Abstract
Study Design
ADAMTS5-deficient and WT mice were chronically exposed to tobacco smoke to investigate effects on intervertebral disc degeneration (IDD).
Objective
To demonstrate a role for ADAMTS5 in mediating tobacco smoking-induced IDD
Summary of Background Data
We previously demonstrated that chronic tobacco smoking causes IDD in mice due, in part, to proteolytic destruction of disc aggrecan. However, it was unknown which matrix proteinase(s) drive these detrimental effects.
Methods
Three-month old Wt (C57BL/6) and ADAMTS5-/- mice were chronically exposed to tobacco smoke (4 cigarettes/day, 5 day/week for 6 months). ADAMTS-mediated cleavage of disc aggrecan was analyzed by Western blot. Disc total glycosaminoglycan (GAG) content was assessed by dimethyl methylene blue assay and safranin O/fast green histology. Vertebral osteoporosity was measured by micro-computed tomography. Human nucleus pulposus (hNP) cell cultures were also exposed directly to tobacco smoke extract (TSE), a condensate containing the water-soluble compounds inhaled by smokers, to measure ADAMTS5 expression and ADAMTS-mediated cleavage of aggrecan. Activation of NF-κB, a family of transcription factors essential for modulating the cellular response to stress, was measured by immunofluorescence assay.
Results
Genetic depletion of ADAMTS5 prevented vertebral bone loss, substantially reduced loss of disc GAG content, and completely obviated ADAMTS-mediated proteolysis of disc aggrecan within its interglobular domain (IGD) in mice following exposure to tobacco smoke. hNP cell cultures exposed to TSE also resulted in upregulation of ADAMTS5 protein expression and a concomitant increase in ADAMTS-mediated cleavage within aggrecan IGD. Activation of NF-κB, known to be required for ADAMTS5 gene expression, was observed in both TSE-treated hNP cell cultures and disc tissue of tobacco smoke-exposed mice.
Discussion
The findings demonstrate that ADAMTS5 is the primary aggrecanase mediating smoking-induced disc aggrecanolysis and IDD. Mouse models of chronic tobacco smoking are important and useful for probing the mechanisms of disc aggrecan catabolism and IDD.
Introduction
Tobacco smoking and intervertebral disc degeneration (IDD)
IDD is responsible for many spine related disorders, including disabling back pain that causes both temporary and permanent disability, resulting in tremendous personal and societal health and economic burden1-4. A growing number of studies, both epidemiological and basic research, demonstrate tobacco smoking as a major risk factor of IDD and back pain5, 6. Tobacco smoking has been reported to exacerbate pre-existing IDD and increase the frequency of IDD and back pain incidence7, 8. Studies using animal models also demonstrate inflammatory and degenerative changes in the intervertebral discs of tobacco smoke-exposed animals6, 9. Hence tobacco smoking greatly impacts personal health through initiating and/or exacerbating degenerative disc disorders and back pain5.
Smoking-induced disc aggrecan catabolism
A hallmark of IDD is loss of proteoglycan (PG), a major structural matrix constituent vital for maintaining disc turgidity to counteract compressive force. Aggrecan is the major disc PG and endows the tissue with its unique capacity to bear load and resist compression10. PG loss is generally attributed to aggrecan catabolism via proteolysis, termed aggrecanolysis, especially under the conditions of genotoxic, oxidative and inflammatory stress10-12. Tobacco smoke, containing numerous genotoxic and oxidative agents, produces DNA mutations and damage in a variety of tissues13. Tobacco smoking also promotes systemic inflammation in many tissues, including the intervertebral discs in which increased interleukin-1β level has been found in smoke-exposed rats9. Using a mouse model of chronic human smoking6, we previously demonstrated disc aggrecanolysis mediated by the action of members of the matrix metalloproteinase (MMP) 14 and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) 15 families. Specifically we showed that chronic exposure of mice to tobacco smoke induces ADAMTS-mediated cleavage within the interglobular domain (IGD) of disc aggrecan6. The IGD consists of ∼150 amino acids residing between the two globular domains G1 and G2 at the N terminus of aggrecan. IGD cleavage is considered pathological as it releases the majority of the aggrecan, including the entire glycosaminoglycan-attachment region essential for counteracting compressive forces, from the cartilage matrix10.
ADAMTS in smoking-induced disc aggrecanolysis
In vivo, the majority of the proteolytic aggrecan IGD cleavage is mediated by the enzymatic activities of MMPs and ADAMTSs10. ADAMTS proteins belong to a family of zinc-dependent enzymes within the metzincin family of metalloproteinases16. ADAMTS proteins comprise an N-terminal prodomain, a catalytic domain, a disintegrin domain, one or more thrombospondin motifs, a cysteine-rich domain and a spacer domain of variable length17. ADAMTS4 and ADAMTS5 are generally considered as the major aggrecanases involved in cartilage erosion16. Moreover, transcription of both ADAMTS4 and ADAMTS5 is dependent on activation of NF-κB, a family of transcription factors that play a central role in cellular stress response18-20. Despite a large body of research demonstrating expression of ADAMTS4 and ADAMTS5 proteins and the presence of aggrecan fragments derived by aggrecanase action in degenerative cartilage and disc tissue, it is still unclear whether ADAMTS4 or ADAMTS5 is the primary aggrecanase that drives degeneration in human cartilage and intervertebral disc16, 21, 22. Hence, the goal of this study is to determine if ADAMTS5 is the primary mediator of smoking-induced disc aggrecanolysis. We focused on ADAMTS5 because recombinant human ADAMTS5 exhibits the highest aggrecanase activity among the different metalloproteinases23 and because mice deficient in ADAMTS5 are protected from cartilage destruction in arthritis models24.
Materials and Methods
Exposure to tobacco smoke
In vivo model
Three-month-old C57BL/6 mice and ADAMTS5-/- mice derived from a pure C57BL6 background (Adamts5tm1Dgen/J, Jackson laboratories, ME) were chronically exposed to tobacco smoke (4 cigarettes/day, 5 day/week for 6 months) and intervertebral disc tissue were isolated as previously described 25. Unexposed age- and sex- matched littermates kept in the same facility environment were used as controls. Nine mice, 4 females and 5 males, were used in each treatment condition. In vitro cell culture model. Human nucleus pulposus (hNP) cells were isolated from disc surgical specimen harvested from non-smoking patients as approved by the human subjects IRB (n=8; male:female= 4:4; mean age 48±5 years; mean Pfirrmann grade of 2.5± 0.2). First passage cultures of hNP cells grown in monolayer (F-12/D-MEM containing 10% FCS, 1% PS, and 25 mg/ml L-ascorbic acid under 5% O2) were treated with tobacco smoke extract (TSE) at 0.1 mg/ml concentrations for 3 days prior to being analyzed for various outcome measures described below. This treatment perturbed matrix homeostasis with minimal cell death26.
Assessment of disc total proteoglycans
Mouse disc proteoglycan content was assessed histologically by safranin O/fast green staining and quantitatively by 1,9-dimethylmethylene blue (DMMB) colorimetric assay as described6.
Immunohistochemistry
Paraffin embedded sections were used to probe for ADAMTS-generated neoeptitope NVTEGE as previously described6.
Immunofluorescence to measure smoking-induced NF-κB activation
Mouse disc tissue
Three month old NF-kB-eGFP reporter mice derived from C57BL6 genetic background were treated with and without tobacco smoke for one week (early NF-κB activation assessment) and six month (chronic NF-κB activation). The NF-kB-eGFP reporter mice contain a knock-in transgene expressing eGFP under the control of an NF-κB regulatory element27. In these mice, green fluorescence from eGFP expression indicates activation of the NF-κB signaling pathway. Lumbar discs were harvested, sectioned and analyzed for cells expressing eGFP by fluorescence microscopy as previously described.
Human NP cell cultures
hNP cells grown in monolayer on chamber slides were treated with and without 0.1 mg/ml concentrations of TSE for 3 days. NF-κB activation was assessed in these cells by nuclear translocation of NF-κB which was visualized by immunofluorescent labeling using an anti-p65 antibody (SC372, Santa Cruz Biotechnology, California). Nuclear translocation of p65, a subunit of NF-κB, was quantified by calculating the percentage of cells immunopositive for nuclear p65 staining. hNP cell cultures treated with 1ng/μl IL-1β were used as a positive control for NF-κB activation.
Western analysis
Detecting aggrecan fragments in mouse disc tissue
Entire lumbar discs removed en bloc were analyzed by immunoblot as previously described6. Antibodies (1:1000 dilution 1° Ab, 1:5000 dilution 2° Ab) raised against aggrecan G1 (Ab2194) or ADAMTS-generated neoepitope NITEGE (Ab1320). The anti-NITEGE neoepitope antibody cross-reacts with the mouse aggrecan NVTEGE neoepitope.
Detecting aggrecan fragments and ADAMTS5 in conditioned media of hNP cell cultures
Conditioned media from TSE-treated and untreated hNP cell cultures were concentrated 3-5X by centrifugal filtering (EMD Millipore UFC900308). Western blotting was performed using Tris-HEPES 4-20% gradient gels (Thermo Scientific 25204) with 1:1000 dilution of the 1° Ab and 1:10000 anti-rabbit goat 2° Ab conjugated with HRP (Thermo Scientific PI-31460). Rabbit polyclonal anti-aggrecan G1 primary antibody (Abcam ab36861) and anti-ADAMTS5 (Abcam ab41037) were used to detect aggrecan fragments and ADAMTS5 protein, respectively. Quantification was performed with densitometry analysis and local background subtraction using the ChemiDoc™ MP system and associated Image Lab 5.2.1 Software (BioRad, USA).
Statistical analysis
Values represent the averages from different experimental samples with 95% confidence intervals calculated to determine statistical significance at p< 0.05. The confidence intervals were calculated based on the t-distribution.
Results
Tobacco smoking induced disc ADAMTS5 protein expression
Smoking-induced disc aggrecanolysis can be catalyzed by several different aggrecanases, including ADAMTS1, ADAMTS4 and ADAMTS5. Given the demonstrated function of ADAMTS5 as a key aggrecanase in cartilage degradation, we measure ADAMTS5 protein expression by disc cells following exposure to TSE. Immunodetection was performed using an antibody that detects the 73 kDa mature form of secreted ADAMTS5 post-furin cleavage. Western analysis revealed the level of ADAMTS5 protein in the conditioned media of hNP cell cultures treated with TSE to be about twice the level of ADAMTS5 in untreated control (Fig. 1A). In addition, immunohistochemistry analysis showed a substantial increase in ADAMTS5 (Fig. 1B) protein levels in NP tissue sections of smoke-exposed mice compared to unexposed controls. These results demonstrated that chronic exposure to tobacco smoking upregulates disc ADAMTS5 expression.
Figure 1. Tobacco smoke upregulated disc cell ADAMTS5 protein expression.
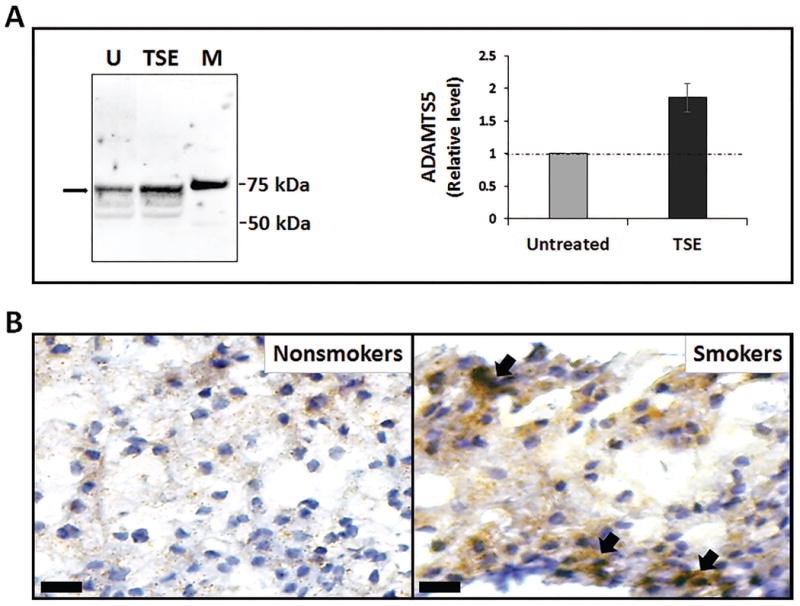
(A) A representative Western blot showing the 73kDa cleaved form of ADAMTS5 protein (arrow) in the conditioned media of untreated (U) and treated hNP cell cultures with TSE for three days. M, protein markers. Right graph shows quantitation of the 73kDa ADAMTS5 band by densitometry (n=4). (B) Immunohistochemical detection of ADAMTS5 (brown stain, arrows) in the nucleus pulposus tissue section of unexposed (non-smokers) and exposed (smokers) WT mice. The bars represent 20 μm.
Tobacco smoking activates disc NF- κB signaling
Induction of ADAMTS5 expression has been shown to be dependent on activation of NF-κB, a central signaling pathway cells utilize to respond to stress. There was an increase in the level of nuclear NF-κB following treatment with TSE, consistent with an increase in NF-κB transcriptional activity (Fig. 2A). To determine if the same phenomenon occurs in vivo, we used knock-in mice expressing eGFP under the control of an NF-κB regulatory element (NF-κB eGFP). In these mice, eGFP expression indicates activation of the NF- κB pathway. Using fluorescent microscopy, an increase in eGFP immunofluorescence was detected in the NP of mice after six month of smoke exposure compared to unexposed control (Fig. 2B), indicating increased NF-κB transcriptional activity in disc tissues of mice exposed to tobacco smoke.
Figure 2. Tobacco smoke activated disc NF-κB signaling.
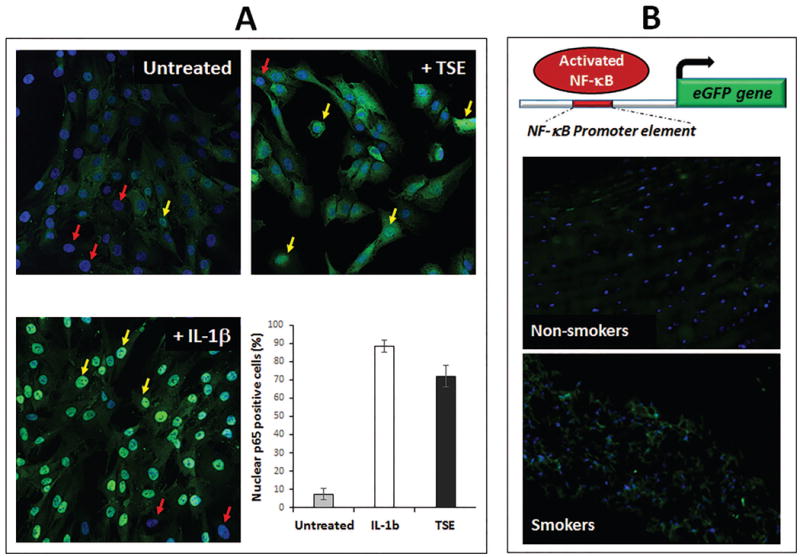
(A) Immunofluorescence revealed increased NF-κB activity, as indicated by an increase in the level of nuclear p65, a subunit of NF-κB, in hNP cell cultures following a fifteen minute treatment with TSE or IL-1β (positive control) compared to untreated control. Absence (red arrows) and presence (yellow arrows) of nuclear p65. (B) Increased NF-κB activation in disc tissue of mice exposed to tobacco smoke for six months. Top, a schematic showing the knock-in reporter mice expressing eGFP under the control of an NF-κB regulatory element (NF-κB eGFP). eGFP expression indicates NF-κB activation. Disc tissue sections from unexposed (non-smokers) or smoke-exposed (smokers) NF-κB eGFP reporter mice were imaged using fluorescent microscopy to detect eGFP expression (green). Nuclei were counter-stained with Hoechst (blue). Representative images of the nucleus pulposus region are shown.
Genetic deletion of ADAMTS5 abolished ADAMTS-mediated cleavage within the interglobular domain of aggrecan in discs of a mouse model of chronic human smoking
Tobacco smoke exposure induced ADAMTS-mediated cleavage of aggrecan IGD in both hNP cell culture model (Fig. 3A) and the intervertebral discs of the WT mice (Fig. 3B, lane 2 vs. lane 3). The increase in ADAMTS5 expression (Fig. 1) suggests a correlative, but not a causative relationship to the increase of these proteolytic cleaved aggrecan fragments. To further demonstrate that ADAMTS5 is the major aggrecanase mediating this cleavage, ADAMTS5-/- mice were used. Western blot was performed using anti-G1 (Fig. 3B, lanes 6-7) and anti-NVTEGE (Fig. 3B, lanes 1-5) antibodies that recognize the neo-epitope in aggrecan IGD fragment generated by the ADAMTS. Depletion of ADAMTS5 completely abolished ADAMTS-mediated cleavage of disc aggrecan IGD in mice. For example, the ADAMTS-generated aggrecan G1 fragments terminating in NVTEGE were detected in the discs of smoke-exposed WT mice (Fig. 3B, lane 3), but not in smoke-exposed ADAMTS5-/- mice (Fig. 3B, lane 5) or in unexposed control WT mice. These results were further confirmed by immunohistochemistry, where aggrecan fragments containing the NVTEGE neo-epitope were detected in high level in the discs of smoke-exposed WT mice, but at a reduced level in ADAMTS5-/- mice (Fig. 3C).
Figure 3. Genetic depletion of ADAMTS5 abrogated ADAMTS-mediated disc aggrecanolysis in mice induced by tobacco smoking.
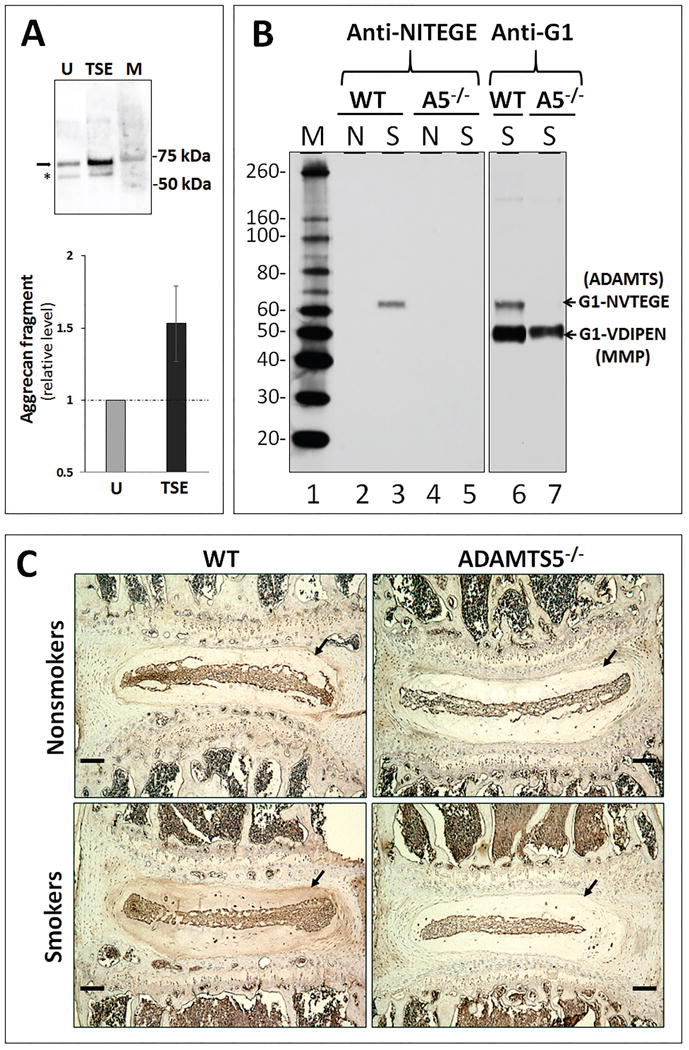
(A) A representative immunoblot detecting the aggrecan fragments generated by ADAMTS (arrow) and MMP (star) in the conditioned media of untreated (U) and TSE-treated hNP cell cultures for three days. M, protein markers. Anti aggrecan G1 antibodies were used. Bottom graph shows quantitation of the ∼70 kDa ADAMTS-generated aggrecan fragment by densitometry (average values ± stdev (n=4)). (B) Immunoblot analysis of G1 fragments bearing the ADAMTS-generated NVTEGE neoepitope were performed using the anti-G1 and anti-NITEGE antibodies. The anti-NITEGE neoepitope antibody cross-reacts with the NVTEGE neoepitope generated from mouse aggrecan. To control for loading, proteins extracted from 1 mg of disc tissue wet weight from unexposed (N = nonsmokers) and six month smoke-exposed (S = smokers) wildtype (WT) and ADAMTS5-deficient (A5-/-) mice were loaded per well. M=Protein size marker. (C) Immunohistochemical detection of aggrecan fragments using antibodies raised against the NITEGE neo-epitope. Arrow indicates nucleus pulposus sites of detection of positive signal (brown). The bars represent 100 μm.
Genetic deletion of ADAMTS5 blunted disc PG loss in a mouse model of chronic human smoking
WT mice chronically exposed to tobacco smoke for 6 months showed about a 40% loss of NP PG matrix content by the quantitative DMMB assay measuring sulfated GAG compared to unexposed WT control (Fig. 4B). In contrast, ADAMTS5-/- mice exposed to smoke for the same period of time demonstrated loss of only 20% NP GAG content compared to unexposed WT control (Fig. 4B). These results were consistent with safranin O staining of sulfated PGs, which revealed a noticeable reduction of PGs in the discs of WT mice exposed to tobacco smoke and a smaller reduction in discs of ADAMTS5-/- mice when compared to disc safranin O staining intensity from non-smoke exposed WT controls (Fig. 4A). These findings suggest that smoking-induced loss of disc PG content is mediated considerably by ADAMTS5.
Figure 4. Depletion of ADAMTS5 reduced disc proteoglycan loss in a mouse model of tobacco smoking.
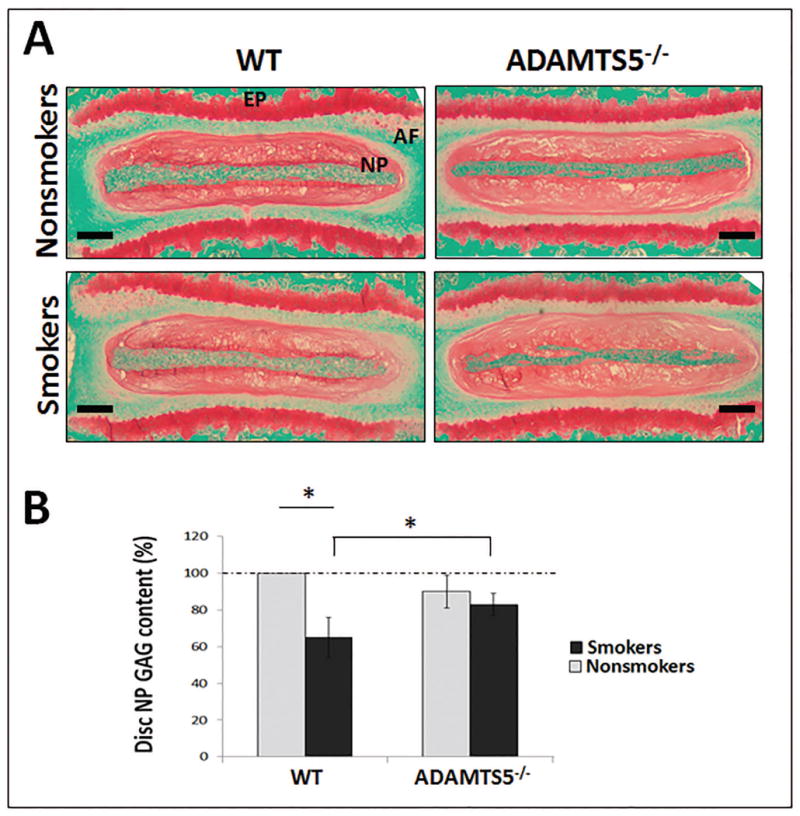
(A) Representative images of Safranin O/fast green stained discs (bar=250 μm) of WT and ADAMTS5-deficient mice treated with or without tobacco smoke for six months. Red stain intensity indicates the level of proteoglycan. Nucleus pulposus (NP), annulus fibrosis (AF), and cartilaginous endplate (EP) regions are indicated. (B) DMMB quantitative assay for total GAG content from NP tissue of smoke-exposed (smokers) WT and ADAMTS5-deficient mice and their respective untreated controls (nonsmokers). Average values ± stdev of eight exposed mice and eight unexposed controls are shown. * denotes p<0.05.
Discussion
Here we focused our efforts in determining if ADAMTS5 is the major aggrecanase responsible for mediating smoking-induced disc aggrecanolysis. Using both animal and disc cell culture model systems, we demonstrated that exposure to tobacco smoke elevated protein expression of ADAMTS5 and proteolytic cleavage within the disc aggrecan IGD. Importantly, we showed that mice genetically deficient in ADAMTS5 were largely protected from disc aggrecanolysis and loss of PG content following chronic exposure to tobacco smoke for a period of six months. Chronic smoke exposure also did not negatively impact vertebral osteoporosity in ADAMTS5-/- mice, in contrast to that of WT mice (unpublished data). Further studies are required to understand biologically how deficiency in ADAMTS5, a cartilaginous aggrecanase, protects smoking-induced vertebral bone loss. Nonetheless, our data suggest that ADAMTS5 is the prominent aggrecanase that mediates smoking-induced disc aggrecanolysis, and that the absence of ADAMTS5 reduces degeneration of the intervertebral discs and vertebral bone in the mouse model of chronic human tobacco smokers.
Smoking induced-disc PG loss
It is important to note that ADAMTS5-deficient mice were not completely protected from smoking-induced disc matrix PG loss. This is due to several possible reasons. Tobacco smoking causes disc PG loss by perturbing disc matrix homeostasis not only by enhancing matrix catabolism but also suppressing matrix anabolism6. New PG synthesis, a marker of matrix anabolism that is measured by the incorporation of 35S-sulfate, has previously been documented to be suppressed in discs of smoke-exposed WT mice6. In the current study, we showed that new PG synthesis in discs of ADAMTS5-/- mice was also suppressed to a similar extent seen in smoke-exposed WT mice, as expected (data not shown). Moreover, tobacco smoking induces not just ADAMTS but also MMP-generated aggrecan fragments from proteolytic cleavage within the disc aggrecan IGD. ADAMTS5 deficiency abrogates only ADAMTS-mediated, but not MMP-mediated aggrecanolysis in the discs of our mouse smokers. Tobacco smoke increases expression of several MMPs, including MMP1, MMP3 and MMP12 in disc cells26. Compared with other MMP members, MMP12 is by far the most efficient at cleaving within the aggrecan IGD23. Moreover, MMP12 is recently reported as an indicator of IDD that is co-expressed with fibrotic markers 28. Tobacco smoking also strongly induces expression of MMP-12, macrophage elastase, in activated macrophages in lung tissues of smokers 25, 29. However MMP12-/- mice did not have a significant reduction in MMP-mediated aggrecan cleavage in our mouse model of tobacco smoking (unpublished data). Thus further investigation is needed to identify the major MMP(s) responsible for mediating smoking-induced aggrecanolysis in disc tissue. Together, our findings suggest that ADAMTS-5 depletion protects smoke-exposed mice from only the ADAMTS-mediated but not MMP-mediated destruction of disc aggrecan; ADAMTS-5 depletion also does not protects smoke-exposed mice from loss of disc PG anabolism.
Mechanism of smoking-induced disc ADAMTS5 expression
The presence of ADAMTS5 has been documented in human intervertebral disc at early and advanced stages of disc degeneration30, 31. Disc ADAMTS5 is also found in various disc cell culture and animal models, especially under degenerative and inflammatory conditions20, 32-34. Upregulation of ADAMTS5 mRNA and protein expression is shown to be dependent on NF-κB signaling in disc cells in the presence of the pro-inflammatory cytokine IL-1β or TNF-α20, 33. NF-κB is a central cell-signaling component in mediating cellular response to damage, stress and inflammation35. It is well know that tobacco smoke contains potent pro-inflammatory agents that cause damage and inflammation in many different body tissues in smokers9, 13, 36. In this current study, we demonstrated elevated inflammation in disc tissue, as evidenced in enhanced activation of NF-κB signaling, in mice chronically exposed to direct inhalation of tobacco smoke (Fig. 2B). This finding is consistent with the reported increase in disc IL-1β in a rat model of passive smokers9. Moreover, TSE treatment of our disc cell cultures also elicited strong inflammatory response, evidenced by the pronounced NF-κB activation (Fig. 2A) and COX-2/PGE2 expression26. In both disc cell culture and mouse models of tobacco smokers, disc NF-κB activation coincides with ADAMTS5 expression. Taken together, these observations suggest that tobacco smoking induces inflammation in disc tissue leading to NF-κB-dependent expression of ADAMTS5.
Disc cellular senescence as a possible source of ADAMTS5 production in smokers
Cellular senescence, defined by growth arrest, is formed as a result of accumulation of unrepaired DNA damage37. Tobacco smoking promotes disc cellular senescence6, likely due to the action of the many mutagenic compounds in tobacco smoke. Stress-induced senescent cells are also inflammatory in nature through the activation of NF-κB as they overproduce and secrete many pro-inflammatory cytokines and matrix metalloproteinases, termed senescence-associated secretory phenotype (SASP)37, 38. Increased ADAMTS5 expression was positively correlated to the level of P16INK4A, a marker of cellular senescence, in human degenerative discs22. We also found that senescent human disc cell cultures produced increased levels of ADAMTS5 and aggrecan fragments (unpublished data). Thus senescent disc cells established from smoke exposure could be the cell source that produces ADAMTS5 in the discs of smokers.
In summary, we demonstrated that disc ADAMTS5 protein expression and aggrecanolysis were upregulated by tobacco smoke in both animal and cell culture models. We also demonstrated that mice genetically deficient in ADAMTS5 are protected from ADAMTS-mediated cleavage within the disc aggrecan IGD following chronic exposure to tobacco smoke for six months. Furthermore, genetic depletion of ADAMTS5 greatly reduces smoking-induced loss of disc PG and vertebral bone. Taken together, these findings demonstrate that ADAMTS5 is the primary aggrecanase mediating smoking-induced disc aggrecanolysis and IDD and thus represents a potential therapeutic target for mitigating IDD severity in smokers when smoking cessation fails. For example, ADAMTS5 silencing by interference RNA has been reported to reduce injury-induced IDD in rabbits 39. Because ADAMTS5 expression is NF-κB dependent, NF-κB signaling also offers an alternative therapeutic target to treat IDD. Indeed, NF-κB inhibition mitigates disc degenerative changes in cell culture and animal models35, 40, 41. Other targets exist which can be further elucidated using the mouse models of chronic tobacco smoking to probe the mechanisms of IDD.
Acknowledgments
The authors thank Yeqing Geng (Western analysis), Lisa Lamplugh (Immunohistochemistry) Qing Dong (Histology), Arvydas Usas (mCT), Vivian Li (NF-κB nuclear translocation scoring) for their technical assistance. This work was supported in part by the PO1 AG043376 to PDR and LJN, FAMRI grant CIA 123062 to PD, Albert B. Ferguson Jr., MD, Orthopaedic Fund of the Pittsburgh Foundation and NIH grant R01 AG044376-01 to NV.
Footnotes
Device Status/Drug Statement: The Manuscript submitted does not contain information about medical devices/drugs
References
- 1.Cheung KM. The relationship between disc degeneration, low back pain, and human pain genetics. Spine J. 10:958–60. doi: 10.1016/j.spinee.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Spine: Low Back and Neck Pain The Burden of Musculoskeletal Diseases in the United States. National Center for Health Statistics: National Health and Nutrition Examination Survey Data, 1998-2004. 2011 [Google Scholar]
- 3.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–5. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 4.Livshits G, et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. 2011;70:1740–5. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogt MT, Hanscom B, Lauerman WC, Kang JD. Influence of smoking on the health status of spinal patients: the National Spine Network database. Spine (Phila Pa 1976) 2002;27:313–9. doi: 10.1097/00007632-200202010-00022. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, et al. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis Cartilage. 2012;20(8):896–905. doi: 10.1016/j.joca.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glassman SD, et al. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine (Phila Pa 1976) 2000;25:2608–15. doi: 10.1097/00007632-200010150-00011. [DOI] [PubMed] [Google Scholar]
- 8.Battie MC, et al. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine (Phila Pa 1976) 1991;16:1015–21. [PubMed] [Google Scholar]
- 9.Ogawa T, et al. Alteration of gene expression in intervertebral disc degeneration of passive cigarette- smoking rats: separate quantitation in separated nucleus pulposus and annulus fibrosus. Pathobiology. 2005;72:146–51. doi: 10.1159/000084118. [DOI] [PubMed] [Google Scholar]
- 10.Roughley PJ. The structure and function of cartilage proteoglycans. Eur Cell Mater. 2006;12:92–101. doi: 10.22203/ecm.v012a11. [DOI] [PubMed] [Google Scholar]
- 11.Vo N, et al. Accelerated aging of intervertebral discs in a mouse model of progeria. J Orthop Res. 2010;28:1600–7. doi: 10.1002/jor.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeMarini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res. 2004;567:447–74. doi: 10.1016/j.mrrev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine (Phila Pa 1976) 1998;23:1612–26. doi: 10.1097/00007632-199807150-00021. [DOI] [PubMed] [Google Scholar]
- 15.Tang BL. ADAMTS: a novel family of extracellular matrix proteases. Int J Biochem Cell Biol. 2001;33:33–44. doi: 10.1016/s1357-2725(00)00061-3. [DOI] [PubMed] [Google Scholar]
- 16.Fosang AJ, Rogerson FM, East CJ, Stanton H. ADAMTS-5: the story so far. Eur Cell Mater. 2008;15:11–26. doi: 10.22203/ecm.v015a02. [DOI] [PubMed] [Google Scholar]
- 17.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seguin CA, Pilliar RM, Roughley PJ, Kandel RA. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine. 2005;30:1940–8. doi: 10.1097/01.brs.0000176188.40263.f9. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi H, et al. Transcriptional induction of ADAMTS5 protein by nuclear factor-kappaB (NF-kappaB) family member RelA/p65 in chondrocytes during osteoarthritis development. J Biol Chem. 2013;288:28620–9. doi: 10.1074/jbc.M113.452169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, et al. TNF-alpha and IL-1beta promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–49. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sztrolovics R, Alini M, Roughley PJ, Mort JS. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326(Pt 1):235–41. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9:R45. doi: 10.1186/ar2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durigova M, Nagase H, Mort JS, Roughley PJ. MMPs are less efficient than ADAMTS5 in cleaving aggrecan core protein. Matrix Biol. 30:145–53. doi: 10.1016/j.matbio.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glasson SS, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 25.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–4. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 26.Vo N, et al. Differential effects of nicotine and tobacco smoke condensate on human annulus fibrosus cell metabolism. J Orthop Res. 2011;29:1585–91. doi: 10.1002/jor.21417. [DOI] [PubMed] [Google Scholar]
- 27.Magness ST, et al. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-kappa B activation using a novel gene-targeted enhanced GFP reporter gene mouse. J Immunol. 2004;173:1561–70. doi: 10.4049/jimmunol.173.3.1561. [DOI] [PubMed] [Google Scholar]
- 28.Lv FJ, et al. Matrix metalloproteinase 12 is an indicator of intervertebral disc degeneration co-expressed with fibrotic markers. Osteoarthritis Cartilage. 2016;24:1826–36. doi: 10.1016/j.joca.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Houghton AM, et al. Elastin fragments drive disease progression in a murine model of emphysema. J Clin Invest. 2006;116:753–9. doi: 10.1172/JCI25617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao CQ, et al. ADAMTS-5 and intervertebral disc degeneration: the results of tissue immunohistochemistry and in vitro cell culture. J Orthop Res. 2011;29:718–25. doi: 10.1002/jor.21285. [DOI] [PubMed] [Google Scholar]
- 31.Patel KP, et al. Aggrecanases and aggrecanase-generated fragments in the human intervertebral disc at early and advanced stages of disc degeneration. Spine (Phila Pa 1976) 2007;32:2596–603. doi: 10.1097/BRS.0b013e318158cb85. [DOI] [PubMed] [Google Scholar]
- 32.Yurube T, et al. Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration. Arthritis Res Ther. 2012;14:R51. doi: 10.1186/ar3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Hutton WC, Yoon ST. Bone morphogenetic protein-7 antagonizes tumor necrosis factor-alpha-induced activation of nuclear factor kappaB and up-regulation of the ADAMTS, leading to decreased degradation of disc matrix macromolecules aggrecan and collagen II. Spine J. 2014;14:505–12. doi: 10.1016/j.spinee.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Vo NV, et al. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13:331–41. doi: 10.1016/j.spinee.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wuertz K, Vo N, Kletsas D, Boos N. Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-kappaB and MAP kinases. Eur Cell Mater. 2012;23:103–19. doi: 10.22203/ecm.v023a08. discussion 119-20. [DOI] [PubMed] [Google Scholar]
- 36.Churg A, Dai J, Tai H, Xie C, Wright JL. Tumor necrosis factor-alpha is central to acute cigarette smoke-induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med. 2002;166:849–54. doi: 10.1164/rccm.200202-097OC. [DOI] [PubMed] [Google Scholar]
- 37.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–9. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011;30:1536–48. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seki S, et al. Effect of small interference RNA (siRNA) for ADAMTS5 on intervertebral disc degeneration in the rabbit anular needle-puncture model. Arthritis Res Ther. 2009;11:R166. doi: 10.1186/ar2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasto LA, et al. ISSLS prize winner: inhibition of NF-kappaB activity ameliorates age-associated disc degeneration in a mouse model of accelerated aging. Spine (Phila Pa 1976) 2012;37:1819–25. doi: 10.1097/BRS.0b013e31824ee8f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, et al. Prolyl-4-hydroxylase domain protein 2 controls NF-kappaB/p65 transactivation and enhances the catabolic effects of inflammatory cytokines on cells of the nucleus pulposus. J Biol Chem. 2015;290:7195–207. doi: 10.1074/jbc.M114.611483. [DOI] [PMC free article] [PubMed] [Google Scholar]


