Abstract
Cardiac fibroblasts, which are abundant in heart tissue, are involved not only in extracellular matrix homeostasis and repair, but also in cardiac remodeling after a myocardial infarction that, in turn, can lead to loss of cardiac function and heart failure. Ca2+ signaling is functionally important in many cell types, but the roles of fibroblast signaling and inflammation in the pathogenesis of heart disease are unclear. Here, we tested the hypothesis that inflammatory activation affects cardiac fibroblasts, both in terms of Ca2+ signaling and their capacity for intercellular communication through the gap junction channel protein connexin 43 (Cx43). We examined Ca2+ responses induced by known modulators of cardiac function such as glutamate, ATP and 5-hydroxytryptamine (5-HT) in human cardiac fibroblasts, under normal and inflammatory conditions. We showed that activation of human cardiac fibroblasts by lipopolysaccharide (LPS) for 24 h altered Ca2+ signaling, increased TLR4 and decreased Cx43 expression. In the fibroblasts, LPS treatment increased glutamate-evoked and decreased 5-HT-evoked Ca2+ signals. LPS activation also induced increased secretion of glutamate and proinflammatory cytokines from these cells. In summary, we propose that inflammatory stimuli can affect intracellular Ca2+ release, Cx43 expression, glutamate release and cytokine secretion in human cardiac fibroblasts. Inflammatory conditions may, therefore, impair intercellular network communication between fibroblasts and cardiomyocytes potentially contributing to cardiac dysfunction.
Keywords: Health sciences, Internal medicine, Medicine, Biochemistry, Biological sciences, Cell biology, Immunology, Cardiology
1. Introduction
Cardiac fibroblasts are abundant in heart tissue and are involved not only in extracellular matrix homeostasis and repair, but also in cardiac remodeling after myocardial infarction that, in turn, can lead to loss of cardiac function and heart failure [1]. Activated cardiac fibroblasts have been implicated in cardiac dysfunction, as mediators of the inflammatory response after myocardial infarction [2]. Activated fibroblasts have also been described as inflammatory supporter cells that can induce heart failure [3]. Mechanical coupling of electrical conduction between fibroblasts and cardiomyocytes was shown to be important for cellular communication and cardiac function [4]. Cardiac fibroblasts can communicate by intercellular Ca2+ signaling through gap junction channel proteins [5]. Connexin 43 (Cx43) is the most abundant gap junction protein in the myocardium [6]. Ca2+ signaling, evoked by ATP, histamine, 5-hydroxytryptamine (5-HT) or glutamate, involves Ca2+ release from the endoplasmatic reticulum [7]. Our previous results shows that intracellular calcium are released from endoplasmic reticulum [8, 9]. Modulation of intracellular Ca2+ is an important parameter because it can influence many cellular functions, including extracellular matrix synthesis and degradation [10]. Increased cytosolic Ca2+ levels can lead to release of signaling molecules, including transmitters, cytokines, prostaglandins, proteins and peptides [11]. Cytosolic Ca2+ is a key second messenger and the control of Ca2+ signaling is, therefore, critical. Elevated Ca2+ levels occur in cellular networks during inflammation, depending on the production and release of ATP through hemi-channel openings in the plasma membrane. This extracellular Ca2+ signaling attenuates intercellular Ca2+ signaling, decreasing communication via gap junctions [12].
The Na+-Ca2+ exchanger, a Ca2+ transporter controlling intracellular Ca2+ concentrations, is driven by the Na+ electrochemical gradient across the plasma membrane. Thus, the Na+ pump, Na+/K+-ATPase, can indirectly modulate Ca2+ signaling [13] and inflammatory stimuli can influence Ca2+ homeostasis in cellular networks [14, 15, 16, 17]. Na+/K+-ATPase is downregulated by inflammatory stimuli [18]. Endogenous glutamate is released by cells, promotes signaling via glutamate receptors and has important functions under both normal and pathological conditions. Extracellular glutamate concentrations at excitatory synapses are regulated by sodium dependent excitatory amino acid transporters (EAATs), including the glutamate/aspartate transporter (GLAST) and glutamate transporter-1 (GLT-1) [19]. A fine-tuned regulation of clearance and maintenance of extracellular glutamate is important for normal metabolism in neurons and impairments of this regulation can lead to excitotoxicity [19]. Increased glutamate concentrations were also reported in synovial fluid from patients with active arthropathies [20] and in serum from patients with fibromyalgia [21]. Cytoskeletal reorganization is pivotal in many cellular processes, especially during inflammation. Dynamic remodeling of the actin cytoskeleton is essential for migration and proliferation of cells [22]. Lipopolysaccharide (LPS) exposure can be used experimentally to generate an inflammatory response, both in vitro and in vivo.
The purpose of our study was to examine effects of inflammatory stimuli on human cardiac fibroblasts, including on intracellular Ca2+ release and Cx43 levels. We also investigated proinflammatory cytokine profiles and glutamate release in these activated fibroblasts.
2. Material and methods
2.1. Cell culture
Human primary cardiac fibroblasts from heart ventricle were from PromoCell GmbH (C-29910, Heidelberg, Germany) and cultured in PromoCell fibroblast growth medium. Cells were seeded at a density of 5000 cells/cm2 and cultured according to the manufacturer's instructions for 10 days until they reached confluency. Cells were stained with anti-discoidin domain receptor 2 (DDR2) antibody (ab63337, Abcam, Cambridge, UK), with antibody against α-smooth muscle actin (α-SMA) (C6198, Sigma-Aldrich, St Louis, MO), phalloidin, a high-affinity F-actin probe (Sigma-Aldrich) and 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) (Hoescht33258, Sigma-Aldrich). Antibody staining was examined with a Zeiss Axioplan 2 Imaging microscope and cells expressed the fibroblast marker discoidin domain receptor 2 and stained with phalloidin, an F-actin marker (Fig. 1). To mimic effects of inflammation, cells were incubated with LPS 10 ng/ml, this concentration was selected according to our earlier results [18], and control cells incubated without LPS, for 24 h. Medium was then collected and cells were harvested. These samples were immediately frozen at −80° and stored at that temperature until analysis.
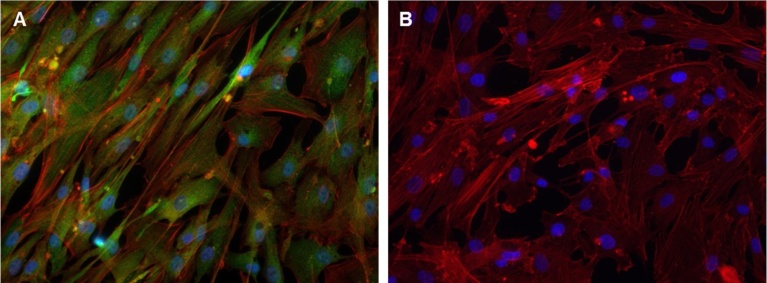
Fig. 1.
Immunocytochemical staining of human cardiac fibroblasts. A) Cells cultured for 10 d and then stained with Discoidin Domain Receptor 2 (DDR2) antibody (green) and counterstained with Alexa™488-conjugated (red) phalloidin probe. B) Isotype control, cells stained with isotype control antibody for DDR2 (green) and phalloidin probe (red). The nuclei, visualized with DAPI (blue).
2.2. Analysis of gene expression
Expression of human CD14 mRNA was determined using quantitative real-time PCR.Total RNA was extracted from cultured human cardiac fibroblasts using RNeasy Kit (Qiagen). The reverse transcription reactions were performed with a cDNA reverse transcription kit (#4368814, Applied Biosystems) using a PCR system (Gene Amp 9700, Applied Biosystems). Real-time PCR amplification was set up using TaqMan gene expression assays for human CD14 (Hs02621496_s1), human HPRT1 (Hs99999909_m1) in combination with Universal PCR master mix (#4324018, Applied Biosystems) and performed for 40 cycles on an ABI Prism 7900HT sequence detection system (Applied Biosystems). We analyzed PCR data using the comparative CT method [23]. The relative quantification of target gene mRNA expression levels were normalized to HPRT1 mRNA expression.
2.3. Cytokine analyses
Levels of cytokines were measured in cell culture medium using a multiplex electrochemiluminescence immunoassay (ELISA) (Meso Scale Discovery (MSD), Rockville, MD, USA). The human V-PLEX Proinflammatory Panel 1, including interferon-γ (IFN-γ), interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13 and tumor necrosis factor- α (TNF-α) was used for these assays, following the manufacturer’s recommendations. Results were read on a QuickPlex SQ120 plate reader (Meso Scale Discovery). The cytokine concentration in fibroblast culture medium of the control cells was set to 100% for each experiment.
2.4. Ca2+ assay
FLIPR Calcium 6 Assay Kit (#R8194 Molecular Devices, LLC, Sunnyvale, CA, USA) was used to detect changes in intracellular Ca2+. Human cardiac fibroblasts were incubated for 1 h at 37° C with the Ca2+ sensitive fluorescent indicator dye, FLIPR Calcium 6, 100 μl dye was added to 100 μl media in 96-well black cell culture plates. All measurements were performed for 120 sec on the FlexStation 3 Multi-Mode Microplate Reader with integrated fluid transfer (Molecular Devices). Human cardiac fibroblasts were incubated with the Ca2+ sensitive fluorescent indicator dye, FLIPR Calcium 6. Where indicated, 5-HT (10−5 M), ATP (10−4 M) or glutamate (10−4 M) (Sigma-Aldrich) were added at the beginning of the experiment in the concentrations that we described previously to give optimal Ca2+ release [9, 18, 24, 25]. The total area under the curve (AUC), reflecting the amount of Ca2+ released, was determined to measure the strength of the Ca2+ responses. The amplitude was expressed as the maximum peak value.
Dose response experiments in cultured cardiac fibroblasts were performed for ATP in the concentration range 10−6 to 10−3 M. The results showed that ATP evoked Ca2+ responses in fibroblasts for all concentrations used, ATP 10−4 M was selected for further experiments (Table 1).
Table 1.
Intracellular Ca2+ changes in ATP activated human cardiac fibroblasts.
| ATP concentration (M) | 10−6 | 10−5 | 10−4 | 10−3 |
|---|---|---|---|---|
| Mean (AUC) | 152,6 | 854,1 | 3320 | 4466 |
| Std. Deviation | 57,82 | 187,1 | 418,5 | 1693 |
| Std. Error of Mean | 33,38 | 108 | 241,6 | 977,4 |
| Lower 95% CI of mean | 8,959 | 389,4 | 2281 | 260,9 |
| Upper 95% CI of mean | 296,2 | 1319 | 4360 | 8672 |
Area under the Ca2+ curve (AUC) was calculated, (n = 3).
2.5. Analysis of protein expression
Western blot analyses were performed according to standard protocols. Briefly, protein extracts were prepared by cell lysis in RIPA buffer (Sigma-Aldrich) supplemented with a mammalian protease inhibitor cocktail (Sigma-Aldrich). Protein concentrations were determined using the Pierce BCA protein assay kit (Life Technologies Carlsbad, CA, USA), according to the manufacturer’s instructions. Equal amounts of extracts (5 μg protein each) were resolved on 4–12% Bis-Tris precast gels (Life Technologies) and bands transferred onto nitrocellulose membranes (GE Healthcare, Uppsala, Sweden). Equal loading and transfer of proteins was confirmed by Ponceau staining (0.1% in acetic acid, Sigma-Aldrich). The membranes were probed with the following primary antibodies: polyclonal rabbit anti-TLR-4 (M-300) (sc-30002, Santa Cruz, Biotech Inc, Dallas, TX, USA), polyclonal rabbit anti-GLT-1 (pab0037, Covalab, Villeurbanne, France), polyclonal rabbit anti-GLAST (pab0036-P, Covalab), polyclonal rabbit anti-Cx43 (71–0700, Life Technologies), monoclonal mouse anti-Na+/K+-ATPase (A276, Sigma-Aldrich), and mouse monoclonal anti-β-actin (A5441, Sigma-Aldrich). The membranes were then probed with horseradish peroxidase conjugated secondary antibodies (JacksonImmuno Research Europe Ltd, Suffolk, UK). Protein bands were detected using Immobilon Western Chemiluminescent HRP substrate (Millipore Corp., Billerica, MA, USA) and a ChemiDoc XRS+ instrument (Bio-Rad, Hercules, CA, USA). The relative intensities of protein bands, in the linear exposure range, were quantified using ImageLab software (Bio-Rad). The intensity of the control protein band was set to 100% for each experiment.
2.6. Glutamate levels in medium
The concentration of glutamate (μmol/l) was measured in the culture medium using the ninhydrin reagent for photometric determination. Duplicate samples from culture medium, from stimulated and unstimulated cells, were analyzed and normalized for protein concentrations. The assay detection limit for glutamate was 5 μmol/l [26].
2.7. Statistics
Data were presented as means and SEM, unless stated otherwise. Levels of significance for differences between group means were determined with Student's t-test. GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA, www.graphpad.com) was used for all statistical calculations. P values < 0.05 (two-sided) were considered statistically significant.
3. Results
3.1. Characterization of human cardiac fibroblasts
Human cardiac fibroblasts were cultured for 10 d then stained with an antibody against discoidin domain receptor 2 (DDR2) and the Alexa™488-conjugated phalloidin probe. Almost all cells expressed the fibroblast marker DDR2 and F-actin filaments (Fig. 1A). No staining was observed in cells stained with isotype control antibody for DDR2 (Fig. 1B).
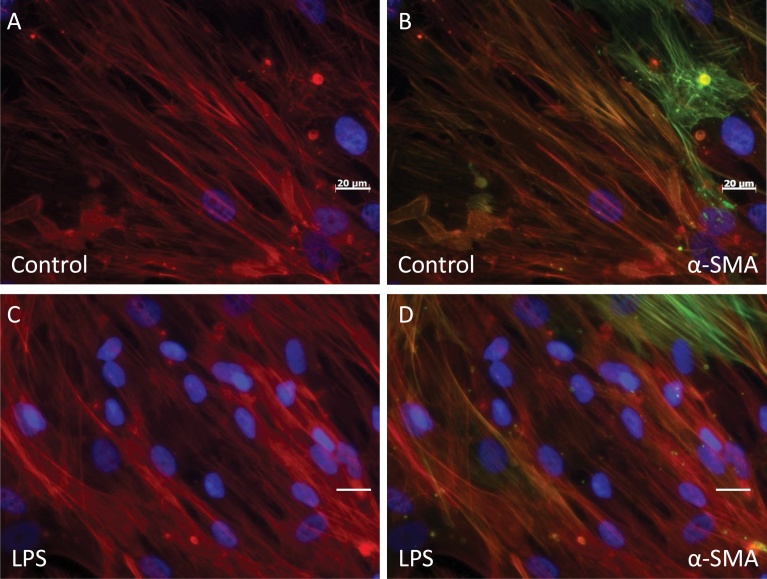
Both unstimulated cells and those stimulated with LPS for 24 h were F-actin positive and demonstrated α-smooth muscle actin (α-SMA) staining in a small number of cells (Fig. 2). Unstimulated cells stained with Alexa™488-conjugated phalloidin probe had F-actin organized into stress fibers (Fig. 2A and B). After incubation with LPS for 24 h, the actin filaments were organized more diffusely (Fig. 2C and D). Staining with the myofibroblast marker α-SMA was unchanged after LPS stimulation (Fig. 2C and D).
Fig. 2.
Staining of actin filaments in inflammatory activated human cardiac fibroblasts. A and B) Unstimulated cultured human cardiac fibroblasts without LPS; C and D) cells stimulated with LPS (10 ng/ml) for 24 h; were stained with Alexa™488-conjugated (red) phalloidin probe; and B and D stained with antibody against α-SMA (green). The nuclei, visualized with DAPI staining (blue).
3.2. Increased expression of CD14 in LPS activated human cardiac fibroblasts
To study whether LPS affects the LPS receptor CD14 in cardiac fibroblasts or not, we cultured human cardiac fibroblasts with and without LPS for 24 h. LPS activation resulted in 1.5-fold increase of CD14mRNA expression (Fig. 3).
Fig. 3.
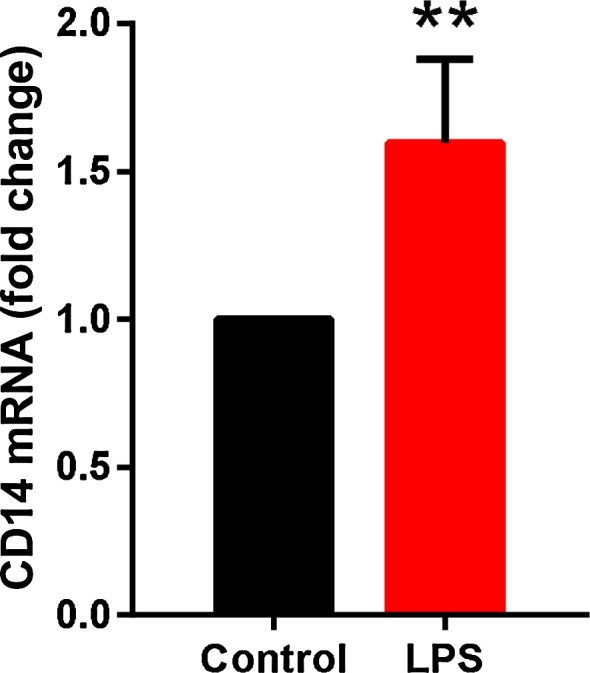
Increased expression of CD14 in inflammatory activated human cardiac fibroblasts. CD14 mRNA expression (relative to the control HPRT1) in unstimulated (control) and LPS stimulated human cardiac fibroblasts analyzed by real-time PCR amplification (n = 3), paired Student’s t-test was used to compare unstimulated and LPS stimulated cells, **P < 0.01.
3.3. Increased cytokine levels released by human primary cardiac fibroblasts cultured under inflammatory conditions
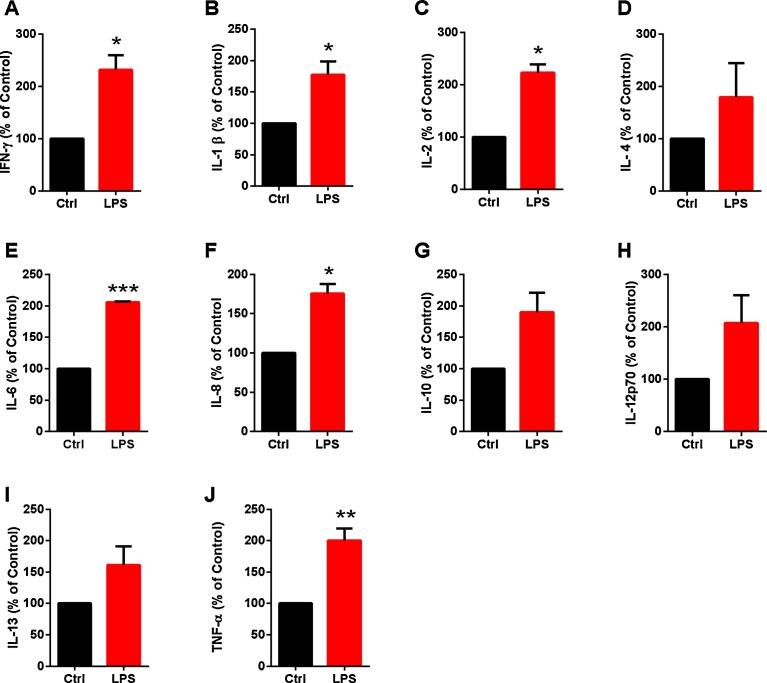
To investigate whether the upregulated CD14 mRNA expression was paralleled by increased cytokine production, we analyzed the effect of LPS on cytokine concentrations in cell culture medium from human cardiac fibroblasts. Levels of IFN-γ, IL-1β, IL-2, IL-6, IL-8, and TNF-α cytokines were increased in fibroblasts after LPS incubation, compared with in cells incubated under normal conditions (Fig. 4). No significant changes in IL-4, IL-10, IL-12p70 or IL-13 levels were observed (Fig. 4).
Fig. 4.
Inflammatory activation increased cytokine production in human cardiac fibroblasts. Concentrations of cytokines were measured with ELISA in culture supernatants of unstimulated and LPS stimulated human cardiac fibroblasts, compared with total amounts of cellular protein. There were significantly higher levels of the following: A) IFN-γ, B) IL-1β, C) IL-2, E) IL-6, F) IL-8, and J) TNF-α in medium from LPS stimulated cells, compared with that from unstimulated control cells. No significant changes were observed for: D) IL–4 G) IL-10, H) IL-12 p70 or I) IL-13. For statistical analysis, a paired Student’s t-test was used to compare unstimulated and LPS stimulated cells (n = 3), *P < 0.05, **P < 0.01, ***P < 0.001.
3.4. Intracellular Ca2+ changes in activated human cardiac fibroblasts
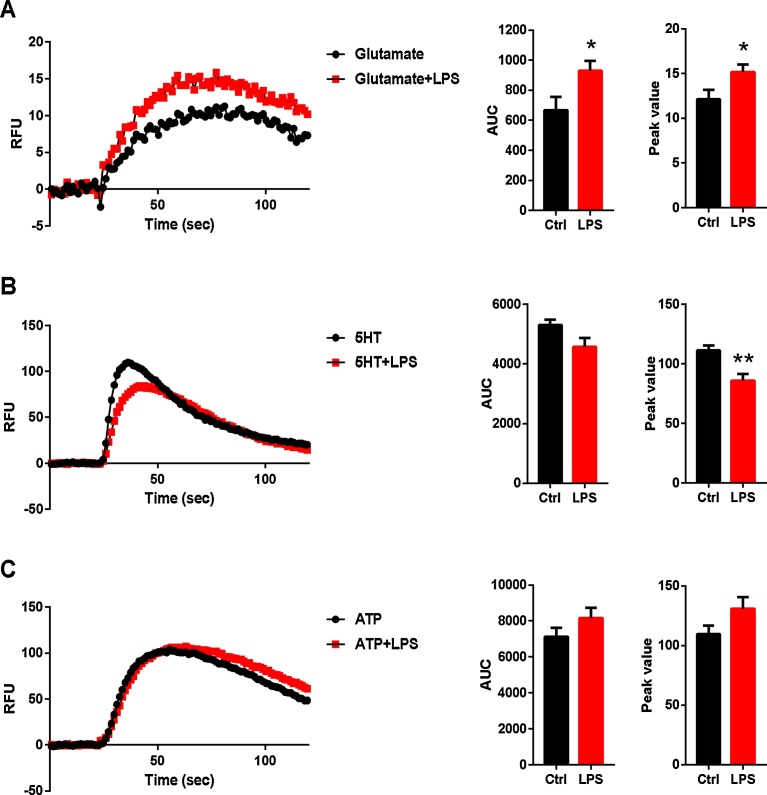
Human cardiac fibroblasts cultured for 10 d and then treated with and without LPS for 24 h, were analysed in Ca2+ release experiments. Area under the Ca2+ curve (AUC) and peak values were calculated to quantify the intracellular Ca2+ concentration (Fig. 5). Glutamate induced increased Ca2+ signaling in cardiac fibroblasts and this was significantly higher in cells stimulated with LPS than in unstimulated controls (Fig. 5A). Stimulation with 5-HT led to significantly decreased peak values in Ca2+ signaling in cardiac fibroblasts stimulated with LPS, compared with in unstimulated controls (Fig. 5B). No significant changes were observed after ATP stimulation (Fig. 5C).
Fig. 5.
Time-dependent changes in intracellular Ca2+. Human cardiac fibroblasts were stimulated, in a fluorescence-based assay for detecting changes in intracellular Ca2+ over time, with the following: A) glutamate (10−4 M), B) 5-HT (10−5 M) or C) ATP (10−4 M). AUC and peak values of Ca2+ transients are shown. The cells were obtained from 4 experiments. A) Ca2+ responses to glutamate and B) 5-HT in cardiac fibroblasts, when stimulated with LPS (10 ng/ml) for 24 h; unstimulated cells were used as controls. Results from a typical experiment are shown and the AUC, reflecting the amount of Ca2+ released, was analyzed to measure strength of each Ca2+ response. The amplitude is expressed as the maximum peak value. For statistical analysis, a paired Student’s t-test was used to compare unstimulated and LPS stimulated cells (n = 4), *P < 0.05, **P < 0.01.
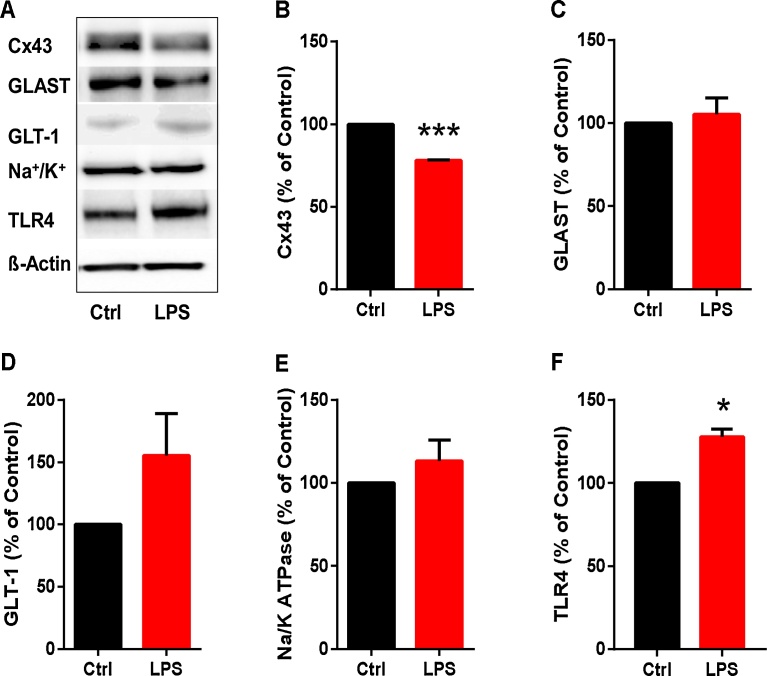
3.5. Effects of LPS treatment on Cx43, GLAST, GLT-1, Na+/K+-ATPase and TLR4 expression in human cardiac fibroblasts
To investigate whether inflammatory activation affected expression of certain relevant proteins in cardiac fibroblasts, we incubated the cells with and without LPS for 24 h. The blots are each representative of four different experiments. Immunoblotting pictures and the mean intensities and standard deviations for four experiments are shown in Fig. 6A–F. By immunoblotting, there were decreased levels of Cx43 protein and increased levels of TLR4 in human cardiac fibroblasts incubated with LPS, compared with in control cells (Fig. 6B and C). No significant differences were found between inflammatory activated cardiac fibroblasts and controls in protein expression of glutamate transporters, GLAST and GLT-1 and Na+/K+-ATPase (Fig. 6C–E).
Fig. 6.
Decreased Cx43 and increased TLR4 expression in LPS stimulated human cardiac fibroblasts. Human cardiac fibroblasts were treated with LPS (10 ng/ml) for 24 h and unstimulated cells were used as controls. The cells were analyzed by western blotting with antibodies against Cx43, GLAST-1, GLT-1, Na+/K+ ATPase, TLR4 and β-actin. A) Immunoblotting pictures Relative intensities of protein bands were quantitated for. B) Cx43, C) GLAST-1, D) GLT-1, E) Na+/K+ ATPase and F) TLR4. Data are representative of four experiments and the intensities of control protein bands were set to 100%. Data are means ± SEM (n = 4). Student’s t-test, *P < 0.05, ***P < 0.001.
3.6. Glutamate release
Glutamate exocytosis is dependent on Ca2+ signaling and glutamate can promote Ca2+ overload. Therefore, we investigated whether inflammatory activation would affect glutamate secretion in human cardiac fibroblasts. We observed that, in medium from LPS-stimulated cells, glutamate concentrations were higher than in that from unstimulated cells (Fig. 7).
Fig. 7.
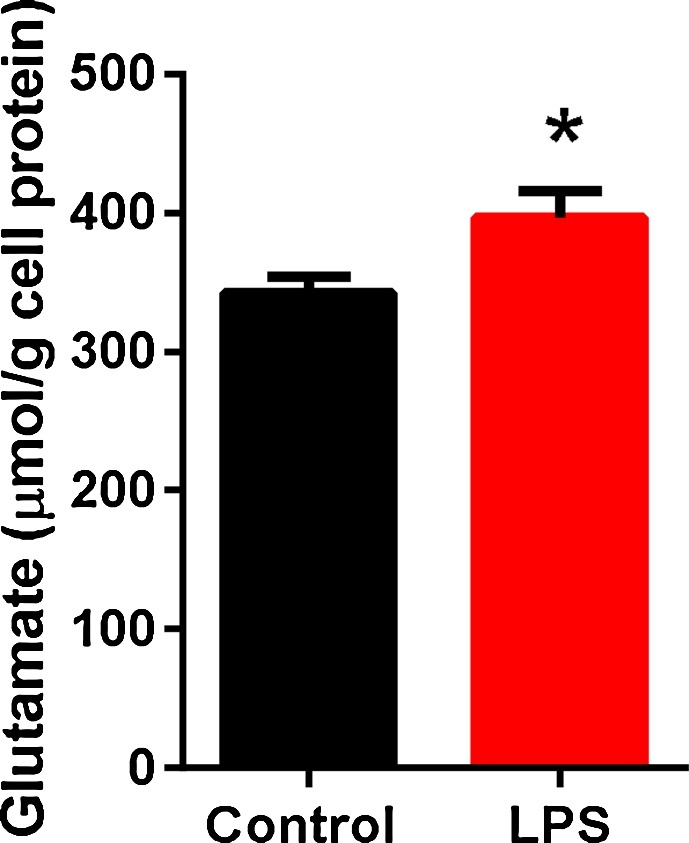
Increased glutamate release in LPS stimulated human cardiac fibroblasts.Human cardiac fibroblasts were treated with LPS (10 ng/ml) for 24 h, and unstimulated cells were used as controls. The concentration of glutamate (μmol/l) was measured in the culture medium using the ninhydrin reagent for photometric determination. Duplicate samples from culture medium were analyzed and correlated to the protein concentrations. Data are means ± SEM (n = 4). Student’s t-test, *P < 0.05.
4. Discussion
Inflammation, both local and systemic, has been implicated in pathogenesis of several cardiovascular diseases [27, 28]. Resident cells in the myocardium (endothelial cells, fibroblasts and dendritic cells) and leukocytes all contribute to the acute inflammatory response. IL-1 is regarded as the major proinflammatory mediator and its levels correlated with progression of atherosclerotic plaques [28]. Our data suggested that inflammatory activation substantially affected cardiac fibroblasts, we observed that LPS increased the LPS receptor CD14 mRNA expression in human primary cardiac fibroblasts indicating that fibroblasts may be important contributors to inflammation-mediated cytokine production. Binding of LPS to CD14 triggers inflammation and activates the transcription factor nuclear factor kappa B and the toll-like receptor pathway, initiating the release of proinflammatory cytokines, a characteristic of cardiovascular diseases [29, 30, 31]. In agreement with our results, in vitro experiments showed that inflammasome activation, stimulated by ischemia, led to IL-1β production in cardiac fibroblasts. This resulted in inflammatory cell infiltration and cytokine expression in the heart after myocardial ischemic injury [32].
An intact cytoskeleton controlling the plasma membrane and the endoplasmatic reticulum complex is important with regard to calcium transients, and is required for the propagation of astrocytic Ca2+ waves [18]. We found a more diffuse organization of actin filaments in cardiac fibroblasts after incubation with LPS. We have observed similar patterns previously and disruption of actin filaments changes the balance between Ca2+-regulating processes [18]. In this study we demonstrated that inflammatory activated human cardiac fibroblasts had altered Ca2+ signaling, increased TLR4 and decreased Cx43 expression, compared with the unstimulated cells. We also detected increased levels of secreted cytokines in cardiac fibroblasts stimulated by LPS. Importantly, data indicate that fibroblasts play a significant role in cardiac inflammation and they represent an abundant cell type in the myocardium [3, 33]. In our study, human cardiac fibroblasts expressed the major gap junction protein Cx43 and its levels were decreased with LPS stimulation, suggesting a link between cardiac fibroblasts and inflammation. Decreased Cx43 protein levels were associated with ventricular arrhythmias and Cx43 depletion may directly affect electrical transmission between cardiac cells [34]. Disturbed myocardial Ca2+ homeostasis was observed in cases of severe heart failure [35]. During inflammation and in cells induced with inflammatory agents, Ca2+ signaling in network-coupled cells may be disturbed. Our data show intracellular Ca2+ release from cardiac fibroblasts, in comparison similar results are shown for astrocytes and are important for network-coupled cells [8, 9].
Increased release of several inflammatory cytokines, including IL-1β, can result in gap junction inhibition [36]. The inflammatory inducer LPS, incubated with fibroblasts for 24 h, increased Ca2+ signaling evoked by glutamate or 5-HT. In contrast, previous studies showed that 5-HT [37] and proinflammatory signaling via the leukotriene pathway increased cytoplasmic Ca2+ in cardiac fibroblasts [38]. Intracellular Ca2+ flux is a critical modulator of many cellular functions, including extracellular matrix synthesis and degradation, potentially explaining such discrepancies in results among studies [39]. However, it should be noted that our results of transient intracellular Ca2+ increase does not necessarily induce long-lasting structural changes.
Increased cytosolic Ca2+ levels can induce exocytosis of cytokines, prostaglandins and transmitters [11]. Na+/K+-ATPase, which can indirectly control Ca2+ signaling, was downregulated in LPS treated astrocytes [40]. However, in our experiments, Na+/K+-ATPase expression was not downregulated by LPS in cardiac fibroblasts.
Inflammatory conditions can lead to activation of Ca2+ signaling in fibroblast networks, influencing extracellular glutamate release. Membrane-bound glutamate transporters, such as GLAST and GLT-1, are responsible for uptake and clearance of extracellular glutamate. We found that human cardiac fibroblasts expressed both GLT-1 and GLAST but levels of these proteins were not affected by LPS. It has been documented that oxidative stress can be induced by LPS or pro-inflammatory cytokines, and activation of primary microglia by LPS induced a release of glutamate that reached concentrations on the order of 10−4 M within 24 h [41]. Therefore we speculate that inflammatory activated human cardiac fibroblasts are an important source of high glutamate levels that may be involved in reperfusion arrhythmias associated with Ca2+ overload [42], and may also mediate cytotoxic effect as glutamate triggers the death of neurons [43].
Fibroblasts are assumed to regulate extracellular matrix homeostasis and myofibroblasts are important in the adverse effects associated with cardiovascular disease [44]. However, we detected only a few α-SMA positive myofibroblasts, with no apparent changes after LPS stimulation. Nonetheless, the fibroblasts secreted several proinflammatory cytokines and glutamate.
In conclusion, this study showed that, in human cardiac fibroblasts, intracellular Ca2+ signaling was altered and expression of the gap junction protein Cx43 was decreased by LPS stimulation. The pronounced effects of LPS on production of inflammatory markers in cardiac fibroblasts suggested that these cells are important mediating inflammatory response.
Declarations
Author contribution statement
Eva Skiöldebrand, Annika Lundqvist, Ulrika Björklund, Mikael Sandstedt, Anders Lindahl, Elisabeth Hansson, Lillemor Mattsson Hultén: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Swedish Research Council, the Swedish Heart-Lung Foundation, ALF Sahlgrenska University Hospital and Laboratory Medicine, Sahlgrenska University Hospital, Edit Jacobson’s Foundation (Gothenburg, Sweden) and AFA Insurance (Stockholm, Sweden).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Porter K.E., Turner N.A. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol. Ther. 2009;123(2):255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Turner N.A. Effects of interleukin-1 on cardiac fibroblast function: relevance to post-myocardial infarction remodelling. Vascul. Pharmacol. 2014;60(1):1–7. doi: 10.1016/j.vph.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Lindner D., Zietsch C., Tank J., Sossalla S., Fluschnik N., Hinrichs S. Cardiac fibroblasts support cardiac inflammation in heart failure. Basic Res. Cardiol. 2014;109(5):428. doi: 10.1007/s00395-014-0428-7. [DOI] [PubMed] [Google Scholar]
- 4.Zhang P., Su J., Mende U. Cross talk between cardiac myocytes and fibroblasts: from multiscale investigative approaches to mechanisms and functional consequences. Am. J. Physiol. Heart Circ. Physiol. 2012;303(12):H1385–96. doi: 10.1152/ajpheart.01167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ongstad E., Kohl P. Fibroblast-myocyte coupling in the heart: Potential relevance for therapeutic interventions. J. Mol. Cell Cardiol. 2016;91:238–246. doi: 10.1016/j.yjmcc.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Kempen M.J., Fromaget C., Gros D., Moorman A.F., Lamers W.H. Spatial distribution of connexin43, the major cardiac gap junction protein, in the developing and adult rat heart. Circ. Res. 1991;68(6):1638–1651. doi: 10.1161/01.res.68.6.1638. [DOI] [PubMed] [Google Scholar]
- 7.Liang W., McDonald P., McManus B., van Breemen C., Wang X. Characteristics of agonist-induced Ca2+ responses in diseased human valvular myofibroblasts. Proc. West Pharmacol. Soc. 2008;51:11–14. [PubMed] [Google Scholar]
- 8.Blomstrand F., Aberg N.D., Eriksson P.S., Hansson E., Ronnback L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience. 1999;92(1):255–265. doi: 10.1016/s0306-4522(98)00738-6. [DOI] [PubMed] [Google Scholar]
- 9.Blomstrand F., Khatibi S., Muyderman H., Hansson E., Olsson T., Ronnback L. 5-Hydroxytryptamine and glutamate modulate velocity and extent of intercellular calcium signalling in hippocampal astroglial cells in primary cultures. Neuroscience. 1999;88(4):1241–1253. doi: 10.1016/s0306-4522(98)00351-0. [DOI] [PubMed] [Google Scholar]
- 10.Berridge M.J., Bootman M.D., Lipp P. Calcium–a life and death signal. Nature. 1998;395(6703):645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 11.Zorec R., Araque A., Carmignoto G., Haydon P.G., Verkhratsky A., Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN Neuro. 2012;4(2) doi: 10.1042/AN20110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karpuk N., Burkovetskaya M., Fritz T., Angle A., Kielian T. Neuroinflammation leads to region-dependent alterations in astrocyte gap junction communication and hemichannel activity. J. Neurosci. 2011;31(2):414–425. doi: 10.1523/JNEUROSCI.5247-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X., Spicarova Z., Rydholm S., Li J., Brismar H., Aperia A. Ankyrin B modulates the function of Na,K-ATPase/inositol 1,4,5-trisphosphate receptor signaling microdomain. J. Biol. Chem. 2008;283(17):11461–11468. doi: 10.1074/jbc.M706942200. [DOI] [PubMed] [Google Scholar]
- 14.Hansson E. Could chronic pain and spread of pain sensation be induced and maintained by glial activation? Acta Physiol. 2006;187(1-2):321–327. doi: 10.1111/j.1748-1716.2006.01568.x. [DOI] [PubMed] [Google Scholar]
- 15.Lundborg C., Hahn-Zoric M., Biber B., Hansson E. Glial cell line-derived neurotrophic factor is increased in cerebrospinal fluid but decreased in blood during long-term pain. J. Neuroimmunol. 2010;220(1-2):108–113. doi: 10.1016/j.jneuroim.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Hansson E., Ronnback L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17(3):341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- 17.Delbro D., Westerlund A., Bjorklund U., Hansson E. In inflammatory reactive astrocytes co-cultured with brain endothelial cells nicotine-evoked Ca(2 +) transients are attenuated due to interleukin-1beta release and rearrangement of actin filaments. Neuroscience. 2009;159(2):770–779. doi: 10.1016/j.neuroscience.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Forshammar J., Block L., Lundborg C., Biber B., Hansson E. Naloxone and ouabain in ultralow concentrations restore Na+/K+-ATPase and cytoskeleton in lipopolysaccharide-treated astrocytes. J. Biol. Chem. 2011;286(36):31586–31597. doi: 10.1074/jbc.M111.247767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y., Danbolt N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. (Vienna) 2014;121(8):799–817. doi: 10.1007/s00702-014-1180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNearney T., Baethge B.A., Cao S., Alam R., Lisse J.R., Westlund K.N. Excitatory amino acids, TNF-alpha, and chemokine levels in synovial fluids of patients with active arthropathies. Clin. Exp. Immunol. 2004;137(3):621–627. doi: 10.1111/j.1365-2249.2004.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggiero V., Mura M., Cacace E., Era B., Peri M., Sanna G. Free amino acids in fibromyalgia syndrome: relationship with clinical picture. Scand. J. Clin. Lab Invest. 2017:1–8. doi: 10.1080/00365513.2016.1269362. [DOI] [PubMed] [Google Scholar]
- 22.Hansson E. Actin Filament Reorganization in Astrocyte Networks is a Key Functional Step in Neuroinflammation Resulting in Persistent Pain: Novel Findings on Network Restoration. Neurochem. Res. 2014 doi: 10.1007/s11064-014-1363-6. [DOI] [PubMed] [Google Scholar]
- 23.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 24.Hansson E., Westerlund A., Bjorklund U., Ronnback L. PACAP attenuates 5-HT, histamine, and ATP-evoked Ca2+ transients in astrocytes. Neuroreport. 2009;20(10):957–962. doi: 10.1097/WNR.0b013e32832ca201. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson M., Hansson E., Ronnback L. Heterogeneity among astroglial cells with respect to 5HT-evoked cytosolic Ca2+ responses: A microspectrofluorimetric study on single cells in primary culture. Life Sci. 1991;49(18):1339–1350. doi: 10.1016/0024-3205(91)90198-k. [DOI] [PubMed] [Google Scholar]
- 26.Moore S., Stein W.H. A Modified Ninhydrin Reagent for the Photometric Determination of Amino Acids and Related Compounds. J. Biol. Chem. 1954;211(2):907–913. [PubMed] [Google Scholar]
- 27.Piccardi B., Giralt D., Bustamante A., Llombart V., Garcia-Berrocoso T., Inzitari D. Blood Markers of Inflammation and Endothelial Dysfunction in Cardioembolic Stroke: Systematic Review and Meta-analysis. Biomarkers. 2017:1–27. doi: 10.1080/1354750X.2017.1286689. [DOI] [PubMed] [Google Scholar]
- 28.Van Tassell B.W., Toldo S., Mezzaroma E., Abbate A. Targeting interleukin-1 in heart disease. Circulation. 2013;128(17):1910–1923. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller Y.I., Viriyakosol S., Worrall D.S., Boullier A., Butler S., Witztum J.L. Toll-like receptor 4-dependent and −independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler. Thromb. Vasc. Biol. 2005;25(6):1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 30.Ryan K.A., Smith M.F., Jr., Sanders M.K., Ernst P.B. Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect. Immun. 2004;72(4):2123–2130. doi: 10.1128/IAI.72.4.2123-2130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Bont N., Netea M.G., Rovers C., Smilde T., Demacker P.N., van der Meer J.W. LPS-induced cytokine production and expression of LPS-receptors by peripheral blood mononuclear cells of patients with familial hypercholesterolemia and the effect of HMG-CoA reductase inhibitors. Atherosclerosis. 1998;139(1):147–152. doi: 10.1016/s0021-9150(98)00074-4. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi M., Takahashi M., Hata T., Kashima Y., Usui F., Morimoto H. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123(6):594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 33.Sinfield J.K., Das A., O'Regan D.J., Ball S.G., Porter K.E., Turner N.A. p38 MAPK alpha mediates cytokine-induced IL-6 and MMP-3 expression in human cardiac fibroblasts. Biochem. Biophys. Res. Commun. 2013;430(1):419–424. doi: 10.1016/j.bbrc.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jansen J.A., Noorman M., Musa H., Stein M., de Jong S., van der Nagel R. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm. 2012;9(4):600–607. doi: 10.1016/j.hrthm.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tank J., Lindner D., Wang X., Stroux A., Gilke L., Gast M. Single-target RNA interference for the blockade of multiple interacting proinflammatory and profibrotic pathways in cardiac fibroblasts. J. Mol. Cell. Cardiol. 2014;66:141–156. doi: 10.1016/j.yjmcc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Kim J.E., Choi H.C., Song H.K., Jo S.M., Kim D.S., Choi S.Y. Levetiracetam inhibits interleukin-1 beta inflammatory responses in the hippocampus and piriform cortex of epileptic rats. Neuroscience Lett. 2010;471(2):94–99. doi: 10.1016/j.neulet.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Hafizi S., Taylor P.M., Chester A.H., Allen S.P., Yacoub M.H. Mitogenic and secretory responses of human valve interstitial cells to vasoactive agents. J. Heart Valve Dis. 2000;9(3):454–458. [PubMed] [Google Scholar]
- 38.Nagy E., Andersson D.C., Caidahl K., Eriksson M.J., Eriksson P., Franco-Cereceda A. Upregulation of the 5-lipoxygenase pathway in human aortic valves correlates with severity of stenosis and leads to leukotriene-induced effects on valvular myofibroblasts. Circulation. 2011;123(12):1316–1325. doi: 10.1161/CIRCULATIONAHA.110.966846. [DOI] [PubMed] [Google Scholar]
- 39.Berridge M.J. Inositol trisphosphate and calcium oscillations. Biochem. Soc. Symp. 2007;74:1–7. doi: 10.1042/BSS0740001. [DOI] [PubMed] [Google Scholar]
- 40.Block L., Bjorklund U., Westerlund A., Jorneberg P., Biber B., Hansson E. A new concept affecting restoration of inflammation-reactive astrocytes. Neuroscience. 2013;250:536–545. doi: 10.1016/j.neuroscience.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 41.Barger S.W., Goodwin M.E., Porter M.M., Beggs M.L. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J. Neurochem. 2007;101(5):1205–1213. doi: 10.1111/j.1471-4159.2007.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun X., Zhong J., Wang D., Xu J., Su H., An C. Increasing glutamate promotes ischemia-reperfusion-induced ventricular arrhythmias in rats in vivo. Pharmacology. 2014;93(1-2):4–9. doi: 10.1159/000356311. [DOI] [PubMed] [Google Scholar]
- 43.Rossi D.J., Oshima T., Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403(6767):316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 44.Goldsmith E.C., Bradshaw A.D., Zile M.R., Spinale F.G. Myocardial fibroblast-matrix interactions and potential therapeutic targets. J. Mol. Cell Cardiol. 2014;70:92–99. doi: 10.1016/j.yjmcc.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]