Abstract
Dysregulated miRNAs play important role in K-ras mutation or smoking caused lung tumorigenesis. Here, we investigate the role and mechanism of miR-124 in K-ras mutation or smoking-caused lung tumorigenesis and evaluate the therapeutic potential of miR-124 agomiR in K-ras mutation or smoking-caused lung cancer treatment. Our data show that smoking suppresses miR-124 expression, and decreased miR-124 expression is inversely correlated with the p-Akt level and predicts poor overall survival in non-small-cell lung cancer (NSCLC) patients. The overexpression of miR-124 suppressed NSCLC growth by inhibiting the Akt pathway by targeting Akt1 and Akt2. In addition, the systemic delivery of miR-124 agomiR dramatically suppressed tumorigenesis in both NNK-induced lung cancer model and K-rasLA1 transgenic mice by increasing apoptosis and inhibiting cell proliferation. Our findings suggest that smoking inhibits the expression of miR-124, and decreased miR-124 contributes to Akt activation, thereby promoting NSCLC progression. Our findings also represent a novel potential therapeutic strategy for lung cancer.
Keywords: lung cancer, miR-124, Akt activation, NNK-induced lung cancer, K-ras mutation-induced lung tumorigenesis
Introduction
Lung cancer remains the most frequently diagnosed cancer and the leading cause of cancer-related death worldwide.1 According to reports, approximately 75% to 85% of lung cancers are non-small-cell lung cancer (NSCLC) at diagnosis.2 However, the overall 5-year survival rate of NSCLC patients is only 16%,3 and the lack of major improvements in the 5-year survival rate of patients with NSCLC has driven the search for new strategies aimed at improving lung cancer management.4 Therefore, a better understanding the biology of lung cancer may lead to the identification of novel targets and strategies for the treatment and chemoprevention of this disease.
Protein kinase B (PKB), also known as Akt, is a serine/threonine protein kinase and one of the most frequently hyperactivated kinases in human lung cancer. The frequency of Akt hyperactivation in NSCLC reportedly ranges from 20% to 89%,2, 5 and the hyperactivation of Akt indicates poor prognosis in patients with NSCLC.4 Accumulating evidence has shown that hyperactivated Akt plays an important role in lung tumorigenesis and progression by stimulating cellular transformation, cancer cell growth, proliferation, and metastasis while also attenuating cancer cell apoptosis.6, 7, 8 In addition, many carcinogens and oncogenes have been reported to activate the Akt pathway, which plays a key role in tumorigenesis. For example, the activation of the oncogene K-ras by mutation9 or the tobacco-specific carcinogen 4-methylnitrosamino-1-3-pyridyl-1-butanone (NNK) can directly induce the hyperactivation of the Akt pathway,10 and the inhibition of the Akt pathway can suppress K-ras mutation-driven11 or NNK-induced lung tumorigenesis.12
MiRNAs are a class of small, non-coding RNAs that repress the expression of multiple target genes by directly binding to the 3′ untranslated region (3′ UTR) of a target gene’s mRNA, thereby inducing mRNA cleavage or translational repression.13 Evidence has shown that the expression of miRNAs is dysregulated in most cancers, and the dysregulation of certain miRNAs plays a key role in cancer progression by activating oncogenic signaling pathways, including the Akt pathway.14, 15 Specifically, downregulated miR-124 expression was identified in several types of cancer,16, 17, 18 and studies have shown that the overexpression of miR-124 suppresses cancer progression by inhibiting the Akt pathway,14, 17 which suggests that miR-124 may play a general role in the regulation of the Akt pathway in different types of cancer. The downregulated expression of miR-124 in lung cancer tissues19 and the negative correlation between miR-124 and p-Akt in lung cancer cells20 have been previously reported. However, the detailed mechanism by which miR-124 regulates Akt phosphorylation is not clear in NSCLC cells. Thus, we herein investigated the effects and mechanism of miR-124 on the Akt pathway and evaluated the restoration of miR-124 expression as a novel strategy for lung cancer therapy in K-rasLA1 transgenic or NNK-induced lung tumor models.
Results
miR-124 Expression Negatively Correlates with Poor Clinical Outcome and p-Akt Expression in NSCLC
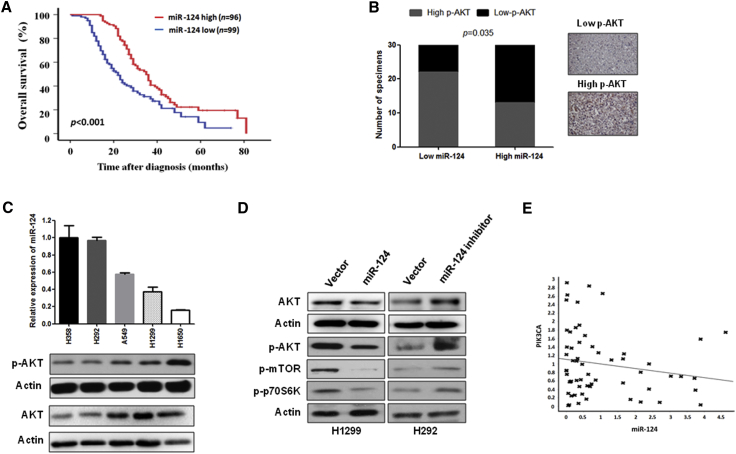
Zhang et al.21 and our clinical data (Figure 1A; Table 1) show that a low level of miR-124 expression correlates with tumor metastasis, clinical stage, and poor overall survival in patients with lung cancer. Furthermore, multivariate analyses show that low miR-124 expression is an independent predictor of prognosis in patients with NSCLC (Table 2). Moreover, previous studies have shown that decreased miR-124 expression contributes to the activation of the Akt pathway in hepatocellular carcinoma and prostate cancer,14, 16 suggesting that miR-124 expression may contribute to NSCLC progression via the activation of Akt. Thus, we first investigated the correlation between the expression of miR-124 and p-Akt in human NSCLC specimens and several lung cancer cell lines. As shown in Figure 1B, the miR-124 expression level negatively correlated with the p-Akt level in specimens of NSCLC. Similar results were also observed in NSCLC cell lines. Specifically, our results show that the expression level of miR-124 inversely correlated with the Akt and p-Akt level in NSCLC cell lines (Figure 1C). In addition, our in vitro data show that Akt, p-Akt, and its downstream signals were suppressed in H1299 cells that stably express miR-124 compared with the respective control cells (Figures 1D and S2A). In contrast, Akt, Akt phosphorylation, and its downstream signals were increased by stably expressing miR-124-antisense in H292 cells compared to the respective control cells (Figures 1D and S2B). Taken together, these findings suggest that miR-124 expression level was negatively correlated with the activation of Akt.
Figure 1.
miR-124 Is Negatively Correlated with Overall Survival and Akt Activation in NSCLC
(A) Kaplan-Meier analysis of overall survival rate for patients with NSCLC with low- and high-miR-124 expression. Patients with NSCLC were divided into high- and low-miR-124 expression, according to the mean fold change of the miR-124 (n = 195). (B) The expression level of miR-124 and p-Akt was negatively correlated in human lung cancer specimens (n = 60). The expression level of p-Akt and miR-124 was determined using immunohistochemistry and qRT-PCR, respectively. (C) miR-124 expression level was inversely correlated with Akt and p-Akt level in several lung cancer cell lines. (D) miR-124 negatively regulates the Akt pathway in NSCLC cells. The indicated proteins associated with the Akt pathway were measured by western blotting in NSCLC cells stably expressing miR-124 or antisense miR-124 and their vector controls. (E) The expression of miR-124 and PIK3CA has no correlation in specimens of NSCLC patients. The expression of miR-124 and PIK3CA was measured in human lung cancer specimens using qRT-PCR (n = 60).
Table 1.
Characteristics of Patients with NSCLC
| Variable | Number of Patients (%) |
p Value | |
|---|---|---|---|
| miR124-Low (n = 99) | miR124-High (n = 96) | ||
| Gender | |||
| Male | 66 (66.67) | 76 (79.17) | 0.55 |
| Female | 33 (33.33) | 20 (20.83) | |
| Age | |||
| ≤60 | 49 (49.49) | 54 (56.25) | 0.35 |
| >60 | 50 (50.51) | 42 (43.75) | |
| Smokinga | |||
| Yes | 84 (84.85) | 8 (8.33) | <0.01 |
| No | 15 (15.15) | 88 (91.67) | |
| Histology | |||
| Adenocarcinoma | 45 (45.45) | 54 (56.25) | 0.68 |
| Squamous cell carcinoma | 31 (31.31) | 22 (22.93) | |
| Bronchioloalveolar carcinoma | 15 (15.15) | 14 (14.58) | |
| Large-cell carcinoma | 5 (5.05) | 2 (2.08) | |
| Mucinous adenocarcinoma | 1 (3.04) | 1 (1.04) | |
| Adenosquamous carcinoma | 1 (1.01) | 2 (2.08) | |
| Carcinoid | 1 (1.01) | 1 (1.04) | |
| Histologic Grade | |||
| Well differentiated | 12 (12.12) | 14 (14.58) | 0.71 |
| Moderately differentiated | 39 (39.39) | 42 (43.75) | |
| Poorly differentiated | 46 (46.46) | 37 (38.54) | |
| Undifferentiated | 2 (2.03) | 3 (3.13) | |
| T Status | |||
| T1 | 23 (23.23) | 28 (29.17) | 0.01 |
| T2 | 12 (12.12) | 25 (26.04) | |
| T3 | 45 (45.45) | 36 (37.50) | |
| T4 | 19 (19.20) | 7 (7.29) | |
| N Status | |||
| N0 | 31 (31.31) | 65 (67.71) | <0.01 |
| N1 | 26 (26.26) | 14 (14.58) | |
| N2 | 30 (30.30) | 12 (12.50) | |
| N3 | 12 (12.13) | 5 (5.21) | |
| M Status | |||
| M0 | 72 (72.73) | 69 (71.88) | 0.04 |
| M1a | 17 (17.17) | 8 (8.33) | |
| M1b | 10 (10.10) | 19 (19.79) | |
Smoker, patients who smoked than one more year and were currently smokers when diagnosed with lung cancer; non-smoker, patients who never smoked.
Table 2.
Multivariable Analyses of Factors Predictive of Poor Overall Survival in Patients with NSCLC
| Variable | HR (95% Confidence Interval) | p Value |
|---|---|---|
| Gender | 0.93 (0.56–1.53) | 0.77 |
| Age | 1.36 (0.96–1.94) | 0.09 |
| Smoking status | 1.48 (1.32–1.71) | 0.04 |
| Histologic type | 0.81 (0.58–1.13) | 0.21 |
| T status | 2.19 (1.89–2.35) | 0.04 |
| N status | 1.32 (1.06–1.65) | 0.01 |
| M status | 2.09 (1.79–2.51) | 0.04 |
| miR124 level | 0.33 (0.19–0.50) | <0.01 |
Furthermore, we investigated the correlation between the expression of miR-124 and PIK3CA in NSCLC specimens because previous reports have shown that miR-124 inactivates the Akt pathway by directly targeting PIK3CA, which is upstream of Akt protein, in hepatocellular carcinoma.14 Unfortunately, we did not observe a significant correlation between the expression of miR-124 and PIK3CA in NSCLC specimens (Figure 1E), suggesting miR-124-induced activation of Akt signaling is not through PIK3CA in NSCLC cells.
Because previous studies show that miR-124 displays anticancer effects in several types of cancer14, 16 and it negatively correlated with Akt activation in NSCLC, here we examined miR-124 anticancer effects on NSCLC cells. Data show that overexpression of miR-124 significantly inhibited NSCLC cell proliferation, invasion, and stimulated apoptosis (Figure S3). Taken together, these findings suggest that miR-124 displays anticancer effects in NSCLC by negatively regulating the Akt pathway.
Akt1 and Akt2 Are Targets of miR-124 in NSCLC
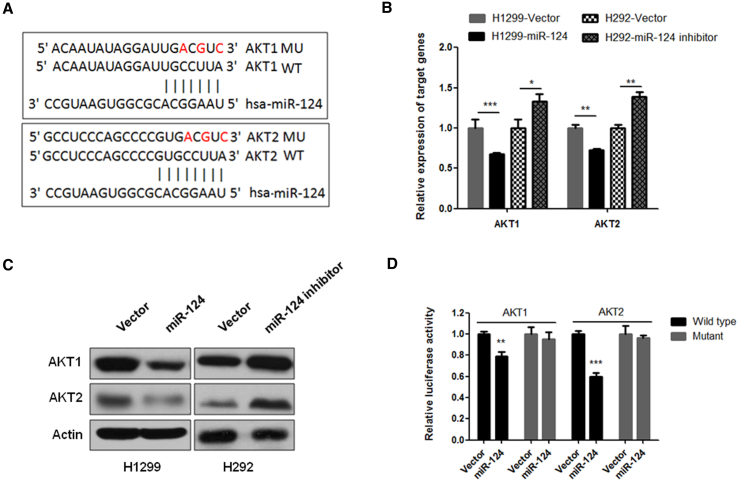
To determine whether miR-124 regulates the Akt pathway in NSCLC, we used algorithms that predict the mRNA target genes of miRNAs (www.targetscan.org) and identified Akt1 and Akt2 as putative target genes of miR-124 (Figure 2A). Because Akt1 and Akt2 are important components for Akt signaling activation and there are no studies that have shown that Akt1 and Akt2 are the target genes of miR-124, to investigate the effects of miR-124 on the expression of Akt1 and Akt2, we measured the expression levels of Akt1 and Akt2 in NSCLC cells that stably expressed miR-124 or miR-124-antisense. As shown in Figures 2B and 2C, the overexpression of miR-124 was significantly suppressed, whereas the inhibition of miR-124 increased the expression of Akt1 and Akt2 at both the mRNA and protein levels compared to the respective control cells. To determine whether the regulation of Akt1 and Akt2 luciferase expression depended on the binding between their complementary 3′ UTR sequences and miR-124, a three-point mutation was generated in the 3′ UTR of Akt1 or Akt2, as indicated in Figure 2A. The results showed that miR-124 overexpression significantly repressed the luciferase activity in both the Akt1 and Akt2 wild-type 3′ UTRs (Figure 2D). In contrast, both 3′ UTR alterations completely abrogated the effect of miR-124 overexpression on luciferase expression (Figure 2D). Cumulatively, these data suggest that miR-124 negatively regulates the expression of Akt1 and Akt2 by directly targeting their 3′ UTR sequences.
Figure 2.
miR-124 Negatively Regulates the Expression of Akt1 and Akt2 by Directly Binding to Their 3′ UTRs
(A) The predicted binding sites of miR-124 in the wild-type 3′ UTRs of Akt1 and Akt2. Mutations in the 3′ UTRs are highlighted in red. (B) miR-124 negatively regulates the mRNA expression of Akt1 and Akt2 in NSCLC cells. The mRNA expression level of Akt1 and Akt2 was measured using qRT-PCR in stably expressing miR-124 H1299 cells and stably expressing miR-124-antisense H292 cells. (C) miR-124 negatively regulates the protein expression of Akt1 and Akt2 in NSCLC cells. The protein expression level of Akt1 and Akt2 was measured using western blot in indicated NSCLC cells that stably express miR-124 (H1299) or miR-124-antisense (H292). (D) 3′ UTR luciferase reporter assay for Akt1 and Akt2. Stably expressing miR-124 H1299 cells were transfected with indicated 3′ UTR luciferase reporter construct. After 48 hr of transfection, luciferase intensity was assessed. The data are presented as the mean ± SD from three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
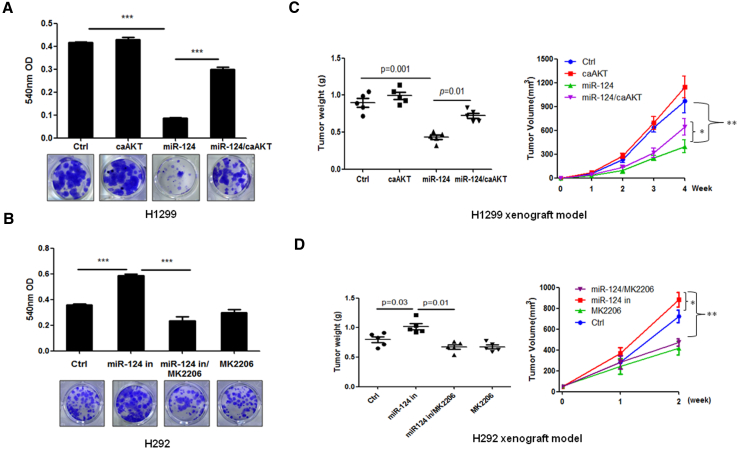
miR-124 Suppressed Tumor Growth by Inhibiting the Akt Pathway in NSCLC
The anticancer effects of miR-124 in lung cancer were previously identified. To determine whether the antitumor effects of miR-124 on NSCLC could partially be explained by targeting the Akt pathway, we performed in vitro and in vivo experiments. Our in vitro data show that the constitutive activation of Akt (Figure S2C) partly blocked the ability of miR-124 to inhibit the growth of H1299 NSCLC cells (Figure 3A). In contrast, the inhibition of Akt by the Akt inhibitor MK2206 (Figure S2D) blunted the stimulation of H292 cell growth by the miR-124 inhibitor in Figure 3B. Consistent with the results obtained from in vitro experiments, the overexpression of constitutively activated Akt or the inhibition of Akt by Akt inhibitor MK2206 inhibited miR-124 overexpression-induced tumor growth inhibition (H1299 xenograft model) or miR-124 inhibition-induced tumor growth stimulation (H292 xenograft model) (Figures 3C and 3D). Taken together, these data suggest that the Akt pathway is a major player in the anticancer effects of miR-124 in NSCLC.
Figure 3.
miR-124 Inhibits NSCLC Growth through the Suppression of the Akt Pathway
(A) The overexpression of miR-124 inhibited colony formation, whereas the constitutive activation of Akt blocked miR-124-induced inhibition of colony formation in H1299 cells. H1299 cells were transfected with indicated plasmid for 24 hr and then subjected to colony-formation assay. (B) The inhibition of miR-124 stimulated colony formation, whereas the inactivation of Akt by MK2206 blocked miR-124 inhibition-induced colony formation in H292 cells. H292 cells were transfected with indicated plasmid for 24 hr then subjected to colony-formation assay. Cells were treated with or without MK2206 every 3 days. (C) Constitutive activation of Akt attenuated miR-124-induced tumor growth inhibition effect in H1299 xenograft models. Stably expressing miR-124 H1299 cells were transfected with plasmid that expression of constitutively active Akt then injected subcutaneously into nude mice (2 × 106 cells per mouse). Tumor volume was measured every week, and after 1 month of cell injection, mice were sacrificed and the tumors were weighed. (D) Inactivation of Akt by MK2206 suppressed miR-124 inhibition-stimulated tumor growth in H292 xenograft models. Control or stably expressing miR-124 antisense H292 cells were injected subcutaneously into nude mice (2 × 106 cells per mouse). When the tumor size was approximately 50 mm3, the mice were administered 6 mg/kg MK2206 or vehicle buffer twice daily by oral gavage. Tumor volume was measured every week, and after 2 weeks of drug treatment, the mice were sacrificed, and the tumor weights were measured. Ctrl, vector control; miR-124 in, miR-124 inhibitor; caAKT, constitutive activation of Akt; *p < 0.05; **p < 0.01; ***p < 0.001.
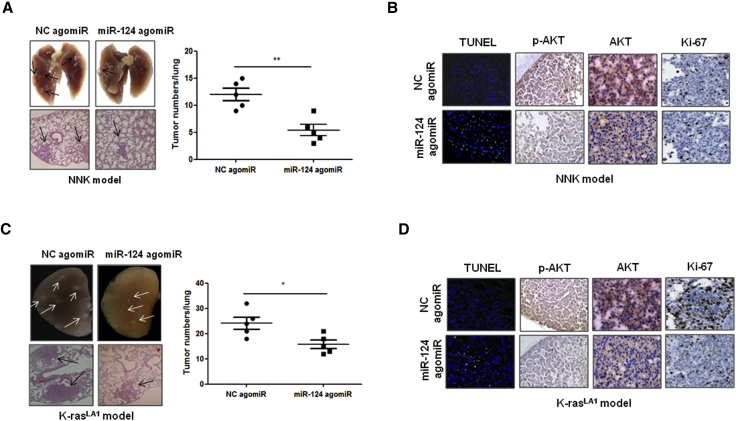
miR-124 agomiR Suppresses K-ras-Driven or NNK-Induced Lung Tumorigenesis in Animal Models
In K-ras mutation-driven22 or NNK-induced12 lung cancer, Akt signaling is an important therapeutic target. Thus, we examined the therapeutic potential of miR-124 agomiR in K-rasLA1 transgenic mice and NNK-induced lung cancer animal models. The results showed that the intravenous injection of agomiR-124 significantly inhibited lung tumorigenesis in both K-rasLA1 transgenic mice and NNK-induced lung cancer animal models (Figures 4A and 4C). Furthermore, an immunohistochemistry (IHC) analysis clearly showed that the administration of miR-124 agomiR significantly suppresses cell proliferation and the expression level of Akt and Akt phosphorylation and promotes apoptosis in both an NNK-induced lung tumor model and K-rasLA1 transgenic mice compared with the respective control groups (Figures 4B and 4D). These findings strongly suggest that the restoration of miR-124 is a useful treatment strategy for K-ras mutation-driven or NNK-induced lung tumors.
Figure 4.
Systemic Delivery of miR-124 agomiR Inhibits Lung Tumorigenesis in NNK-Induced Lung Cancer Model and K-rasLA1 Transgenic Mice
(A) miR-124 agomiR inhibited NNK-induced lung tumorigenesis in mice. Representative gross lung (top) and H&E-stained lung sections (bottom) from NNK-induced lung tumor animal models. Negative control (NC) agomiR or miR-124 agomiR were intravenously injected into NNK-induced lung cancer model mice once a week at a concentration of 10 μM in 100 μL of PBS. After 13 weeks of treatment, mice were sacrificed, and the lungs were collected (n = 5 per group). (B) miR-124 agomiR inhibited Akt, p-Akt, and cell proliferation and induced apoptosis in NNK-induced lung tumor. The expression of Akt, p-Akt, and Ki-67 was detected using immunohistochemistry; apoptotic cells were detected using TUNEL assay. (C) miR-124 agomiR inhibited lung tumorigenesis in K-rasLA1 transgenic mice. Representative gross lung (top) and H&E-stained lung sections (bottom) from K-rasLA1 transgenic mice. Negative control (NC) agomiR or miR-124 agomiR were intravenously injected into 6-week-old female K-rasLA1 transgenic mice once a week at a concentration of 10 μM in 100 μL of PBS. After 5 weeks of treatment, mice were sacrificed and the lungs were collected (n = 5 per group). (D) miR-124 agomiR inhibited Akt, p-Akt, and cell proliferation and induced apoptosis in the lung tumor of K-rasLA1 transgenic mice. The expression of Akt, p-Akt, and Ki-67 was detected using immunohistochemistry; apoptotic cells were detected using TUNEL assay. NC, negative control agomiR; *p < 0.05; **p < 0.01.
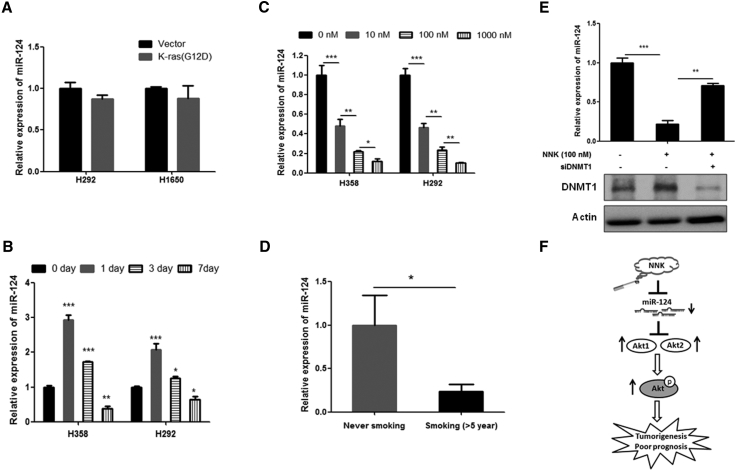
Long-Term Exposure to NNK Suppresses the Expression of miR-124 in NSCLC Cells
Previous studies show that the activation of K-ras23 or NNK treatment24 can alter the expression of some miRNAs. Thus, we investigated the effect of K-ras or NNK treatment on the expression of miR-124 in lung cancer cells. To this end, we overexpressed mutant K-ras (G12D) in the K-ras wild-type lung cancer cell lines H1650 and H292 and subsequently measured the expression of miR-124. The results showed that the overexpression of mutant K-ras does not affect miR-124 expression in NSCLC cells (Figure 5A). However, our data show that NNK treatment can alter miR-124 expression in NSCLC cells. Interestingly, short-term treatment with NNK induces miR-124 expression in NSCLC cells (24 hr), whereas long-term treatment (7 days) with NNK significantly suppressed the expression of miR-124 (Figure 5B). In addition, this suppression was dose dependent (Figure 5C). Consistent with these results, clinical data show that miR-124 expression was significantly decreased in specimens from patients with NSCLC who smoked more than 5 years compared to patients with NSCLC who had never smoked (Figure 5D). In addition, our data show that silencing of DNMT1 can block NNK treatment-induced suppression of miR-124 expression in NSCLC cells (Figure 5E). Overall, we found that decreased miR-124 expression was partially caused by smoking through DNMT1 and that decreased miR-124 contributes to disease progression and the activation of Akt in NSCLC; restoring miR-124 can dramatically inhibit NSCLC by targeting Akt1 and Akt2 (Figure 5F).
Figure 5.
NNK Treatment, but Not K-ras Mutation, Suppressed miR-124 Expression in NSCLC
(A) Overexpression of mutant K-ras does not affect the expression of miR-124 in H292 and H1650 cells. Seventy-two hours after of transfection, the indicated cells were subjected to qRT-PCR analysis. (B) Long time treatment of NNK significantly decreased miR-124 expression in NSCLC cells. The indicated cells were treated with or without 100 nM NNK for indicated times, and subsequently the cells were subjected to qRT-PCR analysis. (C) NNK treatment dose dependently inhibited miR-124 expression in NSCLC cells. Indicated cells were treated with indicated concentration of NNK for 7 days then subjected to qRT-PCR analysis. (D) miR-124 expression was measured by qRT-PCR in specimens of NSCLC patients with never smoking (n = 28) or smoking more than 5 years (n = 28). (E) Silencing of DNMT1 abolished NNK-induced inhibition of miR-124 expression in H358 cells. H358 cells were transfected with siRNA of DNMT1. After 12 hr of transfection, cells were treated with 100 nM NNK for 6 days. Then, cells were subjected to western blot and qRT-PCR analysis. (F) A schematic model of regulation of miR-124 expression and miR-124 effect on NSCLC progression. *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
miR-124 is a useful prognostic marker for patients with cancer, including patients with NSCLC. Moreover, downregulated miR-124 expression was previously identified in patients with breast cancer,25 clear-cell renal cell carcinoma,26 cervical cancer,27 and colorectal cancer,28 and these studies show that decreased miR-124 expression closely correlated with poor clinical outcomes. Consistent with these reports, our clinical data also show that the expression of miR-124 level negatively correlated with the overall survival of NSCLC patients, and our multivariate analysis indicates that miR-124 may be a useful independent biomarker for predicting NSCLC disease progression.
Here, we provide a new molecular mechanism that results in the hyperactivation of Akt in NSCLC. Specifically, the constitutive activation of Akt signaling is closely associated with tumor development and progression in various human cancers, including NSCLC. Thus, understanding the biological basis for the observed dysregulation of Akt hyperactivation is of great value for the future development of novel therapeutic strategies and anticancer agents. Here, our clinical data provide the first evidence to show that decreased miR-124 expression closely correlates with Akt activation in specimens of NSCLC. Furthermore, our in vitro data show that the inhibition of miR-124 can activate Akt signaling in NSCLC cells. In addition, miR-124 expression was previously shown to be significantly decreased in lung cancer.19, 21 Taken together, these and our findings suggest that miR-124 downregulation in NSCLC significantly contributes to activate and sustain Akt signaling.
Furthermore, we clarified the mechanism by which miR-124 regulates the Akt pathway in NSCLC cells. Akt has three isoforms (Atk1, Akt2, and Akt3), and all isoforms contribute to the activation of the Akt pathway. In this study, we for the first time identified Akt1 and Akt2 as miR-124 target genes in NSCLC cells. Our data show that the restoration of miR-124 expression in NSCLC cells suppresses the expression levels of Akt1 and Akt2 at both the mRNA and protein levels and inactivates the Akt pathway; conversely, the inhibition of miR-124 further upregulates Akt1 and Akt2 expression and activates the Akt pathway. In addition, luciferase reporter gene experiments showed that miR-124 directly targets the 3′ UTRs of Akt1 and Akt2, suggesting that miR-124 inhibits the expression of Akt1 and Akt2 by directly binding to their 3′ UTR regions. Furthermore, our data indicate that the constitutive activation of the Akt pathway blocked the miR-124 overexpression-induced inhibition of NSCLC growth both in vitro and in vivo; conversely, the inhibition of Akt activation partly inhibited the miR-124 inhibition-induced stimulation of tumor growth. Taken together, these data suggest that miR-124 inhibits NSCLC growth by inhibiting the Akt pathway via the direct targeting of Akt1 and Akt2. Furthermore, we investigated the correlation between PIK3CA and miR-124 expression in specimens from patients with NSCLC because PIK3CA is an important upstream protein of the Akt pathway, and Lang and Ling reported that PIK3CA is a target gene of miR-124 in hepatocellular carcinoma.14 However, we did not identify a correlation between PIK3CA and miR-124 expression in these specimens, suggesting PIK3CA is not a major target of miR-124 in NSCLC. This discrepancy is possible because one miRNA can target hundreds of genes and plays different roles by targeting different target genes in different cancer types.29 For example, ZEB1 and eIF4B are target genes of miR-216a in NSCLC, but they are not major targets of miR-216a in liver cancer.30
Because the Akt pathway is an important target for cancer therapy, we evaluated the therapeutic potential of miR-124 agomiR for lung cancer using an NNK-induced lung tumor model and K-rasLA1 transgenic mice. Most lung cancers are associated with smoking, and NNK is a potent tobacco-specific carcinogen.10 Studies have shown that smoking increases cancer risk by increasing the somatic mutation load,31 and the K-ras mutation is one of these mutations.32, 33 Specifically, K-ras mutational activation is a common oncogenic event in lung cancer34 found in 20%–30% of patients with NSCLC.35 Interestingly, Akt1 and Akt2 have been identified as key therapeutic targets in NNK-induced and K-ras mutation-driven lung cancer. For example, Hollander et al. reported that the deletion of Akt1 or Akt2 can decrease NNK-induced lung tumor formation by 90% in a mouse model.36 Moreover, Hollander et al.34 and our previous study11 showed that the deletion or suppression of Akt1 significantly inhibits lung tumorigenesis in K-ras transgenic mice. These reports suggest that the ectopic expression of miR-124 may be a useful strategy for lung cancer therapy because miR-124 inhibits Akt1 and Akt2. As expected, the present study showed that the systemic delivery of miR-124 agomiR significantly suppressed lung tumorigenesis in both NNK-induced and K-rasLA1 transgenic lung cancer model mouse by suppressing cancer cell proliferation and stimulating apoptosis via the inhibition of Akt1 and Akt2. In addition, other researchers have found that the restoration of miR-124 may also inhibit lung cancer metastasis,19 glioma angiogenesis, and chemoresistance,17 implying that miR-124 plays a central role in regulating several signaling pathways. This effect confirms its participation in multifarious processes during cancer development. Taken together, these findings suggest that the potential for the development of miR-124 restoration as a new strategy for lung cancer therapy.
Moreover, we provide a novel molecular mechanism that leads to decreased miR-124 expression in lung cancer cells. The data presented herein provide the first direct evidence to show that miR-124 expression was significantly reduced by long-term exposure to NNK in lung cancer cells. Previous studies have shown that miR-124 expression can be epigenetically regulated and thereby contribute to cancer progression.37, 38 Interestingly, a previous report showed that NNK downregulates the expression of tumor suppressor genes by stimulating the hypermethylation of their promoters via an increase in DNA methyltransferase 1 (DNMT1) accumulation in mice and patients with lung cancer.39 Moreover, DNMT1 was shown to negatively regulate miR-124 expression.40 Here, our data show that long-term treatment of NNK decreased miR-124 expression and increased DNMT1 expression in NSCLC cells. However, silencing of DNMT1 abolished NNK-treatment-induced inhibition of miR-124 expression in NSCLC cells. These findings strongly suggest that the long-term treatment of NNK-induced suppression of miR-124 expression may be through upregulation of DNMT1 in lung cancer. Interestingly, our data show that long-term treatment of NNK suppresses, however, short-term treatment of NNK increases the expression of miR-124 in NSCLC cells. Similar with our findings, Izzoti et al.41 also reported that certain miRNAs’ expressions were presented opposite expression pattern in different expose time point of cigarette smoke in mouse.4 However, the mechanism that miRNAs why differently regulated by different expose time of cigarette smoke is not clear and needs further study.
In summary, we demonstrated that the activation of Akt in lung cancer was associated with a decreased expression of miR-124, and NNK can suppress miR-124 expression in lung cancer. The restoration of miR-124 inactivates the Akt pathway by downregulating Akt1 and Akt2 in lung cancer, which inhibits NNK-induced or K-ras mutation-driven lung tumorigenesis. These findings further suggest that the restoration of miR-124 is a novel strategy for the treatment of lung cancer in which the Akt pathway is constitutively activated.
Materials and Methods
Reagent
Fetal bovine serum (FBS), puromycin, 4-(Methylnitrosoamino)-1-(3-pyridinyl)-1-butanone, and cell culture medium were purchased from Sigma (St. Louis, MO, USA). Antibodies against total Akt, Akt1, Akt2, DNMT1, phosphor-Akt (ser473), phosphor-mTOR (ser2448), phosphor-p70S6K (Thr 389), Ki-67, and actin were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Negative control agomiR and miR-124 agomiR were synthesized at Integrated DNA Technologies (Coralville, IA, USA). MK2206 was purchased from Selleckchem (Houston, TX, USA). Plasmid of constitutively active Akt and mutant K-ras (G12D) were kindly gifted from Dr. Cheng (Moffitt Cancer Center). The expression vector of miR-124 and miR-124-antisense were from GeneCopoeia (Rockville, MD, USA). Dual-Luciferase Assay Kit and TUNEL assay kit were purchased from Promega (Madison, WI, USA). SYBR Green PCR kit, Lipofectamine 2000, Taqman MicroRNA Assay kit, TRIzol, Opti MEM, High-Capacity cDNA Reverse Transcription kit, miRNA expression reporter vector, miR-124 mimics, miR-124 and RNU6 primer sets, and antisense of miR-124 were purchased from Life Technologies (Carlsbad, CA, USA). siRNA for target DNMT1 (#1, 5′-CGAGUCUGGUUUGAGAGUTT-3′; #2, 5′-GGAAUGGCAGAUGCCAACAGCTT-3′) was generated by RiboBio (Guangzhou, China).
Cell Culture and Human Specimens
All cell lines used this study were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in RPMI supplemented with 10% FBS.
Human samples were obtained from 195 patients who were diagnosed with NSCLC for the first time at the General Hospital of the People’s Liberation Army. The patients’ characteristics are summarized in Table 1. This research was approved by the Research Ethics Board of the General Hospital of the People’s Liberation Army.
Animal Experiments
For xenograft models, 2 × 106 indicated cells in serum-free medium were injected subcutaneously (s.c.) into 6-week-old female nude mice (n = 5/group). Tumor volume was measured every week. H1299 xenograft model mice were sacrificed at 1 month after cell injection, and the tumor weights were measured. For H292 xenograft model, when the tumor size was approximately 50 mm3, the mice were administered 6 mg/kg MK2206 or vehicle buffer twice daily by oral gavage. After 2 weeks of drug treatment, the mice were sacrificed, and the tumor weights were measured.
The experimental design for the K-rasLA1 transgenic mice and NNK-induced lung tumor model is summarized in Figure S1. Ten 6-week-old K-rasLA1 mice and NNK-induced lung cancer model mice were divided into two groups, respectively. Then, mice were intravenously injected with negative control agomiR or miR-124 agomiR once a week at a concentration of 10 μM in 100 μL of PBS until the end of experiment. For inducing lung tumorigenesis by NNK, 6-week-old A/J female mice were intraperitoneally (i.p.) injected with NNK (100 mg/kg body weight) once a week for 3 weeks. After 2 weeks of NNK injection, mice were started to receive agomiR treatment. At the end of the experiment, the mice were sacrificed and the lungs were collected. The number of tumors on the surface of the lungs was counted. Subsequently, the lung samples were fixed in freshly prepared 10% formaldehyde solution or stored at −80°C for subsequent analysis. All animal experiments were approved by the Animal Care Committee.
Western Blot, IHC, and TUNEL Assay
Western blotting and IHC assays were performed as previously described.11 The TUNEL assay was performed according to the manufacturer’s instructions.
qRT-PCR Analysis
Total RNA was isolated using TRIzol reagent according to the manufacturer’s protocol. Mature miR-124 and RNU6 endogenous control were analyzed using a TaqMan microRNA assay kit. The relative expression of miR-124 was normalized against RNU6 expression using the 2−ΔCt method, and the miR-124 expression fold-change in lung cancer samples matched to non-tumor control samples was evaluated using the 22−ΔΔCt method. Based on the mean fold-change of miR-124 expression, the patients were divided into high (fold-change > mean)- and low (fold-change < mean)-miR-124 expression groups.42
To analyze the expression of other genes, RT and PCR were performed using a High-Capacity cDNA Reverse Transcription kit and QuantiTect SYBR Green PCR kit, respectively. The following primer sequences were used: Akt1, 5′-CTGAGATTGTGTCAGCCCTGGA-3′, 5′-CACAGCCCGAAGTCTGTGATCTTA-3′; Akt2, 5′- GAGGTCATGGAGCACAGGTT-3′, 5′-CTGGTCCAGCTCCAGTA AGC-3′; and GAPDH, 5′-TCAACGACCACTTTGTCAAGCTCAGCT-3′, 5′-GGTGG TCC AGGGGTCTTACT-3′.
Luciferase Report Assay
3′ UTRs of Akt1 and Akt2, containing the predicted miR-124 target sequence, were amplified from human genomic DNA and cloned into the miRNA Expression Reporter Vector at MluI and HindIII sites. For the luciferase reporter experiments, the indicated cells were seeded onto 24-well cell culture plates and co-transfected with the Renilla luciferase plasmid, and indicated reporter plasmids containing firefly luciferase. After 48 hr of transfection, the luciferase activity was measured using the dual-luciferase assay system according to the manufacturer’s instructions. The luciferase activity was normalized to the activity of Renilla luciferase.
Clonogenic Assay and Stable Cell Line Selection
Twenty-four hours after transfection, the cells were seeded onto 24-well cell culture plates at a density of 200 cells/well. After 12 hr, the cells were subsequently incubated with or without 1 μM MK2206, and the medium was changed every 3 days. After 12 days, the cell colonies were fixed with cold methanol, stained with 0.1% crystal violet for 30 min, washed, air-dried, and photographed.
H1299 and H292 cells were transfected with miR-124 and miR-124-antisense expression vector, respectively. After 36 hr, the cells were incubated with 2 μg/mL of puromycin for 1 week. Then, the expression of miR-124 was measured, and the cells were subsequently frozen in aliquots until further use.
Statistical Analyses
All data are presented as the means ± SD. Differences between groups were determined using unpaired Student’s t test or one-way ANOVA using the SAS statistical software package version 6.12 (SAS Institute, Cary, NC). p values less than 0.05 were considered statistically significant.
Author Contributions
H.J. and C.-X.X. contributed to the conception and design of the study. C.-R.L., R.-T.W, and Y.W collected the human samples and performed IHC. H.J., S.-N.W., F.C., and Q.L. performed laboratory and animal experiments. H.J., Q.L., Q.-Y.T., H.Z., C.-X.X., and D.-W. analyzed the data. H.J. and C.-X.X wrote the manuscript.
Conflicts of Interest
The authors declare no competing financial interests.
Acknowledgments
This work was supported by the Startup Fund for Talented Scholars of Daping Hospital and the Research Institute of Surgery, the Third Military Medical University (to H.J. and C.-X.X.), the National Natural Science Foundation of China (81672283, to H.J.), the Natural Science Foundation of Hainan Province (20158329, to R.-T.W.), and the Natural Science Foundation of Chonqing Science and Technology Commission (cstc2313jcyjA10106, to S.-N.W.).
Footnotes
Supplemental Information includes three figures and can be found with this article online at https://doi.org/10.1016/j.omtn.2017.09.005.
Supplemental Information
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Yoshizawa A., Fukuoka J., Shimizu S., Shilo K., Franks T.J., Hewitt S.M., Fujii T., Cordon-Cardo C., Jen J., Travis W.D. Overexpression of phospho-eIF4E is associated with survival through AKT pathway in non-small cell lung cancer. Clin. Cancer Res. 2010;16:240–248. doi: 10.1158/1078-0432.CCR-09-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng W., Ye Z., Cui R., Perry J., Dedousi-Huebner V., Huebner A., Wang Y., Li B., Volinia S., Nakanishi H. MicroRNA-31 predicts the presence of lymph node metastases and survival in patients with lung adenocarcinoma. Clin. Cancer Res. 2013;19:5423–5433. doi: 10.1158/1078-0432.CCR-13-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah A., Swain W.A., Richardson D., Edwards J., Stewart D.J., Richardson C.M., Swinson D.E., Patel D., Jones J.L., O’Byrne K.J. Phospho-akt expression is associated with a favorable outcome in non-small cell lung cancer. Clin. Cancer Res. 2005;11:2930–2936. doi: 10.1158/1078-0432.CCR-04-1385. [DOI] [PubMed] [Google Scholar]
- 5.Tsurutani J., Fukuoka J., Tsurutani H., Shih J.H., Hewitt S.M., Travis W.D., Jen J., Dennis P.A. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J. Clin. Oncol. 2006;24:306–314. doi: 10.1200/JCO.2005.02.4133. [DOI] [PubMed] [Google Scholar]
- 6.Xu C.X., Jin H., Shin J.Y., Kim J.E., Cho M.H. Roles of protein kinase B/Akt in lung cancer. Front. Biosci. (Elite Ed.) 2010;2:1472–1484. doi: 10.2741/e206. [DOI] [PubMed] [Google Scholar]
- 7.Vivanco I., Sawyers C.L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 8.Mende I., Malstrom S., Tsichlis P.N., Vogt P.K., Aoki M. Oncogenic transformation induced by membrane-targeted Akt2 and Akt3. Oncogene. 2001;20:4419–4423. doi: 10.1038/sj.onc.1204486. [DOI] [PubMed] [Google Scholar]
- 9.Okudela K., Hayashi H., Ito T., Yazawa T., Suzuki T., Nakane Y., Sato H., Ishi H., KeQin X., Masuda A. K-ras gene mutation enhances motility of immortalized airway cells and lung adenocarcinoma cells via Akt activation: possible contribution to non-invasive expansion of lung adenocarcinoma. Am. J. Pathol. 2004;164:91–100. doi: 10.1016/S0002-9440(10)63100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West K.A., Brognard J., Clark A.S., Linnoila I.R., Yang X., Swain S.M., Harris C., Belinsky S., Dennis P.A. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J. Clin. Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu C.X., Jere D., Jin H., Chang S.H., Chung Y.S., Shin J.Y., Kim J.E., Park S.J., Lee Y.H., Chae C.H. Poly(ester amine)-mediated, aerosol-delivered Akt1 small interfering RNA suppresses lung tumorigenesis. Am. J. Respir. Crit. Care Med. 2008;178:60–73. doi: 10.1164/rccm.200707-1022OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H.Y., Oh S.H., Woo J.K., Kim W.Y., Van Pelt C.S., Price R.E., Cody D., Tran H., Pezzuto J.M., Moriarty R.M., Hong W.K. Chemopreventive effects of deguelin, a novel Akt inhibitor, on tobacco-induced lung tumorigenesis. J. Natl. Cancer Inst. 2005;97:1695–1699. doi: 10.1093/jnci/dji377. [DOI] [PubMed] [Google Scholar]
- 13.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Lang Q., Ling C. MiR-124 suppresses cell proliferation in hepatocellular carcinoma by targeting PIK3CA. Biochem. Biophys. Res. Commun. 2012;426:247–252. doi: 10.1016/j.bbrc.2012.08.075. [DOI] [PubMed] [Google Scholar]
- 15.Hatley M.E., Patrick D.M., Garcia M.R., Richardson J.A., Bassel-Duby R., van Rooij E., Olson E.N. Modulation of K-Ras-dependent lung tumorigenesis by MicroRNA-21. Cancer Cell. 2010;18:282–293. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W., Mao Y.Q., Wang H., Yin W.J., Zhu S.X., Wang W.C. MiR-124 suppresses cell motility and adhesion by targeting talin 1 in prostate cancer cells. Cancer Cell Int. 2015;15:49. doi: 10.1186/s12935-015-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Z., Chen Q., Li C., Wang L., Qian X., Jiang C., Liu X., Wang X., Li H., Kang C. MiR-124 governs glioma growth and angiogenesis and enhances chemosensitivity by targeting R-Ras and N-Ras. Neuro-oncol. 2014;16:1341–1353. doi: 10.1093/neuonc/nou084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia J., Wu Z., Yu C., He W., Zheng H., He Y., Jian W., Chen L., Zhang L., Li W. miR-124 inhibits cell proliferation in gastric cancer through down-regulation of SPHK1. J. Pathol. 2012;227:470–480. doi: 10.1002/path.4030. [DOI] [PubMed] [Google Scholar]
- 19.Zu L., Xue Y., Wang J., Fu Y., Wang X., Xiao G., Hao M., Sun X., Wang Y., Fu G., Wang J. The feedback loop between miR-124 and TGF-β pathway plays a significant role in non-small cell lung cancer metastasis. Carcinogenesis. 2016;37:333–343. doi: 10.1093/carcin/bgw011. [DOI] [PubMed] [Google Scholar]
- 20.Zhao X., Lu C., Chu W., Zhang B., Zhen Q., Wang R., Zhang Y., Li Z., Lv B., Li H., Liu J. MicroRNA-124 suppresses proliferation and glycolysis in non-small cell lung cancer cells by targeting AKT-GLUT1/HKII. Tumour Biol. 2017;39 doi: 10.1177/1010428317706215. 1010428317706215. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Li H., Han J., Zhang Y. Down-regulation of microRNA-124 is correlated with tumor metastasis and poor prognosis in patients with lung cancer. Int. J. Clin. Exp. Pathol. 2015;8:1967–1972. [PMC free article] [PubMed] [Google Scholar]
- 22.Tolcher A.W., Khan K., Ong M., Banerji U., Papadimitrakopoulou V., Gandara D.R., Patnaik A., Baird R.D., Olmos D., Garrett C.R. Antitumor activity in RAS-driven tumors by blocking AKT and MEK. Clin. Cancer Res. 2015;21:739–748. doi: 10.1158/1078-0432.CCR-14-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang P., Zhu C.F., Ma M.Z., Chen G., Song M., Zeng Z.L., Lu W.H., Yang J., Wen S., Chiao P.J. Micro-RNA-155 is induced by K-Ras oncogenic signal and promotes ROS stress in pancreatic cancer. Oncotarget. 2015;6:21148–21158. doi: 10.18632/oncotarget.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalscheuer S., Zhang X., Zeng Y., Upadhyaya P. Differential expression of microRNAs in early-stage neoplastic transformation in the lungs of F344 rats chronically treated with the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis. 2008;29:2394–2399. doi: 10.1093/carcin/bgn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong L.L., Chen L.M., Wang W.M., Zhang L.M. Decreased expression of microRNA-124 is an independent unfavorable prognostic factor for patients with breast cancer. Diagn. Pathol. 2015;10:45. doi: 10.1186/s13000-015-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butz H., Szabó P.M., Khella H.W.Z., Nofech-Mozes R., Patocs A., Yousef G.M. miRNA-target network reveals miR-124as a key miRNA contributing to clear cell renal cell carcinoma aggressive behaviour by targeting CAV1 and FLOT1. Oncotarget. 2015;6:12543–12557. doi: 10.18632/oncotarget.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong P., Xiong Y., Watari H., Hanley S.J.B., Konno Y., Ihira K., Suzuki F., Yamada T., Kudo M., Yue J., Sakuragi N. Suppression of iASPP-dependent aggressiveness in cervical cancer through reversal of methylation silencing of microRNA-124. Sci. Rep. 2016;6:35480. doi: 10.1038/srep35480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M.J., Li Y., Wang R., Wang C., Yu Y.Y., Yang L., Zhang Y., Zhou B., Zhou Z.G., Sun X.F. Downregulation of microRNA-124 is an independent prognostic factor in patients with colorectal cancer. Int. J. Colorectal Dis. 2013;28:183–189. doi: 10.1007/s00384-012-1550-3. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharya A., Ziebarth J.D., Cui Y. SomamiR: a database for somatic mutations impacting microRNA function in cancer. Nucleic Acids Res. 2013;41:D977–D982. doi: 10.1093/nar/gks1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R.T., Xu M., Xu C.X., Song Z.G., Jin H. Decreased expression of miR216a contributes to non-small-cell lung cancer progression. Clin. Cancer Res. 2014;20:4705–4716. doi: 10.1158/1078-0432.CCR-14-0517. [DOI] [PubMed] [Google Scholar]
- 31.Alexandrov L.B., Ju Y.S., Haase K., Van Loo P., Martincorena I., Nik-Zainal S., Totoki Y., Fujimoto A., Nakagawa H., Shibata T. Mutational signatures associated with tobacco smoking in human cancer. Science. 2016;354:618–622. doi: 10.1126/science.aag0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen B., Liu L., Castonguay A., Maronpot R.R., Anderson M.W., You M. Dose-dependent ras mutation spectra in N-nitrosodiethylamine induced mouse liver tumors and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induced mouse lung tumors. Carcinogenesis. 1993;14:1603–1608. doi: 10.1093/carcin/14.8.1603. [DOI] [PubMed] [Google Scholar]
- 33.Keohavong P., Kahkonen B., Kinchington E., Yin J., Jin J., Liu X., Siegfried J.M., Di Y.P. K-ras mutations in lung tumors from NNK-treated mice with lipopolysaccharide-elicited lung inflammation. Anticancer Res. 2011;31:2877–2882. [PubMed] [Google Scholar]
- 34.Hollander M.C., Maier C.R., Hobbs E.A., Ashmore A.R., Linnoila R.I., Dennis P.A. Akt1 deletion prevents lung tumorigenesis by mutant K-ras. Oncogene. 2011;30:1812–1821. doi: 10.1038/onc.2010.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng D., Yuan M., Li X., Chen L., Yang J., Zhao X., Ma W., Xin J. Prognostic value of K-RAS mutations in patients with non-small cell lung cancer: a systematic review with meta-analysis. Lung Cancer. 2013;81:1–10. doi: 10.1016/j.lungcan.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Hollander M.C., Zhou X., Maier C.R., Patterson A.D., Ding X., Dennis P.A. A Cyp2a polymorphism predicts susceptibility to NNK-induced lung tumorigenesis in mice. Carcinogenesis. 2011;32:1279–1284. doi: 10.1093/carcin/bgr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P., Chen L., Zhang J., Chen H., Fan J., Wang K., Luo J., Chen Z., Meng Z., Liu L. Methylation-mediated silencing of the miR-124 genes facilitates pancreatic cancer progression and metastasis by targeting Rac1. Oncogene. 2014;33:514–524. doi: 10.1038/onc.2012.598. [DOI] [PubMed] [Google Scholar]
- 39.Lin R.K., Hsieh Y.S., Lin P., Hsu H.S., Chen C.Y., Tang Y.A., Lee C.F., Wang Y.C. The tobacco-specific carcinogen NNK induces DNA methyltransferase 1 accumulation and tumor suppressor gene hypermethylation in mice and lung cancer patients. J. Clin. Invest. 2010;120:521–532. doi: 10.1172/JCI40706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng B., Li Z., Chen R., Guo N., Zhou J., Zhou Q., Lin Q., Cheng D., Liao Q., Zheng L., Gong Y. Epigenetic regulation of miR-124 by hepatitis C virus core protein promotes migration and invasion of intrahepatic cholangiocarcinoma cells by targeting SMYD3. FEBS Lett. 2012;586:3271–3278. doi: 10.1016/j.febslet.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 41.Izzotti A., Larghero P., Longobardi M., Cartiglia C., Camoirano A., Steele V.E., De Flora S. Dose-responsiveness and persistence of microRNA expression alterations induced by cigarette smoke in mouse lung. Mutat Res. 2011;717:9–16. doi: 10.1016/j.mrfmmm.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 42.Li J., Huang H., Sun L., Yang M., Pan C., Chen W., Wu D., Lin Z., Zeng C., Yao Y. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin. Cancer Res. 2009;15:3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.