Abstract
A pole estimating for each individual the number of praziquantel tablets needed for treatment according to the height was tested in 20 data sets (25,688 individuals). The tool determined a dose between 30 and 60 mg/kg in more than 98% of the cases and this is within the dose range that has proven efficacious and safe.
Keywords: Schistosomiasis, praziquantel, dosage determination
Introduction
Morbidity and mortality due to schistosomiasis can be controlled in a cost-effective manner by regular treatment with praziquantel directed at vulnerable groups. The core part of the strategy is the coverage of school age children as endorsed by the World Health Assembly in resolution WHA 54.19 in 2001.
One of the major operational drawbacks related to school or community based delivery of praziquantel is the fact that the dosage has to be calculated according to body weight (40 mg/kg –WHO; 1995). Provision and maintenance of weighing scales in the field is difficult and expensive, and their accuracy is often doubtful.
Hall et al. (1999) have developed three “tablet poles” - for Ghana, Tanzania and Malawi respectively - that estimate the number of praziquantel tablets needed to treat each individual according to height. Each “national” pole was developed and tested using data from school age children in one country. Height was found to provide an accurate estimate of weight, as about 75% of children in the three countries would have received a dose of praziquantel within the range of 36-44 mg/kg.
This study attempts to define a single “tablet pole”, suitable to treat schoolchildren in all sub-Saharan Africa, and to assess its validity in different countries.
Materials and methods
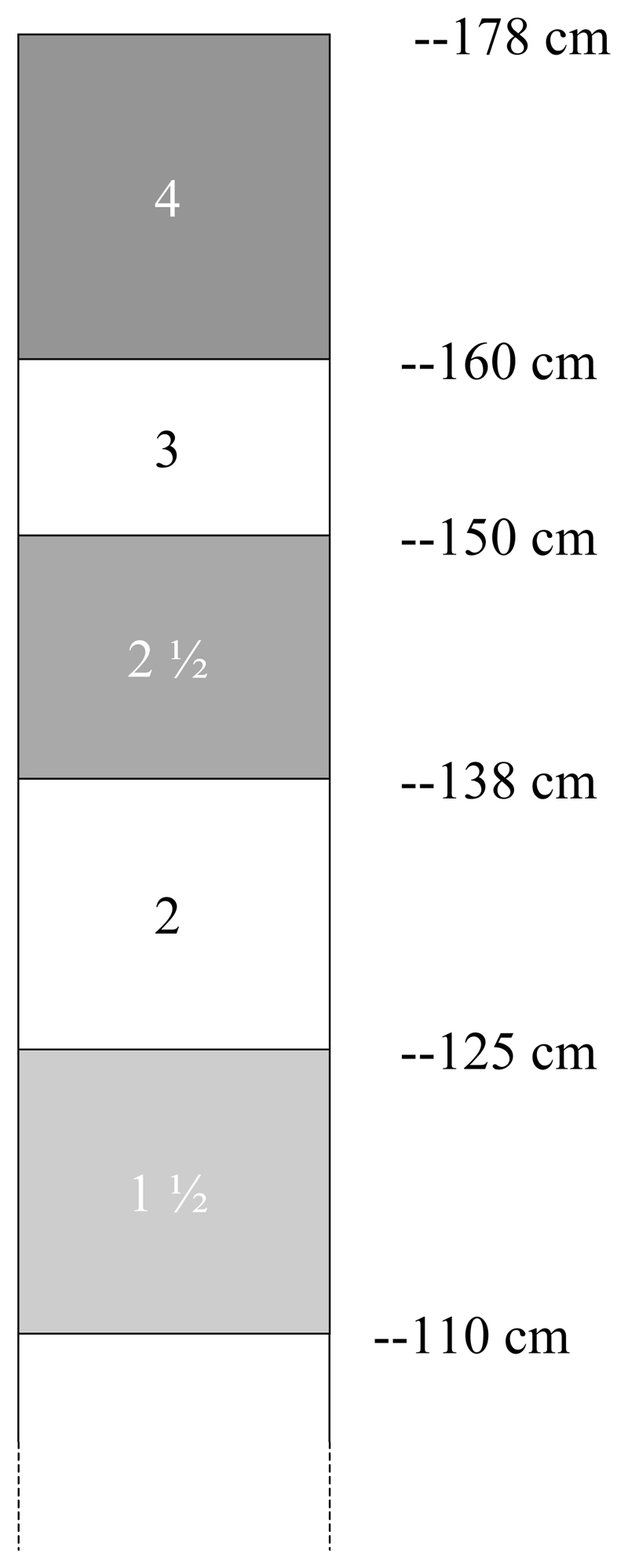
The single “tablet pole” was developed using the method described by Hall et al. (1999), from data collected during a school based survey in Guinea in 2000 (n= 1667 age range 6-19 years). Height was measured to a precision of 1 cm; weight to a precision of 0.1 kg. The pole was developed to deliver a dose of 40-60 mg/kg, in order to avoid under-dosage and was designed to identify 5 height intervals corresponding to 1½, 2, 2 ½, 3 and 4 tablets of praziquantel (Figure).
Figure.
The “tablet pole” consisting of five height intervals and the praziquantel dosage (in tablets) assigned to each interval. Different colours are proposed for each interval in order to facilitate the reading in the field.
The performance of the pole was tested on 20 other data sets (totalling 25,710 individuals in 10 countries). Twenty-two records (0.8%) were excluded from the analysis because mentioning a weight more than 3 standard deviations lower than expected for the height (WHO 1993) and were considered results of data entry errors. The height of each of the remaining 25,688 individuals was classified in one of the intervals identified by the “tablet pole” and the number of tablets that each individual would have received according to the pole was identified. The dosage, in mg/kg, was then calculated by dividing the total dose by the real weight of each individual registered in the data set.
For each data set, the following parameters were calculated: (I) the number of individuals of which the height was within the interval identified by the pole (110-178 cm); (II) the percentage of individuals that would have received appropriate dosages of 30-40 mg/kg, and 40-60 mg/kg, respectively (Taylor et al., 1988; WHO, 1995); (III) the percentage that would have received a dosage of less than 30 mg/kg, considered to be sub-curative and therefore of concern in view of the possible development of drug resistance (Bittencourt 1990); (IV) the percentage of individuals that would have received a dosage of more than 60 mg/kg, considered to be of concern because of the possible occurrence of side effects; (V) the minimum and maximum dose that would have been administered.
Results
Of the 25,688 individuals, 1055 (4.1%) had a height which did not fit the interval identified by the pole (110 - 178 cm).The pole being tested is capable of determining a dose between 30-60 mg/kg - generally considered to be appropriate (Taylor et al., 1988; WHO, 1995a) - in more than 97% of the cases and of indicating a dose between 40-60 mg/kg to more than 80% of individuals with heights ranging between 110 cm and 178 cm. Detail of the results obtained in the different data sets, by country, are presented in the Table.
Table.
The percentage of individuals, by country who would have received a dosage of praziquantel within, below or above the appropriate dosage when the number of drug tablets were calculated with the “tablet pole”.
| Country | Data collected within | Age range | Sample size | within the pole interval | <30 mg/kg | ∃30 - #60 | >60 mg/kg | Minimal dose received | Maximal dose received | |
|---|---|---|---|---|---|---|---|---|---|---|
| ∃30 <40 | ∃40 #60 | |||||||||
| Chad | School surveys | 6-18 | 1017 | 1014 | -- | 71 (7.0%) |
939 (92.6) |
4 (0.4%) |
34 | 68 |
| Ghana (Volta Region) |
8-13 | 2198 | 2179 | 1 (0.0%) |
193 (8.9%) |
1969 (90,3%) |
16 (0.8%) |
27 | 84 | |
| Guinea (data set different from the one used to develop the pole) |
6-20 | 1362 | 1306 | 0 | 167 (12.8%) |
1079 (82.6%) |
60 (4.6%) |
30 | 80 | |
| Kenya (Busia District) |
6-20 | 2123 | 2121 | 17 (0.8%) |
716 (33.7%) |
1382 (65.2%) |
6 (0.3%) |
24 | 69 | |
| Mali | 6-17 | 1195 | 1169 | -- | 102 (8.7%) |
1063 (91%) |
4 (0.3%) |
32 | 65 | |
| Mozambique | 5-18 | 995 | 969 | -- | 198 (20.4%) |
769 (79.4%) |
2 (0.2%) |
30 | 66 | |
| Tanzania (Unguja – Pemba – Mwanza -Tanga) |
1-19 | 11247 | 11165 | 81 (0.7%) |
1318 (11.8%) |
9683 (86.7%) |
83 (0.8%) |
18 | 86 | |
| Sub total School surveys | 0.5% | 13.8%* | 84.8%* | 0.9% | ||||||
| Madagascar (Titikanana and Tananarivo) |
Population surveys | 1-83 | 526 | 434 | 10 (2.4 %) |
202 (46.5%) |
222 (51.1%) |
0 | 21 | 60 |
| Mali (Dogon and Office du Niger) |
1-83 | 1333 | 1120 | 15 (1.3%) |
323 (28.9%) |
763 (68.1%) |
19 (1.7%) |
17 | 80 | |
| Senegal (Ndiangué and Ndombo) |
1-84 | 1193 | 971 | 46 (4.7 %) |
214 (22.0%) |
627 (64.6%) |
84 (8.7%) |
19 | 80 | |
| Tanzania (Ukerewe) |
1-75 | 1112 | 973 | 25 (2.6%) |
319 (32.8%) |
627 (64.4%) |
2 (0.2%) |
22 | 63 | |
| Uganda (Rhino C. and Obonge) |
1-76 | 1387 | 1212 | 7 (0.6 %) |
226 (18.6%) |
971 (80.1%) |
8 (0.7%) |
25 | 71 | |
| Sub total population surveys | 2.1% | 27.3% | 68.2% | 2.4% | ||||||
| TOTAL SAMPLE | 25688 | 24,633 |
202 (0.8%) |
4,049 (16.4%) |
20,094 (81.6%) |
288 (1.2%) |
||||
| 24,143 (98%) | ||||||||||
Significantly different (p<0.001) from the result in population surveys
The pole was capable of determining a dose between 40-60 mg/Kg to more than 84% of the children in school survey while in the population surveys this capacity was of 68% (p<0.001).The pole was capable of determining a dose between 30-60 mg/Kg to more than 98% in school survey and to more than 95% in the population surveys.
In total, only 6 individuals (0.02%) would have received less than 20 mg/kg and 288 (1.2%) would have received more than 60 mg/kg. 4 individuals (0.02%) would have received more than 80 mg/kg.
Discussion
The “dose pole” has been developed based on data from Guinea that were collected with particular accuracy for this purpose. However, the same procedure applied to data sets from other countries and from the combination of all the data sets (n=27,355) gave very similar results in term of threshold (differences ranging between 0.6-2.5 cm if compared with the pole under test) and in the capacity of giving appropriate dosage.
Approximately 4% of the tested individuals had a height outside this range. However, most of them (n=588) were pre-school children, who not targeted for regular treatment campaigns. The tested pole was designed to deliver an average dose of 40-60 mg/kg in order to minimize under-dosage. Its performance therefore cannot strictly be compared with those of the poles developed in Ghana, Tanzania and Malawi.
The better performance of the pole in the school-age population was due to the fact that, following sexual maturity, hormonal influences act on body structure (WHO 1993) and the population weight/height rate became more heterogeneous. In spite of this the performance of the pole in population survey data is considered satisfactory.
An under-dosage in 2.1% of the cases is unlikely to be of concern in relation to the development of drug resistance. A dose of 60-80 mg/kg in another 2.4% of the cases is unlikely to be of concern either, since praziquantel is known to be well tolerated (WHO, 1995a), and daily dosages of 100 mg/kg for 10 days have been safely used for neurocysticercosis (Bittencourt et al., 1990).
Our findings strongly suggest that the proposed pole is suitable for use in most of the sub-Saharan African countries, both in school and community based delivery of praziquantel. Because of the significant advantages of its use. we invite control managers and researchers to further test this tool in the field.
Acknowledgements
We wish to thank the Ministry of Health of Chad, Ghana, Guinea, Kenya, Madagascar, Mali, Mozambique, Senegal, Tanzania, Uganda, Zanzibar, and all the personnel in these countries involved in the data collection.
We also thank: Chad (M.Baboguel M.Beasley, E.Djenguinabe, E Mobele, M. Ndinardmtan), Ghana (Ghana Partnership for Child Development), Guinea (B.Bha, B.Camara), Kenya (T.Miguel, S.Moulin and Busia ICS staff), Madagascar (E. Doehring), Mali (R.Kardoff, F.Ouattara, N.Roschnik, J de Rosso, M.Sacko, M.Traore, U.Vester, and Save the Children Federation), Mozambique (E.Bobrow, J de Rosso, B.J. Mahumane, A. Zacher, and Save the Children Federation), Senegal (R.Kardoff), Tanzania (K.S. Alawi, M. Albonico, M. Beasley, A.Foum, R.Kardoff, N.Lwambo), Uganda (E. Doehring) for having supplied the databases which enabled us to validate the proposed pole. We are further grateful to A. Hall for his suggestions and advice. S.B. is in receipt of a Wellcome Trust Prize Fellowship (#062962).
References
- Bittencourt PR, Gracia CM, Gorz AM, Mazer S, Oliveira TV. High-dose praziquantel for neurocysticercosis: efficacy and tolerability. European Neurology. 1990;30:229–34. doi: 10.1159/000117352. [DOI] [PubMed] [Google Scholar]
- Deenhoff MJ. Is schistosomicidal chemotherapy sub-curative? Implication for drug resistance. Parasitology Today. 1998;14:434–435. doi: 10.1016/s0169-4758(98)01315-5. [DOI] [PubMed] [Google Scholar]
- Hall A, Nokes C, Wen ST, Adjei S, Kihamia C, Mwanri L, Bobrow E, de Graft-Johnson J, Bundy D. Alternatives to bodyweight for estimating the dose of praziquantel needed to treat schistosomiasis. Transaction of the Royal Society of Tropical Medicine and Hygiene. 1999;93:653–8. doi: 10.1016/s0035-9203(99)90087-1. [DOI] [PubMed] [Google Scholar]
- Taylor P, Murare HM, Manomano K. Efficacy of low doses of praziquantel for Schistosoma mansoni and S. haematobium. Journal of Tropical Medicine and Hygiene. 1988;91:13–7. [PubMed] [Google Scholar]
- WHO. Measuring change in nutritional status. World Health Organization; Geneva: 1993. Annex 3: Reference data for the weight and height of children. [Google Scholar]
- WHO. Model Prescribing Information. Drugs used in parasitic diseases. 2nd edition. World Health Organization; Geneva: 1995. [Google Scholar]