Abstract
Functional roles of domestic and wild host populations in infectious keratoconjunctivitis (IKC) epidemiology have been extensively discussed claiming a domestic reservoir for the more susceptible wild hosts, however, based on limited data. With the aim to better assess IKC epidemiology in complex host-pathogen alpine systems, the long-term infectious dynamics and molecular epidemiology of Mycoplasma conjunctivae was investigated in all host populations from six study areas in the Pyrenees and one in the Cantabrian Mountains (Northern Spain). Detection of M. conjunctivae was performed by qPCR on 3600 eye swabs collected during seven years from hunted wild ungulates and sympatric domestic sheep (n = 1800 animals), and cluster analyses of the strains were performed including previous reported local strains. Mycoplasma conjunctivae was consistently detected in three Pyrenean chamois (Rupicapra p. pyrenaica) populations, as well as in sheep flocks (17.0% of sheep) and occasionally in mouflon (Ovis aries musimon) from the Pyrenees (22.2% in one year/area); statistically associated with ocular clinical signs only in chamois. Chamois populations showed different infection dynamics with low but steady prevalence (4.9%) and significant yearly fluctuations (0.0%– 40.0%). Persistence of specific M. conjunctivae strain clusters in wild host populations is demonstrated for six and nine years. Cross-species transmission between chamois and sheep and chamois and mouflon were also sporadically evidenced. Overall, independent M. conjunctivae sylvatic and domestic cycles occurred at the wildlife-livestock interface in the alpine ecosystems from the Pyrenees with sheep and chamois as the key host species for each cycle, and mouflon as a spill-over host. Host population characteristics and M. conjunctivae strains resulted in different epidemiological scenarios in chamois, ranging from the fading out of the mycoplasma to the epidemic and endemic long-term persistence. These findings highlight the capacity of M. conjunctivae to establish diverse interactions and persist in host populations, also with different transmission conditions.
Introduction
Complex systems involving several hosts suppose a challenge for disease ecology studies [1,2]. In the absence of genetics, patterns of incidence and pathogen prevalence are often used to assess the epidemiological roles of host populations, but they have a number of limitations that may infer wrong functional roles [3]. Even with genetics and the evidence of cross-species transmission, low resolution of spatiotemporal data may not provide a comprehensive insight of the host-pathogen system [4]. Furthermore, the identification of reservoir host populations can be especially critical in pathogens that can establish diverse interactions with its hosts, resulting in different clinical outcomes and epidemiological scenarios [5,6].
An illustrative example are infections by Mycoplasma conjunctivae at the wildlife-livestock interface in alpine ecosystems, where there is no clear consensus of the functional roles of wild and domestic hosts [7,8]. Mycoplasma conjunctivae is the causative agent of infectious keratoconjunctivitis (IKC), which is a highly contagious ocular disease that severely affects Caprinae [8]. Clinical signs of IKC are associated with ocular damage and inflammation, which causes visual impairment and blindness [9]. Disease is usually transient and spontaneous clinical recovery is a common course of the infection [10,11]. However, clinical signs may progress to staphyloma and perforation of the cornea. Mortality in wild hosts can range locally from 5 to 27% and is derived from blindness of the animals that either starve or die because of traumatic accidents [12,13].
Historical records of IKC in wild mountain ungulates date back to the beginning of the 20th century in the European Alps [14]. Since then, outbreaks of IKC have been documented in wild populations from almost all European mountain ranges [15–17], and is considered one of the main diseases of mountain ungulates [8]. Infectious keratoconjunctivitis outbreaks are locally perceived as problematic because of the visual impressiveness of the disease and the economic impact in hunting revenues, which usually decrease due to hunting restrictions [15]. Several wild hosts have been described suffering from IKC, chamois (Rupicapra spp.), Alpine ibex (Capra ibex), Iberian ibex (Capra pyrenaica), mouflon (Ovis aries musimon), the Himalayan tahr (Hemitragus jemlahicus) and bighorn sheep (Ovis canadensis) [6,8,18]. Among them, chamois are the most common and widespread species in Europe and the one that have suffered more frequent and severe IKC outbreaks [12,15,17,19,20]. Infectious keratoconjunctivitis can therefore exert strong influence on chamois population dynamics [13,15,21]. Domestic Caprinae, i.e. sheep and goats, also undergo spontaneous IKC outbreaks, although in general the health impact is comparatively lower than in their wild counterparts [22,23].
Among Southern chamois (Rupicapra pyrenaica), IKC was first described in Pyrenean chamois (Rupicapra p. pyrenaica) in mid 20th century [24], but it was not until 1980–1981 when the first severe IKC outbreak arose in the Central Pyrenees spreading throughout all the massifs in successive and massive events [14,25]. Conversely, no similar IKC outbreaks but only sporadic ocular clinical signs have been described in the Cantabrian chamois (Rupicapra p. parva)[24].
In alpine ecosystems, several caprine species that are competent hosts for M. conjunctivae dwell around meadows [26]. Domestic caprines seasonally (May-November) graze free-range in mountain pastures and interactions among wild and domestic ruminants have been broadly documented [27]. Yet the same M. conjunctivae strains were found in sheep and chamois suffering from IKC in the same area from the Swiss Alps, indicating cross-transmission between these species [28]. This finding suggested that epidemiological cycles involving wild and domestic hosts may occur in alpine ecosystems. However, the epidemiology of M. conjunctivae may vary considerably among host species. While M. conjunctivae mostly occurs endemically and at high prevalence in sheep flocks [22,29], wild host populations most commonly suffer from IKC outbreak events [12,17,30], and M. conjunctivae have been reported not to persist in chamois populations from Eastern Switzerland [19]. The health significance of M. conjunctivae infection is also different and a higher proportion of asymptomatic infections occurs in domestic sheep as compared to wild hosts [22,31], which in turn suffer from more severe clinical signs [9,15]. Altogether, these differences suggest domestic sheep as an ideal candidate for being a reservoir host to the more susceptible wild hosts [8], and a key species for the M. conjunctivae maintenance in the alpine ecosystems. Long-term studies that simultaneously consider all competent hosts are needed to yield a comprehensive epidemiological perspective of M. conjunctivae cycles and the relative epidemiological roles.
In this study, an integrative approach consisting in the assessment of long-term infection dynamics and molecular subtyping of M. conjunctivae strains is performed in host-pathogen alpine systems within the Southern chamois (R. pyrenaica) distribution range in Spain, in order to elucidate the relative functional roles of the ungulate species in IKC epidemiology and specifically assess M. conjunctive persistence in wild host populations. The clinical outcome of the M. conjunctivae infection is also evaluated in all the ungulate community.
Materials and methods
Study areas
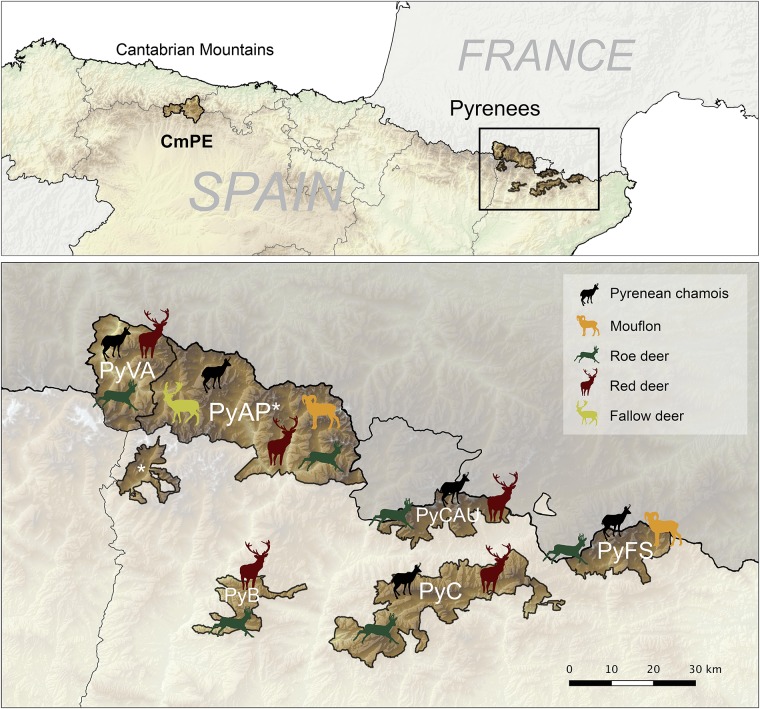
This study was performed within the distribution area of Pyrenean chamois and Cantabrian chamois in the two main mountain ranges from Northern Spain, the Pyrenees and the Cantabrian Mountains, respectively. Within these ranges, seven different geographical units were considered for the study, six from the Eastern and Central Pyrenees (Catalonia, NE Spain): Freser-Setcases National Game Reserve (PyFS), Cadí National Game Reserve (PyC), Cerdanya-Alt Urgell National Game Reserve (PyCAU), Boumort National Game Reserve (PyB), Alt Pallars National Game Reserve (PyAP) and Vall Aran Game Reserve (PyVA); and one in the Eastern Cantabrian Mountains (León, N Spain), the Natural Protected Area of Picos de Europa (CmPE) (Fig 1). In each study area, ungulate populations are managed independently along with hunting plans.
Fig 1. Maps of the study areas and wild ruminant species composition in the Pyrenees.
Location of the study areas in Cantabrian Mountains (Picos de Europa—CmPE) and Eastern and Central Spanish Pyrenees. Wild ruminant species composition by study area from the Pyrenees is showed in detail in the image of the bottom: Vall Aran (PyVA), NGR Alt Pallars (PyAP), NGR Boumort (PyB), NGR Cerdanya-Alt Urgell (PyCAU), NGR Cadí (PyC) and NGR Freser-Setcases (PyFS). The asterisk means the same study area. Chamois in PyB is scarce and not representative of the area (not shown in the map).
These areas are high mountain habitats mostly composed of alpine or subalpine ecosystems with strong seasonal influence, with the exception of PyB which has a dryer climate with a higher Mediterranean and continental influence. Altitude ranges approximately from 800 in the bottom of the valleys to 3100 meters high in the Pyrenees and from 1100 meters to 2600 meters high in the Cantabrian Mountains. Chamois is the most abundant ungulate species in most of the study areas except in PyB, where red deer (Cervus elaphus) is the predominant wild ruminant (Fig 1). Chamois population size is estimated yearly with linear transects performed by the rangers and is calculated to be about 7,500 in the six study areas from the Pyrenees and 3,800 in the study area from the Cantabrian Mountains. The chamois density varies among study areas as a result of differential pestivirus die-offs in the Pyrenees [32,33]. Mean minimum chamois abundances per square Km during the study period, calculated as estimated chamois population/area, were: PyFS, 14.7; PyC, 3.3; PyCAU, 2.7; PyAP, 1.8; PyVA, 1.8; CmPE 4.0. Other wild ungulates that cohabit with chamois include roe deer (Capreolus capreolus), red deer, mouflon, fallow deer (Dama dama) and wild boar (Sus scrofa), but with a different ruminant community composition among study areas from the Pyrenees, shown in Fig 1. Wild boars are present in all study areas and fallow deer and mouflon are not present in Cantabrian Mountains (CmPE). Domestic ruminants (i.e. cattle, sheep, and goats) and domestic horses also share these habitats with the wild species during the grazing period (May-November) in all of the study areas.
Sampling method
A long-term cross-sectional sampling design was performed on alpine wild ungulates hunted during the regular hunting seasons from 2009 to 2015 in the Pyrenees (n = 1556) and from 2010 to 2013 in the Cantabrian Mountains (n = 132). Samples were collected from recently hunted ungulates in proportion to the total number of animals hunted in each study area (Table 1). Four sheep flocks from the Pyrenees that graze in the alpine meadows of three of the study areas (PyVA, PyAP and PyFS) were also sampled in 2014 (around 30 sheep per flock; Table 1).
Table 1. Distribution of animals sampled by study areas and species.
| Study Area | Chamois | Mouflon | Red deer | Roe deer | Fallow deer | Wild boar | Sheep | TOTAL |
|---|---|---|---|---|---|---|---|---|
| Vall Aran (PyVA) | 125 | - | 26 | 25 | - | 3 | 30 | 209 |
| NGR Alt Pallars (PyAP) | 122 | 7 | 7 | 22 | 14 | 3 | 59* | 234 |
| NGR Boumort (PyB) | - | - | 34 | - | - | 8 | - | 42 |
| NGR Cerdanya-Alt Urgell (PyCAU) | 24 | - | 3 | 10 | - | 2 | - | 39 |
| NGR Cadí (PyC) | 343 | - | 40 | 17 | - | 11 | - | 411 |
| NGR Freser-Setcases (PyFS) | 592 | 80 | - | 33 | - | 1 | 23 | 729 |
| Picos de Europa (CmPE) | 88 | - | 25 | 18 | - | 1 | - | 132 |
| Total | 1294 | 87 | 135 | 125 | 14 | 33 | 112 | 1800 |
*Two sheep flocks.
Samples were taken between the third eyelid and the palpebral conjunctiva with sterile cotton swabs without medium from each eye separately and frozen at -20°C within 24 hours from collection. Basic information of the individuals was also registered, including ocular signs, sex, age based on the annual horn segments for chamois and mouflon [34], date and location. Geographic coordinates were also recorded in PyFS from 2012 to 2015. Age was classified in four categories in chamois according to social behavior and aging process, kids (<1 year), yearling (1–2 years), juvenile (2–3 years) and adults (>3 years).
This study accomplish with current guidelines for ethical use of animals in research following the European (2010/63/EU) and Spanish (R.D. 53/2013) legislations. The approval of an ethic committee was not needed since management and sacrifice of animals were not performed for research purposes. Ungulate wild species studied are not endangered, and its abundant populations are managed along hunting plans, regulated by the competent public administrations. Samples were obtained by the rangers from hunted-harvested wild animals during the regular hunting plans from National Game Reserves and Hunting Reserves that belong to public administrations. Both samplings of wild animals and domestic livestock were performed in the frame of health surveillance programs approved by the Departament d′Agricultura, Ramaderia, Pesca, Alimentació i Medi Natural—Generalitat de Catalunya (DARPAMN, the Regional authority in charge of livestock and wildlife management).
Detection of Mycoplasma conjunctivae
Eye swabs were thawed, cut and mixed during one minute with 0.5 mL lysis buffer (100 mM Tris–HCl, pH 8.5, 0.05% Tween 20, 0.24 mg/mL proteinase K) in sterile tubes. The lysates of the cells were obtained by incubating the tubes at 60°C for 60 minutes. Proteinase K was then inactivated at 97°C for 15 minutes [35]. The resulting lysates were directly used as test samples for the molecular detection of M. conjunctivae.
The presence of M. conjunctivae DNA in the samples was assessed with a previously described TaqMan real time PCR (qPCR) using primers LPPS-TM-L, LPPS-TM-R, and probe LPPS-TM-FT [35]. Briefly, 2.5 μL of the sample lysates, 900nM of each forward and reverse primer, 300 nM of the probe, 12.5 μL TaqMan®2x Universal PCR MasterMix (Applied Biosystems, Warrington, UK) and an exogenous internal positive control (IPC; Applied Biosystems, Warrington, UK) were introduced in each reaction well and nuclease-free water up to a total volume of 25 μL. Cycling conditions were set for 40 cycles at 95°C for 15 s and 60°C for one min, with pre-cycling steps of 50°C for 2 min and 95°C for 10 min. The threshold cycle (Ct) of each sample was defined as the number of cycle at which the fluorescent signal of the reaction crossed the threshold that was set to 0.05.
Samples were analyzed per duplicate and were considered valid only if difference between the replicates was less than one Ct. Samples with Ct≤38 were interpreted as qPCR-positive. All PCR reactions were run on Applied Biosystems® 7500 Fast Real-time PCR system (Applied Biosystems, Warrington, UK).
Mycoplasma conjunctivae subtyping and cluster analyses
The lppS gene of M. conjunctivae encodes for a membrane lipoprotein that is involved in adhesion [36], which variable domain can be used for M. conjunctivae subtyping and to perform molecular epidemiology analyses [28]. For cluster analyses, samples from this study with Ct values lower than 33 at the qPCR-M. conjunctivae were considered for sequencing. Available qPCR- M. conjunctivae positive samples from a previous study that analyzed 439 sheep and goats (19 flocks) from the same study areas and period were also included [22]. To obtain lppS gene sequences of these samples, a nested PCR was first performed as described with minor modifications of the primers [28,31] (S1 Table). PCR products were then purified with the High Pure PCR Product Purification Kit (Roche Diagnostics, Rotkreuz, Switzerland). The sequences were determined with the sequencing primers Ser_start2, Ser_start0 and Ser_end0 (S1 Table) using the BigDye termination cycle sequencing kit (Applied Biosystems, Forster City, CA, USA). The resulting sequences were trimmed to contain the region that comprises the nucleotide positions 3935–5035 of the lppS gene from M. conjunctivae type strain HRC/581 (GenBank acc. number AJ318939), which corresponds to the variable lppS domain and flanking regions. Alignment and editing of the sequences were performed with the BioEdit software. A phylogenetic analysis of the sequences was then performed by the generation of cluster analyses trees built by the UPGMA statistical method and performing 1000 bootstrap replications [37]. The generation of the phylogenetic tree was performed using MEGA software [38].
For the tree construction, sequences of M. conjunctivae strains described in previous works from the same study areas were included for comparison (three chamois from PyAP and one mouflon from PyFS) [16], covering a temporal period from 2006 to 2015 (S2 Table). Sequences from other areas (n = 6) and the sequence of the type strain HRC/581 were also included in the tree (S2 Table).
Data management and statistical analyses
Each individual was considered “infected” if the qPCR was positive in one or both eye swabs. When appropriate, database was organized as recommended for proportion data [39]. Mycoplasma conjunctivae apparent prevalence was analyzed to assess 1) the relation between ocular clinical signs and the presence of M. conjunctivae and 2) the trend of M. conjunctivae infection probability during the study period in each study area. For the first analyses, a two-sided Chi-squared test for independence was performed. In the second analysis, generalized additive models (GAMs) were fitted with M. conjunctivae infection as response variable with a binomial distribution and the interaction of year with study area as predictor variables [40]. GAMs can be used to model trends as a nonlinear function of time and provide a framework for testing statistical significance of changes in the response variable frequencies [41]. Known risk factors for M. conjunctivae infection, such as sex and age category [8], were previously tested with Fisher’s exact tests to be equally represented in all the years for each study area. The absence of residual patterns and other general assumptions were confirmed to validate the model once it was fitted [42]. Statistical significance was set at p<0.05 for all the tests. The interval confidence of apparent prevalences were calculated with the “EpiR” package, the graphics were performed with the “ggplot2” package and the GAMs were implemented in the “mgcv” statistical package, all from the R statistical software [43]. The spatial data representation and mapping was made with the software QGIS 2.14 Essen [44].
Results
Prevalence and dynamics of Mycoplasma conjunctivae infections
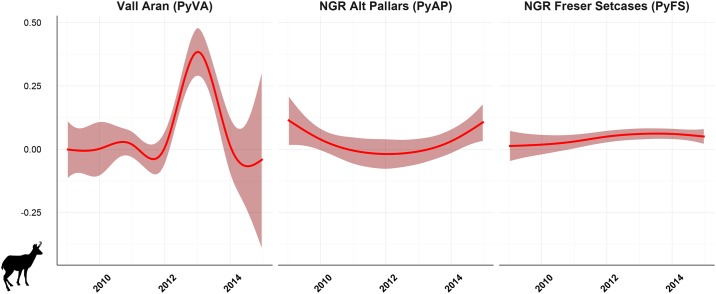
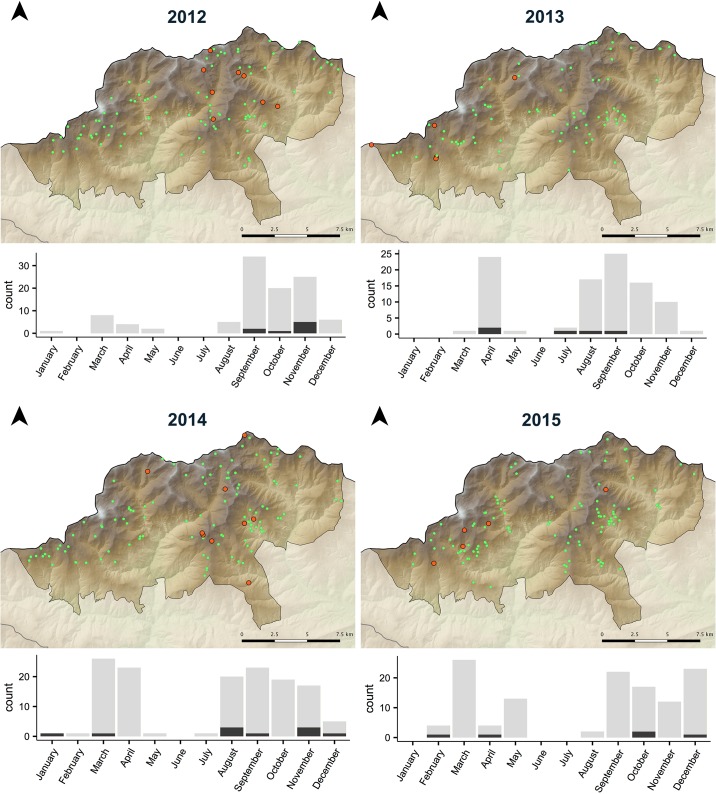
Mycoplasma conjunctivae was detected in eye swabs from Pyrenean chamois in three of the study areas (PyFS, PyAP and PyVA). No M. conjunctivae was detected in Cantabrian chamois (CmPE), as well as in Pyrenean chamois from the rest of study areas from Pyrenees (PyCAU and PyC) (Table 2). Prevalence in Pyrenean chamois population ranged from 2.0% (95% CI 0.1–10.7) to 40.0% (95% CI 19.8–64.2) in years when it was detected (Table 2). The GAM model showed significant differences of M. conjunctivae infection by year in PyVA (p = 0.025) and PyAP (p<0.001), but not in PyFS (8.12 edf; adjusted R2 of 70.4%). Thus, whereas infection indices followed a rather constant trend in PyFS, it changed throughout the study period both in PyAP and more pronouncedly in PyVA, where prevalence peaked in 2013 (Fig 2). No specific M. conjunctivae distribution pattern was found in Pyrenean chamois from PyFS during 2012–2015 as those described in IKC outbreaks [12,20], and infection cases were found distributed in all seasons and months throughout the reserve (Fig 3). Infection cases detected in PyAP and PyVA were localized in some geographic units within the study areas.
Table 2. Temporal trend of M. conjunctivae prevalence in Pyrenean chamois from three study areas where it was detected.
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | Total | |
|---|---|---|---|---|---|---|---|---|
| Vall Aran (PyVA) | ||||||||
| Prev % (Pos/Tot) | 0 (0/10) | 0 (0/12) | 2.0 (1/49) | 0 (0/28) | 40.0 (6/15) | 0 (0/10) | 0 (0/1) | 5.6 (7/125) |
| CI 95% | 0.0–27.8 | 0.0–24.2 | 0.1–10.7 | 0.0–12.1 | 19.8–64.2 | 0.0–27.7 | 0.0–94.9 | 2.7–11.1 |
| NGR Alt Pallars (PyAP) | ||||||||
| Prev % (Pos/Tot) | 20.0 (1/5) | 2.5 (1/40) | 0 (0/14) | 0 (0/6) | 0 (0/21) | 0 (0/22) | 14.3 (2/14) | 3.3 (4/122) |
| CI 95% | 1.1–62.4 | 0.1–12.9 | 0.0–21.5 | 0.0–39.0 | 0.0–15.5 | 0.0–14.9 | 4.1–39.9 | 1.3–8.1 |
| NGR Freser-Setcases (PyFS) | ||||||||
| Prev % (Pos/Tot) | 3.1 (1/32) | 0 (0/8) | 0 (0/87) | 7.6 (8/105) | 5.0 (5/100) | 7.3 (10/137) | 4.1 (5/123) | 4.9 (29/592) |
| CI 95% | 1.8–15.8 | 0.0–32.4 | 0.0–4.2 | 3.9–14.3 | 2.1–11.2 | 4.0–12.9 | 1.7–9.2 | 3.4–6.9 |
Prev, prevalence; Pos, positive; Neg, negative; Tot, Total animals analyzed; CI, confidence interval.
Fig 2. Modeled trend curves of Mycoplasma conjunctivae infection in Pyrenean chamois by generalized additive models.
Infection indices show evident curve differences by study area, in which the interaction of year with the study area resulted statistically significant in NGR Alt Pallars and Vall Aran, but not in NGR Freser Setcases.
Fig 3. Spatio-temporal distribution of Mycoplasma conjunctivae infection in Pyrenean chamois from NGR Freser-Setcasas (PyFS).
Orange dots are M. conjunctivae qPCR-positive chamois and green dots are qPCR-negative chamois. The bar graph at the bottom of each map shows the number of qPCR-positive chamois (dark grey) in total sampled chamois by month that year.
Infection of M. conjunctivae in mouflon was detected only in 2014 in PyFS with a prevalence of 22.2% (95% CI 6.3–54.7). Mycoplasma conjunctivae was confirmed in three out of four sheep flocks sampled with an overall prevalence of 17.0% (95% CI 11.1–25.0%) and a within-flock prevalence that ranged from 6.9% (95% CI 1.9–22.0%) to 33.3% (95% CI 19.2–51.2%). Mycoplasma conjunctivae was not detected in red deer 0.0% (95% CI 0.0–2.8), roe deer 0.0% (95% CI 0.0–2.9), fallow deer 0.0% (95% CI 0.0–21.5) and wild boar 0.0% (95% CI 0.0–10.4).
Cluster analyses of Mycoplasma conjunctivae strains
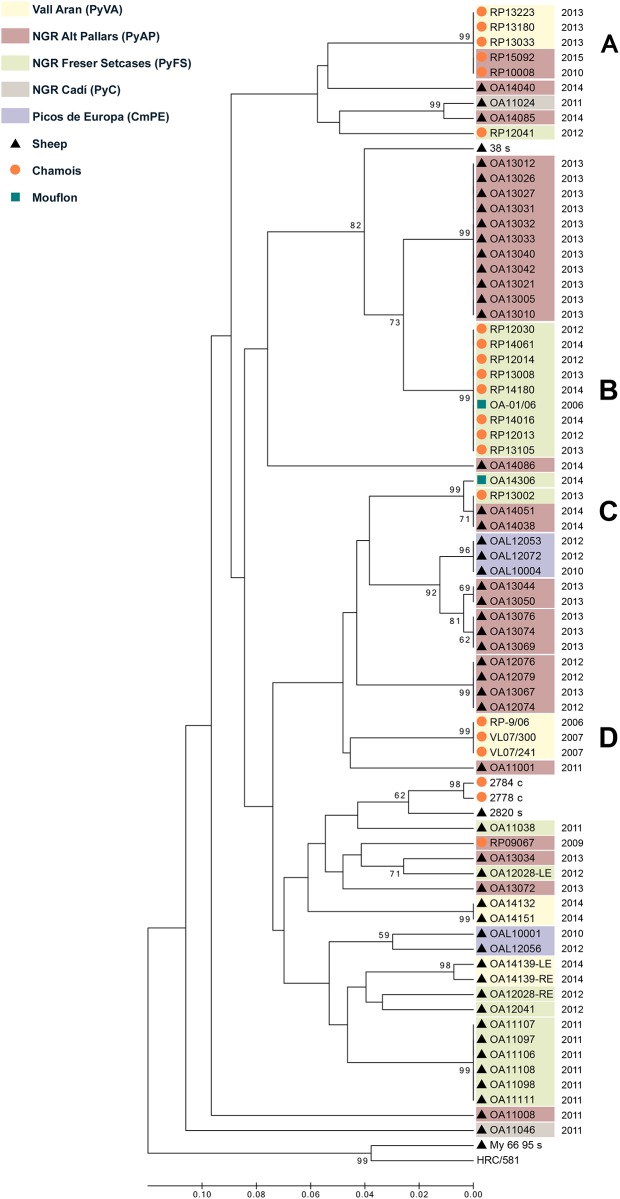
A total of 81 M. conjunctivae qPCR-positive samples were sequenced from 65 ruminants, including sheep (8 sheep from 3 flocks included in this study and 40 sheep from 11 flocks in the frame of a previous study [22], grazing in PyFS, PyAP, PyVA, PyC and CmPE), chamois (n = 16; PyFS, PyAP and PyVA) and mouflon (n = 1; PyFS) (S2 Table). In order to simplify the cluster analyses tree, only one M. conjunctivae strain was considered for the tree construction if the same strain was found in both eyes (n = 67). The tree revealed several clusters of M. conjunctivae strains, following a host species and geographic pattern (Fig 4). Three main clusters were identified that congregate most of the strains found in wild hosts, one cluster formed by chamois and mouflon strains detected during a nine-year period in PyFS (Fig 4B), another cluster with chamois strains detected during a six-year period in PyAP and PyVA altogether (Fig 4A) and a group clustering chamois strains from PyVA (Fig 4D). Sheep showed higher strain diversity and less clustering by study areas than chamois. Main strain clusters of wild hosts and domestic livestock were neither similar nor related by study area. However, similar strains infecting mouflon and chamois from PyFS (Fig 4B), and sheep and chamois from PyAP and PyFS (Fig 4C), were detected (Fig 4).
Fig 4. Cluster analyses tree of Mycoplasma conjunctivae strains.
The tree was inferred using the UPGMA method and including strains identified in chamois, sheep and mouflon during a ten-year period in the Pyrenees and the Cantabrian Mountains. The percentage of replicate trees in which the associated strains clustered together in the bootstrap test (1000 replicates) is shown next to the branches if ≥50%. The four main clusters that include wild ruminants (A-D) are shown. Chamois strains mainly clustered by geographic origin in PyFS (B), and PyAP together with PyVA as a single epidemiological unit (A). Shared strain clusters among different host species is observed between chamois and mouflon (B) and chamois and sheep (C). Sequences from other geographic regions that were included for comparison are showed without background colour. Information associate to each strain is provided in S2 Table.
Clinical outcome of M. conjunctivae infections
Ocular clinical signs were detected in 57/1294 chamois, 3/112 sheep, 1/87 mouflon, 1/125 roe deer, 1/135 red deer, 0/14 fallow deer and 0/33 wild boar, ranging from mild ocular discharge to perforation of the cornea. Mycoplasma conjunctivae was significantly (X2 = 488.5, df = 1, p<0.001) associated with ocular clinical signs in chamois, but not in the other ungulate species (Table 3). Among the M. conjunctivae infected chamois, 25.6% (95%CI 14.6–41.1) were asymptomatic, distributed differently among the study areas (PyAP 0/4; PyFS 9/29; PyVA 1/7).
Table 3. Detection of Mycoplasma conjunctivae by species and ocular clinical signs.
| Total | qPCR Positive | Prevalence % (CI 95%) | |
|---|---|---|---|
| Chamois | |||
| Clinical signs | 57 | 30 | 52.6 (39.9–65.0) |
| No clinical signs | 1238 | 10 | 0.8 (0.4–1.5) |
| Total sampled | 1294 | 40 | 3.1 (2.3–4.2) |
| Sheep | |||
| Clinical signs | 3 | 0 | 0.0 (0.0–56.1) |
| No clinical signs | 109 | 19 | 17.4 (11.4–25.6) |
| Total sampled | 112 | 19 | 17.0 (11.1–25.0) |
| Mouflon | |||
| Clinical signs | 1 | 0 | 0.0 (0.0–94.9) |
| No clinical signs | 86 | 2 | 2.3 (0.6–8.1) |
| Total sampled | 87 | 2 | 2.3 (0.6–8.0) |
Note that clinical signs correspond to any abnormality in the ocular structures or ocular discharge. Species in which M. conjunctivae was not detected are not shown in the table. CI, Confidence Interval.
Discussion
Epidemiological cycles and M. conjunctivae persistence in host populations
With the aim to better assess the relative functional roles in IKC epidemiology, the long-term infection dynamics and molecular subtyping of M. conjunctivae was investigated on all potential host species from alpine ecosystems of North Spain. The results further support the specificity of M. conjunctivae for caprine and ovine hosts [8], being consistently detected in Pyrenean chamois, domestic sheep and occasionally in mouflon. Based on M. conjunctivae subtyping, the low numbers of related strains shared between competent hosts disclosed domestic and sylvatic cycles, with domestic sheep and Pyrenean chamois as the key host species for each cycle. The overall prevalence of M. conjunctivae in domestic sheep was relatively high, as it was expected from previous studies performed in the Spanish Pyrenees [15,22] and in the European Alps [29]. Its detection in most of the sheep flocks sampled reinforce sheep as a proper host for the endemic M. conjunctivae maintenance [23,29]. Specific M. conjunctivae strain clusters were also recurrently detected in chamois populations in two independent epidemiological areas (PyFS and PyAP-PyVA), indicating that M. conjunctivae circulated within the sylvatic system at least along the temporal extent of the strains identified (up to nine years).
Long-term detection of M. conjunctivae clusters has also been demonstrated in wild Caprinae from the Alps, suggesting that M. conjunctivae persistence may also occur in other alpine systems [7,20]. Contrary to this study, host community of sympatric Caprinae seems to be more important for M. conjunctivae maintenance in the ecosystems from the Alps, in which a same cluster of strains has been commonly detected in more than one wild host species (i.e. Alpine chamois and ibex)[7,31], as well as both in domestic and wild hosts (i.e. sheep, Alpine chamois and ibex)[28,45]. Population characteristics and allocation/abundance of resources that mainly drive cross-species interactions in natural systems can widely vary between populations and may explain these differences [27,46]. The results of this study indicate that M. conjunctivae can long-term persist in Pyrenean chamois populations without the significant contribution of other hosts, either domestic or wild, and dismantle the general thought of domestic sheep as the sole reservoir of M. conjunctivae in the alpine host-pathogen systems. However, M. conjunctivae might have also faded out in some chamois populations studied (PyCAU and PyC) where historical IKC outbreaks have been documented [14]. Chamois populations from these areas (PyCAU and PyC) have suffered from prior outstanding pestivirus die-offs [32], which may have drop the population under a critical community size for M. conjunctivae maintenance [47]. The fading out of M. conjunctivae after an IKC outbreak has been also reported in Alpine chamois from some parts of the European Alps [13,19]. Altogether indicate that population characteristics can drive infection dynamics to result either in long-term persistence or fading out of M. conjunctivae in Pyrenean chamois, and reinforce similar observations in wild host communities from the Alps (i.e. Alpine chamois and ibex) [19,20]. Previous controversial hypothesis about reservoir hosts inferred by prevalence estimations and molecular epidemiology in a low spatio-temporal frame or studies performed on partial samplings of the host community should be critically revised [3].
The finding of a related strain in chamois and mouflon (PyFS) and in allopatric sheep more than 100 Km away (PyAP) indicates a wide geographic dispersion of this single cluster (Fig 4C). Despite M. conjunctivae epizootics can cover long distances [15,20], it is unlikely to be caused by a natural spread of M. conjunctivae under the non-epizootic conditions associated [31]. The limited dispersion movements of chamois [48] suggest that livestock trade and/or long-distance movements of livestock in the Pyrenees may favor the introduction of M. conjunctivae strains between distant areas [23]. Since both chamois and sheep can maintain M. conjunctivae as indicated in the present study, cross-transmission between them may have occurred in different past or recent events in the Pyrenees [49], as suggested by the finding of some divergent strains in chamois from each study area (Fig 4). Accordingly, M. conjunctivae was not detected in Cantabrian chamois, where most of the sympatric sheep flocks were free of M. conjunctivae [22]. Similar correspondences between the epidemiological scenarios in sympatric sheep and chamois have been also reported for other pathogens in the study area [50,51], highlighting the significance of spillover events among competent hosts at the wildlife-livestock interface, even if its occurrence is rare [52,53]. Even though independent sylvatic cycles accounted for most of the IKC cases in wild hosts from the Pyrenees, the higher prevalence and diversity of M. conjunctivae strains in sheep suggest that sheep cannot be ruled out as a source of IKC outbreaks in chamois/wild hosts from alpine ecosystems, owing to cross-species transmission of highly virulent strains [28].
Mycoplasma conjunctivae infection dynamics in wild host populations
The different patterns of the M. conjunctivae infection indices observed in chamois populations (Fig 2) indicates that M. conjunctivae persistence entailed different infection dynamics including both epidemic and non-epidemic transmissions. Thus, the low but regular M. conjunctivae detection in PyFS along with the dispersed location of the infection cases agrees with an endemic maintenance of M. conjunctivae [31,54]. Conversely, the prevalence in PyAP and PyVA fluctuated reaching higher incidence in some years followed by the absence of detection, suggesting a sporadic and localized epizootic spread of M. conjunctivae [19]. Similar IKC peaks have been observed every three to eight years in chamois populations from neighboring areas in the Eastern and Central Pyrenees [55,56], as well as in the Alps [14,17], but without leading to the high proportion of mortality that occur at the first disease emergence [12]. The dramatic drop of some chamois populations from the Eastern and Central Pyrenees since the pestivirus arose may have also prevented from extensive IKC outbreaks, as occurred in 1981–1983 in these areas [14], or in other parts of the Pyrenees still not affected by the demographic effects of pestivirus [15,21].
Virulence is positively related with mycoplasma transmission in ocular disease [57], which could suggest that the strain clusters that circulate in the two epidemiological areas identified in chamois (PyFS and PyVA-PyAP) differ in virulence. However, population density, which is clearly different in both areas (eight times higher in PyFS), can be also determinant for transmission by influencing connectivity among individuals, groups and subpopulations [47,58]. Since size and fusion rates of chamois groups increase with density [59], a nearly density-dependent transmission may occur if population density is high and groups/subpopulation units are highly connected [60]. The adaptive immune response of hosts does not always prevent mycoplasma re-infection [61–63], which may eventually be enhanced in denser populations and drive mycoplasma-host interaction to an endemic scenario [6], as observed in PyFS. Low population density in group-living ungulates can however shape pathogen transmission to be frequency-dependent if contacts between social groups or subpopulations are not common [60,64]. The scaling of M. conjunctivae transmission within and between subpopulations may underlay the temporally different disease peaks observed in PyAP and PyVA caused by the same strain cluster (Fig 4)[60,65]. The detection of M. conjunctivae was not constant in these areas, suggesting that both could be part of a bigger epidemiological unit, probably including part of the French Pyrenees, which would enable the epidemic spread of these strains within a subpopulation and its recurrent detection after a temporal fading out [20].
Despite coexistence and spatial overlap of mouflon with chamois and sheep, and the close interaction that may occur among them [66,67], M. conjunctivae was detected only sporadically in mouflon. Therefore, mouflon is probably a spill-over host in the systems studied with self-limiting M. conjunctivae infection in the population.
Ocular clinical signs and Mycoplasma conjunctivae
Mycoplasma conjunctivae infections were associated with ocular clinical signs only in chamois and was probably the main etiological cause of ocular disease in the Pyrenean chamois, as previously suggested [16]. These results further indicate a particular susceptibility of chamois to develop ocular disease by M. conjunctivae infection [7]. Asymptomatic infections in Pyrenean chamois were higher in the endemic M. conjunctivae area PyFS than when detected associated to epidemics in PyVA and PyAP, which may indicate a higher tolerance to the infection associated to the endemic maintenance. However, high number of asymptomatic M. conjunctivae infections have been reported in both epizootics and situations with low but regular incidence of IKC cases [6,31]. Clinical signs compatible with IKC were not registered in Cantabrian chamois, which agrees with the absence of M. conjunctivae detection in this area. Previous reports of keratoconjunctivitis in Cantabrian chamois have been described in the absence of disease outbreaks, but they could not be attributed to M. conjunctivae nor an infectious origin was ascertained [24].
Although sheep and mouflon may develop IKC due to M. conjunctivae infection [16,68,69], both species showed a high rate of asymptomatic infections in this study, agreeing with the lack of statistical association between IKC and M. conjunctivae detection [70]. Altogether highlight differences of susceptibility to the circulating M. conjunctivae strains among hosts [7,20]. Other infectious agents may also have sporadically caused eye disease in negative qPCR-M. conjunctivae [70,71], although IKC healing stages in which M. conjunctivae is not present anymore but evident lesions are still observed probably accounted for most of the Caprinae cases [6].
Conclusions
Independent M. conjunctivae sylvatic and domestic cycles mainly occurred at the wildlife-livestock interface in alpine ecosystems from the Pyrenees, indicating that M. conjunctivae was maintained in some chamois populations without the substantial contribution of other hosts. Furthermore, host population characteristics and M. conjunctivae strains resulted in different epidemiological scenarios in chamois, ranging from the fading out of the mycoplasma to the epidemic and endemic long-term persistence. Altogether, these findings highlight the capacity of M. conjunctivae to establish diverse interactions and persist in host populations, also with different transmission conditions. Population characteristics can therefore shape host-mycoplasma interaction and ultimately its functionality in the system. Host transitions of M. conjunctivae clusters in the alpine ecosystems were also occasionally observed, which indicates that cross-species transmission can be a source of IKC outbreaks.
Supporting information
(DOCX)
Sequences belonged to strains found in Pyrenean chamois (2006–2007; 2009–2015), mouflon (2006 and 2014) and sympatric sheep (2011–2014) from the Pyrenees and the Cantabrian Mountains. Ocular clinical signs associated to the strain are registered in the “IKC” column. Shaded rows are strains from other areas included for comparison.
(DOCX)
Acknowledgments
We are grateful to the rangers and directors of the National Game Reserves for their support in collecting the samples from wild ungulates and to the livestock owners that agreed to the sheep sampling. We also would like to thank the invaluable support of several colleagues from SEFaS research group and students from the Universitat Autònoma de Barcelona that occasionally assist in the sample collection, as well as the technical assistance of Amandine Ruffieux, from the Institute of Veterinary Bacteriology, University of Bern.
Data Availability
All relevant data are within the paper and its Supporting Information files and available from the Genbank database (accession number(s) provided in supplemental table).
Funding Statement
This study was funded by the research projects CGL2009-11631 and CGL2012-40043-C02-02 of the Spanish MICINN (to JRL). X. Fernández-Aguilar and A. Colom-Cadena were supported by the FI-DGR program from the Government of Catalonia. E. Serrano was supported by the postdoctoral programme (SFRH/BPD/96637/2013) of the Fundação para a Ciência e a Tecnologia, Portugal, the University of Aveiro (Department of Biology) and FCT/MEC through financial support to CESAM RU (UID/AMB/50017) and, where applicable, co-financed by the FEDER, within the PT2020 Partnership Agreement.
References
- 1.Fenton A, Pedersen AB. Community epidemiology framework for classifying disease threats. Emerg Infect Dis. 2005;11: 1815–1821. doi: 10.3201/eid1112.050306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. Identifying reservoirs of infection: A conceptual and practical challenge. Emerg Infect Dis. 2002;8: 1468–1473. doi: 10.3201/eid0812.010317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viana M, Mancy R, Biek R, Cleaveland S, Cross PC, Lloyd-Smith JO, et al. Assembling evidence for identifying reservoirs of infection. Trends Ecol Evol. Elsevier Ltd; 2014;29: 270–279. doi: 10.1016/j.tree.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serrano E, Cross PC, Beneria M, Ficapal a, Curia J, Marco X, et al. Decreasing prevalence of brucellosis in red deer through efforts to control disease in livestock. Epidemiol Infect. 2011;139: 1626–1630. doi: 10.1017/S0950268811000951 [DOI] [PubMed] [Google Scholar]
- 5.Citti C, Blanchard A. Mycoplasmas and their host: Emerging and re-emerging minimal pathogens. Trends Microbiol. 2013;21: 196–203. doi: 10.1016/j.tim.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 6.Fernández-Aguilar X, Cabezón O, Granados JE, Frey J, Serrano E, Velarde R, et al. Postepizootic persistence of asymptomatic Mycoplasma conjunctivae infection in Iberian ibex. Appl Environ Microbiol. 2017;83: e00690–17. doi: 10.1128/AEM.00690-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryser-Degiorgis MP, Bischof DF, Marreros N, Willisch C, Signer C, Filli F, et al. Detection of Mycoplasma conjunctivae in the eyes of healthy, free-ranging Alpine ibex: Possible involvement of Alpine ibex as carriers for the main causing agent of infectious keratoconjunctivitis in wild Caprinae. Vet Microbiol. 2009;134: 368–374. doi: 10.1016/j.vetmic.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 8.Giacometti M, Janovsky M, Belloy L, Frey J. Infectious keratoconjunctivitis of ibex, chamois and other Caprinae. Rev Sci Tech. 2002;21: 335–45. Available: http://www.ncbi.nlm.nih.gov/pubmed/11974619 [DOI] [PubMed] [Google Scholar]
- 9.Mayer D, Degiorgis MP, Meier W, Nicolet J, Giacometti M. Lesions associated with infectious keratoconjunctivitis in alpine ibex. J Wildl Dis. 1997;33: 413–419. doi: 10.7589/0090-3558-33.3.413 [DOI] [PubMed] [Google Scholar]
- 10.Giacometti M, Nicolet J, Frey J, Krawinkler M, Meier W, Welle M, et al. Susceptibility of alpine ibex to conjunctivitis caused by inoculation of a sheep-strain of Mycoplasma conjunctivae. Vet Microbiol. 1998;61: 279–288. [DOI] [PubMed] [Google Scholar]
- 11.Ter Laak EA, Schreuder BE, Kimman TG, Houwers DJ. Ovine keratoconjunctivitis experimentally induced by instillation of Mycoplasma conjunctivae. Vet Q. 1988;10: 217–224. doi: 10.1080/01652176.1988.9694175 [DOI] [PubMed] [Google Scholar]
- 12.Degiorgis M-P, Frey J, Nicolet J, Abdo EM, Fatzer R, Schlatter Y, et al. An outbreak of infectious keratoconjunctivitis in Alpine chamois (Rupicapra r. rupicapra) in Simmental-Gruyères, Switzerland. Schweiz Arch Tierheilkd. 2000;142: 520–527. [Google Scholar]
- 13.Loison A, Gaillard J-M, Jullien J. Demographic patterns after an epizootic of keratoconjunctivitis in a chamois population. J Chem Inf Model. 1996;60: 517–527. [Google Scholar]
- 14.Gauthier D. La Kerat-conjonctivite infectieuse du chamois Étude épidémiologique dans le Déprtament de la Savoie 1983–1990. Univsersité Claude Bernard, Lyon, France: 1991. [Google Scholar]
- 15.Arnal MC, Herrero J, de la Fe C, Revilla M, Prada C, Martínez-Durán D, et al. Dynamics of an infectious keratoconjunctivitis outbreak by Mycoplasma conjunctivae on Pyrenean Chamois Rupicapra p. pyrenaica. PLoS One. 2013;8: e61887 doi: 10.1371/journal.pone.0061887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marco I, Mentaberre G, Ballesteros C, Bischof DF, De Veterina F, Auto U, et al. First report of Mycoplasma conjunctivae from wild Caprinae with infectious keratoconjunctivitis in the Pyrenees (NE Spain). J Wildl Dis. 2009;45: 1–5. [DOI] [PubMed] [Google Scholar]
- 17.Tschopp R, Frey J, Zimmermann L, Giacometti M. Outbreaks of infectious keratoconjunctivitis in alpine chamois and ibex in Switzerland between 2001 and 2003. Vet Rec. 2005;157: 13–18. doi: 10.1136/vr.157.1.13 [DOI] [PubMed] [Google Scholar]
- 18.Jansen BD, Heffelfinger JR, Noon TH, Krausman PR, Devos JC. Infectious keratoconjunctivitis in bighorn sheep, Silver Bell Mountains, Arizona, USA. J Wildl Dis. 2006;42: 407–11. doi: 10.7589/0090-3558-42.2.407 [DOI] [PubMed] [Google Scholar]
- 19.Giacometti M, Janovsky M, Jenny H, Nicolet J, Belloy L, Goldschmidt-Clermont E, et al. Mycoplasma conjunctivae infection is not maintained in Alpine chamois in Eastern Switzerland. J Wildl Dis. 2002;38: 297–304. doi: 10.7589/0090-3558-38.2.297 [DOI] [PubMed] [Google Scholar]
- 20.Gelormini G, Gauthier D, Vilei E, Crampe JP, Frey J, Orusa R, et al. Molecular epidemiology of infectious keratoconjunctivitis caused by Mycoplasma conjunctivae in European countries. In: Schumann A, Gudrun W, Greenwood AD, Hofer H, editors. 12th Conference of the European Wildlife Disease Association (EWDA). Berlin: Leibniz Institute for Zoo and Wildlife Research (IZW); 2016. p. 18.
- 21.Serrano E, Colom-Cadena A, Gilot-Fromont E, Garel M, Cabezón O, Velarde R, et al. Border Disease Virus: An Exceptional Driver of Chamois Populations Among Other Threats. Front Microbiol. 2015;6: 1307 doi: 10.3389/fmicb.2015.01307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Aguilar X, Cabezón O, Marco I, Mentaberre G, Frey J, Lavín S, et al. Mycoplasma conjunctivae in domestic small ruminants from high mountain habitats in Northern Spain. BMC Vet Res. 2013;9: 253 doi: 10.1186/1746-6148-9-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández-Aguilar X, Rossi L, Cabezón O, Giorgino A, Victoriano LLopis I, Frey J, et al. Infectious keratoconjunctivitis and ocular coinfection by Mycoplasma conjunctivae and Chlamydiaceae in small domestic ruminants. Vet Rec. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Belda A, Martínez-Ferrando J. Contribution au diagnostique de la keratoconjonctivite du chamois (Rupicapra rupicapra) en Espagne In: Balbo T, Lanfranchi P, Rossi L, Stero P, editors. Atti del Simposio internazionale sulla cheratocongiuntivite infettiva del camoscio. Torino: University of Torino; 1985. pp. 73–77. [Google Scholar]
- 25.Marco I, Lavin S, Gonzalo J, Viñas L. Estudio de un brote de querato-conjuntivitis infecciosa en los rebecos (Rupicapra pyrenaica) del Pirineo leridano. Vet en Prax. 1991;6: 57–62. [Google Scholar]
- 26.Nicolet JN, Freundt EA. Isolation of Mycoplasma conjunctivae from chamois and sheep affected with keratoconjunctivitis. J Veterniary Med Ser B. 1975;22: 302–307. doi: 10.1111/j.1439-0450.1975.tb00591.x [DOI] [PubMed] [Google Scholar]
- 27.Ryser-Degiorgis M-P, Ingold P, Tenhu H, Tebar Less AM, Ryser A, Giacometti M. Encounters between Alpine Ibex, Alpine Chamois and Domestic Sheep in the Swiss Alps. Hystrix. 2002;13: 1–11. [Google Scholar]
- 28.Belloy L, Janovsky M, Vilei EM, Pilo P, Giacometti M, Frey J. Molecular epidemiology of Mycoplasma conjunctivae in Caprinae: Transmission across species in natural outbreaks. Appl Environ Microbiol. 2003;69: 1913–1919. doi: 10.1128/AEM.69.4.1913-1919.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janovsky M, Frey J, Nicolet J, Belloy L, Goldschmidt-Clermont E, Giacometti M. Mycoplasma conjunctivae infection is self-maintained in the Swiss domestic sheep population. Vet Microbiol. 2001;83: 11–22. [DOI] [PubMed] [Google Scholar]
- 30.Grattarola C, Frey J, Abdo E-MM, Orusa R, Nicolet J, Giacometti M. Mycoplasma conjunctivae infections in chamois and ibexes affected with infectious keratoconjunctivitis in the Italian Alps. Vet Rec. 1999;145: 588–589. [DOI] [PubMed] [Google Scholar]
- 31.Mavrot F, Vilei EM, Marreros N, Signer C, Frey J, Ryser-Degiorgis M-P. Occurrence, quantification, and genotyping of Mycoplasma conjunctivae in wild Caprinae with and without infectious keratoconjunctivitis. J Wildl Dis. 2012;48: 619–31. doi: 10.7589/0090-3558-48.3.619 [DOI] [PubMed] [Google Scholar]
- 32.Marco I, Rosell R, Cabezón O, Mentaberre G, Casas E, Velarde R, et al. Border disease virus among chamois, Spain. Emerg Infect Dis. 2009;15: 448–51. doi: 10.3201/eid1503.081155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández-Sirera L, Cabezón O, Allepuz A, Rosell R, Riquelme C, Serrano E, et al. Two Different Epidemiological Scenarios of Border Disease in the Populations of Pyrenean chamois (Rupicapra p. pyrenaica) after the First Disease Outbreaks. PLoS One. 2012;7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corlatti L, Gugiatti A, Imperio S. Horn growth patterns in Alpine chamois. Zoology. 2015;118: 213–219. doi: 10.1016/j.zool.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 35.Vilei EM, Bonvin-Klotz L, Zimmermann L, Ryser-Degiorgis M-P, Giacometti M, Frey J. Validation and diagnostic efficacy of a TaqMan real-time PCR for the detection of Mycoplasma conjunctivae in the eyes of infected Caprinae. J Microbiol Methods. 2007;70: 384–6. doi: 10.1016/j.mimet.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 36.Belloy L, Vilei EM, Giacometti M, Frey J. Characterization of LppS, an adhesin of Mycoplasma conjunctivae. Microbiology. 2003. pp. 185–193. doi: 10.1099/mic.0.25864-0 [DOI] [PubMed] [Google Scholar]
- 37.Sneath PHA, Sokal RR. Numerical Taxonomy The Principles and Practice of Numerical Classification. Freeman W, editor. San Francisco; 1973. [Google Scholar]
- 38.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33: 1870–1874. doi: 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crawley JM. The R Book Second Edition Cran; 2013. [Google Scholar]
- 40.Wood SN. Generalized additive models : an introduction with R Texts Stat Sci. 2006; xvii, 392. [Google Scholar]
- 41.Fewster RM, Buckland ST, Siriwardena GM, Baillie SR, Wilson JD. Analysis of population trends for farmland birds using generalized additive models. Ecology. 2000;81: 1970–1984. [Google Scholar]
- 42.Zuur AF, Ieno EN, Smith GM. Analyzing Ecological Data. Springer; NewYork: Springer; 2007. [Google Scholar]
- 43.R Development Core Team 3.1.3. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; http://www.R-project.org. [Internet]. 2015. [Google Scholar]
- 44.QGIS Development Team. QGIS Geogaphic Information System [Internet]. Open Source Geospatial Foundation Project. 2016. http://qgis.osgeo.org
- 45.Zimmermann L, Jambresic S, Giacometti M, Frey J. Specificity of Mycoplasma conjunctivae strains for alpine chamois Rupicapra r. rupicapra. Wildlife Biol. 2008;14: 118–124. [Google Scholar]
- 46.Richomme C, Gauthier D, Fromont E. Contact rates and exposure to inter-species disease transmission in mountain ungulates. Epidemiol Infect. 2006;134: 21–30. doi: 10.1017/S0950268805004693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swinton J, Woolhouse M, Begon M, Dobson A, Ferroglio E, Grenfell B, et al. Microparasite transmission and Persistence In: Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP, editors. The ecology of wildlife diseases. Oxford University press; 2009. pp. 83–101. [Google Scholar]
- 48.Loison A, Jullien J-M, Menaut P. Subpopulation structure and dispersal in two populations of chamois. J Mammal. 1999;80: 620–632. [Google Scholar]
- 49.Kamath PL, Foster JT, Drees KP, Luikart G, Quance C, Anderson NJ, et al. Genomics reveals historic and contemporary transmission dynamics of a bacterial disease among wildlife and livestock. Nat Commun. Nature Publishing Group; 2016;7: 11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernández-Aguilar X, López-Olvera JR, Marco I, Rosell R, Colom-Cadena A, Soto-Heras S, et al. Pestivirus in alpine wild ruminants and sympatric livestock from the Cantabrian Mountains, Spain. Vet Rec. 2016;178: 586 doi: 10.1136/vr.103577 [DOI] [PubMed] [Google Scholar]
- 51.Fernández-Aguilar X, Pujols J, Velarde R, Rosell R, López-Olvera JR, Marco I, et al. Schmallenberg virus circulation in high mountain ecosystem, Spain. Emerg Infect Dis. 2014;20: 1062–1064. doi: 10.3201/eid2006.130961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cassirer EF, Sinclair ARE. Dynamics of pneumonia in a bighorn sheep metapopulation. J Wildl Manage. 2007;71: 1080–1088. [Google Scholar]
- 53.Luzzago C, Ebranati E, Cabezo O, Rosell R, Fernández-Sirera L, Veo C, et al. Spatial and Temporal Phylogeny of Border Disease Virus in Pyrenean Chamois (Rupicapra p. pyrenaica). PLoS One. 2016;11: e0168232 doi: 10.1371/journal.pone.0168232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holzwarth N, Pospischil A, Mavrot F, Vilei EM, Hilbe M, Zlinszky K, et al. Occurrence of Chlamydiaceae, Mycoplasma Conjunctivae, and Pestiviruses in Alpine Chamois (Rupicapra r. rupicapra) of Grisons, Switzerland. J Vet Diagnostic Investig. 2011;23: 333–337. Available: http://vdi.sagepub.com/lookup/doi/10.1177/104063871102300223 [DOI] [PubMed] [Google Scholar]
- 55.Pañella P, Herrero J, Canut J, García-Serrano A. Long-term monitoring of Pyrenean chamois in a protected area reveals a fluctuating population. Hystrix, Ital J Mammology. 2010;21: 183–188. doi: 10.4404/Hystrix-21.2-4558 [Google Scholar]
- 56.Crampe J-P. Taux de survie et caracteristiques de la mortalite dans une population d’isards (Rupicapra pyrenaica pyrenaica) non chasee du Parc Natinal des Pyrenees. Tarbes, France; 1992. [Google Scholar]
- 57.Williams PD, Dobson AP, Dhondt K V., Hawley DM, Dhondt AA. Evidence of trade-offs shaping virulence evolution in an emerging wildlife pathogen. J Evol Biol. 2014;27: 1271–1278. doi: 10.1111/jeb.12379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boots M, Hudson PJ, Sasaki A. Large Shifts in Pathogen Virulence Relate to Host Population Structure. Science (80-). 2004;303: 842–844. doi: 10.1126/science.1088542 [DOI] [PubMed] [Google Scholar]
- 59.Pépin D, Gerard J-F. Group dynamics and local population density dependence of group size in the Pyrenean chamois, Rupicapra pyrenaica. Anim Behav. 2008;75: 361–369. doi: 10.1016/j.anbehav.2006.09.030 [Google Scholar]
- 60.Manlove KR, Cassirer EF, Cross PC, Plowright RK, Hudson PJ, B PRS. Costs and benefits of group living with disease: a case study of pneumonia in bighorn lambs (Ovis canadensis). Proc R Soc B. 2014;281: 20142331 doi: 10.1098/rspb.2014.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sydenstricker K V., Dhondt AA, Ley DH, Kollias G V. Re-exposure of captive house finches that recovered from Mycoplasma gallisepticum infection. J Wildl Dis. 2005;41: 326–33. doi: 10.7589/0090-3558-41.2.326 [DOI] [PubMed] [Google Scholar]
- 62.Trotter SL, Franklin RM, Baas EJ, Barile MF. Epidemic Caprine Keratoconjunctivitis : Experimentally Induced Disease with a Pure Culture of Mycoplasma conjunctivae. Infect Immun. 1977;18: 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Costa R. Contribution à l’étude étiologique de la kératoconjonctivite du chamois (Rupicapra rupicapra) et du bouquetin (Capra ibex). Université Claude Bernard de Lyon, Lyon, France: 1986. [Google Scholar]
- 64.Begon M, Bennett M, Bowers RG, French NP, Hazel SM, Turner J. A clarification of transmission terms in host-microparasite models: numbers, densities and areas. Epidemiol Infect. 2002;129: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loehle C. Social barriers to pathogen transmission in wild animal popultions. Ecology. 1995;76: 326–335. [Google Scholar]
- 66.Lauvergne J-J, Denis B, Théret M. Hybridation entre un Mouflon de Corse (Ovis ammon musimon Schreber, 1872) et des brebis de divers génotypes: gènes pour la coloration pigmentaire. Ann Génétique Sélection Anim. 1977;9: 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Darmon G, Calenge C, Loison A, Jullien JM, Maillard D, Lopez JF. Spatial distribution and habitat selection in coexisting species of mountain ungulates. Ecography (Cop). 2012;35: 44–53. [Google Scholar]
- 68.Baker SE, Bashiruddin JB, Ayling RD, Nicholas RAJ. Molecular detection of Mycoplasma conjunctivae in English sheep affected by infectious keratoconjunctivitis. Vet Rec. 2001;104: 1998–1999. [DOI] [PubMed] [Google Scholar]
- 69.Ter Laak EA, Schreuder BE, Smith-Buys CM. The occurrence of Mycoplasma conjunctivae in the Netherlands and its association with infectious keratoconjunctivitis in sheep and goats. Vet Q. 1988;10: 73–83. doi: 10.1080/01652176.1988.9694153 [DOI] [PubMed] [Google Scholar]
- 70.Dagnall GJR. An investigation of colonization of the conjunctival sac of sheep by bacteria and mycoplasmas. Epidemiol Infect. 1994;112: 561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilsmore AJ, Dagnall GJ, Woodland RM. Experimental conjunctival infection of lambs with a strain of Chlamydia psittaci isolated from the eyes of a sheep naturally affected with keratoconjunctivitis. Vet Rec. 1990;127: 229–231. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Sequences belonged to strains found in Pyrenean chamois (2006–2007; 2009–2015), mouflon (2006 and 2014) and sympatric sheep (2011–2014) from the Pyrenees and the Cantabrian Mountains. Ocular clinical signs associated to the strain are registered in the “IKC” column. Shaded rows are strains from other areas included for comparison.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files and available from the Genbank database (accession number(s) provided in supplemental table).