Abstract
Tumor-induced osteomalacia (TIO) is a rare paraneoplastic syndrome clinically characterized by bone pain, fractures and muscle weakness. It is caused by tumoral overproduction of fibroblast growth factor 23 (FGF23) that acts primarily at the proximal renal tubule, decreasing phosphate reabsorption and 1α-hydroxylation of 25 hydroxyvitamin D, thus producing hypophosphatemia and osteomalacia. Lesions are typically small, benign mesenchymal tumors that may be found in bone or soft tissue, anywhere in the body. In up to 60% of these tumors, a fibronectin-1(FN1) and fibroblast growth factor receptor-1 (FGFR1) fusion gene has been identified that may serve as a tumoral driver. The diagnosis is established by the finding of acquired chronic hypophosphatemia due to isolated renal phosphate wasting with concomitant elevated or inappropriately normal blood levels of FGF23 and decreased or inappropriately normal 1,25-OH2-Vitamin D (1,25(OH)2D). Locating the tumor is critical, as complete removal is curative. For this purpose, a step-wise approach is recommended, starting with a thorough medical history and physical examination, followed by functional imaging. Suspicious lesions should be confirmed by anatomical imaging, and if needed, selective venous sampling with measurement of FGF23. If the tumor is not localized, or surgical resection is not possible, medical therapy with phosphate and active vitamin D is usually successful in healing the osteomalacia and reducing symptoms. However, compliance is often poor due to the frequent dosing regimen and side effects. Furthermore, careful monitoring is needed to avoid complications such us secondary/tertiary hyperparathyroidism, hypercalciuria, and nephrocalcinosis. Novel therapeutical approaches are being developed for TIO patients, such as image-guided tumor ablation and medical treatment with the anti-FGF23 monoclonal antibody KRN23 or anti FGFR medications. The case of a patient with TIO is presented to illustrate the importance of adequate and appropriate evaluation of patients with bone pain and hypophosphatemia, as well as an step-wise localization study of patients with suspected TIO.
Abbreviations: TIO, tumor-induced osteomalacia; FGF23, fibroblast growth factor 23; 1,25-OH2-vitamin D, 1,25(OH)2D; FGFR1, fibroblast growth factor receptor-1; PTH, parathyroid hormone; TmP/GFR, tubular maximum reabsorption of phosphate to glomerular filtration rate; FDG-PET/CT, fluorodeoxyglucose positron emission tomography with computerized tomography; CT, computerized tomography; MRI, magnetic resonance imaging; PMT, phosphaturic mesenchymal tumor; MAPK, mitogen-activated protein kinase; FN1, fibronectin-1; FISH, fluorescence in situ hybridization; FGF1, fibroblast growth factor 1; TRP, tubular reabsorption of phosphate; SPECT, single-photon emission computed tomography
Keywords: Tumor-induced osteomalacia, FGF23, Phosphaturic mesenchymal tumors
Highlights
-
•
Tumor-induced osteomalacia (TIO) is a paraneoplastic syndrome characterized by bone pain, fractures and muscle weakness.
-
•
It is caused by tumoral overproduction of fibroblast growth factor 23 (FGF23) producing hypophosphatemia and osteomalacia.
-
•
Locating the tumor is critical, as complete removal is curative.
-
•
If resection is not possible, medical therapy is usually successful, but monitoring is needed to avoid complications.
-
•
Novel therapeutical approaches open promising perspectives for the treatment of patients with TIO.
1. Case report
A 55-year-old woman presented for evaluation of fragility fractures in the setting of hypophosphatemia. She described a 12-year history of back and pelvis pain. At age 51, she fell in the bathtub and sustained multiple rib fractures. Following this event, she developed severe lower extremity pain and difficulty walking, rendering her unable to work. She was given a diagnosis of osteoporosis and rheumatoid arthritis and was placed on therapy with teriparatide and etanercept. At age 53, she suffered bilateral femoral fractures after minor falls, requiring bilateral hip replacement and inpatient rehabilitation. Subsequent evaluation showed high serum parathyroid hormone (PTH) with normal calcium, low phosphorus and very low 24-hour urine calcium (Table 1: Initial presentation). The hypophosphatemia was believed to be secondary to hyperparathyroidism. A neck ultrasound revealed a 8 mm right sided posterior nodule, and a SESTAMIBI-Technetium scan was suggestive of a parathyroid adenoma. She was referred for surgery, and underwent a right hemi-thyroidectomy with resection of three parathyroid glands. Pathological analysis revealed only hyperplastic changes. Post-surgically she had significant transient hypocalcemia, requiring intravenous calcium, and persistent hypophosphatemia. She was discharged on calcitriol and phosphate supplements and referred to the Section on Skeletal Disorders and Mineral Homeostasis at the National Institutes of Health for further evaluation.
Table 1.
Laboratory values and treatment doses at different time points.
| Initial presentation | Initial Evaluation at NIH | Pre PMT resection | Post PMT resection | |
|---|---|---|---|---|
| Serum phosphorus (2.5–4.8 mg/dL) | 1.9 | 2.0 | 2.0 | 4.4 |
| Serum calcium (2.05–2.50 mmol/L) | 2.35 | 2.27 | 2.14 | 2.30 |
| Alkaline phosphatase (37–116 U/L) | 570 | 187 | 135 | |
| TmP/GFR (2.8–4.2 mg/dL) | 1.54a | 1.46a | 4.56a | |
| 24-hour urinary calcium (50–250 mg/24 h) | 20 | 191 | ||
| 25-OH-vitamin D (10–100 ng/mL) | 25 | 22 | ||
| 1,25-OH2-vitamin D (18-78 pg/mL) | < 10 | 17 | 160 | |
| PTH (15-65 pg/mL) | 219.0 | 53.1 | 98.6 | 66.5 |
| Intact FGF23 (10–50 pg/mL) | 404 | 42 | ||
| Treatment (dose/24 h) | Calcitriol 2 μg, phosphorus 1 g, calcium 1.6 g | Calcitriol 4.5 μg, phosphorus 2.25 g, calcium 1.5 g | Calcium 1 g, cholecalciferol 1000 IU |
TmP/GFR measurements made off phosphorus supplements.
On admission, a medical history and physical examination were negative for tumors. Medical and family history did not suggest a genetic cause of hypophosphatemia; there was no exposure to medications or toxins associated with acquired hypophosphatemia. Biochemical evaluation showed low serum phosphorus with low tubular maximum reabsorption of phosphate to glomerular filtration rate (TmP/GFR), elevated alkaline phosphatase, undetectable 1,25(OH)2D, and normal PTH. Intact fibroblast growth factor 23 (FGF23, Kainos assay) was significantly elevated (Table 1: Initial evaluation at NIH) and the diagnosis of TIO was established. A skeletal survey revealed signs of osteomalacia with multiple fractures in ribs, vertebrae and long bones and secondary thoracic cage deformity (Fig. 1). Pulmonary function testing showed a severe restrictive disease likely secondary to multiple rib fractures and rib cage collapse. She required high doses of calcitriol (2.25 μg bid), phosphorus (750 mg tid), and calcium (500 mg tid) to maintain low normal serum calcium and phosphorus with normal 24 h urinary calcium.
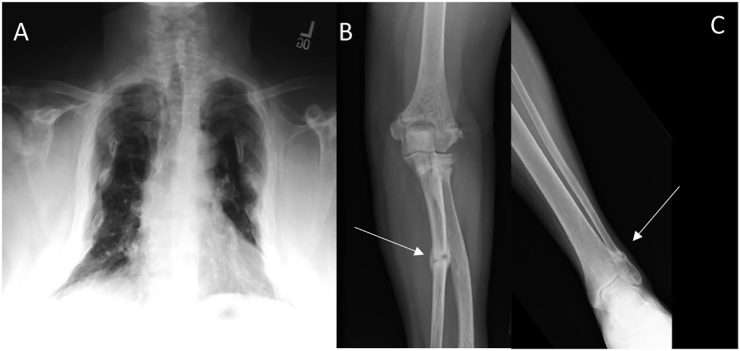
Fig. 1.
Skeletal consequences of tumor-induced osteomalacia.
A) Chest PA radiograph showing significant loss of lung volume and an overall bell-shaped rib cage. B) Fracture in left ulnar shaft (arrow) C) Fracture in left distal fibula (arrow).
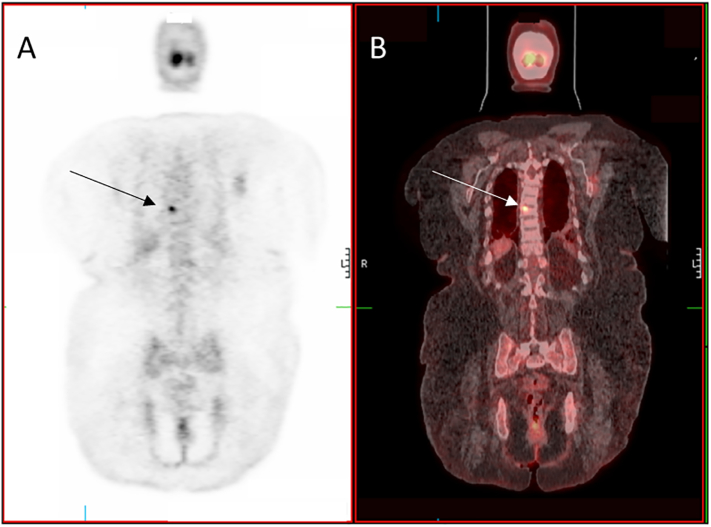
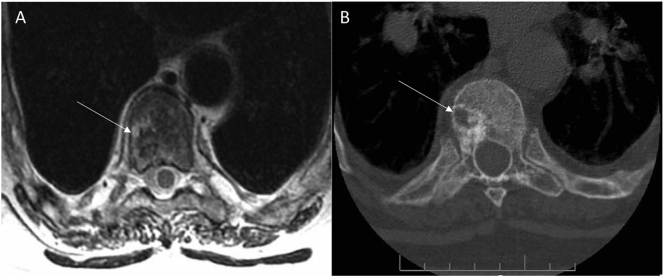
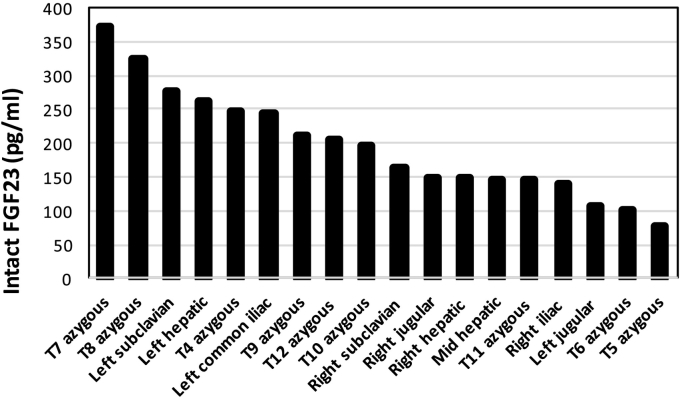
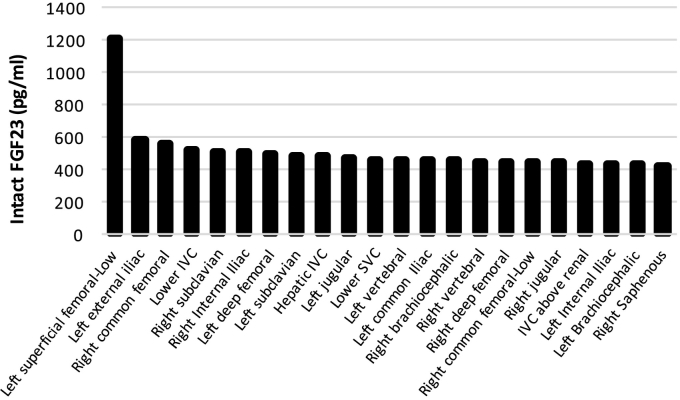
While functional imaging by Single-photon emission computed tomography (SPECT) Octreoscan™ did not identify a tumor, an F-18 fluorodeoxyglucose positron emission tomography with computerized tomography (FDG-PET/CT) revealed a hypermetabolic lesion in the T8 vertebral body (Fig. 2). Anatomical imaging with CT and magnetic resonance imaging (MRI) confirmed a 7 × 10 mm tumor in the posterior right lateral aspect of the vertebral body of T8 (Fig. 3). Given the high surgical risk associated with the tumor's location, selective venous sampling with FGF23 measurement was performed. However, results were not conclusive in confirming the T8 tumor as the source of FGF23 (Fig. 4).
Fig. 2.
Functional Imaging in Tumor-Induced Osteomalacia.
Planar view of an FDG-PET (A) and FDG-PET/CT (B) showing hyper-metabolic lesion in T8 (arrows) consistent with a possible phosphaturic mesenchymal tumor.
Fig. 3.
Anatomical Imaging in Tumor-Induced Osteomalacia.
Axial images through the T8 vertebral body showing a right-sided lesion on a T2-weighted MR image (A) and a CT scan (B) (arrows) (Sciubba et al., 2009).
Fig. 4.
Selective venous sampling to confirm location of phosphaturic mesenchymal tumor.
FGF23 was measured at the indicated sites. While the data were suggestive of a lesion in the anatomical area of the T8/T7 vertebrae, they were not conclusive (e.g. levels in the left subclavian vein were high as well). Overall the findings were not considered to be diagnostic in that there was not a focal anatomical site that demonstrated a ratio > 1.6 over systemic circulation (Andreopoulou et al., 2011).
While biopsies/aspirations of PMTs are discouraged due to the concern for tumor seeding, in this case, given the significant potential morbidity of the proposed operation, greater diagnostic certainty was necessary and a CT-guided biopsy was performed. Intact FGF23 levels in the cell washings of tumor were 1140 pg/mL, significantly higher than the simultaneously measured peripheral plasma levels (177 pg/mL). Histopathology was compatible with a phosphaturic mesenchymal tumor (PMT). The decision was therefore made to resect the T8 vertebral body by en bloc spondylectomy followed by spinal reconstruction to remove all metabolically active tissue (Sciubba et al., 2009). The procedure was well-tolerated, with significant symptomatic improvement, and normalization of intact FGF23 and serum phosphorus levels without supplementation in the first 10 days after resection. (Table 1: Pre and post-operative). Histopathology confirmed that the lesion was a PMT (Fig. 5).
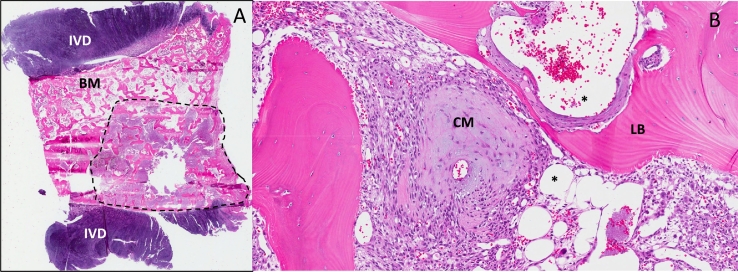
Fig. 5.
Histological features of phosphaturic mesenchymal tumors (PMTs).
Panel A depicts a low power view of the entire T8 vertebral body between the intervertebral discs (IVD), with a 0.5 cm PMT (dotted line), adjacent to preserved bone marrow (BM). A higher power view of the tumor (Panel B) showing typical findings seen in PMTs, including chondroid (“grungy”) matrix (CM), vascularity as demonstrated by abundant venous channels (*),and areas of lamellar bone (LB). Hematoxylin and eosin (H&E).
2. Introduction
Tumor-induced osteomalacia (TIO), also known as oncogenic osteomalacia, is a rare paraneoplastic syndrome characterized by bone pain, muscle weakness and fractures associated with persistent hypophosphatemia due to renal phosphate wasting, inappropriately normal or low 1,25(OH)2D and elevated or inappropriately normal fibroblast growth factor 23 (FGF23). TIO is caused by tumoral overproduction of FGF23 that acts primarily at the proximal renal tubule to inhibit phosphate reabsorption and 1α-hydroxylation of 25-hydroxyvitamin D, which leads to hypophosphatemia and eventually osteomalacia (Chong et al., 2011a, Minisola et al., 2017).
Since the symptoms are relatively non-specific (Jan de Beur, 2005) and phosphate levels are not routinely included in many comprehensive metabolic panels, hypophosphatemia is often overlooked and patients are misdiagnosed with a variety of skeletal, rheumatologic, or neuro-psychiatric diseases (Gonzalez et al., 2017, Lewiecki et al., 2008). Without a timely diagnosis, TIO can lead to a significant decrease in quality of life, and in some patients, severe functional impairment and even prostration. Reported length of time from onset of symptoms to diagnosis ranges from 2.5–28 years (Chong et al., 2011a, Gonzalez et al., 2017). Fortunately, complete tumoral resection leads to restoration of normal mineral metabolism and dramatic resolution of symptoms. These facts highlight the importance of considering TIO and including the measurement of serum phosphate in any patient with persistent bone pain, fractures, or muscle weakness (Jan de Beur, 2005).
This review provides an update on pathophysiological and clinical aspects of TIO, with emphasis on a step-wise approach to evaluation and tumor localization as well as new treatments on the horizon.
3. Epidemiology
Approximately 500 cases of TIO have been reported in literature. The mean age of diagnosis is 40 to 45 years with a wide age range, including cases reported in children. It appears to be a balanced distribution between sexes (Gonzalez et al., 2017, Jiang et al., 2012).
4. Pathophysiology
FGF23 acts primarily on renal proximal tubular cells, binding to an FGF receptor in coordination with its obligate co-receptor, Klotho (Chong et al., 2011a, Razzaque, 2009). Its effect is to reduce expression of the sodium-phosphate cotransporters (NaPi-2a and NaPi-2c) in the proximal renal tubule, leading to decreased renal phosphate reabsorption. In addition, FGF23 inhibits expression of 25-hydroxyvitamin D3 1-alpha-hydroxylase, resulting in inadequate production of 1,25(OH)2D, which is necessary for optimal enteral calcium and phosphate absorption (Chong et al., 2011a, Tsagalis et al., 2009, Bhattacharyya et al., 2012, Fukumoto, 2008). The vast majority of tumors that cause TIO are a distinct entity, namely “phosphaturic mesenchymal tumors (PMTs) of the mixed connective tissue variant” (Minisola et al., 2017, Evans and Azzopardi, 1972, Weidner and Santa Cruz, 1987, Folpe et al., 2004). Histologically, PMTs are characterized by neoplastic cells that are spindle to stellate in shape, normochromatic with small nuclei and indistinct nucleoli. The nuclear grade is low, and mitotic activity is usually absent or very low. The cells are typically embedded within a myxoid or myxochondroid matrix with ‘grungy’ calcification that can resemble chondroid or osteoid. Numerous osteoclast-like giant cells are a frequent finding, and mature fat and even lamellar bone may also be seen. A prominent feature of these tumors is an elaborate intrinsic microvasculature with an admixture of vessel size and vascular pattern (Chong et al., 2011a, Folpe et al., 2004). A key feature of PMTs is the demonstration of FGF23 production (Minisola et al., 2017). Interestingly, recent evidence shows that, in addition to FGF23, PMTs express osteogenic cell markers consistent with having differentiated from an inducible skeletal stem cell (Berglund et al., 2017). PMTs are typically benign, although malignant presentation and metastases have been reported (Chong et al., 2011a).
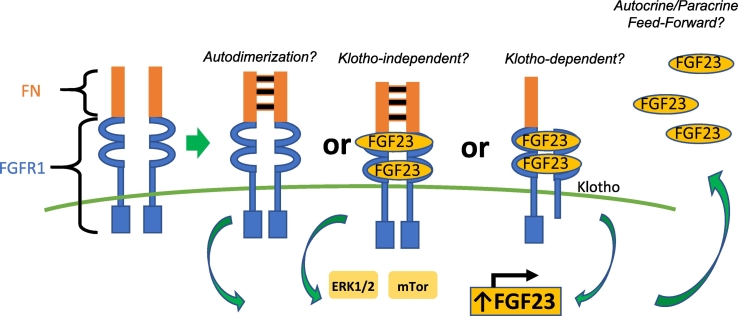
The best-established receptor required for FGF23 signaling is fibroblast growth factor receptor-1 (FGFR1). Binding by the ligand and its co-receptor Klotho activates the mitogen-activated protein kinase (MAPK) pathway, likely regulating cell proliferation, survival, and FGF23 secretion (Urakawa et al., 2006, Fukumoto, 2016). Genetic alterations at FGFR1 loci are associated with various neoplastic disorders (Tanner and Grose, 2016). A very relevant finding in understanding PMTs tumorogenesis was the identification of fibronectin (FN1) and FGFR1 translocations that led to a FN1-FGFR1 fusion protein in 60% of studied PMTs by RNA sequencing or FGFR-specific fluorescence in situ hybridization (FISH) (Lee et al., 2015). Additional studies have confirmed this finding although in a smaller proportion of studied tumors (Berglund et al., 2017, Lee et al., 2016). The FN1-FGFR1 translocation is predicted to produce a chimeric protein that includes the FN1 extracellular domain, and the FGFR1 ligand-binding, transmembrane and intracellular signaling domains. The extracellular domain of the putative chimeric protein includes the FN1 dimerization domain, which, via autodimerization, may facilitate the activation of the FGFR1 kinase domain (Fig. 6) (Lee et al., 2015). Since FN1 is a ubiquitously-expressed extracellular protein and expression is driven by a strong promoter, the FN1-FGFR1 fusion gene would presumably be highly expressed (Minisola et al., 2017, Lee et al., 2015). That the fusion protein is predicted to preserve its ligand-binding domain suggests an autocrine or paracrine role of FGF23 in tumorigenesis (Lee et al., 2015). Whether or not PMTs also express Klotho, in which case FGF23 could bind and signal in a Klotho-dependent manner, or if FGF23 is able to bind to the chimeric FN1-FGFR1 receptor and signal in a Klotho-independent manner, is not known.
Fig. 6.
Fibronectin-fibroblast growth factor receptor 1 translocations in TIO.
Depicted is the putative chimeric protein generated by the fibronectin-fibroblast growth factor 1 (FN1-FGFR1) translocations that were identified in a subset of the phosphaturic mesenchymal tumors that cause tumor-induced osteomalacia (Berglund et al., 2017, Lee et al., 2015, Lee et al., 2016). The chimeric protein includes the fibronectin extracellular autodimerization domain and the FGFR1 ligand binding, transmembrane and intracellular tyrosine kinase signaling domain. As such, the receptor has the potential to autodimerize, resulting in ligand independent signaling. It can also dimerize after FGF23 binding, thus signaling in a ligand-dependent fashion. Transduction continues through the extracellular signal–regulated kinase pathway (ERK1/2) and/or the mechanistic target of rapamycin (mTOR) pathway. If Klotho is also expressed by PMTs, it is possible that FGF23 could bind and signal in a Klotho-dependent manner. This signaling could lead to increased transcription, translation, and secretion of FGF23 by tumor cells via a feed-forward system. (Figure modified by Jason Berglund) (Minisola et al., 2017).
In addition to the description of the FN1-FGFR1 translocation, it has been recently reported that 6% of PMTs present a FN1-fibroblast growth factor 1 (FGF1) translocation. FGF1 protein is a crucial ligand for all FGFRs. It acts as a potent mitogen of fibroblasts and is involved in critical biological functions including development, morphogenesis, and angiogenesis (Lee et al., 2016). FGFR1 expression, as assessed by immunohistochemistry, has been demonstrated in 82% of PMTs, regardless of fusion status (Lee et al., 2016). As stated previously, preliminary data suggests the FGFR1 signaling may be implicated in tumor formation and/or FGF23 secretion (Minisola et al., 2017, Fukumoto, 2016, Martin et al., 2011). The novel FN1–FGF1 protein is expected to be secreted and it might serve as a ligand that activates FGFR1 to achieve an autocrine loop (Lee et al., 2016).
These findings have helped us to understand the oncogenic pathways of PMTs and may also have relevant therapeutic implications (Lee et al., 2015). Several FGFR inhibitors are currently in trials for FGFR-related cancers (Minisola et al., 2017, Parker et al., 2014, Cheng et al., 2017). These drugs been have shown in preclinical models to regulate phosphate homeostasis (Minisola et al., 2017, Wohrle et al., 2013) and may prove to be beneficial in treating unresectable TIO. In fact, the pan FGFR inhibitor, BGJ398, has been effectively used in a patient with metastatic TIO (Collins et al., 2015a).
5. Diagnosis
TIO should be suspected in patients presenting with suggestive symptoms, most commonly bone pain, muscle weakness, and multiple fractures (Chong et al., 2011a, Jan de Beur, 2005) and with persistent acquired hypophosphatemia, isolated PTH-independent renal phosphate wasting, and low or inappropriately normal 1,25(OH)2D (Chong et al., 2011a, Gonzalez et al., 2017). Symptoms are commonly non-specific and often progressive. Pediatric patients can develop rickets and growth retardation (Chong et al., 2011a, Minisola et al., 2017).
Upon the diagnosis of hypophosphatemia, the next step is to confirm renal tubular phosphate wasting by calculation of tubular reabsorption of phosphate (TRP) and/or tubular maximum reabsorption of phosphate to glomerular filtration rate (TmP/GFR) (Chong et al., 2011a). TmP/GFR provides the most accurate assessment of renal phosphate handling, and open access programs that calculate TmP/GFR can be found online. It is higher in children and decreases with age until the adult value is achieved by the age of about 20 years (Minisola et al., 2017, K.U. et al., 1982, Alon and Hellerstein, 1994). TmP/GFR can be cumbersome to perform properly. It requires urine collected over 2 h in an individual fasted overnight with blood sampled at the mid-point of the urine collection. For this reason, TRP is sometimes a more convenient first estimate (Minisola et al., 2017). TRP is calculated from random, simultaneous collections of blood and urine phosphate and creatinine using the following formula:
When the blood phosphate is normal, TRP should be 85–95%; TRP and TmP/GFR are decreased in patients with TIO (Chong et al., 2011a). When calculating TRP or TmP/GFR it's important to use consistent units and to make sure that patients are off phosphate supplements, to avoid falsely low determinations (Chong et al., 2011a).
After confirming renal phosphate wasting, it is recommended to measure PTH, 1,25(OH)2D, calcium, total or bone specific alkaline phosphatase and FGF23 (Minisola et al., 2017). Usually, levels of calcium and PTH are in the normal range, 1,25(OH)2D is low or inappropriately normal and alkaline phosphatase is elevated (Carpenter, 2000). On occasion, secondary hyperparathyroidism is seen even before starting medical therapy. It represents a normal physiological response to low 1,25(OH)2D (Jan de Beur, 2005, Liu and Quarles, 2007). Prolonged secondary hyperparathyroidism in TIO can lead to tertiary hyperparathyroidism (Chong et al., 2011a, Minisola et al., 2017).There are several FGF23 assays available; some measure the intact molecule (intact FGF23) and others measure both the intact hormone plus the carboxy-terminal fragments of the molecule (C-terminal FGF23). Only the C-terminal assays are widely available for commercial use. In most cases the value of “C-terminal FGF23” reflects the values of intact FGF23, as probably all the FGF23 produced by PMTs is intact (Minisola et al., 2017). In the great majority of cases, FGF23 levels are elevated. More than the absolute FGF23 value, it is important to interpret the level in the context of a patient with hypophosphatemia, given that a value in the medium-to-high range of normal is inappropriate in this condition (Chong et al., 2011a, Gonzalez et al., 2017).
Differential diagnosis of hypophosphatemia should include genetic causes of hypophosphatemic rickets. To rule out these diagnoses, it is important to obtain a detailed personal and family history to identify the age of onset of symptoms, the presence of dental abnormalities like enamel hypoplasia or dental abscesses, history of short stature or skeletal deformities, history of nephrocalcinosis or nephrolithiasis, and the presence of other affected family members. In general, the younger the patient is at presentation, the more likely the cause is genetic (Chong et al., 2011a). There are also acquired causes in the differential diagnosis of hypophosphatemia. Most of these are the result of renal tubular damage due to monoclonal gammopathies, exposure to heavy metals, aminoglycoside antibiotics, certain chemotherapeutic agents, or anti-retroviral drugs like tenofovir (Chong et al., 2011a). Unlike TIO, Fanconi-type syndromes are associated with global proximal renal tubular defects with metabolic acidosis and evidence of renal wasting of sodium, bicarbonate, potassium, and amino acids.
6. Localizing the tumor
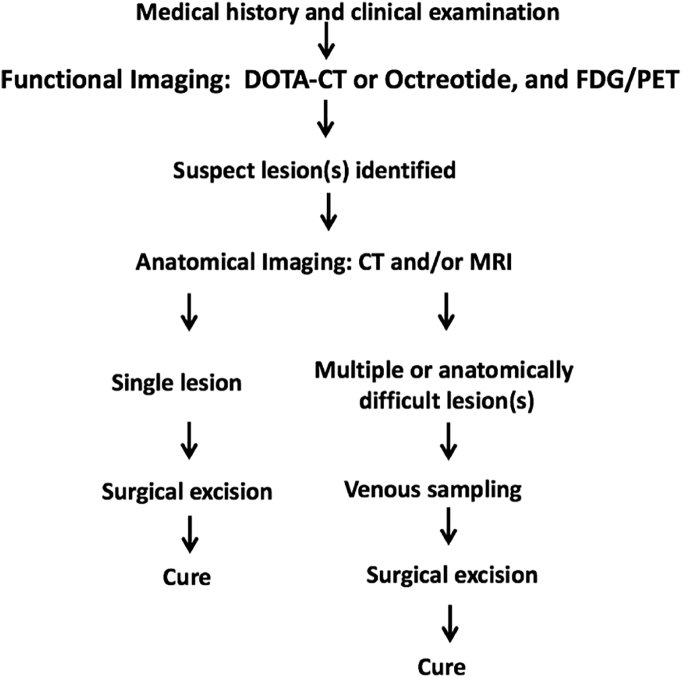
Once the diagnosis of TIO is confirmed, localizing the tumor is critical since its resection leads to complete resolution of the disease. For this purpose, a step-wise approach is recommended (Chong et al., 2011a, Minisola et al., 2017) (Fig. 7).
Fig. 7.
Approach to localization of phosphaturic mesenchymal tumors (Minisola et al., 2017).
The first step is a thorough medical history and physical examination looking for any visible or palpable lesions, including a complete skin and oral cavity evaluation (Chong et al., 2011a).
The second step is functional imaging. Techniques that use somatostatin analogues are the most sensitive, as PMTs express somatostatin receptors (Haeusler et al., 2010, Mussig et al., 2009, Houang et al., 2013). An Octreoscan™ uses pentetreotide conjugated with 111Indium, that can be combined with single photon emission and CT (SPECT/CT) to obtain 3-dimensional views (Chong et al., 2011a). Recently, modified octreotide molecules ((Tyr3)-octreotate) have been combined with chelators as DOTATATE or DOTANOC and then conjugated with positron emitting isotopes like 68Ga. This compound is utilized to generate PET scans such as 68Ga-DOTATATE and combined with CT for anatomical correlation (68Ga-DOTATATE PET/CT) (Chong et al., 2011a, Minisola et al., 2017). Another functional imaging modality utilized is F-18 fluorodeoxyglucose positron emission tomography with CT (FDG-PET/CT), which is based on the increased metabolic activity of PMTs (Roarke and Nguyen, 2007, Hesse et al., 2007a). Studies comparing these functional imaging modalities have shown that Octreoscan™ presents a higher sensitivity, specificity, positive and negative predictive value than FDG-PET/CT (Chong et al., 2013) When comparing 68Ga-DOTATATE PET/CT to Octreoscan-SPECT/CT and 18F FDG-PET in TIO localization, 68Ga-DOTATATE PET/CT demonstrates the greatest sensitivity and specificity, suggesting that it may be the best single study for localization of PMTs (El-Maouche et al., 2016). At times, tumors can be identified on FDG-PET/CT imaging but not seen on somatostatin analog imaging, as in the case presented above (Chong et al., 2013). Therefore somatostatin analog imaging and FDG-based studies can be complementary (Minisola et al., 2017). Regardless of the method, all functional imaging studies must include the entire body from head to toes including the arms and hands (Chong et al., 2011b).
The third step is to better define, with anatomical imaging, the location and quality of the lesion(s) identified on functional imaging. If a single lesion is identified, contrast enhanced CT or MR of the region is generally sufficient to confirm the tumor and to assist in planning the subsequent surgery (Minisola et al., 2017). When multiple suspicious lesions are identified, or the location of a single lesion suggests a surgery with potential significant morbidity, it is necessary to proceed with other modalities that confer greater diagnostic certainty before surgery (Chong et al., 2011a, Minisola et al., 2017). For this purpose, selective venous sampling with FGF23 measurement has been shown to be a sensitive and specific technique for confirming a suspicious lesion (Andreopoulou et al., 2011). When an FGF23 concentration ratio (between the venous drainage of the tumor bed and the general circulation) > 1.6 is considered as diagnostic cut-point, this technique has a sensitivity of 87% and a specificity of 71% (Fig. 8) (Andreopoulou et al., 2011). Sampling without a pre-test suspicious lesion, or “blind” sampling, has not shown to be a useful localizing technique for PMTs (Andreopoulou et al., 2011). Exceptionally, as in our patient's case, biopsy with FGF23 determination of a lesion is needed to confirm a tumor as the source of FGF23. In general, biopsy is not recommended due to the potential for tumor cell seeding (Minisola et al., 2017).
Fig. 8.
Example of a positive result on selective venous sampling.
A suspicious lesion had previously been identified in the fat pad of the left heel (Andreopoulou et al., 2011).
Even after a complete and thorough study, the culprit tumor might not be localized. In these cases, periodic follow-up with medical history, physical examination and imaging is recommended (Chong et al., 2011a).
7. Treatment
The first treatment option is complete resection of the tumor with wide margins. Surgery is considered the only definitive treatment (Chong et al., 2011a, Minisola et al., 2017). It is very important to aim for complete resection of the tumor, as recurrences have been reported (Chong et al., 2011a), including cases with malignant characteristics (Uramoto et al., 2009). While these tumors are often small and not locally aggressive, per se, they are not encapsulated and tend to be locally infiltrative, especially along the trabeculae of bone. Therefore, a wider excision than initially seems necessary is suggested. Post-operative radiotherapy for margin positive tumors has been reported, but data are still limited (Minisola et al., 2017, Tarasova et al., 2013). After resection, there is a rapid clinical recovery, with serum phosphorus and intact FGF23 returning to normal within the first 5 days in most patients (Chong et al., 2013). During the recovery phase, while the skeleton is actively remineralizing, patients may transiently require supplemental calcium to prevent hypocalcemia and secondary hyperparathyroidism in the face of a now robust 1,25(OH)2D level.
On occasion, complete resection is not possible. Tumors may be located in difficult to access areas, or where surgery would likely introduce significant morbidity. This is especially true in patients with poor performance status due to comorbid conditions. Image-guided ablation with radiofrequency or cryoablation is a promising alternative for this group of patients, with little morbidity and short hospital stays. Although surgery remains the treatment of choice, image-guided ablation may be an effective, less invasive, and safe treatment for patients with inoperable TIO (Hesse et al., 2007b, Tutton et al., 2012, Tella et al., 2017). However, long term efficacy of these modalities has not been demonstrated.
In cases where it is not possible to detect and/or completely resect the tumor, medical treatment is indicated. Phosphorus and active vitamin D (calcitriol or alfacalcidol) supplementation is the mainstay of treatment, with the goal of improving symptoms and healing osteomalacia, while maintaining phosphatemia in the lower end of the normal range, and PTH and alkaline phosphatase in the normal range. The treatment regimen is 15–60 mg/kg per day of elemental phosphorus (typically 1–3 g/day) divided into 4–6 doses and calcitriol 15–60 ng/kg per day divided in two doses, with a typical starting dose of 1.5 μg/day in an adult (Chong et al., 2011a). As previously mentioned, secondary hyperparathyroidism can be seen on presentation or it can develop as a result of phosphorus supplementation. Prolonged supplementation can lead to the development of tertiary hyperparathyroidism. Active vitamin D is used to prevent or treat secondary hyperparathyroidism. The goal is to keep the PTH in the normal range. Addition of calcium supplements or increases in active vitamin D are indicated for difficult to normalize PTH, very low urinary calcium or hypocalcemia. This can especially occur at the outset of treatment of osteomalacia as the skeleton begins to mineralize at a high rate. One consequence of over-treatment with active vitamin D is the development of hypercalciuria and the risk for nephrocalcinosis/nephrolithiasis (Chong et al., 2011a). For these reasons, treatment monitoring with serum phosphorus, calcium, alkaline phosphatase, PTH, and 24-hour urine calcium and/or urinary calcium/creatinine ratio should be routinely checked and treatment titrated to fulfill goals avoiding complications (Chong et al., 2011a).
Lowering PTH with the calcimimetic cinacalcet has been reported to decrease phosphaturia, reduce the need for phosphate and calcitriol supplements, and bring about bone healing. The main side effect is the development of hypercalciuria. It appears as a promising treatment that still requires further studies for confirmation (Geller et al., 2007).
8. Future directions
The recent identification of FN1/FGFR1 translocations as a relevant pathophysiological mechanism in TIO has expanded our understanding of the oncogenic pathways of PMTs and will probably increase our understanding of FGF23-mediated mineral homeostasis (Minisola et al., 2017). It also has relevant therapeutic implications. This notion is supported by preliminary in vitro evidence showing that addition of the selective, pan-FGFR inhibitor, BGJ398 with and without the synergistic mTOR-inhibitor rapamycin, decreased FGF23 production by 80% in a FN1/FGFR1 translocation positive tumor, but had a negligible effect on a tumor lacking the translocation (Berglund et al., 2017). Also, BGJ398 was both tumoricidal and significantly inhibited FGF23 secretion in a patient with metastatic TIO (Collins et al., 2015b). However, further studies evaluating FGFR inhibitors are needed. Much remains to be understood on how this translocation drives tumor formation and FGF23 secretion (Minisola et al., 2017), as well as on tumoral pathways involved in PMTs that are translocation negative.
Another promising line of treatment for unresectable TIO is the FGF23 monoclonal antibody drug, KRN23 (Carpenter et al., 2016). It has been shown to decrease the activity of FGF23 interfering with formation of the FGF23-Klotho-receptor complex (Aono et al., 2011). KRN23 appears to significantly and safely increase TmP/GFR, serum phosphate and 1,25(OH)2D in patients with X-linked hypophosphatemic rickets (Carpenter et al., 2014). Preliminary evidence shows that KRN23 treatment increases serum phosphate and 1,25(OH)2D in subjects with TIO (Carpenter et al., 2016). These data suggest that KRN23 will potentially be an effective therapy for patients with TIO who cannot be cured easily with surgery (Minisola et al., 2017, Fukumoto, 2016).
In summary, TIO is a rare paraneoplastic syndrome caused by unregulated over-secretion of FGF23. Causative lesions are benign mesenchymal tumors that may be found anywhere in the body. While the tumors can be difficult to locate, a stepwise approach that involves functional imaging, followed by anatomical imaging, and, if necessary, selective venous sampling or aspiration for confirmation is usually successful. Excision with wide margins is important to avoid late recurrence. When tumors cannot be identified, medical treatment can be successful though periodic surveillance is necessary. Recent pathophysiological findings and novel therapeutical approaches open promising perspectives for the treatment of patients with TIO (Chong et al., 2011a, Minisola et al., 2017).
Acknowledgments
Acknowledgement
Work in the authors' laboratory is supported by the Intramural Research Program of the NIH, NIDCR.
References
- Alon U., Hellerstein S. Assessment and interpretation of the tubular threshold for phosphate in infants and children. Pediatr. Nephrol. 1994;8(2):250–251. doi: 10.1007/BF00865491. [DOI] [PubMed] [Google Scholar]
- Andreopoulou P., Dumitrescu C.E., Kelly M.H., Brillante B.A., Cutler Peck C.M., Wodajo F.M., Chang R., Collins M.T. Selective venous catheterization for the localization of phosphaturic mesenchymal tumors. J. Bone Miner. Res. 2011;26(6):1295–1302. doi: 10.1002/jbmr.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono Y., Hasegawa H., Yamazaki Y., Shimada T., Fujita T., Yamashita T., Fukumoto S. Anti-FGF-23 neutralizing antibodies ameliorate muscle weakness and decreased spontaneous movement of Hyp mice. J. Bone Miner. Res. 2011;26(4):803–810. doi: 10.1002/jbmr.275. [DOI] [PubMed] [Google Scholar]
- Berglund R.G.J., Forsberg J., Molinolo A., Fernandez de Castro L., Ten Hagen K., Tian E., Metwally T., Ovejero Crespo D., Chong W.H., Collins M.T. Insight into the molecular and cellular etiology of the tumors responsible for tumor-induced osteomalacia. Ann. Meet. Endocr. Soc. 2017:2017. (OR07–7) [Google Scholar]
- Bhattacharyya N., Chong W.H., Gafni R.I., Collins M.T. Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol. Metab. 2012;23(12):610–618. doi: 10.1016/j.tem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter T.O. In: Primary Disorders of Phosphate Metabolism. De Groot L.J., Chrousos G., Dungan K., Feingold K.R., Grossman A., Hershman J.M., Koch C., Korbonits M., McLachlan R., New M., Purnell J., Rebar R., Singer F., Vinik A., editors. Endotext; South Dartmouth (MA): 2000. [Google Scholar]
- Carpenter T.O., Imel E.A., Ruppe M.D., Weber T.J., Klausner M.A., Wooddell M.M., Kawakami T., Ito T., Zhang X., Humphrey J., Insogna K.L., Peacock M. Randomized trial of the anti-FGF23 antibody KRN23 in X-linked hypophosphatemia. J. Clin. Invest. 2014;124(4):1587–1597. doi: 10.1172/JCI72829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter T.O., Miller P., Weber T., Peacock M., Ruppe M., Insogna K., Osei S., Luca D., Skrinar A., San Martin J., Jan De Beur S. Annual Meeting of the American Society for Bone and Mineral Research 1098. 2016. Effects of KRN23, and anti-FGF23 antibody, in patients with tumor induced osteomalacia and epidermal nevus syndrome: results from an ongoing phase 2 study. [Google Scholar]
- Cheng W., Wang M., Tian X., Zhang X. An overview of the binding models of FGFR tyrosine kinases in complex with small molecule inhibitors. Eur. J. Med. Chem. 2017;126:476–490. doi: 10.1016/j.ejmech.2016.11.052. [DOI] [PubMed] [Google Scholar]
- Chong W.H., Molinolo A.A., Chen C.C., Collins M.T. Tumor-induced osteomalacia. Endocr. Relat. Cancer. 2011;18(3):R53–77. doi: 10.1530/ERC-11-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong W.H., Yavuz S., Patel S.M., Chen C.C., Collins M.T. The importance of whole body imaging in tumor-induced osteomalacia. J. Clin. Endocrinol. Metab. 2011;96(12):3599–3600. doi: 10.1210/jc.2011-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong W.H., Andreopoulou P., Chen C.C., Reynolds J., Guthrie L., Kelly M., Gafni R.I., Bhattacharyya N., Boyce A.M., El-Maouche D., Crespo D.O., Sherry R., Chang R., Wodajo F.M., Kletter G.B., Dwyer A., Collins M.T. Tumor localization and biochemical response to cure in tumor-induced osteomalacia. J. Bone Miner. Res. 2013;28(6):1386–1398. doi: 10.1002/jbmr.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M.T., Bergwitz C., Aitcheson G., Blau J., Boyce A.M., Gafni R.I., Guthrie L.C., Miranda F., Slosberg E., Graus Porta D., Hopmann C., Welaya K., Isaacs R.E., Miller C. 2015. Striking Response of Tumor-induced Osteomalacia to the FGFR Inhibitor NVP-BGJ398 American Society of Bone and Mineral Research Annual Meeting Seattle, WA. (pp. LB-SA0035 and SA0035) [Google Scholar]
- Collins M.T., Bergwitz C., Aitchenson G., Blau J., Boyce A., Gafni R., Guthrie L., Miranda F., Slosberg E., Graus Porta D., Hopmann C., Welaya K., Isaacs R., Miller C. Annual Meeting of the American Society for Bone and Mineral Research SA0035. 2015. Striking response of tumor-induced osteomalacia to the FGFR inhibitor NVP-BGJ398. [Google Scholar]
- El-Maouche D., Sadowski S.M., Papadakis G.Z., Guthrie L., Cottle-Delisle C., Merkel R., Millo C., Chen C.C., Kebebew E., Collins M.T. 68Ga-DOTATATE for tumor localization in tumor-induced osteomalacia. J. Clin. Endocrinol. Metab. 2016;101(10):3575–3581. doi: 10.1210/jc.2016-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D.J., Azzopardi J.G. Distinctive tumours of bone and soft tissue causing acquired vitamin-D-resistant osteomalacia. Lancet. 1972;1(7746):353–354. doi: 10.1016/s0140-6736(72)92844-9. [DOI] [PubMed] [Google Scholar]
- Folpe A.L., Fanburg-Smith J.C., Billings S.D., Bisceglia M., Bertoni F., Cho J.Y., Econs M.J., Inwards C.Y., Jan de Beur S.M., Mentzel T., Montgomery E., Michal M., Miettinen M., Mills S.E., Reith J.D., O'Connell J.X., Rosenberg A.E., Rubin B.P., Sweet D.E., Vinh T.N., Wold L.E., Wehrli B.M., White K.E., Zaino R.J., Weiss S.W. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am. J. Surg. Pathol. 2004;28(1):1–30. doi: 10.1097/00000478-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Fukumoto S. Physiological regulation and disorders of phosphate metabolism—pivotal role of fibroblast growth factor 23. Intern. Med. 2008;47(5):337–343. doi: 10.2169/internalmedicine.47.0730. [DOI] [PubMed] [Google Scholar]
- Fukumoto S. FGF23-FGF receptor/Klotho pathway as a new drug target for disorders of bone and mineral metabolism. Calcif. Tissue Int. 2016;98(4):334–340. doi: 10.1007/s00223-015-0029-y. [DOI] [PubMed] [Google Scholar]
- Geller J.L., Khosravi A., Kelly M.H., Riminucci M., Adams J.S., Collins M.T. Cinacalcet in the management of tumor-induced osteomalacia. J. Bone Miner. Res. 2007;22(6):931–937. doi: 10.1359/jbmr.070304. [DOI] [PubMed] [Google Scholar]
- Gonzalez G., Baudrand R., Sepulveda M.F., Vucetich N., Guarda F.J., Villanueva P., Contreras O., Villa A., Salech F., Toro L., Michea L., Florenzano P. Tumor-induced osteomalacia: experience from a South American academic center. Osteoporos. Int. 2017 doi: 10.1007/s00198-017-4007-2. [DOI] [PubMed] [Google Scholar]
- Haeusler G., Freilinger M., Dominkus M., Egerbacher M., Amann G., Kolb A., Schlegel W., Raimann A., Staudenherz A. Tumor-induced hypophosphatemic rickets in an adolescent boy—clinical presentation, diagnosis, and histological findings in growth plate and muscle tissue. J. Clin. Endocrinol. Metab. 2010;95(10):4511–4517. doi: 10.1210/jc.2010-0543. [DOI] [PubMed] [Google Scholar]
- Hesse E., Moessinger E., Rosenthal H., Laenger F., Brabant G., Petrich T., Gratz K.F., Bastian L. Oncogenic osteomalacia: exact tumor localization by co-registration of positron emission and computed tomography. J. Bone Miner. Res. 2007;22(1):158–162. doi: 10.1359/jbmr.060909. [DOI] [PubMed] [Google Scholar]
- Hesse E., Rosenthal H., Bastian L. Radiofrequency ablation of a tumor causing oncogenic osteomalacia. N. Engl. J. Med. 2007;357(4):422–424. doi: 10.1056/NEJMc070347. [DOI] [PubMed] [Google Scholar]
- Houang M., Clarkson A., Sioson L., Elston M.S., Clifton-Bligh R.J., Dray M., Ranchere-Vince D., Decouvelaere A.V., de la Fouchardiere A., Gill A.J. Phosphaturic mesenchymal tumors show positive staining for somatostatin receptor 2A (SSTR2A) Hum. Pathol. 2013;44(12):2711–2718. doi: 10.1016/j.humpath.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Jan de Beur S.M. Tumor-induced osteomalacia. JAMA. 2005;294(10):1260–1267. doi: 10.1001/jama.294.10.1260. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Xia W.B., Xing X.P., Silva B.C., Li M., Wang O., Zhang H.B., Li F., Jing H.L., Zhong D.R., Jin J., Gao P., Zhou L., Qi F., Yu W., Bilezikian J.P., Meng X.W. Tumor-induced osteomalacia: an important cause of adult-onset hypophosphatemic osteomalacia in China: report of 39 cases and review of the literature. J. Bone Miner. Res. 2012;27(9):1967–1975. doi: 10.1002/jbmr.1642. [DOI] [PubMed] [Google Scholar]
- K.U., Kruse K., Gopfert G. Renal threshold phosphate concentration (TmPO4/GFR) Arch. Dis. Child. 1982;57:217–223. doi: 10.1136/adc.57.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.C., Jeng Y.M., Su S.Y., Wu C.T., Tsai K.S., Lee C.H., Lin C.Y., Carter J.M., Huang J.W., Chen S.H., Shih S.R., Marino-Enriquez A., Chen C.C., Folpe A.L., Chang Y.L., Liang C.W. Identification of a novel FN1-FGFR1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J. Pathol. 2015;235(4):539–545. doi: 10.1002/path.4465. [DOI] [PubMed] [Google Scholar]
- Lee J.C., Su S.Y., Changou C.A., Yang R.S., Tsai K.S., Collins M.T., Orwoll E.S., Lin C.Y., Chen S.H., Shih S.R., Lee C.H., Oda Y., Billings S.D., Li C.F., Nielsen G.P., Konishi E., Petersson F., Carpenter T.O., Sittampalam K., Huang H.Y., Folpe A.L. Characterization of FN1-FGFR1 and novel FN1-FGF1 fusion genes in a large series of phosphaturic mesenchymal tumors. Mod. Pathol. 2016;29(11):1335–1346. doi: 10.1038/modpathol.2016.137. [DOI] [PubMed] [Google Scholar]
- Lewiecki E.M., Urig E.J., Jr., Williams R.C., Jr. Tumor-induced osteomalacia: lessons learned. Arthritis Rheum. 2008;58(3):773–777. doi: 10.1002/art.23278. [DOI] [PubMed] [Google Scholar]
- Liu S., Quarles L.D. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18(6):1637–1647. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- Martin A., Liu S., David V., Li H., Karydis A., Feng J.Q., Quarles L.D. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25(8):2551–2562. doi: 10.1096/fj.10-177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussig K., Oksuz M.O., Pfannenberg C., Adam P., Zustin J., Beckert S., Petersenn S. Somatostatin receptor expression in an epitheloid hemangioma causing oncogenic osteomalacia. J. Clin. Endocrinol. Metab. 2009;94(11):4123–4124. doi: 10.1210/jc.2009-0927. [DOI] [PubMed] [Google Scholar]
- Minisola S., Peacock M., Fukumoto S., Cipriani C., Pepe J., Tella H.T., Collins M.T. Tumor-induced osteomalacia. Nat Rev Dis Primers. 2017;13(3):17044. doi: 10.1038/nrdp.2017.44. [DOI] [PubMed] [Google Scholar]
- Parker B.C., Engels M., Annala M., Zhang W. Emergence of FGFR family gene fusions as therapeutic targets in a wide spectrum of solid tumours. J. Pathol. 2014;232(1):4–15. doi: 10.1002/path.4297. [DOI] [PubMed] [Google Scholar]
- Razzaque M.S. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009;5(11):611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roarke M.C., Nguyen B.D. PET/CT localization of phosphaturic mesenchymal neoplasm causing tumor-induced osteomalacia. Clin. Nucl. Med. 2007;32(4):300–301. doi: 10.1097/01.rlu.0000257180.03964.51. [DOI] [PubMed] [Google Scholar]
- Sciubba D.M., Petteys R.J., Shakur S.F., Gokaslan Z.L., McCarthy E.F., Collins M.T., McGirt M.J., Hsieh P.C., Nelson C.S., Wolinsky J.P. En bloc spondylectomy for treatment of tumor-induced osteomalacia. J. Neurosurg. Spine. 2009;11(5):600–604. doi: 10.3171/2009.6.SPINE08120. [DOI] [PubMed] [Google Scholar]
- Tanner Y., Grose R.P. Dysregulated FGF signalling in neoplastic disorders. Semin. Cell Dev. Biol. 2016;53:126–135. doi: 10.1016/j.semcdb.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Tarasova V.D., Trepp-Carrasco A.G., Thompson R., Recker R.R., Chong W.H., Collins M.T., Armas L.A. Successful treatment of tumor-induced osteomalacia due to an intracranial tumor by fractionated stereotactic radiotherapy. J. Clin. Endocrinol. Metab. 2013;98(11):4267–4272. doi: 10.1210/jc.2013-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tella B.W.S., Chang R., Levy E., Amalou H., Krishnasamy V., Gafni R., Collins M.T. Annual Meeting of the Endocrine Society OR07–1. 2017. Successful use of CT-guided cryoablation for inoperable tumor-induced osteomalacia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsagalis G., Psimenou E., Manios E., Laggouranis A. Fibroblast growth factor 23 (FGF23) and the kidney. Int. J. Artif. Organs. 2009;32(4):232–239. doi: 10.1177/039139880903200407. [DOI] [PubMed] [Google Scholar]
- Tutton S., Olson E., King D., Shaker J.L. Successful treatment of tumor-induced osteomalacia with CT-guided percutaneous ethanol and cryoablation. J. Clin. Endocrinol. Metab. 2012;97(10):3421–3425. doi: 10.1210/jc.2012-1719. [DOI] [PubMed] [Google Scholar]
- Urakawa I., Yamazaki Y., Shimada T., Iijima K., Hasegawa H., Okawa K., Fujita T., Fukumoto S., Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- Uramoto N., Furukawa M., Yoshizaki T. Malignant phosphaturic mesenchymal tumor, mixed connective tissue variant of the tongue. Auris Nasus Larynx. 2009;36(1):104–105. doi: 10.1016/j.anl.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Weidner N., Santa Cruz D. Phosphaturic mesenchymal tumors. A polymorphous group causing osteomalacia or rickets. Cancer. 1987;59(8):1442–1454. doi: 10.1002/1097-0142(19870415)59:8<1442::aid-cncr2820590810>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Wohrle S., Henninger C., Bonny O., Thuery A., Beluch N., Hynes N.E., Guagnano V., Sellers W.R., Hofmann F., Kneissel M., Graus Porta D. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J. Bone Miner. Res. 2013;28(4):899–911. doi: 10.1002/jbmr.1810. [DOI] [PubMed] [Google Scholar]