Abstract
The kidney's filtration activity is essential for removing toxins and waste products from the body. The vascular endothelial cells of the glomerulus are fenestrated, flattened, and surrounded by podocytes, specialized cells that support glomerular endothelial cells. Mucin-type core 1–derived O-glycans (O-glycans) are highly expressed on both glomerular capillary endothelial cells and their supporting podocytes, but their biological role is unclear. Biosynthesis of core 1–derived O-glycans is catalyzed by the glycosyltransferase core 1 β1,3-galactosyltransferase (C1galt1). Here we report that neonatal or adult mice with inducible deletion of C1galt1 (iC1galt1−/−) exhibit spontaneous proteinuria and rapidly progressing glomerulosclerosis. Ultrastructural analysis of the glomerular filtration barrier components revealed that loss of O-glycans results in altered podocyte foot processes. Further analysis indicated that O-glycan is essential for the normal signaling function of podocalyxin, a podocyte foot process–associated glycoprotein. Our results reveal a new function of O-glycosylation in the integrity of the glomerular filtration barrier.
Keywords: albumin, carbohydrate function, glycobiology, podocyte, renal physiology, O-glycosylation, glomerulosclerosis, podocalyxin, podoplanin
Introduction
The kidney acts as an ultrafiltration unit to remove toxins and waste products from the body. Filtration in the kidney occurs via the glomerulus, which consists of a specialized capillary bed and support cells. The vascular cells of the glomerulus are fenestrated, flattened endothelial cells and are surrounded by podocytes, specialized support cells that envelop the glomerular capillaries by extending foot processes that interdigitate with foot process from adjacent podocytes (1). Glomerular capillary endothelial cells, podocytes, and the fused extracellular matrix of these cells, termed glomerular basement membrane (GBM),3 together comprise the glomerular filtration barrier, which retains blood plasma proteins and results in a urinary product with only trace amounts of protein. A hallmark of compromised glomerular filtration barrier integrity is elevated levels of protein/albumin in the urine, a condition called proteinuria/albuminuria (2).
Glomerulosclerosis, which presents with proteinuria in over 70% of cases, is a major clinical problem because scarred glomeruli cannot be repaired; many patients experience progressive glomerulosclerosis and eventual kidney failure. Therefore, it is imperative to identify factors that perturb the integrity of the glomerular filtration barrier and participate in the etiology of glomerulosclerosis.
Mucin-type O-glycosylation is initiated by addition of a GalNAc to serine or threonine residues on a peptide backbone (3). Core 1–derived O-glycans (O-glycans), a major form of O-glycans, are formed by addition of a galactose to the GalNAc by core 1 β1,3-galactosyltransferase (C1galt1) (4). The core 1 structures can be further extended and modified to form sialylated complex O-glycans (3). O-glycans are highly expressed in different components of the glomerular filtration barrier, with unclear functions (5–7). In this study, we found that mice with deficiency of O-glycans exhibit spontaneous proteinuria and rapidly progressing glomerulosclerosis. Lack of O-glycosylation results in defective podocalyxin signaling function. These results reveal new function of O-glycans in homeostasis of the glomerulus.
Results
Postnatal deletion of C1galt1 leads to spontaneous glomerulosclerosis
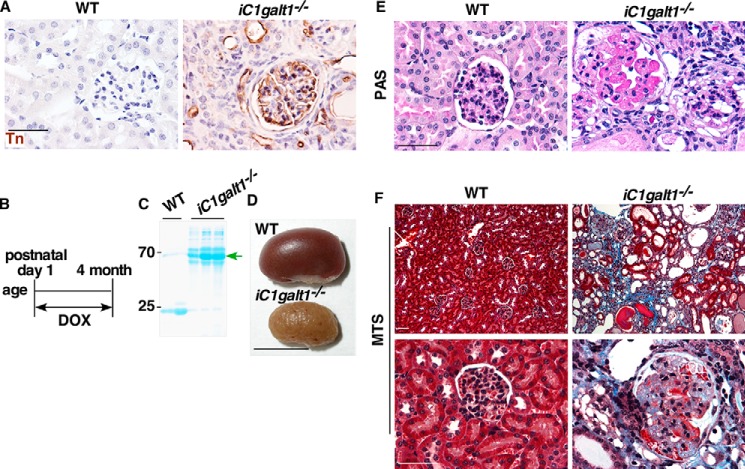
To determine whether O-glycans play a significant role in kidney function, we generated mice with a doxycycline-inducible global deficiency of core 1 O-glycans (iC1galt1−/−), which were generated by first crossing C1galt1f/f mice with Rosa26-rtTA;tetO-Cre Tg mice, and then treated C1galt1f/f;Rosa26-rtTA;tetO-Cre Tg mice with doxycycline postnatally from day 1 for 4 months (8). We confirmed the C1galt1 deletion by immunohistochemical staining for Tn antigen, which is exposed in the absence of core 1 O-glycans. As expected, wild-type controls did not exhibit Tn signals within the glomerular capillary tuft, podocytes covering the tuft, parietal epithelial cells of Bowman's capsule, tubules, or the interstitial blood vessels. In contrast, iC1galt1−/− mice exhibited significant Tn expression in all of these locations (Fig. 1, A and B). iC1galt1−/− mice had significant proteinuria at the time of sacrifice (∼4 months old) (Fig. 1, B and C). Gross analysis revealed that iC1galt1−/− mice showed contracted, paler, and corrugated kidneys (Fig. 1D). Periodic acid-Schiff (PAS) staining, which highlights irregularities of the glomerular architecture, showed that iC1galt1−/− mice had adhesion formation between the glomerular capillary tufts and Bowman's capsule, obliteration of glomerular capillaries with hyalinosis, and severe intra- and periglomerular immune cell infiltrations (Fig. 1E) relative to WT controls. Masson trichrome staining (MTS) further demonstrated glomerular scarring, tubular atrophy, proteinaceous casts, and interstitial fibrosis in iC1galt1−/− mice (Fig. 1F).
Figure 1.
Postnatal deletion of C1galt1 leads to spontaneous glomerulosclerosis. A, Tn expression in iC1galt1−/− mice and littermate controls was examined by immunohistochemistry (IHC). Scale bar = 50 μm. B, schematic of postnatal doxycycline (DOX)-inducible deletion of C1galt1. C, SDS-PAGE analysis of urine from iC1galt1−/− mice and WT littermate controls after induction by DOX for 4 months. D, macroscopic appearance of kidneys from wild-type control and iC1galt1−/− mice after induction by DOX for 4 months. Scale bar = 1 cm. E, PAS staining highlights glomerular changes in iC1galt1−/− mice. Scale bar = 50 μm. F, MTS shows the exposure of collagen within the glomerulus and increased interstitial volume of iC1galt1−/− kidneys, suggestive of severe renal injuries, including glomerulosclerosis. Scale bars = 50 μm.
Adult deletion of C1galt1 leads to spontaneous glomerulosclerosis
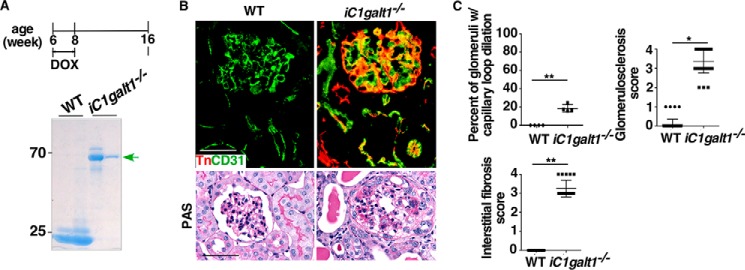
To corroborate our finding, we sought to investigate whether spontaneous glomerulosclerosis occurs in adult mice with deficiency of C1galt1. Global deletion of C1galt1 in adult mice was achieved by administration of doxycycline to 6-week-old C1galt1f/f;Rosa26-rtTA;tetO-Cre Tg mice for 2 weeks. The kidneys of iC1galt1−/− mice expressed Tn antigen in endothelial cells of glomerular capillaries and interstitial blood vessels as well as in podocytes and tubular epithelial cells. Spontaneous albuminuria, glomerulosclerosis, and broad capsular adhesions were observed in these mice by 8 weeks post-induction (Fig. 2, A–C) (9). These results demonstrated that O-glycans play a major role in maintaining the glomerular filtration function in healthy adult mice.
Figure 2.
Adult deletion of C1galt1 leads to spontaneous glomerulosclerosis. A, schematic of adult inducible deletion of C1galt1. SDS-PAGE analysis of urine from iC1galt1−/− mice and WT littermate controls 8 weeks after induction reveals spontaneous albuminuria. B, Tn expression was examined to verify C1galt1 deletion or expression 8 weeks after induction. CD31 was used as an endothelial marker. PAS staining reveals marked changes in global iC1galt1−/− mice. C, quantification as percentage of glomeruli with capillary loop dilation 8 weeks after induction, was analyzed in two groups (n = 4 mice/group). Data are presented as mean ± S.D. (n = 4). For quantitative analysis of kidney histology, 50 full-size glomeruli for each specimen were assessed on PAS-stained sections, and the level of glomerulosclerosis in each glomerulus was semiquantitatively scored as follows: 0, no sclerosis; 1, sclerosis less than 10% of glomeruli; 2, sclerosis 10% to ∼25% of glomeruli; 3, sclerosis 25% to ∼50% of glomeruli; 4, sclerosis more than 50% of glomeruli. Data are presented as mean ± S.D. (n = 50). To evaluate interstitial fibrosis, 20 fields for each section were assessed as follows: 0, no fibrosis; 1, fibrosis less than 10% of areas; 2, fibrosis 10% to ∼25% of areas; 3, fibrosis 25% to ∼50% of areas; 4, fibrosis more than 50% of areas. The averages of the glomerulosclerosis and interstitial fibrosis scores were calculated from the total evaluated glomeruli or interstitial lesions in each section. Data are presented as mean ± S.D. (n = 20). *, p < 0.05; **, p < 0.01. Scale bars = 50 μm.
Alterations of foot processes of podocytes occur prior to development of proteinuria in mice with C1galt1 deficiencies
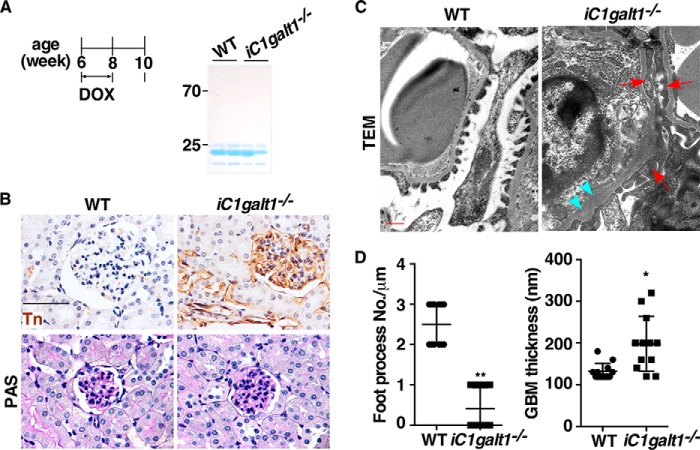
To investigate how a lack of O-glycans leads to abnormal kidney architecture and function, we determined renal defects at early time points after induced deletion of O-glycans. 6-week-old C1galt1f/f;Rosa26-rtTA;tetO-Cre Tg mice and WT littermates were fed food containing doxycycline for 2 weeks. Minimal irregularities of glomerular filtration or architecture were observed in these mice another 2 weeks after doxycycline-induced deletion of C1galt1 (Fig. 3, A and B). However, we found that the ultrastructures of the podocyte–endothelium interface were strikingly different in iC1galt1−/− mice compared with age-matched controls. Early effects of loss of O-glycans were collapse of the space between foot processes and secondary processes and thickening of the glomerular basement membranes, whereas WT littermates displayed podocyte foot process interdigitations (Fig. 3, C and D). These results support the theory that changes in podocyte foot processes in the absence of O-glycans are a prerequisite for development of the renal phenotype.
Figure 3.
Deletion of C1galt1 triggers altered foot processes of podocytes prior to the development of proteinuria. A, SDS-PAGE analysis of urine from iC1galt1−/− mice and WT littermate controls 2 weeks after induction did not reveal detectable albuminuria. B, PAS staining and IHC of Tn expression were carried out before proteinuria was detected in iC1galt1−/− mice. Scale bar = 50 μm. C, representative transmission EM of iC1galt1−/− mice and WT littermate controls corresponding to the light micrographs above. Red arrows indicated the effect of loss of O-glycan to collapse the space between foot processes and secondary processes. Blue arrowheads indicated thickened glomerular basement membranes. Scale bar = 500 nm. D, for quantitative ultrastructural analysis of the glomerulus by transmission EM, the number of podocyte foot processes present in each micrograph was divided by the total length of GBM regions in each image to determine the average density of podocyte foot processes. The GBM thickness in each image was also measured by National Institutes of Health ImageJ software. Data are presented as mean ± S.D. (n = 12). *, p < 0.05; **, p < 0.01.
O-glycosylation of podocalyxin is essential for glomerulus integrity
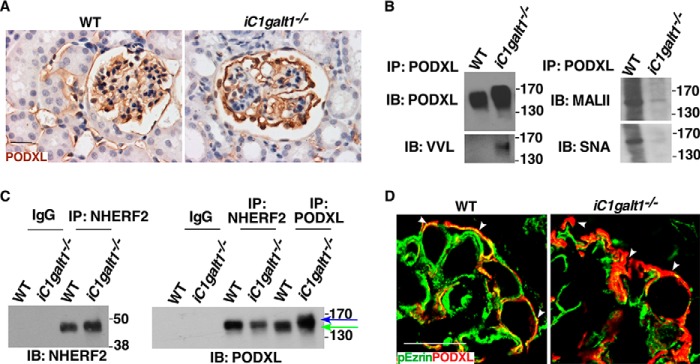
Podocyte foot processes associate with many O-glycosylated sialoproteins such as podoplanin and podocalyxin (10, 11). Podoplanin is a mucin-type transmembrane glycoprotein with extensive O-glycosylation (10, 11). Our laboratory has demonstrated previously that O-glycosylation is critical for the stability and function of podoplanin (8, 12, 13). However, we found that postnatal global deletion of podoplanin only resulted in mild renal disease by 4 months post-induction (Fig. 4). This led us to focus on another O-glycoprotein, podocalyxin, which is expressed predominately at secondary foot processes of podocytes (14). Genetic deletion of Podocalyxin causes complete flattening of foot processes (15), which is compatible with the phenotypes we observed in mice lacking O-glycans. We examined the effect of reduced O-glycosylation on podocalyxin expression and function. Podocalyxin displayed a slower electrophoretic mobility but comparable expression in kidneys from iC1galt1−/− mice compared with WT littermate controls (Fig. 5, A and B). Immunoprecipitated podocalyxin from iC1galt1−/− mice displayed a concomitant increase in VVL staining (binding to Tn antigen) but decreased MALII and SNA staining (binding to sialylated glycans) by Western blotting, suggestive of a dramatic decrease in sialylated O-glycans on podocalyxin (Fig. 5B). In vitro evidence demonstrated that deletion of O-glycosylation sites in the extracellular domain of podocalyxin affects its apical targeting. Podocalyxin and its cytoplasmic binding partner NHERF participate in the formation of an apical scaffold (16–18). Podocalyxin binds to ezrin, directly or indirectly via NHERF, to form a complex triggering RhoA–ROCK signaling, thus inducing actin cytoskeleton reorganization. The complex, in turn, is stabilized by elevated RhoA/ROCK-dependent phosphorylation of Ezrin (19, 20). Given these findings, one plausible mechanism is that O-glycans are required for the correct distribution and signaling function of podocalyxin at the apical surface of podocytes. Intriguingly, we found that loss of O-glycosylation decreased both co-immunoprecipitation of podocalyxin by NHERF2 and co-localization of podocalyxin and pEzrin along the apical plasma membrane of podocytes (Fig. 5, C and D). These data suggest that loss of the podocalyxin interaction with NHERF2/pEzrin is a potential effector mechanism of renal dysfunction following loss of O-glycans.
Figure 4.
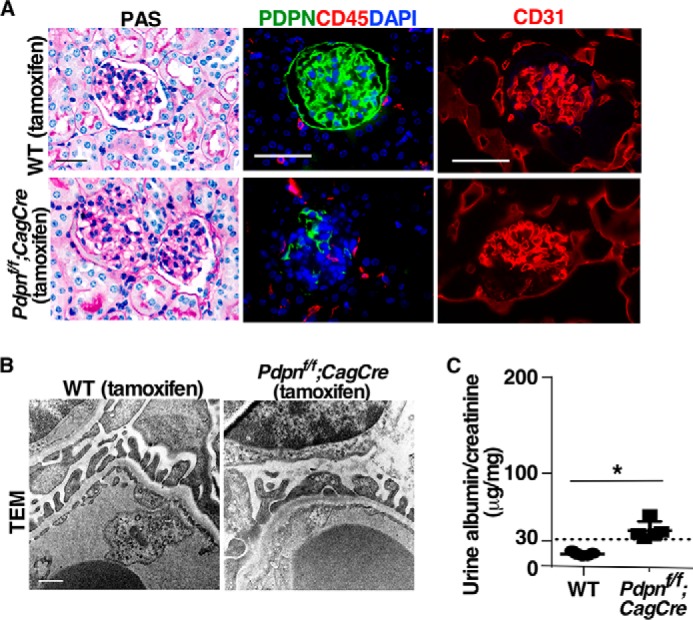
Postnatal deletion of podoplanin (PDPN) does not result in spontaneous glomerulosclerosis. A, representative images of PDPNf/f;CagCre and WT mouse kidney sections with various staining as indicated. Kidney samples from PDPNf/f;CagCre mice 4 months after tamoxifen-induced deletion. Scale bars = 50 μm. B, ultrastructural analysis of the glomeruli from PDPNf/f;CagCre mice and WT littermate controls 4 months after induction by transmission EM. Scale bar = 500 nm. C, functional evaluation of glomerular filtration barrier by analysis of urine albumin:creatinine ratio. The comparison was made between PDPNf/f;CagCre mice and WT littermate controls 4 months after tamoxifen-induced deletion. Data are presented as mean ± S.D. (n = 5). *, p < 0.05.
Figure 5.
Sialoproteins such as podocalyxin are targets of O-glycosylation in glomerulosclerosis. A, IHC of podocalyxin (PODXL) in WT and iC1galt1−/− adult mice after 4 weeks of tamoxifen-induced deletion. B, Western blotting of podocalyxin and evaluation of O-glycosylation/sialylation status of podocalyxin in WT and iC1galt1−/− mice. VVL is a lectin-recognizing Tn antigen. Sialylation of podocalyxin was probed with lectins: MALII recognizes N-acetylneuraminic acid (Neu5Ac)-linked α2,3-Gal; SNA recognizes α2,6-linked sialic acid. IB, immunoblot; IP, immunoprecipitation. C, podocalyxin–NHERF2 interaction was disrupted after loss of O-glycans. Kidney lysates from adult WT and iC1galt1−/− mice 8 weeks post-induction were immunoprecipitated with polyclonal anti-NHERF2 antibody or anti-podocalyxin, followed by immunoblotting. Arrows indicate podocalyxin proteins with differential degrees of O-glycosylation. Blue arrow indicates hypo-O-glycosylated portion of podocalyxin. D, immunofluorescent staining of pEzrin and podocalyxin in glomeruli from WT and iC1galt1−/− mice. Scale bars = 20 μm.
Podocyte-specific deletion of C1galt1 leads to spontaneous glomerulosclerosis
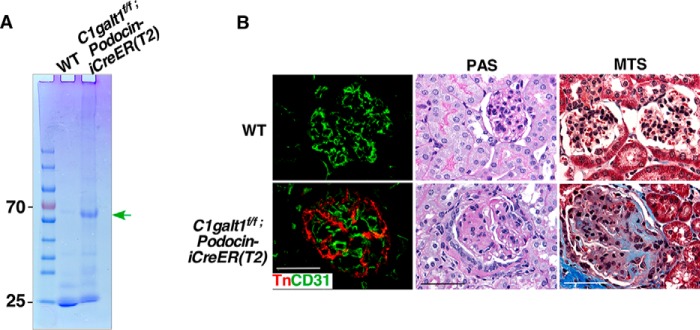
In humans, focal segmental glomerulosclerosis (FSGS) is a frequent and severe glomerular disease characterized by destabilization of the podocyte architecture (21). To evaluate a possible role for podocyte O-glycans in maintaining the glomerular filtration barrier, we determined whether a podocyte-specific deletion of C1galt mimics the renal defects in iC1galt1−/− mice. We generated adult mice with an inducible podocyte-specific deletion of C1galt1 by crossing C1galtf/f mice with podocin-iCreER(T2) mice, which express tamoxifen-inducible Cre in podocytes (22). We found that C1galtf/f;podocin-iCreER(T2) mice treated with tamoxifen postnatally from day 21 for 6 months developed albuminuria spontaneously. The kidneys of tamoxifen-treated mice were analyzed in further detail by PAS and MTS, which revealed gross histological changes, beginning with severely distorted and sclerosed glomeruli, compared with littermate controls (Fig. 6). These findings indicate that O-glycans on podocytes play an essential role in glomerular filtration barrier maintenance.
Figure 6.
Inducible podocyte-specific deletion of C1galt1 leads to spontaneous glomerulosclerosis. A, SDS-PAGE analysis of urine from C1galt1f/f;podocin-iCreER(T2) and WT mice treated with tamoxifen postnatally from day 21 for 6 months. B, immunofluorescent staining of Tn verifies the C1galt1 deletion and the specificity on podocytes. PAS and MTS reveal that loss of podocyte O-glycans leads to glomerulosclerosis. Podocyte-specific deletion of C1galt1 establishes a definitive role of podocyte O-glycans in the maintenance of glomerular filtration barrier integrity. Scale bars = 50 μm.
Discussion
O-glycans are highly expressed on glomerular capillary endothelial cells, their supporting podocytes, and tubular cells in the kidney. We found that global loss of O-glycans in adult iC1galt1−/− mice results in spontaneous proteinuria and glomerulosclerosis. Further analysis indicates that O-glycosylation of podocytes is essential to protect the integrity of the glomerulus. In particular, O-glycosylation is important for podocalyxin to interact with NHERF2/pEzrin, which contribute to glomerular filtration barrier maintenance.
Postnatal Tn exposure because of C1galt1 deficiency may elicit an immune response (23), which may lead to kidney glomerulosclerosis and proteinuria. However, we found that crossing our adult iC1galt1−/− with Rag1−/− mice, which lack T cells and B cells (24, 25), did not ameliorate the renal defects of iC1galt1−/− mice, indicating that an abnormal immune response does not contribute to the kidney phenotypes of C1galt1 deficiency.
Sialylation commonly occurs on O-glycans. Our iC1galt1−/− mice show reduced sialylation. Previously reports show that the morphologic alterations of podocytes are associated with a reduction in the sialic acid content of podocalyxin in puromycin aminonucleoside–treated rats (26, 27), and systemic administration of a sugar analog that eliminates sialylation causes profound kidney dysfunction (28). Together, these data support that loss of sialylation may alter the function of podocalyxin. The mouse genetic approach we used in this study results in prolonged impairment of sialomucins and, thus, convincingly validates the importance of sialomucins in the kidney. The readouts, changes in the distribution of podocalyxin, and changes in podocalyxin signaling are logical outcomes of the loss of C1galt1 function by cells that make podocalyxin.
Most of the data are derived from mice with inducible global deletion of C1galt1. Even though the proteinuria/glomerulosclerosis phenotype is largely reproduced in mice with podocyte-specific deletion of C1galt1, indirect contributions of deficiency of O-glycans in cell types other than podocytes might also contribute to these abnormalities. Future studies are needed to address this issue.
In humans, podocalyxin expression in glomeruli by immunohistochemical evaluation is found to be reduced or lost in FSGS (29). Indeed, we found that adult mice with loss of C1galt1 exhibited reduced podocalyxin levels. However, podocalyxin levels appeared to be normal in mice with earlier stages of renal disease following deletion of C1galt1 (30, 31), suggesting that reduced levels of podocalyxin in glomeruli might be caused by loss of podocytes. On the other hand, excretion of podocalyxin in the urine demonstrates a prognostic value for proteinuria development and kidney function in FSGS (32). Given that O-glycosylation functions as an essential regulatory mechanism of podocalyxin expression and function, further studies of how O-glycans regulates podocalyxin function will provide new insights into the pathogenesis of glomerulosclerosis, and an ability to detect aberrant O-glycosylation will shed new light on the prognosis of glomerulosclerosis.
Experimental procedures
Mice
We previously reported mice in which C1galt1 was flanked by loxP sites (C1galt1f/f mice) (12). We also reported mice with doxycycline-inducible global deficiency of C1galt1 (iC1galt1−/−), which were generated by crossing C1galt1f/f mice with Rosa26-rtTA, tetO-Cre Tg mice (8). To induce postnatal deletion of C1galt1, mice were fed food containing doxycycline starting from postnatal days 1 to 4 months of age. Adult deletion was accomplished by feeding 6-week-old mice for 2 weeks. Wild-type littermates treated with the same regimen were used as controls. Tamoxifen-inducible podocyte-specific C1galt1 knock-out mice were obtained by crossing C1galt1f/f mice with podocin-iCreER(T2) mice that express Cre recombinase specifically in the podocytes (22). Deletion of C1galt1 in C1galt1f/f; podocin-iCreER(T2) adult mice was accomplished by administrating tamoxifen (1 mg per day) for 5 consecutive days beginning at P21 and then once a week thereafter. We previously reported a strategy to generate mice with global inducible deletion of podoplanin (PDPNf/f;CagCre mice) (13). Briefly, Pdpnf/f mice were crossed with CAG-Cre-ERT2 Tg mice. To induce postnatal deletion of podoplanin, tamoxifen (20 μg per day) was administered orally from P1–5. WT littermates (Pdpnf/w;CagCre or Pdpnf/f) treated with the same regimen were used as controls. All vertebrate procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the Oklahoma Medical Research Foundation.
Biochemical measurements of urine albumin and urine creatinine
Urine samples were collected from knock-out mice and littermate controls, briefly centrifuged, diluted, and boiled in 2× Laemmli sample buffer. Albuminuria was assessed by 10% SDS-PAGE followed by Coomassie Blue staining. Urine albumin levels were measured qualitatively and in duplicate using an albumin ELISA quantitation kit according to the protocol of the manufacturer (Exocell), and the absorbance was read at 450 nm. Urine creatinine was measured in duplicate for each sample with a modified Jaffe reaction at an absorbance of 500 nm (Bio-Rad microplate reader).
Histological and ultrastructural analysis
Mouse kidney tissues were harvested, fixed in 4% paraformaldehyde, and embedded in paraffin. All sections (3 μm in thickness) were stained with H&E, PAS (Thermo Scientific) or MTS (Sigma) according to the standard procedures or the instructions of the manufacturer. For transmission EM, kidneys were fixed in Karnovsky fixative containing 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1% cacodylate buffer (pH 7.2) for 2 h at room temperature, followed by post-fixation with 1% osmium tetraoxide in cacodylate buffer for 90 min at 4 °C and mordanting with 1% tannic acid in cacodylate buffer. Ultrathin sections were contrasted with uranyl acetate and lead citrate and examined with a Hitachi H-7600 transmission electron microscope located in the Imaging Core Facility of the Oklahoma Medical Research Foundation. We followed histological scoring methods described previously (9).
Immunohistochemistry and immunofluorescent staining
The study was performed as described previously with modifications (33). For IHC staining, sections were deparaffinized with xylene and alcohol series. After inactivation of endogenous peroxidase with 0.3% hydrogen peroxide and blocking with normal blocking serum, the sections were incubated with glycosylation-independent goat anti-podocalyxin antibody (R&D Systems) or biotinylated mAb against Tn antigen (mouse IgM, clone Ca3638) overnight at 4 °C. After washing, sections were incubated with appropriate secondary antibodies conjugated to horseradish peroxidase. Immunoreactivity was visualized using a peroxidase diaminobenzidine kit (Vector Laboratories). Sections were then washed, counterstained with hematoxylin, and mounted, and photomicrographs were obtained. For IF staining, sections were blocked as described above and then incubated with primary antibody against CD31 (clone MEC13.3, Pharmingen, after treatment with 0.1% trypsin), podocalyxin (R&D Systems), CD45 (clone I3/2.3, Abcam), phosphorylated EzrinThr-567 (pEzrin, Cell Signaling Technology), or biotinylated mAb against Tn antigen (mouse IgM, clone Ca3638) overnight at 4 °C, followed by incubation with the respective secondary antibodies conjugated to fluorescent labels (Alexa Flour 594 or 488, 1:500, Invitrogen) for 1 h at room temperature. Slides were washed in PBS, stained with DAPI, and mounted, and immunofluorescence images were obtained using an Olympus fluorescence microscope equipped with Slidebook 5.0 analysis software.
Immunoprecipitation and Western blotting
For immunoprecipitation, kidney cortices were lysed with RIPA buffer (1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholic acid, 5 mm tetrasodium pyrophosphate, 50 mm sodium fluoride, 5 mm EDTA, 150 mm NaCl, 25 mm Tris (pH 7.5), 4 mm Na3VO4, 5 mm N-ethylmaleimide, and protease inhibitor mixture). Lysates were renatured using 9 volumes of ice-cold RIPA buffer and then prepared for immunoprecipitation as follows. Cell lysates were precleared with protein A+G-Sepharose beads for 1 h at 4 °C, followed by incubation with antibodies against podocalyxin (R&D Systems) or NHERF2 (Santa Cruz Biotechnology) for 4 h at 4 °C. For negative controls, an equal concentration of goat IgG was added instead of specific antibodies. Precipitated proteins were eluted from beads using 2% SDS in 50 mm Tris (pH 7.5) and diluted 1:20 with RIPA buffer, followed by Western blotting. Proteins were resolved by SDS-PAGE (4–15% acrylamide), followed by electroblotting to a polyvinylidene difluoride membrane and blocking with 5% milk (w/v). Primary antibodies against VVL, MALII, or SNA (Vector Laboratories) were incubated at 4 °C overnight, followed by incubation at room temperature with the respective horseradish peroxidase–conjugated secondary antibodies (1:5000) for 1 h. The immunoreactive proteins were detected by enhanced chemiluminescence with autoradiography.
Statistical analysis
Statistical analysis was performed using Student's t test. Differences were considered statistically significant at p < 0.05.
Author contributions
K. S., J. F., and L. X. designed the experiments and acquired and interpreted the data. J. S., K. B., Y. K., J. M. M., S. M., and R. S.-M. performed the experiments or provided important technical support. F. L., H. C., and H. B. interpreted some results. K. S., B. H. H., and L. X. wrote the manuscript.
Acknowledgments
We thank Dr. Rodger P. McEver for insightful discussions. We thank Dr. Farhad Danesh for providing the podocin-iCreER(T2) mice.
This work was supported by National Institutes of Health Grants GM114731, HL128390, HD083418, DK085691, HL093242, and HL118676; National Natural Science Foundation of China Grants 81520108005, 81470825, and 81370617; Jiangsu Province Key Medical Center Grant ZX201102; and Scientist Development Grants from the American Heart Association 11SDG7410022 (to J. F.) and 17SDG33630161 (to K. S.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- GBM
- glomerular basement membrane
- C1galt1
- core 1 β1,3-galactosyltransferase
- PAS
- periodic acid-Schiff
- MTS
- Masson trichrome staining
- FSGS
- focal and segmental glomerulosclerosis
- P
- postnatal day
- RIPA
- radioimmune precipitation assay
- IHC
- immunohistochemistry
- DOX
- doxycycline
- PDPN
- podoplanin
- VVL
- Vicia villosa lectin
- MALII
- Maackia amurensis lectin II
- SNA
- Sambucus nigra lectin
- NHERF
- Na+/H+ exchanger regulatory factor
- ROCK
- Rho-associated protein kinase.
References
- 1. Brinkkoetter P. T., Ising C., and Benzing T. (2013) The role of the podocyte in albumin filtration. Nat. Rev. Nephrol. 9, 328–336 [DOI] [PubMed] [Google Scholar]
- 2. Scott R. P., and Quaggin S. E. (2015) The cell biology of renal filtration. J. Cell Biol. 209, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varki A., Cummings R., Esko J., Freeze H., Hart G., and Marth J. (1999) Essentials of Glycobiology, Chapter 8, pp. 1–26, Cold Spring Harbor Laboratory Press, New York: [PubMed] [Google Scholar]
- 4. Ju T., Cummings R. D., and Canfield W. M. (2002) Purification, characterization, and subunit structure of rat core 1 β 1,3-galactosyltransferase. J. Biol. Chem. 277, 169–177 [DOI] [PubMed] [Google Scholar]
- 5. Liu Z.-H., and He J. C. (eds) (2014) Podocytopathy, Vol. 183, pp. 1–11, Karger, Basel, Switzerland [Google Scholar]
- 6. Kojima K., Davidovits A., Poczewski H., Langer B., Uchida S., Nagy-Bojarski K., Hovorka A., Sedivy R., and Kerjaschki D. (2004) Podocyte flattening and disorder of glomerular basement membrane are associated with splitting of dystroglycan-matrix interaction. J. Am. Soc. Nephrol. 15, 2079–2089 [DOI] [PubMed] [Google Scholar]
- 7. Tran D. T., and Ten Hagen K. G. (2013) Mucin-type O-glycosylation during development. J. Biol. Chem. 288, 6921–6929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan Y., Yago T., Fu J., Herzog B., McDaniel J. M., Mehta-D'Souza P., Cai X., Ruan C., McEver R. P., West C., Dai K., Chen H., and Xia L. (2014) Podoplanin requires sialylated O-glycans for stable expression on lymphatic endothelial cells and for interaction with platelets. Blood 124, 3656–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tian X., Kim J. J., Monkley S. M., Gotoh N., Nandez R., Soda K., Inoue K., Balkin D. M., Hassan H., Son S. H., Lee Y., Moeckel G., Calderwood D. A., Holzman L. B., Critchley D. R., et al. (2014) Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J. Clin. Invest. 124, 1098–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsui K., Breitender-Geleff S., Soleiman A., Kowalski H., and Kerjaschki D. (1999) Podoplanin, a novel 43-kDa membrane protein, controls the shape of podocytes. Nephrol. Dial. Transplant. 14, 9–11 [DOI] [PubMed] [Google Scholar]
- 11. Breiteneder-Geleff S., Matsui K., Soleiman A., Meraner P., Poczewski H., Kalt R., Schaffner G., and Kerjaschki D. (1997) Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol. 151, 1141–1152 [PMC free article] [PubMed] [Google Scholar]
- 12. Fu J., Gerhardt H., McDaniel J. M., Xia B., Liu X., Ivanciu L., Ny A., Hermans K., Silasi-Mansat R., McGee S., Nye E., Ju T., Ramirez M. I., Carmeliet P., Cummings R. D., et al. (2008) Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J. Clin. Invest. 118, 3725–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Herzog B. H., Fu J., Wilson S. J., Hess P. R., Sen A., McDaniel J. M., Pan Y., Sheng M., Yago T., Silasi-Mansat R., McGee S., May F., Nieswandt B., Morris A. J., Lupu F., et al. (2013) Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 502, 105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kerjaschki D., Sharkey D. J., and Farquhar M. G. (1984) Identification and characterization of podocalyxin: the major sialoprotein of the renal glomerular epithelial cell. J. Cell Biol. 98, 1591–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doyonnas R., Kershaw D. B., Duhme C., Merkens H., Chelliah S., Graf T., and McNagny K. M. (2001) Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J. Exp. Med. 194, 13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meder D., Shevchenko A., Simons K., and Füllekrug J. (2005) Gp135/podocalyxin and NHERF-2 participate in the formation of a preapical domain during polarization of MDCK cells. J. Cell Biol. 168, 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Z., Zimmerman S. E., Tsunezumi J., Braitsch C., Trent C., Bryant D. M., Cleaver O., González-Manchón C., and Marciano D. K. (2016) Role of CD34 family members in lumen formation in the developing kidney. Dev. Biol. 418, 66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu C. Y., Chen J. Y., Lin Y. Y., Shen K. F., Lin W. L., Chien C. L., ter Beest M. B., and Jou T. S. (2007) A bipartite signal regulates the faithful delivery of apical domain marker podocalyxin/Gp135. Mol. Biol. Cell 18, 1710–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmieder S., Nagai M., Orlando R. A., Takeda T., and Farquhar M. G. (2004) Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and ezrin in MDCK cells. J. Am. Soc. Nephrol. 15, 2289–2298 [DOI] [PubMed] [Google Scholar]
- 20. Bryant D. M., Roignot J., Datta A., Overeem A. W., Kim M., Yu W., Peng X., Eastburn D. J., Ewald A. J., Werb Z., and Mostov K. E. (2014) A Molecular switch for the orientation of epithelial cell polarization. Dev. Cell. 31, 171–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wiggins R. C. (2007) The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 71, 1205–1214 [DOI] [PubMed] [Google Scholar]
- 22. Wang J., Wang Y., Long J., Chang B. H., Wilson M. H., Overbeek P., and Danesh F. R. (2010) Tamoxifen-inducible podocyte-specific iCre recombinase transgenic mouse provides a simple approach for modulation of podocytes in vivo. Genesis 48, 446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ju T., Otto V. I., and Cummings R. D. (2011) The Tn antigen-structural simplicity and biological complexity. Angew. Chem. Int. Ed. Engl. 50, 1770–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Picarella D. E., Kratz A., Li C. B., Ruddle N. H., and Flavell R. A. (1992) Insulitis in transgenic mice expressing tumor necrosis factor β (lymphotoxin) in the pancreas. Proc. Natl. Acad. Sci. U.S.A. 89, 10036–10040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander W. S., Viney E. M., Zhang J. G., Metcalf D., Kauppi M., Hyland C. D., Carpinelli M. R., Stevenson W., Croker B. A., Hilton A. A., Ellis S., Selan C., Nandurkar H. H., Goodnow C. C., Kile B. T., et al. (2006) Thrombocytopenia and kidney disease in mice with a mutation in the C1galt1 gene. Proc. Natl. Acad. Sci. U.S.A. 103, 16442–16447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kerjaschki D., Vernillo A. T., and Farquhar M. G. (1985) Reduced sialylation of podocalyxin - the major sialoprotein of the rat-kidney glomerulus - in aminonucleoside nephrosis. Am. J. Pathol. 118, 343–349 [PMC free article] [PubMed] [Google Scholar]
- 27. Takeda T., McQuistan T., Orlando R. A., and Farquhar M. G. (2001) Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J. Clin. Invest. 108, 289–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Macauley M. S., Arlian B. M., Rillahan C. D., Pang P. C., Bortell N., Marcondes M. C., Haslam S. M., Dell A., and Paulson J. C. (2014) Systemic blockade of sialylation in mice with a global inhibitor of sialyltransferases. J. Biol. Chem. 289, 35149–35158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gebeshuber C. A., Kornauth C., Dong L., Sierig R., Seibler J., Reiss M., Tauber S., Bilban M., Wang S., Kain R., Böhmig G. A., Moeller M. J., Gröne H. J., Englert C., Martinez J., and Kerjaschki D. (2013) Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat. Med. 19, 481–487 [DOI] [PubMed] [Google Scholar]
- 30. Guo J. K., Menke A. L., Gubler M. C., Clarke A. R., Harrison D., Hammes A., Hastie N. D., and Schedl A. (2002) WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum. Mol. Genet. 11, 651–659 [DOI] [PubMed] [Google Scholar]
- 31. Chau Y. Y., Brownstein D., Mjoseng H., Lee W. C., Buza-Vidas N., Nerlov C., Jacobsen S. E., Perry P., Berry R., Thornburn A., Sexton D., Morton N., Hohenstein P., Freyer E., Samuel K., et al. (2011) Acute multiple organ failure in adult mice deleted for the developmental regulator Wt1. PLoS Genet. 7, e1002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hara M., Yanagihara T., Kihara I., Higashi K., Fujimoto K., and Kajita T. (2005) Apical cell membranes are shed into urine from injured podocytes: a novel phenomenon of podocyte injury. J. Am. Soc. Nephrol. 16, 408–416 [DOI] [PubMed] [Google Scholar]
- 33. Chang B., Tessneer K. L., McManus J., Liu X., Hahn S., Pasula S., Wu H., Song H., Chen Y., Cai X., Dong Y. Z., Brophy M. L., Rahman R., Ma J. X., Xia L., and Chen H. (2015) Epsin is required for Dishevelled stability and Wnt signalling activation in colon cancer development. Nat. Commun. 6, 6380. [DOI] [PMC free article] [PubMed] [Google Scholar]