Abstract
Background
The definitive dietary management of propionic acidaemia (PA) is unknown although natural protein restriction with adequate energy provision is of key importance.
Aim
To describe European dietary practices in the management of patients with PA prior to the publication of the European PA guidelines.
Methods
This was a cross-sectional survey consisting of 27 questions about the dietary practices in PA patients circulated to European IMD dietitians and health professionals in 2014.
Results
Information on protein restricted diets of 186 PA patients from 47 centres, representing 14 European countries was collected. Total protein intake [PA precursor-free L-amino acid supplements (PFAA) and natural protein] met WHO/FAO/UNU (2007) safe protein requirements for age in 36 centres (77%). PFAA were used to supplement natural protein intake in 81% (n = 38) of centres, providing a median of 44% (14–83%) of total protein requirement. Seventy-four per cent of patients were prescribed natural protein intakes below WHO/FAO/UNU (2007) safe levels in one or more of the following age groups: 0–6 m, 7–12 m, 1–10 y, 11–16 y and > 16 y. Sixty-three per cent (n = 117) of patients were tube fed (74% gastrostomy), but only 22% received nocturnal feeds.
Conclusions
There was high use of PFAA with intakes of natural protein commonly below WHO/FAO/UNU (2007) safe levels. Optimal dietary management can only be determined by longitudinal, multi-centre, prospective case controlled studies. The metabolic instability of PA and small patient cohorts in each centre ensure that this is a challenging undertaking.
Keywords: Propionic acidemia, Protein restricted diet, Precursor-free amino acids, Natural protein
1. Introduction
Propionic acidaemia (PA, OMIM #606054) is a rare, life threatening, inherited metabolic disorder with a poor clinical outcome [1]. Movement disorders, cardiomyopathy, prolonged QT, thrombocytopenia and pantocytopenia are common clinical manifestations [2]. PA is caused by deficiency of propionyl-CoA carboxylase (PCC; E.C. 6413) which catalyses the carboxylation of propionyl-CoA to D- methylmalonyl-CoA [3]. Propionyl-CoA is derived from three main sources: 1) catabolism of isoleucine, methionine, valine and threonine, 2) odd-chain fatty acid metabolism and 3) bacterial fermentation of carbohydrate in the gut [4].The main principles of management in PA are to minimise the production of toxic metabolites of organic compounds whilst supporting anabolism, normal growth and good nutritional status. Dietary treatment, together with carnitine and antibiotics are important components of management [5].
In 2014, proposed European guidelines were published on the diagnosis, acute and chronic management of PA [5]. They were developed using SIGN methodology, based on a critical appraisal of all scientific evidence. There were three main dietary recommendations: 1) there should be an adequate energy supply combined with avoidance of prolonged fasting, 2) lower intake of precursor containing amino acids by restricting natural protein intake, and 3) use of PA precursor-free amino acid supplements (PFAA) only if natural protein tolerance is below the WHO/FAO/UNU (2007) safe levels of protein intake [6].
The PA clinical guidelines aimed to improve the consistency of care and provide authoritative recommendations and consensus to reassure practitioners about the appropriateness of treatment practices. In other inherited metabolic conditions, it has already been established that dietary treatment varies widely and is influenced more by geographical region of care [7], [8] than disorder severity [9]. The PA dietary guidelines are based on low grade scientific evidence (mainly level D) and it is possible that these treatment guidelines may have little impact on individual practice.
In order to examine dietetic practices in PA, in a cross-sectional survey we have collected data from health professionals across Europe immediately prior to the publication of the PA guidelines [5].
2. Material and methods
In July to August 2014, a questionnaire (including 27 multiple choice or short answer questions) about 3 types of organic acidaemia's (isovaleric acidaemia [IVA], propionic acidaemia [PA] and methylmalonic acidaemia [MMA]) was sent to all European members of the Society for the Study of Inborn Errors of Metabolism Dietitians Group (SSIEM-DG) and other European dietitians (n = 53) who have previously participated in surveys [7], [9]. They were requested to cascade this questionnaire to dietitians and physicians within their own country. The IVA results have been previously published [8]. All the questions were written in English. Information on patient numbers, prescribed total protein intake, sourced from natural protein and PFAA, was collected by age. Patients were allocated to the following age groups: 0–6 months, 7–12 months, 1–10 years, 11–16 years and > 16 years. Specific questions were asked about treatment criteria, medications, clinical/biochemical monitoring, special low protein foods, energy supplements and use of enteral feeding. In order to evaluate trends in protein prescription, the results were divided into the following geographical regions: Western Europe Group A (The Netherlands, Belgium and France), Western Europe Group B (Germany, Switzerland and Austria), Eastern Europe (Poland), Southern Europe (Italy, Spain and Portugal) and Northern Europe (Denmark, Norway, Sweden and UK). The mean protein intakes in each age category were compared with the WHO/FAO/UNU (2007) safe levels of total protein intake [6].
Ethical approval was not required as clinical outcome data or patient specific data were not included in this survey.
2.1. Statistical analysis
Data was analysed descriptively (percentages, medians, range and means). Answers to open questions were grouped or categorised.
3. Results
Fifty-three centres from 14 countries returned questionnaires between July and August 2014. Six of 53 centres did not manage PA patients in their centre.
3.1. Patient description
One hundred and eighty-six patients with PA on a protein restricted diet were identified. There was a median of only 2 patients (range 1–20) per centre in 47 centres. Only 5 centres had ≥ 10 patients.
Patients were distributed as follows: Western Europe Group A (Netherlands, Belgium and France: n = 14 centres, 61 patients); Western Europe Group B (Germany, Switzerland and Austria: n = 10 centres, 37 patients); Eastern Europe (Poland: n = 1 centre [the only treatment centre responsible for PA management], 10 patients); Southern Europe (Italy, Spain and Portugal: n = 6 centres, 27 patients); and Northern Europe (Denmark, Sweden, Norway and UK, n = 16 centres, 51 patients). The age of presentation was: neonatal, n = 108 (58%); late (> 30 days of age), n = 68 (37%), and unknown, n = 10 (5%). The total number of patients for each age group at the time of questionnaire completion was: < 1 y, n = 13; 1–10 y, n = 85; 11–16 y, n = 48; and > 16 y, n = 40.
3.2. Total protein prescription
Fig. 1 presents the mean total amount of protein (g/kg/day) prescribed in each centre reported by region and age range. WHO/FAO/UNU (2007) safe levels of total protein intake [6] were met by 36 of 47 centres (77%). Table 1 provides the median total protein prescription (g/kg/day) with and without PFAA. The decision about the amount of total protein prescribed was made jointly by the physician and dietitian in 31 centres (66%), physician's only in 14 centres (30%) and dietitian's only in 2 centres (4%). When prescribing total protein, the WHO/FAO/UNU (2007) safe levels of protein intake [6] were used as a benchmark in 26 centres (55%), national references for protein intake were used by 14 centres (30%) and the remaining 7 centres (15%) did not specify which recommendations were used.
Fig. 1.
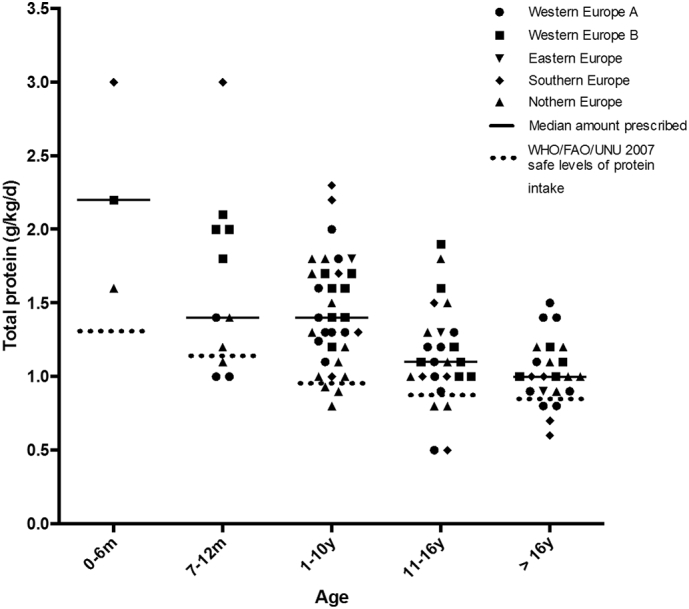
Mean total protein prescription in PA patients by centre in each age range (n = 47 centres).
Table 1.
Descriptive statistics comparing centres (using/not using PFAA) for dietary prescription of: total protein (g/kg), natural protein (g/kg), PFAA (g/kg) and % of protein provided by PFAA compared with total protein prescription.
| Age | Centres using PFAA |
Centres not using PFAA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0-6 m n = 4 |
7-12 m n = 6 |
1-10y n = 64 |
11-16y n = 37 |
> 16y n = 33 |
0-6 m n = 0 |
7-12 m n = 3 |
1-10y n = 21 |
11-16y n = 11 |
> 16y n = 7 |
|
| Total protein (g/kg) | ||||||||||
| Median | 2.2 | 1.9 | 1.6 | 1.2 | 1.0 | None reported | 1.0 | 1.0 | 1.0 | 1.0 |
| Min | 1.6 | 1.1 | 1.0 | 0.5 | 0.7 | 1.0 | 0.8 | 0.5 | 0.6 | |
| Max | 3.0 | 3.0 | 2.3 | 1.9 | 1.5 | 1.2 | 1.5 | 1.8 | 1.0 | |
| Natural protein (g/kg) | ||||||||||
| Median | 1.0 | 1.0 | 0.8 | 0.7 | 0.6 | None reported | 1.0 | 1.0 | 1.0 | 1.0 |
| Min | 0.5 | 0.5 | 0.4 | 0.3 | 0.4 | 1.0 | 0.8 | 0.5 | 0.6 | |
| Max | 1.3 | 1.2 | 1.4 | 1.2 | 0.8 | 1.2 | 1.5 | 1.8 | 1.0 | |
| PFAA (g/kg) | ||||||||||
| Median | 1.2 | 0.9 | 0.7 | 0.6 | 0.5 |
- |
||||
| Min | 0.3 | 0.2 | 0.3 | 0.2 | 0.2 | |||||
| Max | 2.5 | 2.5 | 1.8 | 0.9 | 0.8 | |||||
| Amount of total protein from PFAA used (%) | ||||||||||
| Median | 55 | 44 | 43 | 43 | 50 |
- |
||||
| Min | 19 | 14 | 19 | 20 | 25 | |||||
| Max | 83 | 83 | 78 | 70 | 67 | |||||
PFAA: propionic acidaemia precursor-free amino acid supplements.
n: number of patients.
3.3. Natural protein prescription
Table 1 describes the median natural protein prescription (g/kg/day) of all patients. Mean natural protein prescription of each centre is presented in Fig. 2 by region and age range. The majority of centres (n = 35/47, 74%) prescribed natural protein below the WHO/FAO/UNU (2007) safe levels of protein intake [6] in at least one of the age groups studied. This was higher in centres managing adult patients (91%, 21/23 centres). Twenty-seven of 35 (77%) centres with a very low natural protein ingestion gave PFAA to supplement intake. Seven of 35 (20%) centres achieved safe levels of protein intake without PFAA. The remaining centres failed to achieve safe levels of protein intake. High biological protein (animal) sources were used by most of the centres (77%, 36/47) to provide a source of natural protein.
Fig. 2.
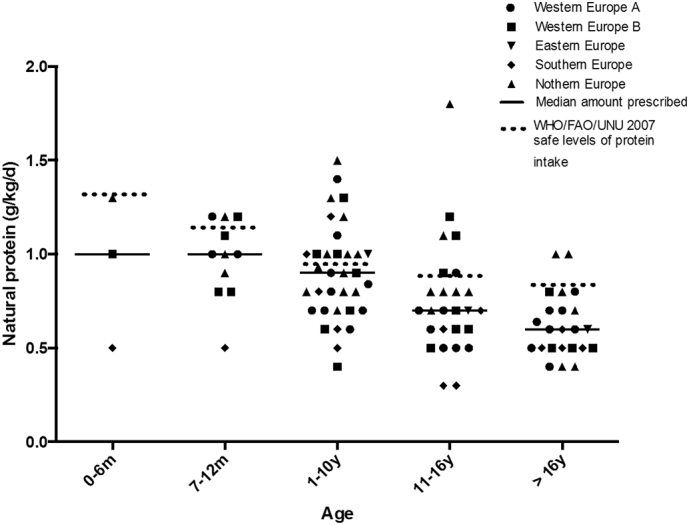
Mean natural protein prescription in PA patients by centre in each age range. (n = 47 centres).
3.4. Prescription of PFAA
PFAA were frequently given to supplement natural protein intake in 38 of 47 centres (81%). 9 centres from Northern Europe did not prescribe PFAA. Fig. 3 gives the mean amount of PFAA prescribed by geographical region and age. Table 1 presents the median amount of PFAA (g/kg/day) prescribed by age. PFAA provided almost half (median of 44%; range 14–83%) of the total protein prescription in all age groups. The contribution of PFAA to the total protein prescription was consistent across different age groups [aged 0 to 12 months, 45% (n = 11 centres); 1 to 10 y, 43% (n = 26 centres), 11 to 16 y, 43% (n = 20 centres) and > 16 y of age, 50% (n = 20 centres)]. In centres prescribing PFAA, 82% (31/38), were prescribing a high protein intake (in excess of 120% of the WHO/FAO/UNU recommendations) in at least one of the age patient groups studied.
Fig. 3.
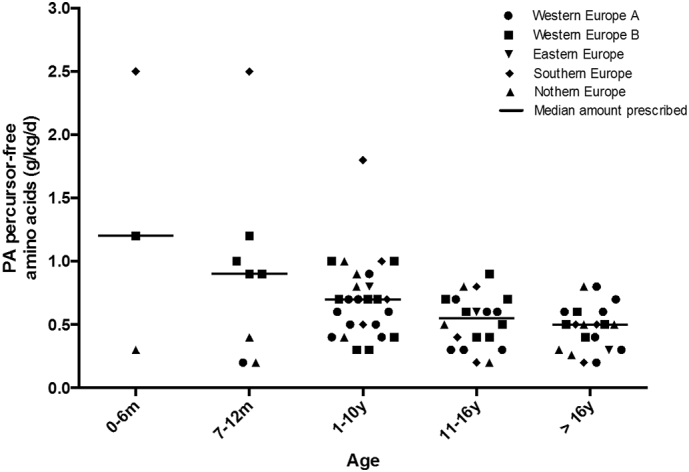
Mean PA precursor free amino acids prescription in PA patients by centre in each age range (n = 38 centres).
PFAA were prescribed in divided doses throughout the day mixed with water/fruit juice or administered via feeding tubes. Formula with carbohydrates, fats, vitamins and minerals was preferred in infancy and PFAA containing carbohydrates, vitamins and minerals in patients aged above 1y. No adherence data on PFAA intake was collected.
3.5. Nutritional support
Sixty-three per cent of patients (n = 117 patients) were given tube feeds. The majority (n = 86 patients) had a gastrostomy and 31 a nasogastric tube. Only 26 patients (22%) were on nocturnal feeds and tubes were used to administer drugs only in 2 patients. Data was not collected about enteral feed composition.
Low protein foods e.g. pasta, rice or biscuits were advocated by 87% (n = 41/47) of centres; and low protein milk was used by 72% (n = 34/47) of centres to replace cow's milk. Only 33% (n = 61/186) of patients were given energy supplements orally.
3.6. Monitoring
Regular assessment of weight, height and biochemical markers was consistent across centres, with infants and young children assessed more frequently. Sixty-four per cent (n = 30) of centres performed nutritional biochemistry including measurement of vitamin B12, plasma MMA, D, A and E, quantitative plasma amino acids, zinc, selenium, haemoglobin and ferritin. Essential fatty acid status was monitored by 67% (n = 31/46) of centres but infrequently. One centre (n = 1) did not answer this question.
3.7. Drug treatment
All centres (n = 46), except one who did not answer the question, prescribed l-carnitine. Laxatives were used to treat/prevent constipation in 41% (n = 19) of the centres. Ammonia scavengers were used to treat hyperammonaemia in 37% (n = 17) of centres. Antibiotics (e.g. metronidazole) were used to prevent bacterial overgrowth in 76% (n = 35) of centres.
4. Discussion
This European survey describes the dietary management of 186 patients with PA from 47 European centres on protein restricted diets prior to the publication of PA guidelines [5]. This is one of the largest PA patient cohort groups reported, although the median number of patients cared for in each centre was only 2. In this study, the majority of centres (74%) prescribed natural protein below WHO/FAO/UNU 2007 safe levels of protein intake [6] in at least one age group studied and with the exception of Northern Europe, practices were very consistent. Although we did not collect clinical data, previous cohort studies [10], showed little difference between clinical outcome and severity of protein restriction and the use of PFAA.
In PA, health professionals appear reluctant to increase natural protein to meet safe levels of protein intake without specific laboratory markers to help guide protein prescription. Instead they advocate very low natural protein intakes and this combined with frequent use of emergency regimens is likely to adversely affect nutritional status and metabolic stability. Very low natural protein diets may have an inappropriate protein:energy ratio and lead to inadequate weight gain [11], [12], [13], poor linear growth [11], [14], lower skeletal muscle mass and an increased weight for height [14]. It may also cause protein malnutrition, persistent catabolism leading to decreased tolerance of PFAA and consequential metabolic decompensations [15]. Isoleucine deficiency causing skin lesions and acrodermatitis enteropathica-like syndrome is not uncommon [16], [17], [18], [19], [20].
The type and balance of natural protein prescribed is an important factor to ensure minimum requirement of essential amino acids and the European PA guidelines recommended some high biological natural protein to ensure a balanced intake of amino acids [5]. Although high biological sources were used by the majority of centres, one quarter (23%, n = 11/47) used plant and cereal protein only which contain lower amounts of essential amino acids when compared with animal protein. An important consideration is the protein digestibility-corrected amino acid score (PDCAAS) (providing an index of availability of amino acids) and this is higher in whey protein, milk or egg (1.0) compared with only 0.59 in cereals and 0.5 in rice [21].
Although there is limited evidence to support the use of PFAA in PA [13], we report widespread and consistent use of PFAA by 81% of centres throughout the age groups studied, with just over half of centres prescribing PFAA giving 50% more protein than advocated by the WHO/FAO/UNU safe levels of protein intake [6]. Some centres gave extra PFAA to compensate for its lower PDCAA score. One centre provided up to 83% of the total protein intake from PFAA, mirroring dietary practices used in PKU and MSUD. Our results on PFAA intake replicate other cross sectional European studies [10], [12], [22], [23]. In PA, the role of high PFAA intake has been questioned [23]. Recent USA dietary management guidelines suggest PFAA combined with natural protein intake should not exceed 120% of total protein USA DRI requirements [24]. In our cohort, 31/38 centres had a median total protein ingested (natural protein plus PFAA) above 120% of WHO/FAO/UNU safe levels of protein intake in at least one age range [6]. Excess PFAA will provide an additional nitrogen load which may contribute to hyperammonaemia, a complication commonly reported in PA [25], [26], associated with N-acetylglutamate synthetase inactivation due to accumulation of propionyl CoA [27].
PFAA designed for PA (without valine, methionine, threonine and isoleucine) contain normal to increased amounts of leucine (e.g. 158 mg of leucine per g of protein equivalent from PFAA vs. approximately 100 mg of leucine per 1 g of protein from egg). Absorption and digestion of L-amino acids are more rapid than those that are protein bound and this may lead to transient amino acid imbalance [28]. In MMA, lower blood concentrations of valine, isoleucine and methionine have been reported in conjunction with the use of PFAA supplements [29]. Any imbalances in the ratio of amino acids particularly the ratio of leucine to the precursor amino acids (valine, isoleucine, threonine and methionine) may alter amino acid transport across the blood brain barrier potentially altering neurotransmitter biosynthesis [29]. However, even when leucine-containing PFAA are prescribed in PA, low plasma leucine concentrations are still reported [14], [23], [30].
Our patient cohort had high use of enteral tube feeding (63%) and this has been reported by others [12], [14], [23], [31], [32]. In PA, tube feeding is associated with improved nutritional status [11], reduced hospital admissions [11], and allows for ‘trouble-free’ administration of PFAA and medications [27]. However, only 22% of our tube fed cohort were given nocturnal feeds. This was unexpected as overnight tube feeding in PA is associated with suppression of propiogenic odd chain fatty acid production from lipolysis [33]. It is estimated that odd-chain fatty acids may contribute approximately 15 μmol/kg/h to propionate production in the fasting state, and overall 25% to propionate production [33], although this has only been studied in a small number of patients with PA and methylmalonic acidaemia, (n = 8).
This survey has several limitations. Some responses were unclear, although every answer was quality checked for any incorrect information. Data was collected about protein prescription practice rather than actual dietary intake. No quantitative information was collected about the type of natural protein consumed by patients, tube feed nutritional composition, clinical outcome, specific drug treatment, severity of disorder, and growth all of which may impact on decision making.
In conclusion, the dietary treatment of patients with PA is problematic as patients quickly and commonly develop metabolic decompensation, particularly in their early years. Due to the condition's rarity, dietitians have limited opportunity to develop expertise in its management. It is possible that natural protein may be excessively restricted with over-usage of PFAA. However, without reliable and direct biochemical markers, natural protein tolerance is difficult to determine with certainty. Due to patient instability, it is challenging to conduct randomized controlled trials to determine optimal dietary treatment. It may be that only multi-centre, prospective, longitudinal case controlled studies together with carefully maintained patient registers collecting data about protein and energy intakes, patient's fasting times as well as metabolic stability will provide the necessary data to achieve optimum dietary management.
Author's roles
All authors participated in data collection, critical revision of the paper and final approval of the version to be published. Anne Daly was involved in questionnaire development, data analysis and writing of the manuscript. Alex Pinto was involved in data quality assessment and development of the manuscript. Sharon Evans was involved in questionnaire design and manuscript development and Anita MacDonald was involved in questionnaire development, interpretation of data and writing of the manuscript.
Source of funding
There was no need for funding to develop this study.
Conflict of interest
Anne Daly has undertaken evaluation work for the nutritional companies – Vitaflo Ltd., Nutricia Ltd. and Metax. Alex Pinto has received an educational grant from Cambrooke Therapeutics and grants from Vitaflo, Merck Serono and Biomarin to attend scientific meetings. Anita MacDonald has received research funding and honoraria from Nutricia, Vitaflo International and Merck Serono. She is a member of the European Nutrition Expert Panel (Biomarin), member of Sapropterin Advisory Board (Biomarin), member of the Advisory Board entitled ELEMENT (Danone-Nutricia), and member of an Advisory Board for Arla and Applied Pharma Research. Allyson Terry has received research honoraria and congress travel allowances from Nutricia and Vitaflo International. Liesbeth van der Ploeg received financial support from Nutricia and Vitaflo to attend conferences. Annemiek van Wegberg received several grants from Nutricia and Vitaflo to visit conferences and received honoraria as a speaker from Excemed and Biomarin. Agnieszka Kowalik received financial support from Nutricia and Vitaflo to attend conferences. Gudrun Elise Kahrs have received support from Vitaflo and SHS/Nutricia to attend meetings. Sharon Evans is a research dietitian funded by Nutricia; financial support from Nutricia and Vitaflo to attend study days and conferences. Corrie Timmer received financial support from Nutricia and Vitaflo to attend conferences. Manuela Ferreira Almeida received grants from Glutamine, Nutricia, Merck Serono, Biomarin, Orphan and Lifediet to attend congress and for education. Júlio César Rocha is member of the European Nutrition Expert Panel (Biomarin) and member of an Advisory Board for Applied Pharma Research. Amaya Bélanger-Quintana, Martine Robert, Esther van Dam, Carina Heidenborg and Margreet. van Rijn are members of the European Nutrition Expert Panel (Biomarin). Fiona White has received honoria from Alexion, Nutricia Metabolics and Vitaflo as well as educational and travel grants from Nutricia Metabolics and Vitaflo. Marjorie Dixon has received research honoraria and congress travel allowances from Nutricia and Vitaflo International. Katharina Dokoupil is member of the European Nutrition Expert Panel (Biomarin) and member of an Advisory Board (Nestlé). She has received honoraria and symposia travel allowances from Nutricia and Vitaflo. Alice Dianin received financial support from Mevalia and DMF to attend conferences.
Acknowledgments
Acknowledgments
The authors would like to thank the following people for the assistance in data collection: Louise Robertson (University Hospitals Birmingham NHS Foundation Trust, UK); Sharan Lowry (Sheffield Children's Hospital, UK); Rychelle Winstone, Kate Stonstreet & Karen van Wyk (Evelina London Children's Hospital, Guy's and St Thomas' NHS Foundation Trust, London, UK); Charlotte Ellerton & Rachel Carruthers (Charles Dent Metabolic Unit National Hospital for Neurology and Surgery, London, UK); Isabelle Nedellec (CHU Angers); Skadi Beblo (Hospital of Children's & Adolescents, University of Leipzig, Germany); Anne-Kathrin Neugebauer (Heinrich-Heine-University, Department of General Pediatrics, Neonatology and Pediatric Cardiology, Dusseldorf); Marianne Diels (Metabolic Center, University Hospitals Leuven and KU Leuven, Belgium); Sophie Defouny (Hôpital Universitaire des Enfants, Reine fabiola, Bruxelles, Belgium); Esmeralda Martins & Anabela Bandeira (Unidade de Doenças Metabólicas, Centro Hospitalar do Porto - CHP, Porto, Portugal); Elisa Leão Teles & Esmeralda Rodrigues (Centro Hospitalar São João — Unidade de Doenças Metabólicas, Porto, Portugal); Mercedes Martinez-Pardo (Unidad de Enfermedades Metabolicas, Servicio de Pediatria, Hospital Ramon y Cajal Madrid, Spain); Jolanta Sykut-Cegielska & Joanna Taybert (Institute of Mother & Child, Poland) and Camilla Caroe & Ann Roskjaer (National University Hospital, Copenhagen, Denmark). We thank the Association “La Vita e’ un Dono” for supporting the fellowship of Giorgia Gallo. We also acknowledge the regional centre for Newborn Screening, Diagnosis and Treatment of Inherited Metabolic Diseases; Ministerial endorsement for the partecipation in the european network for inherited metabolic disorders metabERN.
This article was supported by ERDF through the operation POCI-01-0145-FEDER-007746 funded by the Programa Operacional Competitividade e Internacionalização – COMPETE2020 and by National Funds through FCT - Fundação para a Ciência e a Tecnologia within CINTESIS, R&D Unit (reference UID/IC/4255/2013).
References
- 1.Nizon M., Ottolenghi C., Valayannopoulos V., Arnoux J.B., Barbier V., Habarou F., Desguerre I., Boddaert N., Bonnefont J.P., Acquaviva C., Benoist J.F., Rabier D., Touati G., de Lonlay P. Long-term neurological outcome of a cohort of 80 patients with classical organic acidurias. Orphanet J. Rare Dis. 2013;8:148. doi: 10.1186/1750-1172-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolker S., Garcia-Cazorla A., Valayannopoulos V., Lund A.M., Burlina A.B., Sykut-Cegielska J., Wijburg F.A., Teles E.L., Zeman J., Dionisi-Vici C., Baric I., Karall D., Augoustides-Savvopoulou P., Aksglaede L., Arnoux J.B., Avram P., Baumgartner M.R., Blasco-Alonso J., Chabrol B., Chakrapani A., Chapman K., EC I.S., Couce M.L., de Meirleir L., Dobbelaere D., Dvorakova V., Furlan F., Gleich F., Gradowska W., Grunewald S., Jalan A., Haberle J., Haege G., Lachmann R., Laemmle A., Langereis E., de Lonlay P., Martinelli D., Matsumoto S., Muhlhausen C., de Baulny H.O., Ortez C., Pena-Quintana L., Ramadza D.P., Rodrigues E., Scholl-Burgi S., Sokal E., Staufner C., Summar M.L., Thompson N., Vara R., Pinera I.V., Walter J.H., Williams M., Burgard P. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation. J. Inherit. Metab. Dis. 2015;38:1041–1057. doi: 10.1007/s10545-015-9839-3. [DOI] [PubMed] [Google Scholar]
- 3.Childs B., Nyhan W.L., Borden M., Bard L., Cooke R.E. Idiopathic hyperglycinemia and hyperglycinuria: a new disorder of amino acid metabolism. I. Pediatrics. 1961;27:522–538. [PubMed] [Google Scholar]
- 4.Leonard J.V. Stable isotope studies in propionic and methylmalonic acidaemia. Eur. J. Pediatr. 1997;156:S67–S69. doi: 10.1007/pl00014275. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner M.R., Horster F., Dionisi-Vici C., Haliloglu G., Karall D., Chapman K.A., Huemer M., Hochuli M., Assoun M., Ballhausen D., Burlina A., Fowler B., Grunert S.C., Grunewald S., Honzik T., Merinero B., Perez-Cerda C., Scholl-Burgi S., Skovby F., Wijburg F., MacDonald A., Martinelli D., Sass J.O., Valayannopoulos V., Chakrapani A. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J. Rare Dis. 2014;9:130. doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Technical Report Series. 2007. Protein and amino acid requirements in human nutrition; pp. 1–265. (back cover) [PubMed] [Google Scholar]
- 7.Aguiar A., Ahring K., Almeida M.F., Assoun M., Belanger Quintana A., Bigot S., Bihet G., Blom Malmberg K., Burlina A., Bushueva T., Caris A., Chan H., Clark A., Clark S., Cochrane B., Corthouts K., Dalmau J., Dassy M., De Meyer A., Didycz B., Diels M., Dokupil K., Dubois S., Eftring K., Ekengren J., Ellerton C., Evans S., Faria A., Fischer A., Ford S., Freisinger P., Gizewska M., Gokmen-Ozel H., Gribben J., Gunden F., Heddrich-Ellerbrok M., Heiber S., Heidenborg C., Jankowski C., Janssen-Regelink R., Jones I., Jonkers C., Joerg-Streller M., Kaalund-Hansen K., Kiss E., Lammardo A.M., Lang K., Lier D., Lilje R., Lowry S., Luyten K., MacDonald A., Meyer U., Moor D., Pal A., Robert M., Robertson L., Rocha J.C., Rohde C., Ross K., Saruhan S., Sjoqvist E., Skeath R., Stoelen L., Ter Horst N.M., Terry A., Timmer C., Tuncer N., Vande Kerckhove K., van der Ploeg L., van Rijn M., van Spronsen F.J., van Teeffelen-Heithoff A., van Wegberg A., van Wyk K., Vasconcelos C., Vitoria I., Wildgoose J., Webster D., White F.J., Zweers H. Practices in prescribing protein substitutes for PKU in Europe: no uniformity of approach. Mol. Genet. Metab. 2015;115:17–22. doi: 10.1016/j.ymgme.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Pinto A., Daly A., Evans S., Almeida M.F., Assoun M., Belanger-Quintana A., Bernabei S., Bollhalder S., Cassiman D., Champion H., Chan H., Dalmau J., de Boer F., de Laet C., de Meyer A., Desloovere A., Dianin A., Dixon M., Dokoupil K., Dubois S., Eyskens F., Faria A., Fasan I., Favre E., Feillet F., Fekete A., Gallo G., Gingell C., Gribben J., Kaalund-Hansen K., Horst N., Jankowski C., Janssen-Regelink R., Jones I., Jouault C., Kahrs G.E., Kok I.L., Kowalik A., Laguerre C., Le Verge S., Lilje R., Maddalon C., Mayr D., Meyer U., Micciche A., Robert M., Rocha J.C., Rogozinski H., Rohde C., Ross K., Saruggia I., Schlune A., Singleton K., Sjoqvist E., Stolen L.H., Terry A., Timmer C., Tomlinson L., Tooke A., Vande Kerckhove K., van Dam E., van den Hurk T., van der Ploeg L., van Driessche M., van Rijn M., van Teeffelen-Heithoff A., van Wegberg A., Vasconcelos C., Vestergaard H., Vitoria I., Webster D., White F.J., White L., Zweers H., MacDonald A. Dietary practices in isovaleric acidemia: a European survey. Mol. Genet. Metab. Rep. 2017;12:16–22. doi: 10.1016/j.ymgmr.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adam S., Almeida M.F., Assoun M., Baruteau J., Bernabei S.M., Bigot S., Champion H., Daly A., Dassy M., Dawson S., Dixon M., Dokoupil K., Dubois S., Dunlop C., Evans S., Eyskens F., Faria A., Favre E., Ferguson C., Goncalves C., Gribben J., Heddrich-Ellerbrok M., Jankowski C., Janssen-Regelink R., Jouault C., Laguerre C., Le Verge S., Link R., Lowry S., Luyten K., Macdonald A., Maritz C., McDowell S., Meyer U., Micciche A., Robert M., Robertson L.V., Rocha J.C., Rohde C., Saruggia I., Sjoqvist E., Stafford J., Terry A., Thom R., Vande Kerckhove K., van Rijn M., van Teeffelen-Heithoff A., Wegberg A., van Wyk K., Vasconcelos C., Vestergaard H., Webster D., White F.J., Wildgoose J., Zweers H. Dietary management of urea cycle disorders: European practice. Mol. Genet. Metab. 2013;110:439–445. doi: 10.1016/j.ymgme.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Sass J.O., Hofmann M., Skladal D., Mayatepek E., Schwahn B., Sperl W. Propionic acidemia revisited: a workshop report. Clin. Pediatr. 2004;43:837–843. doi: 10.1177/000992280404300908. [DOI] [PubMed] [Google Scholar]
- 11.Rafique M. Propionic acidaemia: demographic characteristics and complications. J. Pediatr. Endocrinol. Metab. 2013;26:497–501. doi: 10.1515/jpem-2013-0031. [DOI] [PubMed] [Google Scholar]
- 12.van der Meer S.B., Poggi F., Spada M., Bonnefont J.P., Ogier H., Hubert P., Depondt E., Rapoport D., Rabier D., Charpentier C., Parvy P., Bardet J., Kamoun P., Saudubray J.M. Clinical outcome and long-term management of 17 patients with propionic acidaemia. Eur. J. Pediatr. 1996;155:205–210. doi: 10.1007/BF01953939. [DOI] [PubMed] [Google Scholar]
- 13.Yannicelli S., Acosta P.B., Velazquez A., Bock H.G., Marriage B., Kurczynski T.W., Miller M., Korson M., Steiner R.D., Rutledge L., Bernstein L., Chinsky J., Galvin-Parton P., Arnold G.L. Improved growth and nutrition status in children with methylmalonic or propionic acidemia fed an elemental medical food. Mol. Genet. Metab. 2003;80:181–188. doi: 10.1016/j.ymgme.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Daly A., Evans S., Gerrard A., Santra S., Vijay S., MacDonald A. The nutritional intake of patients with organic acidaemias on enteral tube feeding: can we do better? JIMD Rep. 2016;28:29–39. doi: 10.1007/8904_2015_443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burlina A., Cazzorla C., Zanonato E., Viggiano E., Fasan I., Polo G. Clinical experience with N-carbamylglutamate in a single-centre cohort of patients with propionic and methylmalonic aciduria. Mol. Genet. Metab. Rep. 2016;8:34–40. doi: 10.1016/j.ymgmr.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosch A.M., Sillevis Smitt J.H., Van Gennip A.H., Abeling N.G., Schutgens R.B., Bakker H.D., Wijburg F.A. Iatrogenic isolated isoleucine deficiency as the cause of an acrodermatitis enteropathica-like syndrome. Br. J. Dermatol. 1998;139:488–491. doi: 10.1046/j.1365-2133.1998.02415.x. [DOI] [PubMed] [Google Scholar]
- 17.De Raeve L., De Meirleir L., Ramet J., Vandenplas Y., Gerlo E. Acrodermatitis enteropathica-like cutaneous lesions in organic aciduria. J. Pediatr. 1994;124:416–420. doi: 10.1016/s0022-3476(94)70364-7. [DOI] [PubMed] [Google Scholar]
- 18.Lane T.N., Spraker M.K., Parker S.S. Propionic acidemia manifesting with low isoleucine generalized exfoliative dermatosis. Pediatr. Dermatol. 2007;24:508–510. doi: 10.1111/j.1525-1470.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 19.Ozturk Y. Acrodermatitis enteropathica-like syndrome secondary to branched-chain amino acid deficiency in inborn errors of metabolism. Pediatr. Dermatol. 2008;25:415. doi: 10.1111/j.1525-1470.2008.00707.x. [DOI] [PubMed] [Google Scholar]
- 20.Tabanlioglu D., Ersoy-Evans S., Karaduman A. Acrodermatitis enteropathica-like eruption in metabolic disorders: acrodermatitis dysmetabolica is proposed as a better term. Pediatr. Dermatol. 2009;26:150–154. doi: 10.1111/j.1525-1470.2008.00803.x. [DOI] [PubMed] [Google Scholar]
- 21.Food, Nations AOotU, Organization WH . Food and Agriculture Organization of the United Nations; December 1989. Protein Quality Evaluation: Report of the Joint FAO/WHO Expert Consultation, Bethesda, Md., USA, 4-8; p. 1991. [Google Scholar]
- 22.Heaney R.P., Layman D.K. Amount and type of protein influences bone health. Am. J. Clin. Nutr. 2008;87:1567s–1570s. doi: 10.1093/ajcn/87.5.1567S. [DOI] [PubMed] [Google Scholar]
- 23.Touati G., Valayannopoulos V., Mention K., de Lonlay P., Jouvet P., Depondt E., Assoun M., Souberbielle J.C., Rabier D., Ogier de Baulny H., Saudubray J.M. Methylmalonic and propionic acidurias: management without or with a few supplements of specific amino acid mixture. J. Inherit. Metab. Dis. 2006;29:288–298. doi: 10.1007/s10545-006-0351-7. [DOI] [PubMed] [Google Scholar]
- 24.PROP Nutrition Management Guidelines. 2017. [Google Scholar]
- 25.McCrory N.M., Edick M.J., Ahmad A., Lipinski S., Scott Schwoerer J.A., Zhai S., Justice K., Cameron C.A., Berry S.A., Pena L.D. Comparison of methods of initial ascertainment in 58 cases of propionic acidemia enrolled in the inborn errors of metabolism information system reveals significant differences in time to evaluation and symptoms at presentation. J. Pediatr. 2017;180(200–205):e208. doi: 10.1016/j.jpeds.2016.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valayannopoulos V., Baruteau J., Delgado M.B., Cano A., Couce M.L., Del Toro M., Donati M.A., Garcia-Cazorla A., Gil-Ortega D., Gomez-de Quero P., Guffon N., Hofstede F.C., Kalkan-Ucar S., Coker M., Lama-More R., Martinez-Pardo Casanova M., Molina A., Pichard S., Papadia F., Rosello P., Plisson C., Le Mouhaer J., Chakrapani A. Carglumic acid enhances rapid ammonia detoxification in classical organic acidurias with a favourable risk-benefit profile: a retrospective observational study. Orphanet J. Rare Dis. 2016;11:32. doi: 10.1186/s13023-016-0406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Hernandez E., Quijada-Fraile P., Oliveros-Leal L., Garcia-Silva M., Perez-Cerda C., Baro-Fernandez M., Perez-Alonso V., Vivanco J. Nutritional and pharmacological management during chemotherapy in a patient with propionic acidaemia and rhabdomyosarcoma botryoides. JIMD Rep. 2012;6:73–78. doi: 10.1007/8904_2012_137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 29.Manoli I., Myles J.G. A critical reappraisal of dietary practices in methylmalonic acidemia raises concerns about the safety of medical foods. Part 1: isolated methylmalonic acidemias. 2016;18:386–395. doi: 10.1038/gim.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholl-Burgi S., Sass J.O., Zschocke J., Karall D. Amino acid metabolism in patients with propionic acidaemia. J. Inherit. Metab. Dis. 2012;35:65–70. doi: 10.1007/s10545-010-9245-9. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Hernandez E., Lee P.J., Micciche A., Grunewald S., Lachmann R.H. Long-term needs of adult patients with organic acidaemias: outcome and prognostic factors. J. Inherit. Metab. Dis. 2009;32:523–533. doi: 10.1007/s10545-009-1191-12. [DOI] [PubMed] [Google Scholar]
- 32.Shchelochkov O.A., Carrillo N., Venditti C. Propionic acidemia. In: Pagon R.A., Adam M.P., Ardinger H.H., Wallace S.E., Amemiya A., LJH Bean, Bird T.D., Ledbetter N., Mefford H.C., RJH Smith, Stephens K., editors. GeneReviews(R) University of Washington, SeattleUniversity of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle; Seattle (WA): 1993. All rights reserved. [Google Scholar]
- 33.Thompson G.N., Chalmers R.A. Increased urinary metabolite excretion during fasting in disorders of propionate metabolism. Pediatr. Res. 1990;27:413–416. doi: 10.1203/00006450-199004000-00021. [DOI] [PubMed] [Google Scholar]


