Abstract
High-frequency deep brain stimulation (HFS-DBS) of the subcallosal cingulate (SCC) region has been investigated as a treatment for refractory forms of depression with a ~50% remission rate in open label studies. However, the therapeutic mechanisms of DBS are still largely unknown. Using anaesthetized Sprague Dawley rats, we recorded neuronal spiking activity in 102 neurons of the dorsal raphe (DR) before, during and after the induction of a 5-min HFS train in the infralimbic region (IL) of the medial pre-frontal cortex (mPFC), the rodent homologue of the human SCC. The majority of DR cells (82%) significantly decreased firing rate during HFS (P < 0.01, 55.7 ± 4.5% of baseline, 35 rats). To assess whether mPFC-HFS mediates inhibition of DR cellular firing by stimulating local GABAergic interneurons, the GABAA antagonist bicuculline (Bic, 100 μM) was injected directly into the DR during HFS. Neurons inhibited by HFS recovered their firing rate during Bic+HFS (P < 0.01, n = 15, seven rats) to levels not different from baseline. Cells that were not affected by HFS did not change firing rate during Bic+HFS (P = 0.968, n = 7, three rats). These results indicate that blocking GABAA reverses HFS-mediated inhibition of DR neurons. As the cells that were not inhibited by HFS were also unaffected by HFS+Bic, they are probably not innervated by local GABA. Taken together, our results suggest that mPFC-HFS may exert a preferential effect on DR neurons with GABAA receptors.
Keywords: cellular firing, depression, dorsal raphe, high-frequency stimulation, medial prefrontal cortex, rat
Introduction
Deep brain stimulation (DBS) for treatment-resistant depression involves the delivery of a continuous high-frequency electrical stimulus (HFS) to the subcallosal cingulate (SCC) region through surgically implanted electrodes (Mayberg et al., 2005; Lozano et al., 2008; Kennedy et al., 2011). Open label studies have shown that over 50% of patients had a positive clinical response 1 year after surgery (i.e. were 50% better when assessed with validated scales) (Lozano et al., 2008; Holtzheimer et al., 2012). Subsequent follow-ups have shown that these results were stable after 2 and 3 years (Lozano et al., 2008; Kennedy et al., 2011). Our group has recently been able to shed some light on local HFS-mediated plasticity in the SCC of depression patients (Srejic et al., 2014), although the electrophysiological and neurochemical mechanisms underlying downstream effects of DBS are still not well understood. To better understand these mechanisms, research has focused on stimulating homologous regions in experimental animals (Hamani et al., 2010; Challis et al., 2014; Veerakumar et al., 2014). According to neuroanatomical projections and cytoarchitectural structures, the infralimbic (IL) region of the medial prefrontal cortex (mPFC) in rodents has been most commonly suggested as the anatomical correlate of the human SCC (Gabbott et al., 2003; Uylings et al., 2003; Hamani & Temel, 2012). Antidepressant effects of mPFC electrical stimulation in animals have been investigated in some detail (Hamani et al., 2012, 2014; Warden et al., 2012; Veerakumar et al., 2014). However, to our knowledge there have been no in vivo studies examining the real-time effects of high frequency prefrontal stimulation on cellular activity in descending mood pathways implicated in mood disorders.
The mPFC has been shown to exert descending control of the cortical projecting neurons in the dorsal raphe (DR), which suggests an important role for DR afferent systems in the modulation of emotional responses (Peyron et al., 1998; Lee et al., 2003; Vertes, 2004). In vivo studies conducted in anaesthetized rodents showed that brief (0.2 ms) electrical stimulation of the mPFC results in immediate inhibition of putative serotonin (5-HT) neurons in the DR (Celada et al., 2001; Varga et al., 2001). However, these studies did not examine longer train stimulation. To fully clarify DBS effects on the descending prefrontal projections, it is necessary to examine long stimulation trains at clinically relevant high frequencies (100 Hz). Electrophysiological and histological studies also showed that GABAergic interneurons in the DR are preferentially targeted by the glutamatergic projections from the mPFC and exert an inhibitory tone over DR neurons (Celada et al., 2001; Varga et al., 2001, 2003; Jankowski & Sesack, 2004). On the basis of these results, we hypothesize that HFS mediates an inhibitory effect on DR neurons by enhancing the local GABAergic tone. Using microelectrode recordings, we describe the cellular profile of neurons in the DR and demonstrate local inhibition of cellular firing during mPFC-HFS. Lastly, to examine the role of GABA in stimulation-mediated DR neuronal inhibition, we injected the GABAA receptor antagonist bicuculline (Bic) during HFS and show a recovery of cellular firing to baseline levels.
Materials and methods
Animal surgeries
Animal experiments were conducted using 35 adult male Sprague–Dawley rats (280–400 g) in compliance with the ethics protocol of the University Health Network Animal Care Committee (UHN ACC) and in accordance with EU Directive 2010/63/ EU on the protection of animals used for scientific purposes. Every effort was made to minimize the number of experimental animals used. Rats were housed in the animal care facility at the Toronto Western Hospital with food and water available ad libitum. Experiments were performed in the light phase of a 12/12-h light–dark cycle.
Anaesthesia was induced using isoflurane (Pharmaceutical Partners of Canada, Richmond Hill, ON, Canada) and maintained with an intraperitoneal injection of urethane (Sigma, Oakville, ON, Canada) at a dose of 1.2 g/kg. Urethane anaesthesia allows continuous monitoring of cortical electrophysiology over a prolonged period with minimal cardiovascular and respiratory complications (De Wildt et al., 1983). Furthermore, spontaneous potentials measured in rats anaesthetized with urethane have similar characteristics to those generated in the conscious animal (Ebenezer, 1986). Adequate levels of anaesthesia were confirmed every 15 min by absence of a withdrawal reflex to hindlimb pinch and maintained as required by supplemental doses (20% of initial dose) of urethane. Body temperature was maintained at 36–37 °C.
Extracellular recordings of DR neurons
Extracellular recordings were performed with three microelectrodes assembled from Parylene-C-insulated tungsten wires (Micro Probe, Gaithersburg, MD, USA), with a 20-μm tip length. To decrease the initial impedance of 1 MΩ for recording, the electrode tips were electroplated in 24-carat yellow gold electroplating solution (Krohn Technical Products, Carlstadt, NJ, USA) and followed by platinizing solution (VWR Scientific Products, Mississauga, ON, Canada) using a stimulus isolator (A360; World Precision Instruments, Sarasota, FL, USA) and 1 μA of cathodal direct current applied to the electrode for approximately 10 s, giving final impedances of 200–400 kΩ. Finally, the microelectrodes were insulated by a sleeve of polyimide Kapton (Micro ML Tubing, Midway, MA, USA).
To avoid the artery at the sagittal sinus, the microelectrodes were positioned on the midline, 12 mm posterior to bregma. To reach the DR, the trajectory was adjusted and microelectrode angled 32° in the anteroposterior plane, advanced 7–8 mm below dura. The recorded signals were digitized at 12.5 kHz with a CED 1401 data acquisition system (Cambridge Electronic Design, Cambridge, UK) and saved to a computer hard-drive running SPIKE2 software (Cambridge Electronic Design). The acquired neuronal signals were monitored continuously during acquisition by computer display.
Stimulation of the mPFC
Stimulations were performed using a blunted bipolar electrode placed into the infralimbic region of the ventral mPFC (ML +0.5 mm, AP +3.2 mm and DV +5.4 mm relative to bregma), which was modified from previous studies (Hajós et al., 1998; Hamani et al., 2010). Stimulation parameters consisted of clinically relevant high frequency trains (HFS, 100 Hz) of single, square-wave current pulses (60-μA amplitude, 0.2-ms pulse width, 5-min duration). The positions of the stimulating electrodes were verified histologically.
Drug administration
Drugs (dissolved in saline) were infused through a 10-μm stainless steel cannula (Small Parts Inc., Miami, FL, USA), and the microelectrodes were then positioned around the cannula, with the electrode tips extended 200–300 μm from the end of the cannula and were spaced approximately 600 mm from one another. Drugs were injected by using a 10-mL microsyringe (Hamilton Co Inc., Whittier, CA, USA) connected to the microinjection cannula by a ~30-cm length of 26-gauge Teflon tubing (Small Parts Inc.). The stylet of the microsyringe was removed, the assembly was forward-filled with deionized water and visually inspected for leaks, and the stylet was then replaced. An air bubble (~0.5 mL) was introduced into the cannula by drawing back the stylet of the microsyringe and then 3–10 mL of drug solution was back-loaded into the injection cannula. The location of the bubble in the Teflon tubing was marked and used to confirm movement of the solution during microinjection.
The GABAA receptor antagonist bicuculline methobromide (3.25 nM) (Sigma-Aldrich, St Louis, MO, USA) was used to see whether blockade of GABAA receptors in the DR blocks the inhibitory effects of mPFC-HFS on neuronal firing in DR. Bicuculline was prepared in 0.9% saline in a final dose of 75 ng/0.5 μL (100 μM) and infused into the DR 2 min after the onset of mPFC-HFS for a total of 1 min of perfusion time. The doses were chosen on the basis of previous publications as well as pilot studies, using the criteria of pharmacological and behavioural efficacy (Tao & Auerbach, 2003; Tao & Ma, 2012).
Histology
At the end of each experiment, the final position of the stimulating and recording electrode tips was marked with an electrolytic lesion (10 mA for 10 s) to verify location in the tissue. Animals underwent transcardiac perfusion with heparinized saline followed by 4% weight/volume paraformaldehyde in phosphate-buffered saline (PBS). Brains were extracted, post-fixed in 20% sucrose and 4% paraformaldehyde in PBS, processed for 40-μm-thick coronal sections using a cryostat (1720 digital; Leica Microsystems, Wetzlar, Germany) and stained with Cresyl Violet. Recording site locations were confirmed in the DR and stimulating sites in the infralimbic region of the mPFC. Placements of cannulae were verified by comparing the slides with a rat brain atlas (Paxinos & Watson, 2007).
Offline analysis of neuronal activity
The recorded neurons (spikes) were sorted offline by template-matching software (SPIKE2; Cambridge Electronic Design). Neurons were accepted for further analysis if the spikes were of a consistent, distinct shape that could be separated with a high degree of certainty from the spike waveforms of other neurons and background noise or cardiac pulsations. In this study, the artefacts produced by microstimulation were relatively short lasting (0.6 ms) and were removed from the signal, starting from the onset of the artefact to the end. This dead space was then replaced with an equivalent period of neural data immediately prior to the stimulus artefacts but in a reversed order. This had the advantage of producing a smooth transition in the signal at the site where the artefact was removed. The ‘cleaned’ neuronal recordings were then used for spike sorting with the template-matching algorithm, as spike band-pass filtering without removal of the artefact will increase the duration of the artefacts (Bar-Gad et al., 2004; Liu et al., 2012).
The w_hist script in SPIKE 2.0 was used to analyse frequency changes during HFS and upon pharmacological manipulation. Two-minute time windows of stable cellular spiking were taken in for each condition (baseline, HFS, HFS+Bic and post-HFS). Changes in firing rates were assessed on each isolated neuron using Student’s t-test in w_hist. A cell was deemed inhibited or facilitated when P values were greater than 0.05 (significant). Changes in firing rates between each condition across recording sites and animals were compared using one-way ANOVA, Student’s t-test or the Wilcoxon signed rank test.
Results
Cellular characteristics
Extracellular single-unit recordings were taken from a total of 102 well-isolated neurons in the DR of 35 urethane-anaesthetized rats (Fig. 1). This large group of neurons showed a considerable range in firing rate and spike shape. The firing frequency varied widely between 0.2 and 45 spikes per second (average = 11 ± 1 Hz) and spike duration between 0.5 and 1.9 ms (average = 1.08 ± 0.03 ms) (Fig. 2). The distribution of frequencies revealed a significant correlation between neuronal firing rate and spike width, where slower firing cells tended to have broader spikes, while fast spiking neurons were found to have shorter spike width (Fig. 2, R = 0.330, P < 0.001). Neurons firing > 5 Hz were termed ‘fast’ and < 5 Hz as ‘slow’, as previously described (Varga et al., 2003). Recently, multiple studies have shown that the classic electrophysiological features (Vandermaelen & Aghajanian, 1983) extensively used to identify ‘putative’ serotonergic neurons (slow, regular firing rates and wide spike waveforms) were highly variable among both 5-HT and non-5-HT cells (Allers & Sharp, 2003; Kirby et al., 2003; Kocsis et al., 2006; Calizo et al., 2011). Because electrophysiological and pharmacological tests have proven to be insufficient in discerning 5-HT from non-5-HT cells, we examined all DR cells and tested their responses to mPFC-HFS and Bic.
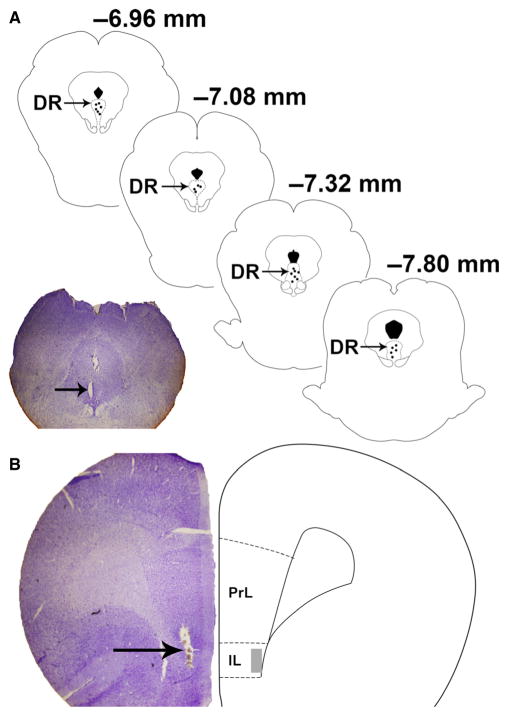
Fig. 1.
(A) Diagram illustrating the location of recording electrodes in the dorsal raphe (DR) (ML 0 mm, AP +12 mm, and DV +7–8 mm relative to bregma in the 32° plane). The section of the brain on the left (40 μm) has been stained with cresyl violet and illustrates the recording electrode lesion (arrow). (B) Illustration (right) of the stimulating electrode location in the infralimbic region (IL) of the medial prefrontal cortex (mPFC) along with cresyl violet histological section (left) of the same region with electrode lesion (arrow).
Fig. 2.
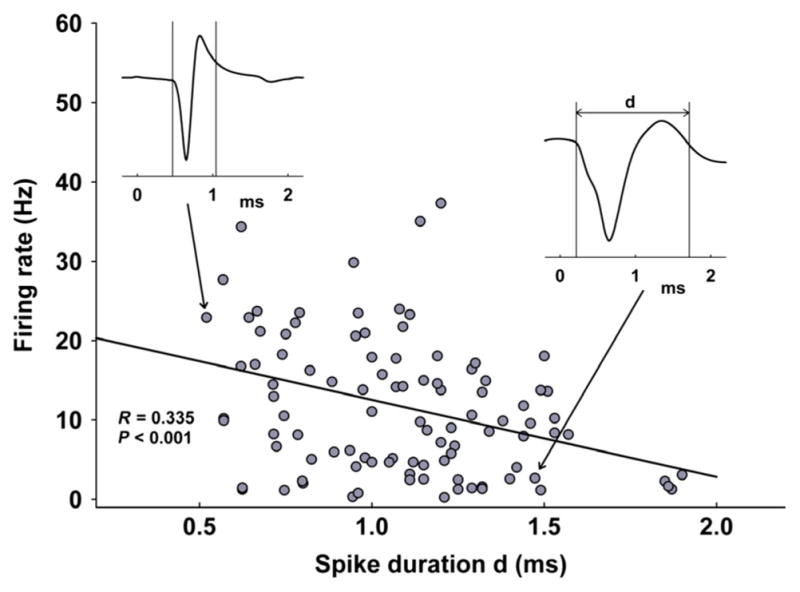
All recorded neurons in the dorsal raphe plotted based on spike duration (d in waveform inset, x-axis, in ms) and baseline firing rate (y-axis, Hz). The two insets are examples of a slow-firing (right) and fast-firing neuron (left). The distribution of frequencies revealed a significant correlation between cellular firing rate and spike width, where slower firing cells tended to have broader spikes, while fast spiking neurons were found to have shorter spike width (R = 0.330, P < 0.001, n = 102, 35 rats).
Effects of mPFC-HFS on neuronal firing in DR
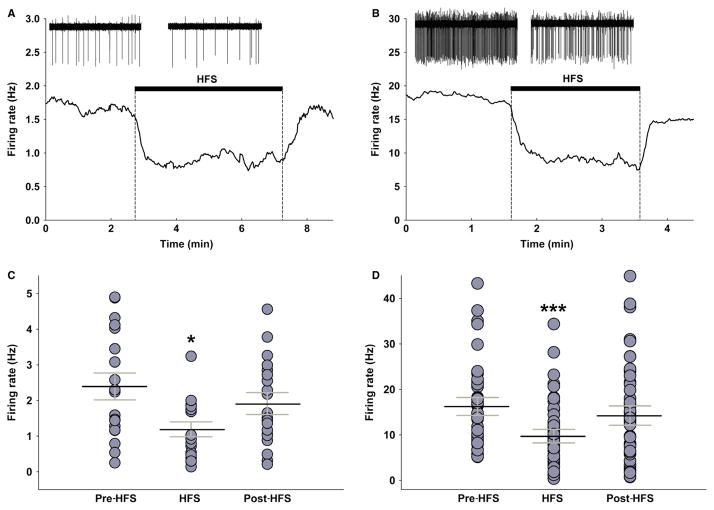
The effect of HFS of the mPFC was studied on all 102 stable firing DR neurons (Table 1). The vast majority of the recorded cells (84 of 102; 82%) responded to the 5-min mPFC-HFS train with a significant decrease in firing rate (P < 0.01, 55.7 ± 4.5% of baseline, 35 rats) (Fig. 3). Following HFS, the firing rate recovered to levels not significantly different from baseline (P = 0.389, 88.6 ± 7.1% of baseline), but significantly different from HFS (P < 0.05). Thirteen neurons (12%) did not show any significant response to HFS and only six neurons (6%) increased firing rate (Table 1). Facilitation of firing might be a non-specific effect of stimulus spread and a motor-like response that activated the cell due to arousal and subsequent movements of the animal. If the animals were administered an additional neuromuscular blockade or ventilated, the facilitation might not have occurred. HFS was not found to preferentially inhibit slow over fast neurons. In total, 24 out of 29 (83%) slow neurons decreased firing rate during HFS (P < 0.05, 53.4 ± 5.7% of baseline, 16 rats), which recovered to baseline after HFS (P = 0.263, 87.5 ± 9.8% of baseline) (Fig. 4A and C). Two slow neurons (7%) increased firing rate, and three (10%) displayed no change during HFS. Sixty out of 75 (80%) fast neurons were inhibited during HFS (P < 0.001, 56.7 ± 4.7% of baseline, 24 rats) and returned to baseline after HFS (P = 0.185, 84.1 ± 7.2% of baseline) (Fig. 4B and D). Ten fast neurons (13%) were facilitated and HFS had no effect on three (5%).
Table 1.
Recordings of all neurons were taken from dorsal raphe (DR) during high-frequency stimulation (HFS) of the medial prefrontal cortex (mPFC); the table lists the responses in firing rate during HFS of slow (< 5 Hz) and fast (> 5 Hz) cells
| Neurons
|
|||
|---|---|---|---|
| Response | All | Slow | Fast |
| Inhibition | 84 (82%) | 24 (83%) | 60 (80%) |
| Facilitation | 13 (12%) | 2 (7%) | 10 (13%) |
| No change | 6 (6%) | 3 (10%) | 3 (4%) |
Fig. 3.
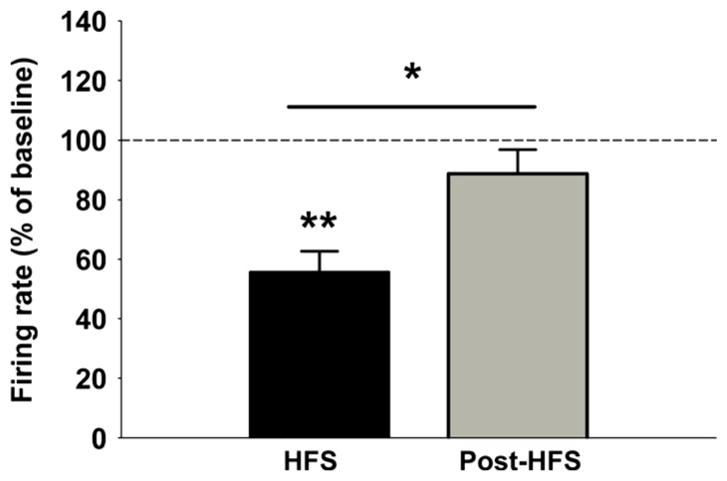
Histograms showing significant decrease in firing rate of cells in the DR during 5 min of mPFC-HFS (P < 0.01, n = 102, 35 rats). Following HFS, the firing rate recovered to levels not significantly different from baseline (P = 0.389), but significantly different from HFS (P < 0.05). *P < 0.05, ** P < 0.01.
Fig. 4.
Examples of slow-firing (A) and fast-firing (B) cells before, during and after HFS. Insets are 30-s segments of raw waveforms illustrating the change in spiking frequency. Note a significant decrease in firing rate during HFS and a recovery to baseline levels in both slow and fast neurons. Bottom graphs are vertical plots of all slow (C) and fast (D) neurons before, during and after HFS. Black lines in the middle of each group indicate mean firing rate, while grey lines are error bars (SEM). Note a significant decrease in firing rate during HFS in both slow (P < 0.05, n = 29) and fast neurons (P < 0.001, n = 73). *P < 0.05, ***P < 0.001.
mPFC-HFS mediates inhibition of DR neurons via local GABAA receptors
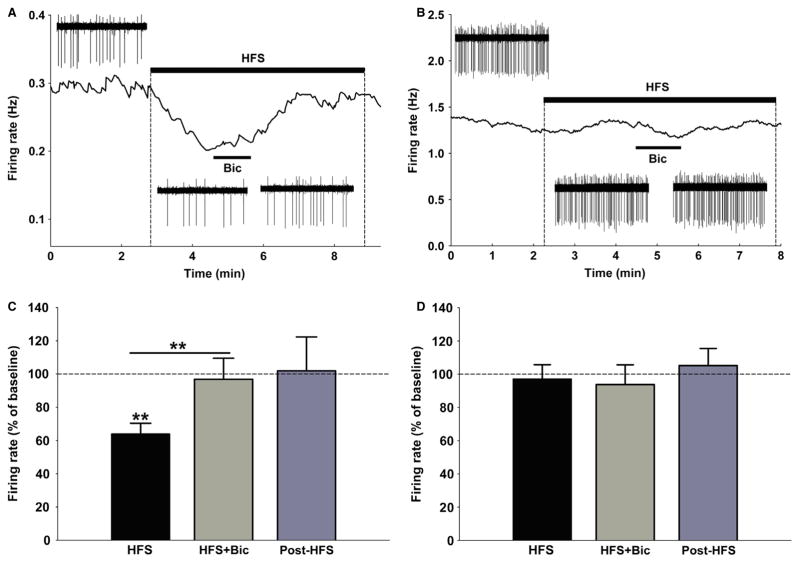
To examine whether mPFC-HFS-mediated inhibition of cellular firing in the DR occurred through the stimulation of local GABAergic interneurons, we tested the effect of focal injections of the GABAA antagonist Bic (100 μM, 75 ng/0.5 μL) during HFS-induced DR inhibition. After a stable baseline of neuronal firing was obtained, HFS was induced for 5 min. Bic was injected in the middle of the HFS train for a total of 1 min of diffusion (Fig. 5A and B). Out of a total of 22 neurons tested, 15 responded by inhibition during HFS (Fig. 5A), one was facilitated and six did not change firing rates (Fig. 5B). Thirteen out of the 15 neurons inhibited by HFS significantly increased their firing rate in response to Bic+HFS (P < 0.01, seven rats) to levels not different from baseline (P = 0.783, 96.8 ± 13% of baseline) (Fig. 5C). The remaining neurons that were not affected by HFS did not change firing rate in response to Bic+HFS (P = 0.968, 93.7 ± 12% of baseline, three rats) (Fig. 5D), indicating that blocking GABAA receptors worked only to cancel HFS-mediated inhibition of DR neurons.
Fig. 5.
(A) Illustration of the effect of bicuculline (Bic, 100 μM direct microinjection) on a DR neuron that was inhibited by mPFC-HFS. Insets are 30-s segments of raw waveforms illustrating the change in spiking frequency. Note the recovery of the firing rate to near-baseline levels upon Bic administration. (B) Illustration of the lack of any effect of Bic on a DR neuron that was not affected by mPFC-HFS. (C) Histogram showing that neurons inhibited by HFS significantly increased their firing rate in response to Bic+HFS (P < 0.01, n = 15, seven rats) to levels not different from baseline (P = 0.783). (D) The neurons that were not inhibited by HFS did not change firing rate in response to Bic+HFS (P = 0.968, n = 7, three rats). **P < 0.01.
Discussion
In the present study we used microelectrodes to record neurons in the region of the DR in anaesthetized rats and report a wide range of firing rates and spike widths. Classic electrophysiological criteria first used to identify putative 5-HT neurons according to their slow-firing, regular patterns and wide spikes (Vandermaelen & Aghajanian, 1983) have become insufficient for gross classification of raphe cells (Allers & Sharp, 2003; Kocsis et al., 2006; Calizo et al., 2011). Several intracellular (Li et al., 2001; Kirby et al., 2003) and juxtacellular labelling (Allers & Sharp, 2003) studies reported that neuronal identification based on electrophysiological criteria alone produced a significant number of false-negative and false-positive results. Allers & Sharp (2003) found that half of the slow-firing DR neurons were non-serotonergic and 20% of fast-spiking putative non-5-HT neurons were actually serotonergic (Allers & Sharp, 2003). Spike width has also been shown to be a poor method of identifying 5-HT neurons, where using morphological and neurochemical evidence found that 45% of non-5-HT neurons were misidentified as 5-HT and 35% of 5-HT neurons were misidentified as non-5-HT (Calizo et al., 2011). In the same study, the authors also looked at the topographical characteristics of raphe neurons and found that homogeneity between 5-HT and non-5-HT neurons also varied according to their location within the DR. Within the ventromedial DR and the lateral wing of the DR, non-5-HT cells had electrophysiological characteristics that were similar to 5-HT neurons. In contrast, the difference in cellular characteristics between 5-HT and non-5-HT cell populations was greatest and most consistent within dorsomedial DR (Calizo et al., 2011). The future use of optogenetic methods to unequivocally identify 5-HT neurons by their response to light stimulation would offer a clear advantage to classic electrophysiological techniques.
The prelimbic (PL) and infralimbic (IL) subdivisions of the mPFC are commonly thought of as the major prefrontal regions that send projections to the DR (Gonçalves et al., 2009). Some retrograde labelling studies show that the PL provides the greatest source of inputs to the DR (Vertes, 2004; Gabbott et al., 2005), whereas others show that a significant glutamatergic innervation to the DR originates from the IL (Hajós et al., 1998; Peyron et al., 1998; Groenewegen & Uylings, 2000; Gonçalves et al., 2009). As the IL has been identified as the closest physiological correlate of the human SCC (Uylings & van Eden, 1990), we chose this region as our stimulation target. We demonstrate that a significant majority of both slow- and fast-firing DR neurons were inhibited during mPFC-HFS. Previous studies used single pulse stimulation of the mPFC and measured post-stimulus latency and duration of inhibition of DR neurons (Hajós et al., 1998; Celada et al., 2001; Varga et al., 2001). Our study is the first to show persistent inhibition of DR firing rates with much longer trains at a clinically relevant stimulation frequency and current intensity (100 Hz, 60 μA). Stimulation of the mPFC has also been shown to elicit preferential inhibition of 5-HT neurons in the DR (Celada et al., 2001; Varga et al., 2001). As electrophysiological methods have proven insufficient to identify 5-HT neurons, we cannot with certainty say that the HFS-induced inhibition did not preferentially target 5-HT neurons in our study. However, because 82% of all cells were inhibited and 5-HT is thought to make up at most 50% of all DR neurons (Fu et al., 2010; Bang & Commons, 2012), it is very unlikely that mPFC-HFS inhibition in our study is 5-HT-specific. Initially, fast firing cells in the DR were thought to be GABAergic (Varga et al., 2003), but subsequent immunohistological studies showed that DR contains many excitatory glutamatergic neurons, which heavily regulate local 5-HT (Pan & Williams, 1989; Lee et al., 2003; Tao & Auerbach, 2003; Lemos et al., 2006; Calizo et al., 2011). Therefore, it is possible that the inhibitory effect of the mPFC-HFS affects a broad range of raphe neurons including glutamatergic neurons, which then in turn modulate 5-HT firing and transmission.
Neuroanatomical and pharmacological studies offer evidence that, together with 5-HT autoreceptors, GABA neurons are crucial mediators of inhibitory control over 5-HT systems (Liu et al., 2000; Varga et al., 2001). Using histological and ultrastructural methods it has been shown that the glutamatergic projections from the mPFC to the DR preferentially target GABA neurons (Jankowski & Sesack, 2004). This has recently been confirmed using whole-cell recording and cFos mapping after direct optogenetic photoactivation of glutamatergic mPFC terminals in the DR (Challis et al., 2014). Various studies have also shown that GABA neurons in the DR may serve as local inhibitory relays for major glutamatergic excitatory inputs originating from the mPFC (Celada et al., 2001; Varga et al., 2001, 2003). Most recently, using optogenetic Archaerhodopsin-mediated silencing, Challis et al. (2013) showed that DR GAD2+ GABA neurons synapse directly onto local 5-HT neurons, providing a source of postsynaptic inhibition. However, it should also be noted that a recent study showed evidence of direct glutamatergic inputs from the mPFC to 5-HT cells in the DR (Pollak Dorocic et al., 2014). In the present study we report that 6% of neurons increased firing rate during HFS, which may indicate that these cells were directly synapsed by the excitatory glutamatergic projections. Taken together, these results point to the heterogeneous nature of inputs from the mPFC to the DRN.
To determine the mechanism of mPFC-HFS-mediated inhibition of DR neurons, we administered the GABAA antagonist Bic during HFS. Bic recovered cellular firing of neurons that were originally inhibited by mPFC-HFS, but did not affect the firing rate of cells that were unchanged or facilitated during HFS. This indicates that HFS-induced inhibition in the DR is mediated by exciting local GABAergic connections (Fig. 6). The cells that were not inhibited by HFS were also unaffected by HFS+Bic, which suggests that these cells are neither synapsed by GABA nor directly influenced by the mPFC. Taken together, our results suggest that mPFC-HFS may have a preferential effect on DR neurons that have GABAA receptors. Another source of inhibition in the DR are 5-HT1A receptors located on the soma and dendrites of serotonin neurons (Riad et al., 2000), where they exert inhibitory feedback on the presynaptic neuron in response to local serotonin release (Wang & Aghajanian, 1977). Release of serotonin in the DR region of the cell body leads to a decrease in firing rate of 5-HT neurons and inhibition of 5-HT synthesis (Blier & de Montigny, 1987). As mPFC-HFS inhibited a large majority of all cells and HFS+Bic recovered firing rates of inhibited neurons, it is unlikely that 5-HT1A-mediated autoinhibition played a significant role in the HFS-mediated inhibition of DR neurons observed in this study.
Fig. 6.
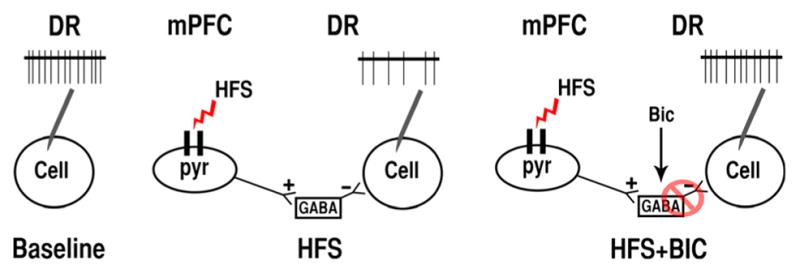
Illustration of the effects of bicuculline (Bic) on neurons in the dorsal raphe (DR) that were inhibited by high-frequency stimulation (HFS) of the medial prefrontal cortex (mPFC). HFS activates the excitatory glutamatergic mPFC projections that preferentially innervate GABA neurons in the DR, thus increasing inhibitory GABAergic tone and decreasing cellular firing of recorded neurons (middle illustration). Local administration of Bic blocks GABAA receptors on the recorded cells, disinhibiting the firing rate of the recorded cell, and thereby cancelling the inhibitory effect of HFS.
The mPFC in rodents and humans has been described as a critical node of emotional, executive and cognitive functions that serves as a regulatory region for affective control of descending inputs to subcortical regions, such as the brainstem raphe (Roy et al., 2012). Multiple animal studies using pharmacological, electrical and optogenetic manipulation have implicated the mPFC as a region that exerts a top-down modulation of DR circuits and regulates behavioural responses to stress (Hamani et al., 2010; Slattery et al., 2011; Warden et al., 2012; Veerakumar et al., 2014). Clinical studies from our group have shown that SCC stimulation is therapeutically beneficial to the chronically depressed population (Hamani et al., 2011; Kennedy et al., 2011; Lozano et al., 2012) and that it may be mediated through the release of 5-HT in forebrain regions (Hamani et al., 2010). We know from our work in human patients that HFS can inhibit cell bodies locally via release of inhibitory transmitters, but also at a significant distance from the stimulation site through the excitation of fibres of passage of inhibitory pathways (Liu et al., 2012). Therefore, although stimulation of the mPFC in the present study inhibited neurons in the DR, it also probably excited the terminals that released 5-HT in the forebrain. Along the same lines, because blocking GABA disinhibited the DR, it stands to reason that it also blocked HFS-mediated 5-HT release in the forebrain.
A recent study demonstrated that selectively activating only those mPFC glutamatergic cells projecting to the DR induced a rapid behavioural reaction during forced swim in defeated rats (Warden et al., 2012). However, disinhibition of 5-HT neurons by pharmacologically blocking DR GABAB but not GABAA receptors has been shown to increase 5-HT output in the mPFC and promote social approach behaviours in defeated mice (Takahashi et al., 2010, 2012). Our results show that HFS of the mPFC has a preferential effect on DR neurons with GABAA receptors, which may indicate that modulating GABAA as one of the factors involved in the anti-depressant mechanism of mPFC-HFS. While these results clearly point to GABA as a mediator of mPFC-HFS-induced inhibition, the involvement of 5-HT is highly probable but remains unproven. Because there is a complicated relationship between 5-HT cellular firing in the DR and 5-HT release in associated regions, future work needs to unequivocally identify 5-HT in the DR and characterize its concentration and reuptake.
Acknowledgments
This research was supported by the Canadian Institute of Health Research (CIHR), MOP 98006 and MOP 110970. C.H. is a consultant for St. Jude Medical, Inc.
Abbreviations
- Bic
bicuculline
- DBS
deep brain stimulation
- DR
dorsal raphe
- HFS
high-frequency stimulation
- IL
infralimbic region
- mPFC
medial prefrontal cortex
- PL
prelimbic region
- SCC
subcallosal cingulate
Footnotes
The authors report no other conflicts of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Commons KG. Forebrain GABAergic projections from the dorsal raphe nucleus identified by using GAD67-GFP knock-in mice. J Comp Neurol. 2012;520:4157–4167. doi: 10.1002/cne.23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J Neurosci. 2004;24:7410–7419. doi: 10.1523/JNEUROSCI.1691-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: electrophysiological studies in the rat brain. Synapse. 1987;1:470–480. doi: 10.1002/syn.890010511. [DOI] [PubMed] [Google Scholar]
- Calizo LH, Akanwa A, Ma X, Pan YZ, Lemos JC, Craige C, Heemstra LA, Beck SG. Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology. 2011;61:524–543. doi: 10.1016/j.neuropharm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABAA, and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, Beck SG, Berton O. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci. 2013;33:13978–13988. 13988a. doi: 10.1523/JNEUROSCI.2383-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis C, Beck SG, Berton O. Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Front Behav Neurosci. 2014;8:43. doi: 10.3389/fnbeh.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wildt DJ, Hillen FC, Rauws AG, Sangster B. Etomidate-anaesthesia, with and without fentanyl, compared with urethane-anaesthesia in the rat. Brit J Pharmacol. 1983;79:461–469. doi: 10.1111/j.1476-5381.1983.tb11019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenezer IS. The generation of cortical slow potentials in the rat anaesthetised with urethane and their modification by nicotine. Neuropharmacology. 1986;25:639–643. doi: 10.1016/0028-3908(86)90217-0. [DOI] [PubMed] [Google Scholar]
- Fu W, Le Maître E, Fabre V, Bernard JF, David Xu ZQ, Hokfelt T. Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J Comp Neurol. 2010;518:3464–3494. doi: 10.1002/cne.22407. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Bacon SJ. Areal and synaptic interconnectivity of prelimbic (area 32), infralimbic (area 25) and insular cortices in the rat. Brain Res. 2003;993:59–71. doi: 10.1016/j.brainres.2003.08.056. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gonçalves L, Nogueira MI, Shammah-Lagnado SJ, Metzger M. Prefrontal afferents to the dorsal raphe nucleus in the rat. Brain Res Bull. 2009;78:240–247. doi: 10.1016/j.brainresbull.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- Hajós M, Richards CD, Székely AD, Sharp T. An electro-physiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4:142rv8. doi: 10.1126/scitranslmed.3003722. [DOI] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Macedo CE, Brandão ML, Shumake J, Gonzalez-Lima F, Raymond R, Lozano AM, Fletcher PJ, Nobrega JN. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiat. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiat. 2011;69:301–308. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hamani C, Machado DC, Hipólide DC, Dubiela FP, Suchecki D, Macedo CE, Tescarollo F, Martins U, Covolan L, Nobrega JN. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiat. 2012;71:30–35. doi: 10.1016/j.biopsych.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Amorim BO, Wheeler AL, Diwan M, Driesslein K, Covolan L, Butson CR, Nobrega JN. Deep brain stimulation in rats: different targets induce similar antidepressant-like effects but influence different circuits. Neurobiol Dis. 2014;71C:205–214. doi: 10.1016/j.nbd.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, Moreines JL, Mewes K, Posse PR, Gutman DA, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiat. 2012;69:150–158. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–529. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, Lozano AM. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiat. 2011;168:502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Pernar L, Valentino RJ, Beck SG. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience. 2003;116:669–683. doi: 10.1016/s0306-4522(02)00584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Varga V, Dahan L, Sik A. Serotonergic neuron diversity: identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc Natl Acad Sci USA. 2006;103:1059–1064. doi: 10.1073/pnas.0508360103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Pan YZ, Ma X, Lamy C, Akanwa AC, Beck SG. Selective 5-HT receptor inhibition of glutamatergic and GABAergic synaptic activity in the rat dorsal and median raphe. Eur J Neurosci. 2006;24:3415–3430. doi: 10.1111/j.1460-9568.2006.05222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Li H, Kaneko T, Mizuno N. Morphological features and electrophysiological properties of serotonergic and non-serotonergic projection neurons in the dorsal raphe nucleus. An intracellular recording and labeling study in rat brain slices. Brain Res. 2001;900:110–118. doi: 10.1016/s0006-8993(01)02272-7. [DOI] [PubMed] [Google Scholar]
- Liu R, Jolas T, Aghajanian G. Serotonin 5-HT2 receptors activate local GABA inhibitory inputs to serotonergic neurons of the dorsal raphe nucleus. Brain Res. 2000;873:34–45. doi: 10.1016/s0006-8993(00)02468-9. [DOI] [PubMed] [Google Scholar]
- Liu LD, Prescott IA, Dostrovsky JO, Hodaie M, Lozano AM, Hutchison WD. Frequency-dependent effects of electrical stimulation in the globus pallidus of dystonia patients. J Neurophysiol. 2012;108:5–17. doi: 10.1152/jn.00527.2011. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiat. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT, Debonnel G, Sadikot AF, Lam RW, Howard AK, Ilcewicz-Klimek M, Honey CR, Mayberg HS. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg. 2012;116:315–322. doi: 10.3171/2011.10.JNS102122. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT. GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro. J Neurophysiol. 1989;61:719–726. doi: 10.1152/jn.1989.61.4.719. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition. Academic Press; New York: 2007. [Google Scholar]
- Peyron C, Petit JM, Rampon C, Jouvet M, Luppi PH. Fore-brain afferents to the rat dorsal raphe nucleus demonstrated by retrograde and anterograde tracing methods. Neuroscience. 1998;82:443–468. doi: 10.1016/s0306-4522(97)00268-6. [DOI] [PubMed] [Google Scholar]
- Pollak Dorocic I, Furth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, Carlén M, Meletis K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014;83:663–678. doi: 10.1016/j.neuron.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID, Cryan JF. Transient inactivation of the infralimbic cortex induces antidepressant-like effects in the rat. J Psychopharmacol. 2011;25:1295–1303. doi: 10.1177/0269881110368873. [DOI] [PubMed] [Google Scholar]
- Srejic LR, Prescott IA, Zhang P, Strauss I, Dostrovsky JO, Giacobbe P, Kennedy SH, Lozano AM, Hamani C, Hutchison WD. Paired pulse depression in the subcallosal cingulate region of depression patients. Biol Psychiat. 2014 doi: 10.1016/j.biopsych.2014.09.018. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABAB receptor modulation of serotonin neurons in the dorsal raphé nucleus and escalation of aggression in mice. J Neurosci. 2010;30:11771–11780. doi: 10.1523/JNEUROSCI.1814-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Schilit AN, Kim J, Debold JF, Koide T, Miczek KA. Behavioral characterization of escalated aggression induced by GABAB receptor activation in the dorsal raphe nucleus. Psychopharmacology. 2012;224:155–166. doi: 10.1007/s00213-012-2654-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Influence of inhibitory and excitatory inputs on serotonin efflux differs in the dorsal and median raphe nuclei. Brain Res. 2003;961:109–120. doi: 10.1016/s0006-8993(02)03851-9. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z. Neural circuit in the dorsal raphe nucleus responsible for cannabinoid-mediated increases in 5-HT efflux in the nucleus accumbens of the rat brain. ISRN Pharmacol. 2012;2012:276902. doi: 10.5402/2012/276902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a pre-frontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Varga V, Székely AD, Csillag A, Sharp T, Hajós M. Evidence for a role of GABA interneurones in the cortical modulation of midbrain 5-hydroxytryptamine neurones. Neuroscience. 2001;106:783–792. doi: 10.1016/s0306-4522(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Varga V, Kocsis B, Sharp T. Electrophysiological evidence for convergence of inputs from the medial prefrontal cortex and lateral habenula on single neurons in the dorsal raphe nucleus. Eur J Neurosci. 2003;17:280–286. doi: 10.1046/j.1460-9568.2003.02465.x. [DOI] [PubMed] [Google Scholar]
- Veerakumar A, Challis C, Gupta P, Da J, Upadhyay A, Beck SG, Berton O. Antidepressant-like effects of cortical deep brain stimulation coincide with proneuroplastic adaptations of serotonin systems. Biol Psychiat. 2014;76:203–212. doi: 10.1016/j.biopsych.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Inhibiton of neurons in the amygdala by dorsal raphe stimulation: mediation through a direct serotonergic pathway. Brain Res. 1977;120:85–102. doi: 10.1016/0006-8993(77)90499-1. [DOI] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. 77. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]