Abstract
A new method for determination of salbutamol sulfate has been developed using poly(4-amino-3-hydroxynaphthalene sulfonic acid/GCE. Cyclic voltammetric investigation of the electrochemical behavior of salbutamol sulfate at the polymer modified glassy carbon unveiled electrocatalytic activity of the modifier towards irreversible oxidation of salbutamol sulfate. Dependence of peak current predominantly on scan rate than on square root of scan rate, and peak potential shift with pH demonstrated that oxidation of salbutamol sulfate at the polymer modified electrode follows adsorption reaction kinetics with proton participation.
Under optimized solution and differential pulse voltammetric parameters, the oxidative peak current showed linear dependence on salbutamol sulfate concentration in the range 0.2 to 8 μM with method detection limit (3s/m) and determination coefficient (R2) of 6.8 × 10−8 M and 0.99786, respectively. Low method detection limit, relatively wide linear range, and recovery results of spiked standard salbutamol sulfate in syrup samples in the range 96.7–98.9% validated the method for determination of salbutamol sulfate in pharmaceutical formulations.
Differential pulse voltammetric analysis of salbutamol sulfate syrup formulation for its salbutamol sulfate content revealed 98.8 to 99.3% of the labeled value confirming the applicability of the developed method for determination of salbutamol sulfate in real samples.
Keywords: Electrochemistry, Analytical chemistry
1. Introduction
β-adrenoceptor (β-AR) agonists which have been classified as β1- and β2-subtypes are considered as first-line medications in the treatment of airway narrowing, the hallmark feature of bronchial asthma and chronic obstructive pulmonary disease (COPD) [1].
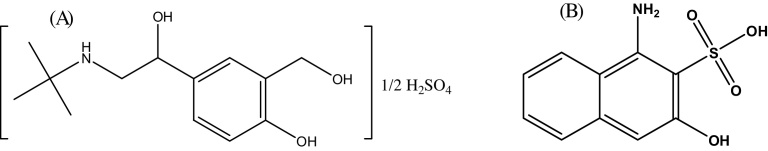
β2-AR agonists represent a mainstay of the management of chronic obstructive pulmonary disease (COPD) and asthma [2, 3]. Salbutamol sulfate (SBS) (Fig. 1(A)), like other β2-adrenergic agonists, is also widely used as bronchodilators in the prevention and treatment of exercise-induced asthma, which is a common entity in high-performance athletes [4]. Even though salbutamol sulfate is prevalently used by athletes, the world anti-doping agency (WADA) has banned oral administration of salbutamol sulfate and other β2-adrenergic agonists due to a presumed ergogenic action [5]. The WADA loosened the restrictions towards salbutamol sulfate in 2010 which is now allowed in therapeutic doses by inhalation as long as the athlete declares the use of it in doping tests, i.e. “declaration of use” [6]. Thus, it is very important to have a sensitive and selective method for effective management and determination of salbutamol sulfate in different pharmaceutical dosage forms, biological fluids and other sample matrixes.
Fig. 1.
Chemical structure of (A) salbutamol sulfate and (B) AHNSA.
Many analytical methods including high performance liquid chromatography [7, 8, 9], spectrophotometry [10, 11, 12, 13, 14], and capillary electrophoresis [15, 16] have been reported for determination of salbutamol sulfate in different samples. However, most of these methods are known to be time consuming, require advanced technical expertise, costly equipment and complex sample pretreatment procedure using organic solvents aggravating environmental pollution. Electrochemical methods have been of great interest due to several advantages, including high sensitivity, simplicity, rapid response and low cost [17]. Some electrochemical methods using modified electrodes have been reported for determination of salbutamol sulfate in pharmaceutical formulations [18, 19, 20, 21, 22]. However, most of the reported modifiers are relatively expensive that require lengthy modification steps. The monomer 4-Amino-3-hydroxynaphthalene sulfonic acid (AHNSA) (Fig. 1(B)) is derivative of aniline which is the most utilized conducting polymer [23]. Poly(AHNSA) modified electrodes showed promising feature for electrochemical determination of number of alkaloids [24, 25, 26]. To the best of our knowledge, glassy carbon electrode (GCE) modified with poly(AHNSA), has not been reported for determination of salbutamol sulfate.
In the present work, the applicability of poly(AHNSA) modified glassy carbon electrode for determination of salbutamol sulfate in pharmaceutical formulations is illustrated.
2. Experimental
2.1. Chemicals and reagents
4-amino-3-hydroxylnaphthalene sulfonic acid (AHNSA) (Sigma-Aldrich); KH2PO4, and K2HPO4 (98–101%) (BDH, England); salbutamol sulfate (99.92%) (Emmellen Biotech Pharmaceuticals Limited); H2SO4 (98%), and HNO3 (69.0–70.0%) (Loba Cheime Pvt. Ltd, India); NaOH and HCl (Riede-De Haen, Germany) were used. All reagents were of analytical grade and were used directly without further purification. Distilled water was used for the preparation of all solutions throughout this work.
Phosphate buffer solutions (PBS) prepared by mixing equi-molar (0.1 M) of K2HPO4 and KH2PO4 were used to prepare salbutamol sulfate solutions. 1.0 M of NaOH and HCl solutions were used to adjust the desired pH of the buffer solutions.
2.2. Instruments and apparatus
All electrochemical measurements were performed using model 700E series Electrochemical Workstation, CH Instruments (Austin, Texas, USA) connected to personal computer. A conventional three-electrode system consisting of unmodified GCE (3 mm in diameter) or poly(AHNSA)/GCE as working electrode, Ag/AgCl, KCl saturated as reference electrode and platinum coil as counter electrode was used. All experiments were carried out at room temperature (about 22 ± 2 °C).
2.3. Procedure
2.3.1. Preparation of polymer modified electrode
The surface of glassy carbon electrode was modified with poly(AHNSA) film following the procedure reported elsewhere [24]. Briefly: The polished and rinsed glassy carbon electrode was dipped in a 2.0 mM AHNSA/0.1 M HNO3 solution and the potential was scanned between −0.8 and +2.0 V at a scan rate of 100 mV s−1 for 15 cycles. Then, the modified electrode was rinsed with distilled water and conditioned by sweeping it in monomer free 0.5 M H2SO4 between −0.8 and +0.8 V at 100 mV s−1 until a stable voltammogram is obtained.
2.3.2. Preparation of salbutamol sulfate Solutions
A 10 mM stock solution of SBS was prepared by dissolving 0.1941 g of SBS in 100 mL of distilled water. A 0.5 mM intermediate SBS solution in pH 7.0 PBS was prepared from the stock solution through dilution. All other working standard solutions of different concentrations (0.2, 0.6, 1, 2, 4, 6, and 8 μM) were also prepared by serial dilution from freshly prepared intermediate standard solution using the appropriate pH buffer solution.
Salbutamol sulfate syrup sample (Pvt. Ltd, India; labeled as 2 mg/5 mL syrup, which is equivalent to 1.387 mol L−1) was purchased from a local pharmacy, Bahir Dar. For recovery and real sample studies, 2 and 4 μM SBS syrup samples in pH 7 PBS were quantitatively prepared through dilution.
2.3.3. Analytical procedure and method validation
While cyclic voltammetry was used to investigate the electrochemical behavior of SBS at unmodified and poly(AHNSA) modified glassy carbon electrodes, and, to study the effect of scan rate and pH on the oxidation peak current and peak potential of SBS at the modified GCE; differential pulse voltammetry was used for quantitative determination of SBS in syrup sample.
Wide dynamic linear range, reasonably high determination coefficient (R2), low method detection limit, and excellent recovery results of spiked SBS in syrup sample demonstrated the applicability of the developed method for determination of salbutamol sulfate in real samples. the developed method was thus applied for determination of SBS content in real syrup sample and the experimental result was compared with the declared value by the manufacturer.
3. Results and discussion
3.1. Electrochemical behavior of salbutamol sulfate at poly(AHNSA)/GCE
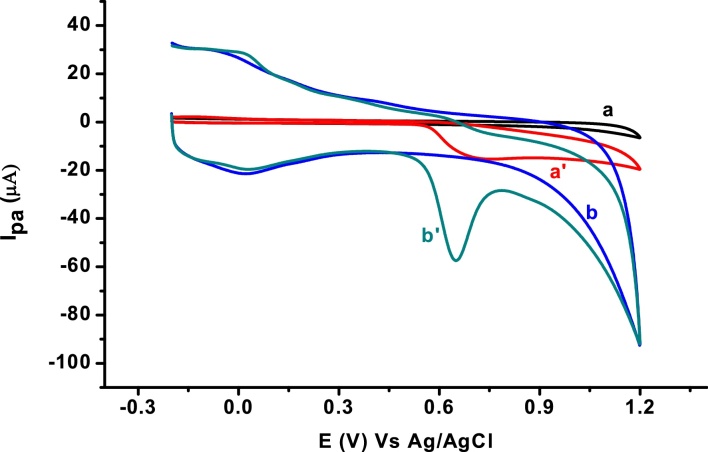
The electrochemical behavior of SBS at unmodified and poly(AHNSA) modified glassy carbon electrodes was investigated using cyclic voltammetry. Fig. 2 presents the cyclic voltammograms unmodified GCE (a and a’) and poly(AHNSA)/GCE (b and b’) in pH 7 PBS containing no (a and b) and 0.5 mM SBS (a’ and b’). Appearance of only an oxidative peak at both the unmodified (a’) and modified (b’) electrodes indicated that SBS undergoes an irreversible oxidation at both electrodes. In contrast to the unmodified electrode, a sharp oxidative peak with a three-fold peak current at 80 mV lower potential at the polymer modified electrode showed the catalytic activity of the modifier towards oxidation of SBS. The catalytic activity of the modified electrode could be attributed to an increased surface area and/or conductivity of the modified electrode surface [24].
Fig. 2.
Cyclic voltammograms of unmodified GCE (a and a’) and poly (AHNSA)/GCE (b and b’) in pH 7.0 PBS containing no (a and b) and 0.5 mM SBS (a’ and b’). Scan rate: 0.1 Vs−1.
3.2. Effect of scan rate on peak current and peak potential of SBS
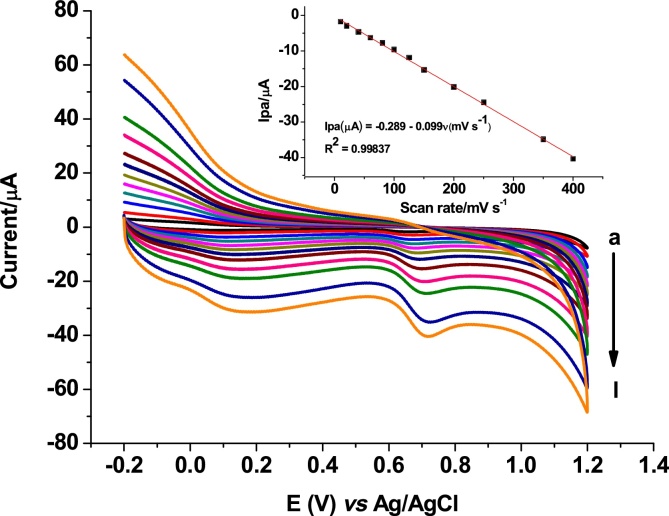
To investigate the oxidative kinetics of SBS at poly(AHNSA)/GCE, effect of scan rate on both the peak potential and peak current was studied. Fig. 3 shows cyclic voltammograms of 0.5 mM SBS at poly(AHNSA)/GCE at various scan rates in the range 10–400 mV/s. As can be seen from the figure, anodic peak potential shifted in the positive direction confirming the irreversibility of the oxidation reaction of SBS at poly(AHNSA)/GCE [19]. A better dependence (R2 = 0.99837) of peak current on scan rate (inset of Fig. 3) than on square root of scan rate showed that the oxidation reaction kinetics of SBS at poly(AHNSA) modified GCE is predominantly adsorption-controlled [19, 20].
Fig. 3.
Cyclic voltammograms of poly(AHNSA)/GCE in pH 7.0 PBS containing 0.5 mM of SBS at different scan rates (a-l: 10, 20, 40, 60, 80, 100, 125, 150, 200, 250, 350, and 400 mV/s, respectively). Inset: plot of oxidative peak current versus scan rate.
3.3. Effect of pH on peak current and peak potential
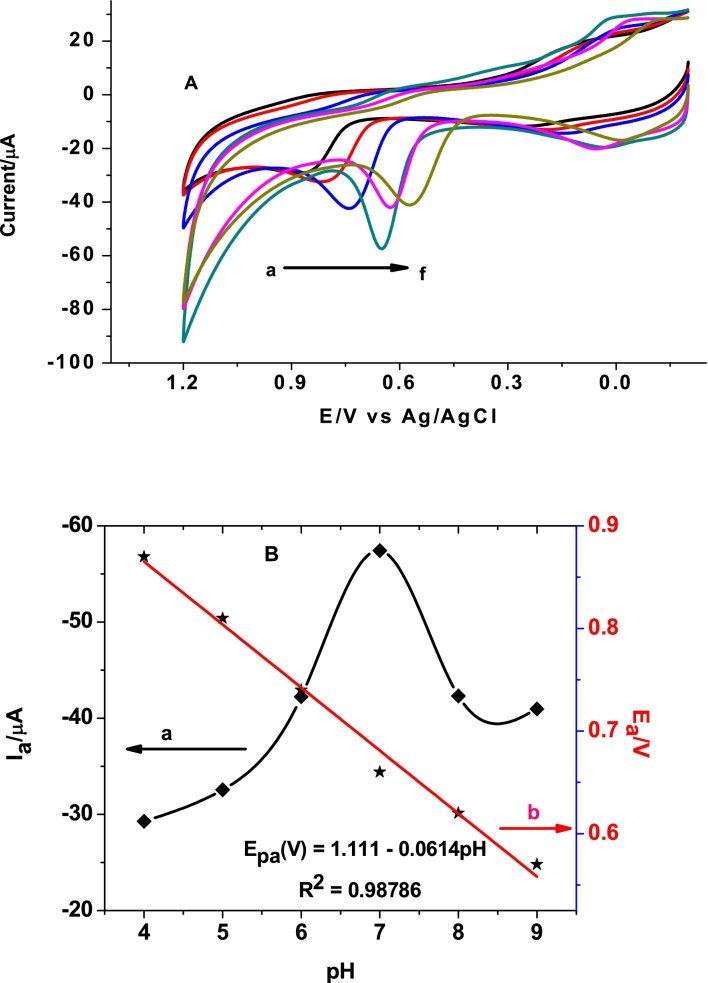
Voltammetric measurements carried out in aqueous solutions are likely to be pH dependent as the addition or removal of an electron from the analyte molecule may induce the uptake or loss of a proton. Fig. 4(A and B) demonstrates the dependence of peak current and peak potential of salbutamol sulfate at poly(AHNSA)/GCE on the pH of buffer solution. As can be seen from Fig. 4(B) (curve a), the oxidative peak current of SBS increased with pH from 4.0 to 7.0 which then decreased indicating pH 7 is the optimum value which is in agreement with reported works [19, 21]. This trend could partly be ascribed to the coulombs force of attraction and repulsion between the electrode modifier (pka ≈4) and SBS (pka ≥ 9) experienced at different pH ranges [24]. Moreover, a potential shift in the negative direction was observed with increasing pH value indicating the participation of protons during oxidation of SBS [19]. The dEp/pH value of 61 mV/pH (curve b of Fig. 4(B)) clearly indicates the involvement of equal number of electrons and protons in the oxidation of SBS [19]. Hence, a reaction mechanism (Fig. 5) that proceeds via dimerization was proposed which is in agreement with previously reported works [19].
Fig. 4.
A) Superimposed cyclic voltammograms of poly(AHNSA)/GCE in PBS of different pH values (a-f: 4.0, 5.0, 6.0, 7.0, 8.0, and 9.0, respectively) containing 0.5 mM SBS. B) Plot of peak current (curve a) and peak potential (curve b) versus pH of PBS containing 0.5 mM SBS.
Fig. 5.
Proposed reaction scheme of SBS at poly(AHNSA)/GCE.
3.4. Differential pulse voltammetric method for determination of SBS
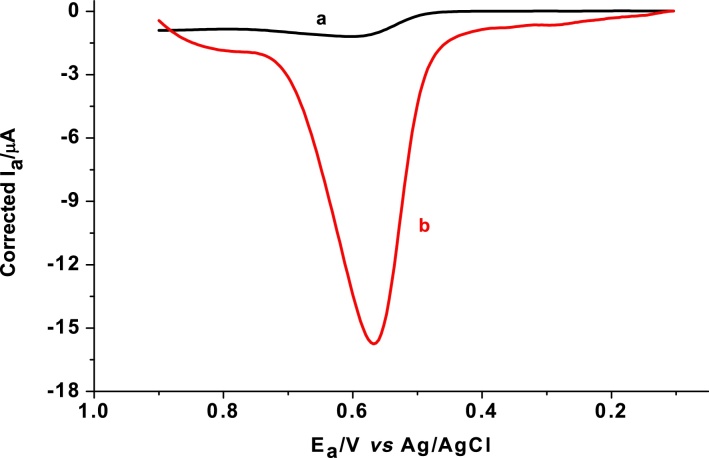
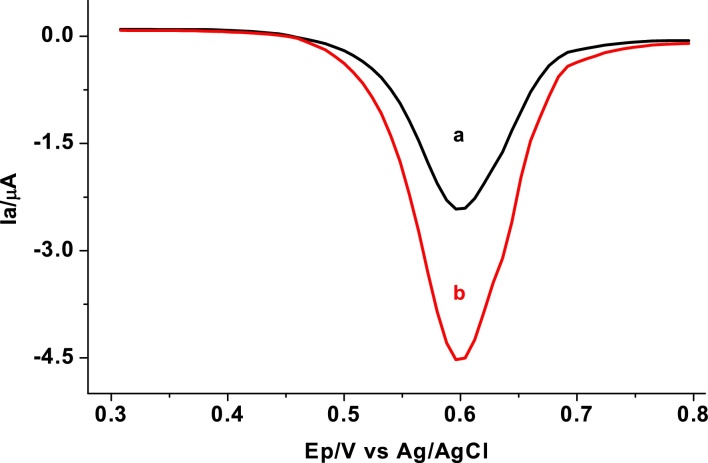
Due to its ability to discriminate faradaic current from non-faradaic current [20], differential pulse voltammetry (DPV) was employed for quantitative determination of SBS in pharmaceutical syrup formulation. Fig. 6 shows differential pulse voltammograms of pH 7 PBS containing 0.5 mM SBS at unmodified and modified GCE. An oxidative peak with one order of magnitude enhanced peak current at the poly(AHNSA)/GCE (curve b of Fig. 6) than at the unmodified GCE (curve a of Fig. 6) signified the catalytic role of the modifier towards the oxidation of SBS.
Fig. 6.
Cyclic voltammograms corrected for blank of unmodified (a) and modified GCE (b) in pH 7.0 PBS containing 0.5 mM SBS. Scan rate: 100 mV s−1.
3.4.1. Optimization of method parameters
3.4.1.1. Optimization of step potential and pulse amplitude
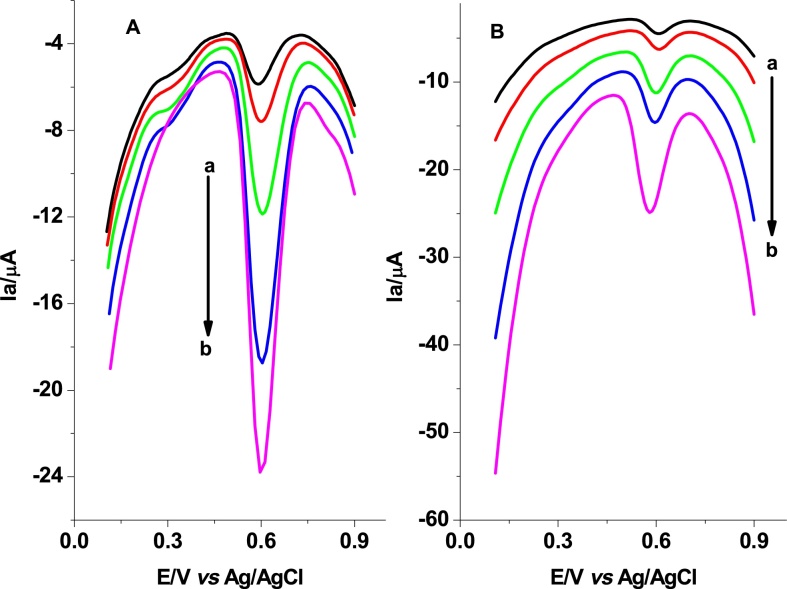
Although the increase of peak current with increasing step potential and pulse amplitude is implicit, these DPV parameters were optimized by making a compromise between the increased faradic peak current and accompanied capacitive current. Fig. 7(A and B) represent the DPVs at different step potentials and pulse amplitudes, respectively. Hence, 8, and 75 mV were taken as the optimum step potential and pulse amplitude, respectively.
Fig. 7.
DPVs of poly(AHNSA)/GCE in pH 7.0 PBS containing 0.5 mM SBS at (A) different step potentials (a-e: 4, 6, 8, 10, and 12 mV, respectively) and (B) different pulse amplitudes (a-e: 25, 35, 50, 60, and 75 mV, respectively).
3.4.1.2. Optimization of accumulation time and potential
Since the study of dependence of peak current on scan rate revealed that the oxidation of SBS at poly(AHNSA)/GCE followed adsorption controlled reaction kinetics, the deposition potential and time were optimized.
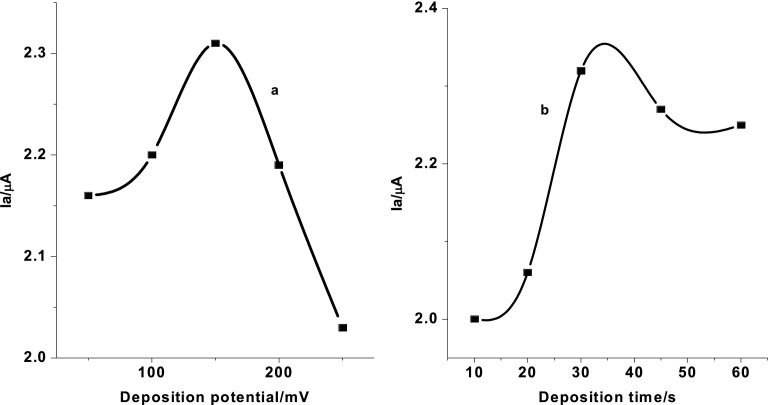
The influence of accumulation potential on oxidation current of 0.5 mM SBS in pH 7 PBS was examined in the range of 50 to 250 mV keeping the accumulation time at 30 s [27]. As can be seen from of Fig. 8(curve a), the peak current increased with the accumulation potential from 50 to 150 mV which then decreased indicating that +150 mV is the optimum accumulation potential. Curve b of Fig. 8(curve b) presents the dependence of the peak current response on the time of accumulation at +150 mV deposition potential. The peak current increased with time up to 30 seconds after which it showed a relative decrease in the peak intensity which could be attributed to the saturation of the active surface of the electrode [28]. Thus, an accumulation time of 30 s was taken as the optimum time.
Fig. 8.
Effect of (a) accumulation potential (+50 to +250 mV), and (b) accumulation time (10–60 s) on oxidative peak current of 0.5 mM SBS at poly(AHNSA)/GCE.
3.4.1.3. Calibration curve and method detection limit
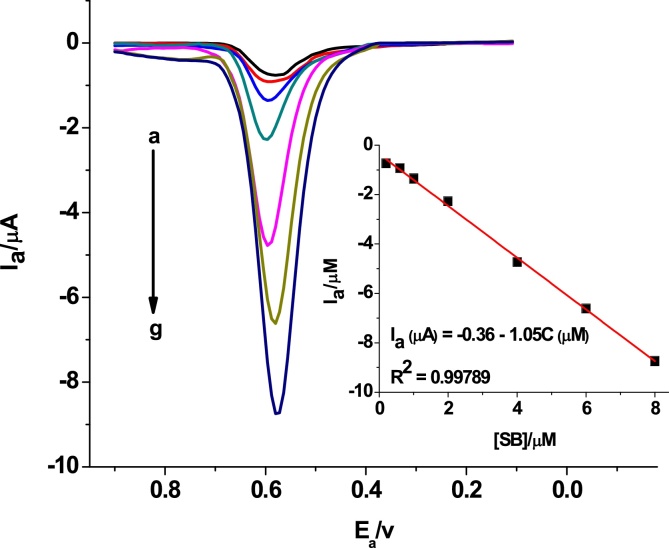
Under the optimized solution and method parameters, the dependence of DPV oxidative peak current response of poly(AHNSA)/GCE for SBS in pH 7 PBS on its concentration was investigated. Fig. 9 shows the DPVs of different concentrations of SBS at poly(AHNSA)/GCE. The oxidative peak current showed linear dependence on the concentration of SBS in the range 0.2 to 8 × 10−6 M with determination coefficient (R2) and method detection limit (MDL = 3S/m for n = 4) of 0.99786, and 0.068 μM, respectively.
Fig. 9.
DPVs of poly(AHNSA)/GCE in pH 7.0 PBS containing different concentrations of SBS (a–g: 0.2, 0.6, 1, 2, 4, 6, and 8 μM, respectively) at step potential, pulse amplitude, deposition time, and deposition potential of 8 mV, 75 mV, 30 s, and 150 mV, respectively. Inset: plot of anodic peak current versus concentration of SBS.
3.4.1.4. Recovery of spiked standard SBS in pharmaceutical samples
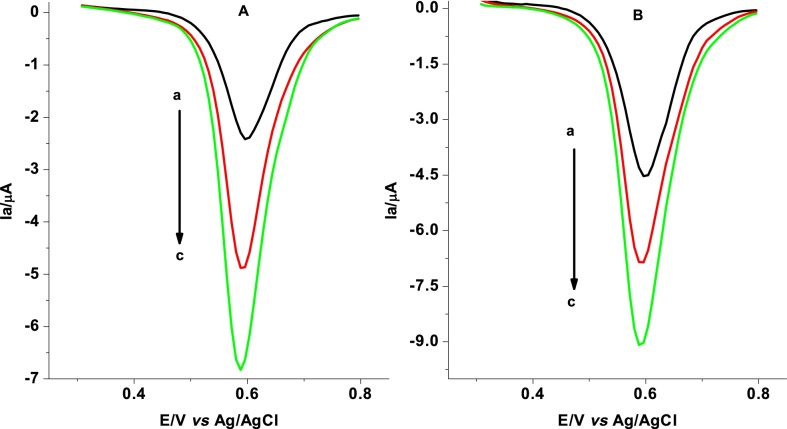
To further validate the developed method, recovery studies were conducted. 2 and 4 μM syrup samples were prepared based on the label of purchased syrup formulations as described in section 2.3.2. Fig. 10(A and B) presents the voltammograms for 2 and 4 μM SBS syrup samples before and after spiking. The oxidative peak current for the unspiked syrup samples, spiked with 2 μM, and with 4 μM of standard SBS are summarized in Table 1. Irrespective of the spike concentration, recovery results in the range 96.7–98.9% of spiked standard SBS in real syrup sample validated the applicability of the developed method for determination of SBS in pharmaceutical formulations.
Fig. 10.
Differential pulse voltammograms of 2 μM, and 4 μM unspiked SBS syrup samples (curve a of figures A and B, respectively), and spiked with 2 μM, and 4 μM (curves b, and c, respectively).
Table 1.
Summary of percentage recoveries of spiked standard SBS in syrup sample solutions.
| SBS in syrup sample (μM) |
Standard added (μM) | Ia/μA | %Recovery ± Er | |
|---|---|---|---|---|
| 2 |
0 | 2.45 | ––- | |
| 2 | 4.86 | 97.9 ± 2.0 | ||
| 4 |
6.86 |
96.7 ± 3.3 |
||
| 4 | 0 | 4.49 | ––- | |
| 2 | 6.88 | 97.2 ± 2.8 | ||
| 4 | 9.0 | 98.9 ± 1.1 | ||
Er relative error.
3.4.2. Application of poly(AHNSA)/GCE for determination of SBS in pharmaceutical syrup formulation
In order to check the applicability of the proposed method for determination of SBS in real samples, 2 and 4 μM SBS syrup samples in pH 7 PBS were prepared from purchased pharmaceutical syrup labeled as “2 mg/5 mL” through dilution.
While the differential pulse voltammograms recorded for the 2 and 4 μM SBS syrup samples are presented in Fig. 11, the respective anodic currents in terms of SBS content in the purchased syrup formulation are summarized in Table 2. As can be seen from the table, detection of SBS content in the range 99.0 to 99.5% of the labeled value showed good agreement with the labeled content (2 mg/5 mL syrups) and hence indicates the applicability of the developed method for the determination of SBS in real samples.
Fig. 11.
DPVs of SBS pharmaceutical syrup samples (a and b: 2, and 4 μM, respectively) at poly(AHNSA)/GCE. Step potential: 8 mV; pulse amplitude: 75 mV; deposition time: 30s; and deposition potential: 150 mV.
Table 2.
Summary of experimental peak current and SBS content per 5 mL syrup sample as compared to the labeled value.
| Declared value (mg/5 mL) |
Prepared syrup solution as per to label (μM) | Expected current (μA) | Experimental current (μA) | Detected SBS (mg/5 mL ± Er) | % of the labeled value |
|---|---|---|---|---|---|
| 2 | 2 | 2.46 | 2.44 | 1.98 ± 0.01 | 99.0 |
| 4 | 4.56 | 4.55 | 1.99 ± 0.05 | 99.5 |
Er relative error.
3.4.3. Comparison of the proposed method with previously reported methods
Table 3 presents summary of performance of the developed method against reported works on electrochemical determination of salbutamol sulfate. As can clearly be seen from the table, the present approach which uses relatively cheap, that does not need complicated modification and cleaning steps, is better in both the detection limit and linear range than most of the previously reported methods.
Table 3.
Comparison between the present reported method and previously reported methods for salbutamol sulfate determination.
4. Conclusion
Cyclic voltammetric investigation of the electrochemical behavior of SBS at poly(AHNSA)/GCE revealed the electrocatalytic roll of the electrode surface towards an irreversible oxidation of SBS.
Under optimized differential pulse voltammetric parameters and solution pH, anodic peak current of salbutamol sulfate at poly(AHNSA)/GCE showed linear dependence on concentration in the range 0. 2–8 μM with MDL (3S/m) of 0.068 μM. The very low detection limit, excellent recovery results of spiked standard SBS in pharmaceutical syrup formulation (98.5 − 100.5%), and agreement of the detected SBS with the labeled value suggested the potential applicability of poly(AHNSA)/GCE for electrochemical determination of salbutamol sulfate in pharmaceutical dosages.
Declarations
Author contribution statement
Meareg Amare: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Getahun Menkir: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Barisione G., Baroffio M., Crimi E., Brusasco V. Beta-Adrenergic Agonists. Pharmaceuticals. 2010;3:1016–1044. doi: 10.3390/ph3041016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balanag V.M., Yunus F., Yang P.C., Jorup C. Efficacy and safety of budesonide formoterol compared with salbutamol in the treatment of acute asthma. Pulm. Pharmacol. Ther. 2006;19(2):139–147. doi: 10.1016/j.pupt.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Ventura R., Damasceno L., Farre M., Cardoso J., Segura J. Analytical methodology for the detection of β-agonists in urine by gas chromatography-mass spectrometry for application in doping control. Anal. Chim. Acta. 2000;418:79–92. [Google Scholar]
- 4.Carlsen K.H., Anderson S.D., Bjermer L., Bonini S., Brusasco V., Canonica W., Cummiskey J., Delgado L., Del Giacco S.R., Drobnic F., Haahtela T., Larsson K., Palange P., Popov T., van Cauwenberge P. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis. Allergy. 2008;63:387–403. doi: 10.1111/j.1398-9995.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- 5.World Anti-Doping Agency . 2008. The Prohibited List, International Standard.https://www.wada-ama.org/sites/default/files/resources/files/WADA_Prohibited_List_2008_EN.pdf 22 September 2007. [Google Scholar]
- 6.The World Anti-Doping Agency . 2013. The prohibited list, international standard.http://www.npc.ir/files/939409293929846.pdf 10 September 2012. [Google Scholar]
- 7.Maithani M., Singh R. Development and validation of a stability-indicating HPLC method for the simultaneous determination of salbutamol sulfate and theophylline in pharmaceutical dosage forms. J. Anal. Bioanal. Tech. 2011;2(1):116. [Google Scholar]
- 8.Chitlange S.S., Chaturvedi K.K., Wankhede S.B. Development and validation of spectrophotometric and HPLC method for the simultaneous estimation of salbutamol sulfate and prednisolone in tablet dosage form. J. Anal. Bioanal. Tech. 2011;2(1):117. [Google Scholar]
- 9.Dubey N., Sahu S., Singh G.N. Development of HPLC method for simultaneous estimation of ambroxol, Guaifenesin and salbutamol in single dosage form. Indian J. Chem. 2012;51B:1633–1636. wos:000311463700007. [Google Scholar]
- 10.Dol I., Knochen M. Flow injection spectrophotometric determination of salbutamol with 4-aminopyrine. Talanta. 2004;64(5):1233–1236. doi: 10.1016/j.talanta.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 11.Dave H.N., Mashru R.C., Thakkar A.R. Simultaneous determination of salbutamol sulfate: bromhexine hydrochloride and etofylline in pharmaceutical formulations with the use of four rapid derivative spectrophotometric methods. Anal. Chim. Acta. 2007;597(1):113–120. doi: 10.1016/j.aca.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Basavaiah K., Somashekar B.C., Ramakrishna V. Rapid titrimetric and spectrophotometric methods for salbutamol sulfate in pharmaceuticals using N-bromosuccinimide. Acta Pharm. 2007;57(1):87–98. doi: 10.2478/v10007-007-0007-7. [DOI] [PubMed] [Google Scholar]
- 13.Mishra A.K., Kumar M., Mishra A., Verma A., Chattopadhyay P. Validated UV spectroscopic method for estimation of salbutamol from tablet formulations. Archives of Applied Science Research. 2010;2(3):207–211. [Google Scholar]
- 14.Montrade M.P., LeBizec B., Monteau F., Siliart B., Andre F. Multi-residue analysis for β-agonistic drugs in urine of meat-producing animals by gas chromatography −mass spectrometry. Anal. Chim. Acta. 1993;275:253–268. [Google Scholar]
- 15.Changguo C., Hong L., Yujing F. Determination of salbutamol sulfate in medicaments by capillary electrophoresis with contactless conductivity detection. Chin. J. chromatogr. 2011;29(2):137–140. doi: 10.3724/sp.j.1123.2011.00137. [DOI] [PubMed] [Google Scholar]
- 16.Sirichai S., Khanatharana P. Rapid analysis of clenbuterol, salbutamol, procaterol, and fenoterol in pharmaceuticals and human urine by capillary electrophoresis. Talanta. 2008;76:1194–1198. doi: 10.1016/j.talanta.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H., Zhao J., Liu H., Wang H., Liu R., Liu J. Application of poly (3-methylthiophene) modified glassy carbon electrode as riboflavin sensor. Int. J. Electrochem. Sci. 2010;5:295–301. www.electrochemsci.org/list10.htm [Google Scholar]
- 18.Goyal R.N., Bishnoi S. Single-Walled-Carbon-Nanotube-Modified Pyrolytic Graphite Electrode Used as a Simple Sensor for the Determination of Salbutamol in Urine. Int. J. Electrochem. 2011;2011:1–8. [Google Scholar]
- 19.Li Y., Ye Z., Luo P., Li Y., Ye B. A sensitive voltammetric sensor for salbutamol based on MWNTs composite nano-Au film modified electrode. Anal. Met. 2014;6:1928–1935. [Google Scholar]
- 20.Wei Y., Zhang Q., Shao C., Li C., Zhang L., Li X. Voltammetric determination of salbutamol on a glassy carbon electrode coated with a nanomaterial thin film. J. Anal. Chem. 2010;65(4):398–403. [Google Scholar]
- 21.Goyal R.N., Oyama M., Singh S.P. Fast determination of salbutamol, abused by athletes for doping, in pharmaceuticals and human biological fluids by square wave voltammetry. J. Electroanal. Chem. 2007;611:140–148. [Google Scholar]
- 22.Goyal R.N., Kaur D., Singh S.P., Pandey A.K. Effect of graphite and metallic impurities of C60 fullerene on determination of salbutamol in biological fluids. Talanta. 2008;75(1):63–69. doi: 10.1016/j.talanta.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 23.Ansari A., Keivani M.B. Polyaniline conducting electroactive polymers: Thermal and environmental stability studies. E-J. Chem. 2006;3(4):202–217. [Google Scholar]
- 24.Amare M., Admassie S. Polymer modified glassy carbon electrode for the electrochemical determination of caffeine in coffee. Talanta. 2012;93:122–128. doi: 10.1016/j.talanta.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 25.Amare M., Lakew W., Admassie S. Poly (4-amino-3-hydroxynaphthalene sulfonic acid) modified glassy carbon electrode for electrochemical detection of ephedrine in human urine. Anal. Bioanal. Electrochem. 2011;3(4):365–378. [Google Scholar]
- 26.Geto A., Amare M., Tessema M., Admassie S. Voltammetric determination of nicotine at poly(4-amino-3-hydroxynaphthalene sulfonic acid)-modified glassy carbon electrode. Electroanalysis. 2012;24(3):659–665. [Google Scholar]
- 27.Yinfeng L., Zhuo Y., Peili L., Ying L., Baoxian Y. A sensitive voltammetric sensor for salbutamol based on MWNTs composite nano-Au film modified electrode. Anal. Met. 2014;6:1928–1935. [Google Scholar]
- 28.Attaran A.M., Javanbakht M., Fathollahi F., Enhessari M. Determination of salbutamol in pharmaceutical and serum samples by adsorptive stripping voltammetry on a carbon paste electrode modified by iron titanate nanopowders. Electroanalysis. 2012;24(10):2013–2020. [Google Scholar]