Abstract
Metastatic melanoma is a highly aggressive disease. Recent progress in immunotherapy (IT) and targeted therapy (TT) has led to significant improvements in response and survival rates in metastatic melanoma patients. The current project aims to determine the benefit of the introduction of these new therapies in advanced melanoma across several regions of Switzerland. This is a retrospective multicenter analysis of 395 advanced melanoma patients treated with standard chemotherapy, checkpoint inhibitors, and kinase inhibitors from January 2008 until December 2014. The 1-year survival was 69% (n=121) in patients treated with checkpoint inhibitors (IT), 50% in patients treated with TTs (n=113), 85% in the IT+TT group (n=66), and 38% in patients treated with standard chemotherapy (n=95). The median overall survival (mOS) from first systemic treatment in the entire study cohort was 16.9 months. mOS of patients treated either with checkpoint or kinase inhibitors (n=300, 14.6 months) between 2008 and 2014 was significantly improved (P<0.0001) compared with patients treated with standard chemotherapy in 2008–2009 (n=95, 7.4 months). mOS of 61 patients with brain metastases at stage IV was 8.1 versus 12.5 months for patients without at stage IV (n=334), therefore being significantly different (P=0.00065). Furthermore, a significant reduction in hospitalization duration compared with chemotherapy was noted. Treatment with checkpoint and kinase inhibitors beyond clinical trials significantly improves the mOS in real life and the results are consistent with published prospective trial data.
Keywords: melanoma, overall survival data, targeted therapy
Introduction
Historically, metastatic melanoma shows a poor prognosis, with a median overall survival (mOS) of less than 1 year and an overall 5-year mortality close to 90% 1. Although surgery and irradiation play a role in the treatment of low burden metastatic disease, systemic therapy is the mainstay for most advanced melanoma patients. Since 1972 and until recently, cytotoxic chemotherapy with dacarbazine has been considered the standard of care in advanced melanoma patients, with an objective response rate in a pooled analysis of 23 randomized clinical trials of 15% 2.
Fortunately, with the development of targeted therapy (TT) and immunotherapy (IT) in the past few years, the standard of care for patients with advanced melanoma has improved considerably 3–11. The introduction and approval of these new treatment options led to a radical and promising change in the treatment landscape and in the outcome of advanced melanoma patients 3,4,6–12.
Approximately 50% of all melanomas harbor BRAF mutations, of which 75% are mutations of BRAF V600E, representing a promising target in melanoma therapy 13–17. Vemurafenib (registered as Zelboraf), a selective BRAF-inhibitor (BRAFi), was the first TT agent on the melanoma market showing a statistically significant improvement both in overall and in progression-free survival (OS, PFS) of advanced melanoma in patients harboring the BRAF V600 mutation 18. However, responses are limited because of acquired drug resistance 13,19–21. Cotargeting the kinase downstream of BRAF in the MAPK-pathway by a combination of MEK and BRAF inhibition delays the emergence of resistance, reduces the cutaneous side effects caused by paradoxical activation of the MAPK-pathway, and significantly improves the OS and PFS compared with vemurafenib or dabrafenib alone 7,13. Although monotherapy with a selective MEK inhibitor (MEKi) in BRAF mutant melanoma appears to be less efficient than BRAF inhibition 8,13,22, it shows promising results in the NRAS mutant melanoma population 23. Currently, BRAFi and MEKi combination treatment belongs to the ‘New Gold Standard’ for BRAF mutant metastatic melanoma 24,25.
Besides being mutagenic, melanoma is considered to be a highly immunogenic tumor on the basis of clinical responses and neoantigen generation 26,27. Ipilimumab (registered as Yervoy; Bristol Meyers Squibb, New York, New York, USA), a fully human monoclonal antibody, blocks CTLA-4, thereby permitting uncontested T-cell proliferation and antitumor immunity 6,12,28. It was the first agent to show a statistically significant benefit in OS in stage IV melanoma patients both in a first-line and in a second-line setting 6. Recently, checkpoint inhibitors interacting with programmed cell death (PD-1) receptor, which is involved in reduction of autoimmunity, changed the fatal history of the disease once again, showing remarkable responses as well as prolonged OS 29–32. PD-1-antibodies alone or in combination with ipilimumab have shown a higher response rate than ipilimumab monotherapy 8,27,33–37. Many other combinations interacting with immune checkpoints are currently being investigated in clinical trials.
The aim of the current study was to evaluate the outcome of stage IV melanoma patients across Switzerland and confirm the longitudinal survival change after the introduction of IT and TT in a real-life setting using a registered-patient cohort standardized database (Swiss Melanoma Registry).
Patients and methods
Patient selection and data acquisition
The study cohort included patients with stage IV metastatic melanoma treated at the Dermatology Department of the University Hospital Zurich, the Department of Medical Oncology of the University Hospital Lausanne, and the Department of Medical Oncology at the Cantonal Hospital Graubünden Chur between January 2008 and December 2014. Stage IV disease was defined according to the current American Joint Committee on Cancer staging system.
Patients were divided into two groups according to the first received treatment during the above period: standard chemotherapy treated between 2008 and 2009 (reference) versus the TT or the IT group from 2008 until 2014. In this period, vemurafenib (Zelboraf, 960 mg twice daily; Roche, Basel, Switzerland) and ipilimumab (Yervoy, 3 mg/kg) were approved by Swiss authorities for the treatment of metastatic melanoma, whereas anti-PD-1 (2 mg/kg) treatment was only accessible in an expanded-access program (EAP). Patients treated with chemotherapy before the approval of TT or IT were still included in the study cohort. In addition, the analysis included antecedent data from 60 patients (15% of the entire study cohort) in the following clinical trials: NCT01511913, NCT01668784, NCT01320085, NCT01436656, NCT01213472, NCT01597908, NCT01682083, NCT01307397, and NCT01704287.
All advanced melanoma patients at the University Hospital Zurich as well as those from Department of Medical Oncology at the Cantonal Hospital Graubünden Chur and Department of Medical Oncology of the University Hospital Lausanne who fulfilled the defined inclusion criteria were registered anonymously into a standardized clinical database. Information was retrospectively collected by reviewing the patient’s electronic medical files. Before analysis, clinical information was anonymized and deidentified. Standard anonymous data collection on the course of disease after the diagnosis of first distant metastases included treatment, development of new metastases, and survival status. Epidemiological, clinicopathological, laboratory, and molecular parameters were also collected. Data were classified with dichotomous variables (yes or no) or coded with the number of treatments and metastatic sites.
The local ethics committee approved written informed consent for tissue storage including retrospective analysis with collection of clinical/laboratory/histological information before collection (Kantonale Ethikkommission Zürich, Biobank/Sammlung von Tumorgewebe, KEK-ZH-Nr. 647, 800).
Definition of baseline and endpoints
The primary endpoint of the study was the percentage of patients surviving 1 year after being treated with standard chemotherapy (reference group) between 2008 and 2009 or with IT or TT from 2008 until 2014. The secondary endpoints included differences in mOS or PFS after the introduction of IT and TT as well as in mOS of patients with or without brain metastases at stage IV disease or those with and without brain metastases during the course of treatment. Furthermore, we analyzed survival with respect to lactate dehydrogenase (LDH) levels and hospitalization time (calculated in days) reflecting the treatment-associated costs.
OS was defined as the time (months) from treatment initiation to death, with censoring on the last known alive date.
Statistical analysis
Descriptive statistics are presented as percentages of total for categorical variables and as median for continuous and ordinal variables. For the entire cohort, OS was estimated with the Kaplan–Meier survival curves. Patients who were alive at the end of the study period were censored at the date of last follow-up. For survival time, summary measures include the mOS and 95% confidence interval (CI). The log-rank test was used to compare the survival time between treatment groups. A Cox proportional hazards model was used to estimate the adjusted hazard ratio (HR). P-value less than 0.05 was considered statistically significant. The analysis was carried out using R 38.
Results
Patient characteristics
Data of 442 American Joint Committee on Cancer Stage IV melanoma patients, who received systemic treatment for the disease from January 2008 until December 2014, were collected in the electronic database.
A total of 395 fulfilled the inclusion criteria and were subanalyzed according to their treatment protocol. Two hundred and thirty-nine (60.5%) patients were men and 156 (39.5%) patients were women; the median age at first diagnosis was 57 years (range: 13.6–88.5 years). Two hundred and eighty-two (71.4%) patients had died by December 2014 and of the 113 patients still alive, 11 were lost to follow-up.
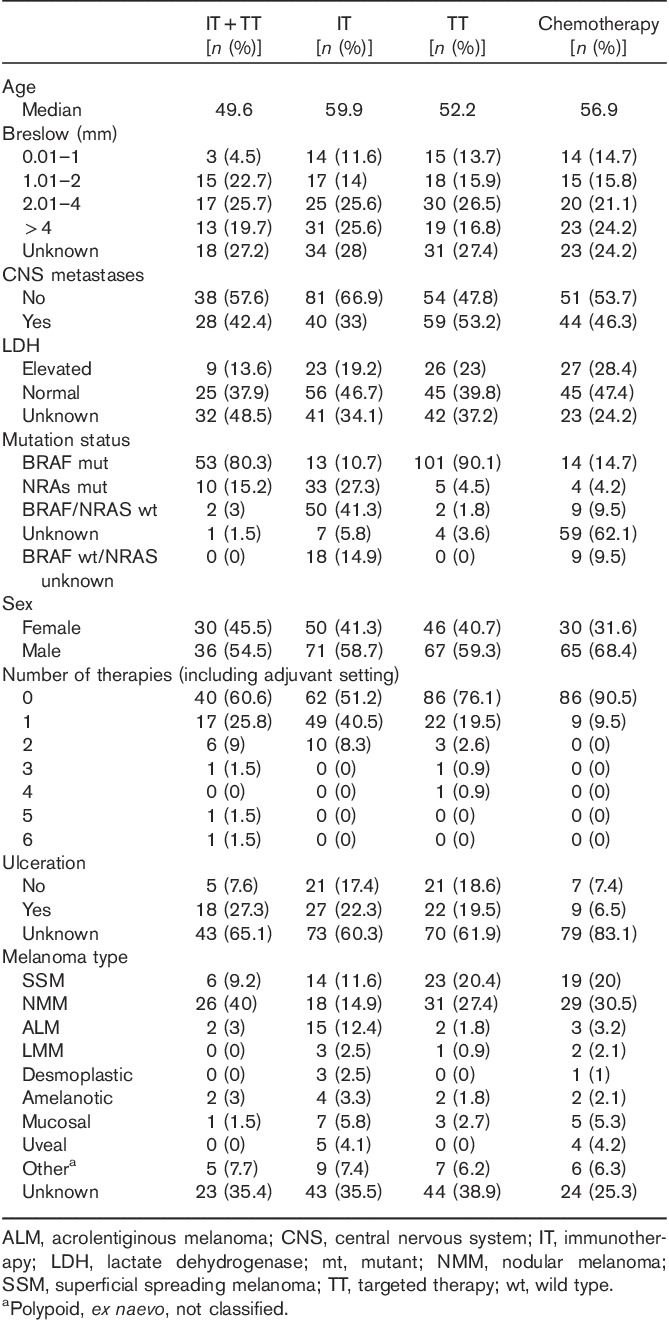
One hundred and twenty-one patients received IT, 113 patients received TT (BRAFi, MEKi or combination), and 66 patients received IT and TT. Six patients in the TT group were treated only with pan-RAF inhibitors. Thus, 95 patients underwent chemotherapy (reference group). In the IT+TT group, 17 (26%) patients received first IT and then upon progression subsequently received TT, whereas 22 (33%) patients were treated with anti-PD-1 antibodies in the EAP.
Before receiving IT or TT, approximately one-third of the patients (n=112) had been treated previously for their advanced disease with alkylating chemotherapeutic agents. Of these, only five patients received more than two systemic treatments (Table 1).
Table 1.
Clinical characteristics of the four treatment groups
Mutation status and patients’ characteristics
For all patients, histopathologic information such as melanoma subtype, localization of primary tumor, and tumor thickness was available. The presence or absence of ulceration was obtainable in 67% of the patients. Patients’ characteristics, demographics, and features of primary metastatic melanoma with respect to mutation status are listed in Table 2.
Table 2.
Patient demographics and primary melanoma characteristics according to mutation status
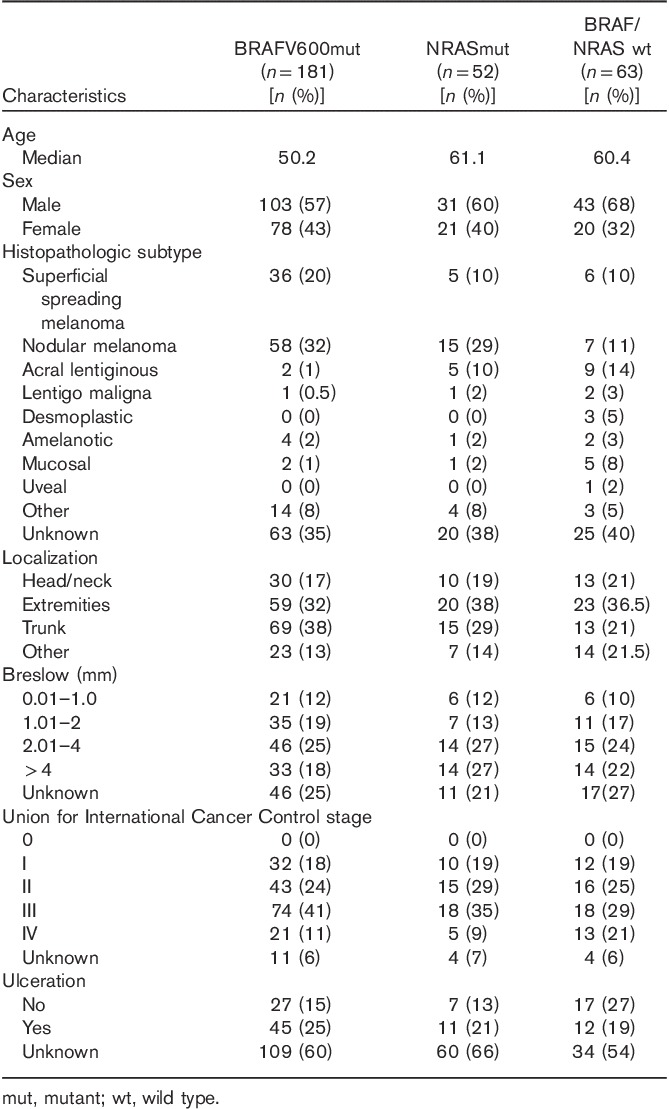
One hundred and eighty-one patients harbored a BRAF mutation, 52 harbored an NRAS mutation, and 63 were BRAF/NRAS wild type (BRAF/NRAS wt), whereas the mutation status of 100 patients was unknown at the time of inclusion.
Patients with BRAF mutant melanoma were generally younger at the time of first diagnosis (median age=50 years) than patients without a BRAF mutation (median age=61 years, P<0.001). Within the BRAF mutated group (n=181), primary melanoma of 69 (38%) patients was found on the trunk, whereas 51 (32%) melanomas were localized on the extremities. Fifty-eight (32%) patients had nodular melanoma (NMM), 36 (20%) patients had superficial spreading melanoma (SSM), and two patients had had acrolentiginous melanoma (ALM) (1%). In the NRAS mutant group (52), 15 (29%) melanomas were located on the trunk and 20 on the extremities (38%). Fifteen (29%) patients had NMM, five (10%) had SSM, and five (10%) had ALM. In the double wild-type group (63), 13 melanomas were located on the trunk (21%) and 23 were located on the extremities (36.5%). Seven (11%) patients had NMM, six (10%) had SSM, and nine (14%) had ALM (Table 2).
Survival data
The primary analysis included 395 patients who have received either chemotherapy or IT or TT. The median follow-up time was 26.3 months (interquartile range: 12.3–86.8 months).
The 1-year survival after first detection of distant metastasis was 59% in the entire study cohort (n=395), amounting to 69% for patients treated with IT (n=121), 50% for the TT group (n=113), 85% for the IT+TT group (n=66), and 38% for patients treated with chemotherapy (n=95). Proportions for patients surviving 2 years were as follows: 30% in the entire study population, 39% in IT, 20% in TT, 55% in those with both IT+TT, and 15% in the reference group.
Compared with the reference group (n=95), there was a statistically significant difference in mOS for patients treated with IT or TT (n=300), with 7.4 months (95% CI: 6–8.5) versus 14.6 months (95% CI: 12.2–18.2), respectively (HR=0.40, P<0.0001) (Table 3). More specifically, mOS was 16.7 months (n=121, 95% CI: 11.8–22.0) in the IT group, 11.2 months (n=113, 95% CI: 8.2–13) in the TT group, and 21.7 in those with IT+TT (n=66, 95% CI: 18.2–37.6) (Fig. 1a and b). The difference remained statistically significant for PFS in all groups, except IT (reference group 2.5 months, IT 2.15, IT/TT 5.4, and TT 7.3, P<0.0001) (Fig. 1c and Table 4).
Table 3.
Median overall survival in months of study subgroups
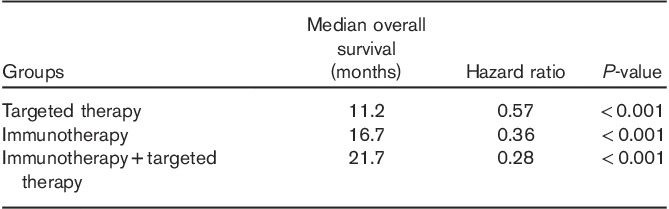
Fig. 1.
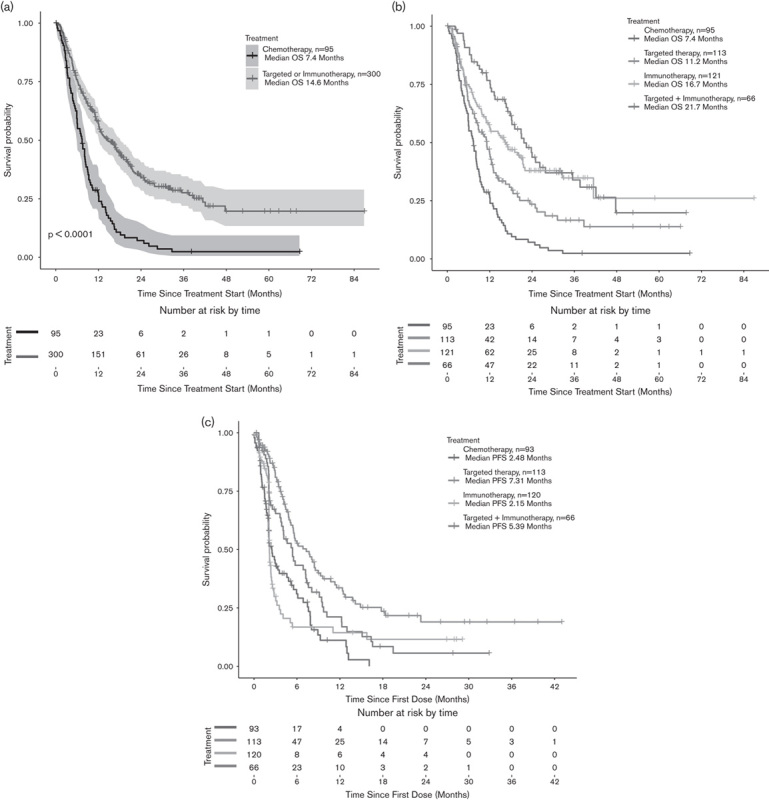
(a) OS from the start of treatment until death or last follow-up between the reference group (patients treated with chemotherapy, n=95) versus patients treated with IT+TT (n=300) (b) OS according to the treatment protocol (c) PFS according to treatment in the patient population. The difference remained statistically significant for PFS in all groups, except IT (reference group 2.5 months, IT 2.15, IT/TT 5.4, and TT 7.3, P<0.0001). IT, immunotherapy; OS, overall survival; PFS, progression-free survival; TT, targeted therapy.
Table 4.
Median progression-free survival in months of study subgroups
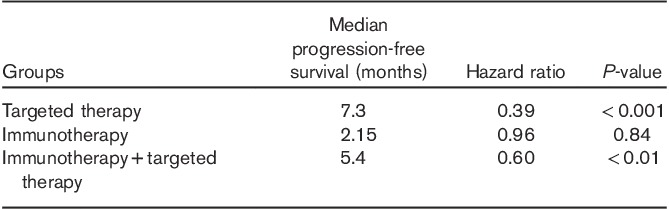
When survival data were subanalyzed for treatment-naive patients (Fig. 2a), mOS was 7.1 for the reference (n=86, 95% CI: 5.9–8.9) versus 17.8 (n=188, 95% CI: 13.5–24.9, HR=0.39, P<0.0001) for IT or TT. Separately, the mOS of IT-treated patients was 41.4 months (n=61), whereas the mOS of TT-treated patients was 11.9 months (n=86) (Fig. 2b. In the TT group, 49 (57%) patients had brain metastasis, whereas only 14 patients were in the IT group.
Fig. 2.
(a, b) OS in treatment-naive patients. mOS was 7.1 for the reference (n=86) versus 17.8 (n=188) for IT or TT. mOS of IT-treated patients was 41.4 months (n=61), whereas the mOS of TT-treated patients was 11.9 months. IT, immunotherapy; mOS, median overall survival; OS, overall survival; TT, targeted therapy.
Survival data with respect to lactate dehydrogenase levels
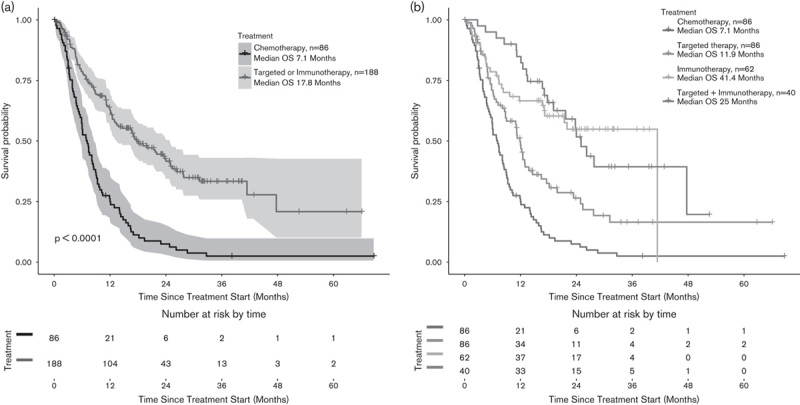
The LDH levels at stage IV disease were unknown in one-third of the study cohort (35%). High LDH levels had a significant negative impact in mOS only in patients treated with TT (mOS 5 months for patients with high LDH levels vs. mOS 12 months for patients with low LDH, HR=0.43, P<0.01) (Fig. 3a). However, LDH levels seemed not to influence the OS of IT-treated patients (mOS 11.8 for high LDH vs. mOS 15.2, HR=0.62, P=0.11) (Fig. 3b).
Fig. 3.
(a, b) Elevated LDH levels had a significantly negative impact on mOS only in patients treated with TT, but not in IT-treated patients. IT, immunotherapy; LDH, lactate dehydrogenase; mOS, median overall survival; TT, targeted therapy.
Brain metastases patients
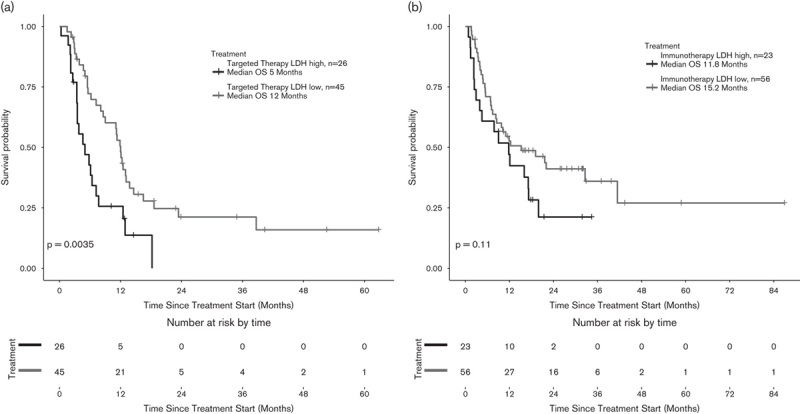
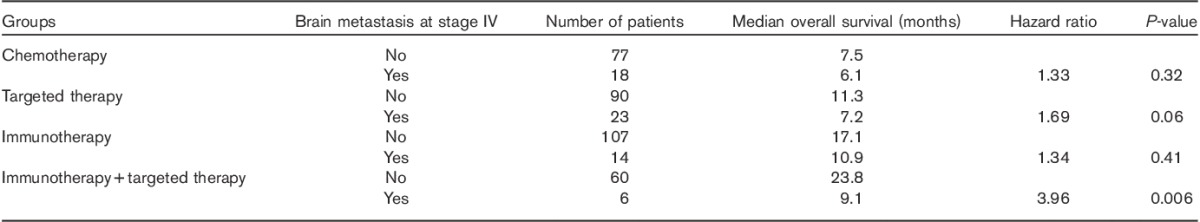
One hundred and seventy-one patients eventually developed cerebral metastases at some point during stage IV disease. OS of patients with brain metastases at stage IV was analyzed separately. mOS of 61 patients with brain metastases was 8.1 versus 12.5 months for patients without evidence of brain metastases at stage IV (n=334), showing a significant difference (HR=1.73, P<0.001) (Fig. 4a). When this subgroup analysis was carried out according to treatment (reference vs. IT vs. TT vs. IT+TT), there was no significant difference in the reference group (Fig. 4b); there was a trend toward improved survival in TT-treated patients (Fig. 4c); there was no significant difference in the IT group (Fig. 4d); and there was a statistically significant difference in mOS in IT+TT (Fig. 4e) (Table 5).
Fig. 4.
(a) mOS in patients with brain metastasis at stage IV (b–e): Subanalysis in the brain-metastasis population according to treatment (reference vs. IT vs. TT vs. IT+TT). There was a trend toward improved survival in TT-treated patients and a statistically significant difference in mOS in the IT+TT group (P=0.32, 0.42, 0.06, and 0.003, respectively). CNS, central nervous system; IT, immunotherapy; mOS, median overall survival; OS, overall survival; TT, targeted therapy.
Table 5.
Median overall survival in months of study subgroups with brain metastasis
Hospitalization time
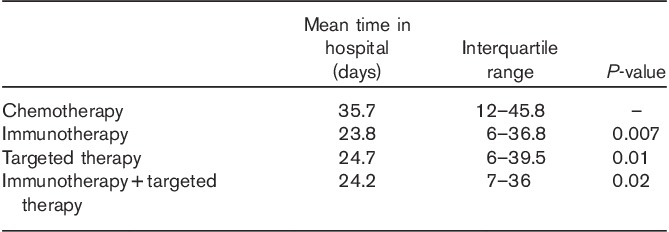
The median time spent in the hospital was investigated for all patients in the subgroups. There was a statistically significant difference in the hospitalization duration within the IT/TT group compared with standard chemotherapy (Table 6).
Table 6.
Mean time in the hospital in terms of the treatment protocol (n=395)
Discussion
In the current study, we report on the survival data of 395 stage IV melanoma patients treated with kinase inhibitors (BRAF or/and MEK) and checkpoint inhibitors (ipilimumab and pembrolizumab/nivolumab) compared with a historical control in the same setting treated with standard chemotherapy. This cohort is well defined and the clinical data are of high quality, being evaluated in a standardized database, and were closely updated every 6 months for the Swiss Melanoma Registry project.
The strengths of our study include the thorough follow-up, the quality of clinical data analyzed by independent clinicians, and the inclusion of patients mostly outside of a clinical trial protocol representing real-life data. To date, this is the largest OS analysis comparing chemotherapy with the new treatment modalities in a real-life setting in metastatic melanoma patients.
The superior outcome of the modern therapies in a real-world setting with a longer follow-up duration in comparison with previous reports could be confirmed. We show that longer survival is possible with MAPK inhibitors, with 1- and 2-year OS rates of 50 and 19.5%. In the TT group, high LDH levels had a significantly negative prognostic impact (P=0.035), suggesting that LDH is an independent parameter associated with clinical outcome in this patient population, which is consistent with prospective published data of the BRAF/MEKi combination treatment 39. Frauchiger et al. 40 also confirmed the predictive value of LDH (mOS for BRAFmut with normal LDH 14.2 vs. 6.95 months for high LDH). Although survival was numerically different in our IT study cohort within LDH low and high patients, the difference did not reach statistical significance, probably due to the low number of IT-treated.
For the checkpoint inhibitors (n=121), the 1- and 2-year OS rates are 69 and 39%, respectively, which are in accordance with the phase III clinical trials and recently published data from the ipilimumab EAP 7,8,11,36,41,42. However, our checkpoint inhibitor cohort included pembrolizumab-treated patients as well (n=36, 30%), reflecting the high 1-year OS of 69% presented here. These results are also in agreement with the recently published ‘real world’ results of 71.2% (95% CI: 71.1–71.3) from Germany 43. The authors commented that this increased survival probability might to be because of the closer radiological follow-up in advanced melanoma patients in Germany and Switzerland every 6 months, which enables an early-stage diagnosis and early initiation of treatment.
The low PFS of 5.4 months in the IT/TT group compared with chemotherapy (PFS=2.5 months) can be explained by the high number of patients treated with ipilimumab in this group, influencing the PFS rates. Accordingly, we report a low PFS (2.15 months) within the IT group, whereas the PFS of TT patients (n=113) was 7.3 months. This is, however, comparable with the median PFS in the ipilimumab-treated arm in the Keynote 006 trial (mPFS 2.8 months) 31. Only 30% of the patients in the IT group had received pembrolizumab (either first or second line), which, despite having influenced the mOS rates, was not the case for the median PFS. In addition, and as is known, patients in clinical trials are commonly filtered (Eastern Cooperative Oncology Group status 0 or 1), often have normal LDH levels, and have no symptomatic brain metastasis as brain metastases are typically an exclusion criterion from clinical trials. A separate subgroup classification of ipilimumab and pembrolizumab was not performed because of the small number of pembrolizumab-treated patients.
However, the development of new lesions in patients receiving ipilimumab may not always indicate progressive disease or treatment failure, reflecting the concept of pseudoprogression, and may not correspond to the sometimes long-lasting responses in a minority of those patients. To overcome this problem, new immune RECIST criteria immune-related response criteria that provide a better correlation between OS and response were proposed 44.
The study also investigated the survival of patients with the presence of brain metastasis at stage IV disease and found a superior outcome to those without as expected (n=334, mOS 8.1 months, vs. mOS 12.5 months’ P=0.00065). These survival outcomes in brain-metastasis melanoma patients are by far superior to the 4-month survival data reported in the literature 45,46. This difference can be explained by the fact that the majority of patients with brain metastasis in our study received subsequent IT or TT after surgery or irradiation, which clearly confounded the results 47,48. We observed a nonsignificant trend toward an improved survival in patients with brain metastases treated with TT (P=0.06) and a significant survival benefit in those treated with IT+TT. Nevertheless, these results should be interpreted with caution because of the low numbers of patients analyzed by treatment. Combining systemic modern therapies for melanoma with conventional treatment of brain metastasis is a field that requires further investigation and large prospective trials are needed to guide future clinical management of this poor-prognosis group.
Another secondary endpoint of our study was to detect any difference in the hospitalization time between patients treated with checkpoint inhibitors or TT and those with standard chemotherapy (reference group), reflecting differences in treatment-association costs. A statistically significant difference was reported (P=0.01 in TT and P=0.007 in IT), despite the presence of sometimes severe immune-related adverse events (e.g. autoimmune-colitis or hypophysitis) documented with ipilimumab. Consistently, and according to a retrospective-single-center English cohort of patients (n=110) treated with ipilimumab, immune-related adverse events do not represent a significant expense in comparison with the drug cost itself 49.
There are clear limitations in our study, including the retrospective setting, with the potential selection bias or time effects. However, patients from different sites including university and nonuniversity hospitals in Switzerland were included, minimizing the risk that the current results are confounded by patient selection or site-specific influences. At the time of the study design and data collection, only ipilimumab (initially in the second-line setting and as of end of 2014, in the first-line setting) and BRAFi monotherapy was approved in Switzerland for the treatment of metastatic melanoma, which might have biased our results. Pembrolizumab was not approved as first-line treatment until May 2016, followed by the approval of combined nivolumab and ipilimumab in summer 2016.
Because of this fact, ∼30% (n=117) of the patients in the IT or the TT group in our study had been pre-treated for their advanced disease with chemotherapy. Also, when analyzing the survival outcomes in treatment-naive patients, there was again a significant difference in the IT or the TT group (mOS 7.1 reference vs. 41 in the IT group and 11.9 in the TT group). The reported mOS for the TT group is consistent with the 13.6 months reported in the literature 50. The fact that the majority of patients under TT develop resistance over time explains this difference between IT and TT. However, resistance after the initial response to anti-PD-1 has been reported recently 51,52.
Furthermore and although we calculated survival outcomes in treatment-naive patients (n=274), we did not differentiate survival data between first-line and second-line or third-line treatments because of the inadequate number of patients.
This study confirms the superior outcome of IT-treated and TT-treated advanced melanoma patients in a real-life setting; however, a safe head-to-head comparison between IT and TT cannot be made. Yet, we did analyze a small subgroup of patients who had both IT+TT, which suggested that the patients who benefit from both treatments might benefit even more from the new drugs. In this context, prospective clinical trials will further elucidate optimal sequencing to improve patients’ counseling. A phase III clinical trial investigating this issue is currently ongoing 53.
Conclusion
Treatment with checkpoint and kinase inhibitors beyond clinical trials significantly improves the mOS in a real-life setting including those patients with brain metastases. These data confirm that national melanoma registries and cancer statistics are useful for monitoring outcomes of approved therapies or newly established treatment protocols across multiple institutions and patient populations.
Acknowledgements
The authors are indebted to Mirja Gautschi for her contribution of patients’ data.
This study was partially funded by Roche, Merck Sharp & Dhome (MSD), and Bristol-Myers Squibb (BMS). The funding was provided by an unrestricted educational grant to the University of Zürich.
Conflicts of interest
Professor Dummer receives research funding to the University of Zürich from Novartis, Merck Sharp & Dhome (MSD), Bristol-Myers Squibb (BMS), Roche, and GlaxoSmithKline (GSK) and has a consultant or advisory board relationship with Novartis, Merck Sharp & Dhome, Bristol-Myers Squibb, Roche, GlaxoSmithKline, and Amgen outside the submitted work. Dr Goldinger has temporary consultant or advisory relationships and receives travel support from Roche, Novartis, Bristol-Myers Squibb (BMS), and Merck Sharp & Dhome (MSD); she receives research funding from the University of Zurich and SAKK. PD Dr.med. Roger von Moos plays a consultant role for Novartis, Roche, BMS, and MSD. For the remaining authors there are no conflicts of interest.
Footnotes
Joanna Mangana, Phil F. Cheng, Simone M. Goldinger, and Reinhard Dummer contributed equally to the writing of this article.
References
- 1.Shah DJ, Dronca RS. Latest advances in chemotherapeutic, targeted, and immune approaches in the treatment of metastatic melanoma. Mayo Clin Proc 2014; 89:504–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lui P, Cashin R, Machado M, Hemels M, Corey-Lisle PK, Einarson TR. Treatments for metastatic melanoma: synthesis of evidence from randomized trials. Cancer Treat Rev 2007; 33:665–680. [DOI] [PubMed] [Google Scholar]
- 3.Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371:1867–1876. [DOI] [PubMed] [Google Scholar]
- 4.Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J, et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011; 305:2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med 2011; 17:1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell 2011; 19:45–57. [DOI] [PubMed] [Google Scholar]
- 7.Ascierto PA, Simeone E, Sileni VC, Pigozzo J, Maio M, Altomonte M, et al. Clinical experience with ipilimumab 3 mg/kg: real-world efficacy and safety data from an expanded access programme cohort. J Transl Med 2014; 12:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dummer R, Daud A, Puzanov I, Hamid O, Schadendorf D, Robert C, et al. A randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab-refractory melanoma. J Transl Med 2015; 13 (Suppl 1):O5. [Google Scholar]
- 9.Ascierto PA, Schadendorf D, Berking C, Agarwala SS, van Herpen CM, Queirolo P, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013; 14:249–256. [DOI] [PubMed] [Google Scholar]
- 10.Dummer R, Schadendorf D, Ascierto PA, Larkin J, Lebbe C, Hauschild A. Integrating first-line treatment options into clinical practice: what’s new in advanced melanoma? Melanoma Res 2015; 25:461–469. [DOI] [PubMed] [Google Scholar]
- 11.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015; 16:522–530. [DOI] [PubMed] [Google Scholar]
- 12.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 2013; 19:5300–5309. [DOI] [PubMed] [Google Scholar]
- 13.Alhumaimess M, Lin Z, Weng W, Dimitratos N, Dummer NF, Taylor SH, et al. Oxidation of benzyl alcohol by using gold nanoparticles supported on ceria foam. Chem Sus Chem 2012; 5:125–131. [DOI] [PubMed] [Google Scholar]
- 14.Ekedahl H, Cirenajwis H, Harbst K, Carneiro A, Nielsen K, Olsson H, et al. The clinical significance of BRAF and NRAS mutations in a clinic-based metastatic melanoma cohort. Br J Dermatol 2013; 169:1049–1055. [DOI] [PubMed] [Google Scholar]
- 15.Spagnolo F, Ghiorzo P, Orgiano L, Pastorino L, Picasso V, Tornari E, et al. BRAF-mutant melanoma: treatment approaches, resistance mechanisms, and diagnostic strategies. Onco Targets Ther 2015; 8:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005; 353:2135–2147. [DOI] [PubMed] [Google Scholar]
- 17.Krauthammer M, Kong Y, Bacchiocchi A, Evans P, Pornputtapong N, Wu C, et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet 2015; 47:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, et al. Vemurafenib: the first drug approved for BRAF-mutant cancer. Nat Rev Drug Discov 2012; 11:873–886. [DOI] [PubMed] [Google Scholar]
- 19.Livingstone E, Zimmer L, Piel S, Schadendorf D. PLX4032: does it keep its promise for metastatic melanoma treatment? Expert Opin Investig Drugs 2010; 19:1439–1449. [DOI] [PubMed] [Google Scholar]
- 20.Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol 2011; 82:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levesque MP, Dummer R, Beerenwinkel N. Perturbing resistance: a network perspective. Pigment Cell Melanoma Res 2016; 29:142–149. [DOI] [PubMed] [Google Scholar]
- 22.Munoz-Couselo E, Garcia JS, Perez-Garcia JM, Cebrian VO, Castan JC. Recent advances in the treatment of melanoma with BRAF and MEK inhibitors. Ann Transl Med 2015; 3:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dummer R, Schadendorf D, Ascierto PA, Fernández AMA, Dutriaux C, Maio M, et al. Results of NEMO: a phase III trial of binimetinib (BINI) vs dacarbazine (DTIC) in NRAS-mutant cutaneous melanoma [abstract]. J Clin Oncol 2016; 34 (Suppl):9500. [Google Scholar]
- 24.Dummer R, Guggenheim M, Arnold AW, Braun R, von Moos R. Project Group Melanoma of the Swiss Group for Clinical Cancer Research. Updated Swiss guidelines for the treatment and follow-up of cutaneous melanoma. Swiss Med Wkly 2011; 141:w13320. [DOI] [PubMed] [Google Scholar]
- 25.Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U. ESMO Guidelines Committee. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26 (Suppl 5):v126–v132. [DOI] [PubMed] [Google Scholar]
- 26.Banaszynski M, Kolesar JM. Vemurafenib and ipilimumab: new agents for metastatic melanoma. Am J Health Syst Pharm 2013; 70:1205–1210. [DOI] [PubMed] [Google Scholar]
- 27.Hughes T, Klairmont M, Sharfman WH, Kaufman HL. Interleukin-2, ipilimumab, and anti-PD-1: clinical management and the evolving role of immunotherapy for the treatment of patients with metastatic melanoma. Cancer Biol Ther 2015. doi: 10.1080/15384047.2015.1095401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackiewicz-Wysocka M, Zolnierek J, Wysocki PJ. New therapeutic options in systemic treatment of advanced cutaneous melanoma. Expert Opin Investig Drugs 2013; 22:181–190. [DOI] [PubMed] [Google Scholar]
- 29.Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, et al. Efficacy and safety results from a phase III trial of nivolumab (NIVO) alone or combined with ipilimumab (IPI) versus IPI alone in treatment-naive patients (pts) with advanced melanoma (MEL) (CheckMate 067). J Clin Oncol 2015; 33:LBA1. [Google Scholar]
- 30.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320–330. [DOI] [PubMed] [Google Scholar]
- 31.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 32.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai KK, Daud AI. Nivolumab plus ipilimumab in the treatment of advanced melanoma. J. Hematol Oncol 2015; 8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faghfuri E, Faramarzi MA, Nikfar S, Abdollahi M. Nivolumab and pembrolizumab as immune-modulating monoclonal antibodies targeting the PD-1 receptor to treat melanoma. Expert Rev Anticancer Ther 2015; 15:981–993. [DOI] [PubMed] [Google Scholar]
- 35.Somasundaram R, Herlyn M. Nivolumab in combination with ipilimumab for the treatment of melanoma. Expert Rev Anticancer Ther 2015; 15:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad SS, Qian W, Ellis S, Mason E, Khattak MA, Gupta A, et al. Ipilimumab in the real world: the UK expanded access programme experience in previously treated advanced melanoma patients. Melanoma Res 2015; 25:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 2010; 107:4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Development Core Team R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 39.Long GV, Weber JS, Infante JR, Kim KB, Daud A, Gonzalez R, et al. Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol 2016; 34:871–878. [DOI] [PubMed] [Google Scholar]
- 40.Frauchiger AL, Mangana J, Rechsteiner M, Moch H, Seifert B, Braun R, et al. Prognostic relevance of LDH and serum S-100 levels in Stage IV melanoma with known BRAF mutation status. Br J Dermatol 2016; 174:823–830. [DOI] [PubMed] [Google Scholar]
- 41.Kelderman S, Heemskerk B, van Tinteren H, van den Brom RR, Hospers GA, van den Eertwegh AJ, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother 2014; 63:449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from phase ii and phase iii trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015; 33:1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forschner A, Eichner F, Amaral T, Keim U, Garbe C, Eigentler TK. Improvement of overall survival in stage IV melanoma patients during 2011–2014: analysis of real-world data in 441 patients of the German Central Malignant Melanoma Registry (CMMR). J Cancer Res Clin Oncol 2017; 143:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15:7412–7420. [DOI] [PubMed] [Google Scholar]
- 45.Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011; 117:1687–1696. [DOI] [PubMed] [Google Scholar]
- 46.Fife KM, Colman MH, Stevens GN, Firth IC, Moon D, Shannon KF, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol 2004; 22:1293–1300. [DOI] [PubMed] [Google Scholar]
- 47.Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012; 13:459–465. [DOI] [PubMed] [Google Scholar]
- 48.Konstantinou MP, Dutriaux C, Gaudy-Marqueste C, Mortier L, Bedane C, Girard C, et al. Ipilimumab in melanoma patients with brain metastasis: a retrospective multicentre evaluation of thirty-eight patients. Acta Derm Venereol 2014; 94:45–49. [DOI] [PubMed] [Google Scholar]
- 49.Yousaf N, Davidson M, Goode E, Thomas C, Hung R, Gore M, et al. The cost of ipilimumab toxicity: a single-centre analysis. Melanoma Res 2015; 25:259–264. [DOI] [PubMed] [Google Scholar]
- 50.McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014; 15:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016; 375:819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov 2017; 7:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.NCT02224781. A randomized phase III trial of dabrafenib+trametinib followed by ipilimumab+nivolumab at progression vs. Ipilimumab+nivolumab followed by dabrafenib+trametinib at progression in patients with advanced BRAFV600 mutant melanoma.


