Abstract
Antiangiogenic tyrosine kinase inhibitors (TKI) that target VEGF receptor-2 (VEGFR2) have not been effective as adjuvant treatments for micrometastatic disease in phase III clinical trials. Angiopoietin-2 (Ang2) is a proangiogenic and proinflammatory vascular destabilizer that cooperates with VEGF. The purpose of this study was to test whether CVX-060 (an Ang2-specific CovX-body) can be combined with VEGFR2-targeting TKIs (sunitinib or regorafenib) to successfully treat postsurgical metastatic disease in multiple orthotopically implanted human tumor xenograft and syngeneic murine tumor models. In the MDA-MB-231.LM2-4 human breast cancer model, adjuvant sunitinib was ineffective, whereas adjuvant CVX-060 delayed the progression of pulmonary or distant lymphatic metastases; however, overall survival was only improved with the adjuvant use of a VEGF-A/Ang2-bispecific CovX-body (CVX-241) but not when CVX-060 is combined with sunitinib. Adjuvant CVX-241 also showed promise in the EMT-6/CDDP murine breast cancer model, with or without an immune checkpoint inhibitor (anti-PD-L1). In the RENCA model of mouse renal cancer, however, combining CVX-060 with sunitinib in the adjuvant setting was superior to CVX-241 as treatment for postsurgical lung metastases. In the HCT116 and HT29 xenograft models of colorectal cancer, both CVX-060 and regorafenib inhibited liver metastases. Overall, our preclinical findings suggest differential strategies by which Ang2 blockers can be successfully combined with VEGF pathway targeting in the adjuvant setting to treat micrometastatic disease—particularly, in combination with VEGF-A blockers (but not VEGFR2 TKIs) in resected breast cancer; in combination with VEGFR2 TKIs in resected kidney cancer; and as single agents or with VEGFR2 TKIs in resected colorectal cancer.
Introduction
Antiangiogenic drugs that target the VEGF signaling pathway have successfully expanded clinical treatment options for many cancer types in the advanced metastatic disease setting. For patients with metastatic colorectal cancer, bevacizumab (an antibody to the VEGF-A ligand) with chemotherapy is an approved first-line treatment, whereas regorafenib [a VEGF receptor-2 (VEGFR2)-targeting tyrosine kinase inhibitor (TKI)] is an approved salvage therapy. For patients with metastatic renal cell carcinoma (mRCC), approved first-line therapies include sunitinib and pazopanib (both VEGFR2-targeting TKIs), as well as bevacizumab plus IFNα. For patients with metastatic breast cancer, bevacizumab with chemotherapy remains an approved treatment in Europe and Asia.
In contrast, these same VEGF pathway–targeted agents have repeatedly failed to improve overall survival (OS) in phase III clinical trials in the adjuvant (postsurgical) setting when directed at micrometastatic disease (Supplementary Table S1). In one of the colorectal cancer trials (AVANT), the OS curves even crossed-over to show a detrimental effect (1). Interestingly, however, the survival analyses sometimes initially appeared favorable for the antiangiogenic therapy groups, but these transient advantages faded over time after adjuvant therapy was stopped (1–3). These results suggest that the transient benefits of adjuvant VEGF inhibition may be significantly improved if therapy was lengthened or combined with certain other drugs.
The negative adjuvant trial results (Supplementary Table S1) have also incited the following questions: In the AVANT trial, could anti-VEGF therapy have inadvertently increased the malignancy of residual disease (1), akin to how in preclinical studies such therapies can have both tumor growth–inhibiting and proinvasive/metastatic effects (4, 5)? Do early-stage micrometastases actually depend on VEGF-driven tumor angiogenesis, or is tumor cell co-option ("hijacking") of existing host blood vessels more relevant when metastatic tumor cells colonize vascular-rich distant organs (6–8)? In the adjuvant antiangiogenic TKI trials, could secondary targeting of the platelet-derived growth factor receptor (PDGFR) and VE-cadherin pathways have compromised the main activity of these broad-spectrum TKIs stemming from VEGFR2 inhibition? At least in preclinical studies, the disruption of pericytes and blood vessel integrity via PDGFR/VE-cadherin inhibition have been observed to counterproductively promote metastatic dissemination even while suppressing tumor growth (9–11).
Angiopoietin-2 (Ang2)—a ligand of the Tie2 receptor—is a proangiogenic, proinflammatory, and prometastatic cytokine that cooperates with VEGF (12). With the above considerations in mind, we hypothesized that Ang2 inhibition might be a superior approach than VEGF/VEGFR2 inhibition for treating postsurgical micrometastatic disease. First, no paradoxical invasion- or metastasis-promoting effects have yet been linked to Ang2-targeted therapies. Second, Ang2 has an implicated role in host vessel co-option (13). Third, pericyte depletion by imatinib (a PDGFR-targeting but not VEGFR2-targeting TKI) in an unresected 4T1 murine breast tumor model was shown to increase lung metastases, a side effect that was counteracted by an Ang2 antibody (LC06; Roche; ref. 14). Fourth, endothelial cell–cell junctions were reinforced in tumor-associated blood vessels in Colo205 colorectal cancer xenografts after Ang2 inhibition [L1-7[N] peptibody; Amgen; ref. 15], as well as in lung metastases–associated blood vessels in a B16-F10 melanoma model after Ang2 inhibition (MEDI3617 antibody; MedImmune; ref. 16). Fifth, although tumor burden tends to be associated with Ang2 elevations (12, 17), tumor debulking by surgery does not necessarily reverse Ang2 dysregulations, as surgical trauma itself induces angiogenic and inflammatory cytokines. Significant elevations in plasma Ang2 and VEGF have been observed in patients after tumor resections compared with preoperative levels, with longer lasting upregulations seen in Ang2 (up to 4 weeks) than in VEGF (18, 19).
The purpose of this study was to evaluate the combination of an anti-Ang2 agent (CVX-060; ref. 20) with antiangiogenic VEGFR2-targeting TKIs (sunitinib or regorafenib) in 5 independent preclinical models of postsurgical micrometastatic disease, using the MDA-MB-231.LM2-4 human mammary, EMT-6/CDDP murine mammary, HCT116 and HT29 human colorectal, and RENCA murine renal adenocarcinoma cell lines.
Materials and Methods
Cell culture
EMT-6/CDDP (a cisplatin-resistant variant of EMT-6) was originally derived in the laboratory of Dr. Beverly Teicher (21) and provided to the Kerbel laboratory in 1991. All other tumor cell lines used were derived as described in our prior publications: LM2-4 (a metastatic variant of MDA-MB-231 derived in 2004; refs. 22, 23); LM2-4luc16 [a luciferase-tagged variant of LM2-4 (5) that had undergone additional in vivo selection for metastasis in 2014]; and HCT116luc, HT29luc, RENCAluc, all luciferase-tagged in 2009 (24, 25). Tumor cell lines were passaged in DMEM high-glucose media with 5% FBS and harvested at 80% confluence for in vivo implantation. Cell lines were tested quarterly for mycoplasma (MycoAlert kit; Lonza), last subjected to DNA authentication testing in 2013 (Genetica DNA Laboratories), and last screened for mouse viruses in 2016 (Charles River Research). See Supplement for details.
Human tumor xenograft and syngeneic murine tumor studies
Animal use protocols were approved by the Sunnybrook Research Institute Animal Care Committee, accredited by the Canadian Council of Animal Care. YFP-SCID mice (26) were bred in-house, and BALB/c mice were purchased from Jackson Laboratory. Breast cancer models involved orthotopically implanting 1 × 106 LM2-4(luc16) cells or 2.5 × 105 EMT-6/CDDP cells suspended in 50 μL of serum-free media (SFM) into the right inguinal mammary fat pads of 8- to 12-week-old female YFP-SCID mice or BALB/c mice, respectively. Breast tumor volumes were calculated as 0.5 × width2 × length. Colorectal cancer models involved orthotopic implantation of 1 mm3-sized HCT116luc or HT29luc tumor fragments into the cecal wall or the intrasplenic implantation of 5 × 105 HCT116luc cells in 5 μL SFM with splenectomy 2 minutes later, using 8- to 12-week-old male YFP-SCID mice (24). Renal cancer models involved orthotopically implanting 1 × 105 RENCAluc cells in 5 μL SFM into the left renal subcapsule of 6- to 8-week-old male BALB/c mice (25). Bioluminescent imaging was performed on an IVIS 200 system (PerkinElmer) and analyzed with Living Image software (v2.50.1, Xenogen) as previously described (27). Tumor resections [mastectomies (22), cecectomies (24), nephrectomies (25)] were performed as previously described. See Supplement for details on endpoint criteria and statistical tests for survival experiments.
Drug preparation for in vivo administration
Sunitinib malate (LC Laboratories) and its vehicle, formulated as previously described (5), were delivered by oral gavage (per os). Regorafenib monohydrate (Bayer HealthCare) was dissolved in a vehicle formulation containing 1,2-propanediol (34% w/v), PEG-400 (34% w/v), Kolliphor P188 (12% w/v), and Super-Q water (20% v/v) for per os administration. CVX-060/CVX-2000/ CVX-241 (Pfizer), DC101 (ImClone/Eli Lilly), and an antibody to mouse PD-L1 (clone 10F.9G2, BioXCell #BE0101) were diluted in PBS for intraperitoneal administration.
ELISA measurement of angiogenic factors
Harvested lung and kidney tissues were snap-frozen in liquid nitrogen and homogenized in RIPA lysis buffer (27). Blood samples obtained via terminal cardiac punctures were collected in heparin-coated plasma-separating tubes (BD Microtainer #365985) and centrifuged for 10 minutes at 1,000 RCF (g) to isolate plasma. Quantikine ELISA kits were used to measure murine Ang2 (R&D #MANG20), VEGF (#MMV00), PDGF-BB (#MBB00), and erythropoietin (EPO; #MEP00) levels in plasma or tissue homogenates.
Immunohistochemistry
To detect human tumor cells in formalin-fixed, paraffin-embedded mouse liver tissues, 5-μm-thick sections were depar-affinized, quenched in 1% H2O2, unmasked in boiling sodium citrate buffer (10 mmol/L, pH 6, 5 minutes), incubated in serum-free protein block (Dako X0909) before staining with a mouse monoclonal antibody to human HLA Class 1 ABC (Abcam ab70328; 1:200 in Dako S3022 diluent), followed by the universal "LSAB+, HRP" detection kit (Dako K0690) and DAB+ chromogen substrate system (Dako K3468) and hematoxylin counterstaining. Serial liver sections (100 μm apart) were visually surveyed in whole for DAB-stained metastatic nodules and imaged at 100× (Leica DM LB2 microscope, DFC300FX camera). Metastatic nodule areas were quantified using ImageJ 1.47v software and normalized to the total liver section area.
Statistical analysis
GraphPad Prism software was used.
Results
Sunitinib versus Ang2-targeting CVX-060 in an orthotopic primary breast tumor model
LM2-4 and LM2-4luc16 are aggressively metastatic variants of the human breast cancer cell line MDA-MB-231 derived through serial in vivo selection of spontaneous lung metastases after resection of orthotopic primary tumors (22, 23).
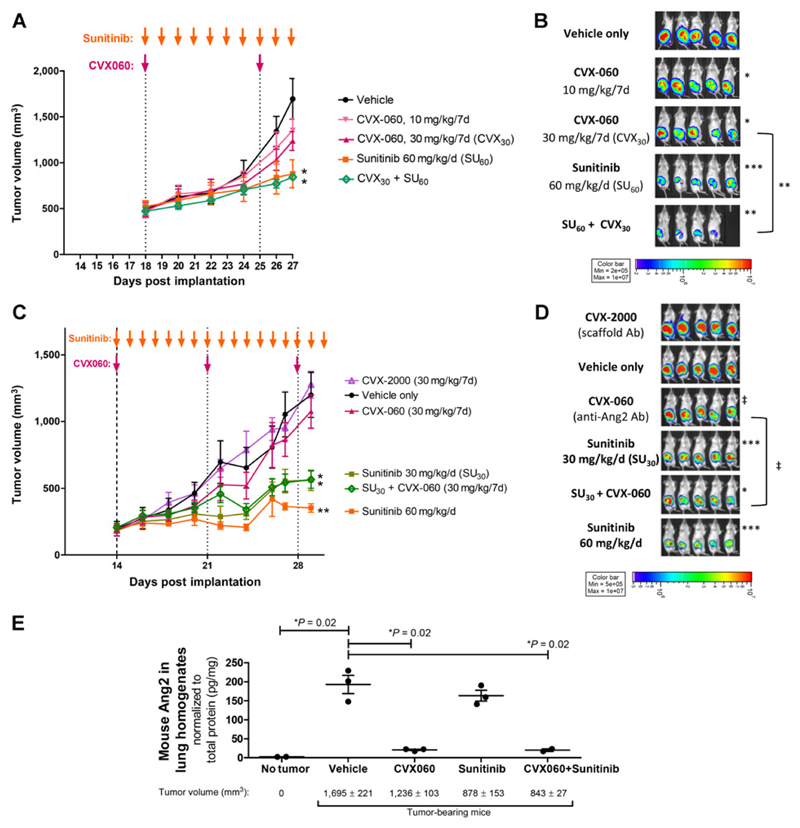
In 2 separate experiments, mice with established orthotopic primary LM2-4luc16 breast tumors were randomized and treated—for 10 days (Fig. 1A and B) and 17 days (Fig. 1C and D), respectively—with vehicle, sunitinib (30 or 60 mg/kg daily), CVX-060 (10 or 30 mg/kg weekly), or the combination. At either dose, single-agent sunitinib was much more potent than single-agent CVX-060 at inhibiting primary tumor growth, as measured by volume or bioluminescence (Fig. 1A–D). Concurrent CVX-060 did not improve the antitumor efficacy of sunitinib (Fig. 1A–D). Gross examination of primary tumors revealed a much less hemorrhagic phenotype after sunitinib treatment compared with CVX-060 treatment, suggesting much weaker antiangiogenic effects of CVX-060 compared with sunitinib (Supplementary Fig. S1).
Figure 1.
Sunitinib versus Ang2-targeting CVX-060 in an orthotopic primary breast tumor model. Two experiments involving 10-day (A and B) or 17-day (C and D) treatment of mice bearing LM2-4luc16 breast tumors with vehicle (negative control), CVX-2000 (scaffold antibody for CVX-060), CVX-060 (Ang2-specific CovX-body), sunitinib (broad-spectrum TKI that potently inhibits VEGFR2), or CVX-060 + sunitinib. A and C, Tumor growth over time. Final tumor volumes were compared by t tests (*, P < 0.05; **, P < 0.01; n = 5; therapy vs. vehicle). Means ± SEM are depicted. Arrows, dosing schedules. B and D, Tumor bioluminescence, measured at 26 and 28 DPI, respectively, were log-transformed prior to t tests (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ‡, P < 0.10; n = 5; vs. vehicle unless otherwise indicated). E, ELISA measurements of murine Ang2 in lung homogenates derived from healthy (tumor-free) control mice versus mice bearing LM2-4luc16 breast tumors after 10-day treatments with vehicle alone, CVX-060 (30 mg/kg/wk), sunitinib (60 mg/kg/d), or CVX-060 + sunitinib. Ang2 protein levels normalized to total protein levels (pg/mg) were compared by t tests. Posttreatment tumor volumes are shown as mean ± SEM.
To confirm "on-target" Ang2-neutralizing activity of CVX-060 at the site of metastases, we measured murine Ang2 protein levels in lung homogenates by ELISA (Fig. 1E). Although basal Ang2 was very low in healthy (tumor-free) mice, the presence of mammary tumors caused a marked upregulation of host Ang2 in the lungs (Fig. 1E). This mirrors clinical observations of higher systemic Ang2 levels in patients with breast cancer than in healthy controls (28, 29). While sunitinib monotherapy effectively suppressed mammary tumor growth (Fig. 1A), it did not provide a proportional correction of the elevated pulmonary Ang2 (Fig. 1E). Instead, CVX-060 was necessary to effectively reverse the tumor-associated upregulation in pulmonary Ang2 (Fig. 1E). This observation, together with the fact that Ang2 is further elevated by surgical trauma (18, 19), provides a rationale for testing sunitinib + CVX-060 in the adjuvant setting.
Although Ang2 was traditionally described as a Tie2 antagonist, recent studies have uncovered contexts in which Ang2 acts agonistically via Tie2 (e.g., in relation to lymphangiogenesis, Tie2-expressing monocytes, and tumor-associated endothelial cells; ref. 30). Under these contexts, sunitinib (being a modest inhibitor of Tie2; Supplementary Fig. S2) will not have significant overlapping activity compared with CVX-060, whereas regorafenib (being a potent inhibitor of Tie2; Supplementary Fig. S2) may mechanistically overlap with CVX-060.
Adjuvant sunitinib versus Ang2-targeting CVX-060 in a model of resected primary breast cancer
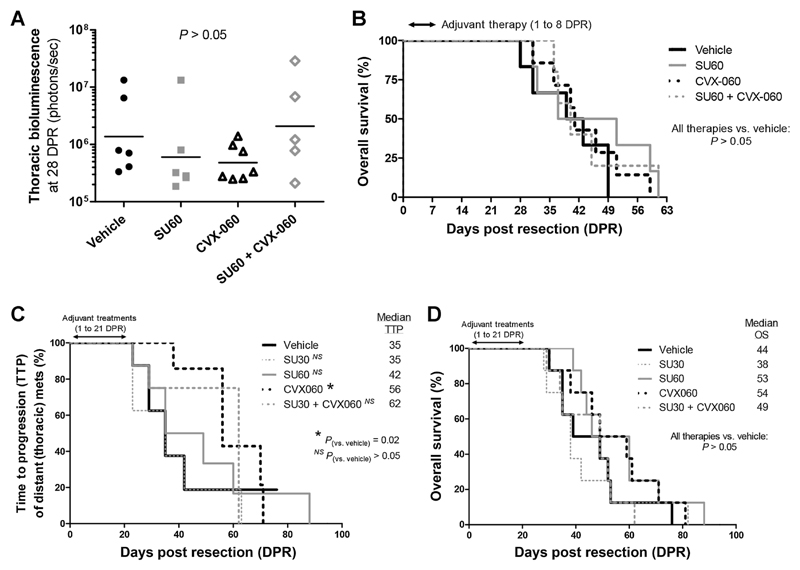
To model postsurgical micrometastatic disease, adjuvant therapies were initiated 1 or 2 days after resecting established orthotopic LM2-4 or LM2-4luc16 primary breast tumors. Adjuvant therapies lasted 7 days (Fig. 2A and B), 21 days (Fig. 2C and D), or 35 days (Fig. 3A), respectively, in 3 separate experiments.
Figure 2.
Effects of adjuvant Ang2-targeting CVX-060 versus sunitinib therapy on distant dissemination of breast cancer and postsurgical OS. Two experiments where orthotopic primary LM2-4luc16 breast tumors averaging 400 mm3 were resected at around 21 DPI to model the adjuvant therapy setting. Sunitinib (SU30, 30 mg/kg/d; SU60, 60 mg/kg/d), CVX-060 (30 mg/kg/wk), and their combinations were given as 1-week (A and B; n = 5–7) or 3-week (C and D; n = 8) adjuvant treatments. A, Bioluminescent quantification of thoracic metastatic burden. Log-transformed fluxes were analyzed by the Mann–Whitney test. Geometric means are depicted. B and D, Kaplan–Meier analysis of OS. C, Kaplan–Meier analysis of TTP of distant thoracic metastases. TTP and OS curves were subjected to the Gehan–Breslow–Wilcoxon test.
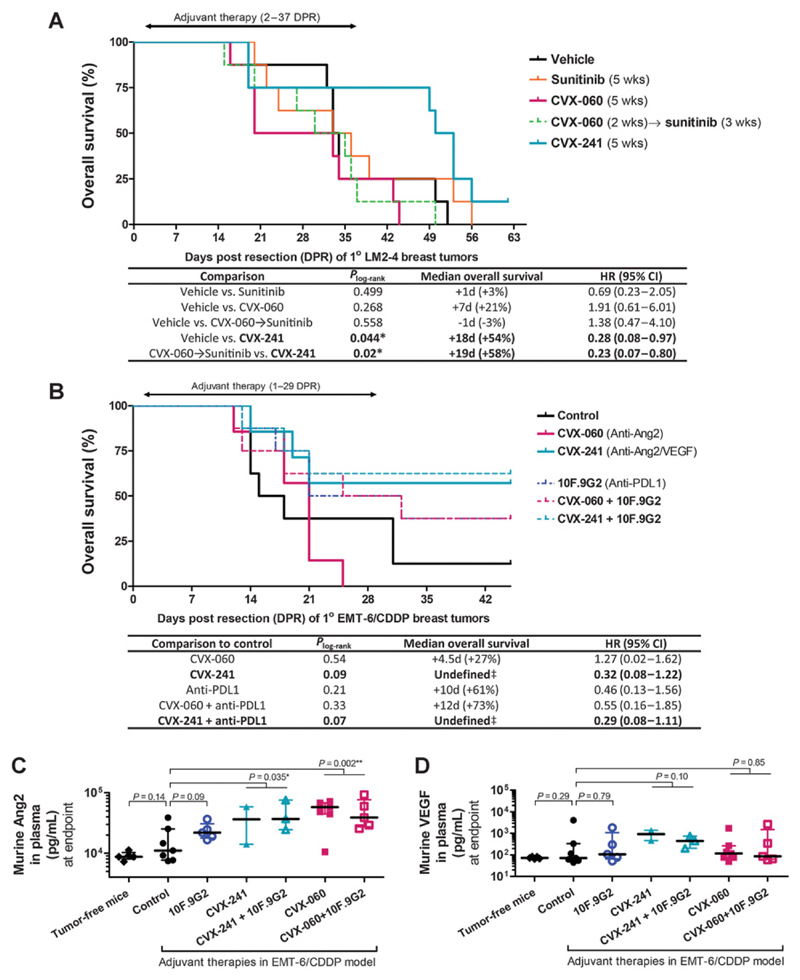
Figure 3.
Adjuvant bispecific targeting of VEGF-A and Ang2 via CVX-241 after resection of primary breast tumors. A and B, Kaplan–Meier analysis of OS. Orthotopic primary breast tumors averaging 400 mm3 were resected at 21 or 10 DPI in the LM2-4 (A; n = 8) or EMT-6/CDDP (B; n = 7–8) models, respectively. Adjuvant therapies were initiated 1 or 2 days later, involving sunitinib (VEGFR2 TKI; 60 mg/kg/d), CVX-060 (anti-Ang2; 30 mg/kg/wk), CVX-241 (anti-VEGF/Ang2; 30 mg/kg/wk), 10F.9G2 (anti-PD-L1; 5 mg/kg, 2×/wk), or combinations thereof. P values were derived using the log-rank test. Of the mice that had survived until the termination date of each experiment (62 and 45 days post-resection in A and B, respectively), none had visible lung metastases by necropsy. C and D, ELISA measurements of circulating murine Ang2 and VEGF protein in endpoint plasma samples from the EMT-6/CDDP experiment above. Medians with interquartile ranges are shown. P values were derived from t tests on log-transformed data.
Unlike in the primary tumor therapy setting (Fig. 1), sunitinib was ineffective as a postsurgical adjuvant therapy in this breast cancer model (Fig. 2). With 7-day adjuvant therapy, single-agent CVX-060 was the most promising in terms of inhibiting metastatic dissemination to the lungs or thoracic lymph nodes, as measured by bioluminescence (Fig. 2A and Supplementary Fig. S3A). These beneficial trends did not, however, translate into OS benefits (Fig. 2B). With extended (21-day) adjuvant CVX-060 monotherapy, a significant delay in the time-to-progression (TTP) of distant thoracic metastases was observed (P = 0.02, Fig. 2C and Supplementary Fig. S3B). This occurred in the absence of a global OS benefit (Fig. 2D), in part due to ineffective control of abdominal tumor regrowths (Supplementary Fig. S3B).
Not only did sunitinib as a single-agent fail to show benefit in terms of inhibiting distant metastases but also its combination with CVX-060 appeared inferior to CVX-060 monotherapy (Fig. 2A and C). We further tested whether the combination might be improved if given sequentially, that is, 2 weeks of CVX-060 followed by 3 weeks of sunitinib, but this too did not yield an OS benefit (Fig. 3A).
Adjuvant bispecific targeting of VEGF and Ang2 via CVX-241 in two models of resected primary breast cancer
Instead, 35-day adjuvant monotherapy with CVX-241, a CovX-body that binds both Ang2 and VEGF-A (31), significantly prolonged OS with an HR of 0.28 [95% confidence interval (CI), 0.08–0.97; Fig. 3A] in the LM2-4 model. This result was reproduced in the EMT-6/CDDP murine breast cancer model, where 28-day adjuvant CVX-241 therapy prolonged OS by a similar magnitude (HR, 0.32; 95% CI, 0.08–1.22, Fig. 3B). Of note, long-term survival was greater in mice receiving CVX-241 (57%) than in mice receiving either CVX-060 (0%) or an antibody to PD-L1 (37.5%), an immune checkpoint protein expressed by EMT-6/ CDDP tumor cells (Fig. 3B). Combining this anti-PD-L1 agent (clone 10F.9G2, BioXCell) with adjuvant CVX-241 therapy only slightly increased long-term survival from 57% to 63% (Fig. 3B).
Consistent with clinical observations of dramatically elevated plasma concentrations of VEGF after anti-VEGF therapies (32) or of Ang2 after anti-Ang2 therapies (33) in patients, we also observed higher total plasma Ang2 after CVX-241 or CVX-060 treatment (∼4- to 6-fold, P < 0.05, Fig. 3C) as well as higher total plasma VEGF after CVX-241 treatment in mice (∼10×, P = 0.10, Fig. 3D). Such elevations are known to consist largely of drug-bound ligands, as opposed to free ligands (33, 34). In contrast, the slightly elevated total plasma Ang2 after anti-PD-L1 treatment (∼2×, P = 0.09, Fig. 3D) would consist entirely of free Ang2.
To recap, our xenograft and syngeneic breast cancer models together predict the ineffectiveness of sunitinib, CVX-060 (anti-Ang2), and sunitinib + CVX-060 as postsurgical adjuvant therapies for breast cancer while identifying CVX-241 (anti-Ang2/VEGF) as the most promising strategy forward with the potential to significantly improve OS with or without concurrent PD-L1 inhibition.
Regorafenib versus Ang2-targeting CVX-060 in two primary orthotopic colorectal tumor models
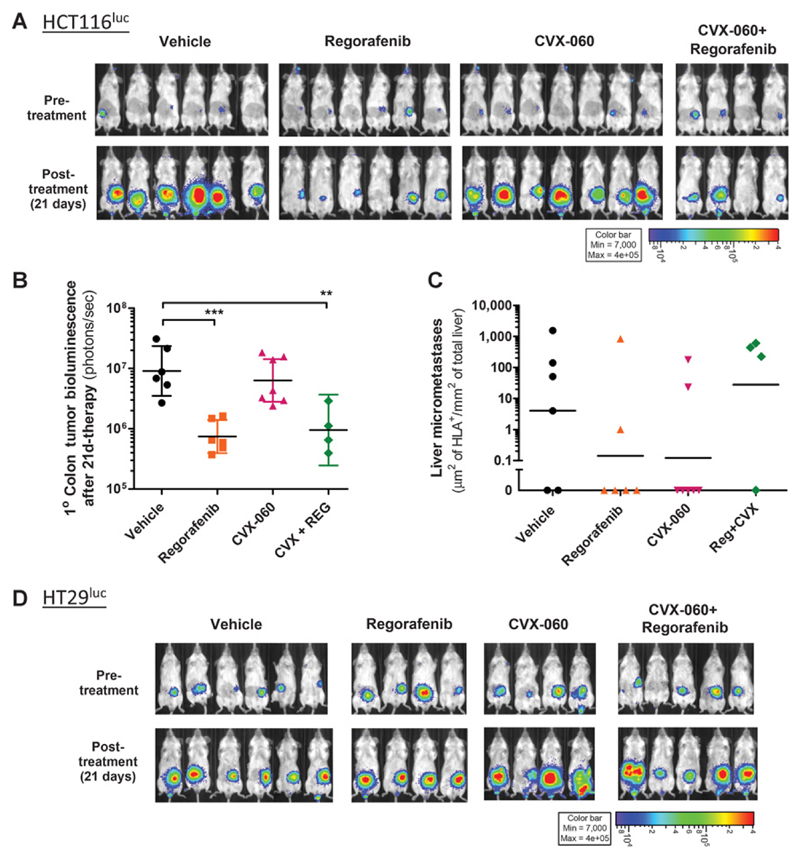
Regorafenib is an antiangiogenic VEGFR2-targeting TKI that also targets Tie2 (35). Using 2 luciferase-tagged human colorectal carcinoma cell lines—HCT116luc, a TP53-wildtype cell line with "microsatellite instability" (MSI), and HT29luc, a TP53-mutant "microsatellite stable" (MSS) cell line (36)—we assessed the effects of CVX-060 versus regorafenib on colorectal cancer primary tumors and metastases.
For primary tumor experiments, HCT116luc or HT29luc tumor fragments were implanted intracecally into YFP-SCID mice. Starting at 12 or 15 days post-implantation (DPI) in the HCT116luc model (Fig. 4A–C) or HT29luc model (Fig. 4D) respectively, mice with established primary cecal tumors were treated for 3 weeks with regorafenib and/or CVX-060. On the basis of bioluminescent imaging, regorafenib monotherapy potently inhibited primary tumor growth in the HCT116luc model (Fig. 4A and B), but not in HT29luc model (Fig. 4D)—similar to our previous finding that p53 deficiency can render colorectal cancer tumors less responsive to a VEGFR2 antibody (37). Single-agent or concurrent CVX-060 did not affect primary cecal tumor growth rates in either model (Fig. 4A and D), although the less hemorrhagic gross appearance of CVX-060–treated primary tumors did suggest a qualitative reduction in tumor blood vessels (Supplementary Fig. S4).
Figure 4.
Regorafenib versus Ang2-targeting CVX-060 in orthotopic primary colorectal tumor models. Mice bearing established orthotopic colorectal HCT116luc tumors (A and C) or HT29luc tumors (D) were treated for 3 weeks with vehicle, regorafenib (15 mg/kg/d), CVX-060 (30 mg/kg/wk), or regorafenib +CVX-060. A, B, and D, Primary cecal tumors were visualized and quantified by bioluminescent imaging. Log-transformed fluxes were compared by one-way ANOVA with Bonferroni correction (**, P ≤ 0.01; ***, P ≤ 0.001). Geometric means ± SEM are shown. C, Spontaneous liver micrometastases as detected by IHC staining of HLA+ tumor cells. Trends of treatment-related reductions were not statistically significant by the Kruskal–Wallis test (P > 0.05). Geometric means are depicted.
Adjuvant regorafenib versus Ang2-targeting CVX-060 in three models of colorectal cancer liver metastases
In the presence of HCT116luc primary tumors, both regorafenib and CVX-060 monotherapies had shown promising trends of reduced spontaneous liver micrometastases by histology (Fig. 4C). Next, using an experimental metastasis model and 2 adjuvant therapy models, we sought to further delineate drug effects on liver metastases, independent of primary colorectal cancer tumors.
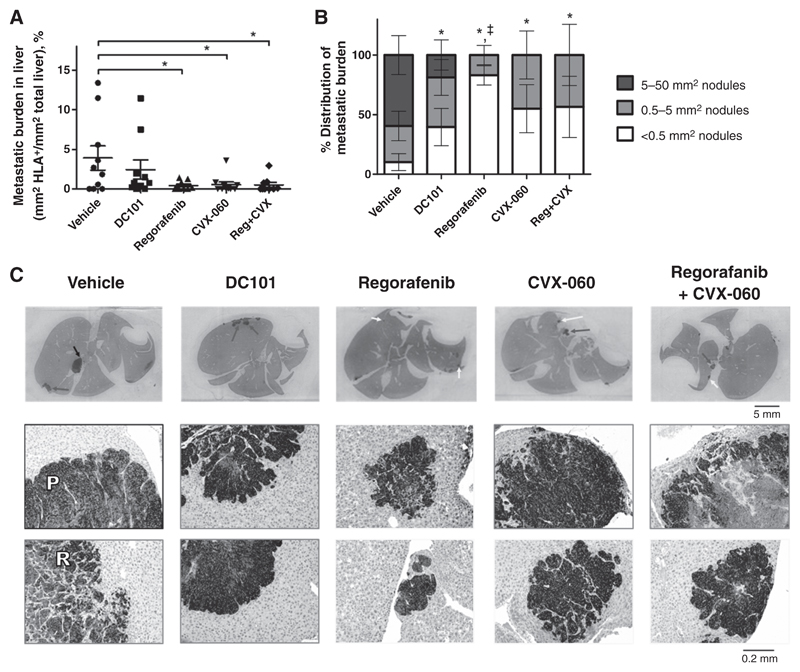
To set up the experimental metastasis model (Fig. 5), 5 × 105 HCT116luc cells were injected intrasplenically into YFP-SCID mice, accompanied by splenectomy 2 minutes later. This approximates the adjuvant therapy setting, whereby liver metastases grow in the absence of primary colorectal cancer tumors. Starting at 3 DPI, mice were given vehicle, DC101, regorafenib, CVX-060, or regorafenib + CVX-060. Therapies were continuous over 31 days, except for regorafenib, which was given discontinuously (15 days on, 7 days off, 5 days on, 4 days off) to minimize drug toxicity. The experiment was terminated at 34 DPI and liver metastases were analyzed by histology. By immunohistochemistry (IHC), regorafenib and CVX-060 as single agents similarly reduced the overall metastatic burden in the liver (Fig. 5A, P < 0.05). Further analyzing the size distribution of metastatic nodules, we found the regorafenib monotherapy group to have a significantly elevated distribution of very small (<0.5 mm2) liver metastases, whereas the CVX-060– treated groups did not (Fig. 5B and C). In contrast to regorafenib, DC101 (our VEGFR2-specific control) only showed a trend of decreasing overall liver metastatic burden (Fig. 5A, P > 0.05). By a different assay, terminal ex vivo bioluminescent imaging of whole livers showed a trend where CVX-060 might have outperformed regorafenib in suppressing liver metastasis (Supplementary Fig. S4C, P > 0.05). It appeared that a mix of "pushing" and "replacement" growth patterns was observable in the liver metastases of this HCT116luc model (Fig. 5C). A histologic study of liver metastases from patients with colorectal cancer had found the "pushing" pattern to be characteristic of angiogenesis-dependent tumor growth, and the "replacement" pattern to be more indicative of tumor growth by co-option of sinusoidal liver blood vessels (38).
Figure 5.
Effects of adjuvant-like regorafenib and Ang2-targeting CVX-060 therapies on colorectal cancer liver metastases. Mice were treated for 31 days with vehicle (n = 10), DC101 (0.8 mg, 2×/wk; n = 10), regorafenib (15 mg/kg/d; n = 10), CVX-060 (30 m/kg/wk; n = 10), or regorafenib + CVX-060 (n = 9) after intrasplenic HCT116luc implantation. Experimental liver metastases were detected by IHC staining for HLA+ tumor cells. A, Overall metastatic burden was subjected to one-way ANOVA with Dunnett posttest (*, P < 0.05); means ± SEM are depicted. B, Size distribution analysis of metastatic nodules was subjected to one-way ANOVA with Dunnett posttest. *, P < 0.05 versus vehicle for large (>5 mm2) nodules; ‡, P < 0.05 versus vehicle for small (<0.5 mm2) nodules; means ± SEM are depicted. C, Representative HLA-stained liver sections. Black, gray, and white arrows, respectively, typical large (>5 mm2), medium (0.5–5 mm2), and small (<0.5 mm2)-sized metastatic nodules, for which ×100 magnifications are shown directly below (with corresponding black, gray, or white borders). "P" and "R" indicate a "pushing growth pattern" and "replacement growth pattern," respectively, as defined (38).
In the adjuvant therapy models (Supplementary Figs. S5 and S6), orthotopic HCT116luc or HT29luc primary tumors were resected between 12 and 14 DPI before mice were randomized and treated with regorafenib, CVX-0060, neither, or both. Extensive adhesion of primary cecal tumors to adjacent tissues/organs resulted in an unexpectedly high rate of animal attrition (∼60%–80%) due to incomplete resections and surgical complications. Given the small remaining sample sizes, definitive conclusions could not be drawn. However, an interesting observation potentially worthy of follow-up was made in the HCT116luc model, whereby postsurgical disease was potently inhibited by regorafenib treatment but dramatically rebounded upon discontinuation of regorafenib (Supplementary Fig. S5). No such rebound was observed in the CVX-060–containing groups (Supplementary Fig. S5).
In summary, both anti-Ang2 therapy (CVX-060) and TKI therapy (regorafenib) demonstrated potential in terms of suppressing colorectal cancer liver metastases. Qualitative differences in CVX-060 versus TKI-treated HCT116luc liver metastases further suggest potentially complementary modes of action.
Sunitinib versus Ang2-targeting CVX-060 in the unresected and postsurgical settings of a lung-metastasizing orthotopic primary renal cancer model
A luciferase-tagged variant of the RENCA murine renal cancer cell line, RENCAluc, was derived in our laboratory and found to metastasize very aggressively to the lungs after orthotopic implantation into BALB/c mice.
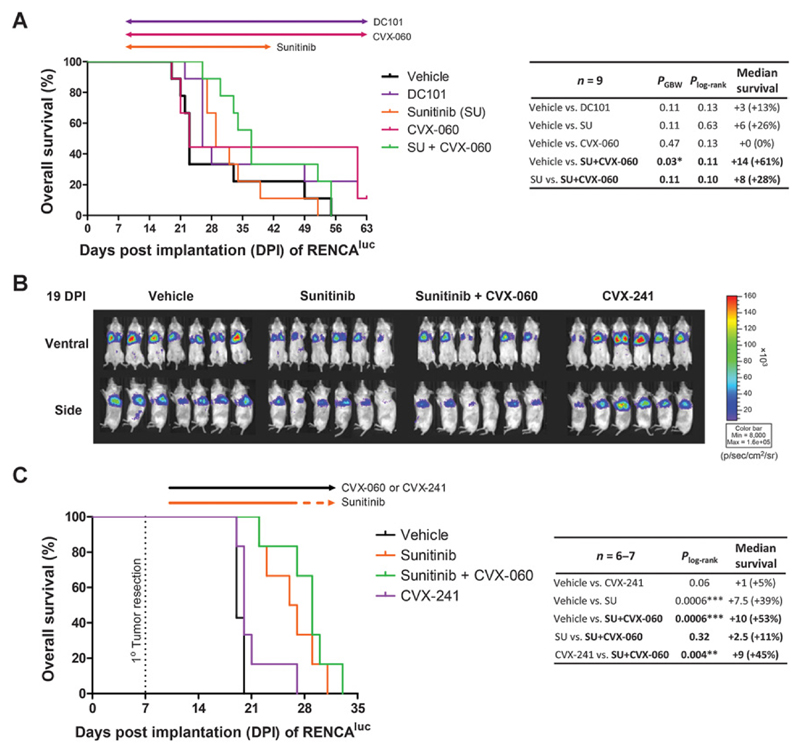
Our first experiment (Fig. 6A) involved treating mice bearing orthotopic primary RENCAluc tumors with vehicle, DC101, sunitinib, CVX-060, or sunitinib + CVX-060. Therapies were initiated early (9 DPI) to ensure that lung metastases were microscopic (Supplementary Fig. S7). Single-agent CVX-060 did not outperform sunitinib or DC101 in terms of extending OS, but when used concurrently, adding CVX-060 to sunitinib showed a trend of improving OS (Fig. 6A).
Figure 6.
Combining Ang2 targeting and VEGF/VEGFR2 targeting in the metastatic (unresected) and adjuvant (postsurgical) settings of a renal cancer model. A, Mice with unresected kidney tumors were treated with vehicle, DC101 (anti-VEGFR2, 0.8 mg, 2×/wk), sunitinib (VEGFR2 TKI, 60 mg/kg/d), CVX-060 (anti-Ang2, 30 mg/kg/wk), or sunitinib plus CVX-060 beginning 9 days after orthotopic implantation of RENCAluc cells. Arrows, treatment durations. Differences in OS were subjected to log-rank (Plog-rank) and Gehan–Breslow–Wilcoxon (PGBW) tests. B and C, Adjuvant therapies began 3 days after resection of orthotopic kidney tumors. Mice received vehicle, sunitinib (60 mg/kg/d), sunitinib + CVX-060 (30 mg/kg/wk), or CVX-241 (anti-Ang2/VEGF-A, 30 mg/kg/wk). Bioluminescent images of postsurgical lung metastases are shown (B). Kaplan–Meier survival analysis is shown (C), with arrows indicating dosing intervals (solid segments indicate continuous therapy; dotted orange segment indicates "2d-off/5d-on" weekly cycles).
A follow-up experiment was performed to delineate the effects of adjuvant therapies on postsurgical lung metastases (Fig. 6B and C). This time, orthotopic primary RENCAluc tumors were resected before therapies were initiated 3 days later, consisting of vehicle, sunitinib, sunitinib + CVX-060, or CVX-241. By bioluminescent imaging, there was again a trend in which the addition of CVX-060 improved adjuvant sunitinib therapy in terms of inhibiting postsurgical metastases (Fig. 6B). However, the corresponding improvement in OS was modest (Fig. 6C), likely because toxicity also increased with combination treatment (Supplementary Fig. S8). Of note, dual neutralization of Ang2 and VEGF-A by CVX-241 was ineffective—and clearly inferior to CVX-060 + sunitinib (P = 0.004)—in this adjuvant RENCA model (Fig. 6B and C), in direct contrast to the adjuvant LM2-4 breast cancer model (Fig. 1E).
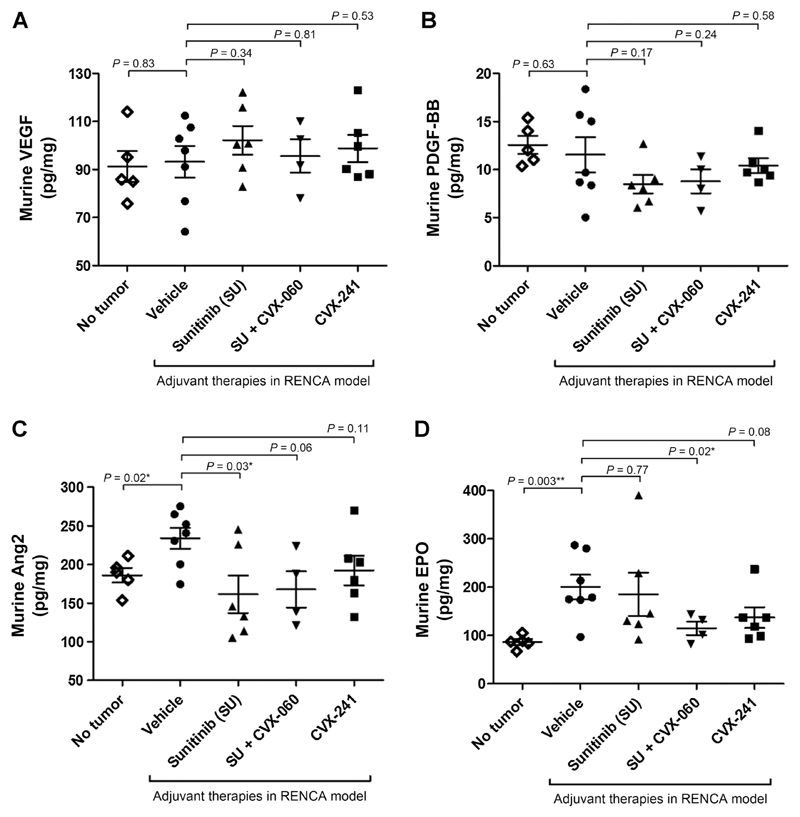
To explore the mechanistic basis behind the superiority of CVX-060 + sunitinib over CVX-241 in the adjuvant RENCA model, we profiled the peripheral concentrations of several angiogenesis-related cytokines—Ang2, VEGF, EPO, and PDGF-BB—that are relevant to renal cancer biology from the right (nontumor–bearing) kidneys at endpoint (Fig. 7). Compared with healthy (tumor-free) controls, vehicle-treated mice with endpoint postsurgical RENCAluc lung metastases had similar levels of VEGF and PDGF-BB (Fig. 7A and B) but showed significant elevations in murine Ang2 and EPO (Fig. 7C and D). The Ang2 elevation was more effectively counteracted by sunitinib adjuvant therapy (with or without CVX-060) than CVX-241 (Fig. 7C), whereas the EPO elevation was most effectively counteracted by sunitinib + CVX-060 (Fig. 7D). Adjuvant sunitinib-containing therapies also showed a trend of decreasing PDGF-BB (Fig. 7B).
Figure 7.
Tissue concentrations of murine VEGF, Ang2, EPO, and PDGF-BB after adjuvant VEGF pathway targeting with or without Ang2 neutralization in a renal cancer model. Left kidneys bearing RENCAluc primary tumors were resected prior to adjuvant administration of vehicle, sunitinib, sunitinib + CVX-060, or CVX-241. Most mice, with the exception of two cases of suspected combination drug toxicity (Supplementary Fig. S8), reached endpoint with comparable metastatic burden in the lungs. From these mice, nontumor–bearing right kidneys were collected at endpoint and homogenized. Murine VEGF (A), PDGF-BB (B), Ang2 (C), and EPO (D) were measured from kidney homogenates by ELISA and normalized to total protein (pg/mg). Mean ± SEM and P values from t tests are depicted.
In summary, in this syngeneic model of lung-metastasizing RCC, concurrent Ang2 inhibition (CVX-060) showed potential for improving the antimetastatic activity of adjuvant sunitinib therapy. However, the survival advantage was limited by concurrently increased drug toxicity. The therapeutic advantage of the sunitinib + CVX-060 combination in this model, compared with CVX-241 therapy, might be related to its greater effectiveness at reducing Ang2, EPO, and PDGF-BB.
Discussion
Across multiple cancer indications (colorectal, breast, liver, and renal cancers), antiangiogenic drugs targeting the VEGF pathway have not been successful at improving OS when used in the adjuvant setting in randomized phase III trials (Supplementary Table S1). The major finding of our preclinical study is that Ang2 targeting has therapeutic potential in postsurgical adjuvant therapy settings. Our study further suggests divergent approaches to combining an anti-Ang2 agent (CVX-060) with VEGF pathway inhibition depending on the cancer indication. Adjuvant Ang2 inhibition was most effective when combined with VEGF-A neutralization (via the bispecific anti-Ang2/VEGF-A agent, CVX-241) in 2 breast cancer models. On the contrary, adjuvant Ang2 inhibition was optimal when combined with VEGFR2 TKI therapy (via CVX-060 + sunitinib) in a renal cancer model. In further contrast, CVX-060 had standalone efficacy similar to VEGFR2 TKI therapy (regorafenib) in 2 colorectal cancer models.
Ang2 neutralization shows greater therapeutic potential in treating early-stage micrometastasis than established primary tumors
There can often be discordant outcomes for a given therapy depending on whether it is used to treat established primary tumors or metastatic disease. For example, although sunitinib effectively suppresses primary tumor growth in various rodent models of breast cancer, it consistently fails to inhibit distant metastases and/or prolong survival in mice treated after breast cancer resection in the adjuvant (micrometastatic disease) or late-stage (macrometastatic disease) setting (5, 10, 39).
Contrary to sunitinib, our present study shows the anti-Ang2 agent, CVX-060, to have greater efficacy against micrometastatic disease than established primary tumors. Despite effective Ang2 sequestration, CVX-060 did not lead to any substantial growth inhibitory or antiangiogenic effects in established orthotopic primary breast (LM2-4luc16) or colorectal (HCT116luc) tumors. Yet, when primary LM2-4luc16 tumors were resected, adjuvant CVX-060 monotherapy delayed the progression of distant lung/lymphatic metastases, although this ultimately did not translate into significant OS benefits unless combined with concurrent VEGF-A neutralization. Moreover, in the HCT116luc experimental metastasis model, CVX-060 monotherapy was as effective as regorafenib in suppressing liver metastases growing in the absence of a primary colorectal tumor.
In line with our findings, a recent preclinical study by Srivastava and colleagues testing a different anti-Ang2 agent (LC06) also reported substantial efficacy when used in the adjuvant setting in combination with metronomic chemotherapy in murine breast and lung carcinoma models (40). Together with our present study, which focused on combination with antiangiogenic TKIs, these 2 studies in sum suggest that clinical evaluation of certain combinations of anti-Ang2–based therapies may be warranted in the adjuvant setting. Even though the anti-Ang2/Ang1 peptibody, AMG386 (trebananib), has been disappointing thus far in clinical trials for various advanced-stage cancers (Supplementary Table S2), it has not yet been evaluated in adjuvant postsurgical settings.
Differential approaches to combining anti-Ang2 with VEGF pathway inhibition in different cancer types
While our results reveal an overall trend of benefit from postsurgically cotargeting the Ang2 and VEGF pathways, we noted variability with respect to drug combinations and tumor types.
For breast cancer, sunitinib has failed in the advanced metastatic setting in 4 phase III clinical trials (41) and neither has it been effective as an adjuvant therapy in preclinical models (10, 41). While we hypothesized that the concurrent CVX-060 might improve the efficacy of adjuvant sunitinib therapy for breast cancer, our experiments using the LM2-4 model suggest otherwise. On the other hand, a significant prolongation in OS was achieved with CVX-241, a bispecific agent against Ang2 and VEGF-A. Our results suggest that when it comes to adding VEGF pathway blockade to anti-Ang2 adjuvant therapy for breast cancer, a ligand-directed approach (VEGF-A targeting by CVX-241) may be superior to a receptor-directed approach (VEGFR2 targeting by sunitinib). Alternatively, a pathway-specific antibody-based approach (via CVX-241) may be superior to a broad-spectrum TKI-based approach (via sunitinib). Unfortunately, CVX-241 never progressed beyond phase I clinical testing, as its development was terminated. There is another bispecific agent, the Ang2-VEGF-A CrossMab (RG7221/RO5520985/vanucizumab; Roche) that is now being evaluated in a phase II trial for metastatic colorectal cancer (42). Our preclinical results from the LM2-4 and EMT-6/CDDP models would suggest that clinical evaluation of this agent in the adjuvant setting for resectable breast cancer could be warranted in the future.
For metastatic colorectal cancer, regorafenib is so far the only clinically approved antiangiogenic TKI. Results from our model of HCT116luc experimental liver metastases suggest that regorafenib may also be efficacious as an adjuvant treatment for resected colorectal cancer. In this same model, Ang2-specific inhibition (CVX-060) was just as effective as regorafenib in terms of inhibiting liver metastases, whereas VEGFR2-specific inhibition (DC101) was inferior to regorafenib. Consistent with our results, the superiority of regorafenib over DC101 was previously observed in another murine colorectal cancer model (CT26) and was attributed at least in part to a greater reduction of tumor-infiltrating Tie2+ macrophages by regorafenib (35). Prior to this present study, preclinical evaluation of Ang2 inhibition with antiangiogenic TKIs in colorectal cancer models has been limited to combinations with vandetanib, cediranib, sorafenib, or sunitinib (none of which are clinically approved for colorectal cancer) in subcutaneous tumor xenografts of various human colorectal cancer cell lines without modeling metastasis (20, 43). There are at least 2 ongoing clinical trials evaluating adjuvant regorafenib for colorectal cancer (clinicaltrials.gov: NCT02425683 and NCT01939223), but none yet to evaluate anti-Ang2 agents in the adjuvant setting for colorectal cancer.
For mRCC, there are several antiangiogenic TKIs approved, including sunitinib, pazopanib, sorafenib, and axitinib. Prior to this study, the addition of an Ang2-specific peptibody (L1-7) was found to improve the antitumor activity of sunitinib in an A498 human RCC xenograft model, although these tumors were subcutaneously implanted and impact on metastasis was not evaluated (17). In our resected orthotopic RENCAluc model, the most promising adjuvant treatment in terms of inhibiting postsurgical lung metastases and prolonging OS was sunitinib + CVX-060, which was superior to CVX-241 therapy. NCT01664182 is an ongoing randomized phase II trial that involves patients whose mRCC has progressed and compares a switch to AMG386 monotherapy versus adding AMG386 on top of their existing VEGF pathway–targeted therapy (sunitinib, sorafenib, pazopanib, or bevacizumab). If this trial is successful and a similar trial design is to be conducted in the adjuvant setting of RCC, our results would predict greater efficacy with AMG386 + sunitinib/sorafenib/pazopanib, compared with AMG386 + bevacizumab or AMG386 alone. We should note that like sunitinib, both sorafenib and pazopanib also effectively inhibit PDGFRβ in addition to VEGFR2 (Supplementary Tables S3 and S4). There was also a phase II trial of CVX-060 with axitinib for mRCC, which was prematurely terminated because of tolerability/toxicity issues (NCT01441414). Thus, if similar combinations are to be clinically evaluated in the adjuvant setting of RCC, the dosing regimens will likely have to be adjusted to reduce combination toxicity and improve therapeutic ratios.
Mechanistic insights: Linking Ang2 to PD-L1 targeting and EPO to sunitinib
In the EMT-6/CDDP breast cancer model, circulating Ang2 was slightly elevated after adjuvant anti-PD-L1 therapy, but the addition of anti-PD-L1 to adjuvant CVX-060 or CVX-241 therapies did not exceptionally improve OS. Nonetheless, this model can be useful for further screening of other immunotherapeutics (e.g., inhibitors of CTLA-4 or PD-1) for drug-induced Ang2 dysregulations and for additive/synergistic efficacy when combined with anti-Ang2 or anti-Ang2/VEGF therapies. Such studies would be timely, given the recent success of a CTLA-4 antibody as an adjuvant therapy for resected melanoma in a phase III trial (44). Several studies have already implicated that targeting Ang2 can improve immunotherapy, for example, by blocking immunosuppressive Tie2-expressing monocytes (45, 46). Thus, it is conceivable that Ang2/Tie2-targeting drugs, including cross-specific antibodies, which also target the VEGF pathway, may enhance the efficacy of adjuvant immunotherapeutics.
With respect to the adjuvant RENCA model, we postulate that the superiority of CVX-060 + sunitinib over CVX-241 might relate to the fact that sunitinib potently inhibits other major angiogenic pathways besides VEGFR2, including PDGFRβ (47). For angiogenic blood vessels, the recruitment of PDGFRβ+ perivascular mural cells is driven by endothelial cell (EC)–derived PDGF-B ligands (48). It was shown recently that tumor-derived PDGF-B can stimulate PDGFRβ+ stromal cells to produce EPO (48, 49). EPO, in turn, is another protumorigenic and proangiogenic factor (49). Its receptor, EpoR, is expressed on ECs and often overexpressed by RCC tumor cells (49). There is even a report of EPO inducing greater expression of VEGFR2 and Tie2 on ECs in the presence of TNFα (49). In our study, adjuvant CVX-060 + sunitinib led to greater decreases in 3 mutually reinforcing angiogenic factors—Ang2, EPO, and PDGF-BB—compared with adjuvant CVX-241 therapy. Thus, CVX-060 + sunitinib might be more effective by virtue of its multipronged targeting of VEGFR2/Tie2/PDGFR/EpoR-mediated angiogenic pathways. Follow-up studies will be needed to confirm whether this sufficiently accounts for the mechanistic advantage of CVX-060 + sunitinib over CVX-241 in RCC.
Summary
In conclusion, evaluation in 5 diverse preclinical models showed that targeting Ang2—while seemingly ineffective on primary tumors—has the potential to improve VEGF pathway targeting in the adjuvant setting to treat postsurgical micrometastatic disease. Our LM2-4 and EMT-6/CDDP models predict the efficacy of dual Ang2 and VEGF-A targeting via a bispecific antibody (CVX-241) in the adjuvant setting of breast cancer. Our RENCA model suggests potential efficacy of combining an anti-Ang2 agent (CVX-060) with lowered doses of antiangiogenic TKIs (ideally ones that target both VEGFR2 and PDGFRβ e.g., sunitinib) in the adjuvant setting of RCC. Our HCT116 and HT29 models suggest that Ang2 neutralization (CVX-060) can be as effective as antiangiogenic TKIs (regorafenib) in the adjuvant setting of colorectal cancer and that further investigation into potential complementary mechanisms is warranted.
Supplementary Material
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Acknowledgments
We thank Profs. Bradly Wouters and Jim Woodgett for their critical input; Dr. Daniel Nolan (Angiocrine Bioscience) for providing VeraVec cells; Dr. Dennis Healy (Bayer HealthCare Pharmaceuticals) for providing regorafenib; Dr. Bronek Pytowski (ImClone Systems, Eli Lilly) for providing DC101; and Cassandra Cheng for excellent secretarial assistance.
Grant Support
This work was mainly funded by research grants awarded to R.S. Kerbel from the Canadian Breast Cancer Foundation (CBCF), Worldwide Cancer Research (formerly known as AICR, the Association of International Cancer Research), and the Canadian Institutes of Health Research (CIHR). F.T.H. Wu received stipend support from the CIHR (Vanier CGS) and the University of Toronto (MD/PhD Studentships; GSEF Dr. James Lepock Memorial Award).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
S.R. Pirie-Shepherd is an employee of Pfizer. U. Emmenegger reports receiving commercial research grants from Johnson and Johnson, Bayer, and Astellas and is a consultant/advisory board member for Bayer, Johnson and Johnson, Amgen, Sanofi, Astellas, Ferring, and Novartis. R.S. Kerbel has received speakers bureau honoraria from Boehringer Ingelheim, Regeneron, and Eli Lilly and is a consultant/advisory board member for Triphase Accelerator and Angiocrine Biosciences. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: F.T.H. Wu, P. Xu, A. Chow, M. Paez-Ribes, U. Emmenegger, R.S. Kerbel
Development of methodology: S. Man, P. Xu
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): F.T.H. Wu, S. Man, P. Xu, A. Chow, M. Paez-Ribes, C.R. Lee
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): F.T.H. Wu, M. Paez-Ribes, U. Emmenegger, R.S. Kerbel
Writing, review, and/or revision of the manuscript: F.T.H. Wu, P. Xu, A. Chow, M. Paez-Ribes, C.R. Lee, S.R. Pirie-Shepherd, U. Emmenegger, R.S. Kerbel
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): S. Man, P. Xu, A. Chow, S.R. Pirie-Shepherd
Study supervision: S. Man, R.S. Kerbel
References
- 1.de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225–33. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 2.Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Petrelli NJ, Lopa SH, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the national surgical adjuvant breast and bowel project C-08 trial. J Clin Oncol. 2013;31:359–64. doi: 10.1200/JCO.2012.44.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387:2008–16. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnem T, Hu J, Ferguson M, Adighibe O, Snell C, Harris AL, et al. Vessel co-option in primary human tumors and metastases: an obstacle to effective anti-angiogenic treatment? Cancer Med. 2013;2:427–36. doi: 10.1002/cam4.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pezzella F, Gatter K. Non-angiogenic tumours unveil a new chapter in cancer biology. J Pathol. 2015;235:381–3. doi: 10.1002/path.4474. [DOI] [PubMed] [Google Scholar]
- 8.Mina LA, Sledge GW., Jr Rethinking the metastatic cascade as a therapeutic target. Nat Rev Clin Oncol. 2011;8:325–32. [Google Scholar]
- 9.Cooke VG, LeBleu VS, Keskin D, Khan Z, O'Connell JT, Teng Y, et al. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung AS, Kowanetz M, Wu X, Zhuang G, Ngu H, Finkle D, et al. Differential drug class-specific metastatic effects following treatment with a panel of angiogenesis inhibitors. J Pathol. 2012;227:404–16. doi: 10.1002/path.4052. [DOI] [PubMed] [Google Scholar]
- 11.Welti JC, Powles T, Foo S, Gourlaouen M, Preece N, Foster J, et al. Contrasting effects of sunitinib within in vivo models of metastasis. Angiogenesis. 2012;15:623–41. doi: 10.1007/s10456-012-9291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575–85. doi: 10.1038/nrc2894. [DOI] [PubMed] [Google Scholar]
- 13.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–8. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 14.Keskin D, Kim J, Cooke VG, Wu CC, Sugimoto H, Gu C, et al. Targeting vascular pericytes in hypoxic tumors increases lung metastasis via angiopoietin-2. Cell Rep. 2015;10:1066–81. doi: 10.1016/j.celrep.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falcon BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol. 2009;175:2159–70. doi: 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holopainen T, Saharinen P, D'Amico G, Lampinen A, Eklund L, Sormunen R, et al. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J Natl Cancer Inst. 2012;104:461–75. doi: 10.1093/jnci/djs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Bullock AJ, Zhang L, Wei L, Yu D, Mahagaokar K, et al. The role of angiopoietins as potential therapeutic targets in renal cell carcinoma. Transl Oncol. 2014;7:188–95. doi: 10.1016/j.tranon.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopczynska E, Dancewicz M, Kowalewski J, Makarewicz R, Kardymowicz H, Kaczmarczyk A, et al. Time-dependent changes of plasma concentrations of angiopoietins, vascular endothelial growth factor, and soluble forms of their receptors in nonsmall cell lung cancer patients following surgical resection. ISRN Oncol. 2012;2012:638352. doi: 10.5402/2012/638352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumara HM, Feingold D, Kalady M, Dujovny N, Senagore A, Hyman N, et al. Colorectal resection is associated with persistent proangiogenic plasma protein changes: postoperative plasma stimulates in vitro endothelial cell growth, migration, and invasion. Ann Surg. 2009;249:973–7. doi: 10.1097/SLA.0b013e3181a6cd72. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Lai JY, Do J, Liu D, Li L, Del Rosario J, et al. Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin Cancer Res. 2011;17:1001–11. doi: 10.1158/1078-0432.CCR-10-2317. [DOI] [PubMed] [Google Scholar]
- 21.Teicher BA, Herman TS, Holden SA, Wang YY, Pfeffer MR, Crawford JW, et al. Tumor resistance to alkylating agents conferred by mechanisms operative only in vivo. Science. 1990;247:1457–61. doi: 10.1126/science.247.4949.1457. [DOI] [PubMed] [Google Scholar]
- 22.Munoz R, Man S, Shaked Y, Lee CR, Wong J, Francia G, et al. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–91. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 23.Man S, Munoz R, Kerbel RS. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer Metastasis Rev. 2007;26:737–47. doi: 10.1007/s10555-007-9087-6. [DOI] [PubMed] [Google Scholar]
- 24.Hackl C, Man S, Francia G, Milsom C, Xu P, Kerbel RS. Metronomic oral topotecan prolongs survival and reduces liver metastasis in improved preclinical orthotopic and adjuvant therapy colon cancer models. Gut. 2013;62:259–71. doi: 10.1136/gutjnl-2011-301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jedeszko C, Paez-Ribes M, Di Desidero T, Man S, Lee CR, Xu P, et al. Postsurgical adjuvant or metastatic renal cell carcinoma therapy models reveal potent anti-tumor activity of metronomic oral topotecan with pazopanib. Sci Transl Med. 2015;7:282ra50. doi: 10.1126/scitranslmed.3010722. [DOI] [PubMed] [Google Scholar]
- 26.Tait LR, Pauley RJ, Santner SJ, Heppner GH, Heng HH, Rak JW, et al. Dynamic stromal-epithelial interactions during progression of MCF10DCIS.com xenografts. Int J Cancer. 2007;120:2127–34. doi: 10.1002/ijc.22572. [DOI] [PubMed] [Google Scholar]
- 27.Wu FT, Lee CR, Bogdanovic E, Prodeus A, Gariepy J, Kerbel RS. Vasculotide reduces endothelial permeability and tumor cell extravasation in the absence of binding to or agonistic activation of Tie2. EMBO Mol Med. 2015;7:770–87. doi: 10.15252/emmm.201404193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caine GJ, Blann AD, Stonelake PS, Ryan P, Lip GY. Plasma angiopoietin-1, angiopoietin-2 and tie-2 in breast and prostate cancer: a comparison with VEGF and flt-1. Eur J Clin Invest. 2003;33:883–90. doi: 10.1046/j.1365-2362.2003.01243.x. [DOI] [PubMed] [Google Scholar]
- 29.Li P, He Q, Luo C, Qian L. Diagnostic and prognostic potential of serum angiopoietin-2 expression in human breast cancer. Int J Clin Exp Pathol. 2015;8:660–4. [PMC free article] [PubMed] [Google Scholar]
- 30.Gerald D, Chintharlapalli S, Augustin HG, Benjamin LE. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res. 2013;73:1649–57. doi: 10.1158/0008-5472.CAN-12-4697. [DOI] [PubMed] [Google Scholar]
- 31.Doppalapudi VR, Huang J, Liu D, Jin P, Liu B, Li L, et al. Chemical generation of bispecific antibodies. Proc Natl Acad Sci U S A. 2010;107:22611–6. doi: 10.1073/pnas.1016478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murukesh N, Dive C, Jayson GC. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br J Cancer. 2010;102:8–18. doi: 10.1038/sj.bjc.6605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stubenrauch K, Wessels U, Essig U, Vogel R, Waltenberger H, Hansbauer A, et al. An immunodepletion procedure advances free angiopoietin-2 determination in human plasma samples during anti-cancer therapy with bispecific anti-Ang2/VEGF CrossMab. J Pharm Biomed Anal. 2015;102:459–67. doi: 10.1016/j.jpba.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Loupakis F, Falcone A, Masi G, Fioravanti A, Kerbel RS, Del Tacca M, et al. Vascular endothelial growth factor levels in immunodepleted plasma of cancer patients as a possible pharmacodynamic marker for bevacizumab activity. J Clin Oncol. 2007;25:1816–8. doi: 10.1200/JCO.2006.10.3051. [DOI] [PubMed] [Google Scholar]
- 35.Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013;12:1322–31. doi: 10.1158/1535-7163.MCT-12-1162. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknaes M, Hektoen M, et al. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71. doi: 10.1038/oncsis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science. 2002;295:1526–8. doi: 10.1126/science.1068327. [DOI] [PubMed] [Google Scholar]
- 38.Vermeulen PB, Colpaert C, Salgado R, Royers R, Hellemans H, Van Den Heuvel E, et al. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol. 2001;195:336–42. doi: 10.1002/path.966. [DOI] [PubMed] [Google Scholar]
- 39.Guerin E, Man S, Xu P, Kerbel RS. A model of postsurgical advanced metastatic breast cancer more accurately replicates the clinical efficacy of antiangiogenic drugs. Cancer Res. 2013;73:2743–8. doi: 10.1158/0008-5472.CAN-12-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srivastava K, Hu J, Korn C, Savant S, Teichert M, Kapel SS, et al. Postsurgical adjuvant tumor therapy by combining anti-angiopoietin-2 and metronomic chemotherapy limits metastatic growth. Cancer Cell. 2014;26:880–95. doi: 10.1016/j.ccell.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Kerbel RS, Guerin E, Francia G, Xu P, Lee CR, Ebos JM, et al. Preclinical recapitulation of antiangiogenic drug clinical efficacies using models of early or late stage breast cancer metastatis. Breast. 2013;22(Suppl 2):S57–65. doi: 10.1016/j.breast.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Sheridan C. Amgen's angiopoietin blocker fails in ovarian cancer. Nat Biotechnol. 2015;33:5–6. doi: 10.1038/nbt0115-5. [DOI] [PubMed] [Google Scholar]
- 43.Brown JL, Cao ZA, Pinzon-Ortiz M, Kendrew J, Reimer C, Wen S, et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in pre-clinical models. Mol Cancer Ther. 2010;9:145–56. doi: 10.1158/1535-7163.MCT-09-0554. [DOI] [PubMed] [Google Scholar]
- 44.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med. 2016 Oct 7; doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coffelt SB, Chen YY, Muthana M, Welford AF, Tal AO, Scholz A, et al. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J Immunol. 2011;186:4183–90. doi: 10.4049/jimmunol.1002802. [DOI] [PubMed] [Google Scholar]
- 46.Ibberson M, Bron S, Guex N, Faes-van't Hull E, Ifticene-Treboux A, Henry L, et al. TIE-2 and VEGFR kinase activities drive immunosuppressive function of TIE-2-expressing monocytes in human breast tumors. Clin Cancer Res. 2013;19:3439–49. doi: 10.1158/1078-0432.CCR-12-3181. [DOI] [PubMed] [Google Scholar]
- 47.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–45. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 48.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao Y. Erythropoietin in cancer: a dilemma in risk therapy. Trends Endocrinol Metab. 2013;24:190–9. doi: 10.1016/j.tem.2012.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.