Abstract
Background
This study aimed to identify the phytochemical content and evaluate the antioxidant, anti-inflammatory, and antiproliferative capacities of various solvent extracts of Ephedra campylopoda stems.
Material/Methods
Fresh stems were suspended in 3 different solvent systems, including distilled water, ethanol, and methanol. The chemical composition was determined using high-performance liquid chromatography (HPLC), and the content of essential oil of this plant species was determined by gas chromatography (GC) coupled with mass spectrometry (MS). Antioxidant activity was determined using DPPH radical scavenging and Fe2+-chelating activity assays. Anti-inflammatory capacity was estimated by both evaluating RAW 264.7 murine macrophage cells-mediated secretion of PGE2 using ELISA technique, and quantifying the mRNA level of the pro-inflammatory cytokines (IL-α, IL-β and IL-6), chemokines (CCL3 and CCL4), and inflammation-inducible COX-2 and iNOS enzymes using quantitative real-time PCR (qRT-PCR). The antiproliferative potential was determined using the XTT viability assay.
Results
Our results showed that the alcoholic extracts were better than the aqueous one in terms of their chemical composition. In parallel, the alcoholic extracts showed more potent antioxidant, anti-inflammatory, and antiproliferative capacities than aqueous extract.
Conclusions
Our observations suggest that Ephedra campylopoda plant could be a promising resource of natural products with antioxidant, anti-inflammatory and antiproliferative capacities.
MeSH Keywords: Anti-Inflammatory Agents, Antioxidants, Chemical Fractionation
Background
Oxidative and inflammatory processes are reported to be associated with a number of chronic diseases, including atherosclerosis, Alzheimer’s disease, cardiovascular diseases, and neurodegenerative disorders, as well as various human cancers [1]. Inflammatory response is a major defensive mechanism against infection, during which, inflammatory cells, such as macrophages, produce reactive oxygen species (ROS) and nitric oxide (NO) [2]. At low concentrations, these molecules serve important physiological roles by acting as second messengers in cell signaling. However, at higher amounts these components can damage cellular lipids, proteins, and DNA, leading to cell death [3–6]. To neutralize the toxic effect of these bioactive molecules, the human body has evolved different defense mechanisms, including the generation of antioxidants [7,8]. Oxidative stress is a result of imbalance between ROS formation and endogenous antioxidant capacity due to excessive ROS production and/or impaired antioxidant system. Identifying exogenous sources of antioxidants and anti-inflammatory molecules is therefore of great importance. Plants have traditionally been used for thousands of years for treating inflammation- and oxidative stress-related disorders. The medicinal value of plants is mainly attributed to their phytochemical component content, especially phenolic compounds and flavonoids, which can exert potent antioxidant and anti-inflammatory effects [9–14]. In the Middle East region, herbal medicines are extensively used and there has been a growing interest in identifying medicinal plants. Indeed, more than 100 plant species known for their medicinal value have been isolated from this region [15,16] and many other plants are yet to be characterized. Lebanon, thanks to its geographic location, varied topography, distinct soil types, and climatic variations, is characterized by a relatively large flora consisting of about 2607 species distributed over 783 genera. Among these, a few hundred species are used to treat various diseases, including gastrointestinal disorders, kidney and urinary diseases, cardiovascular diseases, diabetes, asthma, sexual disorders, hair problems, and various tumors [17,18]. Nowadays, as many studies are focused on characterizing the therapeutic value of Lebanese plants, the list of Lebanese medicinal plants is expected to grow. The plant Ephedra campylopoda belongs to the Ephedraceae family of plants. Those plants are small, leafless, highly branched shrubs, distributed in the dry regions of both hemispheres [19]. In Lebanon, Ephedra campylopoda is found in different regions, mainly rocky ones. In this study, we screened the phytochemical component content and characterized the antioxidant, anti-inflammatory, and antiproliferative capacities of 3 extracts from the stems of Ephedra campylopoda.
Material and Methods
Plant collection and preparation of powders
Fresh plants were gathered in southern Lebanon at 350 m altitude in spring season between March and May in 2011, and the biological authentication was carried out by Professor George Tohme, president of CNRS of Lebanon. After that, they were well-washed, cut into small pieces, and dried in the shade at room temperature, away from sun light. After this period, the dried stems were crushed and ground in a grinder to produce a homogeneous fine powder, which was then kept in a dark place at room temperature until use in various studies.
Apparatus and chemicals
All of the chemicals used were of analytical grade. Absolute ethanol, methanol, n-hexane, sodium hydroxide, ethyl acetate, and dichloromethane were purchased from BDH England. Aluminium chloride, FeSO4•7H2O, and silica gel were purchased from Merck (Germany). Sodium carbonate and hydrogen peroxide were purchased from Unichem (India). Ascorbic acid, gallic acid, rutin, Folin-Ciocalteau reagent, EDTA, Ferrozine, and DPPH were purchased from Sigma Aldrich (USA). PBS was purchased from Gibco (UK). MS spectra were recorded on an Agilent series device, and MSMS spectra were recorded on a Shimadzu series device.
Preparation of crude extracts using water, ethanol, and methanol as solvents
Powdered stems (100 g) were deposited into a flask with 500 ml of the selected solvent (distilled water, ethanol, or methanol). After a period of maceration and stirring for 1 week at room temperature, the macerate was collected and filtered using filter paper. Extracts were then concentrated using a rotary evaporator at 40°C under reduced pressure (for ethanol and methanol extracts). The aqueous extract was prepared using the same steps as for the ethanolic extraction except the temperature of the extraction was 60°C and the filtrates were then frozen before being lyophilized to obtain powders.
Phytochemical Screening
To study the chemical composition of the different extracts from the stems of the studied plant, qualitative tests were done to detect the presence or absence of primary and secondary metabolites, as shown in Table 1. These tests are useful to estimate some biological activities that might be due to the presence of some secondary metabolites in the stems of this plant.
Table 1.
Detection of primary and secondary metabolites in stems of Ephedra campylopoda.
| Metabolites | Added reagent | Expected result |
|---|---|---|
| Alkaloids [32] | Dragendorff reagent | Red or Orange precipitate |
| Tanins [32] | FeCl3 (1%) | Blue coloration |
| Resines [32] | Acetone + water | Turbidity |
| Saponines [33] | Agitation | Formation of foam |
| Phenols [32] | FeCl3 (1%) + K3(Fe(CN)6) (1%) | Green-blue coloration |
| Terpenoids [33] | Chloroform + H2SO4 conc | Reddish brown coloration |
| Flavonoids [34] | KOH (50%) | Yellow coloration |
| Carbohydrates [33] | α-naphtol + H2SO4 | Purple ring |
| Reducing sugars [33] | Fehlings (A+B) | Brownish-red precipitate |
| Quinones [35] | HCl conc | Yellow precipitate |
| Sterols & Steroids (Khandelwal, 2005; [33] | Chloroform + H2SO4 conc | Red color (surface) + fluorescence Greenish-yellow |
| Cardiac glycosides (Khandelwal, 2005; [33] | Glacial acetic acid + FeCl3 (5%) + H2SO4 conc | Ring |
| Diterpenes [32] | Copper acetate | Green coloration |
| Anthraquinones [34] | HCl (10%) + chloroform + Ammonia (10%) | Pink coloration |
| Proteins & aminoacids [36]cinnamate 4-hydroxylase (CsC4H | Ninhydrin 0.25% | Blue coloration |
| Lignines [36]cinnamate 4-hydroxylase (CsC4H | Safranine | Pink coloration |
| Phlabotannins [37] | HCl (1%) | Blue coloration |
| Anthocyanines [38]gene At1g15950 | NaOH (10%) | Blue coloration |
| Flavanones [38]gene At1g15950 | H2SO4 conc | Bluish-red Coloration |
| Fixed oils and fats [33] | Spot test | Oil stain |
Gas chromatography-mass spectrometry (GC/MS) analysis
The GC/MS analysis was performed on an Agilent 7890A-GCMS device. In the separation and identification by GC/MS technique, components were identified on the basis of the retention time and spectral index from the NIST and WILEY library.
Liquid chromatography-mass spectrometry (LC/MS/MS) analysis
The LC/MS/MS analysis was performed on Shimadzu-AB Sciex LCMSMS for detection. In the separation and identification by LC/MS/MS technique, components were identified on the basis of the retention time and mass spectral characteristics.
Biological analysis
DPPH radical scavenging assay
The antioxidant activity was assessed according to the method of Farhan et al [20] using free radical DPPH. Increasing concentrations of extracts (0.05, 0.1, 0.2, 0.4, and 0.5 mg/ml) were prepared, then 1 ml of each prepared dilution of each extract was added to 1 ml of DPPH reagent. The solutions were incubated in the dark at room temperature for 30 min and the absorbance was measured at 517 nm by a Gene Quant 1300 UV-Vis spectrophotometer. The DPPH scavenging ability of peels extracts was calculated according to the following equation:
Control was prepared by mixing 1 ml DPPH with 1 ml of selected solvent. The blank was composed of 1 ml of the selected solvent.
Metal chelating activity
The chelation of ferrous ions by extracts was estimated by the method of Dinis et al. [21]. Briefly, 50 μl of FeCl2 (2 mM) was added to 1 ml of different concentrations of the extract (500, 750, 1000, 1250, and 1500 μg/ml). The reaction was initiated by the addition of 0.2 ml of ferrozine solution (5 mM). The mixture was vigorously shaken and left to stand at room temperature for 10 min. The absorbance of the solution was then measured at 562 nm.
Anti-inflammatory evaluation of the extracts
RAW 264.7, a murine monocyte/macrophage cell line, was grown in DMEM medium supplemented with 10% defined FBS and 1% penicillin G-streptomycin in atmosphere containing 5% CO2/95% air at 37°C. The macrophages were seeded in 12-well plates (1×106 cells/well) using fresh medium. After preincubation for 24 h, plates were cotreated with LPS at 100 ng/ml and 2 different concentrations of the drugs (100 μg/ml and 50 μg/ml) in DMEM without FBS for 24 h (for RNA extraction and COX-2 activity).
PGE2 immuno assay
PGE2 amounts in culture medium were quantified in supernatants by enzyme immune assay using ELISA kits (R&D Systems) following manufacture’s guidelines.
Cell viability
Jurkat cells, corresponding to human leukemic T cell line, were seeded in 96-well plates (8×103 cells/well). The following day, cells were treated with the different extracts at concentrations ranging from 5 to 200 μg/ml for 24, 48, and 72 h and cell viability was detected using the XTT (Gentaur, Belgium) cell proliferation assay as previously described [22]. The XTT (sodium 3′-1 (phenylaminocarbonyl)-3,4-tetrazolium-bis (4-methoxy-6-nitro) benzene sulfonic acid) cell proliferation assay is an effective method to measure cell growth and drug sensitivity in tumor cell lines. XTT is a colorless or slightly yellow compound that when reduced becomes bright orange. Briefly, XTT is cleaved by the mitochondrial dehydrogenase in metabolically active living cells to form an orange formazan dye. The absorbance of each sample was measured with a spectrophotometer at a wavelength of 450 nm.
Quantitative real-time PCR
Total RNA was extracted with Trizol reagent according to the manufacturer’s guidelines (Invitrogen, Merelbeke, Belgium) and first-strand cDNAs were synthesized by reverse transcription (Superscript First-strand Synthesis System for RT-PCR kit; Invitrogen, Merelbeke, Belgium). Quantitative mRNA expression for the different genes was measured by real-time PCR with the PRISM 7900 sequence detection system (Applied Biosystems, Gent, Belgium), and the SYBR Green Master mix kit with β-actin mRNA was used as an internal control. The program used for amplification was: 10 min at 95°C followed by 40 cycles of 15 s at 95°C, and 1 min at 60°C. All qPCR reactions were performed in triplicate. The expression levels (2−ΔΔCt) of mRNAs were calculated as described previously [23].
Statistical analysis
The data are presented as means ±SEM of at least 3 independent experiments and analyzed using Student’s t-test to determine any differences between group means, using SPSS for Windows (Version 21). P-Values <0.05(*), <0.01(**), <0.001(***) were considered significant.
Results
Phytochemical screening of stems of Ephedra campylopoda
Phytochemical screening of the aqueous, ethanolic, and methanolic extracts of fresh Ephedra campylopoda stems identified the presence of different medically active compounds (Table 2). The aqueous crude extract showed high abundance of saponins, phenols, reducing sugars and lignin; average abundance of flavonoids, carbohydrates, and amino acids; low abundance of quinones, cardiac glycosides and diterpenes; and absence of alkaloids, tannins, resins, terpenoids, coumarins, sterols/steroids, anthraquinones, phlobatannins, anthocyanin, flavones, fixed oils, and lipids. On the other hand, the ethanolic crude extract exhibited high amounts of phenols and lignin; moderate amounts of flavonoids, quinones, carbohydrates, amino acids, and sterols/steroids; low amounts of alkaloids, coumarins, and diterpenes; and absence of tannins, resins, saponins, terpenoids, cardiac glycosides, anthraquinones, reducing sugars, phlobatannins, anthocyanin, and fixed oils and lipids. In contrast to the aqueous and ethanolic extracts, more constituents were present in the methanolic extract, which displayed high levels of phenols, carbohydrates, sterols/steroids, flavones and lignin; moderate levels of tannins, quinones, amino acids, cardiac glycosides, and phlobatannins; low levels of resins, terpenoids, flavonoids, coumarins, reducing sugars, and anthocyanins; and absence of only alkaloids, saponins, anthraquinones, and fixed oils and lipids. Altogether, these observations indicate that methanol was the best solvent, in comparison to aqueous and ethanolic solvents, to extract bioactive compounds present in the Ephedra campylopoda stem.
Table 2.
Phytochemical screening of Ephedra campylopoda stem extract using aqueous, methanol or ethanol as extraction solvents. Key: −, absent; +, low in abundance; ++, moderate in abundance; +++, high in abundance.
| Aqueous extract | Methanol extract | Ethanol extract | |
|---|---|---|---|
| Alkaloids | − | − | + |
| Tannins | − | ++ | − |
| Resins | − | + | − |
| Saponins | +++ | − | − |
| Phenol | +++ | +++ | +++ |
| Terpenoids | − | + | − |
| Flavonoids | ++ | + | ++ |
| Quinones | + | ++ | ++ |
| Coumarin | − | + | + |
| Carbohydrates | ++ | +++ | ++ |
| Amino acids | ++ | ++ | ++ |
| Sterols + steroids | − | +++ | ++ |
| Cardiac glycosides | + | ++ | − |
| Diterpenes | + | ++ | + |
| Anthraquinones | − | − | − |
| Reducing sugars | +++ | + | − |
| Phlobatannins | − | ++ | − |
| Anthocyanins | − | + | − |
| Flavones | − | +++ | + |
| Lignin | +++ | +++ | +++ |
| Fixed oil + lipids | − | − | − |
GC/MS analysis of essential oil obtained from the Ephedra campylopoda stem extracts
The GC spectrum of the aqueous, ethanolic and methanolic extracts are shown in Figures 1–3, respectively. A total of 7 compounds present in the aqueous extract, 6 compounds present in the ethanolic extract, and 7 compounds present in the methanolic extract were determined by the chromatographic method with the help of NIST and WILEY library as shown in Tables 3–5, respectively. In the case of ethanolic extract, linolenic acid methyl ester compound was found to be in the highest concentration (77.97%), while other compounds were found in trace amounts (Table 4). In the case of methanolic extract, trans-phytol compound had the highest concentration (39.17%), while other compounds were found in trace amounts (Table 5).
Figure 1.

GC chromatogram of the water extract of E. Campylopoda stem.
Figure 2.
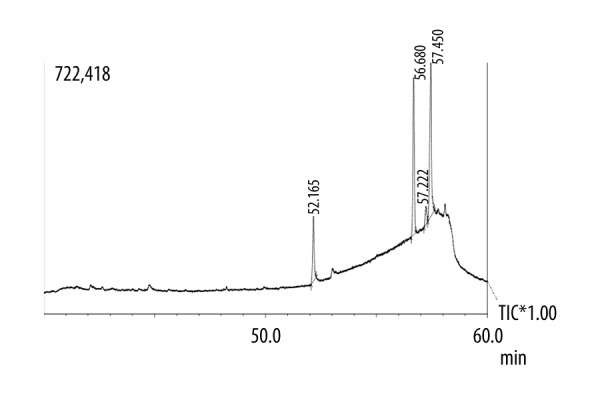
GC chromatogram of the ethanol extract of E. Campylopoda stem.
Figure 3.

GC chromatogram of the methanol extract of E. Campylopoda stem.
Table 3.
Results of the GC-MS analysis of the water extract of the E. campylopoda stem.
| Peak# | RT | Name | MW | Structure | Molecular formula | Area% |
|---|---|---|---|---|---|---|
| 5 | 12.981 | Vinyl guaiacol | 150.17 |

|
C9H10O2 | 1.47 |
| 7 | 13.591 | Syringol | 154.163 |

|
C8H10O3 | 3.48 |
| 8 | 16.107 | di-tert-butylphenol | 206.324 |

|
C14H22O | 0.62 |
| 9 | 17.558 | Antiarol | 184.2 |

|
C9H12O4 | 3.28 |
| 11 | 20.038 | N-Butylbenzenesulfonamide | 213.30 |

|
C10H15NO2S | 0.83 |
| 12 | 20.153 | Pluchidiol | 208 | ----- | C13H20O2 | 7.85 |
| 13 | 22.149 | Cetylic acid | 256.42 |
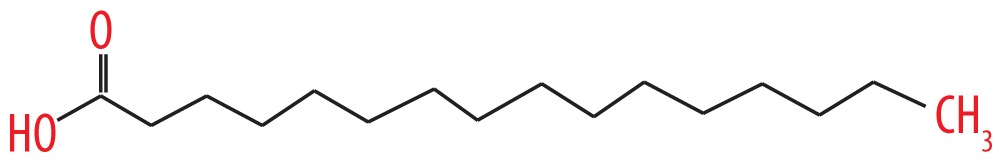
|
C16H32O2 | 4.8 |
Table 4.
Results of the GC-MS analysis of the ethanolic extract of the E. campylopoda stem.
| Peak# | RT | Name | MW | Structure | Molecular formula | Area% |
|---|---|---|---|---|---|---|
| 2 | 52.165 | Palmitic acid | 256.42 |
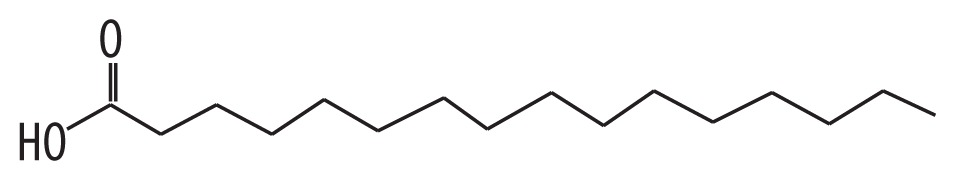
|
C16H32O2 | 2.17 |
| 3 | 56.680 | Phytol | 296.53 |
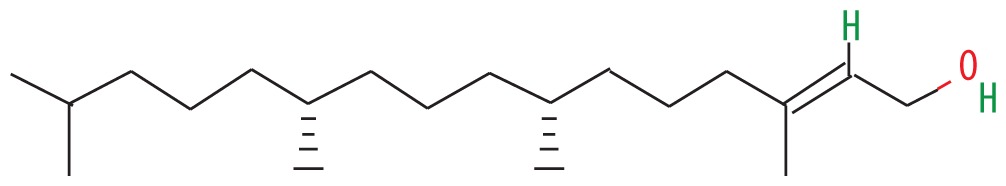
|
C20H40O | 5.04 |
| 4 | 57.222 | Linoleic | 280.452 |

|
C18H32O2 | 0.7 |
| 5 | 57.450 | Linolenic acid methyl ester | 294.479 |
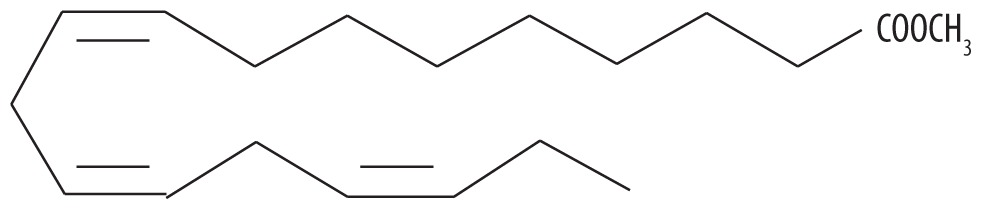
|
C19H34O2 | 6.33 |
| 6 | 81.142 | α-tocopherol-β-d-mannosid | 592.858 |

|
C35H60O7 | 2.82 |
| 7 | 84.636 | γ-sitosterol | 414.71 |

|
C29H50O | 4.97 |
Table 5.
Results of the GC-MS analysis of the methanolic extract of the E. campylopoda stem.
| Peak# | RT | Name | MW | Structure | Molecular formula | Area% |
|---|---|---|---|---|---|---|
| 2 | 24.327 | Coumaran | 120.15 |

|
C8H8O | 6.04 |
| 3 | 28.406 | Vinylguaiacol | 150.17 |

|
C9H10O2 | 4.85 |
| 4 | 36.608 | 2,4-di-tert-butylphenol | 206.324 |

|
C14H22O | 4.15 |
| 5 | 52.114 | Palmitic acid | 256.42 |

|
C16H32O2 | 4.19 |
| 6 | 56.677 | Trans-phytol | 296.53 |
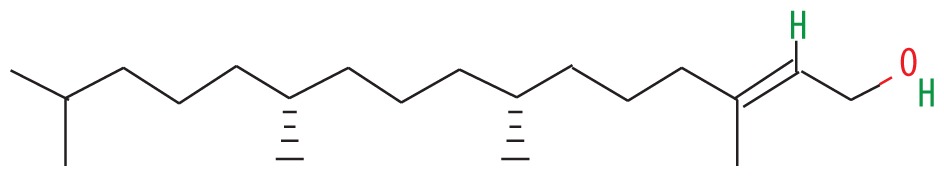
|
C20H40O | 39.17 |
| 7 | 57.383 | Linolenic acid methyl ester | 294.479 |

|
C19H34O2 | 9.6 |
| 8 | 81.15 | Vitamin E | 430.717 |

|
C29H50O2 | 3.31 |
The LC/MS/MS Analysis of the Ephedra campylopoda stem extracts
The LC spectrum results of the E. campylopoda stem extracts are shown in Table 6. A total of 1, 6, and 6 compounds present in the aqueous, ethanolic, and methanolic extracts, respectively, were determined by the chromatographic method based on the retention time and mass characteristics.
Table 6.
Results of LC/MS/MS technique of E. campylopoda plant.
| E. campylopoda | Compounds names | Retention time | Q1 Mass (Da) | Q3 Mass (Da) | CE | DP (V) |
|---|---|---|---|---|---|---|
| Water | Vitexin | 4.96 | 431 | 341 | −30 | −40 |
| Ethanol | Prunin | 5.22 | 433 | 271 | −20 | −40 |
| Quercetin | 5.88 | 301 | 179 | −35 | −40 | |
| Rutin | 4.86 | 609 | 301 | −35 | −40 | |
| Vitexin | 4.93 | 431 | 341 | −30 | −40 | |
| Hyperoside | 4.98 | 463 | 301 | −38 | −40 | |
| Isoorientin | 4.74 | 447 | 429 | −30 | −40 | |
| Methanol | Prunin | 5.24 | 433 | 271 | −20 | −40 |
| Quercetin | 5.89 | 301 | 179 | −35 | −40 | |
| Rutin | 4.91 | 609 | 301 | −35 | −40 | |
| Vitexin | 4.96 | 431 | 341 | −30 | −40 | |
| Hyperoside | 5.02 | 463 | 301 | −38 | −40 | |
| Isoorientin | 4.79 | 447 | 429 | −30 | −40 |
Antioxidant activity of Ephedra campylopoda stem extracts
To investigate the antioxidant activities of the aqueous, methanolic, and ethanolic crude extracts-derived from fresh stems, DPPH free radical scavenging assay was carried out in a first step. As antioxidants can react with the violet colored stable free radical DPPH, and convert it into a yellow-colored α,α-diphenyl-β-picrylhydrazine, this assay is based on quantifying the change of the reaction mixture color as a readout of the scavenging capacity of antioxidants towards DPPH. The different extracts showed varied antioxidant potential and their DPPH scavenging capacities were in the following order: ethanolic extract (IC50=125±4.4 μg/ml) >methanolic extract (IC50=150±5.1 μg/ml) >aqueous extract (IC50=300±4.4 μg/ml) (Table 7).
Table 7.
DPPH free scavenging capacity (IC50, μg/ml) and Ferrous-ion (Fe2+) chelating ability (IC50, mg/ml) of aqueous, methanol or ethanol extracts derived from fresh stems of Ephedra Campylopoda. IC50 value represents the concentration of sample required to scavenge DPPH radical or Ferrous-ion by 50%. Each value represents a mean ±SD (n=3).
| DPPH assay | Fe2+ chelating assay | ||
|---|---|---|---|
| Extract | IC50, μg/ml | IC50, mg/ml | |
| Fresh stems | Aqueous | 300±4.4 | >1.5 |
| Methanol | 150±5.1 | 1±1.2 | |
| Ethanol | 125±4.4 | >1.5 |
Despites its beneficial roles as being required for oxygen transport, respiration, and enzyme activity, iron is a highly reactive metal that can cause oxidative changes in proteins, lipids, and other structural components. Accordingly, in a second step, we characterized the antioxidant activities of the aqueous, methanolic, and ethanolic crude extracts of E. campylopoda stem by performing ferrous (Fe2+) chelating activity assay. As ferrozine can combine with Fe2+ to form a colored complex and since other chelating agents, when present, can disrupt the complex formation and thus reduces the extent of color, the ferrous (Fe2+) chelating assay is based on determination of the change of the reaction mixture color as a readout of the chelating activity of the coexisting chelator. Our results showed that methanolic extract had the most efficient Fe2+ chelating capacity (IC50=1±1.2 mg/ml) in comparison to both the ethanolic and the aqueous extracts, which presented IC50 values of more than 1.5 mg/ml (Table 7).
Anti-inflammatory activity of Ephedra campylopoda stem extracts
Inflammation is one form of host defense strategies to combat pathogenic intruders. Inflammation occurs when different immune cells, mainly macrophages, detect pathogen-associated molecular patterns (PAMPs) such as microbial lipopolysaccharide (LPS) [24]. Once activated, macrophages can then induce the expression of different pro-inflammatory cytokines (including IL-1α, IL-1β, and IL-6), chemokine (such as CCL3 and CCL4) and other pro-inflammatory mediators, including nitric oxide (NO) and prostaglandin E2 (PGE2), which are synthesized by the induced isoforms of NO synthase (iNOS) and cyclooxygenase-2 (COX-2) enzymes [24], respectively. To characterize the anti-inflammatory capacity of E. campylopoda stem extracts, we used RAW 264.7 murine macrophage cells, which upon being stimulated with LPS can produce PGE2. Cells were treated for 24 h with either LPS (100 ng/ml) alone (control), or LPS together with varying concentrations (50 or 100 μg/ml) of either aqueous, methanolic, or ethanolic crude extracts derived from fresh stems. In a first step, quantitative real-time PCR (qRT-PCR) was used to assay the relative iNOS and COX-2 mRNA transcription in extract-treated RAW264.7 cells versus non-treated control cells. In the case of COX2, the mRNA levels were not significantly lowered in response to either of the utilized aqueous extract concentrations (Figure 4A). Interestingly, about 50–60% of COX2 mRNA levels were lost in response to either of the added ethanolic extract concentrations (Figure 4A). However, the methanolic extract was less efficient, as only about 15% of COX2 mRNA levels were absent in response to 50 μg/ml but not 100 μg/ml of extract (Figure 4A). In the case of iNOS, the 3 different extracts were efficient in terms of impairing iNOS transcription, with methanolic extract being the most efficient, followed by ethanolic extract, and finally aqueous extract (Figure 4B).
Figure 4.
Impact of Ephedra campylopoda stem extracts on LPS-induced iNOS, COX-2, PGE2, IL-1α, IL-1-β, IL-6, CCL3, and CCL4 levels in RAW 264.7 cells. Cells were treated for 24 h with 100 ng/ml LPS in the absence or presence of 50 or 100 μg/ml of either aqueous (A), ethanol (E), or methanol (M) extract. Total RNA was isolated and qRT-PCR was carried out to quantify the mRNA levels of COX-2 (A), iNOS (B), IL-1α (D), IL-1β (E), IL-6 (F), CCL3 (G), and CCL4 (H). The presented data correspond to the relative mRNA levels (values obtained in: RAW 264.7 cells treated with both LPS and extract/RAW 264.7 cells treated with only LPS). (C) Cell-free supernatants were harvested and assayed for PGE2 content via ELISA. The data correspond to the relative percentage of PGE2. Reported values represent the averages ±SEM of 3 independent experiments (n=3) each done in triplicate. * p<0.05; ** p<0.01, *** p<0.001 vs. control untreated cells (Student’s t-test).
In a second step, ELISA technique was used to assess the relative PGE2 amounts present in the cell culture media. Intriguingly, the different extracts showed prominent capacity to dampen PGE2 production (Figure 4C). Indeed, about 75%, 60%, and 78% reduction in secreted PGE2 levels were observed upon treating cells with 50 μg/ml of aqueous, ethanolic, and methanolic extracts, respectively (Figure 4C). These levels were further increased to reach 85%, 99%, and 95% in response to 100 μg/ml of aqueous, ethanolic, and methanolic extracts, respectively (Figure 4C).
In a third step, qRT-PCR was applied to assay the relative expression of the pro-inflammatory cytokines IL-1α, IL-1β, and IL-6. In the case of aqueous extract, neither of the 2 utilized concentrations were able to significantly reduce IL-1α (Figure 4D), IL-1β (Figure 4E), or IL-6 (Figure 4F). Interestingly, the ethanolic extract was highly efficient in impairing the transcription of the 3 tested cytokines. Indeed, treating cells with 50 μg/ml of ethanolic extract caused about 75% reduction in mRNA levels of either cytokine, whereas 100 μg/ml of extract resulted in complete loss of transcription of the different cytokines (Figure 4D–4F). Methanolic extract also showed efficiency, even though less than that of ethanolic extract, in lowering IL-1α, IL-1β, and IL-6 transcription levels. For instance, upon treating cells with 50 μg/ml of methanolic extract, the mRNA levels of IL-1α, IL-1β, and IL-6 decreased by 60%, 10%, and 60% respectively (Figure 4D–4F). However, only minimal transcription of either cytokines was detected upon treating cells with 100 μg/ml of methanolic extract (Figure 4D–4F).
In a fourth step, and upon performing qRT-PCR, the mRNA levels of the pro-inflammatory chemokines CCL3 and CCL4 were evaluated. In the case of CCL3, 50 μg/ml of either extract was enough to lose about 75% of mRNA levels (Figure 4G). Although this percentage was only slightly increased to reach about 78% upon treating cells with 100 μg/ml of aqueous extract, it was more strikingly enhanced to reach about 99% and 95% in the case of ethanolic and methanolic extracts, respectively (Figure 4G). In the case of CCL4, aqueous extract moderately reduced CCL4 transcription, as about 45% of mRNA levels were lost upon exposure of cells to 50 but not 100 μg/ml of extract (Figure 4H). On the other hand, both ethanolic and methanolic extracts exhibited prominent efficiency in terms of impairing CCL4 transcription. Indeed, about 75% and 70% of CCL4 mRNA levels were absent following cell treatment with 50 μg/ml of ethanolic and methanolic extracts, respectively (Figure 4H). Moreover, only residual transcription was observed upon treating cells with 100 μg/ml of either extract (Figure 4H).
Antiproliferative activity of Ephedra campylopoda stem extracts
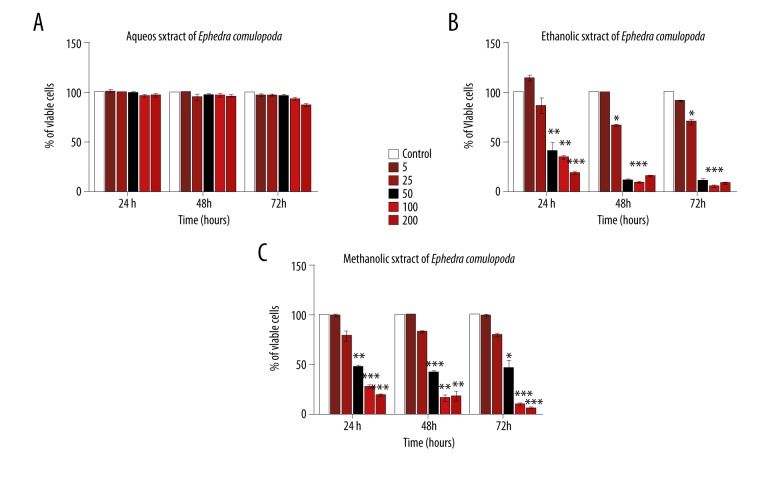
To evaluate the antiproliferative capacity of Ephedra campylopoda stem extracts, XTT assay was performed. This colormetric assay measures cell viability upon determining mitochondrial activity. Indeed, XTT, a yellow water-soluble substrate, can be converted by mitochondrial succinate dehydrogenase enzymes to a highly colored formazan product. This reaction occurs in viable but not dead cells, thus the quantity of the generated formazan is proportional to the amount of viable cells in the sample. Jurkat cells were treated with various fresh stem-derived aqueous, methanolic, or ethanolic extract concentrations (in range from 5 to 200 μg/mL) for different times (24, 48, or 72 h). Neither of the utilized aqueous extract concentrations exerted a significant effect on cell viability at any of the indicated time periods (Figure 5A). However, a dose-dependent inhibitory effect was detected in the case of ethanolic and methanolic extracts (Figure 5B, 5C). Time-impact on the observed inhibition was not striking since, for both extracts, the IC50 values (dose required to inhibit cell growth by 50%) calculated at the different time intervals were comparable. For instance, in the case of ethanolic extract, the IC50 values obtained after 24, 48, and 72 h were 40±2.88, 38 ± 1.54, and 35 ±1.54 μg/ml, respectively (Figure 5B) while in the case of methanolic extract, the IC50 values corresponded to 40±1.45, 48 ±3.51, and 50 ±7.73 μg/ml after 24, 48, and 72 h, respectively (Figure 5C).
Figure 5.
Impact of Ephedra campylopoda stem extracts on Jurkat cells proliferation. Cells were treated with various concentrations (0, 5, 25, 50, 100, 200 μg/ml) of stem extracts for 24, 48, and 72 h, and XTT assay was used to assess their antiproliferative potential. Each value represents a mean ±SEM for 3 independent experiments (n=3), each done in triplicate. Fresh stem-derived aqueous extract (A), ethanol extract (B), and methanol extract (C). * p<0.05; ** p<0.01, *** p<0.001 vs. control untreated cells (Student’s t-test).
Discussion
Humans have used plants as medicine throughout history. People all around the world still rely on herbs to relieve pain and heal sickness. Today, medicinal plants play an important role in modern medicine development since a large number of modern drugs are simple copies or synthetic modifications of natural chemical substances found in plants [25]. Nowadays, substantial research investments are devoted towards identifying and characterizing new medicinal plants. In the present study, we aimed at screening the chemical content of Ephedra campylopoda stem extracts and characterizing their pharmaceutical value. Our phytochemical analysis identified the presence of various important medicinal compounds, such as phenols, flavonoids, carbohydrates, proteins, diterpenes, and lignins in stem extracts prepared using aqueous, ethanolic, or methanolic solvents. Coumarins, sterols/steroids, and flavones were found in both of the alcoholic solvents but not the aqueous one. Alkaloids were detected only in the ethanolic extracts, while tannins, resin, terpenoids, reducing sugars, phlobatannins, and anthocyanin were detected in the methanolic extracts, specifically. Consistent with previous reports [26–28], our data indicate that the methanolic and ethanolic solvents extract are richer than aqueous extract in plant bioactive components. As the medicinal value of Ephedra campylopoda species is yet not fully defined, we therefore assessed their antioxidant, anti-inflammatory, and anti-proliferative activities.
Although they can mediate beneficial physiologic roles, overload of free radicals and reactive biomolecules that cannot be balanced or destroyed generates oxidative stress. This process is harmful for humans since it plays a major role in the initiation and development of chronic and degenerative pathologies such as cardiovascular and neurodegenerative diseases, autoimmune disorders, aging, and cancer [29]. Identifying plants with potent antioxidant potential is therefore of great interest. Here, we assessed the antioxidant capacity of Ephedra campylopoda extracts upon evaluating their ability to scavenge free DPPH radicals or chelate Fe2+ ions. Our data showed a varied antioxidant potential of Ephedra campylopoda extracts, in a manner dependent on the type of utilized solvent. In fact, the alcoholic solvents appeared to exert more potent antioxidant effect than the aqueous extract. This observation parallels the more important chemical content observed in the alcoholic- than water-derived extracts.
Nowadays, a tight association is established between oxidative stress and inflammation since free radicals can be a result or a cause of inflammation. Inflammation has been identified as a primary cause of numerous chronic diseases, including arthritis, atherosclerosis, Alzheimer’s, diabetes, heart disease, and cancer [30,31]. Immune signaling via pro-inflammatory cytokines and mediators is the major mechanism for inflammation initiation and amplification. Inhibiting the expression of these pro-inflammatory molecules is therefore essential to suppress the inflammatory responses. In this study, we evaluated the ability of these plant extracts to inhibit PGE2 secretion and suppress the transcription of pro-inflammatory cytokines (IL-α, IL-β, and IL-6), chemokines (CCL3 and CCL4), and inflammation-responsive COX-2 and iNOS enzymes. Varied plant extract-mediated suppressive capacities on these different pro-inflammatory components were observed. Indeed, alcoholic extracts were more efficient than aqueous extract in impairing PGE2 secretion and inhibiting IL-α, IL-β, IL-6, CCL3, CCL4, COX-2, and iNOS transcription. The prominent anti-inflammatory effect exerted by alcoholic extracts parallels their rich chemical arsenal as well as their substantial antioxidant activity. These observations highlight Ephedra campylopoda as a putative promising resource for designing novel inflammatory suppressive drugs and treating inflammatory disorders.
In the present work, using XTT viability assay, we assessed the ability of Ephedra campylopoda stem extracts to inhibit Jurkat cancer cell proliferation. In line with their low phytochemical content and moderate antioxidant and anti-inflammatory capacities, aqueous extract was inefficient in terms of suppressing cell growth. However, the alcoholic extracts exerted a prominent cytotoxic effect that appeared to be dose-dependent. This robust antiproliferative potential could be attributed to the prominent phytochemical content detected in the alcoholic solvents. So far, a mechanistic understanding of this cytotoxic effect is still lacking. A possible involvement of the apoptotic pathway in the observed impaired cell growth should be addressed in future studies. Moreover, whether Ephedra campylopoda stem extracts are cytotoxic for cancer cells and thus can be used in tumor therapy is the objective of our ongoing work.
Conclusions
In conclusion, the present study identified the presence of various medicinal constituents in Ephedra campylopoda stem extracts. Moreover, this plant exhibited prominent antioxidant, anti-inflammatory, and antiproliferative capacities, and thus can be suggested as a novel and promising therapy for a wide array of human diseases.
Acknowledgments
We thank Professor George Tohme for his valuable help in the identification of this plant, and the Toxicology Department, Faculty of Health Sciences, American University of Science and Technology, Beirut Lebanon, VP Amer Saker for technical support. We thank Dima healthcare company for their financial support.
Footnotes
Source of support: This work was supported by the Lebanese University and the Lebanese National Council for Scientific Research (CNRS-L)
Conflict of interest
All authors declare no conflict of interest.
References
- 1.Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137:183S–85S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 2.Jay Forman H, Torres M. Redox signaling in macrophages. Mol Aspects Med. 2001;22:189–216. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- 3.Valko M, Izakovic M, Mazur M, et al. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 4.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acharya A, Das I, Chandhok D, Saha T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid Med Cell Longev. 2010;3:23–34. doi: 10.4161/oxim.3.1.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Sun YN, Yan XT, et al. Anti-inflammatory and antioxidant activities of phenolic compounds from Desmodium caudatum leaves and stems. Arch Pharm Res. 2014;37:721–27. doi: 10.1007/s12272-013-0241-0. [DOI] [PubMed] [Google Scholar]
- 10.Huang W-Y, Cai Y-Z, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr Cancer. 2010;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 11.Talhouk RS, Karam C, Fostok S, et al. Anti-inflammatory bioactivities in plant extracts. J Med Food. 2007;10:1–10. doi: 10.1089/jmf.2005.055. [DOI] [PubMed] [Google Scholar]
- 12.Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–84. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Ravipati AS, Koyyalamudi SR, et al. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J Agric Food Chem. 2011;59:12361–67. doi: 10.1021/jf203146e. [DOI] [PubMed] [Google Scholar]
- 14.Adebayo SA, Dzoyem JP, Shai LJ, Eloff JN. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Complement Altern Med. 2015;15:159. doi: 10.1186/s12906-015-0669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Said O, Khalil K, Fulder S, Azaizeh H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J Ethnopharmacol. 2002;83:251–65. doi: 10.1016/s0378-8741(02)00253-2. [DOI] [PubMed] [Google Scholar]
- 16.Lev E, Amar Z. Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. J Ethnopharmacol. 2002;82:131–45. doi: 10.1016/s0378-8741(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 17.Marc EB, Nelly A, Annick D-D, Frederic D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J Ethnopharmacol. 2008;120:315–34. doi: 10.1016/j.jep.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Deeb T, Knio K, Shinwari ZK, et al. Survey of medicinal plants currently used by herbalists in Lebanon. Pak J Bot. 2013;45:543–55. [Google Scholar]
- 19.Abourashed EA, El-Alfy AT, Khan IA, Walker L. Ephedra in perspective – a current review. Phytother Res. 2003;17:703–12. doi: 10.1002/ptr.1337. [DOI] [PubMed] [Google Scholar]
- 20.Farhan H, Rammal H, Hijazi A, et al. In vitro antioxidant activity of ethanolic and aqueous extracts from crude Malva parviflora l. grown in Lebanon. Asian J Pharm Clin Res. 2012;5:234–38. [Google Scholar]
- 21.Dinis TC, Maderia VM, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–69. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 22.Scudiero DA, Shoemaker RH, Paull KD, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–33. [PubMed] [Google Scholar]
- 23.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 24.Ashley NT, Weil ZM, Nelson RJ. Inflammation: Mechanisms, costs, and natural variation. Annu Rev Ecol Evol Syst. 2012;43:385–406. [Google Scholar]
- 25.Hosseinzadeh S, Jafarikukhdan A, Hosseini A, Armand R. The application of medicinal plants in traditional and modern medicine: A review of Thymus vulgaris. Int J Clin Med. 2015;6:635–42. [Google Scholar]
- 26.Ahmad I, Mehmood Z, Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol. 1998;62:183–93. doi: 10.1016/s0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 27.Abdallah EM, Khalid AS, Ibrahim N. Antibacterial activity of oleo-gum resins of Commiphora molmol and Boswellia papyrifera against methicillin resistant Staphylococcus aureus (MRSA) Sci Res Essays. 2009;4:351–56. [Google Scholar]
- 28.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman T, Hosen I, Islam MMT, Shekhar HU. Oxidative stress and human health. Adv Biosci Biotechnol. 2012;3:997–1019. [Google Scholar]
- 30.Khan FA, Khan MF, Aziz F, Al KE. Inflammation and acute phase response. Int J Appl Biol Pharm Technol Page. 2010;1:312–21. [Google Scholar]
- 31.Schetter AJ, Heegaard NHH, Harris CC. Inflammation and cancer: Interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aiyegoro OA, Okoh AI. Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement Altern Med. 2010;10:21. doi: 10.1186/1472-6882-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fred-Jaiyesimi A, Ayotunde A. Phytochemical and pharmacognostic studies of Telosma africanum (N.E.Br) colville leaf and stem. IJPSR. 2012:3. [Google Scholar]
- 34.Khandelwal KR. A text book of practical Pharmacognosy. 27th ed. Nirali Prakashan; 2005. [Google Scholar]
- 35.Siddiqui AA, Ali M. Practical pharmaceutical chemistry. 1st ed. CBS publishers and distributors; New Delhi: 1997. [Google Scholar]
- 36.Rani A, Singh K, Ahuja PS, Kumar S. Molecular regulation of catechins biosynthesis in tea [Camellia sinensis (L.) O. Kuntze] Gene. 2012;495:205–10. doi: 10.1016/j.gene.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 37.Krishnaiah D, Devi T, Bono A, Sarbatly R. Studies on phytochemical constituents of six Malaysian medicinal plants. J Med Plants Res. 2009;3:67–72. [Google Scholar]
- 38.Mir Derikvand M, Sierra JB, Ruel K, et al. Redirection of the phenylpropanoid pathway to feruloyl malate in Arabidopsis mutants deficient for cinnamoyl-CoA reductase 1. Planta. 2008;227:943–56. doi: 10.1007/s00425-007-0669-x. [DOI] [PubMed] [Google Scholar]