Abstract
This study examined the impact of eating during simulated night shift on performance and subjective complaints. Subjects were randomized to eating at night (n=5; 23.2 ± 5.5 y) or not eating at night (n=5; 26.2 ± 6.4 y). All participants were given one sleep opportunity of 8 h (22:00 h-06:00 h) before transitioning to the night shift protocol. During the four days of simulated night shift participants were awake from 16:00 h-10:00 h with a daytime sleep of 6 h (10:00 h-16:00 h). In the simulated night shift protocol, meals were provided at ≈0700 h, 1900 h and 0130 h (eating at night); or ≈0700 h, 0930 h, 1410 h and 1900 h (not eating at night). Subjects completed sleepiness, hunger and gastric complaint scales, a Digit Symbol Substitution Task and a 10-min Psychomotor Vigilance Task. Increased sleepiness and performance impairment was evident in both conditions at 0400 h (p<0.05). Performance impairment at 0400 h was exacerbated when eating at night. Not eating at night was associated with elevated hunger and a small but significant elevation in stomach upset across the night (p<0.026). Eating at night was associated with elevated bloating on night one, which decreased across the protocol. Restricting food intake may limit performance impairments at night. Dietary recommendations to improve night-shift performance must also consider worker comfort.
Keywords: Shift-work, Performance, Circadian misalignment, Sleep loss, Psychomotor vigilance, Sleepiness, Timed eating, Hunger
Introduction
There are currently more than 1.5 million shift workers in Australia1) and 29% of workers in the United States, do not work regular daytime shifts, with numbers likely to rise with increasing demand by companies to extend working hours2). Shift workers are often required to work throughout the night, for consecutive nights, when circadian processes typically promote sleep3). Furthermore, night workers are required to sleep during the day, when sleep quality is reduced and sleep length can be between two and four hours shorter, resulting in sleep loss4). The association between circadian misalignment, shortened sleep and impaired glucose has been well established5, 6). This impairment over a long period of time, is associated with the development of a number of health problems including obesity7, 8), type 2 diabetes9) and metabolic syndrome10). However shift work does not only have an impact on health but also performance output.
It has been well established that performance varies across the day, with declines in functioning post habitual bedtime11). This decline worsens over the course of the night with poorest performance in the early morning hours (approximately 0500 h) during the circadian nadir, reflected by minimum body temperature12, 13, 14). Furthermore, restricted sleep or sleep loss, as seen in shift work4), affects many aspects of cognitive performance, particularly alertness15). The psychomotor vigilance task (PVT), a tool sensitive to sleep loss16, 17), is used to assess vigilant attention/alertness. Many sleep restriction studies have shown that sleep loss increases the number of PVT lapses and errors18, 19, 20, 21). In addition, sleep restriction also leads to increased subjective sleepiness21) and impairments in cognitive processing22). Impairments in cognitive processing due to sleep restriction have also been reported using the digit symbol substitution task (DSST)23).
In addition to the effects of sleep loss, a number of studies have shown that performance may be impaired following food intake24, 25, 26). For example, Smith and Miles27), found that the ability to maintain attention and to react quickly to a visual stimulus was impaired post-lunch compared to when a meal was not provided. Furthermore, Smith, et al.28) reported that food intake, regardless of composition, influenced mood, with subjects reporting increased lethargy, clumsiness, dreaminess and decreased mental sharpness post-lunch. Studies examining the effect of food intake on performance during the night are very limited, however another study by Smith and Miles29), looked at the impact of food intake during the day (1230 h-1330 h) and during the night (0130 h-0230 h) on cognitive vigilance and found impairments in the number of correct responses at both time points.
Night shift workers often redistribute food intake from the daytime to the night hours30, 31), which results in food consumption during a time when the body’s normal biological processes are primed for sleep14, 32, 33). Indeed, nutrient absorption, metabolism34, 35), enzyme activity and gastrointestinal motility36, 37) are lower at night. It is perhaps not surprising then, that night shift work is associated with increased gastrointestinal complaints38, 39), including abdominal pain, diarrhoea, constipation, heartburn, and indigestion40).
Previous studies reporting the effects of sleep loss on performance have not reported the timing of food intake and its potential impact on performance or gastric upset. Given performance impairments are greatest at night4) and the previously mentioned effects of food intake, reducing food intake during this time may be a suitable countermeasure to reduce performance impairments and improve gut reaction reported during a night shift. Therefore the aim of this study was to investigate the impact of eating at night vs not eating at night on performance, sleepiness, hunger and gastric upset following four nights of circadian misalignment in healthy young men. We hypothesised that consuming a meal at night would lead to impaired performance and increased sleepiness compared to not eating at night. To test the hypothesis we assessed vigilant attention, cognitive processing, sleepiness, hunger and gastric upset under both conditions, during four consecutive simulated night shifts.
Subjects and Methods
This study was approved by the University of South Australia Human Research Ethics Committee (0000033621) and was conducted in accordance with the Recommendations from the Declaration of Helsinki. This study is registered with the Australian New Zealand Clinical Trials Registry (ACTRN12615001107516). Subjects provided written informed consent prior to entering the laboratory and were provided with an honorarium. The metabolic41) and further performance42) data has been published elsewhere.
Subjects
A total of 13 healthy males, 18–45 y (mean age 24.7 ± 5.6 y, BMI 22.7 ± 1.9 kg/m2), were recruited from the community via local noticeboards and social media. A detailed screening process was conducted to determine eligibility. A general health questionnaire, blood analysis, clinical history, and Beck Depression Inventory (score ≥14)43) were used to determine good physical and mental health. Additionally, subjects reported regular sleep patterns, with the absence of daytime napping and sleepiness or known sleep disorders, confirmed using the general health questionnaire, the Pittsburgh Sleep Quality Index (score >6)44), and the composite morningness- eveningness questionnaire (score of <31 or >69)45). Subjects were excluded if they reported specific food allergies or food habits, such as vegetarianism, BMI was >30 kg/m2 or currently used prescription or over-the-counter medications. Subjects were not smokers, and had not engaged in shift work or trans-meridian travel in the 2 months prior to the study. Participants were also asked to abstain from alcohol and caffeine for the week prior to the study. Females were not eligible for this study, the menstrual cycle and use of oral contraceptives has been shown to alter cognitive function during sleep loss46).
Study design
This study was conducted in the sleep laboratory at the Centre for Sleep Research at the University of South Australia, Adelaide, Australia. The parallel study design was conducted from January to July 2015. The laboratory is windowless and sound attenuated and was kept at an ambient temperature 23 ± 1°C, and light intensity maintained at <50 lux during wake periods and <0.03 lux (complete darkness) during sleep periods. Access to clock and social time cues (i.e., mobile phones, internet, and live television) was restricted.
Randomization was completed at the group level (2–4 participants were in the laboratory at one time, one condition at a time). Subjects were assigned to either the eating at night (control) (n=5; age 23.2 ± 5.5 y; BMI 22.2 ± 1.2 kg/m2) or the not eating at night condition (intervention) (n=5; age 26.2 ± 6.4 y; BMI 23.2 ± 1.3 kg/m2).
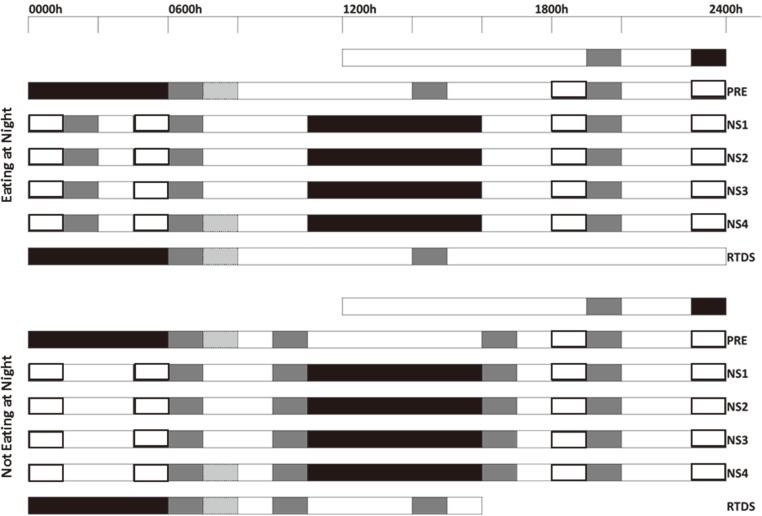
Subjects remained in the sleep laboratory for the duration of the six day protocol. Subjects entered the laboratory and were familiarised with the laboratory environment and cognitive tasks. The protocol (Fig. 1) included a night-time sleep 8 h time in bed (TIB) (2200 h-0600 h), pre simulated night shift (PRE). This was followed by four consecutive nights of simulated night shift with a daytime sleep of 6 h TIB (NS1-NS4; 1000 h-1600 h). During each night of simulated night shift subjects completed a neurobehavioral test battery (NTB) at 1830 h, 2130 h, 2400 h and 0400 h in their individual bedrooms. The NTB consisted of, in order of presentation, a Karolinksa Sleepiness Scale (KSS)47), mood scales, DSST48), and a 10 minute PVT49). Participants were given a 15 min shower opportunity between 16:00–16:30 h on NS1–2 and NS4. A shower was not possible on NS3 due to cannulation. Subjects were then given a nocturnal return to daytime schedule sleep (RTDS) of 8 h TIB (2200 h-0600 h).
Fig. 1.
Protocol schematic.
Black bars; scheduled sleep opportunities, white bars; periods of wake, patterned box; blood testing in response to breakfast meal, grey box; meal times, open black bar; neurobehavioral test battery, PRE; pre night shift, NS1-4; night shift work days, RTDS; return to day shift.
Compliance was monitored throughout the protocol by study personnel. When not completing scheduled tasks, subjects were free to read, watch DVDs, play board games, talk, or listen to music. Vigorous exercise was not permitted in the laboratory.
Food intake
Food timing and composition were strictly controlled throughout the study. Subjects in the eating at night condition consumed “breakfast” at ≈0700 h, “meal 4” at 1900 h and “meal 5” at 0130 h each day, and provided approximately 30%, 30% and 40% of the daily energy requirement, respectively. Subjects in the not eating at night condition consumed “breakfast” at ≈0700 h, “meal 2” at 0930 h, “meal 3” at 1410 h and “meal 4” at 1900 h, providing approximately 30%, 20%, 10%, 40% of the daily energy requirement, respectively. Individual daily energy requirements (kilojoules) were calculated using the Harris Benedict equation, a validated tool [8], with a light/sedentary activity level (laboratory condition). The individual energy requirement was further reduced by 15% to allow for extreme sedentary laboratory conditions. The energy content was increased by 30% on NS1 to allow for the increased time spent awake when transitioning to the night-shift. Macronutrient content of meals was based on the average Australian diet50). An example of the diet provided is shown in Table 1.
Table 1. Food intake.
| Meal 1 (≈07:00 h) ≈30% EER |
Meal 2 (09:30 h) 20% EER |
Meal 3 (14:10 h) 10% EER |
Meal 4 (19:00 h) 30% EER eating at night 40% EER not eating at night |
Meal 5 (01:30 h) 40% EER |
|
|---|---|---|---|---|---|
| Eating at Night Condition | White bread Margarine Strawberry jam Reduced fat milk Cornflakes (cereal) Orange juice |
No meal | No meal |
Roast beef Sandwich: Roast beef Wholemeal bread Lettuce Reduced fat cheese Tomato Apple Small packet of potato crisps Granola bar |
Chicken Salad: Chicken breast Tomato Lettuce Cucumber Parmesan cheese White bread roll Margarine Caesar salad dressing (reduced fat) Egg (hard boiled) Apple juice Small packet of potato crisps |
| Not Eating at Night Condition | White bread Margarine Strawberry jam Reduced fat milk Cornflakes (cereal) Orange juice |
Shortbread cookie Apple |
Apple juice Small bag of potato crisps |
Chicken Salad: Chicken breast Tomato Lettuce Cucumber Parmesan cheese White bread roll Margarine Caesar salad dressing (reduced fat) Egg (hard boiled) Apple juice Small packet of potato crisps Granola bar |
No meal |
*EER: estimated energy requirement
Subjects abstained from caffeine and alcohol intake while in the laboratory. Access to foods and beverages, other than water, was restricted outside of specified meal times.
Cognitive Performance
Psychomotor vigilance task
The PVT is a reaction time (RT) task which is considered a sensitive measure to assess the effects of sleep loss17). Subjects were instructed to respond to a visual stimulus (millisecond counter) displayed on the computer screen as quickly as possible by pressing a button with their dominant thumb. They were asked to respond to the stimulus as quickly as possible without anticipating when the stimulus may appear. The stimulus appeared at random intervals between 2–10 s (inter-stimulus interval). Outcome measures for the PVT include; number of PVT lapses (RT >500 ms), PVT errors (false starts; RT<150 ms or clicking the button when no stimulus) and PVT median response time.
Digit symbol substitution test
The DSST was a computerised version of the cognitive performance test in the Wechsler Adult Intelligence Scale48). Subjects were instructed to match numbers (1–9) with a series of randomly presented symbols based on a code displayed on the screen. Subjects had 3 min to respond as quickly and accurately as possible. The outcome measure was number of correct responses.
Sleepiness, Hunger and Gut Reaction
Karolinksa Sleepiness Scale, hunger and gut reaction scales
The nine point Likert-type scales were completed electronically, with subjects providing a whole number response rather than marking their response on a line. The KSS is a standard subjective measure of sleepiness47). Subjects were asked to rate their sleepiness, using the nine point Likert-type scale, from 1 (“extremely alert”) to 9 (“very sleep, great effort to stay awake”) at the beginning of each NTB. Following the KSS, subjects were asked to complete eight Likert-type mood scales. Subjects rated their hunger (hunger, thoughts of food, fullness and urge to eat) and gut reaction (gassy, bloated, upset stomach and dizziness) on the scale from 1 to 9.
Sleep EEG
Sleep was recorded on PRE: 22:00 h-06:00 h, NS2: 10:00 h-16:00 h and RTDS: 22:00 h-06:00 h, using Compumedics GRAEL recorders (Melbourne, Australia). Data were collected from the F3, F4, C3, C4, O1 and O2 sites with reference to a contralateral mastoid (M1, M2). An experienced sleep technician used an infrared camera to monitor subjects overnight. Sleep studies were scored according to Rechtschaffen and Kales sleep staging criteria51). The sleep variables analysed included total sleep time, wake after sleep onset (WASO), sleep efficiency (SE), sleep onset latency (SOL) and time in minutes of rapid eye movement (REM), stage 1, stage 2, stage 3 and stage 4.
Statistical analyses
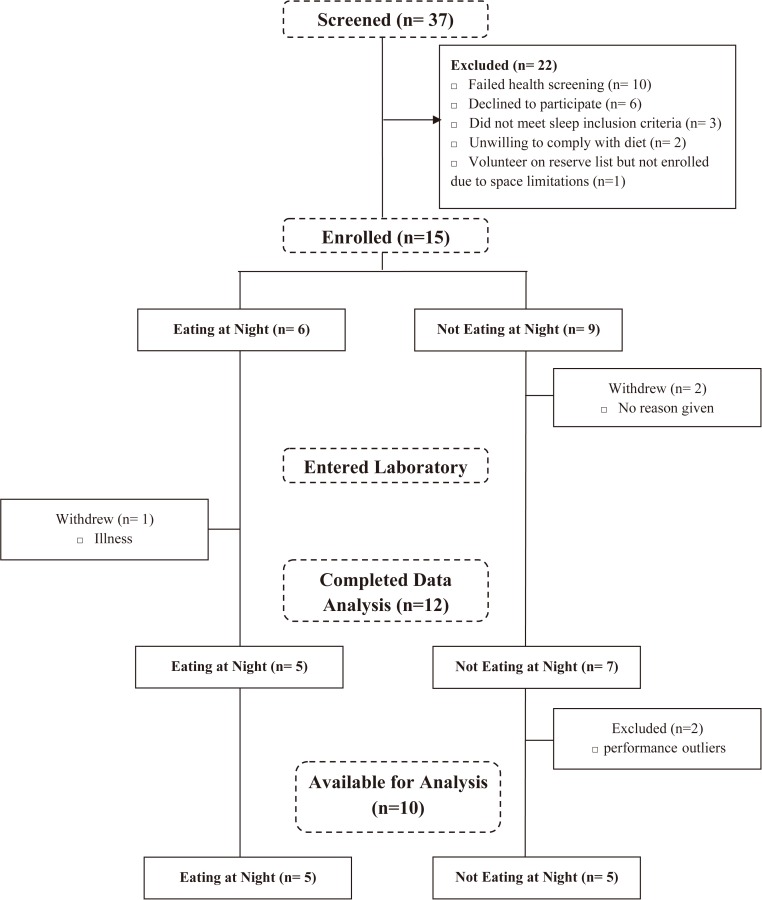
Data analyses were performed using SPSS Statistics Software Version 21.0 (IBM Corp, Armonk, NY, USA). Initially, fifteen subjects were enrolled in the study, two subjects then withdrew prior to entering the laboratory. After commencement of the study one subject withdrew due to illness. Data from two subjects were performance outliers and were withdrawn from analyses. In total data from 10 subjects was included in the final analyses (Fig. 2).
Fig. 2.
Consort diagram of participants who were screened, allocated a condition, completed the laboratory protocol and data analysed.
Mixed-effects ANOVAs were used to analyse the performance (PVT lapses, PVT errors, PVT median response time, DSST number correct responses), KSS, hunger (hunger, fullness, thoughts of food, urge to eat), and gut reaction (gassy, upset stomach, bloated, dizzy) variables with fixed effects of condition (eating at night, not eating at night), day (nightshift 1–4), and time (1830 h 2130 h, 2400 h, 0400 h) their interactions (condition*day, condition*time, condition*day*time), with a random effect of subject on the intercept. Significant interaction effects were explored using within-subjects planned comparisons, comparing all time points to 0400 h and all days to NS1. Basic repeated measures ANOVAs were run separately to estimate effect sizes, with small, medium and large effects determined with thresholds set at 0.01, 0.06, and 0.14, respectively52).
Mixed-effects ANOVA were conducted for the sleep data with fixed effects of condition (eating at night, not eating at night) and day (PRE, NS4, RTDS), and their interaction (condition*day), and a random effect of the subject on the intercept.
Denominator degrees of freedom were corrected with the Satterthwaite approximation and are reported to the nearest whole number. In all analyses, results were considered statistically significant if p<0.05.
Results
Cognitive performance
PVT lapses
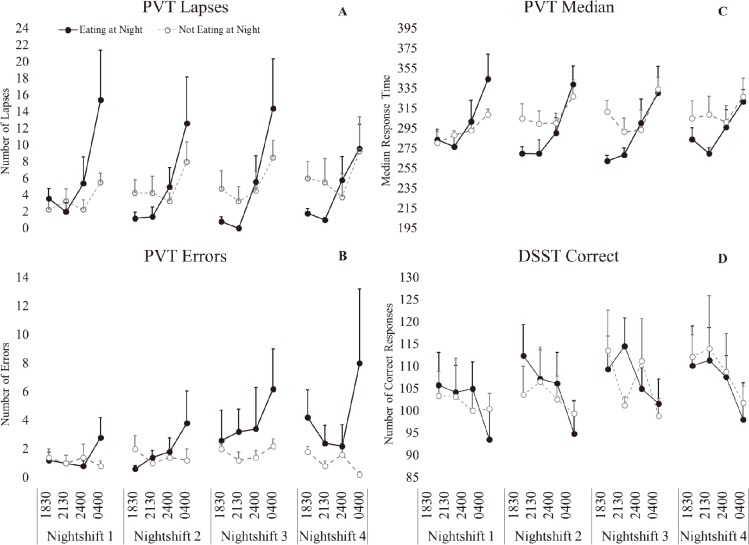
There was a significant main effect of time (p<0.001, ɳ2(partial)=0.561, large effect), such that performance was significantly worse at 0400 h (p<0.001) (Table 2). There was a significant interaction effect of condition*time (p<0.001, ɳ2(partial)=0.289, large effect), such that performance was significantly worse at 0400 h in both conditions, but the effect was stronger in the eating at night condition (Fig. 3).
Table 2. Linear mixed models: performance, hunger and gut reaction outcome measures.
| Outcome Measure | Condition | Day | Time | Condition*Day | Condition*Time | Condition*Day*Time | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fdf | ɳ2(partial) | Fdf | ɳ2(partial) | Fdf | ɳ2(partial) | Fdf | ɳ2(partial) | Fdf | ɳ2(partial) | Fdf | ɳ2(partial) | ||||||
| Performance | |||||||||||||||||
| PVT Lapses | 0.201,8 | 0.024 | 0.093,120 | 0.013 | 27.033,120*** | 0.561 | 1.763,120 | 0.196 | 8.603,120*** | 0.289 | 0.339,120 | 0.078 | |||||
| PVT Errors | 1.361,8 | 0.145 | 2.803,120* | 0.124 | 2.803,120* | 0.231 | 2.373,120 | 0.107 | 4.003,120** | 0.300 | 0.469,120 | 0.112 | |||||
| PVT Median | 0.471,8 | 0.056 | 0.243,120 | 0.028 | 27.543,120*** | 0.587 | 2.763,120* | 0.248 | 5.373,120** | 0.217 | 0.569,120 | 0.120 | |||||
| DSST correct | 0.001,8 | 0.000 | 4.793,120** | 0.328 | 13.683,120*** | 0.556 | 0.673,120 | 0.060 | 1.083,120 | 0.090 | 1.469,120 | 0.185 | |||||
| KSS | 1.191,8 | 0.170 | 8.613,120*** | 0.472 | 61.143,120*** | 0.864 | 1.263,120 | 0.144 | 1.203,120 | 0.189 | 1.239,120 | 0.208 | |||||
| Hunger scales | |||||||||||||||||
| Hunger | 17.191,8** | 0.682 | 2.563,120 | 0.168 | 9.913,120*** | 0.482 | 2.863,120* | 0.184 | 15.563,120*** | 0.594 | 0.549,120 | 0.088 | |||||
| Fullness | 4.391,8 | 0.052 | 1.423,120 | 0.086 | 10.183,120*** | 0.555 | 2.283,120 | 0.132 | 10.923,120*** | 0.572 | 0.179,120 | 0.030 | |||||
| Thoughts of food | 8.2321,8* | 0.507 | 4.853,120** | 0.295 | 14.243,120*** | 0.535 | 1.213,120 | 0.094 | 14.563,120*** | 0.540 | 0.319,120 | 0.055 | |||||
| Urge to Eat | 13.841,8** | 0.634 | 2.543,120 | 0.171 | 16.403,120*** | 0.609 | 0.263,120 | 0.020 | 15.833,120*** | 0.601 | 0.389,120 | 0.062 | |||||
| Gut Reaction | |||||||||||||||||
| Gassy | 1.751,7 | 0.200 | 0.063,105 | 0.007 | 1.033,105 | 0.081 | 1.813,105 | 0.172 | 1.543,105 | 0.117 | 0.439,105 | 0.081 | |||||
| Upset stomach | 0.411,8 | 0.049 | 0.363,120 | 0.056 | 7.153,120*** | 0.353 | 1.643,120 | 0.214 | 4.603,120** | 0.260 | 0.859,120 | 0.109 | |||||
| Bloated | 3.21 1,7 | 0.314 | 3.713,105* | 0.276 | 1.933,105 | 0.221 | 3.953,105* | 0.288 | 2.893,105* | 0.297 | 0.469,105 | 0.069 | |||||
| Dizzy | 0.0551,8 | 0.007 | 1.113,120 | 0.092 | 20.023,120*** | 0.608 | 0.833,120 | 0.071 | 1.113,120 | 0.079 | 0.369,120 | 0.062 | |||||
*p=0.05, **p=0.01, ***p<0.001. KSS: Karolinska Sleepiness Scale, ɳ2(partial): Partial eta2. Results shown are from linear mixed model analyses with main effects of condition: eating at night/not eating at night; time: 1830 h, 2130 h, 2400 h and 0400 h and day: Night shift 1–4 and their interactions (condition*day, condition*time and condition*day*time). Denominator df corrected with Satterthwaite approximation and reported to the nearest whole number (F value with degrees of freedom (df) displayed as subscript). Basic repeated measures ANOVAs were run separately to estimate effect size, with small, medium and large effects valued at 0.01, 0.06, and 0.14, respectively52).
Fig. 3.
Performance following simulated night-shift.
A-PVT Lapses, B- PVT Errors, C- PVT Median Response Time, D- Digit Symbol Substitution Task (DSST) number correct. Eating at night condition- solid black marker, not eating at night condition-open grey circle marker. Bars represent standard error.
PVT errors
There was a significant main effect of day (p=0.043, ɳ2(partial)=0.124, medium effect), such that performance significantly worsened on NS3 (p=0.018) and NS4 (p=0.031) compared to NS1 (Table 2). There was a significant main effect of time (p=0.043, ɳ2(partial)=0.231, large effect), such that performance was significantly worse at 0400 h. There was a significant interaction effect of condition*time (p=0.009, ɳ2(partial)=0.300, large effect), such that performance was significantly worse at 0400 h in the eating at night condition (p<0.001) but not in the not eating at night condition (Fig. 3).
PVT median response
There was a significant main effect of time (p<0.001, ɳ2(partial)=0.587, large effect), such that response time was significantly worse at 0400 h (p<0.001) (Table 2). There was a significant interaction effect of condition*time (p=0.002, ɳ2(partial)=0.217, large effect), such that performance was significantly worse at 0400 h in both conditions, but the effect was stronger in the eating at night condition (Fig. 3).
DSST number correct
There was a significant effect of day (p=0.003, ɳ2(partial)=0.328, large effect), such that performance significantly improved on NS3 (p=0.006) and NS4 (p<0.001) compared to NS1 (Fig. 3). There was a significant main effect of time (p<0.001, ɳ2(partial)=0.556, large effect), such that performance was significantly worse at 0400 h (p<0.001) (Table 2).
Hunger and gut reaction
Hunger scales
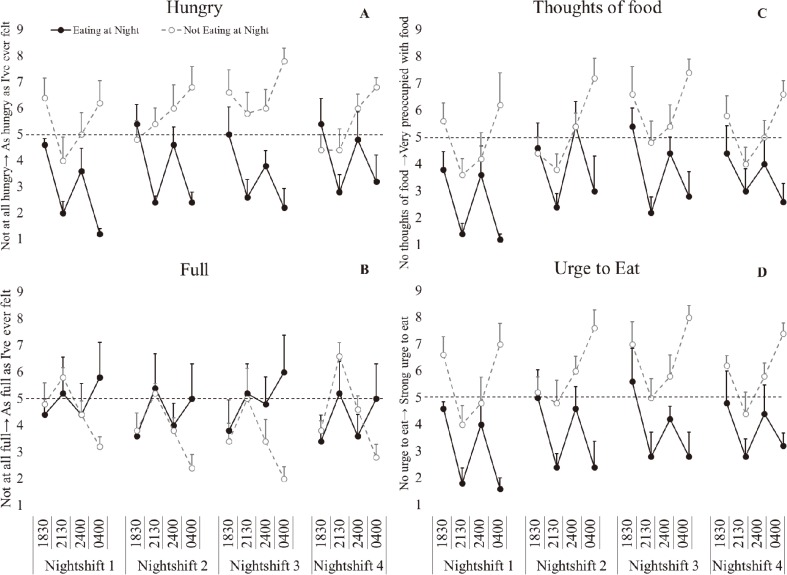
There was a significant main effect of condition with hunger, thoughts of food and urge to eat significantly increased (p<0.021, ɳ2(partial)=0.507–0.982, large effect), but no significant difference in fullness (p=0.526, ɳ2(partial)=0.052, small effect) in the not eating at night condition. There was a significant main effect of day (p<0.05, ɳ2(partial)=0.295, large effect) with thoughts of food significantly increased on NS2 (p=0.010), NS3 (p<0.001) and NS4 (p=0.024) compared to NS1. There was a significant effect of time (p<0.001, ɳ2(partial)=0.482–0.609, large effect) with decreased fullness, and increased hunger, urge to eat and thoughts of food at 0400 h compared to 2130 h (p<0.006) and decreased hunger and urge to eat compared to 1830 h (p<0.049). There was a significant condition*day interaction for hunger (p=0.040, ɳ2(partial)=0.184, large effect), with significantly increased hunger ratings on NS3 compared to NS1 (p=0.012) in the eating at night condition. In the not eating at night condition there was significantly increased hunger ratings on NS4 compared to NS1 (p=0.009). There was a significant condition*time interaction (p<0.001, ɳ2(partial)=0.540–0.601, large effect), with significantly increased hunger ratings, thoughts of food, urge to eat and decreased fullness at 0400 h in the not eating at night condition compared to 1830 h (p<0.006), 2130 h (p<0.001) and 2400 h (p<0.012). In the eating at night condition there was significantly decreased hunger ratings, thoughts of food and urge to eat and increased fullness at 0400 h compared to 1830 h (p<0.001) and 2400 h (p<0.007) (Fig. 4).
Fig. 4.
Hunger ratings following simulated night-shift.
A-hungry, B- full, C- thoughts of food, D- urge to eat. Eating at night condition- solid black marker, not eating at night condition-open grey circle marker. Bars represent standard error. Dash line represents neutral on the rating scale.
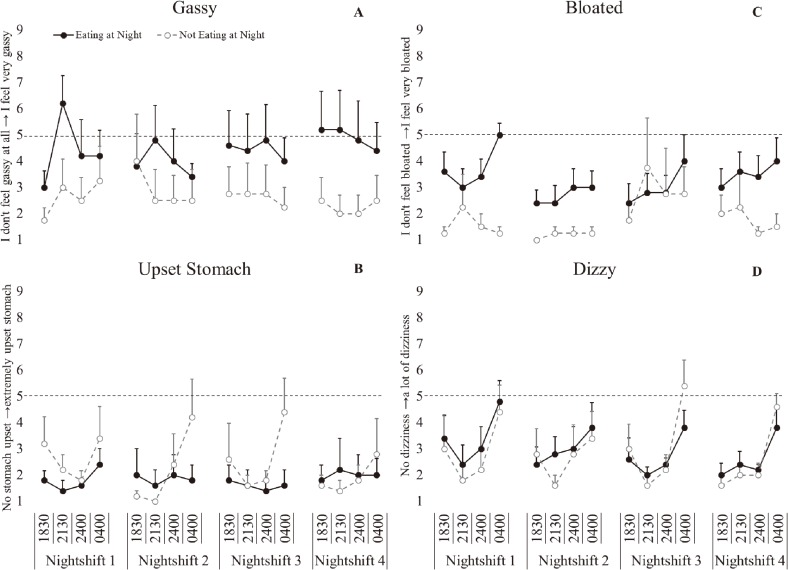
Gut reaction scales
There was a significant main effect of day for bloating (p=0.014, ɳ2(partial)=0.276, large effect), with significantly increased bloating on NS1 compared to NS2 (p=0.018). There was a significant main effect of time with significantly increased stomach upset (p<0.001, ɳ2(partial)=0.353, large effect) and dizziness (p<0.001, ɳ2(partial)=0.608, large effect) but not bloating (p=0.128, ɳ2(partial)=0.221, large effect) or gassiness (p=0.382, ɳ2(partial)=0.081, medium effect) at 0400 h (Fig. 5). There was a significant interaction effect of condition*time (p=0.004, ɳ2(partial)=0.260, large effect), with significantly increased stomach upset reported at 0400 h, compared to all other time points (p<0.001) in the not eating at night condition but not in the eating at night condition (Table 2). There was also a significant condition*day interaction for bloating (p=0.010, ɳ2(partial)=0.288, large effect), with significantly increased bloating on NS1 (p=0.009) compared to NS2 in the eating at night condition. In the not eating at night condition there was significantly decreased bloating on NS1 compared to NS3 (p=0.008).
Fig. 5.
Gut reaction following simulated night-shift.
A-Gassy, B- Upset stomach, C- Bloated, D- Dizzy. Eating at night condition- solid black marker, not eating at night condition- open grey circle marker. Bars represent standard error. Dash line- represents neutral on the rating scale.
Sleep data
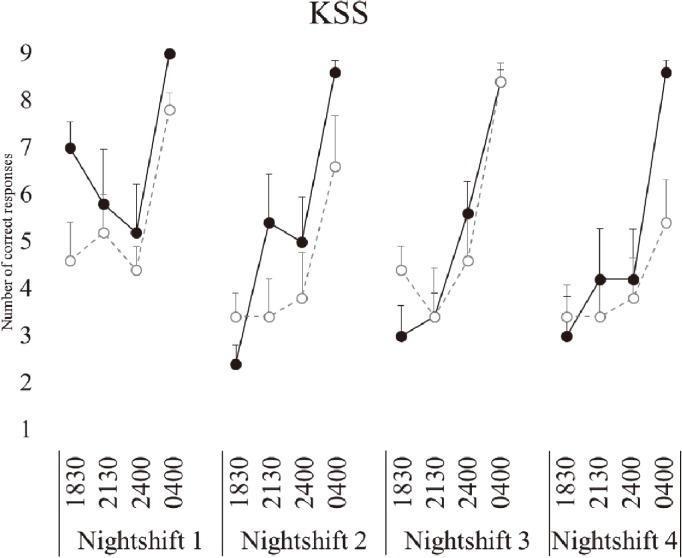
Subjective sleepiness (KSS)
There was a significant effect of day (p<0.001), such that sleepiness was significantly worse on NS1 (p<0.001) compared to NS2–4 (Fig. 6). There was a significant main effect of time (p<0.001), such that sleepiness was significantly worse at 0400 h (p<0.001) (Table 2).
Fig. 6.
Sleepiness following simulated night-shift.
KSS: Karolinska Sleepiness Scale. Eating at night condition- solid black marker, not eating at night condition- open grey circle marker. Bars represent standard error.
Sleep variables
There was no significant effect of condition for any of the sleep variables (Table 3). There was a main effect of day for TST, Stage 1, Stage 2, Stage 3 and Stage 4 (p<0.05), such that subjects had significantly less sleep during the day than at night. There was no significant condition*day interaction effect for any of the sleep variables.
Table 3. Sleep length and architecture.
| Variable | Eating at night (mean ± SE) |
Not Eating at Night (mean ± SE) |
Condition | Day | Condition*Day | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F(df) | pvalue | F(df) | pvalue | F(df) | pvalue | |||||
| TST (min) | 389.25 ± 7.25 | 387.72 ± 5.92 | 0.03(1,24) | 0.872 | 40.58(2,24) | <0.001 | 0.93(2,24) | 0.407 | ||
| WASO (min) | 35.38 ± 8.33 | 33.42 ± 6.80 | 0.03(1,8) | 0.860 | 1.60(2,16) | 0.233 | 0.22(2,16) | 0.806 | ||
| SE (%) | 89.13 ± 1.71 | 88.67 ± 1.40 | 0.04(1,24) | 0.840 | 3.26(2,24) | 0.056 | 0.716(2,24) | 0.499 | ||
| SOL (min) | 13.13 ± 5.79 | 18.17 ± 4.73 | 0.45(1,8) | 0.519 | 0.52(2,16) | 0.011 | 0.70(2,16) | 0.514 | ||
| REM (min) | 74.88 ± 5.51 | 83.28 ± 4.50 | 1.40(1,8) | 0.271 | 3.69(2,16) | 0.048 | 0.08(2,16) | 0.925 | ||
| Stage 1 (min) | 31.92 ± 4.65 | 35.19 ± 3.80 | 0.30(1,8) | 0.600 | 10.51(2,16) | 0.001 | 2.87(2,16) | 0.086 | ||
| Stage 2 (min) | 201.33 ± 7.60 | 185.11 ± 6.20 | 2.74(1,8) | 0.137 | 63.80(2,16) | <0.001 | 0.33(2,16) | 0.724 | ||
| Stage 3 (min) | 23.04 ± 2.65 | 19.69 ± 2.16 | 0.96(1,8) | 0.356 | 5.40(2,16) | 0.016 | 0.72(2,16) | 0.502 | ||
| Stage 4 (min) | 58.08 ± 7.13 | 64.44 ± 5.82 | 0.48(1,8) | 0.509 | 19.87(2,16) | <0.001 | 0.49(2,16) | 0.625 | ||
Data presented (n=10). TST: total sleep time; min: minute; WASO: wake after sleep onset; SE: sleep efficiency; SOL: sleep onset latency; REM: rapid eye movement. Results shown are from linear mixed model analyses with main effects of meal timing; eating at night/not eating at night and day; pre night shift (PRE), the second consecutive night shift (NS2), and return to day shift (RTDS) and their interactions (condition*day). Denominator df corrected with Satterthwaite approximation and reported to the nearest whole number (F value with degrees of freedom (df) displayed as subscript), and significance (pvalue) values were presented.
Discussion
This study examined the effect of eating vs not eating during the nightshift on subjective sleepiness, vigilant attention, processing speed, hunger and gut reaction in a small, all male sample. Eating a meal at 0130 h resulted in poorer vigilant attention at 0400 h compared to not eating a meal at night. Subjective sleepiness was significantly greater at 0400 h compared to other time points, independent of meal condition. The number of DSST correct responses increased across night shifts which was consistent with anticipated learning effects seen in previous studies53). While restricting food intake limited performance impairments at night, subjects that did not eat at 0130 h reported increased hunger and stomach upset, although stomach upset did not rise above neutral on the Likert-type scale. The eating at night group reported elevated bloating and gassiness on the first night-shift, becoming less apparent across days. Even though both groups had increased energy content on NS1 when transitioning to the night-shift and extended wake time on NS1, this may have caused exacerbated gastric complaints when eating at night39).
Regardless of meal timing, the largest impairments in subjective sleepiness, vigilant attention, and information processing were seen at 0400 h. This is consistent with a large number of previous studies showing decreased PVT performance at night, via increased errors of omission (lapses: when the subject fails to respond) and errors of commission (errors: when the subject responds prior to a stimulus appearing)54, 55, 56, 57). The impaired performance at night has largely been reported as a reflection of the circadian low between 0200 h and 0600 h, when the body is primed for sleep14). However, when considering these findings it is important to note that, the previous studies assessing performance provided meals, whether ad libitum or structured, over the night time period and did not address the possible impact of food intake.
This study was the first study to address the impact of food intake on vigilant attention and information processing during the night. In this study we found that restricting food intake to only the daytime hours reduced the number of errors and lapses seen on the PVT. This is in agreement with previously reported findings from this study, in which driving performance (lane variability, time spent in the safe zone, and number of crashes) was significantly impaired by eating at night but not when eating was restricted to only the daytime hours42). Previous literature has shown that there is increased circulating cortisol, decreased core body temperature and reduced glucose tolerance at night13, 58, 59). Changes in circulating cortisol, core body temperature and glucose tolerance have also been associated with poor performance outcomes. In addition, a number of key digestive functions, such as the rate of gastric emptying, colonic motility, intestinal absorption rates and enzymatic activities60) are disturbed at night, although the effect of these changes on cognitive performance have not been tested. Further research is needed to determine the mechanisms involved in decreased postprandial performance at night.
Subjects reported increased sleepiness during the first nightshift compared to the following night shifts. This may be a reflection of the extended wake when transitioning from dayshift to nightshift. In this study we found subjective sleepiness was not affected by eating at night above the effects of remaining awake at night, with subjects in both conditions reporting increased sleepiness at 0400 h. This is in contrast to previous studies which have commonly reported enhanced subjective sleepiness following consumption of meals high in carbohydrate or fats61, 62, 63). However, each of these studies were conducted during the day, which may suggest the time of day (0400 h) effect on sleepiness may overwhelm any additional effect of food intake.
Subjects that did not eat at night reported feeling increased hunger and stomach upset during the night, although the mean rating for stomach upset did not rise above neutral on the Likert-type scale. Previous studies report mixed results regarding appetite during the night shift. Lowden, et al.64) suggests hunger decreases at night during 24 h of constant conditions. Interestingly this study also found time of eating was more important than macronutrient composition (fat/carbohydrates) in determining hunger, although macronutrient content was not addressed in the current study. Decreased hunger at night was supported by a further study which suggested individuals lose their desire to eat when gut function is at its lowest (during the night)65). However, shortened sleep has been associated with elevated appetite and hunger due to significant reductions in the release of leptin and increases in ghrelin66). These hormones were not assessed in the current study, future studies should explore how these hormones change when timing of food intake is altered. It may also be that other factors such as habit and time pressure may play a part in food consumption during the night shift rather than hunger alone67, 68). While this study shows that performance may be better, they may report increased hunger, and stomach upset and bloating (although they did not rise above neutral) when food intake is restricted at night. It is important that future suggestions need to be balanced in the context of subjective symptoms and therefore future work needs to be done to determine whether different variations of food intake i.e. smaller snacks or differences in composition can be used as an intermediate, to reduce hunger, during the night shift.
This study has shown, in a controlled laboratory environment with healthy participants, that limiting food intake to the daytime hours may be an effective strategy to improve some aspects of performance. It is important to note this study only included healthy young men, females and older or shift working populations may be impacted differently. Sample sizes similar to those in this study are common in intensive sleep study protocols of this nature. Effect sizes have been added to assist in the interpretation of the data given the sample size, and are shown in Table 2. Previous findings relating to this study found that restricting food intake at night limited impaired glucose metabolism associated with night shift41). Together, these findings suggest the importance of food timing when discussing the impact of shift work on health and performance. Future studies are needed to address the most suitable balance between food intake, performance, mood, and health. These studies will need to address the varying effects of different meal times, meal content and meal size. Furthermore, although the subjects in this study were sleep restricted (6 h TIB), to simulate sleep restriction observed during shift work, they slept well during the day due to the ideal sleeping environment in the laboratory. Studies have shown that daytime sleep quality and quantity could be reduced due to social and environmental factors including family commitments, light, noise, caffeine and alcohol consumption in the home environment69, 70).
The results of this study may be most generalisable in occupations such as air traffic control, train and truck driving where the majority of the workload is cognitive. Future studies should look at the relationship between food consumption at night and performance when there is an increase in physical demand. Additionally, future research is needed to assess the impact of macronutrient contribution and portion size on performance at night. Previous studies examining the effect of food consumption on performance during the day have shown adverse performance outcomes are more likely following foods with a high fat/carbohydrate content71, 72, 73). Furthermore, a study by Lloyd, Green and Rogers72), suggested that both low fat and high fat meals consumed during the day led to a slower reaction time, compared to a medium fat meal. However, the medium fat meal was closest in size and macronutrient content to the subject’s habitual diet and therefore the change in response time may be a reflection of the disruption in normal intake rather than due to macronutrient content74). This suggests that macronutrient contribution and habitual food intake should also be considered in future studies used to determine dietary recommendations for shift workers.
Conclusions
The findings from this paper suggest eating at night leads to increased errors and delayed response time, leading to an increase in number of lapses. While redistributing food intake to the daytime hours showed some improvements in performance, subjects reported increased hunger and changes in gastric complaints. Therefore, to optimize performance during the night when performance is known to dip, night shift workers could use altered meal timing as an effective countermeasure to improve performance, however further research is needed, particularly into nightshift snacking options, in order to provide recommendations that are for the performance, safety and subjective wellbeing of night shift workers.
Acknowledgments
The authors would like to thank the research staff and students for their contributions. In particular, research assistants; Stephanie Centofanti, Emily Watson, Cassie Hilditch, Alex Agostini, Alex Chatburn, nursing staff; Katja Morsky and student; Kenji Sison. Thank you also to all the participants.
References
- 1.Australian Bureau of Statistics (2010) Working Time Arrangements, Australia 2009: Summary of results. ABS cat.no. 6342.0 Canberra: ABS. [Google Scholar]
- 2.Liira J, Verbeek J, Ruotsalainen J (2015) Pharmacological interventions for sleepiness and sleep disturbances caused by shift work. JAMA 313, 961–2. [DOI] [PubMed] [Google Scholar]
- 3.Borbély AA. (1982) A two process model of sleep regulation. Hum Neurobiol 1, 195–204. [PubMed] [Google Scholar]
- 4.Akerstedt T. (2003) Shift work and disturbed sleep/ wakefulness. Occup Med (Lond) 53, 89–94. [DOI] [PubMed] [Google Scholar]
- 5.Kalsbeek A, la Fleur S, Fliers E (2014) Circadian control of glucose metabolism. Mol Metab 3, 372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences. [DOI] [PMC free article] [PubMed]
- 7.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA (2008) Meta-analysis of short sleep duration and obesity in children and adults. Sleep 31, 619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lorenzo L, De Pergola G, Zocchetti C, L’Abbate N, Basso A, Pannacciulli N, Cignarelli M, Giorgino R, Soleo L (2003) Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord 27, 1353–8. [DOI] [PubMed] [Google Scholar]
- 9.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA (2010) Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 33, 414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsson B, Knutsson A, Lindahl B (2001) Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 58, 747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright KP Jr, Hull JT, Czeisler CA (2002) Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol 283, R1370–7. [DOI] [PubMed] [Google Scholar]
- 12.Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS (1980) Human sleep: its duration and organization depend on its circadian phase. Science 210, 1264–7. [DOI] [PubMed] [Google Scholar]
- 13.Dijk DJ, Duffy JF, Czeisler CA (1992) Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res 1, 112–7. [DOI] [PubMed] [Google Scholar]
- 14.Monk TH, Buysse DJ, Reynolds CF 3rd, Berga SL, Jarrett DB, Begley AE, Kupfer DJ (1997) Circadian rhythms in human performance and mood under constant conditions. J Sleep Res 6, 9–18. [DOI] [PubMed] [Google Scholar]
- 15.Banks S, Dinges DF (2007) Behavioral and Physiological Consequences of Sleep Restriction. J Clin Sleep Med 3: 519–28. [PMC free article] [PubMed] [Google Scholar]
- 16.Balkin TJ, Bliese PD, Belenky G, Sing H, Thorne DR, Thomas M, Redmond DP, Russo M, Wesensten NJ (2004) Comparative utility of instruments for monitoring sleepiness-related performance decrements in the operational environment. J Sleep Res 13, 219–27. [DOI] [PubMed] [Google Scholar]
- 17.Dorrian J, Lamond N, Holmes AL, Burgess HJ, Roach GD, Fletcher A, Dawson D (2003) The ability to self-monitor performance during a week of simulated night shifts. Sleep 26, 871–7. [DOI] [PubMed] [Google Scholar]
- 18.Dinges DF, Kribbs NB (1991) Performing while sleepy: Effects of experimentally-induced sleepiness.
- 19.Kleitman N. (1963) Sleep and wakefulness. University of Chicago Press. [Google Scholar]
- 20.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ (2003) Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res 12, 1–12. [DOI] [PubMed] [Google Scholar]
- 21.Van Dongen HP, Maislin G, Mullington JM, Dinges DF (2003) The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26, 117–26. [DOI] [PubMed] [Google Scholar]
- 22.Herscovitch J, Stuss D, Broughton R (1980) Changes in cognitive processing following short-term cumulative partial sleep deprivation and recovery oversleeping. J Clin Exp Neuropsychol 2, 301–19. [Google Scholar]
- 23.Tietzel AJ, Lack LC (2001) The short-term benefits of brief and long naps following nocturnal sleep restriction. Sleep 24, 293–300. [DOI] [PubMed] [Google Scholar]
- 24.Wells AS, Read NW, Uvnas-Moberg K, Alster P (1997) Influences of fat and carbohydrate on postprandial sleepiness, mood, and hormones. Physiol Behav 61, 679–86. [DOI] [PubMed] [Google Scholar]
- 25.Reyner LA, Wells SJ, Mortlock V, Horne JA (2012) ‘Post-lunch’ sleepiness during prolonged, monotonous driving - effects of meal size. Physiol Behav 105, 1088–91. [DOI] [PubMed] [Google Scholar]
- 26.Orr WC, Shadid G, Harnish MJ, Elsenbruch S (1997) Meal composition and its effect on postprandial sleepiness. Physiol Behav 62, 709–12. [DOI] [PubMed] [Google Scholar]
- 27.Smith AP, Miles C (1986) Effects of lunch on selective and sustained attention. Neuropsychobiology 16, 117–20. [DOI] [PubMed] [Google Scholar]
- 28.Smith A, Leekam S, Ralph A, McNeill G (1988) The influence of meal composition on post-lunch changes in performance efficiency and mood. Appetite 10, 195–203. [DOI] [PubMed] [Google Scholar]
- 29.Smith A, Miles C (1986) Acute effects of meals, noise and nightwork. Br J Psychol 77, 377–87. [DOI] [PubMed] [Google Scholar]
- 30.Lennernäs M, Hambraeus L, Akerstedt T (1995) Shift related dietary intake in day and shift workers. Appetite 25, 253–65. [DOI] [PubMed] [Google Scholar]
- 31.de Assis MAA, Kupek E, Nahas MV, Bellisle F (2003) Food intake and circadian rhythms in shift workers with a high workload. Appetite 40, 175–83. [DOI] [PubMed] [Google Scholar]
- 32.Van Cauter E, Shapiro ET, Tillil H, Polonsky KS (1992) Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol 262, E467–75. [DOI] [PubMed] [Google Scholar]
- 33.Goo RH, Moore JG, Greenberg E, Alazraki NP (1987) Circadian variation in gastric emptying of meals in humans. Gastroenterology 93, 515–8. [DOI] [PubMed] [Google Scholar]
- 34.Zimmet PZ, Wall JR, Rome R, Stimmler L, Jarrett RJ (1974) Diurnal variation in glucose tolerance: associated changes in plasma insulin, growth hormone, and non-esterified fatty acids. Br Med J 1, 485–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carroll KF, Nestel PJ (1973) Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes 22, 333–48. [DOI] [PubMed] [Google Scholar]
- 36.Scheving LA, Russell WE (2007) It’s about time: clock genes unveiled in the gut. Gastroenterology 133, 1373–6. [DOI] [PubMed] [Google Scholar]
- 37.Hoogerwerf WA. (2006) Biologic clocks and the gut. Curr Gastroenterol Rep 8, 353–9. [DOI] [PubMed] [Google Scholar]
- 38.Haus E, Smolensky M (2006) Biological clocks and shift work: circadian dysregulation and potential long-term effects. Cancer Causes Control 17, 489–500. [DOI] [PubMed] [Google Scholar]
- 39.Konturek PC, Brzozowski T, Konturek SJ (2011) Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol 62, 139–50. [PubMed] [Google Scholar]
- 40.Caruso CC, Lusk SL, Gillespie BW (2004) Relationship of work schedules to gastrointestinal diagnoses, symptoms, and medication use in auto factory workers. Am J Ind Med 46, 586–98. [DOI] [PubMed] [Google Scholar]
- 41.Grant CL, Coates AM, Dorrian J, Kennaway DJ, Wittert GA, Heilbronn LK, Pajcin M, Della Vedova C, Gupta CC, Banks S (2017) Timing of food intake during simulated night shift impacts glucose metabolism: A controlled study. Chronobiol Int 2017, 1–11. [DOI] [PubMed] [Google Scholar]
- 42.Gupta CC, Dorrian J, Grant CL, Pajcin M, Coates AM, Kennaway DJ, Wittert GA, Heilbronn LK, Della Vedova CB, Banks S (2016) It’s not just what you eat but when: The impact of eating a meal during simulated shift work on driving performance. Chronobiol Int 34, 66–77. [DOI] [PubMed] [Google Scholar]
- 43.Beck AT, Steer RA, Brown GK (1996) Manual for the Beck Depression Inventory-II. In. Psychological Corporation, San Antonio, TX. [Google Scholar]
- 44.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213. [DOI] [PubMed] [Google Scholar]
- 45.Shahid A, Wilkinson K, Marcu S, Shapiro CM (2012) Composite Morningness Questionnaire. In: Shahid A, Wilkinson K, Marcu S, Shapiro MC (eds) STOP, THAT and One Hundred Other Sleep Scales. Springer New York, New York, NY, pp 137–40. [Google Scholar]
- 46.Wright KP Jr, Badia P (1999) Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res 103, 185–94. [DOI] [PubMed] [Google Scholar]
- 47.Akerstedt T, Gillberg M (1990) Subjective and objective sleepiness in the active individual. Int J Neurosci 52, 29–37. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler D. (1981) WAIS-R manual: Wechsler adult intelligence scale-revised. Psychological Corporation. [Google Scholar]
- 49.Dinges DF, Powell JW (1985) Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput 17, 652–5. [Google Scholar]
- 50.Australian Bureau of Statistics (1997) National Nutrition Survey Selected Highlights, Australia 1995: Summary of Results. ABS cat.no.4802.0, Canberra: ABS. [Google Scholar]
- 51.Rechtschaffen A, Kales A (1968) A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [DOI] [PubMed]
- 52.Richardson JT. (2011) Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev 6, 135–47. [Google Scholar]
- 53.Louca M, Short MA (2014) The effect of one night’s sleep deprivation on adolescent neurobehavioral performance. Sleep 37, 1799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narciso FV, Barela JA, Aguiar SA, Carvalho ANS, Tufik S, de Mello MT (2016) Effects of Shift Work on the Postural and Psychomotor Performance of Night Workers. PLoS One 11, e0151609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graw P, Kräuchi K, Knoblauch V, Wirz-Justice A, Cajochen C (2004) Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav 80, 695–701. [DOI] [PubMed] [Google Scholar]
- 56.Zhou X, Ferguson SA, Matthews RW, Sargent C, Darwent D, Kennaway DJ, Roach GD (2011) Sleep, wake and phase dependent changes in neurobehavioral function under forced desynchrony. Sleep 34, 931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dorrian J, Rogers NL, Dinges DF (2005) Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. In. Marcel Dekker New York.
- 58.Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS (2007) Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch Gen Psychiatry 64, 810–8. [DOI] [PubMed] [Google Scholar]
- 59.Lamport DJ, Lawton CL, Mansfield MW, Dye L (2009) Impairments in glucose tolerance can have a negative impact on cognitive function: a systematic research review. Neurosci Biobehav Rev 33, 394–413. [DOI] [PubMed] [Google Scholar]
- 60.Cagampang FR, Bruce KD (2012) The role of the circadian clock system in nutrition and metabolism. Br J Nutr 108, 381–92. [DOI] [PubMed] [Google Scholar]
- 61.Christie MJ, McBrearty EMT (1979) Psychophysiological investigations of post lunch state in male and female subjects. Ergonomics 22, 307–23. [DOI] [PubMed] [Google Scholar]
- 62.Spring B, Chiodo J, Bowen DJ (1987) Carbohydrates, tryptophan, and behavior: a methodological review. Psychol Bull 102, 234–56. [PubMed] [Google Scholar]
- 63.Spring B, Maller O, Wurtman J, Digman L, Cozolino L (1982–1983) Effects of protein and carbohydrate meals on mood and performance: interactions with sex and age. J Psychiatr Res 17, 155–67. [DOI] [PubMed] [Google Scholar]
- 64.Lowden A, Holmbäck U, Akerstedt T, Forslund A, Forslund J, Lennernäs M (2001) Time of day type of food--relation to mood and hunger during 24 hours of constant conditions. J Hum Ergol (Tokyo) 30, 381–6. [PubMed] [Google Scholar]
- 65.Waterhouse J, Minors D, Atkinson G, Benton D (1997) Chronobiology and meal times: internal and external factors. Br J Nutr 77 Suppl 1, S29–38. [DOI] [PubMed] [Google Scholar]
- 66.Spiegel K, Tasali E, Penev P, Van Cauter E (2004) Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 141, 846–50. [DOI] [PubMed] [Google Scholar]
- 67.Monk TH. (2000) What can the chronobiologist do to help the shift worker? J Biol Rhythms 15, 86–94. [DOI] [PubMed] [Google Scholar]
- 68.Costa G. (1996) The impact of shift and night work on health. Appl Ergon 27, 9–16. [DOI] [PubMed] [Google Scholar]
- 69.Monk TH. (2005) Shift work: basic principles. Principles and practice of sleep medicine 4: 673–9. [Google Scholar]
- 70.Monk TH, Wagner JA (1989) Social factors can outweigh biological ones in determining night shift safety. Hum Factors 31, 721–4. [Google Scholar]
- 71.Cunliffe A, Obeid OA, Powell-Tuck J (1997) Post-prandial changes in measures of fatigue: effect of a mixed or a pure carbohydrate or pure fat meal. Eur J Clin Nutr 51, 831–8. [DOI] [PubMed] [Google Scholar]
- 72.Lloyd HM, Green MW, Rogers PJ (1994) Mood and cognitive performance effects of isocaloric lunches differing in fat and carbohydrate content. Physiol Behav 56, 51–7. [DOI] [PubMed] [Google Scholar]
- 73.Smith AP, Miles C (1986) The effects of lunch on cognitive vigilance tasks. Ergonomics 29, 1251–61. [DOI] [PubMed] [Google Scholar]
- 74.Dye L, Blundell J (2002) Functional foods: psychological and behavioural functions. Br J Nutr 88 Suppl 2, S187–211. [DOI] [PubMed] [Google Scholar]