Abstract
Purpose of review
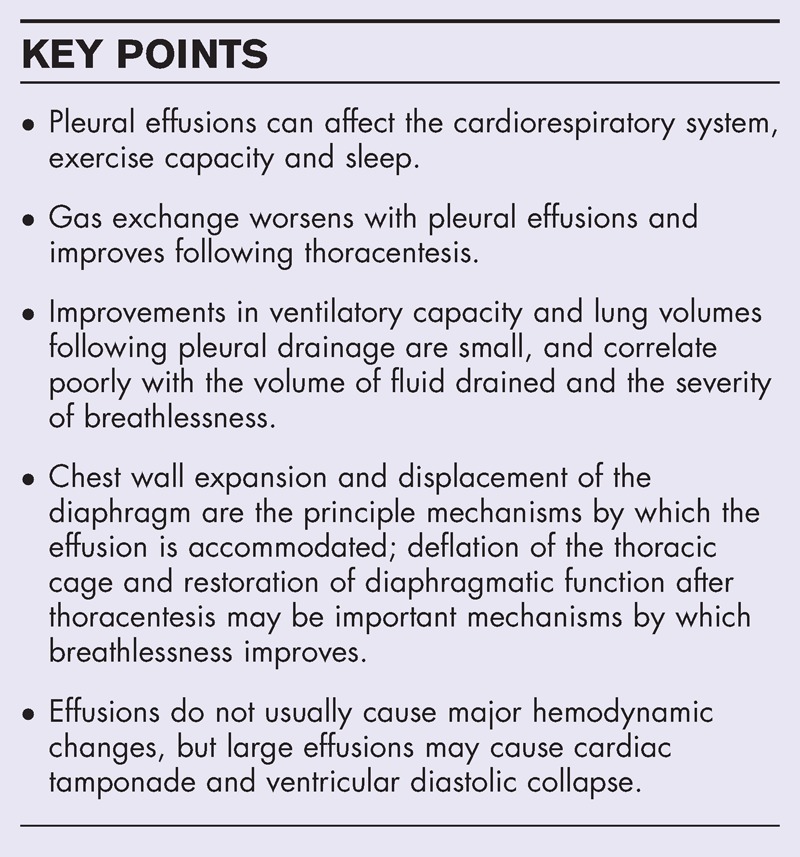
Pleural effusions have a major impact on the cardiorespiratory system. This article reviews the pathophysiological effects of pleural effusions and pleural drainage, their relationship with breathlessness, and highlights key knowledge gaps.
Recent findings
The basis for breathlessness in pleural effusions and relief following thoracentesis is not well understood. Many existing studies on the pathophysiology of breathlessness in pleural effusions are limited by small sample sizes, heterogeneous design and a lack of direct measurements of respiratory muscle function. Gas exchange worsens with pleural effusions and improves after thoracentesis. Improvements in ventilatory capacity and lung volumes following pleural drainage are small, and correlate poorly with the volume of fluid drained and the severity of breathlessness. Rather than lung compression, expansion of the chest wall, including displacement of the diaphragm, appears to be the principle mechanism by which the effusion is accommodated. Deflation of the thoracic cage and restoration of diaphragmatic function after thoracentesis may improve diaphragm effectiveness and efficiency, and this may be an important mechanism by which breathlessness improves. Effusions do not usually lead to major hemodynamic changes, but large effusions may cause cardiac tamponade and ventricular diastolic collapse. Patients with effusions can have impaired exercise capacity and poor sleep quality and efficiency.
Summary
Pleural effusions are associated with abnormalities in gas exchange, respiratory mechanics, respiratory muscle function and hemodynamics, but the association between these abnormalities and breathlessness remains unclear. Prospective studies should aim to identify the key mechanisms of effusion-related breathlessness and predictors of improvement following pleural drainage.
Keywords: breathlessness, pathophysiology, pleural effusion, thoracentesis
INTRODUCTION
Over 1 million patients develop a pleural effusion annually in the United States alone [1]. Pleural effusions have more than 60 different causes, and vary in size and risk (and rates) of recurrence. Congestive heart failure (CHF), malignancy, pulmonary infection and embolism account for over 90% of pleural effusions [2].
Pleural effusions can impact profoundly on the cardiorespiratory system. Breathlessness, the commonest presentation, is often debilitating and significantly impairs quality of life. Relief of breathlessness often necessitates therapeutic pleural interventions with associated discomfort, risks of infection, bleeding, pneumothorax and even death. Management of pleural effusions represents a significant healthcare burden worldwide.
To date, research on, and hence our understanding of, the effects of pleural effusions on respiratory physiology and breathlessness has been limited. The conventional belief that pleural effusions cause breathlessness through compression of the lung is overly simplistic. The severity of breathlessness often correlates poorly with the size of the effusion [3▪,4,5] Conversely, symptom reduction from fluid drainage varies significantly between patients, and no reliable predictors exist to identify those who will benefit. Most previous studies have involved small cohorts and examined specific etiological factors in isolation; few have compared physiological changes with symptom benefits in patients.
This article reviews published literature on the pathophysiological effects of pleural effusions (and their therapeutic evacuation). Key knowledge gaps are highlighted which may guide future research.
Box 1.
no caption available
LITERATURE SEARCH
Medline, Excerpta Medica dataBASE (EMBASE) and the Cochrane Database of Systematic Reviews were interrogated using the following search terms, such as ‘pleural effusion’ and ‘oxygenation’ (gas exchange, hypoxemia and oxygen desaturation), ‘pulmonary function test’ (lung function test, respiratory function test and spirometry), ‘pleural pressure’ (Ppl, pleural elastance), ‘exercise’ [6-min walk test (6MWT), cardiopulmonary exercise test], ‘sleep’, ‘cardiac’ (hemodynamic effects, hypotension) and ‘dyspnea’ (breathlessness). References and their citation lists were scrutinized. Studies pertaining to the effects of pleural effusion on pulmonary physiology and breathlessness were included.
PATHOPHYSIOLOGICAL EFFECTS OF A PLEURAL EFFUSION
Most studies on the pathophysiologic effects of effusions have included small numbers of patients who are heterogeneous in their underlying pleural and systemic diseases, and many have been conducted in different settings (e.g. ventilated vs. spontaneous breathing patients). Therefore, care must be executed in interpreting their findings.
Gas exchange
Pleural effusions can worsen gas exchange. Instillation of physiological saline into both pleural spaces of mechanically ventilated pigs has been shown to induce early and dose-related hypoxemia [6]. Intrapulmonary shunt was shown to underlie the hypoxemia when Agusti et al.[7] examined patients (n = 9) with recent-onset pleural effusions using the multiple inert gas elimination technique.
Thoracentesis appears to improve gas exchange. A meta-analysis of 19 studies (1124 patients) [8▪] on thoracentesis in mechanically ventilated patients showed an average improvement in PaO2:FiO2 of 18%. Improvements in gas exchange are more consistently found at 24 h [5,9], rather than immediately [7,10–12] after thoracentesis. Hypoxemia has been shown to worsen up to 2 h after thoracentesis [13]. These observations have been attributed to re-expansion pulmonary edema or delayed pulmonary re-expansion.
Several factors may influence the improvement in gas exchange with thoracentesis. In mechanically ventilated patients, improvements in PaO2:FiO2 have been associated with the volume drained [14] and the increase in end-expiratory lung volume [15▪]; those with lower PaO2:FiO2 ratios appeared to have greater benefit. Patients with acute respiratory distress syndrome appeared to have less improvement in gas exchange [15▪,16]. In mechanically ventilated patients with CHF effusions, the improvement in PaO2:FiO2 after thoracentesis correlated inversely with pleural elastance [17]. Improvements in oxygenation are greater in patients with diaphragm paradox [18]. No evidence links abnormal gas exchange caused by pleural effusions (and their improvements postthoracentesis) to the symptom of breathlessness.
Pulmonary function and respiratory mechanics
Studies of pleural effusion and pulmonary function are often heterogeneous in their designs and physiological indices measured. Nonetheless, most studies agree that increases in lung volumes are small and correlate poorly [19] (or not at all [10]) with the volume of pleural fluid drained [4,5,10,19,20] irrespective of whether the effusion is a transudate or exudates [21].
Animal and human studies suggest that expansion in the thoracic cage is the principle mechanism by which extra volume is generated to accommodate the effusion, and helps preserve lung volumes. In anesthetized dogs, infusion of saline intrapleurally increased thoracic cage volume by two-thirds of the total volume instilled but only reduced the functional residual capacity (FRC) by one-third of the total volume instilled [22,23]. The increase in thoracic cage volume was achieved mainly through downward displacement of the diaphragm [23,24]. In rats, bilateral pleural effusions also increased both the anteroposterior and lateral rib cage diameters [24]. Acute pleural effusions increase respiratory system elastance by increasing lung elastance [24,25], likely via lung distortion and decreases in FRC. The effect of pleural effusions on lung resistance is unclear. [24,25] Pleural effusions do not appear to alter chest wall elastance or resistance [24,25].
Our knowledge on the effect of effusion on lung volume in humans comes mainly from changes measured prethoracentesis and postthoracentesis. Although thoracentesis can improve the forced expiratory volume in 1 s (FEV1), vital capacity and lung volumes [3▪,4,5,10,18] the magnitudes of increase are highly variable and often do not correlate with the volume of fluid drained [3▪,10]. (Table 1 and Fig. 1) In 26 patients who had thoracentesis (1.74 ± 0.90 l), the vital capacity and total lung capacity increased by 0.41 ± 0.39 and 0.70 ± 0.20 l, respectively, after 24 h. The improvement correlated best with the Ppl after 0.80 l of fluid had been withdrawn [19]. The effect of thoracentesis on FEV1 and vital capacity appears to be greater in patients with paradoxical movement of their hemidiaphragm [18]. Another small study (n = 9) [4] also found similar modest improvements of vital capacity (median 0.30 l) and total lung capacity (0.64 l) compared with the volume of fluid evacuated (1.82 ± 0.60 l). These human data are consistent with findings in animal studies that effusions are accommodated mainly through expansion of the thoracic cage rather than lung compression.
Table 1.
Pulmonary function tests before and after thoracentesis of 1.3 l in a 62-year-old man with a large malignant right pleural effusion
| Pulmonary function test (l) | Predicted (l) | Prethoracentesis (l) | % Predicted | Postthoracentesis (l) | % Predicted | Difference (ml) |
| FEV1 | 3.87 | 1.07 | 27 | 1.67 | 43 | 600 |
| FVC | 5.14 | 1.35 | 26 | 2.01 | 39 | 660 |
| TLC | 7.65 | 2.89 | 37 | 3.48 | 45 | 590 |
| RV | 2.50 | 1.56 | 62 | 1.37 | 54 | −190 |
The improvements in vital capacity and total lung capacity (TLC) were less than half the volume of pleural fluid removed. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; RV, residual volume.
FIGURE 1.
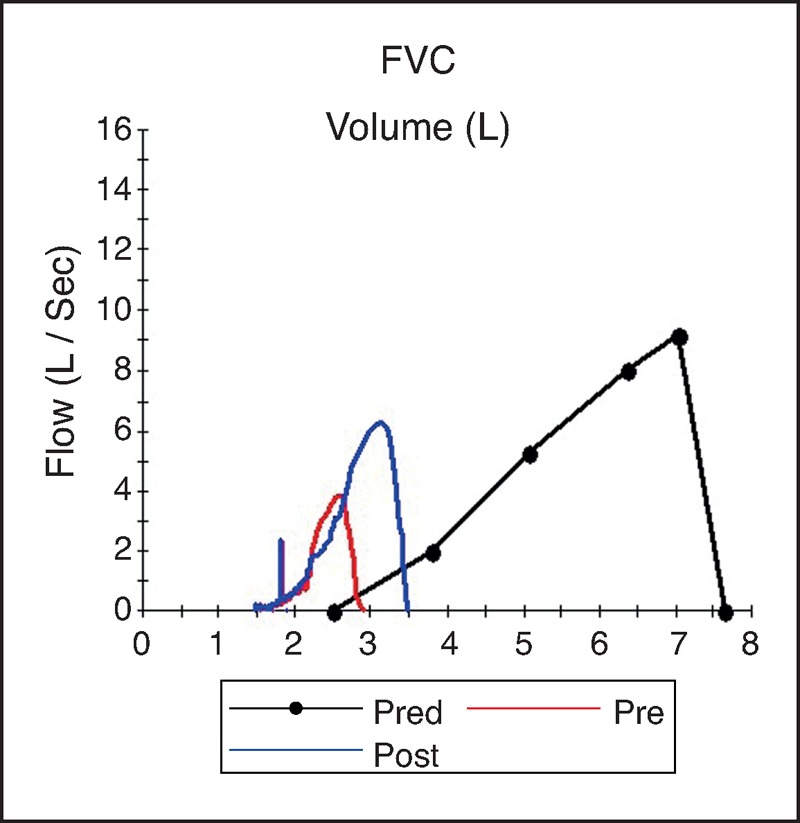
Expiratory flow-volume loops before (red) and 4 h after (blue) thoracocentesis of 1.3 l in a 62-year-old man with large malignant right pleural effusion. Thoracocentesis resulted in increases in vital capacity, total lung capacity and peak expiratory flow, but they remain severely reduced due to underlying pulmonary and pleural disease. FVC, forced vital capacity.
A study of 129 acute respiratory distress syndrome patients [16] found that small (median 287 ml) pleural effusions were associated with greater increases in thoracic cage volume than reduction in lung volume and had no significant effects on respiratory mechanics. Two studies of mechanically ventilated patients [15▪,26] with acute respiratory failure showed that thoracentesis improved compliance and respiratory resistance within 24 h. However, the improvement in respiratory compliance, respiratory resistance, elastic lung recoil and intrinsic positive end-expiratory pressure may not be apparent within the initial 2 h after thoracentesis [4,11,15▪]. Thoracentesis does not appear to significantly alter the diffusing capacity of lung for carbon monoxide [7,21] specific airway conductance or the physiological dead space, tidal volume ratio [9,21].
Taken together these data suggest that symptomatic benefits of pleural drainage may result from deflation of the thoracic cage and associated improvements in inspiratory muscle function.
Pleural pressure
In health, Ppl at FRC is determined by the balance of elastic recoil pressures of the lung and chest wall, and is usually subatmospheric (−3 to −5 cmH2O). In mechanically ventilated pigs with an artificially created pleural effusion, the Ppl progressively increases toward the most dependent part of the effusion [6]. However, Ppl is highly variable in patients with pleural effusions and may be either increased or reduced [27,28]. In a study of 52 patients with pleural effusions, Light et al.[27] found that Ppl ranged from −21 to +8 cmH2O. This difference may be explained by concurrent pulmonary pathologies in patients. Ppl can be reduced (<−5 cmH2O) particularly in patients with a trapped lung [28].
Thoracentesis lowers Ppl [7,19,27,28] and the ratio of the fall in Ppl to the volume drained defines the pleural space elastance. During thoracentesis, Ppl usually falls most rapidly in the initial and final phases of drainage. The magnitude of the fall in Ppl with thoracentesis and pleural space elastance is highly variable [27]. Differences in Ppl prior to thoracentesis and pleural space elastance have been proposed to have diagnostic and prognostic values, and three patterns have been described. First, in patients with pleural effusion and normal lung elastic recoil, airway patency and visceral pleura, Ppl is elevated and pleural space elastance is low. Second, a reduced Ppl with high pleural space elastance (>25 cmH2O/l) [27,28] is often seen in patients whose lung is trapped (e.g. by malignancy), and is associated with pleurodesis failure [29]. A third pattern is seen in the presence of nonexpandable lungs due to airway obstruction, increased lung elastic recoil or pleural inflammation in which Ppl is often increased at baseline and elastance is also raised.
A high baseline Ppl (and smaller reductions in Ppl postthoracentesis) was associated with greater increase in vital capacity in one study (n = 26) [19] but with less increase in end-expiratory lung volume in another (n = 20 ventilated patients) [15▪]. Patients with low pleural space elastance had the greatest improvement in gas exchange in a study of 26 ventilated patients with CHF. Light et al.[27] proposed that Ppl below −20 cmH2O postthoracentesis was associated with a higher likelihood of re-expansion pulmonary edema but this has not been confirmed by other studies [30].
Whether Ppl and pleural space elastance changes correlate with changes in breathlessness remains unknown.
Cardiovascular function
Small or moderate effusions, and their drainage, generally do not cause major hemodynamic changes. Even in mechanically ventilated patients fluid evacuation does not significantly alter blood pressure, cardiac output (CO) or catecholamine dose requirements [15▪]. Detailed description of the effects of pleural effusion on cardiovascular function is beyond the scope of this article.
Large effusions do not usually reduce CO at rest but can limit its rise during exercise [31]. When saline up to 40 ml/kg was infused into both hemithoraces of dogs, pulmonary artery occlusion pressure and central venous pressure increased in proportion to the pleural fluid added. The CO did not change until a larger volume (40–80 ml/kg) was instilled when the CO dropped significantly and caused death in many animals [6]. The hemodynamic changes of large bilateral effusions in dogs mimic those of pericardial tamponade: elevated intrapericardial pressure, impaired cardiac filling and stroke volume and right ventricular diastolic collapse [32]. Ventricular diastolic collapse can also be seen with large, unilateral effusions and resolves quickly postthoracentesis [33].
Exercise
Breathlessness in patients with pleural effusions is most prominent during exertion. Only two studies have examined the effect of pleural effusion and thoracentesis on exercise capacity. Both studies showed impairment of baseline exercise capacity in patients with effusions and benefits from thoracentesis.
One study (n = 25) employed the 6MWT [3▪] and found an average baseline distance of 432 ± 78 m (73% predicted). The other (n = 15) used incremental cardiopulmonary exercise test [34] which revealed a mean maximum oxygen consumption per minute (VO2max) of only 37% of the predicted values.
The 6MWT distance improved by 15% (63 m) after thoracentesis (1.56 ± 0.70 l). This improvement correlated with increases in ventilatory capacity, but neither correlated with the volume of fluid removed. Breathlessness improved significantly at rest (Borg scores from 2.7 to 1.5) and after exercise (from 5.1 to 2.4), even though oxygenation did not improve.
In the study using cardiopulmonary exercise test [34], improvements in exercise tolerance, maximum workload and VO2max following therapeutic thoracentesis (mean 1.6 l removed) were variable. Change in VO2max postthoracentesis correlated with changes in FEV1 (r = 0.58), forced vital capacity (r = 0.61) and maximum O2 pulse (r = 0.78). Again, no correlation with the amount of fluid removed was found.
Sleep
Only one published study has examined the effect of pleural effusion on sleep [35▪] and found poor sleep quality and efficiency in patients (n = 19) with a large pleural effusion at baseline as measured by the Pittsburgh Sleep Quality Index and overnight sleep studies.
Thoracentesis improved total sleep time, sleep efficiency (from 76 to 81%) and rapid eye movement sleep, and reduced stage 1 sleep and Borg score (from 2.3 to 0.8). No correlation existed between the volume of pleural fluid removed (mean 1.6 l) and the improvement in sleep or dyspnea scores.
THE DIAPHRAGM IN EFFUSION-RELATED BREATHLESSNESS
Breathlessness from pleural effusions is likely multifactorial. The contribution of concurrent extrapleural diseases to breathlessness must be considered when evaluating patients with a pleural effusion.
As discussed earlier, pleural effusions are accommodated by a greater expansion of the thoracic cage (Fig. 2) than reduction in lung volume [16,22,23]. Greater compliance of the thoracic cage reduces the impact of the effusion on lung mechanics and gas exchange. A considerable proportion of the expansion of the thoracic cage occurs in the vertical dimension [23,24]. In erect humans, this would result in distortion of the hemidiaphragm causing a downward displacement of the dome of the diaphragm (Fig. 3), and reductions in muscle length and area of apposition of the diaphragm with the rib cage. Moderate or large effusions commonly cause flattening (Figs 4 and 5) or even inversion of the diaphragm (Fig. 6), and this is associated with severe breathlessness [5]. These changes are likely to render the diaphragm less effective and efficient. The shortened diaphragm has a reduced capacity to develop tension, requires higher neural activation to develop an equivalent tension [36] and is less effective at translating tension to transdiaphragmatic pressure [37]. The smaller area of apposition reduces the capacity for increases in abdominal pressure during inspiration to expand the rib cage. The finding of paradoxical movement of the hemidiaphragm in response to a unilateral pleural effusion [18] is supportive evidence of the reduced effectiveness of the hemidiaphragm when loaded with a large effusion. These effects of pleural effusions on the diaphragm are likely to result in a reduced ventilatory output relative to neural drive of the diaphragm (‘neuromechanical uncoupling’). There is considerable evidence that such neuromechanical uncoupling generates afferent neural impulses from mechanoreceptors throughout the respiratory system (muscle, chest wall, airways and lung parenchyma) to the sensorimotor cortex and results in the sensation of breathlessness [38,39].
FIGURE 2.
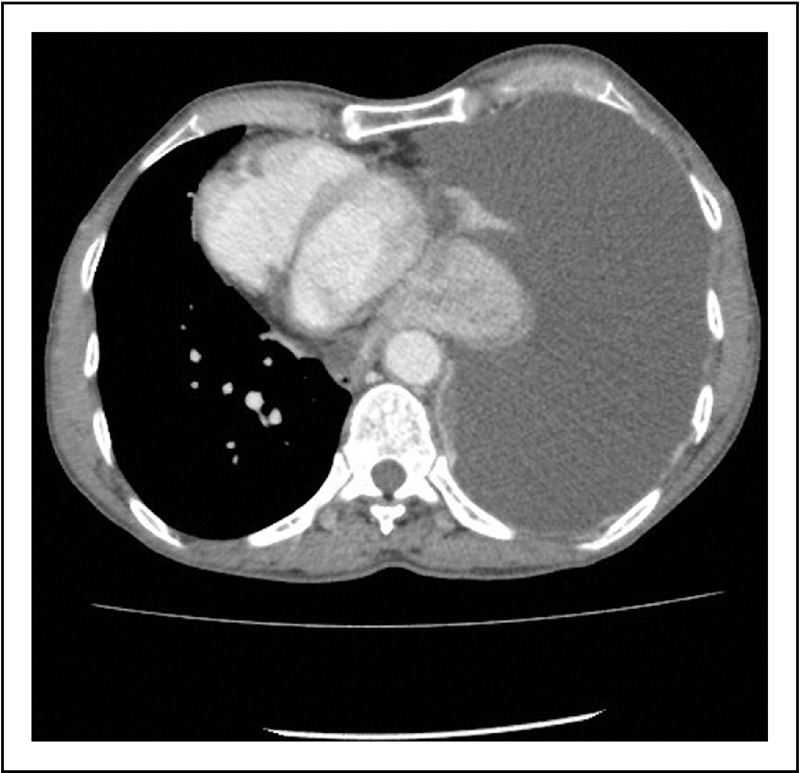
Computed tomography scan (axial view) shows expansion of the right thoracic cage (with increased anteroposterior diameter and widened intercostal space) to accommodate a large pleural effusion.
FIGURE 3.
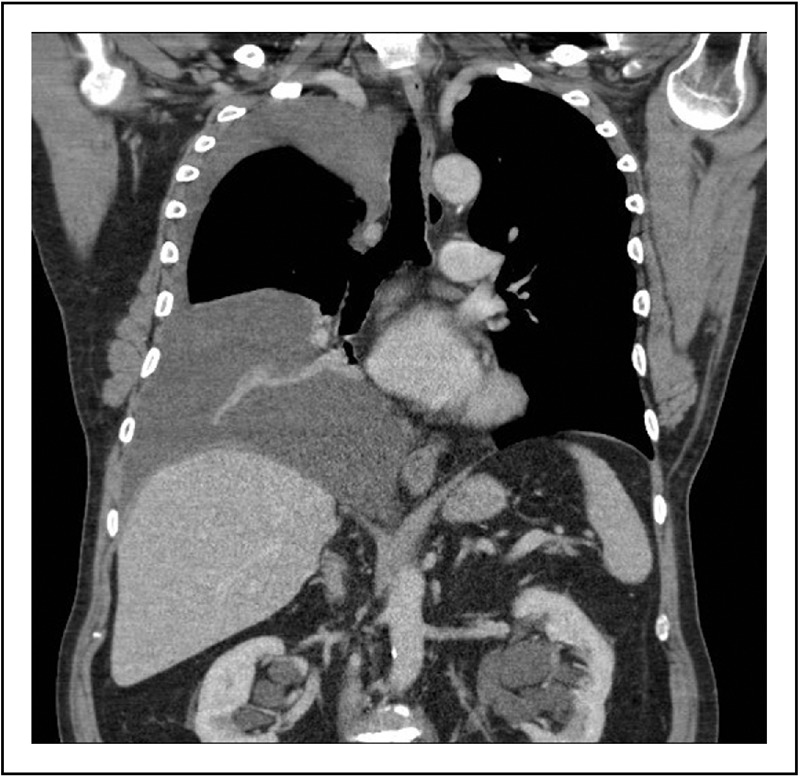
Computed tomography scan (coronal view) shows the right diaphragm that is pushed down by a moderate pleural effusion to a level lower than the left diaphragm. It shows the effect of the weight of fluid on the diaphragm even when the patient is in the supine position. This is likely to be worse when the patient is in an upright position.
FIGURE 4.
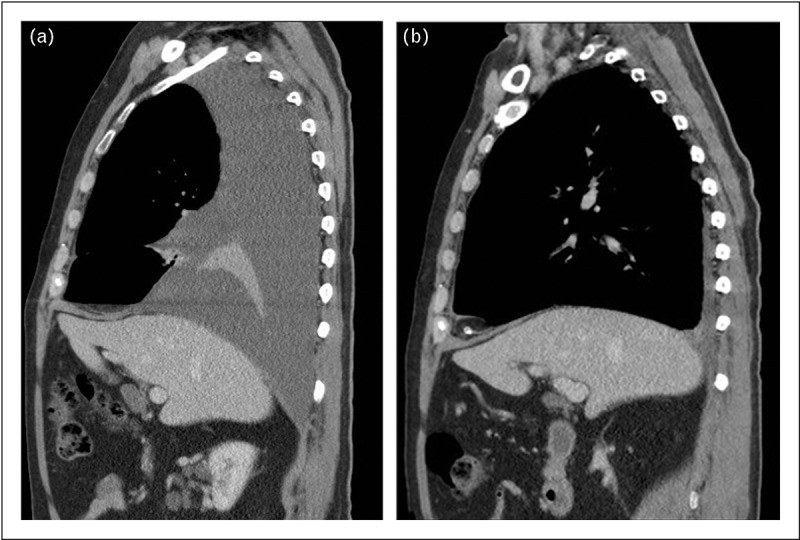
(a, b) Computed tomography scan (sagittal view) and ultrasound showing flattened right diaphragm caused by a moderate pleural effusion.
FIGURE 5.
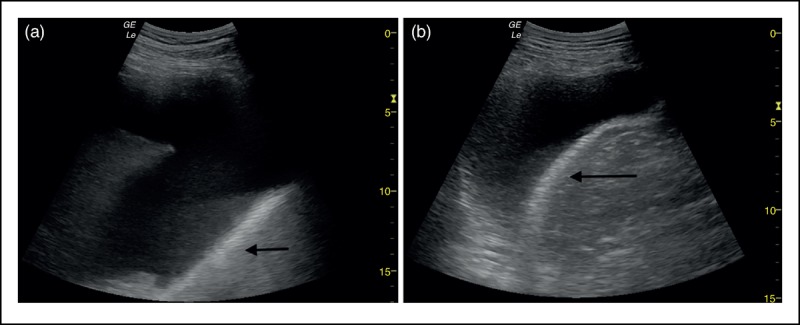
(a, b) The normal shape of the right diaphragm is restored following therapeutic drainage of 1 l of pleural fluid.
FIGURE 6.
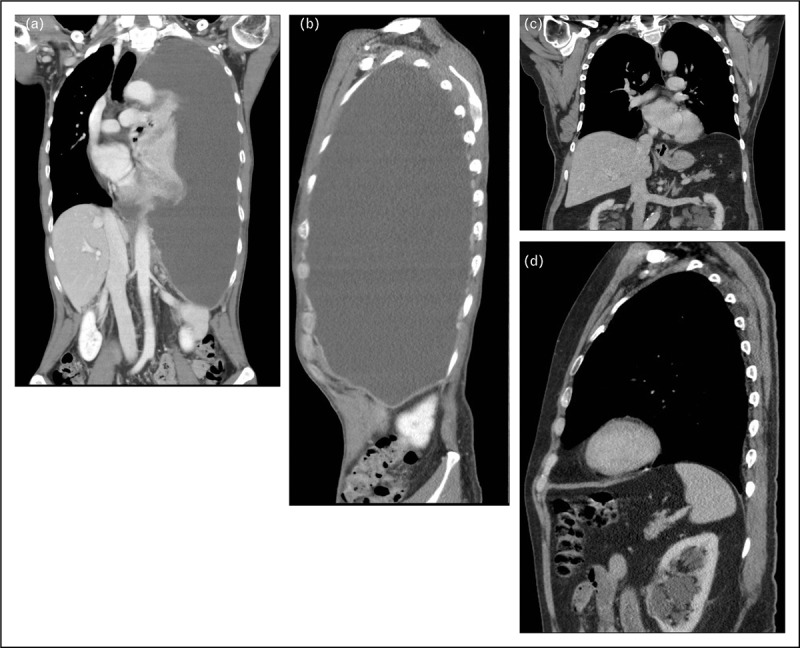
(a, b) CT scan (coronal and sagittal views) shows a large pleural effusion pushing down and inverting the left diaphragm. (c, d) The left diaphragm reverts to its normal shape and position following complete evacuation of the effusion.
Impairment of hemidiaphragm function caused by a large pleural effusion, for reasons discussed above, may also cause ‘pendulum breathing’ where paradoxical movement of the diaphragm allows the movement of air from the abnormal hemithorax to the normal hemithorax during inspiration. This will impair ventilatory efficiency further and may contribute to breathlessness [40,41]. Breathlessness from pendulum breathing is only relieved on lying supine or after fluid removal [40].
Thoracentesis often results in an immediate and dramatic improvement in breathlessness and exercise tolerance that cannot be explained on the basis of improvements in ventilatory capacity, gas exchange or mechanical properties of the lung. It is likely that thoracentesis restores respiratory muscles, in particular the diaphragm, to a more normal length and shape immediately (Figs 4–6). This immediate improvement in neuromechanical coupling may be the basis for a corresponding immediate improvement in symptoms. This may explain why patients with paradoxical diaphragmatic movements are significantly more breathless than those without (Borg scores 5.1 vs. 2.9, respectively), and are more likely to improve in their breathlessness [18] and exercise capacity [5] after thoracentesis.
KNOWLEDGE GAP AND FUTURE DIRECTIONS
Pleural effusion is common and therapeutic drainages are performed worldwide everyday despite there being limited understanding of the mechanism of breathlessness and no accurate predictors to guide patient selection.
Definitive studies to examine the cause of breathlessness in pleural effusion are challenging to perform. Prior attempts have been limited by small and markedly heterogeneous patient cohorts that include a wide range of pleural effusions of different causes, size and the acuity of accumulation in patients of varying severity of breathlessness/hypoxemia and concurrent cardiorespiratory diseases. Variations in study protocols and endpoints also make comparisons difficult. For example, different assessment times after drainage (ranging from immediately to 48 h postdrainage) complicate result interpretation. Differences in fluid volume removed further confound the results.
Defining the optimal tool to assess breathlessness in pleural effusion must be a priority. Prospective studies to identify key factors governing breathlessness with pleural effusions and to develop predictors of improvement following pleural drainage are needed. Ideally a comprehensive approach incorporating clinical, physiological and radiological parameters in a large unbiased patient population should be employed.
The ability to select and limit pleural interventions to patients who are likely to have symptomatic benefits from fluid drainage would represent a major advance in pleural medicine.
CONCLUSION
Pleural effusions have major effects on the cardiorespiratory system and cause abnormalities in gas exchange, respiratory mechanics and muscle function and hemodynamics. The association between these abnormalities and effusion-related breathlessness, and the response following thoracentesis, remains uncertain. Future research should aim to identify the key mechanisms driving breathlessness in patients with a pleural effusion and the important predictors of improvement following pleural drainage.
Acknowledgements
None.
Financial support and sponsorship
All the authors receive a research project grant from Cancer Council Western Australia. R.T. is supported by scholarships from the National Health & Medical Research Council (NH&MRC), the Lung Institute of Western Australia and WA Cancer & Palliative Care Network. Y.C.G.L. receives research funding from the NH&MRC, the New South Wales Dust Disease Board and the Sir Charles Gairdner Research Advisory Committee. P.R.E. is funded by an NH&MRC Senior Research Fellowship (No. 1042341).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Light RW. Pleural diseases. 4th ed.Philadelphia:Lippincott Williams & Wilkins; 2001. 89. [Google Scholar]
- 2.Marel M, Zrustova M, Stasny B, et al. The incidence of pleural effusion in a well defined region. Epidemiologic study in central Bohemia. Chest 1993; 104:1486–1489. [DOI] [PubMed] [Google Scholar]
- 3▪.Cartaxo AM, Vargas FS, Salge JM, et al. Improvements in the 6-min walk test and spirometry following thoracentesis for symptomatic pleural effusions. Chest 2011; 139:1424–1429. [DOI] [PubMed] [Google Scholar]; This prospective study of 25 patients is the only one to assess the effect of pleural effusions on exercise capacity, and showed that thoracentesis significantly improves breathlessness and the 6-min walk distance.
- 4.Estenne M, Yernault JC, De Troyer A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am J Med 1983; 74:813–819. [DOI] [PubMed] [Google Scholar]
- 5.Wang JS, Tseng CH. Changes in pulmonary mechanics and gas exchange after thoracentesis on patients with inversion of a hemidiaphragm secondary to large pleural effusion. Chest 1995; 107:1610–1614. [DOI] [PubMed] [Google Scholar]
- 6.Nishida O, Arellano R, Cheng DC, et al. Gas exchange and hemodynamics in experimental pleural effusion. Crit Care Med 1999; 27:583–587. [DOI] [PubMed] [Google Scholar]
- 7.Agusti AG, Cardus J, Roca J, et al. Ventilation-perfusion mismatch in patients with pleural effusion: effects of thoracentesis. Am J Respir Crit Care Med 1997; 156:1205–1209. [DOI] [PubMed] [Google Scholar]
- 8▪.Goligher EC, Leis JA, Fowler RA, et al. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care 2011; 15:R46. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a large systematic review and meta-analysis of 19 studies that involved 1124 mechanically ventilated patients with pleural effusion. The study did not find definite evidence to either support or refute the practice of pleural drainage to improve duration of ventilation and length of stay outcomes.
- 9.Perpina M, Benlloch E, Marco V, et al. Effect of thoracentesis on pulmonary gas exchange. Thorax 1983; 38:747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown NE, Zamel N, Aberman A. Changes in pulmonary mechanics and gas exchange following thoracocentesis. Chest 1978; 74:540–542. [DOI] [PubMed] [Google Scholar]
- 11.Doelken P, Abreu R, Sahn SA, et al. Effect of thoracentesis on respiratory mechanics and gas exchange in the patient receiving mechanical ventilation. Chest 2006; 130:1354–1361. [DOI] [PubMed] [Google Scholar]
- 12.Karetzky MS, Kothari GA, Fourre JA, et al. Effect of thoracentesis on arterial oxygen tension. Respiration 1978; 36:96–103. [DOI] [PubMed] [Google Scholar]
- 13.Brandstetter RD, Cohen RP. Hypoxemia after thoracentesis. A predictable and treatable condition. JAMA 1979; 242:1060–1061. [PubMed] [Google Scholar]
- 14.Roch A, Bojan M, Michelet P, et al. Usefulness of ultrasonography in predicting pleural effusions > 500 mL in patients receiving mechanical ventilation. Chest 2005; 127:224–232. [DOI] [PubMed] [Google Scholar]
- 15▪.Razazi K, Thille AW, Carteaux G, et al. Effects of pleural effusion drainage on oxygenation, respiratory mechanics, and hemodynamics in mechanically ventilated patients. Ann Am Thorac Soc 2014; 11:1018–1024. [DOI] [PubMed] [Google Scholar]; This prospective study of mechanically ventilated patients with pleural effusion demonstrated that significant improvements in oxygenation, end-expiratory lung volume and respiratory mechanics occur following large volume pleural fluid drainage.
- 16.Chiumello D, Marino A, Cressoni M, et al. Pleural effusion in patients with acute lung injury: a CT scan study. Crit Care Med 2013; 41:935–944. [DOI] [PubMed] [Google Scholar]
- 17.Chen WL, Chung CL, Hsiao SH, et al. Pleural space elastance and changes in oxygenation after therapeutic thoracentesis in ventilated patients with heart failure and transudative pleural effusions. Respirology 2010; 15:1001–1008. [DOI] [PubMed] [Google Scholar]
- 18.Wang LM, Cherng JM, Wang JS. Improved lung function after thoracocentesis in patients with paradoxical movement of a hemidiaphragm secondary to a large pleural effusion. Respirology 2007; 12:719–723. [DOI] [PubMed] [Google Scholar]
- 19.Light RW, Stansbury DW, Brown SE. The relationship between pleural pressures and changes in pulmonary function after therapeutic thoracentesis. Am Rev Respir Dis 1986; 133:658–661. [DOI] [PubMed] [Google Scholar]
- 20.Gilmartin JJ, Wright AJ, Gibson GJ. Effects of pneumothorax or pleural effusion on pulmonary function. Thorax 1985; 40:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Light RW, Stanssbury DW, Brown SE. Changes in pulmonary function following therapentic thoracocentesis (Abstract). Chest 1981; 80:341. [Google Scholar]
- 22.Dechman G, Mishima M, Bates JH. Assessment of acute pleural effusion in dogs by computed tomography. J Appl Physiol 1994; 76:1993–1998. [DOI] [PubMed] [Google Scholar]
- 23.Krell WS, Rodarte JR. Effects of acute pleural effusion on respiratory system mechanics in dogs. J Appl Physiol 1985; 59:1458–1463. [DOI] [PubMed] [Google Scholar]
- 24.Sousa AS, Moll RJ, Pontes CF, et al. Mechanical and morphometrical changes in progressive bilateral pneumothorax and pleural effusion in normal rats. Eur Respir J 1995; 8:99–104. [DOI] [PubMed] [Google Scholar]
- 25.Dechman G, Sato J, Bates JH. Effect of pleural effusion on respiratory mechanics, and the influence of deep inflation, in dogs. Eur Respir J 1993; 6:219–224. [PubMed] [Google Scholar]
- 26.Talmor M, Hydo L, Gershenwald JG, et al. Beneficial effects of chest tube drainage of pleural effusion in acute respiratory failure refractory to positive end-expiratory pressure ventilation. Surgery 1998; 123:137–143. [PubMed] [Google Scholar]
- 27.Light RW, Jenkinson SG, Minh VD, et al. Observations on pleural fluid pressures as fluid is withdrawn during thoracentesis. Am Rev Respir Dis 1980; 121:799–804. [DOI] [PubMed] [Google Scholar]
- 28.Villena V, Lopez-Encuentra A, Pozo F, et al. Measurement of pleural pressure during therapeutic thoracentesis. Am J Respir Crit Care Med 2000; 162:1534–1538. [DOI] [PubMed] [Google Scholar]
- 29.Lan RS, Lo SK, Chuang ML, et al. Elastance of the pleural space: a predictor for the outcome of pleurodesis in patients with malignant pleural effusion. Ann Intern Med 1997; 126:768–774. [DOI] [PubMed] [Google Scholar]
- 30.Feller-Kopman D, Berkowitz D, Boiselle P, et al. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg 2007; 84:1656–1661. [DOI] [PubMed] [Google Scholar]
- 31.Altschule MD, Zamcheck N. The effects of pleural effusion on respiration and circulation in man. J Clin Invest 1944; 23:325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaska K, Wann LS, Sagar K, et al. Pleural effusion as a cause of right ventricular diastolic collapse. Circulation 1992; 86:609–617. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan LM, Epstein SK, Schwartz SL, et al. Clinical, echocardiographic, and hemodynamic evidence of cardiac tamponade caused by large pleural effusions. Am J Respir Crit Care Med 1995; 151:904–908. [DOI] [PubMed] [Google Scholar]
- 34.Light RW, Lee YC. Textbook of pleural diseases. Hodder Arnold, 2nd ed.London, UK:2008. [Google Scholar]
- 35▪.Marcondes BF, Vargas F, Paschoal FH, et al. Sleep in patients with large pleural effusion: impact of thoracentesis. Sleep Breath 2012; 16:483–489. [DOI] [PubMed] [Google Scholar]; This study is the first one to report on the effects of pleural effusions on patients’ sleep quality. It showed in 19 patients with large effusions that sleep quality was poor and improved after thoracentesis.
- 36.Finucane KE, Panizza JA, Singh B. Efficiency of the normal human diaphragm with hyperinflation. J Appl Physiol (1985) 2005; 99:1402–1411. [DOI] [PubMed] [Google Scholar]
- 37.Decramer M. Effects of hyperinflation on the respiratory muscles. Eur Respir J 1989; 2:299–302. [PubMed] [Google Scholar]
- 38.Burki NK, Lee LY. Mechanisms of dyspnea. Chest 2010; 138:1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scano G, Innocenti-Bruni G, Stendardi L. Do obstructive and restrictive lung diseases share common underlying mechanisms of breathlessness? Respir Med 2010; 104:925–933. [DOI] [PubMed] [Google Scholar]
- 40.Cooper JC, Elliott ST. Pleural effusions diaphragm inversion, and paradox: new observations using sonography. AJR Am J Roentgenol 1995; 164:510. [DOI] [PubMed] [Google Scholar]
- 41.Mulvey RB. The effect of pleural fluid on the diaphragm. Radiology 1965; 84:1080–1086. [DOI] [PubMed] [Google Scholar]


