Abstract
Introduction
Linked-color imaging (LCI) is a recently developed system used in endoscopy. It creates clear and bright endoscopic images using short-wavelength, narrow-band laser light combined with white laser light. The illuminating light and signal processing emphasize slight color differences in abnormal regions that approximate the normal color of the mucosa. As a result, regions initially appearing red become a deeper shade of red, while regions originally appearing white become brighter, yet with natural tones. This process facilitates recognition of slight differences in the color of the mucosa and clarifies the boundaries of the mucosal pit.
Aim
To determine whether LCI of the colon can improve the correlation between endoscopic findings and pathological diagnosis.
Methods
Consecutive patients who underwent colonoscopy requiring polypectomy or removal by biopsy forceps if possible were recruited. Probable polyp histology was assessed by two endoscopists using the Narrow-band imaging International Colorectal Endoscopic (NICE) classification and LCI data. All detected polyps were sent to the pathology department for pathological diagnosis by two pathologists.
Results
In total, 94 polyps were found in 43 patients. The sensitivity, specificity, positive predictive value, and negative predictive value for neoplastic lesion prediction (NICE type2/3) were 96.5%, 83.8%, 90.2%, and 93.9%, respectively.
Conclusion
LCI combined with the NICE classification system is a powerful tool for predicting probable histology of colon polyps.
Keywords: linked-color imaging, NICE classification, colon polyps, histology, sensitivity, specificity
Introduction
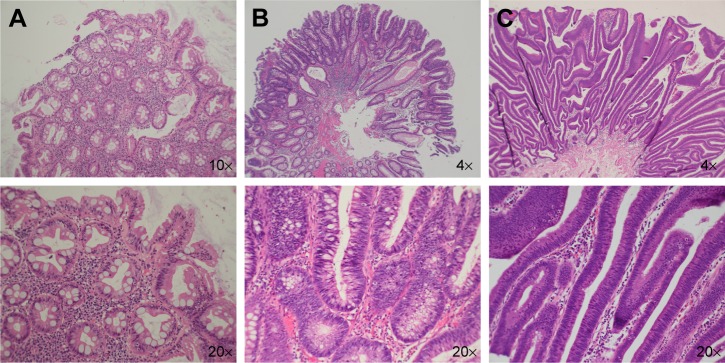
Colonoscopy to survey polyps requiring polypectomy is considered to be the most effective method of improving colorectal cancer-related morbidity and mortality.1 There are several types of polyps, which can be divided into nonneoplastic (ie, hyperplastic) and neoplastic (ie, adenomatous) lesion types. The typical pathologic image of nonneoplastic lesions and neoplastic lesions are shown in Figure 1. Removal of all adenomatous polyps during colonoscopy has been the most effective method of preventing colon cancer. Pathological examination, however, is the most accurate method of characterizing and diagnosing polyps. Endoscopists are currently searching for more effective methods of distinguishing different types of polyps during colonoscopy. This has prompted the development of several types of imaging techniques and colonoscopy devices to enhance surface and vascular patterns to improve diagnostic yield. Narrow-band imaging (NBI), image-enhanced endoscopy (i-scan, Pentax, Montvale, NJ, USA), and blue laser imaging and linked-color imaging (LCI) systems are currently in use. In addition to these new technologies, there are also several classification systems used for pathological prediction of colon polyps, including the Hiroshima, Sano, Showa, and the NBI International Colorectal Endoscopic (NICE) classifications. Of these methods, the NICE classification system is the most convenient for daily practice because it can be applied using colonoscopes with or without optical (ie, zoom) magnification capability. The NICE classification was first introduced by The Colon Tumor NBI Interest Group in 2009.2 The NICE classification system was the first NBI classification that did not require magnifying endoscopy; this has facilitated its widespread use all over the world. Although the classification scheme was initially designed for use with NBI, it has also demonstrated utility in other image-enhanced endoscopy methods, such as i-scan.3
Figure 1.
Photomicrographs (hematoxylin & eosin stain) of different types of colon polyps.
Notes: Histologically, a hyperplastic polyp shows epithelial hyperplasia characterized by a serrated appearance without nuclear atypia (A); a tubular adenoma contains elongated crypts lined by columnar epithelial cells with nuclear atypia, including nuclear elongation, pleomorphism, and hyperchromasia (B); a villous adenoma is characterized by a villiform architecture lined by columnar epithelial cells with nuclear atypia similar to that seen in a tubular adenoma (C). Nx: picture taken with Nx objective lens.
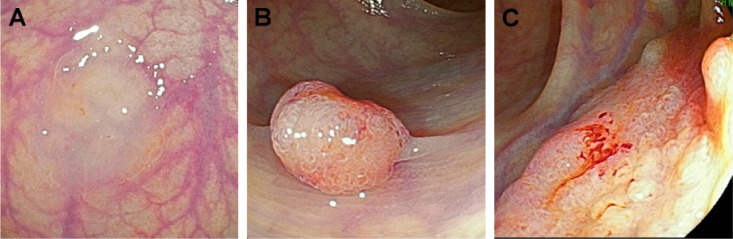
Recently, a new image-enhanced endoscopy system, known as LCI, has been developed that provides clear and bright endoscopic images using short-wavelength, narrow-band laser light combined with white laser light. The LCI system enables red areas to appear redder and white areas to appear brighter. Thus, it is an appropriate tool for recognizing color differences in the mucosa because it enhances clarity and recognition of the mucosal pit, which in turn facilitates pathological prediction of colon polyps (Figure 2). However, there have been no studies investigating the use of the NICE classification with data obtained using LCI. Accordingly, the aim of our study was to determine the feasibility of using the NICE classification combined with data obtained using an LCI system without magnification to predict colon polyp pathology.
Figure 2.
Endoscopic findings using LCI.
Notes: Lesion classified as NICE type 1 (A); lesion classified as NICE type 2 (B); lesion classified as NICE type 3 (C).
Abbreviations: LCI, linked-color imaging; NICE, Narrow-band imaging International Colorectal Endoscopic.
Methods
Patients
Chang Gung Memorial Hospital’s Institutional Review Board Committee approved the study protocol (Code of IRB: 201600789B0). This retrospective study recruited consecutive patients who underwent colonoscopy requiring polypectomy or endoscopic biopsy based on LCI findings. The institutional review board waived patient written informed consent due to deidentified medical records.
Colonoscopy procedure
For bowel preparation, patients ingested 1.5–2 L of polyethylene glycol or 90 mL of a split-dose phospho-soda solution (Fleet; CB Fleet Co, Lynchburg, VA, USA) before the procedure. Two colonoscopists performed all colonoscopy procedures up to the cecum using high-resolution endoscopy (EC-L590ZW/L; Fujifilm, Tokyo, Japan) and a laser light source (Lasereo; Fujifilm).
Endoscopic diagnosis using the NICE classification
All lesions were initially detected using conventional viewing methods and were then examined using the LCI system without magnification to evaluate endoscopic surface features. All lesions were subsequently classified into one of three types based on the NICE classification (Table 1).
Table 1.
NICE classification summarya
| Characteristic | Type 1 | Type 2b | Type 3 |
|---|---|---|---|
| Color | Same or lighter than background | Browner relative to background (verify color arises from vessels) | Brown to dark brown relative to background; sometimes patchy whiter areas |
| Vessels | None, or isolated, lacy vessels coursing across the lesion may be present | Brown vessels surrounding white structuresc | Area(s) of disrupted or missing vessels |
| Surface pattern | Dark or white spots of uniform size, or homogeneous absence of pattern | Oval, tubular, or branched white structures surrounded by brown vessels | Amorphous or absent |
| Most likely pathology | Hyperplastic | Adenoma | Deep submucosal invasive cancer |
Notes:
Can be applied using colonoscopes with or without optical (zoom) magnification capability;
Type 2 consists of Vienna classification types 3, 4, and superficial 5 (all adenomas with either low- or high-grade dysplasia, or with superficial submucosal carcinoma). The presence of high-grade dysplasia or superficial submucosal carcinoma may be suggested by an irregular vessel or surface pattern and is often associated with atypical morphology (eg, depressed area);
these structures (regular or irregular) may represent the pits and the epithelium of the crypt opening.
Abbreviation: NICE, Narrow-band imaging International Colorectal Endoscopic.
Clinicopathological evaluation
Medical records for lesion size, shape (according to Paris classification),4 NICE classification category, and pathological diagnosis were reviewed. Two pathologists blinded to the clinical information diagnosed all cases together with consistency consent. Using this information, the relationship between LCI data, NICE classification, and the histopathological findings were examined.
Results
A total of 94 polyps were detected in 43 patients (32 male, 11 female; mean age 54.2 years [range 42–75 years]). The mean polyp size was 17 mm (range 2–25 mm). Seven polyps were pedunculated, 63 were sessile, and 24 were flat. Thirty-three (35%) polyps were classified as NICE type 1 and, based on pathology, 31 (93.9%) of these were hyperplastic and sessile serrated adenomas/polyps, and two (6.1%) were low-grade adenomas (Table 2). Fifty-nine polyps were classified as NICE type 2, 49 (83.1%) of these were low-grade adenomas, six (10.1%) were hyperplastic or sessile serrated adenomas/polyps, and four (6.7%) were high-grade adenomas. The remaining two polyps included a high-grade adenoma and an invasive carcinoma, both of which exhibited endoscopic features classified as NICE type 3 (Table 3).
Table 2.
Clinicopathological features
| Feature | |
|---|---|
| Patients, n | 94 |
| Sex, male: female, n:n | 32:11 |
| Age, years, mean (range) | 54.2 (42–75) |
| Polyps | |
| Size, mm, mean (range) | 17 (2–25) |
| Shape | Pedunculated (n=7); sessile (n=63); flat (n=24) |
| Histology, n | |
| Nonneoplastic | 37 (Hyperplastic [n=32]; sessile serrated adenoma/polyp [n=5]) |
| Adenoma | 56 (Low-grade dysplasia [n=51]; high-grade dysplasia [n=5]) |
| Invasive cancer | 1 |
Table 3.
Relationship between NICE classification system and histological findings using LCI data
| NICE classification | HP & SSA/P | LGD | HGD | Carcinoma |
|---|---|---|---|---|
| Type 1 | 31 (93.9) | 2 (6.1) | ||
| Type 2 | 6 (10.1) | 49 (83.1) | 4 (6.7) | |
| Type 3 | 1 (50.0) | 1 (50.0) |
Note: Data presented as n (%).
Abbreviations: NICE, Narrow-band Imaging International Colorectal Endoscopic; LCI, linked-color imaging; HP, hyperplastic polyp; SSA/P, sessile serrated adenoma/polyp; LGD, low-grade dysplasia; HGD, high-grade dysplasia.
Histologically, colorectal polyps are classified as either neoplastic (NICE type 2/3) or nonneoplastic (NICE type 1). The endoscopist in this study correctly predicted neoplastic histology in 55 of 61 polyps and nonneoplastic histology in 31 of 33 polyps. Therefore, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for neoplastic lesion prediction were 96.5%, 83.8%, 90.2%, and 93.9%, respectively (Table 4).
Table 4.
Performance characteristics of LCI in predicting neoplastic lesions
| Lesion | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| NICE type 2/3 | 96.5 (87.5–99.6) |
83.8 (67.9–93.8) |
90.2 (79.8–96.3) |
93.9 (79.8–99.2) |
Note: Data presented as % (95% confidence interval).
Abbreviations: NICE, Narrow-band imaging International Colorectal Endoscopic; LCI, linked-color imaging; PPV, positive predictive value; NPV, negative predictive value.
Discussion
The adenoma–carcinoma sequence was described by Morson5 in 1974. Based on this study, endoscopists worldwide recognized that removing all adenomatous polyps is among the most important goals of colonoscopy. However, the “resect and discard” policy is currently advocated,6,7 not only to reduce the risk for bleeding or perforation during the polyp removal procedure but also to eliminate the associated costs of pathological examination. For this reason, various image-enhanced endoscopy systems are available in clinical practice to improve detection and histological prediction of lesions. The LCI system used in the present study is an additional image processing technique that enhances the separation of red colors to clearly depict deeper shades of red and to brighten white colors. Recently, Suzuki et al8 reported an LCI high-magnification endoscopy method, combined with crystal violet staining, to yield images that closely approximate histopathological findings. This is expected to improve the accuracy of endoscopic assessment of the depth of invasion for early-stage colorectal cancer. The LCI system can be used without optical magnification and is an effective adjunct in daily practice. Although there are several methods for the pathological prediction of colon polyps, the NICE classification system is the only method that can be used without optical magnification.
The results of this study demonstrated the sensitivity, specificity, PPV, and NPV of classifying neoplastic lesions according to the NICE system using LCI data. Rex7 recruited 451 consecutive colorectal polyps and reported high-confidence predictions of adenoma and hyperplastic histology (correct for 91% and 95%, respectively) using NBI without optical magnification. Kim et al9 reported a sensitivity, specificity, PPV, and NPV for neoplastic lesion prediction of 97.5%, 85.1%, 91.7%, and 95.2%, respectively, using the NBI system without optical magnification in 126 colorectal polyps. Our results are similar to those of previous studies investigating the application of the NICE classification using an NBI system without optical magnification. LCI combined with the NICE classification system is a powerful tool for prediction of probable histology of colon polyps. In addition, a recent study showed that LCI improved polyp visibility for both expert and nonexpert endoscopists and was also found to be useful for improving polyp visibility in any location and with any size, morphology, histology, and preparation level.10
Limitation
The major limitation of this study was the small number of polyps assessed. Moreover, most of the patients were referred from a health management center and had no clinical symptoms. The proportion of advanced polyps or invasive cancers found in this study was lower than that found in previous studies. Thus, definitive conclusions cannot be drawn from these data and larger studies are warranted.
In a study by Rex,7 large polyps (>10 mm) had better pathological prediction rates compared with small polyps (<10 mm). Because we placed no restrictions on the size of polyps included in our study (most were <10 mm in size), polyp size may have influenced our pathological prediction rates.
Conclusion
In conclusion, LCI combined with the NICE classification system is a powerful tool for predicting probable histology of colon polyps. LCI appears to be a convenient and practical tool, even without optimal magnification. Moreover, it maintains natural color tones; therefore, it could replace the need for white light endoscopy during the entire procedure.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka S, Sano Y. Aim to unify the narrow band imaging (NBI) magnifying classification for colorectal tumors: current status in Japan from a summary of the consensus symposium in the 79th annual meeting of the Japan Gastroenterological Endoscopy Society. Dig Endosc. 2011;23(Suppl 1):131–139. doi: 10.1111/j.1443-1661.2011.01106.x. [DOI] [PubMed] [Google Scholar]
- 3.Pigo F, Bertani H, Manno M, et al. i-Scan high-definition white light endoscopy and colorectal polyps: prediction of histology, interobserver and intraobserver agreement. Int J Colorectal Dis. 2013;28(3):399–406. doi: 10.1007/s00384-012-1583-7. [DOI] [PubMed] [Google Scholar]
- 4.The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(Suppl 6):S3–S43. doi: 10.1016/s0016-5107(03)02159-x. [DOI] [PubMed] [Google Scholar]
- 5.Morson B. President’s address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974;67(6 Pt 1):451–457. [PMC free article] [PubMed] [Google Scholar]
- 6.Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 2009;10(12):1171–1178. doi: 10.1016/S1470-2045(09)70329-8. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136(4):1174–1181. doi: 10.1053/j.gastro.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Hara T, Kitagawa Y, Yamaguchi T. Magnified endoscopic observation of early colorectal cancer by linked color imaging with crystal violet staining (with video) Gastrointest Endosc. 2016;84(4):726–729. doi: 10.1016/j.gie.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Kim JJ, Hong KS, Kim JS, Jung HC. A Randomized Controlled Clinical Study comparing the diagnostic accuracy of the histologic prediction for colorectal polyps depending on the use of either magnified or non-magnified narrow band imaging. Clin Endosc. 2015;48(6):528–533. doi: 10.5946/ce.2015.48.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida N, Naito Y, Murakami T, et al. Linked color imaging improves the visibility of colorectal polyps: a video study. Endosc Int Open. 2017;5(6):E518–E525. doi: 10.1055/s-0043-105495. [DOI] [PMC free article] [PubMed] [Google Scholar]