Summary
Perceptual decisions arise after considering the available sensory evidence [1]. When sensory information is unreliable, a good strategy is to rely on previous experience in similar situations to guide decisions [2–6]. It is well-known that patients with Parkinson’s disease (PD) are impaired at value-based decision-making [7–11]. How patients combine past experience and sensory information to make perceptual decisions is unknown. We developed a novel, perceptual decision-making task and manipulated the statistics of the sensory stimuli presented to patients with PD and healthy participants to determine the influence of past experience on decision-making. We show that patients with PD are impaired at combining previously learned information with current sensory information to guide decisions. We modeled the results using the drift-diffusion model (DDM) and found that the impairment corresponds to a failure in adjusting the amount of sensory evidence needed to make a decision. Our modeling results also show that two complementary mechanisms operate to implement a bias when two sets of priors are learned concurrently. Asymmetric decision threshold adjustments, as reflected by changes in the starting point of evidence accumulation, are responsible for a general choice bias, whereas the adjustment of a dynamic bias that develops over the course of a trial, as reflected by a drift rate offset, provides the stimulus-specific component of the prior. A proper interplay between these two processes is required to implement a bias based on concurrent, stimulus-specific priors in decision-making. We show here that patients with PD are impaired in these across-trial decision threshold adjustments.
Keywords: Perception, Basal Ganglia, Cognition, Implicit Learning, Glass patterns, Decision-Making, Drift Diffusion Model, Bias, Expectancy, Memory
RESULTS
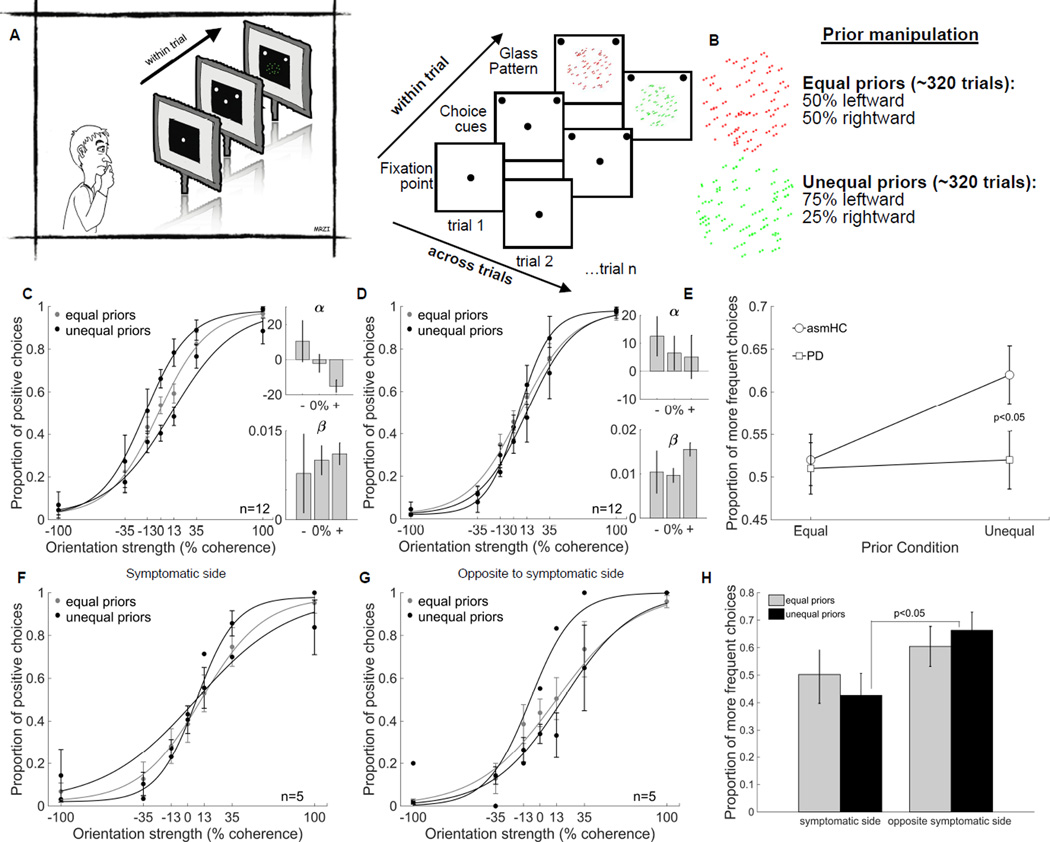
Patients with PD and healthy age- and sex-matched control participants (asmHCs) performed our Glass pattern decision-making task (Figure 1A and B; see also Table S1). Both groups performed the task well, reaching >90% correct performance when the sensory stimulus was strong (Figure 1C and D grey data). This indicates that in both patients and asmHC, visual perceptual performance and motor performance were intact. Manipulation of prior information revealed differences in performance between the groups. asmHCs used the prior information to guide their decisions when sensory information was less informative as indicated by the shift in the response bias between the equal and unequal prior conditions (Figure 1C, grey versus black lines; see also Figure S1). Confirming this statistically, we found that the α parameters from the logistic fits were significantly different between the equal and unequal prior conditions in asmHCs (t(11)= 3.49, p<0.01; Figure 1C) indicating that the prior manipulation altered the response bias but not the sensitivity (β, t(11)=.10, p>0.05; Figure 1C). Patients with PD, however, failed to use the prior information to inform their choices when sensory information was ambiguous (Figure 1D black). Neither the α parameters nor the β parameters from the logistic fits of the patients’ data were significantly different between the equal and unequal prior conditions (α, t(11)=1.47 p>0.05; β, t(11)=-1.16, p>0.05; Figure 1D). To compare performance and the use of priors across both asmHCs and patients, we quantified the proportion of choosing the more frequently shown orientation (“more frequent choices”) for the most difficult stimulus conditions (0% and 13%), because the use of the prior was most prominent in conditions of high sensory uncertainty in healthy participants. Compared to asmHCs, patients with PD had a similar proportion of more frequent choices for the equal prior condition (Parkinson’s: 0.51±0.03; asmHCs: 0.52±0.03, cf., Figure 1E squares vs circles: Equal prior), but a had smaller proportion of more frequent choices for the unequal prior condition (Parkinson’s: 0.52±0.03; asmHCs: 0.62±0.03 cf., Figure 1E squares vs circles: Unequal prior). This difference was confirmed using a 2x2 mixed ANOVA comparing choice performance for patients and asmHCs in the equal and unequal prior conditions. We found a main effect of prior condition (F(1,70)=7.63, p<0.01) and a significant interaction between groups and prior condition (F(1,70)=5.82, p<0.05). Subsequent post-hoc comparisons showed that patients and asmHCs did not differ in the equal prior condition (t(70)=0.27, p>0.05; Figure 1E), but patients and asmHCs differed in their performance in the unequal prior condition (t(70)=2.03, p<0.05; Figure 1E). The result is consistent with the hypothesis that patients are impaired in using prior information to guide perceptual decisions when faced with sensory uncertainty and that this impairment is due neither to sensory nor motor impairments nor age or sex.
Figure 1. Patients with PD are impaired in using priors to guide decisions.
(A) Participants performed a two-alternative forced choice task in which they discriminated between two possible stimulus orientations (rightward or leftward). Initially, a centrally-located fixation spot appeared and then one choice target appeared in each hemifield. On randomly interleaved trials, either a red or a green Glass pattern appeared replacing the fixation point and varying in dot pair coherence, making the orientation discrimination more or less difficult. The coherence of the Glass pattern was also randomly chosen on every trial. Participants indicated what direction they perceived by making an eye movement to one of the two choice targets (left or right) or by pressing the “O” (leftward) or the ‘P” (rightward) key on a computer keyboard using one hand. Participants heard a beep for every correct trial and received no feedback for incorrect trials. No explicit reward was provided.
(B) The certainty of the sensory information contained in the Glass pattern was manipulated by varying the dot pair coherence (orientation strength), which ranged from 0% (no information about orientation) to 100% (all pairs sharing same orientation). To assess the influence of past experience on decision-making performance we manipulated the statistics of the task. The two directions of orientation of the Glass patterns occurred with unequal frequencies for stimuli of one color (75:25) and occurred with the same frequency for stimuli of the other color (50:50) counterbalanced across participants.
(C) Proportion of leftward (positive) choices is plotted against orientation strength for n = 12 asmHC (5 males, 7 females, mean age 63yrs). These data are fitted with a logistic function of the form, p(P) = λ + (1–2* λ)/(1+exp(-β (C-α))); where p(P) is the proportion of positive choices and C is dot pair coherence. α and β are free parameters determined using maximum likelihood methods, and provide a measure of the response bias (α) and the slope or sensitivity of the psychometric function (β). The lapse rate λ is the difference between the asymptote of the function and perfect performance. It is assumed to arise from transient lapses in attention during task performance. A description of the fit and parameters is provided in Figure S1A. The grey points and lines show the data and the logistic fits in the equal prior trials (50:50) whereas the black points and lines show the data for unequal prior trials (75:25). The direction and color of the stimulus with different priors were counterbalanced across participants so that all participants contributed to the grey data and different subsets of participants contributed to the two black curves. The insets show the α and β parameters for the fits in the negative, equal and positive prior trials. Error bars are ±SEM. Since this task has not been used before, we validated performance and use of the stimulus-specific priors in young healthy control participants (yHC). These results are shown in Figure S1B-D. We further showed that trial-by-trial feedback is required to learn the priors to perform this task correctly (Figure S1E).
(D) Proportion of positive choices is plotted against orientation strength for patients with PD. N = 12 (7 males, 5 females, mean age 66yrs).
(E) Proportion of more frequent choices for the most difficult stimuli (0% and 13% coherence) is plotted for asmHC (circles) and patients with Parkinson’s (squares) in the Equal and Unequal prior condition. Error bars are ±SEM.
(F) Proportion of positive choices is plotted against orientation strength for patients who experienced the prior on their symptomatic side (n=5, 2 males 3 females). Grey points and lines show data and fits for all 5 patients whereas the black lines show the data and fits for 4 patients for the leftward prior and 1 patient for the rightward prior.
(G) Proportion of positive choices is plotted against orientation strength for patients who experienced the prior on the side opposite their symptoms (n=5, 4 males 1 female). Grey points and lines show data and fits for all 5 patients whereas the black lines show the data and fits for 1 patient for the leftward prior and 4 patients for the rightward prior.
(H) Proportion of more frequent choices for the most difficult stimuli is plotted for the equal (grey) and unequal prior (black) conditions for the symptomatic and opposite sides. Error bars are ±SEM. See also Figure S1 and Table S1.
Our patient participants were all early stage and mostly presented with motor symptoms unilaterally, in spite of being on medication (see Table S1). We reasoned that decision-making impairments might be asymmetric in patients who presented with unilateral symptoms and that these differences might explain some of the variability in performance between patients. We sorted patients with unilateral symptoms into two groups: those who experienced the prior direction toward the same side as their motor symptoms (Figure 1F) and those who experienced the prior direction toward the side opposite their motor symptoms (Figure 1G). In general, when patients experienced the priors for the direction that corresponded to the side of their motor symptoms, they used the priors less (cf., Figure 1F and 1G). The proportion of more frequent choices made in the equal and unequal prior conditions for the symptomatic side and the opposite side groups were 0.50±0.05 vs 0.60±0.05 and 0.43±0.05 vs 0.66±0.05 (Figure 1H grey and black bars). A 2x2 mixed ANOVA revealed a significant main effect of side (F(1,28)=6.32, p<0.05) and a significant side × prior condition interaction (F(1,28)=7.33, p<0.05). Post-hoc analyses confirmed that there were no differences in the proportion of choosing the more frequent orientation between sides for the equal prior condition (Figure 1H grey bars; t(28)=−1.38, p>0.05), but there were significant differences for trials with unequal priors (Figure 1H black bars; t(28)= −3.39, p<0.01). These results suggest that the inability to use the directional priors is pronounced when the prior direction corresponds to the side of the motor symptoms, consistent with the unilateral representation of directional priors in the striatum [12]. The lack of differences in leftward and rightward performance on the equal prior trials shows that perceptual processing and motor responding per se are independent of the side of symptoms. The difference appears only when combining the priors with sensory information to guide decisions. Although interesting, we interpret this result cautiously due to the small sample size.
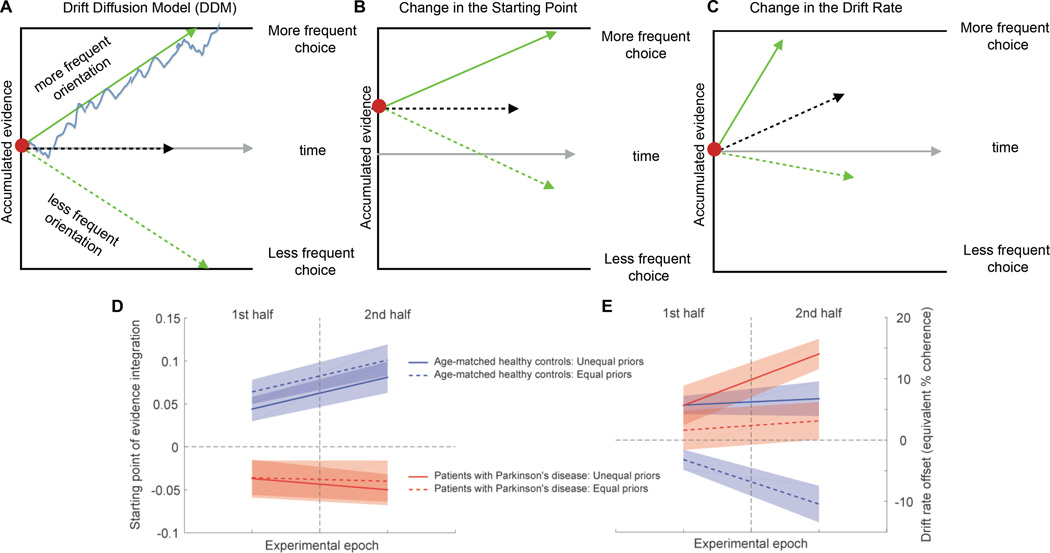
The behavioral results show that patients are impaired at adjusting their response bias (α parameter), but they do not shed light on the mechanism that underlies this impairment. Integration-to-threshold models, formalized as a drift-diffusion process, are successful in capturing choice data from a variety of perceptual decision tasks and provide mechanistic insight (Figure 2A) [13]. The idea is that the brain accumulates noisy sensory evidence until one of two decision thresholds is reached which terminates the decision process and determines the choice. The momentary evidence signal has a deterministic component, the “drift rate”, which we assumed to be proportional to stimulus coherence [14, 15] and a stochastic component, which is reflected in the diffusion process. This framework, with the addition of an urgency component in the form of collapsing decision bounds, largely captured decision behavior in our task (see Supplementary Information). There are two different mechanisms that can lead to biased decisions in this framework. One is to start the evidence accumulation process closer to one of the bounds, which increases the frequency of choosing the option associated with the closer bound (Figure 2B). The other is to offset the drift rate. In this case, even in the absence of any sensory information, the decision process drifts towards one decision bound and away from the other, which biases decisions in favor of the option associated with the default bound (Figure 2C). In asmHC, an increase in overall decision bias corresponded to starting point adjustments (chi-square variance test, p=5∙10−6), but this adjustment was not stimulus-specific (Figure 2D blue solid and dashed lines; see also Figure S2 and Table S2 and S5). The stimulus-specific bias was provided by the drift rate offset (Figure 2E blue solid and dashed lines). The positive drift rate offset for the stimuli with unequal priors further amplifies the decision bias that is introduced by the starting point offset, whereas the negative drift rate offset for the stimuli with equal priors counteracts it, resulting in a situation where mostly only decisions about stimuli with a particular color are biased.
Figure 2. Patients with Parkinson’s fail to adjust the starting point of evidence accumulation.
(A) The drift diffusion model (DDM) explains choice performance and reaction times (RTs) in 2-alternative forced choice decision tasks. Noisy sensory evidence is accumulated (blue line) until one of two decision bounds (black lines) is reached, terminating the decision process. The distance between the starting point (red dot) and the bound is the amount of evidence required for a decision and is referred to as the decision threshold. Each bound corresponds to one of the two choice alternatives. The average rate at which evidence accumulates, referred to as the drift rate, is determined by the strength of the sensory stimulus. The green arrows reflect situations with strong stimuli, in which case decisions tend to be fast and accurate. The black dashed arrow reflects a situation with a very weak stimulus, in which case uncertainty is high and decisions tend to be slow and inaccurate. The grey arrow indicates advancing time. Trial-to-trial variability in the accumulation process leads to variability in choice and reaction time.
(B) Prior information leading to a decision bias can be implemented in two possible ways in the DDM framework. In one, the sensory evidence accumulation process starts closer to the bound that is associated with the choice that should be more frequent according to the priors. An initial value of +1 on the accumulated evidence axis indicates that the process starts right at this decision bound, and a negative value indicates that the process starts closer to the opposite bound, leading to a decision bias that would be inconsistent with the priors. A starting point offset of zero indicates that the accumulation process starts at an equal distance from both decision bounds. Shifting the starting point of evidence accumulation closer to one of the bounds leads to the corresponding option being chosen more frequently.
(C) Prior information leading to a decision bias can also be implemented by adding an offset to the drift rate such that, even in the absence of sensory evidence, the process drifts towards one of the decision bounds (and away from the opposite bound), which also results in one of the options being chosen more frequently. Although both mechanisms have similar effects on biasing choices, they have different effects on RTs, which provides the basis for computational modeling being able to estimate the contributions of both mechanisms to the decision behavior [13, 17, 38]. Note that the schematics shown are idealized model representations. Our model included an urgency component in the form of collapsing decision bounds to capture the RT distributions in our data set. See Supplemental Information for model details.
(D) We modeled the data from the dichromatic task and allowed the starting point of evidence accumulation and the drift rate offset to be stimulus-specific and to change over time. We estimated the parameters for two different task epochs: first half and second half of the trials, reasoning that it requires time for the influence of the priors to appear (see Figure S1E). All other model parameters remained fixed. Blue lines show parameter estimates for the starting point of evidence accumulation (mechanism shown in B) in the two epochs for the group of 12 asmHC of Figure 1C, red lines for the group of 12 patients of Figure 1D. Dashed lines are for the equal prior stimuli, solid lines for the stimuli with unequal priors. Shaded areas indicate 95% confidence intervals.
(E) Parameter estimates for the drift rate offset (mechanism shown in C), otherwise as in D. A positive value of the drift rate offset indicates that the process drifts towards the bound associated with the more frequent choice according to the priors. The size of the offset is provided in terms of equivalent coherence. For example, a value of 10% means that, in the absence of any sensory evidence, the decision process drifts towards one of the bounds with a rate equivalent to what would normally be observed in the presence of a stimulus with 10% coherence. See also Figure S2, Table S1, Table S2 and Table S5.
Interestingly, like the asmHC participants, patients with PD developed a stimulus-specific drift rate offset (Figure 2E red solid and dashed lines), indicating that they were able to learn the stimulus statistics for each color. However, their starting point of evidence accumulation was set incorrectly, and opposite in sign to that indicated by the priors (Figure 2D red solid and dashed lines). The apparent failure to adjust the starting point (chi-square variance test, p=0.78) resulted in an inability to express the decision bias appropriately. The negative starting point counteracted the bias that would have been introduced by the positive drift rate offset leading to a failure to implement a choice bias.
Drift rate offset compensates for starting point adjustment impairments in PD
The behavioral data indicate that patients with PD are unable to apply prior information to make decisions when sensory information is uncertain. The modeling reveals that patients with PD can learn priors, but that they cannot use them; they are impaired at adjusting their starting point of evidence accumulation to express an appropriate bias. To test whether the inability to adjust the starting point of evidence accumulation is due to the requirement of tracking multiple sets of priors, we designed a simpler task without this requirement. We also added two epochs with equal priors, one before and one after, exposing participants to trials with unequal priors to be able to assess baseline decision behavior.
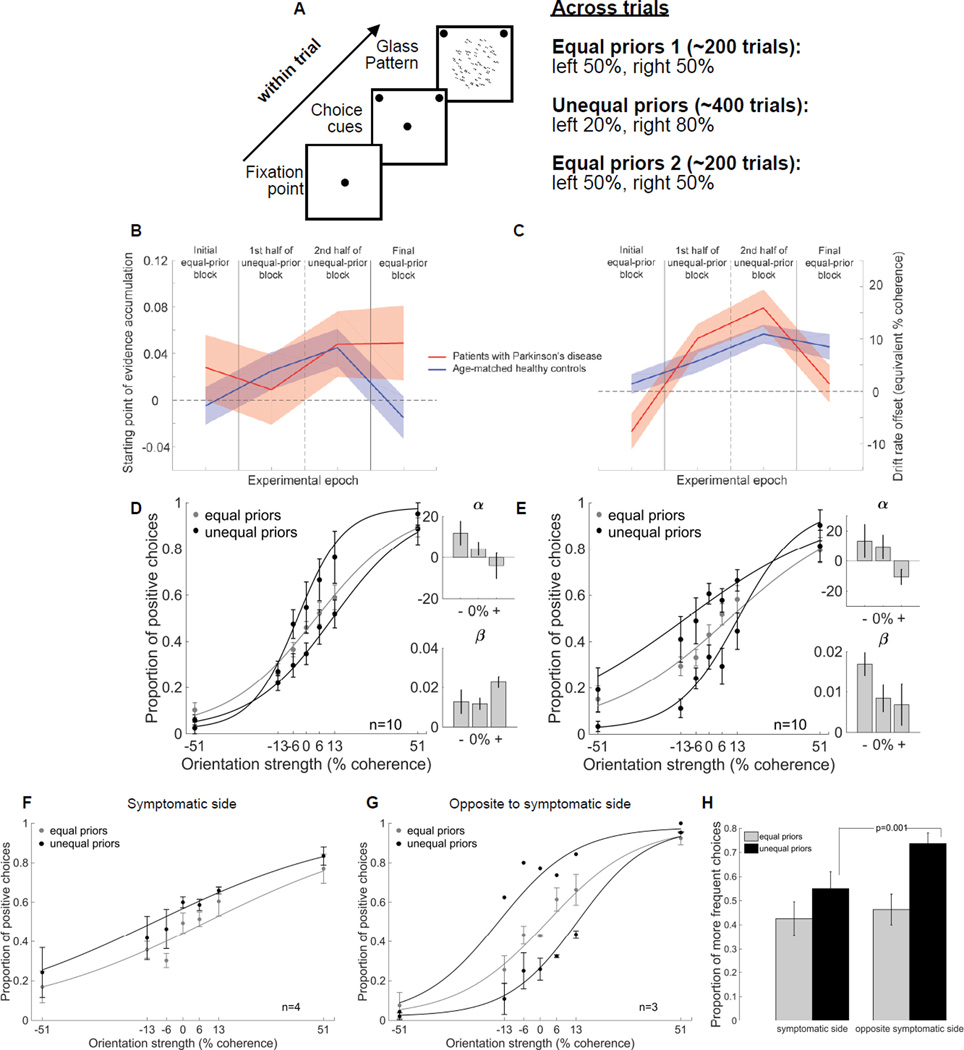
We tested patients with PD and asmHCs in the monochromatic task and modeled their data as we did for the dichromatic task (Figure 3). asmHCs tracked the priors with their starting point of evidence accumulation as in the dichromatic task (Figure 3B, blue; chi-square variance test, p<10−6; see also Figure S3 and Table S3 and S5). In contrast, patients with PD showed little adjustment of their starting point (Figure 3B, red; chi-square variance test, p=0.18). In the monochromatic task, patients showed large changes in their drift rate offset (Figure 3C, red). The asmHC, in contrast, made much smaller adjustments to their drift rate offset (Figure 3C, blue). These results point to the idea that the larger change in the drift rate offset may help patients overcome some of the deficits caused by an impaired ability to adjust the starting point of evidence integration.
Figure 3. Drift rate offsets can compensate for impaired starting point adjustments in patients with PD.
(A) To determine whether the patients showed impairments in starting point adjustments even in an easier version of the task, we designed a simpler task that did not require tracking multiple sets of priors and that also had an initial, equal prior condition, before introducing unequal priors. This allowed us to determine the baseline starting point of evidence accumulation in patients. Participants performed three blocks of trials. During the first block we presented 200 randomly interleaved stimuli, half of the stimuli were leftward oriented Glass patterns and half were rightward oriented Glass Patterns. The next block consisted of 400 stimuli unevenly distributed between leftward and rightward Glass patterns (unequal priors): either 80% leftward and 20% rightward (80:20) or vice versa. The orientation associated with the higher probability of occurrence was counterbalanced across participants. The last block was the same as the first having an equal number of leftward and rightward Glass patterns (50:50). We made this task simpler by using only a monochromatic Glass pattern and therefore refer to this task as the monochromatic task. We validated the performance and the use of priors in this task in yHC (Figure S1F) and also the requirement of feedback for learning the priors (Figure S1G).
(B) Using the DDM, we estimated the starting point of evidence accumulation for four different task epochs: the initial control block with equal priors, the first half of the block with unequal priors, the second half of this block (reasoning that it would take time for the influence of the priors to develop - see Figure S1G), and the final block of trials with equal priors. The four epochs are demarcated by the vertical lines. The blue line shows parameter estimates for asmHC. The red line shows parameter estimates for patients with PD. Shaded areas indicate 95% confidence intervals.
(C) Parameter estimates for drift rate offset, otherwise like B.
(D) Proportion of positive choices is plotted against orientation strength for 10 patients with PD (5 males and 5 females, mean age 63yrs). The grey points and lines show the data and the logistic fits in the equal prior trials (50:50) whereas the black points and lines show the data for unequal prior trials (80:20). The direction and color of the stimulus with different priors were counterbalanced across participants so that all participants contributed to the grey data and different subsets of participants contributed to the black data.
(E) Same as in (D) for 10 asmHC (4 males, 6 females, mean age 56yrs).
(F) Proportion of positive choices is plotted against orientation strength for patients who experienced the prior on their symptomatic side (n=4, 1 male 3 females). Grey points and lines as well as black points and lines show data and fits for all 4 patients who experienced a leftward bias.
(G) Proportion of positive choices is plotted against orientation strength for patients who experienced the prior on their symptomatic side (n=3, 2 males 1 females). Grey points and lines show data and fits for all 3 patients whereas black points and lines show the data and fits for 1 patient who experienced the leftward bias and 2 patients who experienced the rightward bias.
(H) Proportion of more frequent choices for the most difficult stimuli (0% and 13% coherence) is plotted for the equal (grey) and unequal prior (black) conditions for the symptomatic and opposite sides. Error bars in panels D-H are ±SEM. See also Figure S1, Figure S3, Table S1, Table S3 and Table S5
Comparison of the performance of patients and asmHC participants in the monochromatic task is consistent with the idea that the drift rate offset compensates for the starting point adjustment problems seen in patients. In the equal prior condition, with strong sensory information (51%), the performance of patients was ~80% correct, indicating no problems in orientation discrimination or motor performance (cf., Figure 3D grey). Similarly, in the block with unequal priors, patients with PD shifted their response bias towards the prior direction while keeping their sensitivity unchanged (cf., Figure 3D black versus grey; α t(9)=2.87, p<0.05; β, t(9)= 0.43, p>0.05; see also Figure S1). asmHC also showed shifts in the α parameter, but not the β parameter of the logistic fits with prior information, indicating a change in response bias, but not in sensitivity (cf., Figure 3E black versus grey; α, t(9)= 2.83, p<0.05; β, t(9)= 1.93, p>0.05). Comparing the proportion of choosing the more frequent orientation between patients and asmHC participants, as we did for the dichromatic task, revealed a similar proportion of choosing the more frequent orientation for patients and asmHCs in the equal and the unequal prior condition (0.50±0.03 vs. 0.54±0.03 and 0.62±0.03 vs. 0.61±0.03; data not shown). A 2x2 mixed ANOVA showed a significant main effect of prior condition (F(1,58)=22.85, p<0.0001) due to the differences in the equal and unequal prior conditions, but no significant main effect of group (F(1,58)=0.14, p>0.05) nor group×prior interaction (F(1,58)=2.37, p>0.05), consistent with the better ability of patients to perform this simpler task compared to their ability to perform the dichromatic task. The behavioral data together with the modeling indicate that the enhanced drift rate offset compensates for the starting point adjustment problems in patients performing the monochromatic task, allowing them to better express the choice bias.
In spite of the better performance of patients in the monochromatic task, there was a measurable difference in the ability of patients to use the priors and this depended upon whether or not the prior direction coincided with the patients’ symptomatic side. We again divided our patient participants into a symptomatic side group (Figure 3F) and an opposite to symptomatic side group (Figure 3G) depending on the side of their symptoms relative to the direction of the priors they experienced. The symptomatic side group used the priors less (0.56±0.04) compared to the opposite symptomatic side group (0.73± 0.04) in the unequal prior condition (Figure 3H, black bars) but there were no differences in the proportion of choosing the more frequent orientation in the equal prior condition (0.43±0.05 vs. 0.46±0.04). A 2x2 mixed ANOVA revealed a significant main effect of prior, F(1,19)=10.79 p<0.01 and side×prior interaction (F(1,19)=25.77 p<0.001). Post hoc analyses revealed that the differences were significant in the unequal prior condition (t(19)=-4.1 p=0.001) but not in the equal prior condition (t(19)=1.86 p>0.05). As we saw in the dichromatic task, the results from the monochromatic task suggest that the inability to use the directional priors is more pronounced when the prior direction corresponds to the side of the motor symptoms and the dysfunction is independent of motor or perceptual processing and because it is unilateral, may not result from dopamine medication. Again, due to the small sample size, we interpret this result cautiously.
Failure to combine priors and sensory information is independent of feedback learning
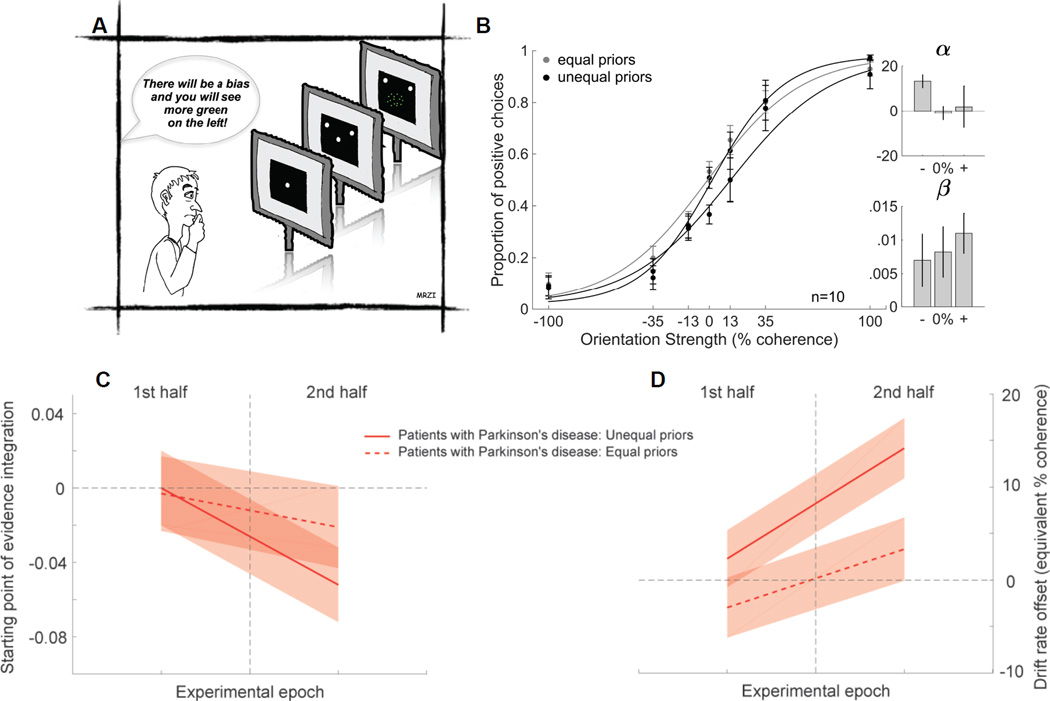
We tested patients using the dichromatic task as above except that information about the prior probability was given to the participants verbally. This eliminated the need for learning from trial feedback (Figure 4A; see also Figure S1). We reasoned that if the impairment in using priors seen in patients with PD results from faulty feedback learning, eliminating the need for feedback learning should eliminate or minimize the impairment [8, 16]. We found that patients performed well when the sensory information was strong (Figure 4B, grey), indicating no perceptual or motor impairment. With ambiguous sensory information, patients failed to use the verbal instruction about the priors to inform their choices (Figure 4B black). This result was confirmed statistically by the lack of a significant shift in the α parameter of the logistic fit between performance on the equal and unequal prior trials (Figure 4B, α t(9)=1.27, p>0.05). We next compared the proportion of more frequent choices between patients performing the dichromatic task (Figure 1C) and patients performing the dichromatic task with explicit instructions (Figure 4B) and found that they were similar in both the equal and unequal prior conditions (equal: 0.51±0.03 versus 0.51±0.04; unequal: 0.52±0.03 versus 0.54±0.04). The differences were statistically indistinguishable as determined by a 2x2 mixed ANOVA showing a lack of significant effects of groups (F(1,64)=0.02, p>0.05), prior (F(1,64)=0.98, p>0.05) or group×prior interaction (F(1,64)=0.49, p>0.05). Thus, providing explicit instructions about the prior direction is insufficient to improve the ability of patients to use priors in conditions of sensory uncertainty. We modeled the data to assess whether a similar impairment in adjusting the starting point was present even when priors were provided explicitly. We found that the poor performance of patients could again be explained by problems in adjusting the starting point of evidence accumulation (Figure 4C; see also Table S4). Patients were able to adjust their drift rate offset in a stimulus-specific manner (Figure 4D, red solid and dashed lines). However, the starting point adjustment was inappropriate for the direction of the prior, explaining their poor performance (Figure 4C). Similar to what we observed in the dichromatic task before, the starting point was positioned in the direction opposite to the prior manipulation. This “repulsion” might be specific to situations in which different priors are tracked for different stimulus features. Future work is required to determine the mechanism that underlies this inappropriate starting point adjustment. Taken together, we conclude that even with explicit prior information, patients with PD fail to use them to express a response bias.
Figure 4. Patients with PD do not benefit from explicit knowledge of prior probabilities.
(A) Schematic of the dichromatic Glass pattern task showing that participants were informed of the priors explicitly (75:25) by instruction (“explicit task”). This eliminates the need for learning. We first verified the validity of this manipulation by testing a group of 16 naïve yHCs performing the dichromatic task without feedback but with explicit, verbal instructions and we concluded that providing explicit instructions about the priors is a valid way to induce a decision bias in the absence of feedback learning (Figure S1H).
(B) Proportion of positive choices is plotted against the orientation strength from 10 patients with PD (4 females, 6 males, mean age 62 yrs) as in Figure 1C. The grey points and lines show the data and logistic fits from the equal prior conditions whereas the black points and lines show the data and fits for the unequal prior conditions. The prior direction was counterbalanced across participants so different subsets of patients contribute to the two prior direction conditions. All patient data contribute to the equal prior condition. Six of the 10 patients also performed the original version of the dichromatic task ~5 months before performing the explicit task. Only 2 of the patients experienced opposite priors in the two tasks, minimizing the likelihood of learning conflicting priors and maximizing the chances that if patients could use the prior they would. After completing this task, 8 patients reported following the instructions and all 10 reported being aware of the unequal priors even though they failed to use them. This observation rules out an interpretation based on faulty working or short-term memory for the priors.
(C) Starting point of evidence accumulation from the drift-diffusion model during the two experimental epochs (separated by the vertical line) of the explicit task for patients. The solid red line is for the stimuli with unequal priors, the dashed red line for the stimuli with equal priors. Shaded areas indicate 95% confidence intervals.
(D) Parameter estimates for drift rate offset, otherwise like C.
DISCUSSION
Using a novel task, we assessed perceptual decision-making performance in early stage, medicated patients with PD compared to that of age- and sex-matched healthy participants. We manipulated task statistics to assess the ability of participants to combine prior experience with visual information to guide decisions. We modeled the data using the DDM to reveal the mechanisms used to incorporate prior information into perceptual decision-making. All participants performed the perceptual decision-making task and their choice accuracy increased with the strength of the sensory information. However, with prior information available to guide choices, healthy participants used the priors whereas patients with PD were impaired. Modeling revealed that the impairment resulted from a failure to adjust the starting point of evidence accumulation, consistent with an impairment in adjusting decision thresholds asymmetrically in such a way that less sensory evidence needs to be collected before committing to the choice associated with the more frequently presented alternative.
A change in the starting point of evidence accumulation relative to the decision bounds is usually considered the primary mechanism for incorporating priors into perceptual decisions [6, 17]. Our modeling results show that two mechanisms interact when combining sensory information and priors: starting point adjustments and drift rate offsets [5, 18]. Our novel results show that starting point adjustments provide only a global choice bias, whereas drift rate offsets incorporate stimulus-specific prior information when multiple sets of priors are tracked. Patients with PD fail to express an appropriate choice bias. In the DDM framework, this impairment corresponds to a failure in adjusting the distances between the starting point of evidence accumulation and the decision bounds. The ability to adjust the drift rate offset remains intact and, in some cases, can compensate for impaired starting point adjustments. Future experiments, measuring neuronal activity, will be able to test these insights gained from computational modeling.
Our results are broadly consistent with models suggesting that basal ganglia (BG) nuclei play a role in setting decision thresholds [19–26]. However, our patients showed impairments in the ability to combine prior information with sensory information to guide decisions. As far as we are aware no other group has addressed this in patients with PD. Therefore, our results extend the model of the BG in perceptual decision-making in a critical way. In addition to setting within-trial adjustments of thresholds when faced with conflict [27, 28], our results implicate the BG in across-trial decision threshold adjustments that take place gradually as the prior information is learned and the memory of the priors is formed.
It is well-known that patients with PD on dopamine medication are capable of learning from positive feedback during value-based decision-making [7, 11, 29, 30], so we expected that our patient sample could learn and use the priors in our task. Our surprising finding showed that patients could learn the priors, but were impaired at using them, consistent with suggestions that patients are impaired at performance rather than learning [9, 10]. Furthermore, we found impairments in patient performance even when they were explicitly instructed about the priors. Therefore, our results point toward differences in the mechanisms underlying value-based decisions involving reinforcement learning and incorporating priors for perceptual decisions [31].
Our results also suggest that the BG play a role in linking the neuronal circuits that encode past experience and sensory information and those controlling decision-making. However, PD is a multisystem disease involving many brain areas and neuromodulatory systems [32–34] and all of our experiments were performed in patients on dopamine medication, calling into question the role of dopamine in these behaviors [35–37]. Future experiments will be required to determine whether the decision-making dysfunction we report here stems from alterations in BG circuits or whether they depend upon other circuits and modulatory systems affected by PD.
Supplementary Material
Acknowledgments
We are grateful to Dr. Tony Movshon for providing code for the Glass pattern stimuli, Dr. Xueqi Cheng for programming support, Adam Myers for administrative support and MRZI for illustrations. We would like to thank Dr. Joaquin Fuster for his support and Drs. Sham and Knowlton for helpful discussions at early stages of this work and Drs. Alice Powers, Aaron Seitz and Peggy Series for critical comments on a previous version of the manuscript. We thank all our participant volunteers and Drs. Pouratian, Bordelon and Wu for their help in recruiting patients. This work was supported by the Dana Foundation and NIH EY13692 (MAB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes three figures, five supplemental items and Supplemental Experimental Procedures.
AUTHOR CONTRIBUTIONS
Conceptualization, A.P. and M.A.B.; Methodology, A.P. and M.A.B.; Formal Analysis, A.P., J.D., M.A.B., Investigation, A.P.; Writing – Original Draft, A.P., J.D., M.A.B Writing – Review & Editing, A.P., J.D., M.A.B; Funding Acquisition, M.A.B.; Resources, M.A.B.; Supervision, J.D., M.A.B.
REFERENCES
- 1.Gold JI, Shadlen MN. The neural basis of decision making. Annual Review of Neuroscience. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 2.Mulder MJ, Wagenmakers EJ, Ratcliff R, Boekel W, Forstmann BU. Bias in the brain: a diffusion model analysis of prior probability and potential payoff. J Neurosci. 2012;32:2335–2343. doi: 10.1523/JNEUROSCI.4156-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leite F, Ratcliff R. What cognitive processes drive response biases? A diffusion model analysis. Judgement and Decision Making. 2011;6:651–687. [Google Scholar]
- 4.Summerfield C, Egner T. Expectation (and attention) in visual cognition. Trends in Cognitive Sciences. 2009;13:403–409. doi: 10.1016/j.tics.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Hanks TD, Mazurek ME, Kiani R, Hopp E, Shadlen MN. Elapsed Decision Time Affects the Weighting of Prior Probability in a Perceptual Decision Task. The Journal of Neuroscience. 2011;31:6339–6352. doi: 10.1523/JNEUROSCI.5613-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao V, DeAngelis GC, Snyder LH. Neural Correlates of Prior Expectations of Motion in the Lateral Intraparietal and Middle Temporal Areas. The Journal of Neuroscience. 2012;32:10063–10074. doi: 10.1523/JNEUROSCI.5948-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank MJ, Seeberger LC, O'Reilly RC. By Carrot or by Stick: Cognitive Reinforcement Learning in Parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 8.Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA, Poldrack RA. Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. 2004;127 doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- 9.Shiner T, Seymour B, Wunderlich K, Hill C, Bhatia KP, Dayan P, Dolan RJ. Dopamine and performance in a reinforcement learning task: evidence from Parkinson’s disease. Brain. 2012;135:1871–1883. doi: 10.1093/brain/aws083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smittenaar P, Chase HW, Aarts E, Nusselein B, Bloem BR, Cools R. Decomposing effects of dopaminergic medication in Parkinson’s disease on probabilistic action selection – learning or performance? European Journal of Neuroscience. 2012;35:1144–1151. doi: 10.1111/j.1460-9568.2012.08043.x. [DOI] [PubMed] [Google Scholar]
- 11.Foerde K, Shohamy D. The role of the basal ganglia in learning and memory: Insight from Parkinson’s disease. Neurobiology of Learning and Memory. 2011;96:624–636. doi: 10.1016/j.nlm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forstmann BU, Brown S, Dutilh G, Neumann J, Wagenmakers E-J. The neural substrate of prior information in perceptual decision making: a model-based analysis. Frontiers in Human Neuroscience. 2010;4 doi: 10.3389/fnhum.2010.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratcliff R, McKoon G. The Diffusion Decision Model: Theory and Data for Two-Choice Decision Tasks. Neural computation. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer J, Huk AC, Shadlen MN. The effect of stimulus strength on the speed and accuracy of a perceptual decision. Journal of Vision. 2005;5:376–404. doi: 10.1167/5.5.1. [DOI] [PubMed] [Google Scholar]
- 15.Ditterich J. Stochastic models of decisions about motion direction: Behavior and physiology. Neural Networks. 2006;19:981. doi: 10.1016/j.neunet.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Knowlton BJ, Mangels JA, Squire LR. A Neostriatal Habit Learning System in Humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 17.White CN, Poldrack RA. Decomposing bias in different types of simple decisions. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2014;40:385–398. doi: 10.1037/a0034851. [DOI] [PubMed] [Google Scholar]
- 18.Moran R. Optimal decision making in heterogeneous and biased environments. Psychonomic Bulletin & Review. 2015;22:38–53. doi: 10.3758/s13423-014-0669-3. [DOI] [PubMed] [Google Scholar]
- 19.Wei W, Rubin JE, Wang X-J. Role of the Indirect Pathway of the Basal Ganglia in Perceptual Decision Making. The Journal of Neuroscience. 2015;35:4052–4064. doi: 10.1523/JNEUROSCI.3611-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogacz R, Wagenmakers E-J, Forstmann BU, Nieuwenhuis S. The neural basis of the speed–accuracy tradeoff. Trends in Neurosciences. 2010;33:10–16. doi: 10.1016/j.tins.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Bogacz R, Gurney K. The Basal Ganglia and Cortex Implement Optimal Decision Making Between Alternative Actions. Neural computation. 2007;19:442–477. doi: 10.1162/neco.2007.19.2.442. [DOI] [PubMed] [Google Scholar]
- 22.Lo CC, Wang XJ. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat. Neurosci. 2006;9:956. doi: 10.1038/nn1722. [DOI] [PubMed] [Google Scholar]
- 23.Ding L, Gold Joshua I. The Basal Ganglia’s Contributions to Perceptual Decision Making. Neuron. 2013;79:640–649. doi: 10.1016/j.neuron.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratcliff R, Frank MJ. Reinforcement-based decision making in corticostriatal circuits: mutual constraints by neurocomputational and diffusion models. Neural computation. 2012;24:1186–1229. doi: 10.1162/NECO_a_00270. [DOI] [PubMed] [Google Scholar]
- 25.Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci. 2011;14:1462–1467. doi: 10.1038/nn.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Networks. 2006;19:1120. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Zavala BA, Tan H, Little S, Ashkan K, Hariz M, Foltynie T, Zrinzo L, Zaghloul KA, Brown P. Midline frontal cortex low-frequency activity drives subthalamic nucleus oscillations during conflict. J Neurosci. 2014;34:7322–7333. doi: 10.1523/JNEUROSCI.1169-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herz Damian M, Zavala Baltazar A, Bogacz R, Brown P. Neural Correlates of Decision Thresholds in the Human Subthalamic Nucleus. Current Biology. 26:916–920. doi: 10.1016/j.cub.2016.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA. The role of dopamine in cognitive sequence learning: evidence from Parkinson’s disease. Behavioural brain research. 2005;156:191–199. doi: 10.1016/j.bbr.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Chong TTJ, Bonnelle V, Manohar S, Veromann K-R, Muhammed K, Tofaris GK, Hu M, Husain M. Dopamine enhances willingness to exert effort for reward in Parkinson's disease. Cortex; a journal devoted to the study of the nervous system and behavior. 2015;69:40–46. doi: 10.1016/j.cortex.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shadlen Michael N, Kiani R. Decision Making as a Window on Cognition. Neuron. 2013;80:791–806. doi: 10.1016/j.neuron.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 33.Robbins TW, Cools R. Cognitive deficits in Parkinson's disease: A cognitive neuroscience perspective. Movement Disorders. 2014;29:597–607. doi: 10.1002/mds.25853. [DOI] [PubMed] [Google Scholar]
- 34.Halliday GM, Leverenz JB, Schneider JS, Adler CH. The neurobiological basis of cognitive impairment in Parkinson's disease. Mov Disord. 2014;29:634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or Impaired Cognitive Function in Parkinson's Disease as a Function of Dopaminergic Medication and Task Demands. Cerebral Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- 36.Huang DY-T, Georgiev D, Foltynie T, Limousin P, Speekenbrink M, Jahanshahi M. Different effects of dopaminergic medication on perceptual decision-making in Parkinson's disease as a function of task difficulty and speed–accuracy instructions. Neuropsychologia. 2015;75:577–587. doi: 10.1016/j.neuropsychologia.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Nagano-Saito A, Cisek P, Perna AS, Shirdel FZ, Benkelfat C, Leyton M, Dagher A. From anticipation to action, the role of dopamine in perceptual decision making: an fMRI-tyrosine depletion study. J Neurophysiol. 2012;108:501–512. doi: 10.1152/jn.00592.2011. [DOI] [PubMed] [Google Scholar]
- 38.Ratcliff R. A theory of memory retrieval. Psychological review. 1978;85:59. doi: 10.1037/0033-295x.95.3.385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.