Abstract
The steadily increasing knowledge regarding pathogenetic mechanisms in autoimmune rheumatic diseases has paved the way to different therapeutic approaches. In particular, the market entry of biologics has dramatically modified the natural history of rheumatic chronic inflammatory diseases with a meaningful impact on patients’ quality of life. Among the wide spectrum of available biological treatments, rituximab (RTX), first used in the treatment of non-Hodgkin’s lymphoma, was later approved for rheumatoid arthritis and anti-neutrophil cytoplasmic antibodies-associated vasculitis. Nowadays, in rheumatology, RTX is also used with off-label indications in patients with systemic sclerosis, Sjögren’s syndrome and systemic lupus erythematosus. RTX is a monoclonal antibody directed to CD20 molecules expressed on the surfaces of pre-B and mature B lymphocytes. It acts by causing apoptosis of these cells with antibody- and complement-dependent cytotoxicity. As inflammatory responses to cell-associated immune complexes are key elements in the pathogenesis of several autoimmune rheumatic diseases, such an approach might be effective in these patients. In fact, RTX, by promoting the rapid and long-term depletion of circulating and lymphoid tissue-associated B cells, leads to a lower recruitment of these effector cells at sites of immune complex deposition, thus reducing inflammation and tissue damage. RTX is of the most interest to rheumatologists as it represents an important additional therapeutic approach. Thus, the advent in clinical practice of approved RTX biosimilars, such as CT-P10, may be of help in improving treatment access as well as in reducing costs.
Keywords: rituximab, rheumatoid arthritis, ANCA-associated vasculitis, systemic sclerosis, Sjögren’s syndrome, systemic lupus erythematosus, biologics, biosimilars, myositis, pregnancy, vaccination
Introduction
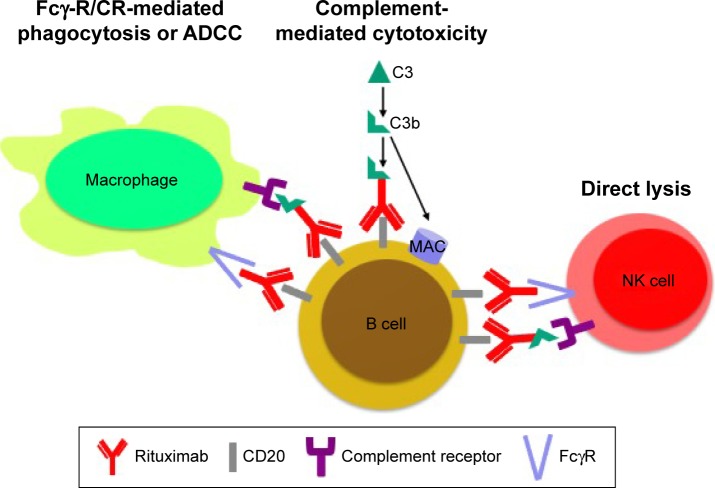
Rituximab (RTX) is a chimeric mouse/human monoclonal antibody that targets the transmembrane protein CD20 molecule on the surfaces of some but not all B cells. RTX by binding to CD20, that is expressed on pre-B and mature B lymphocytes, leads to apoptosis of these cells with antibody- and complement-dependent cytotoxicity (Figure 1). This mechanism of action leads, in most patients, to a selective peripheral B cell depletion for more than 24 weeks. However, other niches of B cells (eg, those in the synovium) are variably depleted. RTX has no or little effects on autoantibody levels, which are mainly secreted by mature plasma cells, but it is active on memory and mature B cells. Repopulation of peripheral B cells occurs after 6–9 months from RTX course, and it can be of particular utility in patients with scarce adherence to daily therapy.
Figure 1.
RTX has different mechanisms of action through activation of the complement cascade which leads to a direct lyse B cells by complement-mediated cytotoxicity, the recognition by both Fcγ receptors and complement receptors 1 and 3 on macrophages causes phagocytosis and antibody-dependent cell-mediated cytotoxicity and interaction with NK cells via FcγRIII and complement receptor 3.
Abbreviations: ADCC, antibody-dependent cell-mediated cytotoxicity; NK, natural killer; RTX, rituximab.
Nowadays, RTX is a well-established biologic agent for the treatment of some rheumatic autoimmune diseases such as refractory rheumatoid arthritis (RA)1,2 and anti-neutrophil cytoplasmic antibodies (ANCAs)-associated vasculitis (AAV).3
At the moment, RTX regimen is intravenous (IV) with slightly different dosages in rheumatic diseases ranging from 1,000 mg administered 2 weeks apart in RA to 375 mg/m2 weekly for 4 weeks in AVV. In all patients, premedication before each infusion with methylprednisolone 100 mg IV, acetaminophen and antihistamines is highly recommended.
This review provides insight into the current on- and off-label use of RTX in rheumatic diseases with a focus on the advent of biosimilars.
RTX in RA
In 2004, the first randomized double-blind placebo-controlled trial in patients with long-standing active RA, despite methotrexate treatment, demonstrated that a single course of two infusions of RTX, alone or in combination with either cyclophosphamide or continued methotrexate, provided significant improvement in clinical response at weeks 24 and 48.4
The efficacy and safety of different RTX doses plus methotrexate, with or without glucocorticoids, in patients with active RA who did not respond to disease-modifying antirheumatic drugs (DMARDs) were tested in the DANCER study.5 Both RTX doses (ie, 500 mg or 1,000 mg on days 1 and 15) were effective and well tolerated.5
Moreover, the MIRROR study showed that RTX dose escalation from two doses of 500 mg to two doses of 1,000 mg did not improve clinical response. Retreatment strategy from week 24 supported a sustained suppression of disease activity through to week 48.6
The Phase III SERENE study showed the efficacy and safety of RTX plus methotrexate in patients with active RA who were naive to prior biological treatment. RTX both 2×500 mg and 2×1,000 mg plus methotrexate significantly improved clinical outcomes at weeks 24 and 48.7
Further studies in patients with RA with inadequate response to antitumor necrosis factor (anti-TNF) therapies showed that a single course of RTX associated with methotrexate therapy provided significant improvements in disease activity and progression of radiological damage.8–10 A sustained clinical efficacy was better maintained after two courses of RTX about 6 months apart.10
In 2011, a Phase IIIb open-label prospective study (RESET) confirmed that RTX is an effective treatment option for patients who have not responded to a single TNF-α inhibitor, particularly for seropositive patients.11–13
The MIRAR study and real-life data indicate that switching to RTX is a successful treatment option for patients with RA failing on TNF antagonists.12,14,15
Treatment with RTX (2×1,000 mg) in combination with MTX has been shown to be an effective treatment for patients with MTX-naive RA, leading to sustained improvements in radiographic, clinical and functional outcomes over 2 years.16–18
RTX in AAV
AAV are rare diseases classified on the basis of both vascular inflammation distribution and the presence or absence of granulomatosis and asthma. AAV includes microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA; also known as Wegener’s granulomatosis) and eosinophilic GPA (also known as Churg–Strauss syndrome).19
RTX was approved by the Food and Drug Administration (FDA) for the treatment of patients with GPA and MPA in 2011.3
Two retrospective open-label studies reported remission (Birmingham Vasculitis Activity Score Modified for Wegener’s Granulomatosis: 0) in all the 21 AAV patients enrolled.20,21 Based on these successful results, the first seminal multicenter randomized double-blind controlled trial on RTX in AAV (RAVE) trial was designed.22 This study demonstrated that RTX therapy was not inferior to daily cyclophosphamide for induction of remission in severe AAV as a higher percentage of remission occurred in RTX-treated patients (64% vs 53%).22 Moreover, RTX appeared to be superior in patients with relapsing RA.
Results from 18-month extension of the RAVE trial demonstrated that a single course of RTX was as effective as continuous conventional immunosuppressive therapy for the induction and maintenance of remission in AAV.23
Further analysis of the RAVE trial showed that an increase in PR3-ANCA levels during remission was related to an increased risk of relapse, particularly among patients with renal involvement or alveolar hemorrhage.24
RTX was also studied for remission maintenance. The randomized controlled studies MAINRITSAN and RITAZEREM demonstrated that RTX was superior to azathioprine for remission maintenance in AAV, without increasing the adverse event rate.25,26
RTX in systemic lupus erythematosus (SLE)
Since B cells play a critical role in SLE, in the past 10 years, targeted B cell therapies have been proposed in these patients.27
B cell depletion therapy based on RTX is still unlicensed for SLE, but it is used to treat early onset and refractory disease. The most important studies on RTX in SLE are reported in Table 1. RTX has not been designed for SLE patients, but many uncontrolled studies described its utility in SLE patients who are refractory to conventional treatments.28–33 In fact, RTX is a recommended option in SLE nephritis in both European League Against Rheumatism (EULAR) and American College of Rheumatology guidelines.34
Table 1.
Results from the off-label use of RTX in SLE
| Studies | Study design | Number of patients | Drug regimen | Results |
|---|---|---|---|---|
| Merrill et al35 | Prospective Randomized (2:1) Double-blind Placebo-controlled |
257 SLE | RTX 1 g or placebo on days 1, 15, 168 and 182 | Extra-renal manifestations: no difference between RTX and placebo |
| Rovin et al36 | Prospective Randomized (1:1) Double-blind Placebo-controlled |
144 SLE | RTX 1 g or placebo on days 1, 15, 168 and 182 | Primary end point: no renal response at week 52 Reduction in anti-dsDNA and C3/C4 levels |
| Leandro et al28 | Prospective Open-label study | 24 SLE | In most cases: RTX 1 g, CYC 750 mg and MPD 250 mg 2 weeks apart | At 6 months: BILAG, anti-dsDNA and C3 improved |
| Lu et al29 | Retrospective | 50 SLE (45 with available follow-up at 6 months) | 46 of 50: RTX 1 g, CYC 750 mg and MPD 100–250 mg 2 weeks apart | BILAG Remission: 42% Partial remission: 47% Anti-dsDNA antibody titers: decreased C3: increased |
| Diaz-Lagares et al30 | Retrospective Multicenter Registry |
164 biopsy-proven lupus nephropathy | RTX with corticosteroids (99%) and immunosuppressive agents (76%, CYC and MMF) | At 6 and 12 months: Complete response: 27% and 30% Partial response: 40% and 37% No response: 33% At 12 months, significant improvement in proteinuria, albumin and protein/creatinine ratio Better response in type III lupus nephropathy Worse response in nephrotic syndrome and renal failure at the time of RTX administration |
| Condon et al32 | Cohort study Prospective Observational Monocentric |
50 biopsy-proven lupus nephropathy | RTX 1 g and MPD 500 mg 2 weeks apart, with MMF as maintenance therapy | At 52 weeks: Responders: 90% Complete biochemical remission: 52% Partial biochemical remission: 34% Relapses after 65.1 weeks (20–112) from remission: 12 Systemic flares: 6 |
| Witt et al33 | Registry Retrospective Multicenter Noninterventional |
85 active SLE | RTX 1 g 2 weeks apart 67: 1 course 6: 2 courses 2: 3 courses | Complete response: 46.8% Partial response: 34.2% No response: 19.0% SLEDAI: 12.2 → 3.3 Clinical (tender and swollen joint counts, fatigue, myalgia, general well-being, Raynaud’s phenomenon) and laboratory (anti-dsDNA, complement factors, hematologic parameters, proteinuria): improvement |
| Albert et al37 | Prospective Open-label Multicenter |
24 mild and moderate SLE without concomitant immunosuppressive therapy | RTX 1 g 2 weeks apart | 1-year follow-up In 18 patients, B cell levels in peripheral blood were available: Effective CD19+ B cell depletion: 17 B cell return before 24 weeks: 6 SLEDAI: improvement by week 55 in 70% Approximately one-third of the patients developed human anti-chimeric antibody titers correlated with poor B cell depletion |
| Lindholm et al38 | Retrospective Monocentric |
26 SLE with active nephritis (17) or autoantibody-mediated cytopenias (thrombocytopenia: 10 and hemolytic anemia: 4) refractory to conventional immunosuppressive treatment | RTX 375 mg/m2/week for 4 weeks added to conventional immunosuppressive therapy | Complete B cell depletion in all patients Complete or partial response in 11 patients with lupus nephritis was achieved after 6–12 months Significant increase in platelet count after 1 month Complete platelet count normalization at 6 weeks in five patients |
| Ramos-Casals et al39 | Multicenter Registry |
196 with systemic autoimmune diseases refractory to standard therapies 107 SLE |
91: RTX 375 mg/m2/week for 4 weeks 16: RTX 1 g 2 weeks apart |
Mean follow-up of 26.05±1.62 months Complete response: 47% Partial response: 34% No response: 24% Relapses in responders: 25% Deaths: 5% |
| Vital et al40 | Open-label Monocentric Observational |
39 active SLE | RTX 1 g 2 weeks apart | BILAG: significantly reduced Major clinical response: 51% Partial clinical response: 31% Relapse after 6–18 months: 50% B cell numbers: no response in 21 patients after RTX (included seven patients with no response) Memory B cell and plasmablast repopulation after 26 weeks faster in patients with earlier relapse |
| Fernandez-Nebro et al41 | Multicenter Retrospective Longitudinal study |
116 SLE nonresponder to standard therapy | 65%: RTX 1 g 2 weeks apart 30%: RTX 375 mg/m2/week for 4 weeks 5%: others |
After 6 months: Complete response: 17% Partial response: 44% After a mean follow-up of 20.0±15.2 months: Responses: 77.6% Relapses: 38% |
| Terrier et al42 | Registry Observational Prospective |
136 SLE | 60%: RTX 1 g 2 weeks apart 36%: RTX 375 mg/m2/week for 4 weeks 4%: others |
Safety of Estrogens in Lupus Erythematosus: National Assessment (SELENA)-SLEDAI: improvement in 71% Relapses in 41% of responders with a good response in 91% to retreatment |
| Pinto et al45 | Prospective Observational Multicenter |
42 severe and refractory SLE | RTX 1 g 2 weeks apart | Reduction in steroid requirement at 24 months At 12-month follow-up, remission according to proteinuria: Complete: 28% Partial: 36% At 12-month follow-up, remission according to creatinine clearance: Complete: 12.5% Partial: 33% No RTX reinfusion required: 80% |
Abbreviations: BILAG, British Isles Lupus Assessment Group; CYC, cyclophosphamide; MMF, mycophenolate mofetil; MPD, methylprednisolone; RTX, rituximab; SLE, systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
RTX failed primary end points in two randomized clinical trials (RCTs; EXPLORER in non-renal SLE and LUNAR in renal SLE).35,36 In recent years, interest in B cell therapies has been maintained as demonstrated by the approval of belimumab.
RTX is currently used for more severe forms and to achieve disease control rather than corticosteroid-sparing strategy in patients with lupus nephritis. Moreover, probably due to its efficacy in idiopathic autoimmune hemolytic anemia and idiopathic thrombocytopenia purpura (ITP), RTX is also used in patients with SLE complicated by thrombocytopenia and hemolytic anemia. RTX is less used in cutaneous and musculoskeletal SLE involvement. The efficacy of RTX in mucocutaneous manifestations is unclear, while RTX seems to be effective in articular manifestations.37–42
RTX has been also described as an effective therapy in anti-phospholipid syndrome secondary to SLE in the prevention of recurrent thrombotic events.43 Scarce and non-conclusive data are available on neuropsychiatric SLE.39–41,44,45
A study demonstrated that a single infusion of RTX was as effective as multiple doses with a reduction in cost therapy.46 Reports suggest RTX as an induction therapy followed by belimumab as maintenance.47 Two prospective clinical trials (NCT02260934 and NCT02284984 on ClinicalTrials.org) are currently ongoing to assess the efficacy of the sequential therapy with RTX followed by belimumab in SLE patients.
RTX is used differently all across Europe also for economical reasons.48
RTX in Sjögren syndrome (SS)
Traditional immunosuppressive therapies did not show effectiveness in RCTs. Nowadays, SS therapy is essentially based on symptomatic and supportive measures. As B cells play a pivotal role in SS pathogenesis, RTX has been suggested to be potentially useful.49 The most important studies on RTX in SS are listed in Table 2.
Table 2.
Results from the off-label use of RTX in SS
| Studies | Study design | Number of patients | Drug regimen | Results |
|---|---|---|---|---|
| Dass et al50 | Randomized Double-blind Placebo-controlled |
17 pSS and fatigue VAS >50 | RTX 1 g 2 weeks apart or placebo | At 6-month follow-up: Fatigue VAS: reduction >20% in RTX HRQOL: SF-36 better in RTX |
| Meijer et al51 | Randomized (2:1) Double-blind Placebo-controlled |
30 active pSS and a rate of SWS secretion ≥0.15 mL/minute | RTX 1 g 2 weeks apart or placebo | Follow-up at 5, 12, 24, 36 and 48 weeks Primary end point: SWS: better in RTX Secondary end points: Laboratory (B cell and RF): better in RTX Subjective variables (MFI and VAS): better in RTX Extra-glandular manifestations: better in RTX Better when compared to baseline values: SWS, B cell, RF, UWS, lacrimal gland function, MFI, SF-36 and sicca VAS |
| Devauchelle-Pensec et al52 | Randomized (1:1) Placebo-controlled Multicenter |
120 recent-onset or systemic pSS with 50 mm or greater on at least 2 of 4 VAS (global disease, pain, fatigue, dryness) | RTX 1 g 2 weeks apart or placebo | At 24 weeks Primary end points: Improvement of at least 30 mm in 2 of 4 VAS by week 24:no difference Some subjective efficacy with RTX before 24 weeks |
| Carubbi et al54 | Prospective Multicenter |
41 pSS with early and active disease (ESSDAI ≥6) | RTX or DMARDs | Follow-up for 120 weeks (at weeks 12, 24, 48, 72, 96 and 120): ESSDAI: better in RTX Other clinical parameters (self-reported global disease activity pain, sicca symptoms and fatigue VAS, UWS and Schirmer’s I test): better with RTX Minor salivary gland biopsies at baseline and at week 120: glandular infiltrate receded with RTX |
| Jousse-Joulin et al56 | Randomized (1:1) Double-blind Placebo-controlled Multicenter |
28 recent-onset or systemic pSS with 50 mm or greater on at least 2 of 4 VAS (global disease, pain, fatigue, dryness) | RTX 1 g 2 weeks apart or placebo | At 6-week follow-up: Salivary gland echostructure: better in RTX (50% vs 7%) Gland sizes: no change Vascularization: no change |
| Gottenberg et al61 | Registry Prospective |
78 pSS with systemic or severe glandular involvement | 86%: RTX 1 g 2 weeks apart 14%: RTX 375 mg/m2/week for 4 weeks |
Follow-up every 6 months for 5 years (78 patients with at least one follow-up) ESSDAI: decreased Median dosage of corticosteroid: decreased 41 retreatments |
| Meiners et al62 | Retrospective | 15 pSS | RTX 1 g 2 weeks apart for two courses Median interval between courses: 103 weeks |
Follow-up at 24 and 48 weeks after RTX treatment Better after both courses with RTX: ESSDAI, B cells, RF, MFI, IgG Improved significantly after first course but with a trend after second one: patient GDA and oral dryness VAS Improved significantly only after first course: ocular dryness VAS SWS: stable during the first 24 weeks of both courses, but with a significant at week 48 of the first course Less pronounced deterioration after the treatment course |
| Cornec et al63 | Open-label (group I) Placebo (group II) |
45 pSS | Group I (14): low-dose RTX (two 375 m2) Group II: full-dose RTX (two 1,000 g) (17) vs placebo (14) | At 24 weeks: SSRI-30: 50% in both RTX groups BCD duration: similar in both groups BCD duration: not associated with clinical response Responders: lower baseline proportions of SG B cells Baseline serum BAFF: correlated with the proportion of SG B cells and clinical response (higher levels in nonresponders) |
| Delli et al64 | Randomized (2:1) Double-blind Placebo-controlled |
20 RTX-treated and 10 placebo-treated pSS | RTX 1 g 2 weeks apart or placebo | Biopsies at baseline and 12 weeks after treatment: B cells, number and the severity of lymphoepithelial lesions and germinal centers: reduced in RTX T cells (CD3+): no change CD20+ higher in responders |
Abbreviations: BAFF, B cell-activating factor; BCD, B cell depletion; DMARDs, disease-modifying antirheumatic drugs; ESSDAI, EULAR Sjögren’s syndrome disease activity index; EULAR, European League Against Rheumatism; GDA, global disease activity; HRQOL, health-related quality of life; MFI, multidimensional fatigue inventory; pSS, primary SS; RF, rheumatoid factor; RTX, rituximab; SF-36, 36-Item Short Form Health Survey; SG, salivary gland; SS, Sjögren syndrome; SSRI, SS responder index; SWS, stimulated whole saliva; UWS, unstimulated whole saliva; VAS, Visual Analog Scale.
A meta-analysis published in 2016 evaluated 276 subjects (145 RTX and 131 placebo) from four RCTs: no statistically significant change regarding lacrimal gland function, as assessed by Schirmer test, was noted while an improvement in salivary gland production and fatigue were described at 24 weeks.50–53
Carubbi et al54 reported on 41 patients with SS an improvement at 120 weeks in unstimulated saliva flow rate and a decrease in labial salivary gland lymphocytic infiltration as assessed by focus score in patients treated with RTX compared to patients treated with conventional therapies.
RTX has been demonstrated to be effective at 6 months as assessed by both the SS responder index and ultrasonography.55,56
According to recently published SS treatment recommendations, RTX should be used in selected patients who have not responded to conventional therapies for sicca syndrome and for some extra-glandular manifestations (vasculitis, arthritis, lung involvement, peripheral neuropathy and parotid involvement).57
Treatment with belimumab could decrease B cell-activating factor (BAFF) levels, B cell hyperactivation and salivary gland B cell infiltration. Sequential treatment with belimumab and RTX has been suggested.58,59 Synergic action of RTX and belimumab is now under investigation also in other rheumatic conditions (NCT02260934, NCT02631538 and NCT02284984 on ClinicalTrials.org).
A retrospective study by a Taiwanese group on 10 patients with SS complicated by interstitial lung disease treated with RTX reported pulmonary involvement stabilization.60
RTX retreatment seems to be reasonable in patients who responded to first course with RTX, as reported by two different groups.61,62
It would be extremely important to identify predictor factors for RTX response. Moreover, the most adequate RTX regimen should be assessed throughout a specific trial.
Salivary gland B cell infiltration would be important to determine the efficacy of RTX even if its role has not yet been completely elucidated; the studies published are difficult to be compared as they report opposite results but they do significantly differ about methodology.63,64
Many reasons could be evoked to explain why biological therapies are ineffective in SS randomized trials. In a recent paper, the authors gave many possible explanations: incorrect diagnosis, nonrepresentative SS population enrolled in clinical trials, antinuclear antibody (ANA) false negativity, lack of marker for fatigue and other benign symptoms, and an unknown link between immune system and central nervous system.65
RTX in systemic sclerosis (SSc)
B cells play a central role in SSc pathogenesis. A mounting quantity of evidences provides a rationale for the use of RTX in SSc patients.66–68 The most significant studies on RTX in SSc are reported in Table 3.
Table 3.
Results from the off-label use of RTX in SSc
| Studies | Study design | Number of patients | Drug regimen | Results |
|---|---|---|---|---|
| Lafyatis et al71 | Open-label Observational |
15 dcSSc | RTX 1 g 2 weeks apart | Primary outcome: Change in mRSS at 6 months: no change Secondary outcomes: PFTs: stable Organ involvement: stable B cell infiltrates: depleted (vs baseline) Autoantibodies: modest changes |
| Bosello et al72 | Open-label | 9 SSc | RTX 1 g 2 weeks apart | Follow-up up to 36 months (skin biopsy at baseline and during the follow-up): After 6 months; skin score, disease activity index and disease severity index: decreased IL-6: reduced Serum B cells: reduced in seven patients B cells at baseline in three patients |
| Daoussis et al73 | Open-label | 8 dcSSc with ILD | RTX 375 mg/m2/week for 4 weeks | Long-term (2 years) safety and efficacy: Lung involvement (PFTs and HRCT): improved Skin involvement (mRSS and myofibroblast): improved |
| Smith et al74 | Open-label | 8 dcSSc | RTX 1 g 2 weeks apart | 24-week follow-up: Peripheral CD19+: reduced Skin sclerosis score: reduced Biopsies (dermal hyalinized collagen content and dermal myofibroblast numbers): change |
| Smith et al75 | Open-label | 8 dcSSc | RTX 1 g 2 weeks apart at baseline and after 6 months | 2-year follow-up: mRSS: decreased DAS: decreased Internal organ involvement: stable B cell depletion Biopsies (hyalinized collagen score): change |
| Moazedi-Fuerst et al76 | Open-label | 5 SSc with ILD nonresponders to CYC | RTX 500 mg 2 weeks apart every 3 months for 1 year | mRSS: decreased DLCO and FVC: increased Lung fibrosis (three patients): decreased Digital ulcerations: healed Severity of Raynaud’s phenomenon and vascular pain: decreased Number of capillary bleeds and megacapillaries: decreased B-lymphocyte count decreased Serum immunoglobulins, autoantibody titers or CRP levels: no change |
| Giuggioli et al77 | Open-label | 10 SSc | One or more cycles of RTX 375 mg/m2/week for 4 weeks | Follow-up at 6 months and at last follow-up (up to 72 months): mRSS: decreased at 6 months Other cutaneous manifestations (hypermelanosis, pruritus, calcinosis): improved Arthritis: improved ILD: stable in 6 and worsened in 2 Pro-inflammatory cytokines: a more or less pronounced reduction after the first RTX cycle |
| Daoussis et al78 | Randomized | 14 SSc | 8: RTX 375 m2 weekly for 4 weeks at baseline and at 24 weeks plus standard therapy 6: standard treatment alone |
1-year follow-up: FVC, DLCO and skin involvement: increased |
| Jordan et al81 | Registry Case–control |
88 SSc | 63: RTX 1 g 2 weeks apart 25: controls |
Primary end point: mRSS: reduced better in RTX Secondary end points: FVC: no further decline Safety measures: good |
| Bosello et al82 | Open-label | 29 dcSSc with or without ILD | RTX 1 g 2 weeks apart (more courses when needed) | Follow-up up to 68.9 months: Skin score, activity and severity indices improved significantly after 12 months and at final follow-up compared to baseline FVC and TLC: increased DLCO: stable HRCT: stable in 80% of patients |
| Daoussis et al83 | Multicenter Open-label |
51 SSc with ILD | 33: RTX 375 m2 weekly for 4 weeks 18: conventional therapy |
Median follow-up 4 years (up to 7 years): FVC: increased at 2-year follow-up, results confirmed at 7 years mRSS: outcome favorable to RTX at all times |
Abbreviations: CRP, C-reactive protein; CYC, cyclophosphamide; DAS, Disease Activity Score; dcSSc, diffuse cutaneous SSc; DLCO, carbon monoxide diffusing capacity; FVC, forced vital capacity; HRCT, high-resolution computed tomography; IL-6, interleukin-6; ILD, interstitial lung disease; mRSS, Rodnan skin thickness score; PFTs, pulmonary function tests; RTX, rituximab; SSc, systemic sclerosis; TLC, total lung capacity.
RTX was initially administered in patients affected by chronic graft-versus-host disease with a good response on skin fibrosis but not on extra-cutaneous manifestations.69
Uncontrolled studies and case reports described the efficacy of RTX in SSc patients with regard to pulmonary function, skin fibrosis, and less frequently arthritis, calcinosis and quality of life.70–80
A retrospective case–control analysis performed by the European Scleroderma Trial and Research Group described 63 SSc patients treated with RTX matched to 25 controls; authors described an improvement in skin involvement as assessed by modified Rodnan skin score and a stabilization of lung function as assessed by pulmonary lung function.81
Bosello et al82 described, in a cohort of 20 SSc patients, the effectiveness of RTX with regard to skin fibrosis and disease activity.
A recent published work by Daoussis et al83 showed a beneficial effect on lung involvement of RTX on 33 patients with a follow-up up to 7 years.
Due to heterogeneity of these studies (different dosages and modalities of administration, number of cycles and follow-up period, indications and end points) it would be very problematic to draw definitive conclusions. Not enough data are currently available in the literature to prescribe RTX in SSc patients who are naive to conventional therapy. RTX treatment seems to be promising in lung, skin and articular involvement secondary to SSc. There are little data on calcinosis, where RTX can be considered as a rescue therapy. A prospective, placebo-controlled, randomized trial is needed to definitively assess the efficacy of RTX in SSc. Meanwhile, RTX can be considered as a valid option in those patients who cannot tolerate or have contraindications for conventional therapies (ie, cyclophosphamide) or in patients where conventional therapies have already failed. RTX would be useful in pulmonary involvement as a maintenance therapy after induction with cyclophosphamide.
RTX in spondyloarthritis
The efficacy of RTX has also been tested in spondyloarthritis. A prospective open-label study showed that, among 20 patients with ankylosing spondylitis, 40% of anti-TNF-naive patients (N=10) achieved an improvement in Assessment of SpondyloArthritis international Society (ASAS) and 50% in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), while RTX did not seem to be effective in the TNF failure (N=10).84 Moreover, the same authors reported that five patients who flared on follow-up responded again when retreated with RTX.85
Thus, these studies include a small number of patients and are open label, and no clear conclusions can be drawn. Further studies are needed to ascertain the real therapeutic role of RTX.
Idiopathic inflammatory myopathies (IIMs)
IIMs include adult polymyositis (PM) and dermatomyositis (DM), juvenile PM and DM, anti-synthetase syndrome (ASS) and inclusion body myositis.
According to 2012 Cochrane review focused on therapy in DM and PM, no adequately designed study is present in the literature to assess which immunosuppressive drug is the best corticosteroid-sparing agent.86 As a result, the drug choice is often based on empirical considerations.
Since up to 80% of patients with IIMs show circulating autoantibodies and B cells that are found within inflamed muscle fibers, RTX therapy seems to be reasonable.87
Although the use of RTX in IIMs is rational and several uncontrolled trials suggested its utility, the RTX in myositis (RIM) trial, conducted on 195 patients, failed to reach both primary and secondary end points;88 however, almost 80% of patients responded to RTX treatment.
A subanalysis of RIM trial also demonstrated RTX as effective in refractory skin involvement in patients with both adult and juvenile DM.89
Some evidence suggests that RTX might be useful in interstitial lung disease secondary to IIMs, especially when related to ASS.90–93
The presence of antibodies predicts a good response to RTX.94,95 Moreover, their titers decrease after therapy with variable correlation with disease activity and muscle enzyme.96
RTX biosimilars
RTX patents expired in Europe in 2013 and in the USA in 2016. Various Phases I, II and III clinical trials are ongoing (JHL1101, ABP 798, MabionCD20, PF-05280586, RTXM83, SAIT101, CT-P10, GP-2013).
European Medicines Agency (EMA) has recently approved the first RTX biosimilar, CT-P10, in RA. In the pivotal trial, patients with active RA were randomly assigned (2:1), to receive CT-P10 1,000 mg or RTX 1,000 mg 2 weeks apart. Patients were randomized to receive the treatment (50 patients for each group). Additional 50 patients were recruited to the CT-P10 group to better assess its safety. CT-P10 was demonstrated to be equivalent regarding pharmacokinetics and efficacy with similar immunogenicity and safety profiles as the originator.97
Moreover, patients who completed the follow-up at 72 weeks (N=87: 58 in the CT-P10 group and 29 in the RTX group) entered into the open-label extension study for 56 weeks. Patients of each group received CT-P10 according to DAS28. Patients who switched from RTX to CT-P10 demonstrated comparable efficacy and safety profiles compared to those who maintained CT-P10. In RA patients, maintained CT-P10 was also well tolerated and effective up to 2 years.98
EMA is also currently evaluating GP2013 in RA. GP2013 has been demonstrated to be comparable to the originator in a trial recently published as an abstract.99
PF-05280586 was proven to be similar to the EU and US originator with regard to pharmacokinetics, CD19 depletion, antidrugs antibodies production and adverse events in RA patients.100
Moreover, RTX biosimilars (BCD-020, Baball and MabTas) have been licensed in countries where regulatory processes are not as strict as FDA and EMA recommendations.
Of note, other biosimilars (ie, infliximab and etanercept) have been successfully introduced in the treatment of RA. Biosimilars have no clinical meaningful differences, in terms of efficacy and safety with respect to the originator; thanks to cost saving, they should be considered and their use should be promoted. The availability of biosimilars would allow patients to receive medications that might otherwise be unaffordable to them.101
RTX in pregnancy
RTX was shown not to have any teratogenic effect in animals.102 In human beings, when RTX is administered during the second and third trimester, similar levels are found in mother and cord blood.103,104
Chakravarty et al105 reported 153 pregnancies exposed to RTX in patients affected by RA, non-Hodgkin lymphoma and other autoimmune diseases: 90 live births (22 premature and one extremely premature births), 33 miscarriages, 28 elective terminations, one late fetal loss and one maternal death due to cerebral hemorrhage in idiopathic thrombocytopenic purpura. Among live births, two congenital malformations, one death for unknown causes (at 6 months), 11 hematological abnormalities without infectious complication and four neonatal infections were reported.105 In particular, 21 patients received RTX during the second or third trimester, among them no maternal death, neonatal death or congenital malformations were noted, whereas cytopenia was reported in seven newborns.105
RTX exposure before conception or during early pregnancy does not provoke B cell depletion in newborns, whereas during the late stage of pregnancy (second and third trimester) RTX is able to reduce B cells that usually normalize after 3–6 months. Mothers and newborns, exposed to RTX during second and third trimester, should be monitored for the risk of infections since neutropenia and B cell depletion have been described in newborns.104,106–108
Although no fetus damage has been reported in pregnancies exposed to RTX during the first trimester, this therapy, according to EULAR recommendations, should be considered only when no other therapeutic option is available.
According to the British Society of Rheumatology (BSR) and British Health Professionals in Rheumatology(BHPR) guidelines on prescribing drugs in pregnancy and breastfeeding, an effective contraception is recommended while taking RTX and for 12 months following treatment.109
According to EULAR recommendations, when RTX is administered before week 22, vaccinations can be performed according to local guidelines (live vaccines included). When administered later in pregnancy, live vaccines should be avoided till 6 months of life. Due to the lack of data, lactation should be avoided.110
Miscellaneous
RTX has been shown to impact on vaccine immunogenicity, thus highlighting the importance of the right timing of vaccines in relation to RTX administration.111 For this reason, the better results in terms of humoral response are reported 6 months or more after RTX dosing.112,113 Vaccinations should be considered at least 4 weeks before RTX administration. In particular, a significant humoral response impairment has been reported for influenza and pneumococcal vaccinations.112–117 No data are available on the effects of RTX on hepatitis B virus (HBV), human papilloma virus or yellow fever vaccines. Safety for live vaccines has not been studied in patients treated with RTX; thus, these vaccines are considered contraindicated in this setting.
Screening serologies for HBV and hepatitis C virus (HCV) must be undertaken even if resolved HBV hepatitis reactivation has been rarely reported.118,119
In patients with HBsAg and anti-HBc negativity, vaccination should be considered before RTX initiation. By contrast, patients who are HBsAg and/or anti-HBc positive should be referred to a hepatologist for consideration of a prophylactic therapy, and HBV DNA levels have to be closely monitored if RTX is administered.118,119
With regard to HCV, RTX is used in the treatment of HCV-induced cryoglobulinemia. HCV should be screened, and for chronic HCV carriers, collaboration with a hepatologist is mandatory to plan a treatment strategy.18,120
Before RTX administration, routine screening for tuberculosis is suggested, even if it is not currently believed to be necessary. Patients with active tuberculosis should be appropriately treated and RTX should not be initiated.121
The long-term RTX safety report highlighted that serious opportunistic infections were rare. Among these, the reactivation of the John Cunningham (JC) virus leading to progressive multifocal leukoencephalopathy has been reported in patients with autoimmune diseases who should be informed of this risk.122
Finally, it is well known that long-term RTX administration is associated with hypogammaglobulinemia whose consequences are still unclear. It is recommended to evaluate baseline immunoglobulin levels and to consider cessation of therapy when the IgG level drops progressively.123
Moreover, attention should be paid to late-onset neutropenia that has been described as a potential RTX-related adverse event.124
Conclusion
RTX is currently considered useful and a relatively safe biological agent in the treatment of some rheumatic diseases.
Although RTX has been demonstrated to be relatively safe for infections, particular attention should be paid in the presence of HBV for the risk of reactivation.
Pregnancy during RTX treatment should be avoided since RTX, especially when administered during second and third trimester, increases the risk of infection in the mother and in the newborn.
RTX has been demonstrated useful in RA and AAV, and it is currently approved in many countries with these indications. RTX is also administered in other rheumatic conditions, such as SLE, SS and SSc, refractory to conventional therapies, but its utility in these conditions has not yet been completely and fully elucidated.
Moreover, further studies are needed to clarify some controversial points such as the association with concomitant DMARDs, RTX dosage and the optimal interval for retreatment. The availability of approved RTX biosimilars, such as CT-P10, would allow a widespread access of this treatment with cost saving. More likely, the harmonization of guidelines and recommendations on the use of biosimilars will be of help in clinical practice.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dorner T, Burmester GR. The role of B cells in rheumatoid arthritis: mechanisms and therapeutic targets. Curr Opin Rheumatol. 2003;15(3):246–252. doi: 10.1097/00002281-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Olsen NJ, Stein CM. New drugs for rheumatoid arthritis. N Engl J Med. 2004;350(21):2167–2179. doi: 10.1056/NEJMra032906. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration [webpage on the Internet] Questions and Answers on Rituximab (added 12/19/2006) [Accessed August 9, 2017]. Available from: https://wayback.archive-it.org/7993/20170722191606/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109107.htm.
- 4.Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 5.Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54(5):1390–1400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 6.Rubbert-Roth A, Tak PP, Zerbini C, et al. Efficacy and safety of various repeat treatment dosing regimens of rituximab in patients with active rheumatoid arthritis: results of a phase III randomized study (MIRROR) Rheumatology. 2010;49(9):1683–1693. doi: 10.1093/rheumatology/keq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery P, Deodhar A, Rigby WF, et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)) Ann Rheum Dis. 2010;69(9):1629–1635. doi: 10.1136/ard.2009.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SB, Keystone E, Genovese MC, et al. Continued inhibition of structural damage over 2 years in patients with rheumatoid arthritis treated with rituximab in combination with methotrexate. Ann Rheum Dis. 2010;69(6):1158–1161. doi: 10.1136/ard.2009.119222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mease PJ, Cohen S, Gaylis NB, et al. Efficacy and safety of retreatment in patients with rheumatoid arthritis with previous inadequate response to tumor necrosis factor inhibitors: results from the SUNRISE trial. J Rheumatol. 2010;37(5):917–927. doi: 10.3899/jrheum.090442. [DOI] [PubMed] [Google Scholar]
- 11.Haraoui B, Bokarewa M, Kallmeyer I, Bykerk VP, Investigators R. Safety and effectiveness of rituximab in patients with rheumatoid arthritis following an inadequate response to 1 prior tumor necrosis factor inhibitor: the RESET Trial. J Rheumatol. 2011;38(12):2548–2556. doi: 10.3899/jrheum.110444. [DOI] [PubMed] [Google Scholar]
- 12.Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis. 2015;74(6):979–984. doi: 10.1136/annrheumdis-2013-203993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers. 2013;35(6):727–734. doi: 10.1155/2013/726598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Reino JJ, Maneiro JR, Ruiz J, et al. Comparative effectiveness of switching to alternative tumour necrosis factor (TNF) antagonists versus switching to rituximab in patients with rheumatoid arthritis who failed previous TNF antagonists: the MIRAR Study. Ann Rheum Dis. 2012;71(11):1861–1864. doi: 10.1136/annrheumdis-2012-201324. [DOI] [PubMed] [Google Scholar]
- 15.Ingegnoli F, Favalli EG, Meroni PL. Does polymorphism of genes coding for pro-inflammatory mediators predict the clinical response to tnf alpha blocking agents? A review analysis of the literature. Autoimmun Rev. 2011;10(8):460–463. doi: 10.1016/j.autrev.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Tak PP, Rigby WF, Rubbert-Roth A, et al. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann Rheum Dis. 2011;70(1):39–46. doi: 10.1136/ard.2010.137703. [DOI] [PubMed] [Google Scholar]
- 17.Tak PP, Rigby W, Rubbert-Roth A, et al. Sustained inhibition of progressive joint damage with rituximab plus methotrexate in early active rheumatoid arthritis: 2-year results from the randomised controlled trial IMAGE. Ann Rheum Dis. 2012;71(3):351–357. doi: 10.1136/annrheumdis-2011-200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mok CC. Rituximab for the treatment of rheumatoid arthritis: an update. Drug Des Devel Ther. 2013;8:87–100. doi: 10.2147/DDDT.S41645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 20.Keogh KA, Limper AH. Characterization of lymphocyte populations in nonspecific interstitial pneumonia. Respir Res. 2005;6:137. doi: 10.1186/1465-9921-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial. Am J Respir Crit Care Med. 2006;173(2):180–187. doi: 10.1164/rccm.200507-1144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Specks U, Merkel PA, Seo P, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369(5):417–427. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fussner LA, Hummel AM, Schroeder DR, et al. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol. 2016;68(7):1700–1710. doi: 10.1002/art.39637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371(19):1771–1780. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 26.Gopaluni S, Smith RM, Lewin M, et al. Rituximab versus azathioprine as therapy for maintenance of remission for anti-neutrophil cytoplasm antibody-associated vasculitis (RITAZAREM): study protocol for a randomized controlled trial. Trials. 2017;18(1):112. doi: 10.1186/s13063-017-1857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6(6):326–337. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leandro MJ, Cambridge G, Edwards JC, Ehrenstein MR, Isenberg DA. B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology. 2005;44(12):1542–1545. doi: 10.1093/rheumatology/kei080. [DOI] [PubMed] [Google Scholar]
- 29.Lu TY, Ng KP, Cambridge G, et al. A retrospective seven-year analysis of the use of B cell depletion therapy in systemic lupus erythematosus at University College London Hospital: the first fifty patients. Arthritis Rheum. 2009;61(4):482–487. doi: 10.1002/art.24341. [DOI] [PubMed] [Google Scholar]
- 30.Diaz-Lagares C, Croca S, Sangle S, et al. Efficacy of rituximab in 164 patients with biopsy-proven lupus nephritis: pooled data from European cohorts. Autoimmun Rev. 2012;11(5):357–364. doi: 10.1016/j.autrev.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Lightstone L. The landscape after LUNAR: rituximab’s crater-filled path. Arthritis Rheum. 2012;64(4):962–965. doi: 10.1002/art.34362. [DOI] [PubMed] [Google Scholar]
- 32.Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013;72(8):1280–1286. doi: 10.1136/annrheumdis-2012-202844. [DOI] [PubMed] [Google Scholar]
- 33.Witt M, Grunke M, Proft F, et al. Clinical outcomes and safety of rituximab treatment for patients with systemic lupus erythematosus (SLE) – results from a nationwide cohort in Germany (GRAID) Lupus. 2013;22(11):1142–1149. doi: 10.1177/0961203313503912. [DOI] [PubMed] [Google Scholar]
- 34.Wilhelmus S, Bajema IM, Bertsias GK, et al. Lupus nephritis management guidelines compared. Nephrol Dial Transplant. 2016;31(6):904–913. doi: 10.1093/ndt/gfv102. [DOI] [PubMed] [Google Scholar]
- 35.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62(1):222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 37.Albert D, Dunham J, Khan S, et al. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Ann Rheum Dis. 2008;67(12):1724–1731. doi: 10.1136/ard.2007.083162. [DOI] [PubMed] [Google Scholar]
- 38.Lindholm C, Borjesson-Asp K, Zendjanchi K, Sundqvist AC, Tarkowski A, Bokarewa M. Longterm clinical and immunological effects of anti-CD20 treatment in patients with refractory systemic lupus erythematosus. J Rheumatol. 2008;35(5):826–833. [PubMed] [Google Scholar]
- 39.Ramos-Casals M, Garcia-Hernandez FJ, de Ramon E, et al. Off-label use of rituximab in 196 patients with severe, refractory systemic autoimmune diseases. Clin Exp Rheumatol. 2010;28(4):468–476. [PubMed] [Google Scholar]
- 40.Vital EM, Dass S, Buch MH, et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 2011;63(10):3038–3047. doi: 10.1002/art.30466. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Nebro A, de la Fuente JL, Carreno L, et al. Multicenter longitudinal study of B-lymphocyte depletion in refractory systemic lupus erythematosus: the LESIMAB study. Lupus. 2012;21(10):1063–1076. doi: 10.1177/0961203312446627. [DOI] [PubMed] [Google Scholar]
- 42.Terrier B, Amoura Z, Ravaud P, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum. 2010;62(8):2458–2466. doi: 10.1002/art.27541. [DOI] [PubMed] [Google Scholar]
- 43.Wang CR, Liu MF. Rituximab usage in systemic lupus erythematosus-associated antiphospholipid syndrome: a single-center experience. Semin Arthritis Rheum. 2016;46(1):102–108. doi: 10.1016/j.semarthrit.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Tokunaga M, Saito K, Kawabata D, et al. Efficacy of rituximab (anti-CD20) for refractory systemic lupus erythematosus involving the central nervous system. Ann Rheum Dis. 2007;66(4):470–475. doi: 10.1136/ard.2006.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinto LF, Velasquez CJ, Prieto C, Mestra L, Forero E, Marquez JD. Rituximab induces a rapid and sustained remission in Colombian patients with severe and refractory systemic lupus erythematosus. Lupus. 2011;20(11):1219–1226. doi: 10.1177/0961203311409273. [DOI] [PubMed] [Google Scholar]
- 46.Kotagiri P, Martin A, Hughes P, Becker G, Nicholls K. Single-dose rituximab in refractory lupus nephritis. Intern Med J. 2016;46(8):899–901. doi: 10.1111/imj.13136. [DOI] [PubMed] [Google Scholar]
- 47.Simonetta F, Allali D, Roux-Lombard P, Chizzolini C. Successful treatment of refractory lupus nephritis by the sequential use of rituximab and belimumab. Joint Bone Spine. 2016;84(2):235–236. doi: 10.1016/j.jbspin.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Ryden-Aulin M, Boumpas D, Bultink I, et al. Off-label use of rituximab for systemic lupus erythematosus in Europe. Lupus Sci Med. 2016;3(1):e000163. doi: 10.1136/lupus-2016-000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cornec D, Devauchelle-Pensec V, Tobon GJ, Pers JO, Jousse-Joulin S, Saraux A. B cells in Sjogren’s syndrome: from pathophysiology to diagnosis and treatment. J Autoimmun. 2012;39(3):161–167. doi: 10.1016/j.jaut.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Dass S, Bowman SJ, Vital EM, et al. Reduction of fatigue in Sjogren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann Rheum Dis. 2008;67(11):1541–1544. doi: 10.1136/ard.2007.083865. [DOI] [PubMed] [Google Scholar]
- 51.Meijer JM, Meiners PM, Vissink A, et al. Effectiveness of rituximab treatment in primary Sjogren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(4):960–968. doi: 10.1002/art.27314. [DOI] [PubMed] [Google Scholar]
- 52.Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, et al. Treatment of primary Sjogren syndrome with rituximab: a randomized trial. Ann Intern Med. 2014;160(4):233–242. doi: 10.7326/M13-1085. [DOI] [PubMed] [Google Scholar]
- 53.Souza FB, Porfirio GJ, Andriolo BN, Albuquerque JV, Trevisani VF. Rituximab effectiveness and safety for treating primary Sjogren’s syndrome (pSS): systematic review and meta-analysis. PLoS One. 2016;11(3):e0150749. doi: 10.1371/journal.pone.0150749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carubbi F, Cipriani P, Marrelli A, et al. Efficacy and safety of rituximab treatment in early primary Sjogren’s syndrome: a prospective, multi-center, follow-up study. Arthritis Res Ther. 2013;15(5):R172. doi: 10.1186/ar4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cornec D, Devauchelle-Pensec V, Mariette X, et al. Development of the Sjogren’s Syndrome Responder Index, a data-driven composite endpoint for assessing treatment efficacy. Rheumatology. 2015;54(9):1699–1708. doi: 10.1093/rheumatology/kev114. [DOI] [PubMed] [Google Scholar]
- 56.Jousse-Joulin S, Devauchelle-Pensec V, Cornec D, et al. Brief report: ultrasonographic assessment of salivary gland response to rituximab in primary Sjogren’s syndrome. Arthritis Rheumatol. 2015;67(6):1623–1628. doi: 10.1002/art.39088. [DOI] [PubMed] [Google Scholar]
- 57.Carsons SE, Vivino FB, Parke A, et al. Treatment guidelines for rheumatologic manifestations of Sjogren’s syndrome: use of biologic agents, management of fatigue, and inflammatory musculoskeletal pain. Arthritis Care Res. 2016;69(4):517–527. doi: 10.1002/acr.22968. [DOI] [PubMed] [Google Scholar]
- 58.De Vita S, Quartuccio L, Salvin S, et al. Sequential therapy with belimumab followed by rituximab in Sjogren’s syndrome associated with B-cell lymphoproliferation and overexpression of BAFF: evidence for long-term efficacy. Clin Exp Rheumatol. 2014;32(4):490–494. [PubMed] [Google Scholar]
- 59.Mariette X, Seror R, Quartuccio L, et al. Efficacy and safety of belimumab in primary Sjogren’s syndrome: results of the BELISS open-label phase II study. Ann Rheum Dis. 2015;74(3):526–531. doi: 10.1136/annrheumdis-2013-203991. [DOI] [PubMed] [Google Scholar]
- 60.Chen MH, Chen CK, Chou HP, Chen MH, Tsai CY, Chang DM. Rituximab therapy in primary Sjogren’s syndrome with interstitial lung disease: a retrospective cohort study. Clin Exp Rheumatol. 2016;34(6):1077–1084. [PubMed] [Google Scholar]
- 61.Gottenberg JE, Cinquetti G, Larroche C, et al. Efficacy of rituximab in systemic manifestations of primary Sjogren’s syndrome: results in 78 patients of the AutoImmune and Rituximab registry. Ann Rheum Dis. 2013;72(6):1026–1031. doi: 10.1136/annrheumdis-2012-202293. [DOI] [PubMed] [Google Scholar]
- 62.Meiners PM, Arends S, Meijer JM, et al. Efficacy of retreatment with rituximab in patients with primary Sjogren’s syndrome. Clin Exp Rheumatol. 2015;33(3):443–444. [PubMed] [Google Scholar]
- 63.Cornec D, Costa S, Devauchelle-Pensec V, et al. Blood and salivary-gland BAFF-driven B-cell hyperactivity is associated to rituximab inefficacy in primary Sjogren’s syndrome. J Autoimmun. 2016;67:102–110. doi: 10.1016/j.jaut.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Delli K, Haacke EA, Kroese FG, et al. Towards personalised treatment in primary Sjogren’s syndrome: baseline parotid histopathology predicts responsiveness to rituximab treatment. Ann Rheum Dis. 2016;75(11):1933–1938. doi: 10.1136/annrheumdis-2015-208304. [DOI] [PubMed] [Google Scholar]
- 65.Fox RI, Fox CM. Sjogren syndrome: why do clinical trials fail? Rheum Dis Clin North Am. 2016;42(3):519–530. doi: 10.1016/j.rdc.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Bosello S, De Luca G, Tolusso B, et al. B cells in systemic sclerosis: a possible target for therapy. Autoimmun Rev. 2011;10(10):624–630. doi: 10.1016/j.autrev.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 67.Ingegnoli F, Trabattoni D, Saresella M, Fantini F, Clerici M. Distinct immune profiles characterize patients with diffuse or limited systemic sclerosis. Clin Immunol. 2003;108(1):21–28. doi: 10.1016/s1521-6616(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 68.Ingegnoli F, Gualtierotti R, Schioppo T, et al. Fibrosis biomarkers in isolated Raynaud’s phenomenon: too little, too soon? Ann Rheum Dis. 2014;73(5):940–941. doi: 10.1136/annrheumdis-2013-204009. [DOI] [PubMed] [Google Scholar]
- 69.Okamoto M, Okano A, Akamatsu S, et al. Rituximab is effective for steroid-refractory sclerodermatous chronic graft-versus-host disease. Leukemia. 2006;20(1):172–173. doi: 10.1038/sj.leu.2403996. [DOI] [PubMed] [Google Scholar]
- 70.McGonagle D, Tan AL, Madden J, et al. Successful treatment of resistant scleroderma-associated interstitial lung disease with rituximab. Rheumatology. 2008;47(4):552–553. doi: 10.1093/rheumatology/kem357. [DOI] [PubMed] [Google Scholar]
- 71.Lafyatis R, Kissin E, York M, et al. B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2009;60(2):578–583. doi: 10.1002/art.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bosello S, De Santis M, Lama G, et al. B cell depletion in diffuse progressive systemic sclerosis: safety, skin score modification and IL-6 modulation in an up to thirty-six months follow-up open-label trial. Arthritis Res Ther. 2010;12(2):R54. doi: 10.1186/ar2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daoussis D, Liossis SN, Tsamandas AC, et al. Effect of long-term treatment with rituximab on pulmonary function and skin fibrosis in patients with diffuse systemic sclerosis. Clin Exp Rheumatol. 2012;30(2 suppl 71):S17–S22. [PubMed] [Google Scholar]
- 74.Smith V, Van Praet JT, Vandooren B, et al. Rituximab in diffuse cutaneous systemic sclerosis: an open-label clinical and histopathological study. Ann Rheum Dis. 2010;69(1):193–197. doi: 10.1136/ard.2008.095463. [DOI] [PubMed] [Google Scholar]
- 75.Smith V, Piette Y, van Praet JT, et al. Two-year results of an open pilot study of a 2-treatment course with rituximab in patients with early systemic sclerosis with diffuse skin involvement. J Rheumatol. 2013;40(1):52–57. doi: 10.3899/jrheum.120778. [DOI] [PubMed] [Google Scholar]
- 76.Moazedi-Fuerst FC, Kielhauser SM, Brickmann K, et al. Rituximab for systemic sclerosis: arrest of pulmonary disease progression in five cases. Results of a lower dosage and shorter interval regimen. Scand J Rheumatol. 2014;43(3):257–258. doi: 10.3109/03009742.2013.869617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giuggioli D, Lumetti F, Colaci M, Fallahi P, Antonelli A, Ferri C. Rituximab in the treatment of patients with systemic sclerosis. Our experience and review of the literature. Autoimmun Rev. 2015;14(11):1072–1078. doi: 10.1016/j.autrev.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 78.Daoussis D, Liossis SN, Tsamandas AC, et al. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology. 2010;49(2):271–280. doi: 10.1093/rheumatology/kep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gualtierotti R, Ingegnoli F, Scalone L, et al. Feasibility, acceptability and construct validity of EQ-5D in systemic sclerosis. Swiss Med Wkly. 2017;146:w14394. doi: 10.4414/smw.2016.14394. [DOI] [PubMed] [Google Scholar]
- 80.Ghrenassia E, Avouac J, Khanna D, et al. Prevalence, correlates and outcomes of gastric antral vascular ectasia in systemic sclerosis: a EUSTAR case-control study. J Rheumatol. 2014;41(1):99–105. doi: 10.3899/jrheum.130386. [DOI] [PubMed] [Google Scholar]
- 81.Jordan S, Distler JH, Maurer B, et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. 2015;74(6):1188–1194. doi: 10.1136/annrheumdis-2013-204522. [DOI] [PubMed] [Google Scholar]
- 82.Bosello SL, De Luca G, Rucco M, et al. Long-term efficacy of B cell depletion therapy on lung and skin involvement in diffuse systemic sclerosis. Semin Arthritis Rheum. 2015;44(4):428–436. doi: 10.1016/j.semarthrit.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 83.Daoussis D, Melissaropoulos K, Sakellaropoulos G, et al. A multi-center, open-label, comparative study of B-cell depletion therapy with Rituximab for systemic sclerosis-associated interstitial lung disease. Semin Arthritis Rheum. 2016;46(5):625–631. doi: 10.1016/j.semarthrit.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Song IH, Heldmann F, Rudwaleit M, et al. Different response to rituximab in tumor necrosis factor blocker-naive patients with active ankylosing spondylitis and in patients in whom tumor necrosis factor blockers have failed: a twenty-four-week clinical trial. Arthritis Rheum. 2010;62(5):1290–1297. doi: 10.1002/art.27383. [DOI] [PubMed] [Google Scholar]
- 85.Song IH, Heldmann F, Rudwaleit M, et al. One-year follow-up of ankylosing spondylitis patients responding to rituximab treatment and re-treated in case of a flare. Ann Rheum Dis. 2013;72(2):305–306. doi: 10.1136/annrheumdis-2012-201926. [DOI] [PubMed] [Google Scholar]
- 86.Gordon PA, Winer JB, Hoogendijk JE, Choy EH. Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev. 2012;(8):CD003643. doi: 10.1002/14651858.CD003643.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shinjo SK, de Souza FH, de Moraes JC. Dermatomyositis and polymyositis: from immunopathology to immunotherapy (immunobiologics) Rev Bras Reumatol. 2013;53(1):101–110. doi: 10.1016/s2255-5021(13)70010-5. [DOI] [PubMed] [Google Scholar]
- 88.Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65(2):314–324. doi: 10.1002/art.37754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aggarwal R, Loganathan P, Koontz D, Qi Z, Reed AM, Oddis CV. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2017;56(2):247–254. doi: 10.1093/rheumatology/kew396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lambotte O, Kotb R, Maigne G, Blanc FX, Goujard C, Delfraissy JF. Efficacy of rituximab in refractory polymyositis. J Rheumatol. 2005;32(7):1369–1370. [PubMed] [Google Scholar]
- 91.Marie I, Dominique S, Janvresse A, Levesque H, Menard JF. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med. 2012;106(4):581–587. doi: 10.1016/j.rmed.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 92.Rider LG, Yip AL, Horkayne-Szakaly I, et al. Novel assessment tools to evaluate clinical and laboratory responses in a subset of patients enrolled in the Rituximab in Myositis trial. Clin Exp Rheumatol. 2014;32(5):689–696. [PMC free article] [PubMed] [Google Scholar]
- 93.Andersson H, Sem M, Lund MB, et al. Long-term experience with rituximab in anti-synthetase syndrome-related interstitial lung disease. Rheumatology. 2015;54(8):1420–1428. doi: 10.1093/rheumatology/kev004. [DOI] [PubMed] [Google Scholar]
- 94.Mahler EA, Blom M, Voermans NC, van Engelen BG, van Riel PL, Vonk MC. Rituximab treatment in patients with refractory inflammatory myopathies. Rheumatology. 2011;50(12):2206–2213. doi: 10.1093/rheumatology/ker088. [DOI] [PubMed] [Google Scholar]
- 95.Reed AM, Crowson CS, Hein M, et al. Biologic predictors of clinical improvement in rituximab-treated refractory myositis. BMC Musculoskelet Disord. 2015;16:257. doi: 10.1186/s12891-015-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aggarwal R, Oddis CV, Goudeau D, et al. Autoantibody levels in myositis patients correlate with clinical response during B cell depletion with rituximab. Rheumatology. 2016;55(6):991–999. doi: 10.1093/rheumatology/kev444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoo DH, Suh CH, Shim SC, et al. A multicentre randomised controlled trial to compare the pharmacokinetics, efficacy and safety of CT-P10 and innovator rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76(3):566–570. doi: 10.1136/annrheumdis-2016-209540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoo DH, Park W, Suh CH, et al. Efficacy and safety of switched CT-10 from innovator rituximab compared to those of maintained CT-10 in patients with rheumatoid arthritis up to 56 weeks. [Accessed July 20, 2017];Arthritis Rheumatol. 2015 67(suppl 10) webpage on the Internet. Available from: http://acrabstracts.org/abstract/efficacy-and-safety-of-switched-ct-p10-from-innovator-rituximab-compared-to-those-of-maintained-ct-p10-in-patients-with-rheumatoid-arthritis-up-to-56-weeks/ [Google Scholar]
- 99.Smolen J, Scheinberg M, Tony H, et al. FRI0222 pharmacokinetics, pharmacodynamics, safety and efficacy of proposed rituximab biosimilar (GP2013) vs EU-approved rituximab (RTX) in patients with rheumatoid arthritis: results from a randomized controlled trial (GP13-201) over 52 weeks. Ann Rheum Dis. 2016;75:512–513. [Google Scholar]
- 100.Cohen S, Emery P, Greenwald M, et al. A phase I pharmacokinetics trial comparing PF-05280586 (a potential biosimilar) and rituximab in patients with active rheumatoid arthritis. Br J Clin Pharmacol. 2016;82(1):129–138. doi: 10.1111/bcp.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dorner T, Strand V, Cornes P, et al. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis. 2016;75(6):974–982. doi: 10.1136/annrheumdis-2016-209166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schroder C, Azimzadeh AM, Wu G, Price JO, Atkinson JB, Pierson RN. Anti-CD20 treatment depletes B-cells in blood and lymphatic tissue of cynomolgus monkeys. Transpl Immunol. 2003;12(1):19–28. doi: 10.1016/S0966-3274(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 103.Decker M, Rothermundt C, Hollander G, Tichelli A, Rochlitz C. Rituximab plus CHOP for treatment of diffuse large B-cell lymphoma during second trimester of pregnancy. Lancet Oncol. 2006;7(8):693–694. doi: 10.1016/S1470-2045(06)70797-5. [DOI] [PubMed] [Google Scholar]
- 104.Friedrichs B, Tiemann M, Salwender H, Verpoort K, Wenger MK, Schmitz N. The effects of rituximab treatment during pregnancy on a neonate. Haematologica. 2006;91(10):1426–1427. [PubMed] [Google Scholar]
- 105.Chakravarty EF, Murray ER, Kelman A, Farmer P. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117(5):1499–1506. doi: 10.1182/blood-2010-07-295444. [DOI] [PubMed] [Google Scholar]
- 106.Ostensen M, Forger F. How safe are anti-rheumatic drugs during pregnancy? Curr Opin Pharmacol. 2013;13(3):470–475. doi: 10.1016/j.coph.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 107.Klink DT, van Elburg RM, Schreurs MW, van Well GT. Rituximab administration in third trimester of pregnancy suppresses neonatal B-cell development. Clin Dev Immunol. 2008;2008:271363. doi: 10.1155/2008/271363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gualtierotti R, Ingegnoli F, Meroni PL. Pre-conceptional exposure to rituximab: comment on the article by Ojeda-Uribe et al. Clin Rheumatol. 2013;32(5):727–728. doi: 10.1007/s10067-013-2241-3. [DOI] [PubMed] [Google Scholar]
- 109.Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology. 2016;55(9):1693–1697. doi: 10.1093/rheumatology/kev404. [DOI] [PubMed] [Google Scholar]
- 110.Gotestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75(5):795–810. doi: 10.1136/annrheumdis-2015-208840. [DOI] [PubMed] [Google Scholar]
- 111.Friedman MA, Winthrop KL. Vaccines and disease-modifying antirheumatic drugs: practical implications for the rheumatologist. Rheum Dis Clin North Am. 2017;43(1):1–13. doi: 10.1016/j.rdc.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 112.Rehnberg M, Brisslert M, Amu S, Zendjanchi K, Hawi G, Bokarewa MI. Vaccination response to protein and carbohydrate antigens in patients with rheumatoid arthritis after rituximab treatment. Arthritis Res Ther. 2010;12(3):R111. doi: 10.1186/ar3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Assen S, Holvast A, Benne CA, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62(1):75–81. doi: 10.1002/art.25033. [DOI] [PubMed] [Google Scholar]
- 114.Arad U, Tzadok S, Amir S, et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine. 2011;29(8):1643–1648. doi: 10.1016/j.vaccine.2010.12.072. [DOI] [PubMed] [Google Scholar]
- 115.Oren S, Mandelboim M, Braun-Moscovici Y, et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis. 2008;67(7):937–941. doi: 10.1136/ard.2007.077461. [DOI] [PubMed] [Google Scholar]
- 116.Crnkic Kapetanovic M, Saxne T, Jonsson G, Truedsson L, Geborek P. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15(5):R171. doi: 10.1186/ar4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bingham CO, 3rd, Looney RJ, Deodhar A, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62(1):64–74. doi: 10.1002/art.25034. [DOI] [PubMed] [Google Scholar]
- 118.Ghrenassia E, Mekinian A, Rouaghe S, Ganne N, Fain O. Reactivation of resolved hepatitis B during rituximab therapy for rheumatoid arthritis. Joint Bone Spine. 2012;79(1):100–101. doi: 10.1016/j.jbspin.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 119.Pyrpasopoulou A, Douma S, Vassiliadis T, Chatzimichailidou S, Triantafyllou A, Aslanidis S. Reactivation of chronic hepatitis B virus infection following rituximab administration for rheumatoid arthritis. Rheumatol Int. 2011;31(3):403–404. doi: 10.1007/s00296-009-1202-2. [DOI] [PubMed] [Google Scholar]
- 120.Ennishi D, Yokoyama M, Terui Y, et al. Does rituximab really induce hepatitis C virus reactivation? J Clin Oncol. 2008;26(28):4695–4696. doi: 10.1200/JCO.2008.18.7609. author reply 4696. [DOI] [PubMed] [Google Scholar]
- 121.Buch MH, Smolen JS, Betteridge N, et al. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(6):909–920. doi: 10.1136/ard.2010.144998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clavel G, Moulignier A, Semerano L. Progressive multifocal leukoencephalopathy and rheumatoid arthritis treatments. Joint Bone Spine. 2017 Mar 18; doi: 10.1016/j.jbspin.2017.03.002. Epub. [DOI] [PubMed] [Google Scholar]
- 123.Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13(2):106–111. doi: 10.1016/j.clml.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Breuer GS, Ehrenfeld M, Rosner I, et al. Late-onset neutropenia following rituximab treatment for rheumatologic conditions. Clin Rheumatol. 2014;33(9):1337–1340. doi: 10.1007/s10067-014-2562-x. [DOI] [PubMed] [Google Scholar]