Abstract
Patient: Male, 54
Final Diagnosis: Guillain-Barré syndrome
Symptoms: Paresthesia of extremities • unilateral facial palsy
Medication: —
Clinical Procedure: —
Specialty: Neurology
Objective:
Unusual clinical course
Background:
A rare variant of Guillain-Barré syndrome (GBS) consists of facial diplegia and paresthesia, but an even more rare association is with facial hemiplegia, similar to Bell’s palsy. This case report is of this rare variant of GBS that was associated with IgG antibodies to galactocerebroside and phosphatidic acid.
Case Report:
A 54-year-old man presented with lower left facial palsy and paresthesia of his extremities, following an upper respiratory tract infection. Physical examination confirmed lower left facial palsy and paresthesia of his extremities with hyporeflexia of his lower limbs and sensory loss of all four extremities. The differential diagnosis was between a variant of GBS and Bell’s palsy. Following initial treatment with glucocorticoids followed by intravenous immunoglobulin (IVIG), his sensory abnormalities resolved. Serum IgG antibodies to galactocerebroside and phosphatidic acid were positive in this patient, but not other antibodies to glycolipids or phospholipids were found. Five months following discharge from hospital, his left facial palsy had improved.
Conclusions:
A case of a rare variant of GBS is presented with facial diplegia and paresthesia and with unilateral facial palsy. This rare variant of GBS may which may mimic Bell’s palsy. In this case, IgG antibodies to galactocerebroside and phosphatidic acid were detected.
MeSH Keywords: Bell Palsy, Facial Paralysis, Guillain- Barré Syndrome, Paresthesia, Phosphatidic Acids
Background
A rare variant of Guillain-Barré syndrome (GBS) consists of facial diplegia and paresthesia, which may cause diagnostic difficulty [1,2]. Patients with this rare variant of GBS usually present with limb numbness, followed by bilateral facial nerve palsy [1,2]. However, an even more rare variant of GBS also includes facial hemiplegia, which may mimic Bell’s palsy [1–4].
This report is of a case of a rare variant of GBS consisting of facial diplegia and paresthesia with left facial hemiplegia that was associated with IgG antibodies to galactocerebroside and phosphatidic acid.
Case Report
A 54-year-old man was admitted to our hospital with paresthesia of his extremities and a left facial palsy. Two days before hospital admission, he developed a left facial palsy. Two weeks before hospital admission, he had an upper respiratory tract infection. Six days before hospital admission, he developed paresthesia of the extremities. Four days before hospital admission, the patient had visited a hospital near to his office.
The patient had no history of medical diseases, operations, use of medications, or previous hospitalization. He was an ex-smoker until ten years before hospital admission, when he smoked up to twelve cigarettes a day. He regularly consumed 350 mL of beer per day.
On physical examination, he was alert, and his general appearance, cardiovascular and respiratory system were normal. His lower abdomen was non-distended and anal sphincter tone was normal. His temperature of 36.5°C, his blood pressure was 165/126 mmHg, his heart rate was 78 beats per minute, and his oxygen saturation was 99% breathing ambient room air. Neurological examination showed a left-sided facial palsy (Figure 1). The rest of the cranial nerve examination was normal. His peripheral neurological examination showed normal tone of his extremities, and manual muscle testing was 5/5 bilaterally throughout. However, patellar and Achilles deep tendon reflexes were reduced, but other reflexes were normal. Cutaneous nociception, thermosensation, and vibratory sensation in his extremities were also reduced (Figure 2). Other neurological examinations were normal.
Figure 1.
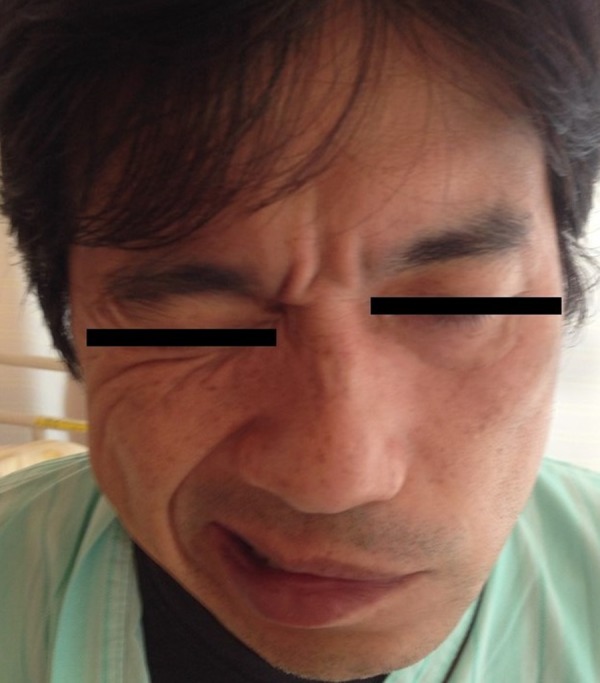
Facial nerve palsy in a patient with Guillain-Barré syndrome (GBS). A photographic image of the lower face of the patient on hospital admission shows the appearance of a complete lower motor neuron facial nerve palsy.
Figure 2.
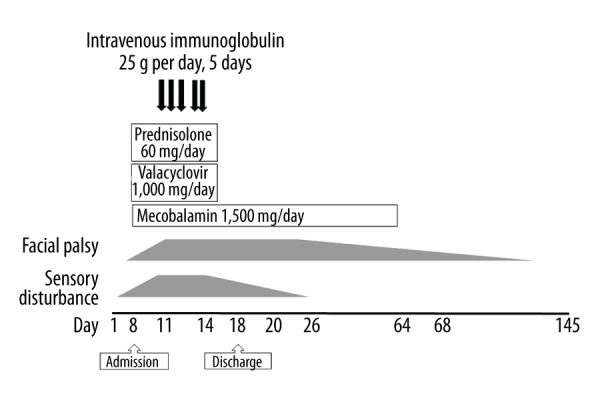
The clinical course of a patient with Guillain-Barré syndrome (GBS).
On admission, a non-contrast head computed tomography (CT) scan and nerve conduction velocity studies of his extremities were undertaken, both of which showed no abnormalities. Chest X-ray was normal. A non-contrast brain magnetic resonance imaging (MRI) scan for central neurological disease, showed no abnormality.
Laboratory investigations showed peripheral blood leukocytes of 7,700/μL, hemoglobin of 16.2 g/dL, platelets of 170,000/μL, total protein of 7.5 g/dL, albumin of 4.9 g/dL, creatinine of 1.1 mg/dL, C-reactive protein of 0.05 mg/dL, and hemoglobin A1c of 5.6%. A urinalysis revealed no protein, leukocytes or erythrocytes. A cerebrospinal fluid (CSF) examination showed a total leukocyte count of 16/μL, which was monocyte-predominant (11/μL monocytes; 5/μL polymorphonuclear cells), and protein of 71.7 mg/dL (normal range; 10–40 mg/dL).
On the second day after his admission, treatment with oral prednisolone 60 mg/day, valacyclovir 1,000 mg/day, and methylcobalamin (Vitamin B12) 1,500 μg/day were initiated due to a possible diagnosis of Bell’s palsy. However, his neurological symptoms persisted.
On the fourth day following hospital admission, repeated examination of the cerebrospinal fluid (CSF) showed a total leukocyte count of 9/μL, which was monocyte-predominant (9/μL monocytes; 0/μL polymorphonucleocytes) and protein of 82.7 mg/dL (normal range; 10–40 mg/dL). On the fifth day following hospital admission, a nerve conduction study revealed mildly prolonged distal motor latencies and mildly reduced distal motor amplitude with poor responses in both of his legs (Table 1). Bilateral sural nerves also showed markedly reduced sensory nerve action potentials (Table 1). These results were consistent with demyelination and excluded Bell’s palsy. Therefore, on the eighth day after admission, pooled intravenous immunoglobulin (IVIG) treatment was also commenced on suspicion of a diagnosis of a variant of Guillain-Barré syndrome (GBS) for his left facial palsy and peripheral nerve signs.
Table 1.
Nerve conduction study.
| Sensory nerves (left/right) | TL, ms | A, μV | NCV, m/sec |
|---|---|---|---|
| Median | 4.3/4.3 N (3.5) | 22.1/17.8 N (10) | 61.4/63.0 N (35–40) |
| Ulnar | 5.1/4.1 N (3.5) | 19.9/19.9 N (2) | 50.4/70.9 N (35–40) |
| Sural | PR/PR N (4.4) | PR/PR N (6) | PR/PR N (35–40) |
| Motor nerves (left/right) | TL, ms | A, mV | NCV, m/sec | F-wave, ms |
|---|---|---|---|---|
| Median | 4.6/4.6 N (4.4) | 10.0/17.9 N (4) | 58.6/56.5 N (49) | PR/PR N (31) |
| Ulnar | 4.4/4.7 N (3.3) | 10.0/9.5 N (5) | 61.0/61.1 N (53) | PR/PR N (32) |
| Tibial | 10.1/10.0 N (5.8) | 7.6/6.5 N (4) | 37.7/40.4 N (41) | PR/PR N (56) |
| Peroneal | 8.0/9.3 N (5.8) | 6.3/3.2 N (2) | 50.1/47.8 N (44) | PR/PR N (57) |
TL – teminal latency; A – amplitude; NCV – nerve conduction velocity; N() – normal value; PR – poor response.
Two weeks after admission, his abnormal sensations had improved. At the beginning of the admission, his blood pressure was elevated to 180/130 mmHg, and which decreased to approximately 140/90 mmHg after initiation of the IVIG treatment. He was discharged on the 18th hospital day. His left facial palsy gradually recovered and was almost back to a premorbid state by five months after the onset of his symptoms (Figure 3).
Figure 3.
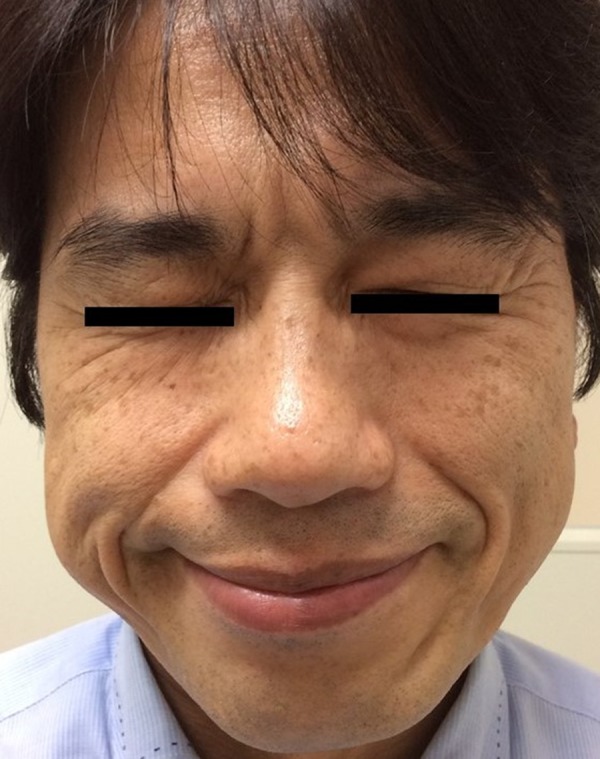
Resolution of facial nerve palsy in a patient with Guillain-Barré syndrome (GBS), five months on from presentation. A photographic image of the lower face of the patient five months on from presentation shows a normal appearance.
To identify the potential cause of his neurological illness, further tests were undertaken. Blood and CSF culture were negative. Stool culture was not performed. A cytological examination of the CSF showed few lymphocytes without malignant cells. Vitamin B12 level was normal (891 pg/mL; normal range, 249–938 pg/mL). Human immunodeficiency virus (HIV) 1 and 2 antibodies were negative and Mycoplasma IgG antibody was negative (particle agglutination test). Epstein-Barr virus (EBV) IgM antibody was negative, but EBV IgG antibody was 80 times (normal range, 0–10 times), EBV nuclear antigen was 40 times (normal range, 0–10 times) indicating previous infection. Cytomegalovirus (CMV) IgM was negative, CMV IgG was 34.4 arbitrary units (AU)/mL (normal range, 0–6 AU/mL) showing prior infection.
The patient’s blood sample was sent to an immunology reference laboratory for the analysis of anti-glycolipid antibodies. IgG to phospholipid acid, and IgM antibodies to glycolipids (GM1, GM2, GM3a, GD1a, GD1b, G3, GT1b, GQ1b, galactocerebroside, and GalNAc-GD1a) were analyzed. In addition, serum IgG to glycolipids Gd1a/Gd1b was assessed. IgG antibodies to galactocerebroside and phosphatidic acid were positive, but the rest of the anti-glycolipid antibodies were negative.
Discussion
This case report is of a rare variant of Guillain-Barré syndrome (GBS), consisting of facial diplegia and paresthesia with left facial hemiplegia that was associated with IgG antibodies to galactocerebroside and phosphatidic acid.
On initial presentation, the patient’s symptoms were diagnosed either as a variant of GBS or as left unilateral Bell’s palsy. This patient had a respiratory tract infection that preceded his neurological symptoms. The symptoms of paresthesia in his extremities were associated with decreased deep tendon reflexes, and sensory disturbance on physical examination. Increased protein levels and a decreased cell count in the CSF were consistent with a diagnosis of a variant of GBS, with facial palsy and sensory disturbance.
GBS is known to be a post-infectious polyneuropathy characterized by acute lower limb weakness [2], but in between 24–60% of cases, patients may develop facial nerve palsy [3]. In 1994 Ropper, for the first time, described a new variant of GBS with features of facial diplegia, limb paresthesia, decreased deep tendon reflexes, an elevation in CSF protein (>0.55 g/L) but without an increase in white blood cells in more than 75% of cases, and demyelination on nerve conduction studies, as facial diplegia and paresthesia [2–5]. Most patients with facial diplegia and paresthesia initially present with limb numbness, followed by bilateral facial nerve palsy [1]. Almost all patients have antecedent infection within four weeks prior to the onset of neurological symptoms and many patients have demyelination findings in their limbs on nerve conduction investigations [1,2].
Anti-GM2 ganglioside antibodies are commonly, but not always, detected in GBS [1]. The variant of GBS with facial diplegia and paresthesia sometimes occurs unilaterally and then subsequently becomes bilateral [1]. Thus, it can be difficult to diagnose variant GBS with facial diplegia and paresthesia and to distinguish it from Bell’s palsy. Facial palsy in this variant of GBS is usually bilateral, but it may be asymmetrical and is uncommonly unilateral [6]. In our patient, the clinical presentation of GBS was a rare cause of facial hemiplegia and it is possible that the patient’s history of hypertension may have been caused by this variant of GBS, as hypertension is a recognized comorbid condition in GBS and in this patient, hypertension reduced after treatment [2]. However, hypertension has been rarely reported in patients with the GBS with facial diplegia and paresthesia [6].
GBS is associated with a history of infection, including Campylobacter jejuni, influenza, Mycoplasma pneumoniae, Epstein-Barr virus (EBV), and other pathogens [1–4]. In this patient, a positive serum anti-galactocerebroside antibody suggested a preceding history of Mycoplasma infection [7–11]. Among patients with variant GBS with facial diplegia and paresthesia, an upper respiratory infection is a common preceding infection and anti-CMV antibodies are frequently positive [1,12]. In our case, the patient had a preceding viral upper respiratory tract infection, which was supported by the negative culture studies for bacterial pathogens.
In patients with variant GBS with facial diplegia and paresthesia, there have been a few cases reported with positive anti-glycolipid antibodies [1,13]. However, in this case, the potential effects of antibodies to galactocerebroside and phosphatidic acid may be useful as a diagnostic marker for variant GBS, but this requires further study. In particular, antibodies to GM1, GQ1b, GM1b, GD1a, GalNAc-GD1a, LM1, and galactocerebroside are specific for GBS [5]. Also, antibodies of GD1a/GD1b, GD1b/GT1b, and GM1/GalNAc-GD1a are also associated with GBS [14,15]. Among the proteins eliciting an antibody response in GBS, galactocerebroside is a major glycolipid antigen that is present in myelin, and antibodies to galactocerebroside may have a role in the pathogenesis of autoimmune demyelinating neuropathies including GBS [11,14,15]. Anti-glycolipid antibodies in variant GBS with facial diplegia and paresthesia such as, anti-GM2 antibody have been reported to be positive in a few patients [1], but most patients with the disease have no specific anti-glycolipid antibodies detected. In previous studies, the detection of antibodies to phosphatidic acid in combination with anti-glycolipid antibodies was more useful for the diagnosis of GBS [16,17]. To the best of our knowledge, there has been no report of serum immunological investigations of antibodies to galactocerebroside and phosphatidic acid patients with GBS, including the variant with facial diplegia and paresthesia. However, because assays for antibodies to galactocerebroside and phosphatidic acid are not commercially available and need to be done in specialized referral laboratories, these immunological tests are not commonly available at present.
Therefore, assays for antibodies to galactocerebroside and phosphatidic acid should be considered as an additional test that may help to confirm variant GBS with facial diplegia and paresthesia. Treatment of patients with variant GBS with facial diplegia and paresthesia remains controversial [18]. Patients with GBS may be treated with plasmapheresis or intravenous immunoglobulin (IVIG) [19,22–26]. However, treatment is not always needed if the patient remains mobile one week after the initial onset of symptoms [18,20]. Almost all patients reported with variant GBS with facial diplegia and paresthesia have preserved mobility throughout the duration of their illness [21].
Glucocorticoid treatment has been also reported and found to be variably effective in patients with this rare variant of GBS [22], while patients with IgM antibodies to N-acetylgalactosaminyl GD1a (GalNAc-GD1a) and other gangliosides have responded to glucocorticoids [27,28]. Therefore, glucocorticoids may be useful for improvement of facial palsy in cases of GBS with facial diplegia and paresthesia, the action of which is thought to be due to its anti-inflammatory effects, and reduction of edema [27]. In the present case, because the symptoms were initially similar to those of Bell’s palsy, the patient was initially prescribed oral prednisolone, valacyclovir, and vitamin B12 [29,30], which was followed by IVIG treatment for his persistent facial palsy [25,26]. Five months after his presentation, the patient had almost returned to normal (Figure 2). However, it is not clear whether this improvement was due to glucocorticoids, and/or IVIG treatment, or that the symptoms could have improved spontaneously.
Conclusions
Variant Guillain-Barré syndrome (GBS) with facial diplegia and paresthesia is a rare manifestation, that occasionally presents with facial hemiplegia, and which may mimic the more common Bell’s palsy. For the diagnosis of future cases, detection of antibodies to galactocerebroside and phosphatidic acid may assist in differentiating the disease from other pathologies.
Acknowledgments
The authors thank Dr. Susumu Kusunoki (Department of Neurology, Kindai University School of Medicine) for performing the antibody testing and Dr. Izumi Kitagawa (Department of General Internal Medicine, Shonan Kamakura General Hospital) who supervised the preparation of the article.
Footnotes
Conflict of interest
None.
References:
- 1.Susuki K, Koga M, Hirata K, et al. A Guillain-Barré syndrome variant with prominent facial diplegia. J Neurol. 2009;256(11):1899–905. doi: 10.1007/s00415-009-5254-8. [DOI] [PubMed] [Google Scholar]
- 2.Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366(9497):1653–66. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 3.Ropper AH. Further regional variants of acute immune polyneuropathy. Bifacial weakness or sixth nerve paresis with paresthesia, lumbar polyradiculopathy, and ataxia with pharyngeal-cervical-brachial weakness. Arch Neurol. 1994;51(7):671–75. doi: 10.1001/archneur.1994.00540190051014. [DOI] [PubMed] [Google Scholar]
- 4.Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366:2294. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 5.Nishimoto Y, Odaka M, Hirata K, Yuki N. Usefulness of anti-GQ1b IgG antibody testing in Fisher syndrome compared with cerebrospinal fluid examination. J Neuroimmunol. 2004;148:200–5. doi: 10.1016/j.jneuroim.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Smith N, Grattan-Smith P, Andrews IP, Kainer G. Acquired facial palsy with hypertension secondary to Guillain-Barré syndrome. J Pediatrics Child Health. 2010;46(3):125–27. doi: 10.1111/j.1440-1754.2009.01650.x. [DOI] [PubMed] [Google Scholar]
- 7.Kusunoki S, Chiba A, Hitoshi S, et al. Anti-Gal-C antibody in autoimmune neuropathies subsequent to mycoplasma infection. Muscle Nerve. 1995;18(4):409–13. doi: 10.1002/mus.880180407. [DOI] [PubMed] [Google Scholar]
- 8.Susuki K, Odaka M, Mori M, et al. Acute motor axonal neuropathy after Mycoplasma infection: Evidence of molecular mimicry. Neurology. 2004;62(6):949–56. doi: 10.1212/01.wnl.0000115123.42929.fd. [DOI] [PubMed] [Google Scholar]
- 9.Kusunoki S, Shiina M, Kanazawa I. Anti-Gal-C antibodies in Guillain-Barré syndrome subsequent to mycoplasma infection: Evidence of molecular mimicry. Neurology. 2001;57(4):736–38. doi: 10.1212/wnl.57.4.736. [DOI] [PubMed] [Google Scholar]
- 10.Arakawa H, Yuhara Y, Todokoro M, et al. Immunoadsorption therapy for a child with Guillain-Barré syndrome subsequent to Mycoplasma infection: A case study. Brain Dev. 2005;27(6):431–33. doi: 10.1016/j.braindev.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Meyer Sauteur PM, Huizinga R, Tio-Gillen AP, et al. Mycoplasma pneumoniae triggering Guillain-Barré syndrome: A case-control study. Ann Neurol. 2016;80(4):566–80. doi: 10.1002/ana.24755. [DOI] [PubMed] [Google Scholar]
- 12.Visser LH, van der Meche FG, Meulstee J, et al. Cytomegalovirus infection and Guillain-Barré syndrome: The clinical, electrophysiologic, and prognostic features. Dutch Guillain-Barré Study Group. Neurology. 1996;47(3):668–73. doi: 10.1212/wnl.47.3.668. [DOI] [PubMed] [Google Scholar]
- 13.Kusunoki S, Kaida K, Ueda M. Antibodies against gangliosides and ganglioside complexes in Guillain-Barré syndrome: New aspects of research. Biochimica Biophysica Acta. 2008;1780(3):441–44. doi: 10.1016/j.bbagen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Kaida K, Morita D, Kanzaki M, et al. Anti-ganglioside complex antibodies associated with severe disability in Guillain-Barré syndrome. J Neuroimmunol. 2007;182(1–2):212–18. doi: 10.1016/j.jneuroim.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Kaida K, Sonoo M, Ogawa G, et al. GM1/GalNAc-GD1a complex: A target for pure motor Guillain-Barré syndrome. Neurology. 2008;71(21):1683–90. doi: 10.1212/01.wnl.0000335160.72184.7d. [DOI] [PubMed] [Google Scholar]
- 16.Kusunoki S, Morita D, Ohminami S, et al. Binding of immunoglobulin G antibodies in Guillain-Barre syndrome sera to a mixture of GM1 and a phospholipid: Possible clinical implications. Muscle Nerve. 2003;27(3):302–6. doi: 10.1002/mus.10307. [DOI] [PubMed] [Google Scholar]
- 17.Hirakawa M, Morita D, Tsuji S, Kusunoki S. Effects of phospholipids on antiganglioside antibody reactivity in Guillain-Barré. J Neuroimmunol. 2005;159(1–2):129–32. doi: 10.1016/j.jneuroim.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Verma R, Chaudhari TS, Giri P. Unilateral facial palsy in Guillain-Barré syndrome (GBS): A rare occurrence. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-2012-007077. pii: bcr2012007077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Lancet. 1997;349(9047):225–30. [PubMed] [Google Scholar]
- 20.Green DM, Ropper AH. Mild Guillain-Barré syndrome. Arch Neurol. 2001;58(7):1098–101. doi: 10.1001/archneur.58.7.1098. [DOI] [PubMed] [Google Scholar]
- 21.Tatsumoto M, Odaka M, Hirata K, Yuki N. Isolated abducens nerve palsy as a regional variant of Guillain-Barré syndrome. J Neurology Sci. 2006;243(1–2):35–38. doi: 10.1016/j.jns.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann HC, Macht S, Jander S, et al. Guillain-Barré syndrome variant with prominent facial diplegia, limb paresthesia, and brisk reflexes. J Neurol. 2012;259(2):370–71. doi: 10.1007/s00415-011-6169-8. [DOI] [PubMed] [Google Scholar]
- 23.Barbi F, Ariatti A, Funakoshi K, et al. Parvovirus B19 infection antedating Guillain-Barre’ syndrome variant with prominent facial diplegia. J Neurol. 2011;258(8):1551–52. doi: 10.1007/s00415-011-5949-5. [DOI] [PubMed] [Google Scholar]
- 24.Yardimci N, Avci AY, Kayhan E, Benli S. Bilateral facial nerve enhancement demonstrated by magnetic resonance imaging in Guillain-Barré syndrome. Neurol Sci. 2009;30(5):431–33. doi: 10.1007/s10072-009-0120-0. [DOI] [PubMed] [Google Scholar]
- 25.Inaloo S, Katibeh P. Guillain-Barré syndrome presenting with bilateral facial nerve palsy. Iran J Child Neurol. 2014;8(1):70–72. [PMC free article] [PubMed] [Google Scholar]
- 26.Prabhat K, Riyaz C, Anish B, et al. Facial diplegia with paresthesia: An uncommon variant of Guillain-Barré syndrome. J Clin Diagn Res. 2016;10(7):1–2. doi: 10.7860/JCDR/2016/19951.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi R, Yamaguchi S. Guillain-Barré syndrome variant with facial diplegia and paresthesia associated with IgM anti-GalNAc-GD1a antibodies. Intern Med (Tokyo, Japan) 2015;54(3):345–47. doi: 10.2169/internalmedicine.54.2972. [DOI] [PubMed] [Google Scholar]
- 28.Takiyama Y, Sato Y, Sawada M, et al. An unusual case of facial diplegia. Muscle Nerve. 1999;22(6):778–79. doi: 10.1002/(sici)1097-4598(199906)22:6<778::aid-mus17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Gagyor I, Madhok VB, Daly F, et al. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2015;9(11):1–54. doi: 10.1002/14651858.CD001869.pub9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jalaludin MA. Methylcobalamin treatment of Bell’s palsy. Methods Find Exp Clin Pharmacol. 1995;17:539–44. [PubMed] [Google Scholar]


