Abstract
Diffuse alveolar hemorrhage (DAH) is a rare but potentially fatal complication in systemic lupus erythematosus (SLE). DAH is typically characterized by hemoptysis, dyspnea, new infiltrates on chest x-rays or CT-scans and a drop in hemoglobin. DAH is seen in less than 2% of patients with SLE and carries a high acute mortality risk of up to 70–90%.
The current treatment of DAH is high-dose intravenous corticosteroids, cyclophosphamide and extensive supportive care. Plasmapheresis is also often considered in the treatment.
A few case reports have described patients with SLE and DAH in whom a single series of Rituximab (RTX), a specific anti-CD20-antigen B-cell antibody, successfully has been used to treat DAH.
We here present the first case of a patient with combined SLE, antiphospholipid syndrome (APS) and recurrent DAH who was successfully controlled by continued treatment with RTX.
1. Introduction
Diffuse alveolar hemorrhage (DAH) is a rare but potentially fatal complication in systemic lupus erythematosus (SLE). DAH is typically characterized by hemoptysis, dyspnea, new infiltrates on chest x-rays or CT-scans and a drop in hemoglobin [1], [2], [3]. DAH is seen in less than 2% of patients with SLE and carries a high acute mortality risk of up to 70–90% [3].
The current treatment of DAH is high-dose intravenous corticosteroids, cyclophosphamide and extensive supportive care. Plasmapheresis is also often considered in the treatment [3].
A few case reports have described patients with SLE and DAH in whom a single series of Rituximab (RTX), a specific anti-CD20-antigen B-cell antibody, successfully has been used to treat DAH [1], [2], [4], [5], [6], [7], [8].
We here present the first case of a patient with combined SLE, antiphospholipid syndrome (APS) and recurrent DAH who was successfully controlled by continued treatment with RTX.
2. Case
Our patient is a 24-year old caucasian male diagnosed with SLE and associated antiphospholipid syndrome (APS) in 2001 at the age of 9. Initial symptoms were arthralgia, livedo reticularis and a decrease in complement. In 2005 he developed a class 4a glomerulonephritis. He was treated with prednisolone and cyclophosphamide as induction therapy and with azathioprine as maintenance treatment. Azathioprin was later changed to mycophenolate mofetil (MMF). His renal function normalized with an estimated glomerular filtration rate (eGFr) of 71 ml/min but permanent albuminuria of 2–3g/24 h. The diagnosis of APS was made in 2009 based on deep vein thrombosis, positive lupus anticoagulant >150 s (<42 s), elevated β2-glycoprotein IgM 51·103 units/l (<20 units/l) and β2-glycoprotein IgG 75·103 units/l (<20 units/l) and an increased APTT of 138 s (25–38 s) and he was therefore treated with warfarin (INR 2.0–3.5).
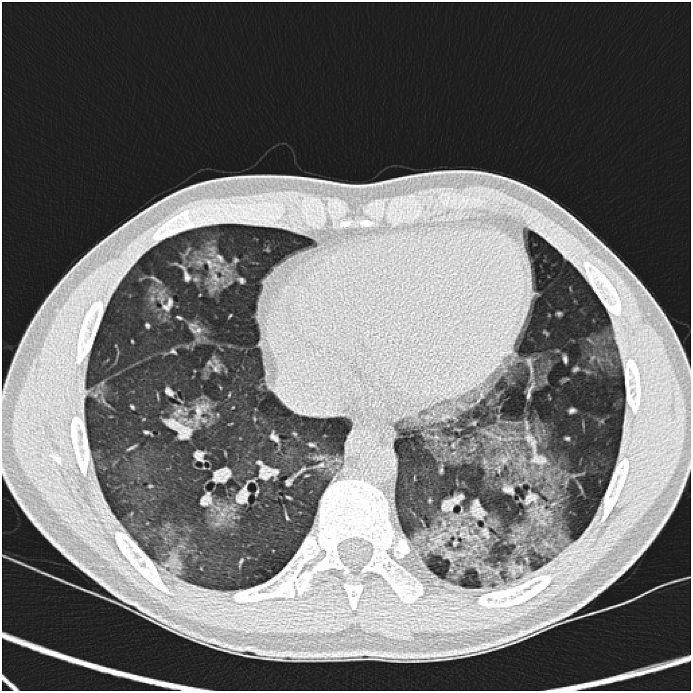
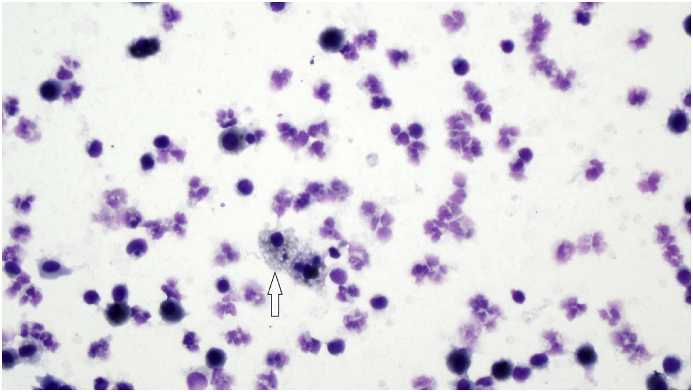
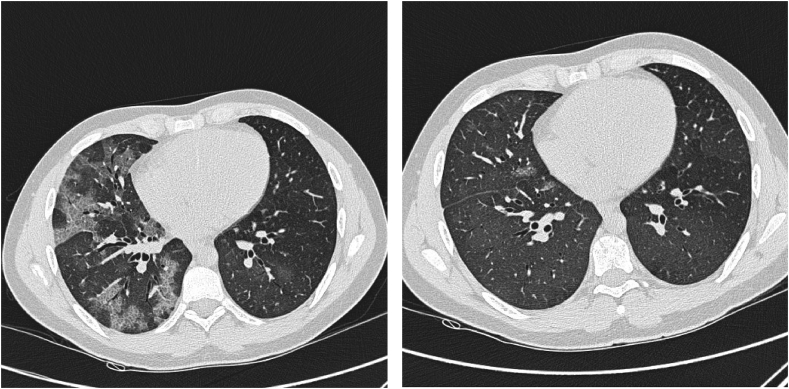
In spite of the heavy immunosuppressive treatment, his SLE worsened. In 2008, he was seen in our department for the first time due to an increased cough, dyspnea and hemoptysis. He was in a physical good shape playing football in spite of his kidney and lung symptoms even when his haemoptysis worsened. A high-resolution computed tomography (HRCT) showed lobular ground glass attenuation compatible with alveolar bleeding (Fig. 1), confirmed by a bronchoscopy showing hemorrhagic lavage fluid and hemosiderin-laden macrophages (Fig. 2). Culture was without mycobacteria or other microorganisms. During the DAH episode he had low C3c 5.5 μmol/l (6.7–13.4 μmol/l), thrombocytopenia 84·109/l (145–350·109/l), low hemoglobin 6.8 mmol/l (8.3–10.5 mmol/l) and a hematocrite of 0.37 (0.40–0.50). The findings were interpreted as an activation of his SLE and the mycophenolate dosage was increased. Due to recurrent haemoptysis, RTX was added to his treatment. As induction therapy, 1g of RTX was administered twice two weeks apart. His condition improved rapidly. Forced expiratory volume in 1 second (FEV1) increased from 2.47L to 2.93L and forced vital capacity (FVC) increased from 2.72L to 3.28L. His haemoptysis decreased significantly. HRCT show significantly regression in ground glass attenuation (Fig. 3).
Fig. 1.
High-resolution computed tomography (HRCT) showing lobular ground-glass opacities and in some areas interlobular septal thickening superimposed on ground-glass opacity (crazy paving pattern) compatible with alveolar bleeding.
Fig. 2.
Hemosiderin laden macrophages from hemorrhagic lavage fluid from bronchoscopy. 400x.
Fig. 3.
High-resolution computed tomography (HRCT) a few months apart. The first is during a DAH-episode, showing lobular ground-glass opacities. The second HRCT-scan is post-Rituximab treatment, showing significant regression in ground-glass opacities.
However, after 3 months, the dyspnea and haemoptysis returned and he again required RTX followed by continuous RTX 1g initially once a year. Due to recurrent and increasing hemoptysis after two years of treatment, the intervals were shortened to 6 months between each series of RTX.
During the 8 years with DAH, the patient was only hospitalized once due to a pulmonary infection. He has never been hospitalized due to DAH and has never needed mechanical ventilation.
His current treatment consists of prednisolone 10 mg x 1, mycophenolate mofetil 500 mg x 3, hydroxychloroquin 200 mg x 1 and 1 g of RTX every 4 months.
3. Discussion
This is the first case of a patient with combined SLE and associated APS with recurrent DAH treated successfully with continuous RTX. In previous SLE cases, DAH has presented without APS. APS usually presents with thromboembolisms such as deep venous thromboembolisms (DVT), lung embolisms or miscarriages and other events during pregnancy in females while bleeding is typical [9]. Patients with APS have elevated antiphospholipid antibodies that bind to phospholipids in cell membranes and stimulate the coagulation and the complement system. Standard therapy for APS is oral anticoagulation, often with vitamin K antagonists such as warfarin. In the present case, the combination of anticoagulant therapy and recurrent haemoptysis presented a differential diagnostic problem i.e. was the alveolar bleeding caused by SLE and DAH or due to a side effect of his warfarin treatment? The patient had his first episode of DAH before warfarin was initiated and the recurrent episodes were not related to fluctuations in warfarin treatment or INR. Obviously, concomitant anticoagulant treatment demands careful monitoring in order to prevent increased hemorrhage. The patients' INR was closely monitored and low in therapeutic range during the entire period of warfarin treatment. Anti-DNA was elevated compatible with SLE activity. We therefore believe that his DAH events were caused by SLE and not by his APS and warfarin treatment. Also, if DAH had been caused by Warfarin, we would not expect RTX to have had any beneficial impact.
DAH is a well known but rare complication to SLE although a more common complication to SLE than to APS. However, a few cases of APS patients with DAH have been reported [10], [11], [12], [13], [14]. Only a single case report has previously described RTX as treatment of DAH in a patient with APS without SLE [12]. The patient was a 32-year old male with primary APS who was treated with anticoagulation before having episodes of DAH. The patient developed recurrent DAH insufficiently treated with standard DAH immunosuppressive treatment. A single series of RTX (1g 2 weeks apart) was sufficient for remission of DAH in a two-year follow-up period despite continued anticoagulation therapy.
DAH is normally a life threatening condition. At the time of onset of DAH, all patients were critically ill and hospitalized in an intensive care unit requiring mechanical ventilation in addition to the immunosuppressive treatment [1], [2], [4], [5], [6], [7], [8]. Contrary to these cases, our patient was in a good physically shape. At first presentation with haemoptysis, he was only bothered during physical activity such as football. His only hospitalization was due to an infection and not to DAH and he has never needed mechanical ventilation during DAH events.
The current recommended treatment of DAH is high-dose intravenous corticosteroids, cyclophosphamide and extensive supportive care. Plasmapheresis is also often considered [3]. Since intensification of the immunosuppressive treatment with mycophenolate mofetil and corticosteroids was not sufficient to control the episodes of DAH in our patient, RTX was initiated.
B cell activation is thought to play an important role in SLE pathophysiology. Autoantibody production, cytokine secretion and T-B cell interaction are some of the functions that are depressed by depleting B cell targeted therapies in SLE [8]. RTX is an anti-CD20 monoclonal antibody. All B cells express CD20 and RTX reduces cytokine secretion and autoantibody production by specifically targeting these B cells [1].
All previous cases with SLE and DAH have just needed a single RTX series for complete remission of DAH [1], [2], [4], [5], [6], [7], [8]. The disease of our patient was more aggressive and he is the first case where RTX maintenance therapy was necessary. He has now been treated with RTX continuously for 8 years with varying intervals guided by the burden of his symptoms, allowing him an almost normal youth. He has not suffered any side effects to RTX and opposed to long-term treatment with prednisone we find his RTX treatment justified. Future RTX treatment will slowly be tapered by increasing the RTX intervals, guided by his pulmonary function tests and symptom burden.
The present case shows that maintenance RTX treatment is a rational choice in SLE patients with recurrent DAH when conventional treatment is not sufficient to maintain disease control.
References
- 1.Tse J.R., Schwab K.E., Mcmahon M., Simon W. Case report rituximab: an emerging treatment for recurrent diffuse alveolar hemorrhage in systemic lupus erythematosus. Lupus. 2015;24:756–759. doi: 10.1177/0961203314564235. [DOI] [PubMed] [Google Scholar]
- 2.Na J.O., Chang S.H., Seo K.-H., Choi J.S., Lee H.S., Lyu J.W. Successful early rituximab treatment in a case of systemic lupus erythematosus with potentially fatal diffuse alveolar hemorrhage. Respiration [Internet] 2015;89(1):62–65. doi: 10.1159/000369038. http://www.karger.com?doi=10.1159/000369038 Available from: [DOI] [PubMed] [Google Scholar]
- 3.Ednalino C., Yip J., Carsons S.E. Systematic review of diffuse alveolar hemorrhage in systemic lupus erythematosus. Focus on outcome and therapy. JCR J. Clin. Rheumatol. [Internet] 2015;21(6):305–310. doi: 10.1097/RHU.0000000000000291. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00124743-201509000-00005 Available from: [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Martínez M.U., Abud-Mendoza C. Recurrent diffuse alveolar haemorrhage in a patient with systemic lupus erythematosus: long-term benefit of rituximab. Lupus [Internet] 2012;21(10):1124–1127. doi: 10.1177/0961203312444171. http://www.ncbi.nlm.nih.gov/pubmed/22460294 Available from: [DOI] [PubMed] [Google Scholar]
- 5.Narshi C.B., Haider S., Ford C.M., Isenberg D.A., Giles I.P. Rituximab as early therapy for pulmonary haemorrhage in systemic lupus erythematosus. Rheumatology. 2010;49(2):392–394. doi: 10.1093/rheumatology/kep356. [DOI] [PubMed] [Google Scholar]
- 6.Nellessen C.M., Pöge U., Brensing K.A., Sauerbruch T., Klehr H.-U., Rabe C. Diffuse alveolar haemorrhage in a systemic lupus erythematosus patient successfully treated with rituximab: a case report. Nephrol. Dial. Transpl. [Internet] 2008;23(1):385–386. doi: 10.1093/ndt/gfm701. http://www.ncbi.nlm.nih.gov/pubmed/17933839 Available from: [DOI] [PubMed] [Google Scholar]
- 7.Pinto L.F., Candia L., Garcia P., Marín J.I., Pachón I., Espinoza L.R. Effective treatment of refractory pulmonary hemorrhage with monoclonal anti-CD20 antibody (Rituximab) Respiration. 2009;78(1):106–109. doi: 10.1159/000156965. [DOI] [PubMed] [Google Scholar]
- 8.Pottier V., Pierrot M., Subra J.F., Mercat A., Kouatchet A., Parrot A. Successful rituximab therapy in a lupus patient with diffuse alveolar haemorrhage. Lupus. 2011;20(6):656–659. doi: 10.1177/0961203310386276. [DOI] [PubMed] [Google Scholar]
- 9.Tektonidou M.G., Laskari K., Panagiotakos D.B., Moutsopoulos H.M. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum. 2009;61(1):29–36. doi: 10.1002/art.24232. [DOI] [PubMed] [Google Scholar]
- 10.Asherson R., Greenblatt M. Recurrent alveolar hemorrhage and pulmonary capillaritis in the “primary” antiphospholipid syndrome. JCR J. Clin. Rheumatol. 2001;7:30–33. doi: 10.1097/00124743-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Deane K.D., West S.G. Antiphospholipid antibodies as a cause of pulmonary capillaritis and diffuse alveolar hemorrhage: a case series and literature review. Semin. Arthritis Rheum. 2005;35(3):154–165. doi: 10.1016/j.semarthrit.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Elazary A., Klahr P., Hershko A., Dranitzki Z., Rubinow A., Naparstek Y. Rituximab induces resolution of recurrent diffuse alveolar hemorrhage in a patient with primary antiphospholipid antibody syndrome. Lupus. 2012;21(4):438–440. doi: 10.1177/0961203311422713. [DOI] [PubMed] [Google Scholar]
- 13.Isshiki T., Sugino K., Gocho K., Furuya K., Shimizu H., Sekiya M. Primary antiphospholipid syndrome associated with diffuse alveolar hemorrhage and pulmonary thromboembolism. Intern Med. [Internet] 2015;54(16):2029–2033. doi: 10.2169/internalmedicine.54.4058. http://libaccess.mcmaster.ca/login?url=http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emed13&AN=2015308208 Available from: [DOI] [PubMed] [Google Scholar]
- 14.Rangel M.L., Alghamdi I., Contreras G., Harrington T., Thomas D.B., Barisoni L. Catastrophic antiphospholipid syndrome with concurrent thrombotic and hemorrhagic manifestations. Lupus [Internet] 2013;22(8):855–864. doi: 10.1177/0961203313491024. http://www.ncbi.nlm.nih.gov/pubmed/23722230 Available from: [DOI] [PubMed] [Google Scholar]