Abstract
Objective
Preeclampsia (PE) is a complication affecting pregnant women worldwide, which usually manifests as severe maternal hypertension. Resveratrol (RESV), a naturally existing polyphenol, is known to exhibit beneficial effects in cardiovascular disease including hypertension. We evaluated the outcome of treatment combining oral nifedipine (NIFE) and RESV against PE.
Design and methods
Using a randomized group assignment, 400 PE patients were enrolled and received oral treatments of either NIFE + RESV or NIFE + placebo. Primary endpoints were defined as time to control blood pressure and time before a new hypertensive crisis. Secondary endpoints were defined as the number of doses needed to control blood pressure, maternal and neonatal adverse effects.
Results
Compared with the NIFE + placebo group, the time needed to control blood pressure was significantly reduced in NIFE + RESV group, while time before a new hypertensive crisis was greatly delayed in NIFE + RESV group. The number of treatment doses needed to control blood pressure was also categorically lower in NIFE + RESV group. No differences in maternal or neonatal adverse effects were observed between the two treatment groups.
Conclusion
Our data support the potential of RESV as a safe and effective adjuvant of oral NIFE to attenuate hypertensive symptoms among PE patients.
Keywords: preeclampsia, resveratrol, nifedipine, hypertension, pregnancy
Introduction
Preeclampsia (PE) is a severe disease that manifests in multiple systems, and uniquely occurs during pregnancy, usually after 20 weeks of gestation (1). PE remains the leading cause of mortality and morbidity during pregnancy for women worldwide, and its most detrimental symptoms are severe elevated blood pressure and proteinuria. Thus, to monitor and control blood pressure of PE patients are important in the clinical management of PE (2, 3), in which anti-hypertensive drugs are commonly found to be of clinical efficacy in relieving hypertension (4).
Nifedipine (NIFE) is among several first-line anti-hypertensive treatments among PE patients (3, 5, 6). NIFE is a Ca2+ channel inhibitor (5) and functions to reduce vascular resistance by improving renal blood flow, as well as elevate urine output by repressing release of anti-diuretic hormones. Therefore, NIFE has become a potent anti-hypertensive agent for effective blood pressure control during pregnancy (7). Importantly, NIFE has been proven to be clinically safe, especially for pregnant patients (8). When prescribed in the 3rd trimester, administration of NIFE was able to effectively control high blood pressure, whereas it did not cause any serious adverse effects on either maternal or fetal development (9). In addition, NIFE has also been employed for treating pre-term labor without showing any perinatal adverse effects (10). Of particular relevance to our current study, in severe PE patients, NIFE was reported to improve arterial pressure and urine output in immediate puerperium (11).
Resveratrol (3,4,5-trihydroxy-trans-stilbene, RESV), a natural polyphenol extracted from fruits such as grapes and cranberries, has exhibited beneficial effects in various cardiovascular diseases (12). For instance, in rat model of PE, RESV was reported to reduce blood pressure (13), as well as inhibit trophoblast apoptosis through oxidative stress (14). In a pregnant mouse model with reduced blood supply to the uterus, RESV increased uterine artery blood flow velocity and fetal weight (15). In spontaneously hypertensive rats, RESV could lower blood pressure through production of calcium-dependent endothelial NO (16). In addition, in a recent clinical trial among patients with primary hypertension, RESV was able to supplement standard anti-hypertensive therapy to effectively reduce blood pressure to normal levels (17). Altogether these above studies support the promising potential of RESV as a potent anti-hypertensive agent.
In the current clinical trial, we aimed to use RESV as an adjuvant of oral NIFE treatment against pregnancy-induced severe PE and examine the treatment efficacy as well as potential adverse effects.
Materials and methods
Ethics
This is an intent-to-treat study. Design of the current clinical trial followed the guidelines of Declaration of Helsinki, and this study protocol was approved by the Ethical Committee in the Maternal and Child Health Care Hospital of Shandong Province. All patients enrolled in the study have signed written informed consent forms.
Patient selection
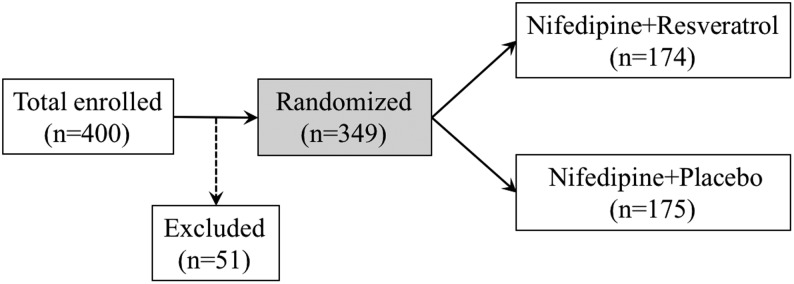
From June 2013 to November 2016, a total of 400 women carrying singleton pregnancy, aged between 21 and 32 years, were diagnosed of severe PE in hospital and subsequently agreed to participate in the current study. All patients required blood pressure control treatments, among which 51 patients were excluded due to exclusion criteria: (1) history of treatment with anti-hypertensive drugs; (2) history of heart failure during the course of the pregnancy.
Randomization process and drug treatment
349 patients were eventually eligible for the randomization process, who were randomly assigned, using a permuted-block design stratified according to their diastolic blood pressure, into two treatment groups: (1) NIFE + RESV group (n = 174), administered oral NIFE capsule (Quancheng Pharmaceuticals, Shandong, China; 10 mg each, up to 5 dosages) and RESV capsule (Yixin Pharmaceuticals, Zhejiang, China; 50 mg each, up to 5 dosages) every 15 min until blood pressure ≤150/100 mmHg; (2) NIFE + placebo group (n = 175), administered oral NIFE capsule (10 mg each, up to 5 dosages) plus glucose capsule (50 mg each, up to 5 dosages) as placebo every 15 min until blood pressure ≤150/100 mmHg. Once blood pressure of the patient reached ≤150/100 mmHg, no further dose was administered. Investigators blind to the group assignment prepared the RESV and placebo capsules identical in appearance to mask the content to patients.
Definition of endpoints
Primary endpoints were defined as: (1) time needed to bring blood pressure to no higher than 150/100 mmHg; (2) time before a new hypertensive crisis. Secondary endpoints were defined as: (1) the number of doses needed to bring blood pressure to no higher than 150/100 mmHg; (2) maternal and neonatal adverse effects. All measurements were performed by investigators blind to the group assignment.
Anthropometrics
Body weight was measured with patients in light clothing and no shoes standing straight on a digital scale with 0.1 kg accuracy. Body height was measured with patients standing straight without shoes standing straight on a stadiometer with 0.1 cm accuracy. Body mass index (BMI) was defined as body weight/(body height)2 in kg/m2. Blood pressure was measured by a digital blood pressure monitor with 0.1 mmHg accuracy.
Adverse effects
At the end of the trial, all patients were asked to complete a questionnaire on the symptoms of nausea, vomiting, maternal tachycardia, mild headache, dizziness, chest pain, hypotension and shortness of breath they might had in the process of the study. During the treatment, the maternal and fetal heart rates were under constant monitoring.
Statistical analysis
Student t test and Pearson chi-square test were performed appropriately to address differences in data between the two treatment groups. The 95% confidence intervals (CI) were calculated, and P < 0.05 indicated statistically significant difference. All statistical analyses were performed using SPSS 18.0 (SPSS).
Results
As shown in Fig. 1, from June 2013 to November 2016, 400 pregnant PE patients, carrying singleton pregnancy aged between 21 and 32 years and, were initially enrolled for the study. Fifty-one participants were subsequently excluded, and the remaining eligible 349 participants were randomly assigned to two treatment groups. 174 patients were instructed to administer oral NIFE + RESV, and 175 patients were instructed to administer oral NIFE + placebo, with one dose every 15 min until their blood pressure was controlled to ≤150/100 mmHg. Table 1 compared the anthropometrics, including maternal age, gestational age, systolic and diastolic blood pressures, heart rate, body weight, body height and BMI, of all 349 participants between the two treatment groups. We did not observe any significant differences between patients from the two treatment groups.
Figure 1.
Illustration of study design.
Table 1.
Characteristics of patients from the two treatment groups.
| Characteristics | NIFE + RESV (n = 174) | NIFE + placebo (n = 175) | P value |
|---|---|---|---|
| Maternal age (year) | 27.1 ± 6.2 | 26.9 ± 7.1 | 0.41 |
| Gestation age (week) | 34.7 ± 3.4 | 33.4 ± 4.1 | 0.52 |
| Systolic blood pressure (mmHg) | 169.1 ± 15.2 | 171.4 ± 16.1 | 0.28 |
| Diastolic blood pressure (mmHg) | 107.9 ± 9.6 | 112.4 ± 10.1 | 0.13 |
| Heart rate (/min) | 84.1 ± 10.2 | 86.4 ± 9.4 | 0.27 |
| Body weight (kg) | 64.8 ± 3.6 | 63.1 ± 4.8 | 0.31 |
| Body height (m) | 1.67 ± 0.12 | 1.65 ± 0.11 | 0.20 |
| BMI (kg/m2) | 23.5 ± 3.1 | 24.4 ± 3.8 | 0.37 |
BMI, body mass index; NIFE, nifedipine; RESV, resveratrol.
Table 2 summarized primary endpoints of the two treatment groups. First, the time required to effectively control blood pressure in the NIFE + RESV group was 35.6 ± 18.7 min. While in the NIFE + placebo group it was 51.1 ± 22.4 min, significantly longer than the NIFE + RESV group (P = 0.01; 95% CI 4.7–10.9). Next, the time before a new hypertensive crisis following effective blood pressure control in the NIFE + RESV group was 8.0 ± 2.1 h. While it was 5.5 ± 1.8 h in the NIFE + placebo group, significantly shorter than the NIFE + RESV group (P = 0.02; 95% CI 0.3–2.4).
Table 2.
Efficacy of the two treatments in controlling blood pressure among preeclampsia patients.
| Primary endpoints | NIFE + RESV (n = 174) | NIFE + placebo (n = 175) | P value |
|---|---|---|---|
| Time to control blood pressure (min) | 35.6 ± 18.7 | 51.1 ± 22.4 | 0.01 |
| Time before a new hypertensive crisis (h) | 8.0 ± 2.1 | 5.5 ± 1.8 | 0.02 |
NIFE, nifedipine; RESV, resveratrol.
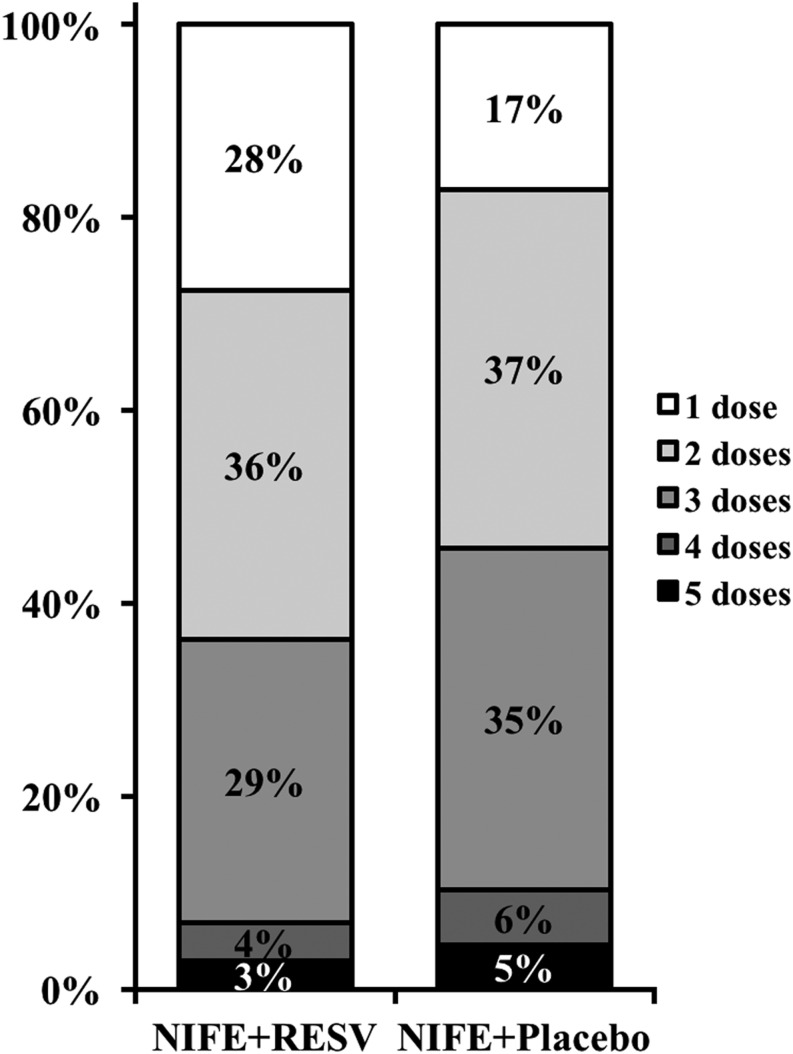
We next evaluated secondary endpoints of both the treatment groups. As shown in Fig. 2, the number of doses needed to control blood pressure was significantly lower in the NIFE + RESV group than the NIFE + placebo group. At last, at the end of trial, maternal and neonatal adverse effects were summarized in Table 3. Between the two treatment groups, we did not observe any significant differences for maternal adverse effects, in terms of nausea, vomiting, maternal tachycardia, mild headache, dizziness, chest pain, hypotension or shortness of breath. In addition, we did not find any differences in neonatal adverse effects between the two treatment groups, in terms of birth weight and Apgar scores of the newborns.
Figure 2.
Number of doses needed to control blood pressure in two groups of patients. Percentages do not add up to 100 due to rounding.
Table 3.
Adverse effects and neonatal complications of the two treatments.
| Secondary endpoints | NIFE + RESV (n = 174) | NIFE + placebo (n = 175) | |
|---|---|---|---|
| No adverse effect | 151 (86.8%) | 147 (84.0%) | |
| Nausea | 8 (4.6%) | 8 (4.6%) | |
| Vomiting | 6 (3.4%) | 9 (5.1%) | |
| Maternal tachycardia | 3 (1.7%) | 2 (1.1%) | |
| Mild headache | 3 (1.7%) | 3 (1.7%) | |
| Dizziness | 1 (0.6%) | 2 (1.1%) | |
| Chest pain | 2 (1.1%) | 2 (1.1%) | |
| Hypotension | 0 (0%) | 1 (0.6%) | |
| Shortness of breath | 0 (0%) | 1 (0.6%) | |
| Birth weight (kg) | 2.95 ± 0.61 | 3.07 ± 0.58 | P = 0.41 |
| Apgar scores | |||
| >6 | 141 (81.0%) | 138 (78.9%) | |
| 4–6 | 33 (19.0%) | 37 (21.1%) |
NIFE, nifedipine; RESV, resveratrol.
Discussion
Anti-hypertensive therapies are critical for controlling severe hypertension during pregnancy-induced PE (18) and also important for lowering incidence rate of both maternal and fetal complications (19, 20). Currently, clinical choices of proper use of drugs for hypertension management among PE patients are often made empirically, among which NIFE is one of first-line drugs commonly prescribed for controlling severe high blood pressure during pregnancy (3, 6). Compared with other anti-hypertensive alternatives, oral NIFE is cheaper, safer and more effective in achieving similar anti-hypertensive efficacy against pregnancy-induced PE (5, 21).
Due to extra diligence and considerations for pregnancy, PE patients often find themselves with a lack of available anti-hypertensive drugs, despite the fact that these drugs are widely used for hypertension patients without pregnancy. Therefore, a novel and safe drug with clinical efficacy to complement oral NIFE could greatly benefit PE patients. In line with the above notion, RESV is a natural extract from fruits such as grapes, and also enriched in drinks such as red wine. Due to its natural and safe origin, the use of RESV has been reported in complications during pregnancy (22), including gestational diabetes mellitus (23). Of particular interest to our current study, the anti-hypertension property of RESV has also been demonstrated in a number of studies employing both animal and clinical studies (13, 14, 15, 16, 17). Given its widely reported anti-hypertensive function, we therefore also employed RESV in the current clinical trial among pregnant women affected by PE, with the hypothesis that RESV could also exhibit benefits in relieving hypertension in pregnancy-induced PE.
Results from our clinical trial have demonstrated that, the combinational treatment of oral NIFE + RESV has significantly improved both the primary and secondary endpoints, compared with NIFE + placebo treatment. Compared with the NIFE + placebo group, the time needed to control blood pressure was significantly reduced in NIFE + RESV group, while time before a new hypertensive crisis was greatly delayed in NIFE + RESV group. The number of treatment doses needed to control blood pressure was also categorically lower in NIFE + RESV group. Most importantly concerning the pregnant PE patients, we did not find any severe adverse effects, maternal or neonatal, associated with RESV administration, suggesting the clinical safety of RESV among pregnant women.
Despite the observed clinical efficacy of RESV in complementing oral NIFE, the underlying mechanism of RESV action in relieving hypertension is still unknown. It has been reported that RESV could inhibit the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta (22). It is possible that sFlt-1 may be the molecular target underlying the anti-hypertensive property of RESV, since sFlt-1 itself is a reliable biomarker for clinical prediction of PE (24, 25, 26, 27, 28, 29). Besides sFlt-1, matrix metalloproteinase (MMP)-2 and MMP-9 were also reported to be involved in the pathology of PE, both as predictive biomarkers and as drug targets (30, 31, 32, 33). Given the great body of studies reporting the function of RESV in regulating levels and activities of both MMP-2 and MMP-9 (34, 35, 36, 37), these two MMPs are also likely to be the mediating factor of RESV in relieving hypertension of PE patients. Further studies are currently underway to evaluate the above potential mechanisms, particularly a larger scale multicenter trial is needed.
To conclude, our current randomized, placebo-controlled and double-blinded clinical trial is the first to report the potency, as well as safety, of RESV as a promising adjuvant to complement oral NIFE therapy, which could greatly enhance the clinical efficacy of treatments against hypertension of severe pregnancy-induced PE.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Bijvank SW, Visser W, Duvekot JJ, Steegers EA, Edens MA, Roofthooft DW, Vulto AG, Hanff LM. Ketanserin versus dihydralazine for the treatment of severe hypertension in early-onset preeclampsia: a double blind randomized controlled trial. European Journal of Obstetrics and Gynecology and Reproductive Biology 2015. 189 106–111. ( 10.1016/j.ejogrb.2015.02.002) [DOI] [PubMed] [Google Scholar]
- 2.Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2014. 2 CD002252 ( 10.1002/14651858.CD002252.pub3) [DOI] [PubMed] [Google Scholar]
- 3.Magee LA, Helewa M, Moutquin JM, von Dadelszen P, Hypertension Guideline C & Strategic Training Initiative in Research in the Reproductive Health Sciences S. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Journal of Obstetrics and Gynaecology Canada 2008. 30 S1–S48. ( 10.1016/S1701-2163(16)32776-1) [DOI] [PubMed] [Google Scholar]
- 4.Belfort MA, Anthony J, Buccimazza A, Davey DA. Hemodynamic changes associated with intravenous infusion of the calcium antagonist verapamil in the treatment of severe gestational proteinuric hypertension. Obstetrics and Gynecology 1990. 75 970–974. [PubMed] [Google Scholar]
- 5.Fenakel K, Fenakel G, Appelman Z, Lurie S, Katz Z, Shoham Z. Nifedipine in the treatment of severe preeclampsia. Obstetrics and Gynecology 1991. 77 331–337. [PubMed] [Google Scholar]
- 6.Duley L, Meher S, Jones L. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2013. 7 CD001449 ( 10.1002/14651858.CD001449.pub3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannubilo SR, Bezzeccheri V, Cecchi S, Landi B, Battistoni GI, Vitali P, Cecchi L, Tranquilli AL. Nifedipine versus labetalol in the treatment of hypertensive disorders of pregnancy. Archives of Gynecology and Obstetrics 2012. 286 637–642. ( 10.1007/s00404-012-2371-x) [DOI] [PubMed] [Google Scholar]
- 8.Clark SM, Dunn HE, Hankins GD. A review of oral labetalol and nifedipine in mild to moderate hypertension in pregnancy. Seminars in Perinatology 2015. 39 548–555. ( 10.1053/j.semperi.2015.08.011) [DOI] [PubMed] [Google Scholar]
- 9.Childress CH, Katz VL. Nifedipine and its indications in obstetrics and gynecology. Obstetrics and Gynecology 1994. 83 616–624. ( 10.1097/00006250-199404000-00024) [DOI] [PubMed] [Google Scholar]
- 10.Ferlinz J. Nifedipine in myocardial ischemia, systemic hypertension, and other cardiovascular disorders. Annals of Internal Medicine 1986. 105 714–729. ( 10.7326/0003-4819-105-5-714) [DOI] [PubMed] [Google Scholar]
- 11.Barton JR, Hiett AK, Conover WB. The use of nifedipine during the postpartum period in patients with severe preeclampsia. American Journal of Obstetrics and Gynecology 1990. 162 788–792. ( 10.1016/0002-9378(90)91011-Z) [DOI] [PubMed] [Google Scholar]
- 12.Bonnefont-Rousselot D. Resveratrol and cardiovascular diseases. Nutrients 2016. 8 250 ( 10.3390/nu8050250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moraloglu O, Engin-Ustun Y, Tonguc E, Var T, Tapisiz OL, Ergun H, Guvenc T, Gacar A. The effect of resveratrol on blood pressure in a rat model of preeclampsia. Journal of Maternal-Fetal and Neonatal Medicine 2012. 25 845–848. ( 10.3109/14767058.2011.599081) [DOI] [PubMed] [Google Scholar]
- 14.Zou Y, Zuo Q, Huang S, Yu X, Jiang Z, Zou S, Fan M, Sun L. Resveratrol inhibits trophoblast apoptosis through oxidative stress in preeclampsia-model rats. Molecules 2014. 19 20570–20579. ( 10.3390/molecules191220570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poudel R, Stanley JL, Rueda-Clausen CF, Andersson IJ, Sibley CP, Davidge ST, Baker PN. Effects of resveratrol in pregnancy using murine models with reduced blood supply to the uterus. PLoS ONE 2013. 8 e64401 ( 10.1371/journal.pone.0064401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Dai Y, Yan S, Shi Y, Li J, Liu J, Cha L, Mu J. Resveratrol lowers blood pressure in spontaneously hypertensive rats via calcium-dependent endothelial NO production. Clinical and Experimental Hypertension 2016. 38 287–293. ( 10.3109/10641963.2015.1089882) [DOI] [PubMed] [Google Scholar]
- 17.Theodotou M, Fokianos K, Mouzouridou A, Konstantinou C, Aristotelous A, Prodromou D, Chrysikou A. The effect of resveratrol on hypertension: a clinical trial. Experimental and Therapeutic Medicine 2017. 13 295–301. ( 10.3892/etm.2016.3958) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Q, Leng W, Yao Q, Mi C, Xing A. Oral nifedipine versus intravenous labetalol for the treatment of severe hypertension in pregnancy. International Journal of Cardiology 2015. 178 162–164. ( 10.1016/j.ijcard.2014.10.111) [DOI] [PubMed] [Google Scholar]
- 19.Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J, Peek MJ, Rowan JA, Walters BN. & Austalasian Society of the Study of Hypertension in Pregnancy. The detection, investigation and management of hypertension in pregnancy: full consensus statement. Australian and New Zealand Journal of Obstetrics and Gynaecology 2000. 40 139–155. ( 10.1111/j.1479-828X.2000.tb01137.x) [DOI] [PubMed] [Google Scholar]
- 20.Rey E, LeLorier J, Burgess E, Lange IR, Leduc L. Report of the Canadian Hypertension Society Consensus Conference: 3. Pharmacologic treatment of hypertensive disorders in pregnancy. CMAJ 1997. 157 1245–1254. [PMC free article] [PubMed] [Google Scholar]
- 21.Aali BS, Nejad SS. Nifedipine or hydralazine as a first-line agent to control hypertension in severe preeclampsia. Acta Obstetricia et Gynecologica Scandinavica 2002. 81 25–30. ( 10.1034/j.1600-0412.2002.810105.x) [DOI] [PubMed] [Google Scholar]
- 22.Cudmore MJ, Ramma W, Cai M, Fujisawa T, Ahmad S, Al-Ani B, Ahmed A. Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. American Journal of Obstetrics and Gynecology 2012. 206 253.e210–253.e255. ( 10.1016/j.ajog.2011.11.010) [DOI] [PubMed] [Google Scholar]
- 23.Tran HT, Liong S, Lim R, Barker G, Lappas M. Resveratrol ameliorates the chemical and microbial induction of inflammation and insulin resistance in human placenta, adipose tissue and skeletal muscle. PLoS ONE 2017. 12 e0173373 ( 10.1371/journal.pone.0173373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dragan I, Wright D, Fiolna M, Leipold G, Nicolaides KH. Development of pre-eclampsia within 4 weeks of sFlt-1/PlGF ratio >38: comparison of performance at 31–34 vs 35–37 weeks’ gestation. Ultrasound in Obstetrics and Gynecology 2016. 49 209–212. ( 10.1002/uog.17310) [DOI] [PubMed] [Google Scholar]
- 25.Frusca T, Gervasi MT, Paolini D, Dionisi M, Ferre F, Cetin I. Budget impact analysis of sFlt-1/PlGF ratio as prediction test in Italian women with suspected preeclampsia. Journal of Maternal-Fetal and Neonatal Medicine 2016. 30 2166–2173. ( 10.1080/14767058.2016.1242122) [DOI] [PubMed] [Google Scholar]
- 26.Klein E, Schlembach D, Ramoni A, Langer E, Bahlmann F, Grill S, Schaffenrath H, van der Does R, Messinger D, Verhagen-Kamerbeek WD, et al. Influence of the sFlt-1/PlGF ratio on clinical decision-making in women with suspected preeclampsia. PLoS ONE 2016. 11 e0156013 ( 10.1371/journal.pone.0156013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perales A, Delgado JL, De La Calle M, Garcia-Hernandez JA, Escudero AI, Campillos JM, Sarabia MD, Laiz B, Duque M, Navarro M, et al. sFlt-1/PlGF for early-onset pre-eclampsia prediction: STEPS (Study of Early Pre-eclampsia in Spain). Ultrasound in Obstetrics and Gynecology 2016. 50 373–382. ( 10.1002/uog.17373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vatish M, Strunz-McKendry T, Hund M, Allegranza D, Wolf C, Smare C. sFlt-1/PlGF ratio test for pre-eclampsia: an economic assessment for the UK. Ultrasound in Obstetrics and Gynecology 2016. 48 765–771. ( 10.1002/uog.15997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennstrom M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. New England Journal of Medicine 2016. 374 13–22. ( 10.1056/NEJMoa1414838) [DOI] [PubMed] [Google Scholar]
- 30.Lavee M, Goldman S, Daniel-Spiegel E, Shalev E. Matrix metalloproteinase-2 is elevated in midtrimester amniotic fluid prior to the development of preeclampsia. Reproductive Biology and Endocrinology 2009. 7 85 ( 10.1186/1477-7827-7-85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montagnana M, Lippi G, Albiero A, Scevarolli S, Salvagno GL, Franchi M, Guidi GC. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. Journal of Clinical Laboratory Analysis 2009. 23 88–92. ( 10.1002/jcla.20295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K, Ni W, Kalluri R, Werb Z. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. PNAS 2013. 110 11109–11114. ( 10.1073/pnas.1309561110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palei AC, Granger JP, Tanus-Santos JE. Matrix metalloproteinases as drug targets in preeclampsia. Current Drug Targets 2013. 14 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gweon EJ, Kim SJ. Resveratrol attenuates matrix metalloproteinase-9 and -2-regulated differentiation of HTB94 chondrosarcoma cells through the p38 kinase and JNK pathways. Oncology Reports 2014. 32 71–78. ( 10.3892/or.2014.3192) [DOI] [PubMed] [Google Scholar]
- 35.Pandey AK, Bhattacharya P, Shukla SC, Paul S, Patnaik R. Resveratrol inhibits matrix metalloproteinases to attenuate neuronal damage in cerebral ischemia: a molecular docking study exploring possible neuroprotection. Neural Regeneration Research 2015. 10 568–575. ( 10.4103/1673-5374.155429) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Chen Z, Hu L, Lu M, Shen Z. Resveratrol reduces matrix metalloproteinases and alleviates intrahepatic cholestasis of pregnancy in rats. Canadian Journal of Physiology and Pharmacology 2016. 94 402–407. ( 10.1139/cjpp-2015-0454) [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Bai Q, Zhao Z, Sui H, Xie X. Resveratrol improves delayed r-tPA treatment outcome by reducing MMPs. Acta Neurologica Scandinavica 2016. 134 54–60. ( 10.1111/ane.12511) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a