Abstract
Pediatric cataract is a leading cause of childhood blindness. This study aimed to determine the genetic cause of pediatric cataract in Australian families by screening known disease-associated genes using massively parallel sequencing technology. We sequenced 51 previously reported pediatric cataract genes in 33 affected individuals with a family history (cases with previously known or published mutations were excluded) using the Ion Torrent Personal Genome Machine. Variants were prioritized for validation if they were predicted to alter the protein sequence and were absent or rare with minor allele frequency <1% in public databases. Confirmed mutations were assessed for segregation with the phenotype in all available family members. All identified novel or previously reported cataract-causing mutations were screened in 326 unrelated Australian controls. We detected 11 novel mutations in GJA3, GJA8, CRYAA, CRYBB2, CRYGS, CRYGA, GCNT2, CRYGA, and MIP; and three previously reported cataract-causing mutations in GJA8, CRYAA, and CRYBB2. The most commonly mutated genes were those coding for gap junctions and crystallin proteins. Including previous reports of pediatric cataract-associated mutations in our Australian cohort, known genes account for >60% of familial pediatric cataract in Australia, indicating that still more causative genes remain to be identified.
Keywords: Ion Torrent, PGM, congenital cataract, pediatric cataract, massively parallel sequencing, Mutant Screen Report
Pediatric cataract is one of the leading causes of blindness in children. Approximately 200,000 children worldwide are blind from this condition (Chan et al. 2012). In industrialized countries, the incidence is 1–6 per 10,000 live births (Santana and Waiswo 2011). In Australia, the incidence is 2.2 per 10,000 live births, making the condition one of the most common causes of visual impairment in children (Wirth et al. 2002). Intrauterine infection, drug exposure, metabolic disorders, malnutrition, and heredity are known risk factors for pediatric cataract (Churchill and Graw 2011). Pediatric cataract is often referred to as congenital or infantile cataract when it presents at birth or in the first year of life, and juvenile cataract when it presents later during childhood (Yi et al. 2011).
Around 25–33% of pediatric cataracts are inherited (Santana et al. 2009). It is thought that 28% of bilateral pediatric cataract cases have a genetic basis while only 2% of unilateral cases are genetic (Rahi and Dezateux 2001; Santana and Waiswo 2011). Genetic and clinical heterogeneity have been reported in inherited pediatric cataracts (Lorenz 2007). Inherited cataracts can be transmitted as autosomal recessive, autosomal dominant, or X-linked traits, with autosomal dominant being the most common mode of inheritance. They can be isolated (nonsyndromic) or combined with other phenotypic features (syndromic) (Hejtmancik 2008).
Changes in lens development including lens fiber cell differentiation, protein solubility and stability, and defects in lens structure can lead to the development of cataract (Huang and He 2010). Mutations in genes involved in these functions have been reported to cause pediatric cataract. The known genes include at least 10 crystallin genes (Churchill and Graw 2011; Berry et al. 2001; Kannabiran et al. 1998; Riazuddin et al. 2005; Willoughby et al. 2005; Litt et al. 1997, 1998; AlFadhli et al. 2012; Heon et al. 1999), as well as membrane proteins [MIP, LIM2 (Pras et al. 2002; Berry et al. 2000)]; gap junction proteins [GJA8, GJA3 (Beyer and Berthoud 2014)], cytoskeletal proteins [BFSP1, BFSP2 (Ramachandran et al. 2007; Jakobs et al. 2000)]; stress response genes [HSF4 (Bu et al. 2002)], cell signaling proteins [EPHA2 (Shiels et al. 2008)], and transcription factors [PITX3, PAX6, EYA1, FOXE3, VSX2, FTL and MAF (Churchill and Graw 2011; Burdon et al. 2007; Shiels et al. 2007; Santana and Waiswo 2011)]. Crystallin and gap junction protein-encoding genes are the most commonly reported classes of genes for nonsyndromic pediatric cataracts, accounting for 50 and 25% of reported mutations, respectively (Hejtmancik 2008).
The large number of genes known to cause pediatric cataract and the limited genotype-phenotype correlations make clinical testing using traditional sequencing technologies challenging and expensive. Massively parallel (next-generation) sequencing (MPS) technologies are now accessible and are cost-effective tools to screen many candidate genes in parallel. In this study, we screened our repository of South Eastern Australian individuals with familial pediatric cataract for mutations in known causative genes using the Ion Torrent Personal Genome Machine (PGM). We hypothesized that a significant proportion of familial pediatric cataract cases would be accounted for by mutations in known genes, and that screening genes in parallel would be an effective method for genetic testing in this heterogeneous disease.
Materials and Methods
Participants’ recruitment and DNA extraction
The study adhered to the tenets of the Declaration of Helsinki and was approved by the Southern Adelaide Clinical Human Research Ethics Committee, Adelaide, Australia, and the Royal Victorian Eye and Ear Hospital (RVEEH) Human Research and Ethics Committee, Melbourne, Australia. The probands in each family were recruited from the eye clinic at Flinders Medical Centre (Adelaide), the Women’s and Children’s Hospital (Adelaide), the Royal Children’s Hospital (Melbourne), or the Royal Victorian Eye and Ear Hospital (Melbourne). Written informed consent was obtained from all participants or their guardians if they were under 18 years old. A detailed family history was obtained and additional affected and unaffected family members were invited to participate in the study. An ophthalmologist examined all available family members.
Genomic DNA was extracted from either peripheral whole blood using a QiaAmp DNA Blood Maxi Kit (Qiagen, Hilden, Germany) or from saliva using an Oragene DNA saliva collection kit (DNA Genotek, Ontario, Canada) according to the manufacturers’ protocols. Participants were included in this study if they reported a family history of pediatric cataract and if a causative mutation had not previously been detected in the family by other means.
Gene selection, primer design, library preparation, and sequencing
Fifty-one genes known to cause pediatric cataract in human or mouse were selected through review of the literature (Supplemental Material, Table S1 in File S1 (Chen et al. 2011; Churchill and Graw 2011; Hughes et al. 2011; Lachke et al. 2011; Aldahmesh et al. 2012; Pras et al. 2004; Azuma et al. 2000; Nonnenmacher et al. 2011; Jamieson et al. 2007; Shiels et al. 2007; Zhou et al. 2010; Ferda Percin et al. 2000; Reis et al. 2012). The strategy for generating the list included all genes covered in a review of pediatric cataract genes (Churchill and Graw 2011) except the mouse gene gjf1, which does not have a human homolog (n = 39). A search in PubMed using the terms “paediatric cataract” or “congenital cataracts” in combination with “genetic” or “gene” was used to include additional genes from more recent publications and those genes not covered by the review (n = 12). We focused on those genes known to be associated with nonsyndromic pediatric cataract, as this was the predominant phenotype in our cohort. In addition, we included some syndromic pediatric cataract genes known to cause a predominantly ocular syndrome (e.g., PAX6 and PITX3) or where pediatric cataract is a major diagnostic feature of the syndrome (NHS) as such genes may also contribute to nonsyndromic cataract (Gillespie et al. 2014).
PCR primers to amplify coding, 3′-, and 5′-untranslated regions of the 51 genes were designed with the Ion AmpliSeq Designer tool v1.22 (Life Technologies, www.ampliseq.com). The final design consisted of a total of 1216 amplicons ranging from 125 to 225 bp, covering 94.26% of the target sequence. Primers were supplied in two 100 nM pools (Life Technologies, Carlsbad, CA). Briefly, the concentration of genomic DNA was determined using the dsDNA HS Assay Kit on a Qubit fluorometer (Life Technologies), and libraries were prepared with the Ion AmpliSeq library kit version 2.0 according to manufacturer’s protocols. Libraries were prepared in two pools per individual, and the amplified pools were combined before partially digesting the primers and barcode adaptor ligation. The amplified library was diluted to 10 pM, and 25 μl of the diluted library was used for template preparation using Ion PGM Template OT2 200 Kit (Life Technologies) and the manufacturer’s protocol. The clonally amplified library was then enriched on an Ion OneTouch enrichment system. Samples were barcoded during library preparation using Ion Xpress Barcode Adapters 1–16 kit (Life Technologies) and pooled in groups of 3–5 during template preparation on the Ion OneTouch.
Libraries were quantified either with a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA) using the High Sensitivity DNA Kit (Agilent Technologies), or by qPCR using the Ion library TaqMan quantification kit (Life Technologies). Sequencing was performed on an Ion Torrent PGM using The Ion PGM Sequencing 200 Kit v2 and an Ion 318 chip (Life Technologies).
Torrent Suite (version 3.6) was used to align reads to human genome reference sequence 19 (hg19). The Coverage Analysis plugin (v4.0-r77897) was used to calculate the number of mapped reads, the percentage of on-target reads, and the mean depth of reads. Variants were called using the Variant caller plugin (V4.0-r76860) with the germline algorithm [allele frequency of 0.15, minimum read quality of 10, and minimum coverage of 20 were set as cut-offs for both indels and single nucleotide polymorphisms (SNPs)]. For annotation, variant call format (VCF) files were uploaded to Ion Reporter V4.0 (https://ionreporter.lifetechnologies.com/ir/) using the Ion Reporter Uploader plugin for Torrent Suite (v4.1-r79929). Variants were prioritized for further analysis if they were predicted to be protein-changing, and were absent or rare with minor allele frequency (MAF) <1% in dbSNP137 (http://www.ncbi.nlm.nih.gov/projects/SNP/), the Exome Aggregation Consortium (ExAC) (http://exac.broadinstitute.org/), and gnomAD (http://gnomad.broadinstitute.org/). In addition, identified variants were compared with an in-house list of common sequencing errors previously detected with this gene panel.
Validation, segregation analysis, and evaluating potential functional effects of mutations
Direct Sanger sequencing was used to confirm the detected protein-changing mutations in probands and to evaluate the segregation of the mutation in families. Forward and reverse primer sequences were designed using Primer3 (Untergasser et al. 2012; Koressaar and Remm 2007) and are listed in Table S2 in File S1.
PCR reactions of 20 μl final volume consisted of 1× Coraload PCR buffer (Qiagen), which gave a final concentration of 1.5 mM Mg2+, 0.1 mM dNTPs (Roche Diagnostics, Risch-Rotkreuz, Switzerland), 0.5 μM of each primer, 0.5 U Hot Star Plus Taq Polymerase (Qiagen), and 40 ng of gDNA. Five times Q Solution (Qiagen) was included at a final concentration of 1× as required, and water volume was adjusted accordingly. PCR was performed on a Palm Cycler (Corbett Life science, Qiagen) with one cycle at 95° for 5 min, followed by 30 or 35 cycles (Table S2 in File S1) at 95° for 30 sec, 57−65° (annealing temperature, Table S2 in File S1) for 30 sec, and 72° for 30 sec, and a final extension step at 72° for 5 min. To clean the PCR products for sequencing, 5 μl of PCR product, 2 μl shrimp alkaline phosphatase (SAP; 1 unit/μl) and 0.5 μl (20 units/μl) of exonuclease 1 (Exo1) (New England Biolabs, Massachusetts) were mixed. Reactions were incubated at 37° for 1 hr, followed by incubation at 80° for 20 min to inactivate the enzymes.
Sequencing reactions were prepared with the respective forward primer at 5 μM and purified PCR product at 10 ng/100 bp (i.e., 30 ng for 300 bp product) combined with BigDye Terminator v3.1 (Life Technologies) and 5× Sequencing Buffer (Life Technologies), and made up to 20 μl with water. Reactions were taken through a cycle sequencing PCR protocol on a MasterCycler thermal cycler (Eppendorf, Hamburg, Germany). PCR extension products were purified using Agencourt CleanSeq Magnetic Beads and a SPRI plate, according to the manufacturer’s protocol (Beckman Coulter, California). Purified extension products were then resolved using POP-7 polymer on the 3130xl Genetic Analyzer (Life Technologies) in the Flinders Sequencing Facility (Flinders Medical Centre, Adelaide, Australia).
Sequence chromatograms of affected and unaffected individuals were compared to each other and the reference sequence using Sequencher v.5 (GeneCodes Corporation, Ann Arbor, MI).
Each confirmed segregating novel mutation was assessed for a potential functional effect on the predicted protein sequence using SIFT (Sorting Intolerant from Tolerant, http://sift.jcvi.org/) (Kumar et al. 2009) and Polyphen-2 (version 2.2.2; the default HumDiv model was used) (Adzhubei et al. 2010) (http://genetics.bwh.harvard.edu/pph2/). The conservation of each altered amino acid was calculated using PhyloP as implemented in Mutation Taster (http://www.mutationtaster.org/) and available through the University of California Santa Cruz (UCSC) genome browser. PhyloP values between −14 and +6 indicate conservation at individual nucleotides, ignoring the effects of their neighbors. Amino acid conservation across species was visualized using the Mutation Taster website. Clinical interpretation of genetic variants by the 2015 ACMG/AMP guideline was determined using InterVar (http://wintervar.wglab.org) (Li and Wang 2017; Richards et al. 2015).
Screening novel or previously reported variants in control population
Novel variants were screened in 326 unrelated normal Australian controls recruited from Flinders Medical Centre, Adelaide, using the MassArray platform and iPlEX chemistry (Sequenom) at the Australian Genome Research Facility (Brisbane, Australia). Variants identified in families CRCH139, CSA133, and CSA95 were screened in controls using custom TaqMan SNP genotyping assays (Life Technologies) on a StepOne Plus real-time PCR instrument (Life Technologies) using standard manufacturer’s protocols. All variants reported in this study have been submitted to the ClinVar database (http://www.ncbi.nlm.nih.gov/clinvar/; ClinVar accessions SCV000297746–SCV000297762).
Data availability
Table S1 in File S1 contains a list of reported pediatric cataract genes selected for sequencing. PCR primers used to validate novel or rare coding mutations detected by next-generation sequencing are listed in Table S2 in File S1. Table S3 in File S1 contains systemic features of the five participants with syndromic pediatric cataract included in the study. Figure S1 in File S1 shows amplicons with <20 fold coverage. Figure S2, A–C in File S1) shows protein sequence alignments demonstrating the conservation of the altered amino acid in families with causative mutations.
Results and Discussion
We sequenced 51 known pediatric cataract genes in 33 unrelated probands using Ion Torrent MPS technology. Syndromic cataract was present in 5/33 (15%) probands (syndromic features described in Table S3 in File S1) while 28/33 (85%) probands had isolated pediatric cataract. Primers were designed for 1216 amplicons in the size range of 125–225 bp to cover the 51 known causative genes. In total, 154.1 kb of target sequence was included in the design process with amplicons designed for 94.3% of the target sequence (8.8 kb not covered). The presence of repetitive sequence, unacceptable GC content, and melting temperatures of the primers outside the optimal range were the main factors limiting primer design for target regions not covered.
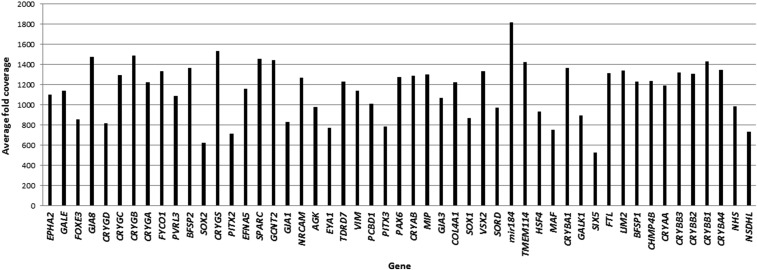
The mean number of mapped reads per sample was 1,536,538, with 91% of reads on target. A mean of 1155 reads was achieved per amplicon, with a coverage uniformity of 89%. Of all the amplicons, 96 and 91% were covered at least 20 and 100 fold, respectively. The average coverage per gene is shown in Figure 1. Of the 1216 amplicons, 30 amplicons (2%) across 17 genes were covered <20 fold (Figure S1 in File S1).
Figure 1.
Average fold coverage of target genes sequenced from AmpliSeq libraries in 33 probands with pediatric cataract.
A total of 4726 variants were annotated (an average of 139 variants per individual). In total, 178 variants were absent/rare (MAF <1%) in publically referenced databases, of which 56 were nonsynonymous exonic variants. Twenty-three variants were selected for validation using Sanger sequencing after filtering out the variants in an in-house list of sequencing artifacts (33 variants). Seventeen variants were validated and six were false positives.
Of the 17 validated variants (Table 1), 14 appeared to be the likely cause of cataract in the respective families, accounting for 42% of the 33 screened probands. The validated mutations were considered to be pathogenic if the protein change was predicted to be pathogenic by SIFT (Kumar et al. 2009) and/or Polyphen-2 (Adzhubei et al. 2010), the variant segregated with the phenotype in the family, and was absent from all screened local controls. Two of the 17 validated variants did not segregate, and one was considered benign by both SIFT and Polyphen-2. All segregating mutations were highly conserved across species (Figure S2, A–C in File S1 and Table 1). We have used Polyphen-2 and SIFT predictions as a pathogenicity indicator guide; however, the predictions based on ACMG (Richards et al. 2015; Li and Wang 2017) guideline were also included for clinical purposes. The variants predicted to have uncertain significance under ACMG guideline look promising based on the evidence generated in this study; however, additional studies will be needed to confirm their pathogenicity.
Table 1. List of mutations detected in families.
| Family | Reported MAF in Public Databases | Novel/Known | Gene | Position in hg19 | Nucleotide Change | Protein Change | PhyloP Score | Polyphen-2 (HumDiv) | SIFT | Segregation/Penetrance | Inheritance | ACMG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSA95 | 0 | Novel | GJA3 | chr13:20717372 | c.56C > T | p.(Thr19Met) | 6.141 | PD | D | Yes/ Full | AD | LP |
| CSA109 | 0 | Novel | GJA3 | chr13:20716962 | c.466A > C | p.(Lys156Gln) | 3.268 | PD | D | Yes/ Full | AD | US |
| CRCH20 | 0 | Novel | GJA8 | chr1:147380155 | c.73T > C | p.(Trp25Arg) | 4.833 | PD | D | Yes/ Incomplete | AD | LP |
| CSA125 | 0 | Novel | GJA8 | chr1:147380566 | c.484G > A | p.(Glu162Lys) | 5.784 | PD | D | Yes/ Full | AD | US |
| CSA162 | 0 | Known (Vanita et al. 2008) (Ma et al. 2016) | GJA8 | chr1:147380216 | c.134G > C | p.Trp45Ser | 5.786 | PD | D | Yes/ Full | AD | P |
| CSA159 | 0 | Novel | CRYAA | chr21:44592307 | c.440delA | p.(Gln147Argfs*48) | NA | NA | NA | Yes/ Full | AD | P |
| CRVEEH111 | gnomAD: 0.00003231 | Known (Khan et al. 2007) (Devi et al. 2008) | CRYAA | chr21:44589369 | c.160C > T | p.(Arg54Cys) | 4.982 | PD | T | Yes/ Full | AD | P |
| CSA94 | 0 | Novel | CRYGS | chr3:186257377-78 | c.30_31delCTinsAA | p.(Phe10_Tyr11delinsLeuAsn) | NA | PD | D | Yes/ Full | AD | US |
| CRCH139 | ExAc:0.00428 gnomAD: 0.003861 dbSNP:0.0022 (rs139353014) | Novel | CRYGA | chr2:209027941 | c.239G > A | p.(Arg80His) | 0.799 | PD | T | Yes/ Incomplete | AD | LB |
| ExAc: 0.001816 gnomAD: 0.001904 dbSNP: 0.0006 (rs79006549) | Novel | PVRL3 | chr3:110841054 | c.886A > C | p.(Asn296His) | 4.027 | PD | D | No | US | ||
| CSA133 | 0 | Known (Litt et al. 1997) | CRYBB2 | chr22:25627584 | c.463C > T | p.(Gln155*) | NA | NA | D | Yes/ Full | AD | P |
| CRVEEH85 | 0 | Novel | CRYBB2 | chr22:25627684 | c.563G > T | p.(Arg188Leu) | 5.11 | PD | D | Yes/ Full | AD | LP |
| ExAC: 8.489 × 10−6 gnomAD:4.085 × 10−6 | Novel | BFSP2 | chr3:133191301 | c.1136C > A | p.(Ala379Glu) | 0.366 | B | T | Yes/ Full | US | ||
| CRCH89 | 0 | Novel | GCNT2 | chr6:10626722 | c.1091T > C | p.(Phe364Ser) | 4.256 | PD | D | Yes/ homozygous in cases/Full | AR | LP |
| CRCH136 | 0 | Novel | GCNT2 | chr6:10626796 | c.1169_1172delATCA | p.(Asn388Arg*20) | NA | NA | NA | Yes/ heterozygous in cases/NA | AR | US |
| CSA131 | 0 | Novel | MIP | chr12:56845225 | c.631G > T | p.(Gly211*) | NA | NA | NA | Yes/Full | AD | US |
| gnomAD: 0.00002439 | Novel | FYCO1 | chr3:46009288 | c.1538G > A | p.(Arg513Gln) | B | T | No | US |
MAF, minor allele frequency; PD, probably damaging; D, damaging; AD, autosomal dominant; LP, likely pathogenic; US, uncertain significance; P, pathogenic; NA, not applicable; T, tolerated; LB, likely benign; B, benign; AR, autosomal recessive. GenBank accession numbers are shown in Table S1 in File S1. Zero in second column indicates that the variant was not present in all three databases (ExAC, genome ID and dbSNP).
In total, as shown in Table 1, we detected 11 novel mutations in eight different genes (GJA3, GJA8, CRYAA, CRYBB2, CRYGS, CRYGA, GCNT2, CRYGA, and MIP), three previously reported cataract-causing mutations in three different genes (GJA8, CRYAA, and CRYBB2). The phenotype in each of the 14 families is given in Table 2, and representative clinical photos where available are shown in Figure 2.
Table 2. Observed phenotypes in families with causative mutations identified in pediatric cataract associated genes.
| Family | Gene | Affected Members | Phenotype | Age at Diagnosis | Age at Surgery | Age at Surgery |
|---|---|---|---|---|---|---|
| Right Eye | Left Eye | |||||
| CSA95 | GJA3 | CSA95.01 | — | 0 yr | 0 yr | 0 yr |
| CSA95.02 | — | 20 yr | — | — | ||
| CSA109 | GJA3 | CSA109.01 | Fetal nuclear cataract | 3 yr | — | — |
| CSA109.02 | Fetal nuclear/lamellar cataract | 5 yr | 16 yr | 17 yr | ||
| CRCH20 | GJA8 | CRCH20.02 | Bilateral congenital nuclear | — | 35 yr | — |
| CRCH20.07 | Bilateral minor lens opacities | — | — | — | ||
| CSA125 | GJA8 | CSA125.01 | Nuclear | 10 yr | — | — |
| CSA125.02 | Posterior polar | — | 6 yr | — | ||
| CSA162 | GJA8 | CSA162.01 | — | — | — | — |
| CSA162.02 | — | — | — | — | ||
| CSA159 | CRYAA | CSA159.01 | Severe congenital | 0 yr | 1 mo | 2 mo |
| CSA159.02 | Nuclear and cortical, blue-dot component: mild | 19 yr | 25 yr | 25 yr | ||
| CSA159.04 | Lamellar: mild | 4 yr | NA | NA | ||
| CRVEEH111 | CRYAA | CRVEEH111.01 | Bilateral | — | — | — |
| CRVEEH111.04 | Bilateral | — | 17 mo | 17 mo | ||
| CRVEEH111.05 | Central, anterior polar rider, faint nuclear opacity only | — | — | — | ||
| CRVEEH111.06 | Central nuclear opacity | — | — | — | ||
| CSA94 | CRYGS | CSA94.01 | Lamellar cortical-nuclear clear | 6 yr | 6 yr | 5 yr |
| CSA94.02 | Cortical | 4 yr | 6 yr | 5 yr | ||
| CSA94.03 | Lamellar | 2 yr | 3 yr | 4 yr | ||
| CSA94.04 | Lamellar | 2 yr | 5 yr | 5 yr | ||
| CRCH139 | CRYGA | CRCH139.02 | Congenital | — | — | — |
| CSA133 | CRYBB2 | CSA133.01 | — | — | — | — |
| CSA133.03 | — | — | — | — | ||
| CRVEEH85 | CRYBB2 | CRVEEH85.01 | Congenital | — | — | — |
| CRVEEH85.02 | Congenital | 2–3 yr | 3 yr | 3 yr | ||
| CRVEEH85.03 | Congenital | — | — | — | ||
| CRCH89 | GCNT2 | CRCH89.01 | Bilateral congenital | — | 3 wk | 3 wk |
| CRCH89.02 | Bilateral congenital | — | 1 yr | 1 yr | ||
| CRCH89.05 | Bilateral congenital | — | — | — | ||
| CRCH89.07 | Bilateral congenital | — | — | — | ||
| CRCH136a | GCNT2 | CRCH136.01 | Bilateral dense central opacity | — | — | — |
| CRCH136.02 | Bilateral dense central opacity | — | — | — | ||
| CSA131 | MIP | CSA131.01 | White dots | 20 yr | NA | NA |
| CSA131.02 | White dots | 22 yr | NA | NA | ||
| CSA131.04 | Cortical and nuclear sclerotic, multiple cortical dots as well as anterior cortical spokes | 45 yr | 46 yr | 46 yr |
Missing data are indicated by “—”. NA indicates the individual has not had surgery to date.
One heterozygous deletion detected in affected members of this family with autosomal recessive inheritance pattern.
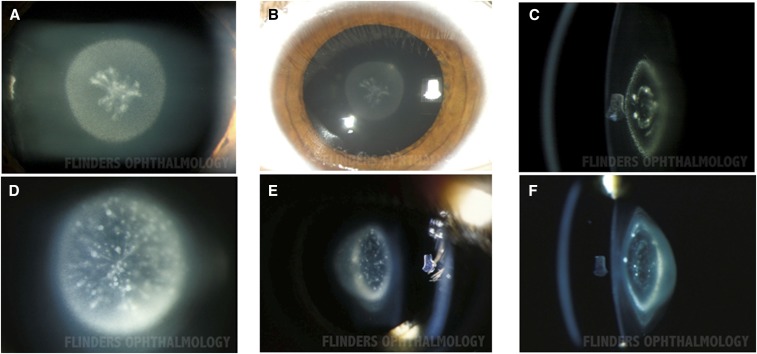
Figure 2.
Phenotype of pediatric cataract in family CSA109 carrying causative mutations in GJA3. Photographs of individual CSA109.01 A–C show fetal nuclear cataract. D–F show fetal nuclear/lamellar cataract in individual CSA109.02.
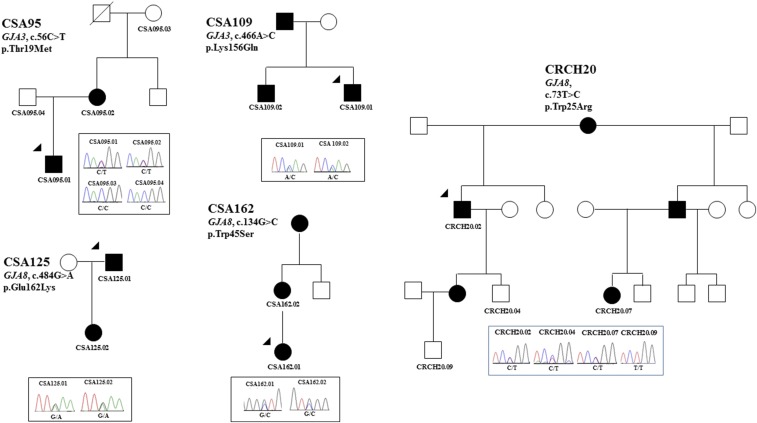
We identified novel mutations in two gap junction genes (GJA3 and GJA8) in five families. Of the two families (CSA95 and CSA109) with mutations in GJA3, phenotypic information was not available for family CSA95, but variant p.Thr19Met was predicted to be pathogenic and segregated in the two affected individuals (Figure 3). Both tested individuals in family CSA109 had fetal nuclear lamellar cataracts (Figure 2) and the variant p.(Lys156Gln) was predicted to be damaging and segregated in two affected siblings. The affected father was not available for testing.
Figure 3.
Pedigree and Sanger sequencing analysis of families with variants in gap junction genes (GJA3 and GJA8). The chromatograms below each pedigree show the sequence detected via Sanger sequencing for each variant in families, and the gene names and mutation at cDNA and protein level have been mentioned on each pedigree. The penetrance of mutations in family CRCH20 (GJA8, c.73T > C) is incomplete. The arrowheads indicate the proband sequenced on the gene panel by AmpliSeq. Solid circles indicate affected females and solid squares show the affected males.
Two of the three families with GJA8 mutations (CSA125 and CRCH20) had cataracts described as nuclear, with no phenotype information available for family CRCH162 (Table 2). In family CRCH20 the damaging mutation [p.(Trp25Arg)] segregated in two generations and appeared to have incomplete penetrance, as individual CRCH20.04 carries the mutation but as yet does not have cataract. Mutations in families CSA162 [p.(Trp45Ser)] and CSA125 [p.(Glu162Lys)] were inherited from the affected mother and the affected father, respectively (Figure 3), and segregated with the disease.
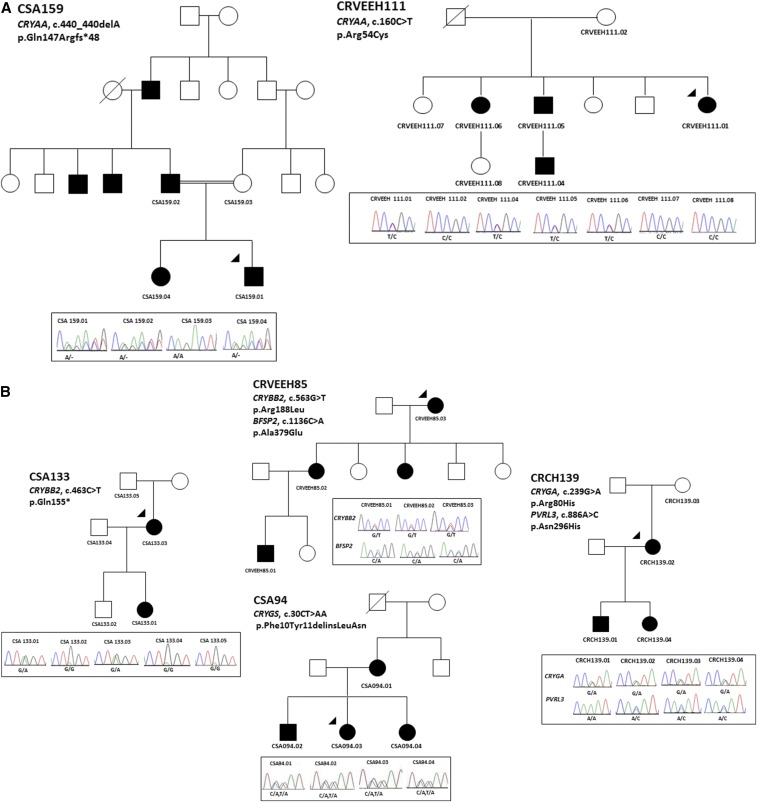
Six mutations were identified in crystallin genes. A previously reported mutation (Khan et al. 2007; Devi et al. 2008) in CRYAA [p.(Arg54Cys)] was detected in family CRVEEH111 that segregated with the disease (Figure 4A). SIFT predicted this variant to be tolerated, but Polyphen-2 predicted it to be pathogenic (Table 1). Family CRVEEH111 had central nuclear cataract with varying severity in affected family members. The second mutation detected in CRYAA is a novel frameshift deletion [p.(Gln147Argfs*48)] in a consanguineous family (CSA159) displaying autosomal dominant inheritance (Figure 4A). The father and both children carried the mutation; however, the severity of the phenotype varied between affected members. The proband (CSA159.01) was diagnosed at birth and underwent cataract surgery at 1 month of age. His sister (CSA159.04) was diagnosed with a milder lamellar cataract with a similar appearance to that in the father.
Figure 4.
Pedigree and Sanger sequencing analysis of families with variants in (A) α crystallins (CRYAA); (B) β and γ crystallins (CRYBB2, CRYGA, and CRYGS). The penetrance of mutations in family CRCH139 (CRYGA, c.239G > A) is incomplete. The variants in PVRL3 in CRCH139 do not segregate with the phenotype. The segregating variant in BFAP2 in CRVEEH85 was predicted to be nonpathogenic by both SIFT and Polyphen-2. The arrowheads indicate the proband sequenced on the gene panel by AmpliSeq. Solid circles indicate affected females and solid squares show the affected males. Diagonal lines indicate the person is deceased. The chromatograms below each pedigree show the Sanger sequencing result of each detected variant in family members. The gene names and mutation at cDNA and protein level have been mentioned on each pedigree.
One novel and one previously reported mutation were detected in CRYBB2. Three affected individuals from family CRVEEH85 carried a novel mutation [p.(Arg188Leu)]. These individuals also carried a variant in BFSP2 [p.(Ala379Glu)]. However, this variant was reported to be benign by both SIFT and Polyphen-2 and was less conserved. It was therefore considered not pathogenic. A previously reported truncating mutation, p.(Gln155*) (rs74315489), in the CRYBB2 gene was identified in two affected individuals in family CSA133. No information was available regarding the phenotype in this family. This variant has not been reported in normal populations and was not detected in our local controls, thus is likely pathogenic.
Three mutations were detected in two different γ-crystallin genes. Family CSA94 (Figure 4B) had a novel dinucleotide substitution (c.30_31delCTinsAA) resulting in the substitution of two amino acids [p.(Phe10_Tyr11delinsLeuAsn]) in CRYGS which was predicted to be damaging (Table 1). Affected members of this family had a juvenile onset cortical lamellar cataract and all the members had surgery by 6 yr of age (Table 2). Family CRCH139 had a missense variant [p.(Arg80His), rs139353014] in CRYGA segregating with the phenotype in three individuals. This variant was predicted to be damaging by Polyphen-2 and the residue was conserved across species. However, it had a MAF of 0.2% in dbSNP, 0.4% in the ExAC database, and 0.1% in our Australian controls. The variant was also present in an unaffected individual CRCH139.03 in this family (Figure 4B). The reduced penetrance is consistent with this variant being present in the population at lower frequency. A second variant was detected in this family in PVRL3; however, it did not segregate with the phenotype. Although it is not clear whether the rare CRYGA variant is responsible for the disease in this family, it remains the best candidate mutation observed to date in family CRCH139 and may represent a deleterious variant of lower penetrance.
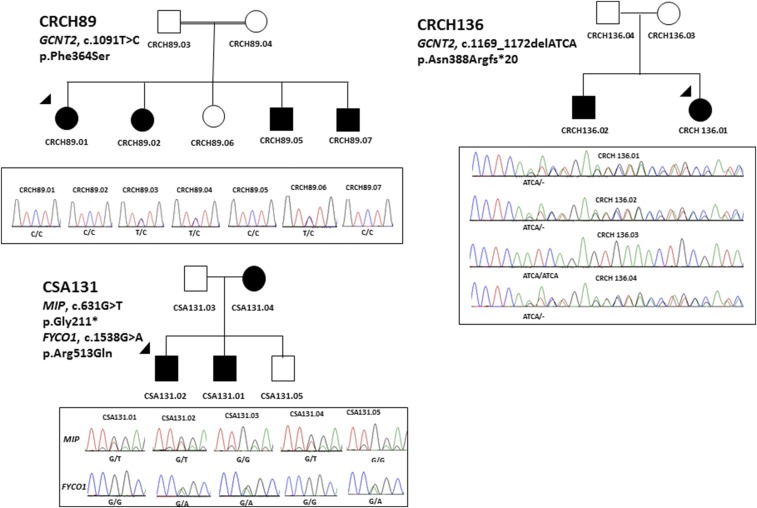
Families CRCH89 and CRCH136 displayed an autosomal recessive inheritance pattern of cataract. Affected members of the consanguineous family, CRCH89, were homozygous for a novel variant [p.(Phe364Ser)] in GCNT2 predicted to be pathogenic (Figure 5). The four affected siblings all had bilateral pediatric cataracts with surgery in the first few weeks to 1 year of age (Table 2). A single heterozygous variant in GCNT2 resulting in a premature stop codon [p.(Asn388Argfs*20)] was detected in family CRCH136 and was inherited from the unaffected mother. No other variant was identified in GCNT2 in this family.
Figure 5.
Pedigree and Sanger sequencing analysis of families with variants in GCNT2 and MIP. The arrowheads indicate the proband sequenced on the gene panel by AmpliSeq. Solid circles indicate affected females and solid squares show the affected males. The double line in CRCH89 shows consanguinity. The chromatograms below each pedigree show the segregation analysis of the variants in families. The gene names and mutation at cDNA and protein level have been mentioned on each pedigree.
A segregating stop mutation [p.(Gly211*)] in MIP was detected in family CSA131 (Figure 5). The mother (CSA131.04) had cortical and nuclear sclerotic cataracts with multiple cortical dots, while two other affected members (CSA131.01 and CSA131.02) had anterior cortical spokes and white dot cataracts (Table 2). This family also carried a mutation in FYCO1; however, the latter variant was predicted to be benign and did not segregate with the disease.
Mutations meeting the criteria for potential pathogenicity were not identified in the remaining 19 families (57%) or the other 43 genes screened on this gene panel.
In this study, we used targeted massively parallel sequencing to identify genetic variants associated with inherited pediatric cataract. We identified likely causative variants in 42% of previously unsolved familial cases. We detected 11 novel mutations contributing to pediatric cataract, confirmed three previously reported mutations, and identified rare coding variants that may be important in the disease. We have previously identified mutations in genes included in this panel in other families in our repository (Sharma et al. 2008; Burdon et al. 2004a,b, 2007; Reches et al. 2007; Dave et al. 2013; Craig et al. 2003; McLeod et al. 2002; Javadiyan et al. 2016). When considered together with our earlier published work, these 51 genes account for 62% of familial pediatric cataract, a proportion comparable to that reported in a similar study of patients from the UK (Gillespie et al. 2014) and another Australian cohort (Ma et al. 2016).
The phenotypes observed in these families, where detailed information is available, are similar to previous reports of mutations in these genes. For example, we describe predominantly nuclear phenotypes related to mutations in the gap junction genes GJA3 and GJA8 (Shiels et al. 2010). Similarly, nuclear or total cataracts are observed in CRYAA mutation carriers (Khan et al. 2007; Devi et al. 2008) while mutations in CRYBB2 give rise to cortical and lamellar cataracts (Devi et al. 2008; Faletra et al. 2013).
Homozygous or compound heterozygous mutations in GCNT2 have been reported in families with autosomal recessive cataract (Borck et al. 2012). Affected individuals in family CRCH136 carry a single copy of a 4 bp frameshift deletion [p.(Asn388Argfs*20)] in GCNT2. Although segregation in this family is consistent with autosomal recessive inheritance (Figure 5), a second mutation in GCNT2 or in any other gene in the panel could not be identified in the proband. The affected individuals had bilateral dense central opacities, similar to those reported by Borck et al. (2012) in other families with a homozygous mutation in this gene, and are also similar to those seen in family CRCH89 in which a homozygous GCNT2 mutation was identified. It is possible that the second mutation in family CRCH136 was not detectable by the methods employed in this study, which may include partial gene deletions or mutations affecting noncoding regions. The possibility that this mutation does not contribute to the disease in this family cannot be excluded but is considered less likely.
This work highlights the not-infrequent occurrence of mutations with incomplete penetrance in pediatric cataract. We observed reduced penetrance with mutations in GJA8 (CRCH20) and CRYGA (CRCH139). This suggests the involvement of modifier genes altering the penetrance of these mutations and the severity of the cataract; however, such genes have not yet been identified (Maeda et al. 2001). There have been previous reports of reduced penetrance with mutations in GJA3 in families with total cortical (Devi et al. 2005) and nuclear lamellar pulverulent (Burdon et al. 2004b) cataracts. Furthermore, there has been one report of reduced penetrance of a mutation in CRYBB2 associated with congenital zonular cataract (Santhiya et al. 2010).
The possible involvement of rare variants present in public databases in pediatric cataract pathogenesis is suggested by this study. Family CRCH139 has a segregating rare variant in CRYGA (rs139353014) with incomplete penetrance. Other factors, including high level of conservation at this residue and Polyphen-2 prediction of a functional effect, provide support for contribution of this mutation to cataract in this family. The incomplete penetrance is consistent with the presence of this variant at very low levels in public databases and our local controls. If this variant does not always lead to disease, it would be expected to accumulate in such databases. As public databases increase in size, and are generated from unscreened individuals, we expect that, increasingly, disease-causing variants will be found at low frequency in these resources. This scenario of disease-causing variants being represented in public databases of genetic variation due to the large numbers of individuals sequenced is well exemplified by the p.Gln368* mutation in the MYOC gene, which leads to primary open-angle glaucoma with high penetrance and is present in the 1000 Genomes database with a frequency of 0.06% as rs74315329. Such rare variation should not be automatically discounted when evaluating variants as pathogenic in disease cohorts. SIFT and ACMG predictions of benign for the Arg80His variant of CRCH139 make it difficult to comment on the pathogenicity of this variant without any functional studies. Other possibilities, such as the causative variant being intronic, in a novel not-yet-reported gene (or not included in this panel), or a large structural variant should not be ignored.
In the current study, 2% of amplicons were covered <20 fold, potentially limiting the ability to detect heterozygous mutations in these amplicons. By chance, the majority of the 16 genes containing these low coverage amplicons are involved in syndromic forms of pediatric cataract. In this study, only five probands had syndromic pediatric cataracts and no mutations were identified in these individuals. It may be that mutations were missed due to low coverage, or that these individuals have mutations in genes not targeted by this panel. Furthermore, it is important to comment that this study is not able to detect large insertions or deletions or any structural variants such as copy number variation (CNV) within screened genes. Such variants have been reported in the literature to be associated with the disease (Siggs et al. 2017; Burdon et al. 2007; Van Esch et al. 2007).
One of the main advantages of the AmpliSeq method on the Ion Torrent PGM is that it only requires 10–40 ng of DNA as starting material. Many of the DNA samples used in this study were >10 years old, and the limited quantity of DNA available was somewhat degraded. The successful sequencing of these samples and identification of likely causative mutations suggests that quality of DNA is not necessarily a crucial factor in obtaining reliable sequencing results using this methodology.
Pediatric cataract is a clinically and genetically heterogeneous condition, which makes accurate molecular diagnosis difficult. Genetic linkage analysis and candidate gene screening are the conventional methods for detecting disease-causing genes for familial diseases, but this approach is limited by the size of the families. In small families, as in this current cohort, gene identification has been difficult due to lack of power for linkage and the excessive cost of screening large numbers of candidate genes using traditional Sanger sequencing. MPS platforms are able to target numerous genes in parallel and are cost-effective tools for gene identification in heterogeneous conditions such as pediatric cataract. In this study, we aimed to determine the genetic contribution of the known pediatric cataract genes to inherited pediatric cataract in an Australian cohort. In our experience, both ophthalmologists and their patients are highly motivated to know the genetic basis of their disease. Developing genetic diagnostic panels increases the chance of obtaining a molecular diagnosis, which allows patients and their families to be better educated about the mode of inheritance, and facilitates more accurate genetic counseling about the risk of recurrence for future pregnancies, enabling discussion regarding possible reproductive options. For example, the parents in family CSA159 enrolled in this research study during a pregnancy with a desire to understand the likelihood of the child being affected with the severe disease observed in their son.
Although some degree of genotype/phenotype correlation is beginning to emerge for some pediatric cataract genes, the clinical evaluation of a patient is often insufficient to establish which genes are most likely involved in order to initiate specific genetic testing. This is particularly the case in historic samples where surgery was performed prior to enrolment in this study. These genotype-phenotype correlations are worthy of further study, as genetic counseling in the future should take account of the likely severity of affected status, the likelihood of systemic manifestation, and the chance of incomplete penetrance. Gene panel testing, as has been shown in previous studies (Gillespie et al. 2014) and here in an Australian cohort, is therefore an efficient way to rapidly determine the genetic cause of heterogeneous diseases such as pediatric cataract. In our cohort of cases, the chosen panel of 51 genes would cover 62% of causative mutations.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.300109/-/DC1.
Acknowledgments
This work was supported by funding from the Channel 7 Children’s Research Foundation, the Ophthalmic Research Institute of Australia, and an Australian National Health and Medical Research Council (NHMRC) Centres of Research Excellence Grant 1023911 (2012F2016). The Centre for Eye Research Australia receives operational infrastructure support from the Victorian Government. J.E.C. is supported by an NHMRC Practitioner Fellowship, and K.P.B. by an NHMRC Senior Research Fellowship. A.W.H. was supported by an NHMRC Early Career Fellowship 1037838 (2012–2015).
Footnotes
Communicating editor: R. Cantor
Literature Cited
- Adzhubei I. A., Schmidt S., Peshkin L., Ramensky V. E., Gerasimova A., et al. , 2010. A method and server for predicting damaging missense mutations. Nat. Methods 7: 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldahmesh M. A., Khan A. O., Mohamed J. Y., Alghamdi M. H., Alkuraya F. S., 2012. Identification of a truncation mutation of acylglycerol kinase (AGK) gene in a novel autosomal recessive cataract locus. Hum. Mutat. 33: 960–962. [DOI] [PubMed] [Google Scholar]
- AlFadhli S., Abdelmoaty S., Al-Hajeri A., Behbehani A., Alkuraya F., 2012. Novel crystallin gamma B mutations in a Kuwaiti family with autosomal dominant congenital cataracts reveal genetic and clinical heterogeneity. Mol. Vis. 18: 2931–2936. [PMC free article] [PubMed] [Google Scholar]
- Azuma N., Hirakiyama A., Inoue T., Asaka A., Yamada M., 2000. Mutations of a human homologue of the Drosophila eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Hum. Mol. Genet. 9: 363–366. [DOI] [PubMed] [Google Scholar]
- Berry V., Francis P., Kaushal S., Moore A., Bhattacharya S., 2000. Missense mutations in MIP underlie autosomal dominant ‘polymorphic’ and lamellar cataracts linked to 12q. Nat. Genet. 25: 15–17. [DOI] [PubMed] [Google Scholar]
- Berry V., Francis P., Reddy M. A., Collyer D., Vithana E., et al. , 2001. Alpha-B crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans. Am. J. Hum. Genet. 69: 1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C., Berthoud V. M., 2014. Connexin hemichannels in the lens. Front. Physiol. 5: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck G., Kakar N., Hoch J., Friedrich K., Freudenberg J., et al. , 2012. An Alu repeat-mediated genomic GCNT2 deletion underlies congenital cataracts and adult i blood group. Hum. Genet. 131: 209–216. [DOI] [PubMed] [Google Scholar]
- Bu L., Jin Y., Shi Y., Chu R., Ban A., et al. , 2002. Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat. Genet. 31: 276–278. [DOI] [PubMed] [Google Scholar]
- Burdon K. P., Wirth M. G., Mackey D. A., Russell-Eggitt I. M., Craig J. E., et al. , 2004a. Investigation of crystallin genes in familial cataract, and report of two disease associated mutations. Br. J. Ophthalmol. 88: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon K. P., Wirth M. G., Mackey D. A., Russell-Eggitt I. M., Craig J. E., et al. , 2004b. A novel mutation in the Connexin 46 gene causes autosomal dominant congenital cataract with incomplete penetrance. J. Med. Genet. 41: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon K. P., Sharma S., Chen C. S., Dimasi D. P., Mackey D. A., et al. , 2007. A novel deletion in the FTL gene causes hereditary hyperferritinemia cataract syndrome (HHCS) by alteration of the transcription start site. Hum. Mutat. 28: 742. [DOI] [PubMed] [Google Scholar]
- Chan W. H., Biswas S., Ashworth J. L., Lloyd I. C., 2012. Congenital and infantile cataract: aetiology and management. Eur. J. Pediatr. 171: 625–630. [DOI] [PubMed] [Google Scholar]
- Chen J., Ma Z., Jiao X., Fariss R., Kantorow W. L., et al. , 2011. Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am. J. Hum. Genet. 88: 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill A., Graw J., 2011. Clinical and experimental advances in congenital and paediatric cataracts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366: 1234–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. E., Clark J. B., McLeod J. L., Kirkland M. A., Grant G., et al. , 2003. Hereditary hyperferritinemia-cataract syndrome: prevalence, lens morphology, spectrum of mutations, and clinical presentations. Arch. Ophthalmol. 121: 1753–1761. [DOI] [PubMed] [Google Scholar]
- Dave A., Laurie K., Staffieri S. E., Taranath D., Mackey D. A., et al. , 2013. Mutations in the EPHA2 gene are a major contributor to inherited cataracts in South-Eastern Australia. PLoS One 8: e72518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi R. R., Reena C., Vijayalakshmi P., 2005. Novel mutations in GJA3 associated with autosomal dominant congenital cataract in the Indian population. Mol. Vis. 11: 846–852. [PubMed] [Google Scholar]
- Devi R. R., Yao W., Vijayalakshmi P., Sergeev Y. V., Sundaresan P., et al. , 2008. Crystallin gene mutations in Indian families with inherited pediatric cataract. Mol. Vis. 14: 1157–1170. [PMC free article] [PubMed] [Google Scholar]
- Faletra F., d’Adamo A. P., Pensiero S., Athanasakis E., Catalano D., et al. , 2013. A novel CRYBB2 missense mutation causing congenital autosomal dominant cataract in an Italian family. Ophthalmic Genet. 34: 115–117. [DOI] [PubMed] [Google Scholar]
- Ferda Percin E., Ploder L. A., Yu J. J., Arici K., Horsford D. J., et al. , 2000. Human microphthalmia associated with mutations in the retinal homeobox gene CHX10. Nat. Genet. 25: 397–401. [DOI] [PubMed] [Google Scholar]
- Gillespie R. L., O’Sullivan J., Ashworth J., Bhaskar S., Williams S., et al. , 2014. Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmology 121: 2124–2137 e2121–2122. [DOI] [PubMed] [Google Scholar]
- Hejtmancik J. F., 2008. Congenital cataracts and their molecular genetics. Semin. Cell Dev. Biol. 19: 134–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heon E., Priston M., Schorderet D. F., Billingsley G. D., Girard P. O., et al. , 1999. The gamma-crystallins and human cataracts: a puzzle made clearer. Am. J. Hum. Genet. 65: 1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., He W., 2010. Molecular characteristics of inherited congenital cataracts. Eur. J. Med. Genet. 53: 347–357. [DOI] [PubMed] [Google Scholar]
- Hughes A. E., Bradley D. T., Campbell M., Lechner J., Dash D. P., et al. , 2011. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am. J. Hum. Genet. 89: 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs P. M., Hess J. F., FitzGerald P. G., Kramer P., Weleber R. G., et al. , 2000. Autosomal-dominant congenital cataract associated with a deletion mutation in the human beaded filament protein gene BFSP2. Am. J. Hum. Genet. 66: 1432–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson R. V., Farrar N., Stewart K., Perveen R., Mihelec M., et al. , 2007. Characterization of a familial t(16;22) balanced translocation associated with congenital cataract leads to identification of a novel gene, TMEM114, expressed in the lens and disrupted by the translocation. Hum. Mutat. 28: 968–977. [DOI] [PubMed] [Google Scholar]
- Javadiyan S., Craig J. E., Souzeau E., Sharma S., Lower K. M., et al. , 2016. Recurrent mutation in the crystallin alpha A gene associated with inherited paediatric cataract. BMC Res. Notes 9: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannabiran C., Rogan P. K., Olmos L., Basti S., Rao G. N., et al. , 1998. Autosomal dominant zonular cataract with sutural opacities is associated with a splice mutation in the betaA3/A1-crystallin gene. Mol. Vis. 4: 21. [PubMed] [Google Scholar]
- Khan A. O., Aldahmesh M. A., Meyer B., 2007. Recessive congenital total cataract with microcornea and heterozygote carrier signs caused by a novel missense CRYAA mutation (R54C). Am. J. Ophthalmol. 144: 949–952. [DOI] [PubMed] [Google Scholar]
- Koressaar T., Remm M., 2007. Enhancements and modifications of primer design program Primer3. Bioinformatics 23: 1289–1291. [DOI] [PubMed] [Google Scholar]
- Kumar P., Henikoff S., Ng P. C., 2009. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4: 1073–1081. [DOI] [PubMed] [Google Scholar]
- Lachke S. A., Alkuraya F. S., Kneeland S. C., Ohn T., Aboukhalil A., et al. , 2011. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science 331: 1571–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wang K., 2017. InterVar: clinical interpretation of genetic variants by the 2015 ACMG-AMP guidelines. Am. J. Hum. Genet. 100: 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt M., Carrero-Valenzuela R., LaMorticella D. M., Schultz D. W., Mitchell T. N., et al. , 1997. Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human beta-crystallin gene CRYBB2. Hum. Mol. Genet. 6: 665–668. [DOI] [PubMed] [Google Scholar]
- Litt M., Kramer P., LaMorticella D. M., Murphey W., Lovrien E. W., et al. , 1998. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum. Mol. Genet. 7: 471–474. [DOI] [PubMed] [Google Scholar]
- Lorenz B., 2007. Genetische Untersuchungen bei kongenitaler Katarakt. Ophthalmologe 104: 559–565. [DOI] [PubMed] [Google Scholar]
- Ma A. S., Grigg J. R., Ho G., Prokudin I., Farnsworth E., et al. , 2016. Sporadic and familial congenital cataracts: mutational spectrum and new diagnoses using next-generation sequencing. Hum. Mutat. 37: 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y. Y., Funata N., Takahama S., Sugata Y., Yonekawa H., 2001. Two interactive genes responsible for a new inherited cataract (RCT) in the mouse. Mamm. Genome 12: 278–283. [DOI] [PubMed] [Google Scholar]
- McLeod J. L., Craig J., Gumley S., Roberts S., Kirkland M. A., 2002. Mutation spectrum in Australian pedigrees with hereditary hyperferritinaemia-cataract syndrome reveals novel and de novo mutations. Br. J. Haematol. 118: 1179–1182. [DOI] [PubMed] [Google Scholar]
- Nonnenmacher L., Langer T., Blessing H., Gabriel H., Buchwald H. J., et al. , 2011. Hereditary hyperferritinemia cataract syndrome: clinical, genetic, and laboratory findings in 5 families. Klin. Padiatr. 223: 346–351. [DOI] [PubMed] [Google Scholar]
- Pras E., Levy-Nissenbaum E., Bakhan T., Lahat H., Assia E., et al. , 2002. A missense mutation in the LIM2 gene is associated with autosomal recessive presenile cataract in an inbred Iraqi Jewish family. Am. J. Hum. Genet. 70: 1363–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras E., Raz J., Yahalom V., Frydman M., Garzozi H. J., et al. , 2004. A nonsense mutation in the glucosaminyl (N-acetyl) transferase 2 gene (GCNT2): association with autosomal recessive congenital cataracts. Invest. Ophthalmol. Vis. Sci. 45: 1940–1945. [DOI] [PubMed] [Google Scholar]
- Rahi J. S., Dezateux C., 2001. Measuring and interpreting the incidence of congenital ocular anomalies: lessons from a national study of congenital cataract in the UK. Invest. Ophthalmol. Vis. Sci. 42: 1444–1448. [PubMed] [Google Scholar]
- Ramachandran R. D., Perumalsamy V., Hejtmancik J. F., 2007. Autosomal recessive juvenile onset cataract associated with mutation in BFSP1. Hum. Genet. 121: 475–482. [DOI] [PubMed] [Google Scholar]
- Reches A., Yaron Y., Burdon K., Crystal-Shalit O., Kidron D., et al. , 2007. Prenatal detection of congenital bilateral cataract leading to the diagnosis of Nance-Horan syndrome in the extended family. Prenat. Diagn. 27: 662–664. [DOI] [PubMed] [Google Scholar]
- Reis L. M., Tyler R. C., Volkmann Kloss B. A., Schilter K. F., Levin A. V., et al. , 2012. PITX2 and FOXC1 spectrum of mutations in ocular syndromes. Eur. J. Hum. Genet. 20: 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin S. A., Yasmeen A., Yao W., Sergeev Y. V., Zhang Q., et al. , 2005. Mutations in betaB3-crystallin associated with autosomal recessive cataract in two Pakistani families. Invest. Ophthalmol. Vis. Sci. 46: 2100–2106. [DOI] [PubMed] [Google Scholar]
- Richards S., Aziz N., Bale S., Bick D., Das S., et al. , 2015. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17: 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana A., Waiswo M., 2011. The genetic and molecular basis of congenital cataract. Arq. Bras. Oftalmol. 74: 136–142. [DOI] [PubMed] [Google Scholar]
- Santana A., Waiswol M., Arcieri E. S., Cabral de Vasconcellos J. P., Barbosa de Melo M., 2009. Mutation analysis of CRYAA, CRYGC, and CRYGD associated with autosomal dominant congenital cataract in Brazilian families. Mol. Vis. 15: 793–800. [PMC free article] [PubMed] [Google Scholar]
- Santhiya S. T., Kumar G. S., Sudhakar P., Gupta N., Klopp N., et al. , 2010. Molecular analysis of cataract families in India: new mutations in the CRYBB2 and GJA3 genes and rare polymorphisms. Mol. Vis. 16: 1837–1847. [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Burdon K. P., Dave A., Jamieson R. V., Yaron Y., et al. , 2008. Novel causative mutations in patients with Nance-Horan syndrome and altered localization of the mutant NHS-A protein isoform. Mol. Vis. 14: 1856–1864. [PMC free article] [PubMed] [Google Scholar]
- Shiels A., Bennett T. M., Knopf H. L., Yamada K., Yoshiura K., et al. , 2007. CHMP4B, a novel gene for autosomal dominant cataracts linked to chromosome 20q. Am. J. Hum. Genet. 81: 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A., Bennett T. M., Knopf H. L., Maraini G., Li A., et al. , 2008. The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol. Vis. 14: 2042–2055. [PMC free article] [PubMed] [Google Scholar]
- Shiels A., Bennett T. M., Hejtmancik J. F., 2010. Cat-Map: putting cataract on the map. Mol. Vis. 16: 2007–2015. [PMC free article] [PubMed] [Google Scholar]
- Shiels A. H. J., 2007. Genetic origins of cataract. Arch. Ophthalmol. 125: 165–173. [DOI] [PubMed] [Google Scholar]
- Siggs O. M., Javadiyan S., Sharma S., Souzeau E., Lower K. M., et al. , 2017. Partial duplication of the CRYBB1-CRYBA4 locus is associated with autosomal dominant congenital cataract. Eur. J. Hum. Genet. 25: 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B. C., et al. , 2012. Primer3–new capabilities and interfaces. Nucleic Acids Res. 40: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esch H., Jansen A., Bauters M., Froyen G., Fryns J. P., 2007. Encephalopathy and bilateral cataract in a boy with an interstitial deletion of Xp22 comprising the CDKL5 and NHS genes. Am. J. Med. Genet. A. 143: 364–369. [DOI] [PubMed] [Google Scholar]
- Vanita V., Singh J. R., Singh D., Varon R., Sperling K., 2008. A mutation in GJA8 (p.P88Q) is associated with “balloon-like” cataract with Y-sutural opacities in a family of Indian origin. Mol. Vis. 17: 1171–1175. [PMC free article] [PubMed] [Google Scholar]
- Willoughby C. E., Shafiq A., Ferrini W., Chan L. L., Billingsley G., et al. , 2005. CRYBB1 mutation associated with congenital cataract and microcornea. Mol. Vis. 11: 587–593. [PubMed] [Google Scholar]
- Wirth M. G., Russell-Eggitt I. M., Craig J. E., Elder J. E., Mackey D. A., 2002. Aetiology of congenital and paediatric cataract in an Australian population. Br. J. Ophthalmol. 86: 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J., Yun J., Li Z. K., Xu C. T., Pan B. R., 2011. Epidemiology and molecular genetics of congenital cataracts. Int. J. Ophthalmol. 4: 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Zhou N., Hu S., Zhao L., Zhang C., et al. , 2010. A missense mutation in CRYBA4 associated with congenital cataract and microcornea. Mol. Vis. 16: 1019–1024. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Table S1 in File S1 contains a list of reported pediatric cataract genes selected for sequencing. PCR primers used to validate novel or rare coding mutations detected by next-generation sequencing are listed in Table S2 in File S1. Table S3 in File S1 contains systemic features of the five participants with syndromic pediatric cataract included in the study. Figure S1 in File S1 shows amplicons with <20 fold coverage. Figure S2, A–C in File S1) shows protein sequence alignments demonstrating the conservation of the altered amino acid in families with causative mutations.