Abstract
Background
As the capabilities and reach of technology have expanded, there is an accompanying proliferation of digital technologies developed for use in the care of patients with mental illness. The objective of this review was to systematically search published literature to identify currently available health technologies and their intended uses for patients with serious mental illness.
Materials and methods
The Medline, Embase, and BIOSIS Previews electronic databases were searched to identify peer-reviewed English language articles that reported the use of digital, mobile, and other advanced technology in patients with schizophrenia/schizoaffective disorder, bipolar disorder, and major depressive disorder. Eligible studies were systematically reviewed based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
Eighteen studies that met the inclusion criteria were identified. Digital health technologies (DHTs) assessed in the selected studies included mobile applications (apps), digital medicine, digital personal health records, and an electronic pill container. Smartphone apps accounted for the largest share of DHTs. The intended uses of DHTs could be broadly classified as monitoring to gain a better understanding of illness, clinical assessment, and intervention. Overall, studies indicated high usability/feasibility and efficacy/effectiveness, with several reporting validity against established clinical scales. Users were generally engaged with the DHT, and mobile assessments were deemed helpful in monitoring disease symptoms.
Conclusion
Rapidly proliferating digital technologies seem to be feasible for short-term use in patients with serious mental illness; nevertheless, long-term effectiveness data from naturalistic studies will help demonstrate their usefulness and facilitate their adoption and integration into the mental health-care system.
Keywords: serious mental illness, health technology, mHealth, smartphone applications, digital medicine
Introduction
The global burden of mental illness is substantial.1 Serious mental illnesses (SMIs), such as bipolar disorder, schizophrenia, and major depressive disorder (MDD), are chronic conditions associated with significant disability, and often require continuous long-term treatment and care. In addition to the mental health-care system being overburdened, access, cost, and stigma are among several reported barriers to obtaining adequate mental health care.2–5 Emerging digital health technologies (DHTs) have provided a means to potentially overcome some of these obstacles and improve care.
Digital technology has rapidly pervaded and transformed the daily lives of people. According to the Pew Research Center, 72% of the US population owned a mobile phone in 2015.6 In the same year, a report by the IMS Institute for Healthcare Informatics noted that 165,000 mobile health (mHealth) applications (apps) were available, two-thirds of which were focused on general wellness.7 There are fewer apps for mental health, but the numbers are growing.8 Developing DHTs that can be used by people with SMI can be particularly challenging because of illness-related factors, such as cognitive impairments,9 and thus requires specific design considerations.10,11 Nevertheless, results of several surveys indicate that patients with SMI are receptive to the use of technology.12–15 There are several examples of DHTs that have been developed for and used in psychiatry, ranging from computerized cognitive behavior therapy (CBT)16 to symptom-monitoring apps17 to ingestible sensors.18
The potential advantages of technology in enhancing mental health care have been recognized and appreciated in several recent reviews.19–21 DHTs have created opportunities to develop a better understanding of MI and potentially improve the outcomes for patients with chronic mental health conditions in a variety of ways. Real-time longitudinal patient data can guide diagnosis and treatment decisions or facilitate timely interventions before a crisis develops. Remote on-demand provision of therapy allows for more consistent and accessible treatment. Objective measures of medication adherence allow for better-informed treatment decisions.
Previous reviews have focused on specific types of technology in specific populations. For example, Donker et al systematically reviewed studies of mental health apps for mobile devices, noting that there was insufficient scientific evidence to support their efficacy.22 The usability of mental health apps was perceived to be moderate–high, but evidence for their sustained use was not considered adequate, because most studies were of a short duration. Huguet et al reviewed available CBT and behavioral activation apps for depression, and concluded that there was not enough evidence available to support their use.23 Firth and Torous reviewed smartphone apps for schizophrenia. Although based on a small number of studies, the preliminary results indicated high rates of retention and adherence.24 While demonstrating the usability of DHTs, these reviews have emphasized the urgent need for data demonstrating efficacy. As noted earlier, these reviews have been limited in the type of technology or the disease state being evaluated. Moreover, studies on the use of advanced technology, such as wearable sensors, have not been addressed in previous reviews. HT is evolving at a rapid pace; therefore, to guide other developers of DHTs and to facilitate the adoption of various DHTs in clinical practice, it is important to review up-to-date available data on the usability and effectiveness of novel DHTs. The purpose of this systematic review is to identify currently available DHTs, encompassing mobile apps, eHealth, and other advanced technologies, and their intended use in patients with SMI, including schizophrenia, MDD, and bipolar disorders.
Materials and methods
A qualitative literature review was conducted based on the guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.25
Literature search
A search of the electronic databases Medline, Embase, and BIOSIS Previews was conducted to identify peer-reviewed English language articles published within 10 years of the search date of November 8, 2016. Because technology is evolving at a rapid pace, to avoid discussion of any technology that is now potentially obsolete, we limited our search to the previous 10 years. The search terms were designed to capture specific types of DHT intended for use in patients with SMI: (“digital medicine” OR “digital health” OR ehealth OR mhealth OR “mobile health” OR “mobile app*” OR “ingestible *sensor*” OR wearable OR “wireless system” OR “electronic adherence”) AND (schizophrenia OR psychosis OR MDD OR depressi* OR bipolar) NOT (review OR meta-analysis OR diabetes OR hypertension OR cancer OR obesity OR prevention OR alcohol OR dementia OR Alzheimer* OR protocol OR stroke OR editor* OR Parkinson* OR cardi* OR “traumatic brain injury” OR “multiple sclerosis” OR “stress management” OR lupus OR HIV OR COPD OR adolescent OR PTSD OR “mobile health unit*” OR “conference abstract” OR note OR letter OR chapter OR “meeting poster” OR patent OR “short survey” OR “case report” OR “conference paper” OR “meeting abstract” OR news). To meet the criteria for inclusion, an article had to be a primary report of a prospective study involving ≥20 participants, include patients with a diagnosis of SMI, defined here as bipolar disorder, schizophrenia, or MDD, and incorporate an intervention or assessment using digital, mobile, or other advanced technology.
Article selection process
Titles and abstracts of articles retrieved from the search were screened for eligibility by all authors. All authors also participated in full-text review, with each selected article being reviewed by two authors to identify those meeting the predefined criteria for inclusion. Review articles and commentaries were excluded. Randomized and nonrandomized studies and studies with and without control groups were included. Included articles reported technologies that required the participants to use and interact with the technology. Passive technology, such as videoconferencing, was excluded.
Results
Article selection
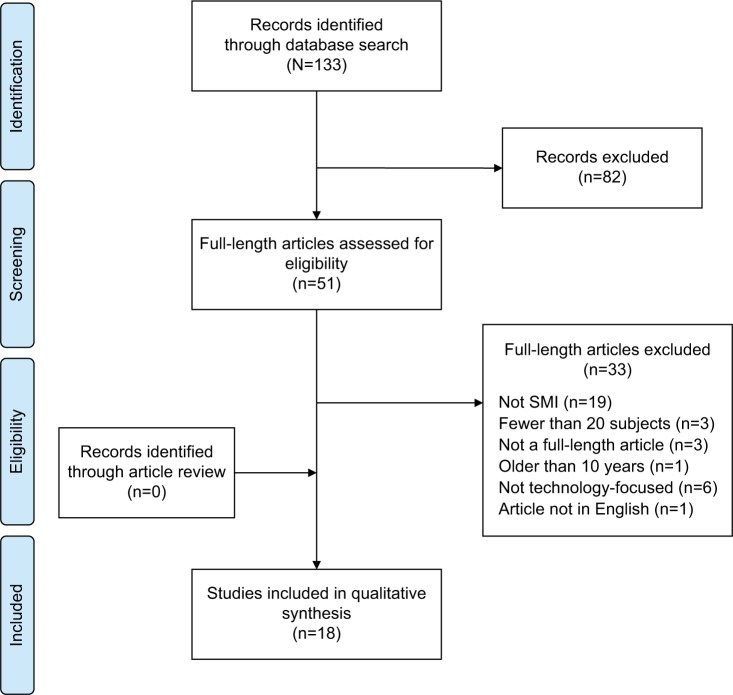
The search retrieved 133 records. After abstracts had been screened for eligibility, 82 were excluded. A full-text review of the remaining 51 potentially eligible articles was conducted. A total of 18 articles were included in the analysis. The most common reason for exclusion was that the article reported on a study conducted in a non-SMI study population (Figure 1).
Figure 1.
Article selection process.
Abbreviation: SMI, serious mental illness.
Information on study design, patient population, and types of DHTs and their intended use was collected. Because of variability in the study populations, study designs, and outcomes, a descriptive analysis of data was performed.
Characteristics of included studies and patient populations
Characteristics of the studies reported in the selected articles are summarized in Table 1. Five articles discussed the results of randomized studies.26–30 A majority were nonrandomized, single-arm studies (n=11),18,31–40 and two were parallel-arm studies.41,42 A total of 1,000 participants were recruited across the selected studies. The studies included patients diagnosed with schizophrenia and/or schizoaffective disorder (n=9),18,31–33,35,36,39–41 bipolar disorder (n=5),18,26,34,37,38 MDD (n=2),28,30 and psychotic disorder with suicidality (n=2).27,42 Where reported, patients’ mean disease duration ranged from 6 to 19 years. A majority of the studies were conducted in predominantly male population, with the exception of two studies, in which ~70% of the study sample was female.26,28 Study duration ranged from 2 hours for usability testing33 to over 1 year;27 participants were followed for ≥1 year in only three studies.26,27,37 Either the study completion rate or adherence to the technology was reported. Overall, the study completion rate was high across the selected studies (Table 1). Five studies assessed the usability and feasibility of DHTs,27,33,34,36,42 three tested feasibility and validity,37,39,40 eight evaluated efficacy or effectiveness,26,28–30,32,35,38,41 and two evaluated both usability and effectiveness.18,31 In all but three studies,28,35,37 training or support was provided during initial use of the technologies.
Table 1.
Study characteristics of selected articles
| Study | Design | Location | Population diagnosis (n) | Duration | Digital technology | Completion rate |
|---|---|---|---|---|---|---|
| Randomized studies | ||||||
| Faurholt-Jepsen et al26 | Randomized, controlled | Denmark | Bipolar I and II disorder (33) • 71% women, illness duration 9.8 years |
6 months | MONARCA smartphone app for monitoring | Mean use of the system 93% |
| Forchuk et al27 | Randomized, mixed-method, qualitative | Canada | Mood or psychotic disorder (95) | 12–18 months | Lawson SMART record, a smartphone- and iPad-based electronic personal health record | 99% |
| Ly et al28 | Randomized, open-label | Sweden | Major depressive disorder and depression (81) • 70% women, 63% college educated, 80% employed/studying |
8 weeks | Smartphone app for psychoeducation for behavior activation and mindfulness intervention | 85.2% |
| Velligan et al29 | Randomized, controlled | USA | Schizophrenia or schizoaffective disorder (132) • ~50% men |
9 months | PharmCAT, Med-eMonitor, and treatment-as-usual | 82% |
| Watts et al30 | Randomized, controlled | Australia | Major depressive disorder (35) • 80% women |
3 months | Get Happy Program, a mobile app version of clinician-assisted treatment program | 68.6% |
| Nonrandomized studies | ||||||
| Ben-Zeev32 | Nonrandomized, single-arm | USA | Schizophrenia or schizoaffective disorder (24) • Mild positive and negative symptoms • 71% men, ~75% high school-graduated or higher, illness duration 17 years |
1 week | Mobile EMA software package | 100% |
| Ben-Zeev et al41 | Nonrandomized, parallel-arm | USA | Schizophrenia or schizoaffective disorder (24) and nonclinical (26) • Mild psychotic symptoms, illness duration 17 years • 71% men in experimental group, 65% women in control group |
1 week | Mobile EMA software package | 100% |
| Ben-Zeev et al33 | Nonrandomized, single-arm | USA | Schizophrenia or schizoaffective disorder (12)a • 67% men • ~50% had less than high-school diploma |
2 h | FOCUS, a smartphone app | 100% |
| Ben-Zeev et al31 | Nonrandomized, single-arm | USA | Schizophrenia or schizoaffective disorder (32) • 61% men, average education level eighth grade • Moderate illness severity at baseline |
1 months | FOCUS, a system comprising three smartphone apps | 97% |
| Depp et al38 | Nonrandomized | USA | Bipolar I or II (41) • Patients had mild illness |
11 weeks | Smartphone app for EMA of mood and related experiences | Adherence to the program 65.1% |
| Hidalgo-Mazzei et al34 | Nonrandomized, single-arm | Spain | Bipolar disorder (49) • Patients were euthymic • 57% men, 61% high education level • Illness duration, 12.7 years |
3 months | SIMPLe smartphone app to collect EMAs | App use 94% at 1 month, 82% at 2 months, and 74% at 3 months |
| Husky et al42 | Nonrandomized, parallel-arm | France | Mood disorder with suicidality (83) and healthy controls (13) • 73.8% women in recent suicide-attempt group, all women in other groups |
1 week | EMA delivered via a mobile device (PDA) | 73.8% in experimental group, 85.7% in the control group |
| Kane et al18 | Nonrandomized, single-arm, observational | USA | Schizophrenia and bipolar disorder (28) • 64% men, majority had completed high school or college |
4 weeks | DHFS, which electronically confirms ingestion of oral medication embedded with an ingestion sensor and acquires physiological metrics | 96% |
| Moore et al39 | Nonrandomized, observational | USA | Schizophrenia/schizoaffective disorder (21) and healthy controls (13) | 1 visit | Mobile app version of a scale for functioning capacity assessment (UPSA-M) | NS |
| Osipov et al35 | Nonrandomized, observational | NS | Healthy adults (19), schizophrenia (16) • Patients were in relative symptomatic remission • 58% men in schizophrenia group and 75% in control group • Illness duration 6.4 years |
4 weeks | Adhesive patch to monitor locomotor activity and heart rate paired to a mobile device | NS |
| Palmier-Claus et al40 | Nonrandomized, observational | UK | Schizophrenia and related disorders (44) • 77.8% men |
1 week | ClinTouch, a mobile app for assessment of mood symptoms | Met criteria for compliance with methodology 82% |
| Peters-Strickland et al36 | Nonrandomized, Phase II, open-label | USA | Schizophrenia (49) • Majority of patients mildly ill • 74.6% men • Illness duration 19.3 years |
8 weeks | Digital medicine system comprising medication embedded with an ingestible sensor, a wearable sensor, and software apps | 73.1% |
| Tsanas et al37 | Nonrandomized, observational | UK | Bipolar disorder or borderline personality disorder and healthy controls (130) • 74% women in experimental group |
3 months | Smartphone app Mood Zoom, a clinical questionnaire for daily mood monitoring | Adherence to program 87%–93% at 3 months |
Note:
904 participated in the stage 1 needs assessment part of the study.
Abbreviations: App, application; DHFS, digital health feedback system; EMA, ecological momentary assessment; NS, not specified; PDA, personal digital assistant; UPSA-M, University of California, San Diego performance-based skills assessment – mobile.
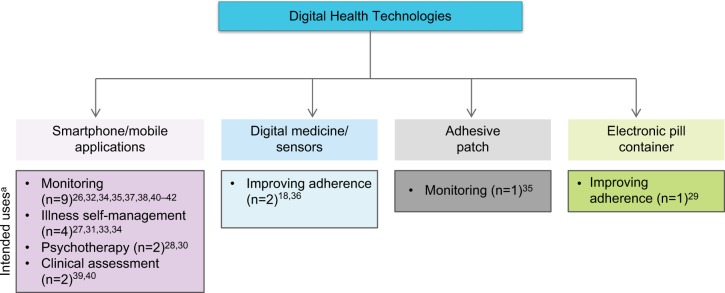
Characteristics of digital health technologies
The DHTs evaluated across the studies could be broadly classified as mobile/smartphone apps (n=14), digital medicine (n=2), adhesive patches paired with mobile devices (n=1), or electronic pill containers (n=1). Key findings from the studies are described in Table 2. The majority of DHTs described in the selected studies (14 of 18 [77.8%]) were mobile/smartphone apps that collected patient data in real time. In five studies, mobile device apps were used to collect ecological momentary assessments (EMAs).32,34,38,41,42 In three studies, smartphone apps were used for daily mood and symptom monitoring.26,37,40 One study tested a mobile version of a skill assessment scale for assessing functional capacity of patients.39 Forchuk et al evaluated the Mental Health Engagement Network, a web-based electronic personal health record and smartphone intervention.27 The purpose of the program was to help patients self-manage their illness and improve their communications with care providers. Smartphones were used to deliver psychosocial/behavioral therapy in four studies.28,30,31,33 A frequently used format in smartphone apps was completion of self-reports or questionnaires when prompted by an auditory signal generated by the app.26,32–34 These questionnaires aimed to assess domains, such as mood, sleep, activity, and symptoms.
Table 2.
Key findings from selected studies
| Study | Technology and assessment | Intended use of technology | Training/prompts | Results | |
|---|---|---|---|---|---|
| Mobile apps | |||||
| Ben-Zeev et al32 | PDA programmed to run Experience Sampling Program 4.0, an EMA package Participants completed self-report questionnaire that assessed multiple domains, including current location, company, activity, positive affect, negative affect, psychotic symptoms, and self-stigma |
Monitoring and understanding disease | Yes/yes | Participants spent 63% of their time at home, were often alone (60%) or with family (20%), spent much time inactive (39%) and eating (21%), or engaged in other activities (20%) Self-stigma and symptom ratings varied both between and within individuals Average (SD) 0.3 (0.12)-point increase in level of self-stigma when doing unspecified “other” activities; change was significantly greater compared with 0.11 (0.04)-point decrease in self-stigma when eating (P=0.005); no other statistically significant differences |
|
| Ben-Zeev et al41 | Mobile EMA or ESM software package ESM ratings on questionnaires were compared with retrospective reports |
Monitoring | Yes/yes | Retrospective ratings were higher than ESM ratings for both groups; however, not all differences were statistically significant Mean retrospective ratings were lower than mean peak ESM ratings (P<0.01); thus, the magnitude of exaggeration was small In clinical group, overestimations were lower for psychotic symptoms |
|
| Ben-Zeev et al33 | FOCUS system comprising three smartphone apps • Usability testing was performed in a lab setting |
Monitoring and illness management | Yes/NA | All participants were confident in using the system Majority were satisfied with ease of use |
|
| Ben-Zeev et al31 | FOCUS, a smartphone app • Treatment target included medication adherence and two of the following domains: social, mood, auditory hallucinations, or sleep difficulties • Participants completed assessments three times daily; responses determined the deployment of interventions by the FOCUS app |
Monitoring and illness management | Yes/yes | 93.7% of patients were overall satisfied with ease of use of the app and thought components of FOCUS worked well together • Reduction in symptoms was observed at the end of trial, indicated by improvements in PANSS total (P=0.002), PANSS positive (P<0.001), PANSS general psychopathology (P<0.001), and BDI-II (P=0.003) • Improvement in BDI-II scores significantly associated with the use of FOCUS (P<0.05) • Beliefs about medication did not change |
|
| Depp et al38 | Smartphone app for EMA of mood and related symptoms • Program prompted completion of web-enabled survey of current momentary mood experience and daily life activities • Symptoms, functioning, and adherence were measured by standardized clinical scales |
Monitoring and understanding disease | Yes/yes | Higher impulsivity associated with more severe baseline manic symptoms, increased suicide risk, problems with medication adherence, and lower baseline cognitive function | |
| Faurholt-Jepsen et al26 | MONARCA smartphone system for self-monitoring • Mood, sleep, medication taken, activity level, stress level, irritability, and alcohol consumption were evaluated • Clinical assessments for symptoms were performed once a month |
Monitoring and understanding disease | NA/yes | Patients with bipolar II disorder experienced significantly lower mean mood level (P=0.02) and lower time in euthymia (P=0.03) compared with patients with bipolar I disorder Patients with bipolar II disorder had a mean of 0.7 more mood changes per week than patients with bipolar I disorder |
|
| Forchuk et al27 | LSR, a smartphone- and iPad-based electronic personal health record • Focus-group sessions were conducted to gather information on usability, ethical issues, and recommendations on improvement of LSR |
Monitoring and Illness management | Yes/NA | Overall, participants recognized versatile functionality of LSR and smartphone, identified technology-associated barriers, and provided suggestions for improvement Technology perceived to have positive outcomes on lives of the participants; many reported enhanced mood awareness |
|
| Hidalgo-Mazzei et al34 | SIMPLe smartphone app • Prompts the user to answer daily and weekly screening tests; it shows a mood chart based on the answers |
Monitoring and intervention for illness management | Yes/yes | 86% were satisfied with the app, 82% found it useful for management of their condition, and 98% found it to be user-friendly Mean weekly mood score on the SIMPLe app significantly correlated with YMRS (P=0.001) and HDRS (P=0.01) scores |
|
| Husky et al42 | EMA delivered via a mobile device (PDA) • EMA included activity, location, mood states, and suicidal ideation |
Monitoring | Yes/yes | Baseline HAM-D scores predicted sad mood (P<0.001) Social interaction variables were not affected by duration of study, except being with friends more frequently 7.8% of patients with history of recent suicide attempt reported suicidal ideation No significant reactive effects were seen with increasing duration of study |
|
| Ly et al28 | Smartphone apps for behavioral activation and mindfulness-based self-help Primary outcome measures were BDI-II and PHQ-9 administered in an online format |
Psychotherapy | NA/no | Outcome measures were not significantly different between treatment groups Recovery rates were not significantly different between treatment groups overall; however, there was a trend favoring behavioral activation over mindfulness in severely depressed patients |
|
| Moore et al39 | Mobile app of UPSA-M and a brief version of UPSA-B (finances and communication subset of full UPSA) • Validity of UPSA-M was checked against full UPSA and standard clinical scales for symptom assessment • Computer questionnaire was used to assess tablet use |
Clinical assessment | Yes/no | Patients with schizophrenia found the device somewhat difficult to operate Controls achieved significantly higher scores on UPSA-M vs patients with schizophrenia UPSA-M scores correlated with clinical assessment scales UPSA-M was able to accurately differentiate adults with schizophrenia from controls 80% of the time |
|
| Palmier-Claus et al40 | ClinTouch, a mobile app for retrospective momentary assessment of mood and symptoms • Assessment questions were related to items on clinical scales • Validity was tested against PANSS and CDS |
Clinical assessment | Yes/yes | 82% completed ≥33% of all entries in mobile assessment Correlations with standard clinical scales were generally good, although there was variability; positive symptoms showed moderate–strong correlation with corresponding items on CDS and PANSS scales Mobile assessments showed some instability across time |
|
| Tsanas et al37 | Smartphone app MZ, a clinical questionnaire for daily mood monitoring • MZ questionnaire asked users to rate their mood on a Likert scale (1–7) on the following descriptors: anxious, elated, sad, angry, irritable, and energetic • Adherence was defined as the proportion of prompted responses completed • MZ questionnaire was validated against established self-report questionnaires: QIDS, ASRM, GAD-7, and EQ-5D • Mood variability between healthy adults; bipolar-disorder and borderline personality-disorder groups were compared |
Monitoring and understanding disease | NA/yes | Median adherence for MZ was 81.2% MZ items of negative mood showed high correlation with QIDS and GAD-7 Compared with bipolar disorder, patients with borderline personality disorder had higher median scores for QIDS, GAD-7, and MZ items of negative mood and lower scores for EQ-5D and MZ irritability items |
|
| Watts et al30 | Get Happy, a mobile app version of clinician-assisted treatment program, compared with computer version • The program comprised six lessons, upon completion of which participants were assigned homework activities • Other assessments included PHQ-9, K-10, BDI-II, CEQ, SDS, and ERS |
Psychotherapy | Yes/NA | Adherence to program similar between mobile and computer groups (P>0.05) Significant benefits of both computer and mobile programs observed on PHQ-9, BDI-II, and K-10 SDS scores showed a significant reduction in the number of days lost and underproductive days 45% met criteria for depression posttreatment 54% of patients in mobile group and 64% in computer group were satisfied with program |
|
| Digital medicine with sensors | |||||
| Kane et al18 | DHFS, which electronically confirms ingestion of oral medication embedded with an ingestion sensor using a wearable sensor and acquires physiological metrics Assessments included detection accuracy, physiological metrics, and usability |
Adherence intervention | Yes/no | Positive detection accuracy of DHFS was 94% Physiological metrics did not differ between schizophrenia and bipolar groups (P>0.05) 70% found DHFS is easy to understand and 89% thought it could be useful |
|
| Peters-Strickland et al36 | DMS, comprising medication embedded with an ingestible sensor, a wearable sensor, and software apps • Assessments included the ability of the patients to use the DMS, adherence metrics, patient and HCP satisfaction with the DMS, and call-center support |
Adherence intervention | Yes/no | 82.1% of patients independently or with minimal assistance were able to complete tasks associated with app of wearable sensor and pairing with smartphone app Mean (SD) adherence was 73.9% (23.3%) 78% were somewhat to extremely satisfied with DMS Number of patients seeking support from call center highest in the first week and decreased over study period Five device-related adverse events led to study discontinuation |
|
| Adhesive patch/wearable sensor | |||||
| Osipov et al35 | Adhesive patch to monitor locomotor activity and heart rate paired to a mobile device • Using a machine-learning framework, algorithms were used to extract heart rate and activity features; classifiers were used to map these features into schizophrenia and healthy controls |
Monitoring and understanding disease | NA | Combination of heart rate and locomotor activity provided 95.3% classification accuracy vs heart rate (78.5%) or locomotor activity (85.5%) alone | |
| Electronic pill container | |||||
| Velligan et al29 | PharmCAT (app of environmental supports maintained on weekly home visits by a case worker), MM (smart-pill container capable of cueing the taking of medication and alerting staff of missed medication), and treatment as usual • Medication adherence was measured by electronic monitor and unannounced pill counts in participants’ homes • Symptoms and functioning were assessed using BPRS and SOFAS, respectively • Use of hospital and emergency services was recorded |
Adherence intervention | NA | Average adherence was 91% for MM, 90% for PharmCAT, and 72% for treatment-as-usual group PharmCAT and MM were significantly better at improving adherence than treatment as usual (P<0.0001), but not significantly different from each other (P>0.43) No significant differences noted in symptoms, functioning, or health-care utilization |
|
Abbreviations: App, application; ASRM, Altman Self-Rating Mania (scale); BDI, Beck Depression Inventory; BPRS, Brief Psychiatric Rating Scale; CDS, Calgary Depression Scale; CEQ, Credibility/Expectancy Questionnaire; DHFS, digital health-feedback system; DMS, digital medicine system; EMA, ecological momentary assessment; ERS, Environment Rating Scale; ESM, Experience Sampling Method; EQ, EuroQoL; GAD, Generalized Anxiety Disorder; HAM-D, Hamilton Depression (rating scale); HCP, health-care professional; HDRS, Hamilton Depression Rating Scale; K-10, Kessler 10-item psychological distress scale; LSR, Lawson Smart Record; MM, Med-eMonitor; MZ, Mood Zoom; NA, not available; PANSS, Positive and Negative Syndrome Scale; PDA, personal digital assistant; PHQ, Patient Health Questionnaire; QIDS, Quick Inventory of Depressive Symptomatology Self (-report); SDS, Sheehan Disability Scale; SOFAS, Social and Occupational Functioning Assessment Scale; UPSA-M, University of California, San Diego Performance-Based Skills Assessment – mobile; YMRS, Young Mania Rating Scale.
Two studies described the development of a digital medicine system that consisted of oral medication embedded with an ingestible event marker, a wearable sensor that detected the ingestion, and smartphone apps that allowed collection and sharing of data.18,36 The wearable sensor also collected physiological metrics. In one study, an adhesive patch that continuously monitored locomotor activity and heart rate was paired with a mobile phone to collect and save data to a secure server.35 In another example of digital technology for use in psychiatry, a randomized study compared the efficacy of an electronic smart-pill container, Med-eMonitor (MM; InforMedix, Rockville, MD, USA), with other interventions for improving patients’ medication adherence.29 The MM was programmed and set up by a therapist in the patient’s home to prompt the patient to take his or her medication, while recording missed medication and side effects.
Usability and effectiveness of health technologies
As previously mentioned, usability is of particular interest in technologies used by patients with SMI. Four studies reported following a user-centered design approach:30,31,33,34 one study indicated that the program was not specifically designed for the intended user,28 and the rest did not specify their design approach.
Eight studies included in the analysis tested the usability or feasibility of digital tools and technologies in the target population of patients with SMI. In 2-hour laboratory-based usability testing, after a brief tutorial all patients with schizophrenia were able to learn to use the FOCUS system,33 which comprised three apps installed on a smartphone. Additionally, a majority of users found the system easy to use and considered it helpful.33 After refinement of some of the features based on user recommendations, the usability of FOCUS was further confirmed in a field trial, in which patients with schizophrenia used the system in their day-today surroundings for >1 month.31 Preliminary assessment of efficacy showed that use of the FOCUS system was associated with reduction in positive symptoms, general symptoms of psychopathology, and depression. Similarly, in another study, patients with mood or psychotic disorders found the use of a mobile personal health record engaging, and believed that its use had a positive effect on their lives.27 Study participants also suggested improvements to the functionality of the program. In a study testing the usability of the SIMPLe app, which monitors symptoms and offers personalized psychoeducation, 98% of patients with bipolar disorder considered its use to be simple and 82% considered it relevant to self-management of their condition.34 However, the use of the app decreased over time; the number of active users dropped from 94% at 1 month to 74% at 3 months. The feasibility of ClinTouch, another smartphone app for monitoring symptoms, was demonstrated in a group of patients with schizophrenia and related disorders.40
The feasibility and acceptability of a digital medicine system was assessed in two studies. In a pilot two-site study that recruited patients with schizophrenia, Kane et al demonstrated 94% positive detection accuracy with a digital health feedback system (DHFS) when compared with directly observed ingestions.18 Of the users, 70% found the DHFS concept easy to understand, and 89% considered it useful. In the other study, 82% of patients were able to complete various tasks associated with the use of the digital medicine system independently or with minimal assistance.36 Overall, 78% of patients were satisfied with the system, and 65% found it to be somewhat easy, easy, or extremely easy to use. Husky et al demonstrated the feasibility of using mobile EMA in monitoring suicidal ideation.42 Study acceptance rates were high: 66.7%–87.5% across the different study groups. Overall, compliance with the EMA procedures was 73.8%–85.7%.42
The effectiveness of smartphone-delivered behavioral and mindfulness treatments for MDD was demonstrated in a randomized controlled trial.28 Although the benefits of the two programs were not different overall from each other, subgroup analysis indicated that behavioral activation had a more favorable effect for patients with a higher severity of depression, whereas a mindfulness program was more effective for mildly depressed patients. Overall adherence to the entire treatment was 70%. Another randomized study compared the efficacy of a mobile format of CBT for major depression with a computer format.30 Both formats of the intervention resulted in significant benefits, indicated by scores on clinical assessment scales. Overall adherence to the programs was similar, with 68.6% of participants completing all assessments. Among the users, 54% in the mobile group and 64% in the computer group were very satisfied with the program.
Velligan et al compared the effectiveness of the smart-pill container MM with the Pharmacogenomics Clinical Annotation Tool (PharmCAT; a subset of techniques from cognitive adaptation training) and treatment as usual to improve medication adherence in patients with schizophrenia.29 Both the MM and PharmCAT significantly improved adherence compared with treatment as usual. However, study dropout rates were higher in the MM group.
Intended use of digital health technologies
The primary intended uses of DHTs included in the selected studies were monitoring to gain an understanding of disease, clinical assessment, and intervention. Interventions were further divided into illness self-management, adherence intervention, and psychoeducation/CBT (Figure 2).
Figure 2.
Digital health technologies and their intended uses.
Note: aDigital technologies may have had more than one intended use.
Use of digital health technologies for monitoring
All the tools intended for patient monitoring employed smartphone apps. Ben-Zeev et al conducted two studies with a mobile device programmed to run experience sampling, an EMA package.32 The program generated auditory prompts for completing a self-report questionnaire that assessed various domains in real time. One study examined the relations among external/contextual factors, illness symptoms, and self-stigma in patients with schizophrenia or schizoaffective disorder. Activity was found to be a significant predictor of change in self-stigma. In the other study, average ratings of mobile MAs were found to be comparable to those from retrospective reports, with the added dimension of variability in experience over time.41 Together, both studies demonstrated the utility of mobile EMA in monitoring disease symptoms.
Another mobile device-based EMA app asked users to describe their social interaction variables, such as location, activity, and social company. Users also rated their mood states and noted any negative feelings.42 It has been hypothesized that repeated questioning about negative cognition may be harmful for participants and eventually contribute to suicide risk, a phenomenon referred to as reactive effect. However, in people at risk of suicide, no increase in reactive effects was observed with increasing duration of mobile assessments in this study.
Three studies described mobile device-based monitoring programs developed for patients with bipolar disorder. MONARCA (monitoring, treatment, and prediction of bipolar disorder episodes)26 is a smartphone app for daily monitoring of such variables as mood, sleep, activity level, and stress level in real time. In a randomized study, daily electronic self-monitoring data from MONARCA was used to differentiate between bipolar-I disorder (BP-I) and bipolar-II disorder (BP-II).26 Patients with BP-II showed more mood fluctuations than those with BP-I. Mood Zoom (MZ),37 a clinical questionnaire, was also developed as a smartphone app for longitudinal assessment of mood symptoms in patients with bipolar disorder. Users were asked to assess their mood using the predefined descriptors anxious, elated, sad, angry, irritable, and energetic.
In an observational study, the MZ questionnaire was able to differentiate between bipolar disorder and borderline personality disorder, with borderline personality disorder showing greater variability in mood scores, particularly for irritability.37 Scores on MZ items were validated against established self- report questionnaires. In the third example of a smartphone-based app for monitoring patients with bipolar disorder, users completed a web-enabled survey of current momentary mood and related experiences.38 The data were used to assess impulsivity. Higher impulsivity was associated with greater manic symptoms, suicide risk, and adherence problems.
A disposable adhesive patch paired to a mobile phone was developed to collect electrocardiogram-derived heart rate and accelerometry-derived locomotor data in patients with schizophrenia.35 In a study conducted to identify predictors of schizophrenia compared with healthy controls, a combination of locomotor activity and heart rate provided better classification accuracy than either feature alone for predicting schizophrenia.
Use of digital health technologies for clinical assessment
Two studies reported on smartphone apps used for clinical assessments. Moore et al developed a mobile app version of University of California, San Diego Performance-Based Skills Assessment – mobile (UPSA-M) for evaluating the functional capacity of patients with schizophrenia.39 A tutorial and user manual were provided to familiarize patients with the app, following which the app was administered at a visit with minimal examiner involvement. Findings from the study demonstrated that UPSA-M correlated well with the standardized clinical assessment scale and was able to differentiate between healthy adults and patients with schizophrenia 80% of the time. In another study, a smartphone-based self-report assessment scale for psychosis was developed, which included items that were designed to be equivalent to some items on standard clinical assessment scales.40 The majority of symptom scores collected with the app showed good correlation with standard clinical symptom scales and were internally consistent. Additionally, the scores were sensitive to shifts in symptomatology across time.
Use of digital health technologies for intervention
Illness self-management
Three programs have been designed to assist SMI patients with self-management of their illness. The first of these – FOCUS, a smartphone app for illness management – was evaluated in two studies.31,33 Users were asked to self-assess several domains, such as adherence, mood, sleep, social functioning, and coping with symptoms. Targeted illness management interventions were suggested based on the domains that were “flagged”. The program also included on-demand resources for illness management. Additionally, users could share data with their health-care providers. During the usability study, participants used some of the coping strategies suggested by the program.33
The second DHT intended for illness self-management comprised an electronic personal health record with a smartphone intervention that was tested in a group of patients with mood or psychotic disorders to help them manage their mental health.27 Users considered features of the program useful, such as a mood-tracking function and appointment reminders. Some users described enhanced self-awareness due to mood tracking. However, the authors noted that simply owning a smartphone (provided by the investigators) might have biased the users’ positive perception of the intervention program. The third smartphone app for enabling self-management of bipolar disorder was SIMPLe, which detected mood changes based on EMA and offered psychoeducational messages to cope with the situation.34 Mean mood scores generated by the app showed significant correlation with standardized clinical scales of mania and depression.
Medication adherence
Three studies described the use of DHTs for improving medication adherence in patients with schizophrenia and bipolar disorder. Of these, two reported the development of digital medicine systems to measure medication ingestion objectively and acquire physiological metrics.18,36 In a 4-week observational study in patients with schizophrenia or bipolar disorder, positive detection accuracy with the DHFS was 94% when compared with directly observed ingestions. Physiological metrics did not show any significant differences between patients with schizophrenia and bipolar disorder.18 The mean adherence estimate obtained with a digital medicine system in an 8-week usability study was 73.9%.36 A randomized trial compared PharmCAT and the MM with treatment as usual for improving medication adherence in patients with schizophrenia.29 The study examined adherence, symptoms, and functioning over 3, 6, and 9 months. Average adherence was 90% and 91% with PharmCAT and MM, respectively, compared with 72% for the treatment-as-usual group. Improved adherence, however, was not associated with improvements in symptoms or functioning.
Therapy
Two randomized controlled trials investigated the use of smartphone apps to deliver psychotherapy to patients with MDD. Ly et al compared behavioral activation with mindfulness treatment delivered via smartphones.28 A behavior-activation app included a database of 54 behaviors for the user to perform and reflect on. Behavior data were available to the therapist, who could send encouraging messages to the patient. At the 6-month follow-up, patients with higher severity of depression benefited more from behavioral activation, whereas mindfulness was more effective for patients with mild symptoms.
Watts et al compared a mobile format of CBT, the Get Happy program, with a computer format.30 The program consisted of lessons in the form of a comic book that narrated case stories, after completion of which users were assigned homework activities. At the end of the study, both mobile and computer formats of the program were associated with significant improvement in symptoms, as indicated by standardized clinical scales for measuring symptoms.
Discussion
A review of the selected studies identified mobile apps, an electronic pill container, a personal DH record, digital medicine, and wearable sensors as DHTs being explored for use in people with SMI. Mobile/smartphone apps were the most common among the digital technologies being developed, and the most common intended use of DHTs was patient monitoring. Overall, the selected studies indicated high feasibility and acceptability of technologies for mental health care. The studies also demonstrated the potential applicability of these technologies in monitoring symptoms to gain a better understanding of disease, providing psychotherapy, assessing or improving medication adherence, and assessing clinical symptoms.
Testing usability in target users is particularly critical for developing digital tools for patients with SMI, because mental illness-related factors, such as cognitive impairments, can limit the use of DHTs in this population.10,43 As suggested by Rotondi et al,10 the needs of users with SMI differ from those of the general population, and DHTs that reduce the cognitive effort required on the part of users may be more appropriate for persons with SMI. However, it is noteworthy that most of the studies reviewed here did not specify following a user-centered approach for designing the DHT. This underscores the need for further emphasis on tailoring the design elements to user needs. Additionally, a large number of the selected studies included patients with mild illness severity. Although the selection of less impaired subjects is essential to rule out the effect of impairments on technology use, it is important to test usability in a population more representative of the real world to improve generalizability of the results.
In addition, the characteristics of patients included in the studies differed from one another and may have differed from the intended user populations. Whether sex differences affect the use of DHTs for mental health is not known, but the study populations were predominantly male. In an online survey, women were more engaged than men in finding health-related information on the web, whereas men more frequently used apps.44 Education level may also affect the use of and receptivity to health technology. The education level of participants varied between studies, ranging from eighth grade to college. In addition, most of the studies were conducted in small populations under controlled conditions. Collectively, the results may not be generalizable to patients encountered in routine clinical practice. Nevertheless, reviewed studies overall indicated high feasibility and usability of DHTs among patients with SMI, at least those with mild–moderate disease severity. This is consistent with findings in other reviews.16,24
Study completion rates and adherence to the technology were high among the selected studies, when reported. However, in the study by Hidalgo-Mazzei et al, use of the SIMPLe app decreased over time.34 This highlights challenges with sustained user engagement and should be kept in mind when designing DHTs for mental health. Several apps used auditory prompts to remind users of assessments. In a published systematic review, various means of prompts were shown to be helpful in promoting engagement with digital technologies,45 thus suggesting that this might be a useful feature to incorporate into the functionality of a DHT.
The majority of DHTs assessed in the selected studies were mobile apps. Mobile apps offer novel approaches, such as real-time monitoring of mental and physical health variables and remote access to information for patients and providers. Such information has the potential to improve our understanding of the illness and offers an opportunity to intervene in a timely manner when predictors of disease worsening are identified. Indeed, several studies noted a correlation between mobile self-assessments and disease symptoms.26,34,35,37,38,41,46 For example, higher impulsivity was shown to be associated with worse baseline cognitive function, prior suicide attempts, medication-adherence problems, and more severe manic symptoms.38 Mobile apps also allow patients access to therapy outside in-person treatment sessions. Two studies demonstrated the efficacy of delivering psychotherapy via mobile apps.28,30 However, a previously published systematic literature review found little evidence to support the efficacy of CBT apps,23 although a computerized version was found to be effective for anxiety and depression.16 Where evaluated, good correlation between mobile assessment scores and standard clinical assessments was observed, supporting the clinical applicability of the apps.37,39,40 Further support was provided by a Cochrane review that evaluated questionnaire responses obtained from mobile apps and found them to be equivalent to other modes of delivery.47 Additionally, records from apps were more complete than paper. However, for implementation in clinical practice, standardization of various assessments and their interpretation needs to be established.
Digital technology holds promise to overcome other barriers in mental health. For example, obtaining an accurate measure of medication adherence can be challenging in clinical practice.48 However, wearable sensors enable measurements of accurate, real-time estimates of medication ingestion.18,36 The data provided by these technologies may allow for more informed treatment decisions and potentially avoid unnecessary medication changes.
The duration of most studies reviewed here was short. Therefore, there was a lack of sufficient data on long-term use of DHTs for mental health. Results obtained for the short term look promising, but whether the large volume of data obtained from DHTs can indeed transform mental health care and improve patient outcomes remains to be determined. Considering the rapid proliferation of health technologies and increasingly widespread adoption, digital technologies have the potential to change the nature of clinical practice and health care. However, to integrate them into a health-care delivery system, clinicians need an evidence base that demonstrates the effectiveness of these technologies in improving the outcomes in larger patient populations. In a systematic review and meta-analysis of digital health interventions for children and young people with mental health disorders, Hollis et al noted a clinical benefit, particularly for computerized CBT for depression and anxiety.49 However, the authors did not find quality evidence to support the clinical benefit of technology in attention deficit/hyperactivity disorder, autism, psychosis, or eating disorders. Other systematic reviews have also pointed out that scientific evidence to support the efficacy of technology interventions is inadequate.22–24 Most DHTs are tested in pilot studies in a small population under controlled conditions, as also evidenced in this review. Additionally, owing to a lack of standardized evaluation and reporting, comparison of technologies is challenging.
Several efforts based on literature reviews and surveys of experts in the field have been undertaken to standardize the quality of evidence for mHealth interventions. The World Health Organization mHealth Technical Evidence Review Group recently developed a checklist of items for developing and reporting mHealth interventions.50 CONSORT-eHealth (Consolidated Standards of Reporting Trials of electronic and mHealth applications and online telehealth) is another tool recommended for use in reporting web-based and mHealth interventions.51 Bakker et al provided evidence-based recommendations for developing smartphone apps for mental health.52 These initiatives should lead to harmonization of evaluation and reporting methods, thus allowing clinicians to better understand and compare options to make informed choices.
Conclusion
There are increasing numbers of DHTs available for use in persons with SMI. Studies summarized in this review indicate that overall short-term usability and feasibility of technology are high in the SMI population, suggesting the potential utility of digital technology for incorporation into treatment of SMI. Real-world, naturalistic studies using larger samples will further facilitate the integration of digital technology into everyday mental health care.
Acknowledgments
Editorial support for development of this manuscript was provided by Vandana Sharma, PhD, at C4 MedSolutions LLC (Yardley, PA), a CHC Group company, and funded by Otsuka Pharmaceutical Development and Commercialization Inc.
Footnotes
Disclosure
SB is a pharmaceutical medicine fellow at Rutgers Robert Wood Johnson Medical School. RAB, TW, FF, FD, and TPS are employees of Otsuka Pharmaceutical Development and Commercialization Inc. The authors report no other conflict of interest in this work.
References
- 1.Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One. 2015;10(2):e0116820. doi: 10.1371/journal.pone.0116820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huxley CJ, Atherton H, Watkins JA, Griffiths F. Digital communication between clinician and patient and the impact on marginalised groups: a realist review in general practice. Br J Gen Pract. 2015;65(641):e813–e821. doi: 10.3399/bjgp15X687853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pepin R, Segal DL, Coolidge FL. Intrinsic and extrinsic barriers to mental health care among community-dwelling younger and older adults. Aging Ment Health. 2009;13(5):769–777. doi: 10.1080/13607860902918231. [DOI] [PubMed] [Google Scholar]
- 4.Rowan K, McAlpine DD, Blewett LA. Access and cost barriers to mental health care, by insurance status, 1999-2010. Health Aff (Millwood) 2013;32(10):1723–1730. doi: 10.1377/hlthaff.2013.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunnigham PJ. Beyond parity: primary care physicians’ perspectives on access to mental health care. Health Aff (Millwood) 2013;28(3):w490–w501. doi: 10.1377/hlthaff.28.3.w490. [DOI] [PubMed] [Google Scholar]
- 6.Poushter J. Smartphone ownership and internet usage continues to climb in emerging economies. 2016. [Accessed May 9, 2017]. Available from: http://www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climb-in-emerging-economies.
- 7.Quintiles IMS IMS health study: patient options expand as mobile healthcare apps address wellness and chronic disease treatment needs. 2015. [Accessed May 9, 2017]. Available from: http://www.imshealth.com/en/about-us/news/ims-health-study:-patient-options-expand-as-mobile-healthcare-apps-address-wellness-and-chronic-disease-treatment-needs.
- 8.Anthes E. Mental health: there’s an app for that. Nature. 2016;532(7597):20–23. doi: 10.1038/532020a. [DOI] [PubMed] [Google Scholar]
- 9.Vöhringer PA, Barroilhet SA, Amerio A, et al. Cognitive impairment in bipolar disorder and schizophrenia: a systematic review. Front Psychiatry. 2013;4:87. doi: 10.3389/fpsyt.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotondi AJ, Eack SM, Hanusa BH, Spring MB, Haas GL. Critical design elements of e-health applications for users with severe mental illness: singular focus, simple architecture, prominent contents, explicit navigation, and inclusive hyperlinks. Schizophr Bull. 2015;41(2):440–448. doi: 10.1093/schbul/sbt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depp CA, Mausbach B, Granholm E, et al. Mobile interventions for severe mental illness: design and preliminary data from three approaches. J Nerv Ment Dis. 2010;198(10):715–721. doi: 10.1097/NMD.0b013e3181f49ea3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Zeev D, Davis KE, Kaiser S, Krzsos I, Drake RE. Mobile technologies among people with serious mental illness: opportunities for future services. Adm Policy Ment Health. 2013;40(4):340–343. doi: 10.1007/s10488-012-0424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borzekowski DL, Leith J, Medoff DR, et al. Use of the Internet and other media for health information among clinic outpatients with serious mental illness. Psychiatr Serv. 2009;60(9):1265–1268. doi: 10.1176/ps.2009.60.9.1265. [DOI] [PubMed] [Google Scholar]
- 14.Robotham D, Satkunanathan S, Doughty L, Wykes T. Do we still have a digital divide in mental health? A five-year survey follow-up. J Med Internet Res. 2016;18(11):e309. doi: 10.2196/jmir.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gay K, Torous J, Joseph A, Pandya A, Duckworth K. Digital technology use among individuals with schizophrenia: results of an online survey. JMIR Ment Health. 2016;3(2):e15. doi: 10.2196/mental.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews G, Cuijpers P, Craske MG, McEvoy P, Titov N. Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: a meta-analysis. PLoS One. 2010;5(10):e13196. doi: 10.1371/journal.pone.0013196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beiwinkel T, Kindermann S, Maier A, et al. Using smartphones to monitor bipolar disorder symptoms: a pilot study. JMIR Ment Health. 2016;3(1):e2. doi: 10.2196/mental.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kane JM, Perlis RH, DiCarlo LA, Au-Yeung K, Duong J, Petrides G. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533–e540. doi: 10.4088/JCP.12m08222. [DOI] [PubMed] [Google Scholar]
- 19.Luxton DD, McCann RA, Bush NE, Mishkind MC, Reger GM. mHealth for mental health: integrating smartphone technology in behavioral healthcare. Prof Psychol Res Pr. 2011;42(6):505–512. [Google Scholar]
- 20.Price M, Yuen EK, Goetter EM, et al. mHealth: a mechanism to deliver more accessible, more effective mental health care. Clin Psychol Psychother. 2014;21(5):427–436. doi: 10.1002/cpp.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke G, Yarborough BJ. Evaluating the promise of health IT to enhance/expand the reach of mental health services. Gen Hosp Psychiatry. 2013;35(4):339–344. doi: 10.1016/j.genhosppsych.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donker T, Petrie K, Proudfoot J, Clarke J, Birch MR, Christensen H. Smartphones for smarter delivery of mental health programs: a systematic review. J Med Internet Res. 2013;15(11):e247. doi: 10.2196/jmir.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huguet A, Rao S, McGrath PJ, et al. A systematic review of cognitive behavioral therapy and behavioral activation apps for depression. PLoS One. 2016;11(5):e0154248. doi: 10.1371/journal.pone.0154248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firth J, Torous J. Smartphone apps for schizophrenia: a systematic review. JMIR Mhealth Uhealth. 2015;3(4):e102. doi: 10.2196/mhealth.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faurholt-Jepsen M, Ritz C, Frost M, et al. Mood instability in bipolar disorder type I versus type II: continuous daily electronic self-monitoring of illness activity using smartphones. J Affect Disord. 2015;186:342–349. doi: 10.1016/j.jad.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Forchuk C, Reiss JP, O’Regan T, Ethridge P, Donelle L, Rudnick A. Client perceptions of the Mental Health Engagement Network: a qualitative analysis of an electronic personal health record. BMC Psychiatry. 2015;15:250. doi: 10.1186/s12888-015-0614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ly KH, Trüschel A, Jarl L, et al. Behavioural activation versus mindfulness-based guided self-help treatment administered through a smartphone application: a randomised controlled trial. BMJ Open. 2014;4(1):e003440. doi: 10.1136/bmjopen-2013-003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Velligan D, Mintz J, Maples N, et al. A randomized trial comparing in person and electronic interventions for improving adherence to oral medications in schizophrenia. Schizophr Bull. 2013;39(5):999–1007. doi: 10.1093/schbul/sbs116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watts S, Mackenzie A, Thomas C, et al. CBT for depression: a pilot RCT comparing mobile phone vs. computer. BMC Psychiatry. 2013;13:49. doi: 10.1186/1471-244X-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Zeev D, Brenner CJ, Begale M, Duffecy J, Mohr DC, Mueser KT. Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophr Bull. 2014;40(6):1244–1253. doi: 10.1093/schbul/sbu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Zeev D, Frounfelker R, Morris SB, Corrigan PW. Predictors of self-stigma in schizophrenia: new insights using mobile technologies. J Dual Diagn. 2012;8(4):305–314. doi: 10.1080/15504263.2012.723311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Zeev D, Kaiser SM, Brenner CJ, Begale M, Duffecy J, Mohr DC. Development and usability testing of Focus: a smartphone system for self-management of schizophrenia. Psychiatr Rehabil J. 2013;36(4):289–296. doi: 10.1037/prj0000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hidalgo-Mazzei D, Mateu A, Reinares M, et al. Psychoeducation in bipolar disorder with a SIMPLe smartphone application: feasibility, acceptability and satisfaction. J Affect Disord. 2016;200:58–66. doi: 10.1016/j.jad.2016.04.042. [DOI] [PubMed] [Google Scholar]
- 35.Osipov M, Behzadi Y, Kane JM, Petrides G, Clifford GD. Objective identification and analysis of physiological and behavioral signs of schizophrenia. J Ment Health. 2015;24(5):276–282. doi: 10.3109/09638237.2015.1019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters-Strickland T, Pestreich L, Hatch A, et al. Usability of a novel digital medicine system in adults with schizophrenia treated with sensor-embedded tablets of aripiprazole. Neuropsychiatr Dis Treat. 2016;12:2587–2594. doi: 10.2147/NDT.S116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsanas A, Saunders KE, Bilderbeck AC, et al. Daily longitudinal self-monitoring of mood variability in bipolar disorder and borderline personality disorder. J Affect Disord. 2016;205:225–233. doi: 10.1016/j.jad.2016.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Depp CA, Moore RC, Dev SI, Mausbach BT, Eyler LT, Granholm EL. The temporal course and clinical correlates of subjective impulsivity in bipolar disorder as revealed through ecological momentary assessment. J Affect Disord. 2016;193:145–150. doi: 10.1016/j.jad.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore RC, Fazeli PL, Patterson TL, et al. UPSA-M: feasibility and initial validity of a mobile application of the UCSD performance-based skills assessment. Schizophr Res. 2015;164(1–3):187–192. doi: 10.1016/j.schres.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmier-Claus JE, Ainsworth J, Machin M, et al. The feasibility and validity of ambulatory self-report of psychotic symptoms using a smartphone software application. BMC Psychiatry. 2012;12:172. doi: 10.1186/1471-244X-12-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben-Zeev D, McHugo GJ, Xie H, Dobbins K, Young MA. Comparing retrospective reports to real-time/real-place mobile assessments in individuals with schizophrenia and a nonclinical comparison group. Schizophr Bull. 2012;38(3):396–404. doi: 10.1093/schbul/sbr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Husky M, Olié E, Guillaume S, Genty C, Swendsen J, Courtet P. Feasibility and validity of ecological momentary assessment in the investigation of suicide risk. Psychiatry Res. 2014;220(1–2):564–570. doi: 10.1016/j.psychres.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Doherty G, Coyle D, Matthews M. Design and evaluation guidelines for mental health technologies. Interact Comput. 2010;22(4):243–252. [Google Scholar]
- 44.Bidmon S, Terlutter R. Gender differences in searching for health information on the Internet and the virtual patient-physician relationship in Germany: exploratory results on how men and women differ and why. J Med Internet Res. 2015;17(6):e156. doi: 10.2196/jmir.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alkhaldi G, Hamilton FL, Lau R, Webster R, Michie S, Murray E. The effectiveness of prompts to promote engagement with digital interventions: a systematic review. J Med Internet Res. 2016;18(1):e6. doi: 10.2196/jmir.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmier-Claus JE, Rogers A, Ainsworth J, et al. Integrating mobile-phone based assessment for psychosis into people’s everyday lives and clinical care: a qualitative study. BMC Psychiatry. 2013;13:34. doi: 10.1186/1471-244X-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belisario JS, Jamsek J, Huckvale K, O’Donoghue J, Morrison CP, Car J. Comparison of self-administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database Syst Rev. 2015;(7):MR000042. doi: 10.1002/14651858.MR000042.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sajatovic M, Velligan DI, Weiden PJ, Valenstein MA, Ogedegbe G. Measurement of psychiatric treatment adherence. J Psychosom Res. 2010;69(6):591–599. doi: 10.1016/j.jpsychores.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollis C, Falconer CJ, Martin JL, et al. Annual research review: digital health interventions for children and young people with mental health problems – a systematic and meta-review. J Child Psychol Psychiatry. 2017;58(4):474–503. doi: 10.1111/jcpp.12663. [DOI] [PubMed] [Google Scholar]
- 50.Agarwal S, LeFevre AE, Lee J, et al. Guidelines for reporting of health interventions using mobile phones: mobile health (mHealth) evidence reporting and assessment (mERA) checklist. BMJ. 2016;352:i1174. doi: 10.1136/bmj.i1174. [DOI] [PubMed] [Google Scholar]
- 51.Eysenbach G, CONSORT-EHEALTH Group CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health interventions. J Med Internet Res. 2011;13(4):e126. doi: 10.2196/jmir.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bakker D, Kazantzis N, Rickwood D, Rickard N. Mental health smartphone apps: review and evidence-based recommendations for future developments. JMIR Ment Health. 2016;3(1):e7. doi: 10.2196/mental.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]