Dedemadi G, et al. Regional chemoimmunotherapy for nonresectable metastatic liver disease of colorectal origin. A prospective randomized study. Hepatogastroenterology. 2001;48:1085–1087. [PubMed] [Google Scholar]
- 7.D’Angelica MI, Correa-Gallego C, Paty PB, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg. 2015;261:353–360. doi: 10.1097/SLA.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konstantinidis IT, Do RK, Gultekin DH, et al. Regional chemotherapy for unresectable intrahepatic cholangiocarcinoma: a potential role for dynamic magnetic resonance imaging as an imaging biomarker and a survival update from two prospective clinical trials. Ann Surg Oncol. 2014;21:2675–2683. doi: 10.1245/s10434-014-3649-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstantinidis IT, Groot KB, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122:758–765. doi: 10.1002/cncr.29824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 11.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 12.Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 13.Cheng J, Hong D, Zhu G, et al. Laparoscopic placement of hepatic artery infusion pumps: technical considerations and early results. Ann Surg Oncol. 2004;11:589–597. doi: 10.1245/ASO.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Feliciotti F, Paganini A, Guerrieri M, et al. Laparoscopic intra-arterial catheter implantation for regional chemotherapy of liver metastasis. Surg Endosc. 1996;10:449–452. doi: 10.1007/BF00191639. [DOI] [PubMed] [Google Scholar]
- 15.Franklin ME, Jr, Gonzalez JJ., Jr Laparoscopic placement of hepatic artery catheter for regional chemotherapy infusion: technique, benefits, and complications. Surg Laparosc Endosc Percutan Tech. 2002;12:398–407. doi: 10.1097/00129689-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Hellan M, Pigazzi A. Robotic-assisted placement of a hepatic artery infusion catheter for regional chemotherapy. Surg Endosc. 2008;22:548–551. doi: 10.1007/s00464-007-9496-1. [DOI] [PubMed] [Google Scholar]
- 17.Urbach DR, Herron DM, Khajanchee YS, et al. Laparoscopic hepatic artery infusion pump placement. Arch Surg. 2001;136:700–704. doi: 10.1001/archsurg.136.6.700. [DOI] [PubMed] [Google Scholar]
- 18.Allen PJ, Stojadinovic A, Ben-Porat L, et al. The management of variant arterial anatomy during hepatic arterial infusion pump placement. Ann Surg Oncol. 2002;9:875–880. doi: 10.1007/BF02557524. [DOI] [PubMed] [Google Scholar]
- 19.Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. 2005;201:57–65. doi: 10.1016/j.jamcollsurg.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Curley SA, Hohn DC, Roh MS. Hepatic artery infusion pumps: cannulation techniques and other surgical considerations. Langenbecks Arch Chir. 1990;375:119–124. doi: 10.1007/BF00713397. [DOI] [PubMed] [Google Scholar]
- 21.DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–937. doi: 10.1097/01.sla.0000246856.03918.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.BREEDIS C, YOUNG G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 23.Perer E, Lee DI, Ahlering T, et al. Robotic revelation: laparoscopic radical prostatectomy by a nonlaparoscopic surgeon. J Am Coll Surg. 2003;197:693–696. doi: 10.1016/S1072-7515(03)00723-3. [DOI] [PubMed] [Google Scholar]
- 24.Dhir M, Zenati MS, Padussis JC, et al. Robotic assisted placement of hepatic artery infusion pump is a safe and feasible approach. J Surg Oncol. 2016;114:342–347. doi: 10.1002/jso.24325. [DOI] [PubMed] [Google Scholar]
- 25.Dhir M, Magge D, Novak S, et al. Robotic-Assisted Placement of an Hepatic Artery Infusion Pump and Catheter for Regional Chemotherapy of the Liver. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5484-9. [DOI] [PubMed] [Google Scholar]