Abstract
Ethanol exposure at the time of burn injury is a major contributor to post-burn pathogenesis. Many of the adverse effects associated with ethanol and burn injury are linked to an impaired intestinal barrier. The combined insult causes intestinal inflammation, resulting in tissue damage, altered tight junction expression, and increased intestinal permeability. microRNAs play a critical role in maintaining intestinal homeostasis including intestinal inflammation and barrier function. Specifically, miR-150 regulates inflammatory mediators which can contribute to gut barrier disruption. The present study examined whether ethanol and burn injury alters expression of microRNA processing enzymes (Drosha, Dicer, and Argonaute-2) and miR-150 in the small intestine. Male mice were gavaged with ethanol (~2.9g/kg) 4 hours prior to receiving a ~12.5% total body surface area full thickness burn. One or three days after injury, mice were euthanized and small intestinal epithelial cells (IECs) were isolated and analyzed for expression of microRNA biogenesis components and miR-150. Dicer mRNA and protein levels were not changed following the combined insult. Drosha and Argonaute-2 mRNA and protein levels were significantly reduced in IECs one day after injury; which accompanied reduced miR-150 expression. To further determine the role of miR-150 in intestinal inflammation, young adult mouse colonocytes were transfected with a miR-150 plasmid and stimulated with LPS (100ng/mL). miR-150 overexpression significantly reduced IL-6 and KC protein levels compared to vector control cells challenged with LPS. These results suggest that altered microRNA biogenesis and associated decrease in miR-150 likely contributes to increased intestinal inflammation following ethanol and burn injury.
Keywords: Alcohol, miRs, microRNA processing, inflammation, gut, trauma
Introduction
Nearly 500,000 burn injuries are reported each year in the United States resulting in 40,000 hospitalizations(1). Approximately, 50% of these injuries occur under the influence of alcohol (ethanol) (2–6). Studies have shown that ethanol exposure at the time of burn injury further confounds the post-burn pathogenesis by delaying wound healing, resulting in longer hospitalization, and increasing susceptibility to infections(4, 7, 8). Furthermore, ethanol intoxication at the time of burn injury increases the risk of sepsis and multiple organ failure (8, 9). Additional findings suggest that patients who ingested ethanol prior to burn injury had higher mortality from smaller burn injuries (2, 3). Several lines of evidence suggest a role of gut barrier dysfunction in these co-morbidities associated with ethanol and burn injury (10–16).
Recent findings have demonstrated that the gut barrier disruption following ethanol and burn injury is widely associated with excess inflammation (13, 16–18). These studies suggest that increases in intestinal inflammatory mediators such as IL-18, IL-6 or other chemokines can directly or via recruitment of neutrophils cause intestinal tissue damage and alter tight junction protein expression (13, 16, 17, 19–21). Many studies have shown a role for microRNAs in regulating tissue inflammation following ethanol exposure or tissue injury (22, 23). However, whether microRNAs play a role following ethanol and burn injury remains unknown.
MicroRNAs are small noncoding RNA sequences, which regulate gene expression at the post-transcriptional level (24–27). Biogenesis of microRNAs occurs in several steps, starting with transcription by RNA polymerase II forming primary microRNA (pri-miRNA), which is then cleaved by Drosha (an RNase III enzyme) resulting in a precursor miRNA (pre-miRNA). The pre-miRNA is exported from the nucleus by exportin-5, where it is cleaved by Dicer (24, 25). Cleavage by Dicer results in a duplex miRNA complex containing both the guide and passenger strand. The guide strand is loaded onto an Argonaute (Ago) protein forming the miRNA induced silencing complex (miRISC); while the passenger stand is usually degraded. The guide miRNA uses partial base pairing to guide miRISC to its target mRNA. Binding of miRISC to the target mRNA allows for miRNA mediated gene regulation (24, 25, 28).
Numerous studies have illustrated the importance of microRNAs in maintenance of the intestinal barrier (29–31). Thus, altered expression of microRNAs could negatively affect the intestinal barrier. Changes in microRNAs expression as a result of ethanol and burn injury could potentially alter the levels of pro-inflammatory cytokines, which have been associated with excessive tissue damage and altered tight junction expression (13, 16, 19–21). Specifically, miR-150 is predicted to target the inflammatory mediator IL-18 and is down-regulated in sepsis patients and following exposure of cells to bacterial lipopolysaccharide (LPS) in vitro(32, 33). Furthermore, Liu et al demonstrated miR-150 overexpression reduces inflammatory mediators (TNF-α, IL-1β, and IL-6) in monocytes (34).
We examined whether ethanol and burn injury modulates miR-150 expression and microRNA biogenesis in intestinal epithelial cells (IECs). We hypothesized that microRNA expression and biogenesis would be diminished following ethanol and burn injury. Our data suggest that ethanol and burn injury diminishes Drosha and Ago-2 expression. Furthermore, ethanol combined with burn injury reduces expression of miR-150. In vitro, overexpression of miR-150 illustrates that the microRNA is particularly important in regulation of inflammatory mediators. Taken together, these results demonstrate that ethanol and burn injury negatively affects microRNA biogenesis and miR-150 expression which influences levels of pro-inflammatory mediators and these observations provide a mechanism behind the elevated inflammatory response following ethanol and burn injury.
Materials and Methods
Mouse model of acute ethanol intoxication and burn injury
Adult male C57BL/6 mice (22–25g) were purchased from Charles River Laboratories (Wilmington, MA, USA). Mice were randomly divided into four groups: sham vehicle; sham ethanol; burn vehicle and burn ethanol. Mice were gavaged with 0.4ml of 25% ethanol in water (~2.9g/Kg) or water (vehicle). Four hours following the gavage, mice were anesthetized by intraperitoneal injection with ketamine and xylazine (80 mg/Kg and 1.25 mg/Kg, respectively). The mice were transferred to a template fabricated to expose ~12.5% of the total body surface area. For burn injury, mice were immersed in 85–90°C water bath for 7–8 sec. Mice were dried and resuscitated with an intraperitoneal injection of 1.0 ml physiological saline and returned to their cages and given water and food ad libitum (18). All animal procedures were carried out in accordance with the Institutional Animal Care and Use Committee at Loyola University Chicago Health Sciences Division.
Small Intestinal Epithelial Cell Isolation & RNA isolation
Days one and three after injury, mice were euthanized and small intestines were harvested and washed in ice cold Phosphate-buffered saline (PBS) containing a cocktail of Gentamycin (50mg/ml) (Hyclone, Logan, UT) and 1% penicillin-streptomycin (Corning, Manassas, VA). As described by Weigmann et al (35), the small intestines were cut into small pieces and incubated in a pre-digestion solution (Hank’s Balanced Salt Solution, 5% heat-inactivated fetal bovine serum (Life Technologies,Carlsbad, CA), 1% penicillin-streptomycin (Corning, Manassas, VA), 1% HEPES, 0.5% Gentamycin (Hyclone, Logan, UT), 5mM ethylenediaminetetraacetic acid and 1mM dithiothreitol (Sigma Aldrich, St. Louis, MO) and incubated at 37 °C for 20 minutes. Following incubation, the supernatant was passed through a 100 μm strainer to collect epithelial cells. The incubation in the pre-digestion solution was performed twice to maximize cell count. The collected cells were centrifuged for 10 minutes at 4°C at 1500 RPM and washed in 1x PBS. Isolated small intestinal epithelial cells (IECs) were lysed and processed for large and small RNA extraction using MirVana miRNA isolation Kit (Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. A nanodrop spectrophotometer (Thermo Scientific, Bannockburn, IL) was used to determine RNA concentration.
Determination of microRNA biogenesis components (Drosha, Dicer and Argonaute-2) mRNA expression
Large RNA was used to make cDNA using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies,Carlsbad, CA). Expression of Drosha, Dicer and Argonaute-2 (Ago-2) was assessed by qRT-PCR using their respective primers (Life Technologies,Carlsbad, CA). GAPDH was used as the endogenous control for qRT-PCR experiments. The target genes Ct cycle values were normalized to GAPDH Ct values. Data were calculated using the ΔΔCT method and expressed relative to the average of sham vehicle group (6).
Determination of microRNA biogenesis components (Drosha, Dicer and Argonaute-2) protein expression
To determine protein levels of microRNA biogenesis components (Drosha, Dicer and Argonaute-2) IECs were lysed and the lysates were analyzed by SDS-PAGE and were transferred to either PVDF or nitrocellulose membranes. The membrane was blocked for 1 hour at room temperature with 5% BSA in TBS-T (0.05% Tween 20 in TBS). Following this, the membrane was incubated with a desired antibody (e.g., anti-Dicer, Santa Cruz Biotechnology, Santa Cruz, CA; anti-Drosha and anti-Argonaute-2, Cell Signaling Technology, Danvers, MA) overnight at 4°C. Membranes were washed with TBS-T and incubated with secondary antibody conjugated with horseradish peroxidase for one hour. After the incubation in the secondary antibody, the membrane was washed five times for five minutes in TBS-T and one time for 10 minutes in TBS. Following the final wash the membranes were probed using Western Lightning™ Chemiluminescence Reagent Plus (PerkinElmer, Norwalk, CT). The membrane was visualized using a ChemiDoc System.
Assessment of miR-150 expression
Enriched small RNAs were used for cDNA synthesis using miScript II RT Kit (Qiagen,Valencia, CA). miR-150 expression was examined by qRT-PCR using Qiagen miScript Primer Assays. Snord68 was used as an endogenous control for microRNA qRT-PCR experiments. miR-150 Ct cycle values were normalized to snord68 Ct values. Data were calculated using the ΔΔCT method and expressed relative to the average of sham vehicle group.
miR-150 overexpression and assessment of IL-6 and KC
To establish the role of miR-150 on intestinal inflammation, 6 × 105 young adult mouse colonocytes (YAMCs) were seeded in RPMI 1640 containing 2mM glutamine, 1% Antibiotic-Antimycotic Solution (Hyclone, Logan, UT), 1% ITS+ Premix (Corning, Manassas, VA), and 5% fetal bovine serum (Life Technologies,Carlsbad, CA). One day after plating, the cells were transfected with 250ng of mir-150 expression plasmid (miR-150 plasmid) or PCMVMIR empty vector control (vector) from Origene (Rockville, MD) and Lipofectamine 2000 from Invitrogen (Carlsbad, CA). The cells incubated for 48 hours in the Lipofectamine 2000/DNA mixture at 37°C. Following the incubation, the cells were washed with PBS and then treated with lipopolysaccharide (LPS) (100ng/ml) for 6 hours at 37°C. After the 6 hour of LPS treatment, both the supernatant and cells were collected. The cells were lysed and used for RNA isolation; total and enriched RNA were used for cDNA synthesis and subsequent qRT-PCR (miR-150, IL-6 and KC). Snord68 was used as an endogenous control for miR-150 qRT-PCR; while β-actin was used as an endogenous control for IL-6 and KC qRT-PCR. IL-6 (BD Biosciences, Bedford, MA) and KC (R&D Systems; Minneapolis, MN) levels in the supernatant were determined by enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions. Transfection experiments were performed in 3 individual experiments and each experiment was carried out in duplicate. Due to variability between IL-6 and KC protein levels on different days; IL-6 and KC values were normalized to vector LPS.
Statistical analysis
The results are presented as mean ± standard error of the mean (SEM). The data was analyzed using One-Way analysis of variance (ANOVA) Tukey-Kramer Multiple Comparisons Test or Student’s t test (GraphPad Prism6 La Jolla, CA, USA). A p<0.05 was considered statistically significant.
Results
Drosha mRNA and protein levels are significantly reduced in IECs following ethanol and burn injury
To determine whether ethanol and burn injury modulates expression of microRNA biogenesis components, we examined expression of Drosha. The first step in microRNA maturation is cleavage of pre-miRNA by Drosha in the nucleus (24). Drosha expression was significantly diminished (32%) day one following ethanol and burn injury in IECs (p=0.009) (Figure 1A). Drosha expression however, was normalized by day three following ethanol combined with burn injury compared to sham injured animals (Figure 1B). Consistent with our qRT-PCR data, Drosha protein levels were significantly reduced (52%) in IECs day one following the combined insult compared to shams (Figure 1C).
Figure 1. Drosha mRNA and protein levels are significantly reduced in IECs following ethanol and burn injury.
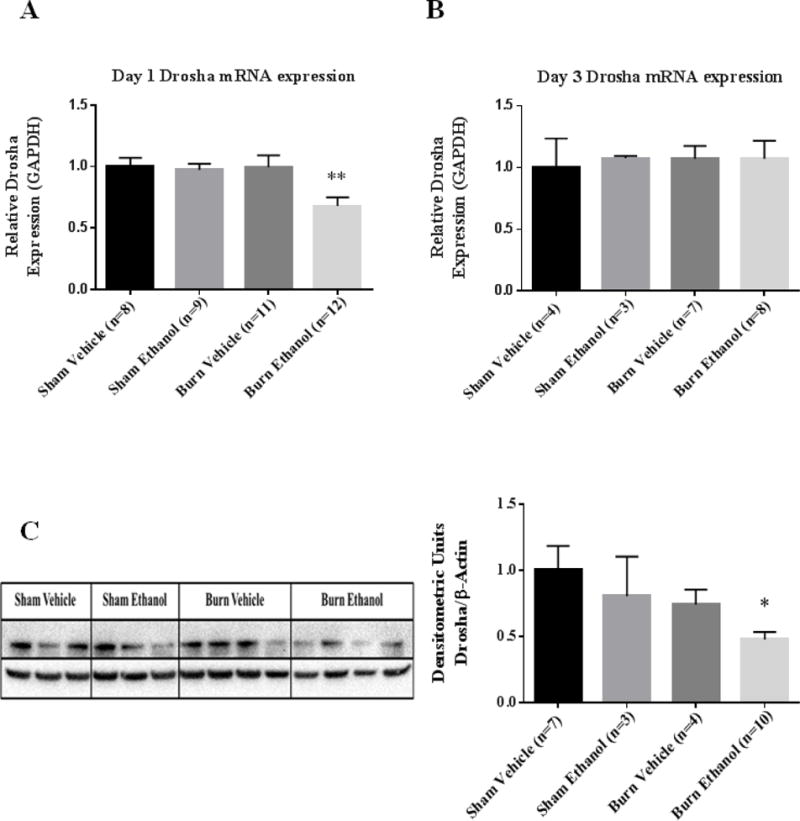
IECs were used to examine Drosha expression day one (A) and day three (B) following ethanol and burn injury. Values were calculated using a ΔΔCT method and normalized to sham vehicle animals. GAPDH was used as an endogenous control. (C) Homogenates from IECs harvested one day after ethanol and burn injury were used to examine Drosha protein levels. Densitometry measurements for Drosha are given as a ratio of Drosha density to β-actin, normalized to sham vehicle animals and the values are shown as means+ standard error of the means of duplicate experiments. *p < 0.05, **p < 0.01 by One-Way ANOVA compared to sham vehicle.
Dicer mRNA and protein levels are not changed in IECs following ethanol and burn injury
Cytoplasmic cleavage of the pre-miR by Dicer is the next maturation step in microRNA biogenesis (24). There were no changes in Dicer gene expression in IECs on day one or three following the combined insult compared to animals in the sham vehicle groups (Figure 2A–B). Additionally, Dicer protein levels were not changed day one following ethanol and burn injury in IECs compared to sham injured animals (Figure 2C).
Figure 2. Dicer mRNA and protein levels are not altered in IECs following ethanol and burn injury.
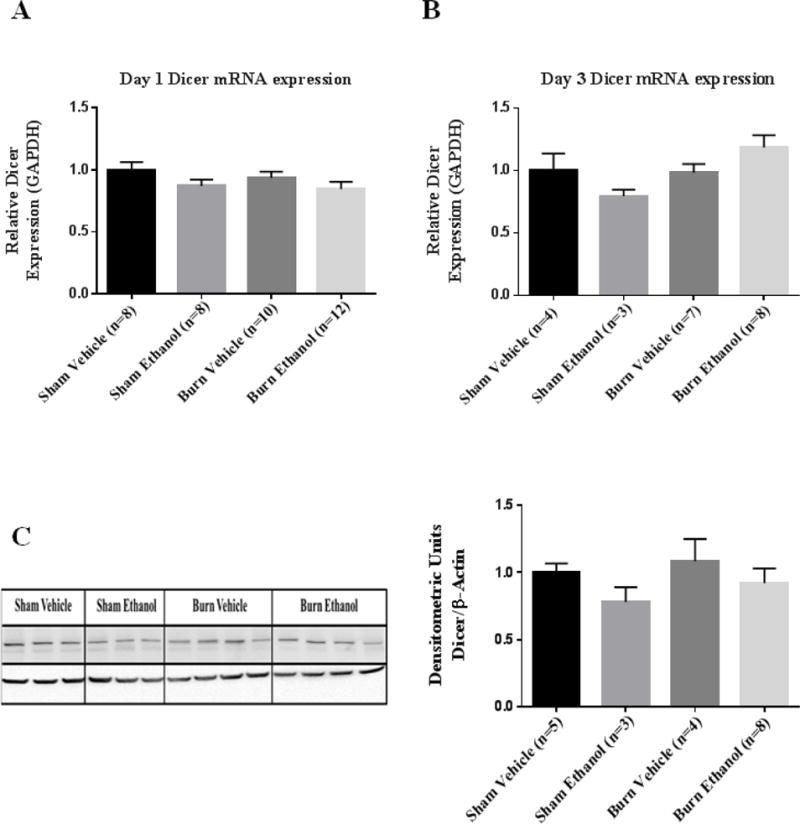
IECs were used to examine Dicer expression day one (A) and day three (B) following ethanol and burn injury. Values were calculated using a ΔΔCT method and normalized to sham vehicle animals. GAPDH was used as an endogenous control. (C) Homogenates from IECs harvested one day after ethanol and burn injury were used to examine Dicer protein levels. Densitometry measurements for Dicer are given as a ratio of Dicer density to β-actin, normalized to sham vehicle animals and the values are shown as are means+ standard error of the means of duplicate experiments.
Ago-2 mRNA and protein levels are significantly reduced in IECs following ethanol and burn injury
Next, we examined whether the combined insult affects Ago-2 expression. Ago-2 is the core component of the miRISC complex which protects the single stranded mature microRNA from cleavage by ribonucleases (36, 37). Therefore, altered expression of Ago-2 could influence microRNA levels. There was a significant decrease (22%) in Argonaute-2 expression day one following the combined insult in IECs compared to shams (p=0.002) (Figure 3A). There was no change in Ago-2 expression in IECs day three following ethanol and burn injury compared to sham injured animals (Figure 3B). Furthermore, there was a 49% reduction in Ago-2 protein levels day one following the combined insult compared to sham vehicle group (Figure 3C).
Figure 3. Ago-2 mRNA and protein levels are significantly reduced in IECs following ethanol and burn injury.
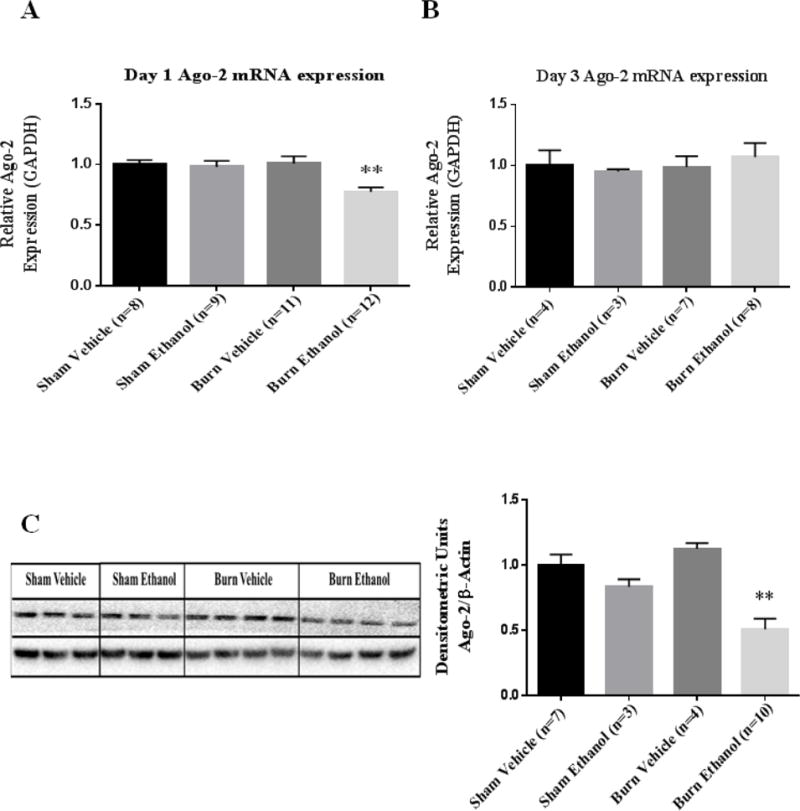
IECs were used to examine Ago-2 expression day one (A) and day three (B) following ethanol and burn injury. Values were calculated using a ΔΔCT method and normalized to sham vehicle animals. GAPDH was used as an endogenous control. (C) Homogenates from IECs harvested one day after ethanol and burn injury were used to examine Ago-2 protein levels. Densitometry measurements for Ago-2 are given as a ratio of Ago-2 density to β-actin, normalized to sham vehicle animals and the values are shown as means + standard error of the means of duplicate experiments. **p < 0.01 by One-Way ANOVA compared to sham vehicle.
miRNA expression in IECs following ethanol and burn injury
Next, we sought to determine whether the observed reduction in microRNA processing enzymes (Drosha and Ago-2) resulted in a reduction in microRNA expression. We chose to examine expression of miR-150 due its observed involvement in inflammation (33, 34, 38), which is a major adverse effect following ethanol and burn injury (18). miR-150 expression was significantly reduced by 64% (p=0.008) following the combined insult compared to sham vehicle mice (Figure 4A). There was a trend of increased expression of miR-150 on day three following the combined insult compared to sham vehicle, however this was not found to be significantly different (Figure 4B).
Figure 4. miRNA expression IECs days one and three following ethanol and burn injury.
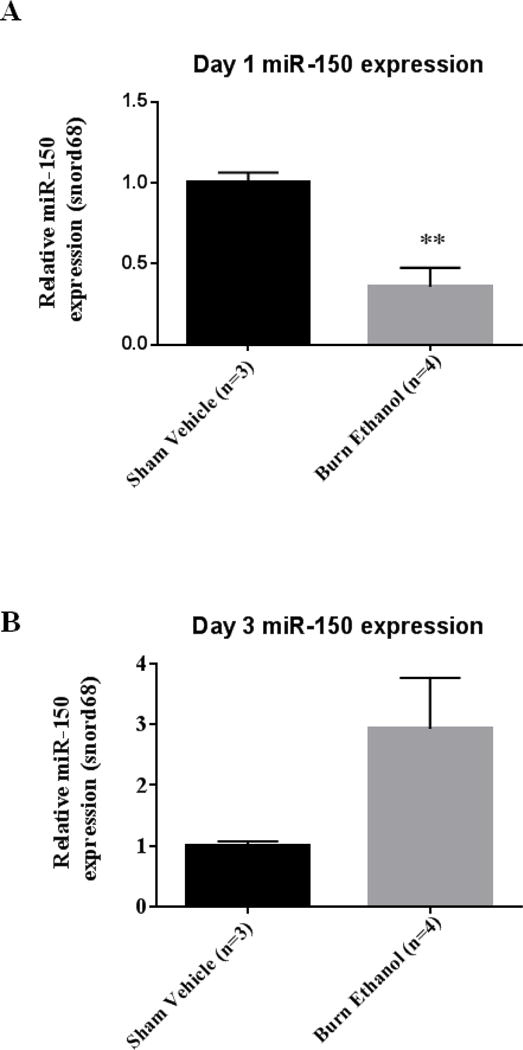
miR-150 expression was analyzed by qRT-PCR from IECs day one (A) and day three (B) following the combined insult. Values were calculated using a ΔΔCT method and normalized to sham vehicle animals. Snord68 was used as an endogenous control. Data shown are means +standard error of the means. **p < 0.01 by student’s t-test compared to sham vehicle.
miR-150 regulates the levels of pro-inflammatory mediators IL-6 and KC
To further investigate the role of miR-150 in increased inflammation after ethanol and burn injury we used an in vitro approach in which young adult mouse colonocytes were transfected with a miR-150 plasmid for 48 hours prior to a 6 hour LPS (100ng/ml) treatment (Figure 5A). We observed that the 48-hour transfection significantly increased miR-150 expression in cells transfected with the miR-150 plasmid compared to cells transfected with the empty vector (Figure 5B). Treatment of cells containing the empty vector with LPS resulted in a significant increase in (12-fold) IL-6 and (48-fold) KC expression. Overexpression of miR-150 did not affect IL-6 or KC mRNA expression (Figure 5C and 5E). However, IL-6 protein levels were significantly reduced following transfection with miR-150 compared to vector LPS treated cells (Figure 5D). Furthermore, we observed a significant reduction in KC in cells transfected with the miR-150 plasmid and challenged with LPS compared to vector LPS cells (Figure 5F).
Figure 5. miR-150 regulates levels of pro-inflammatory mediators IL-6 and KC.
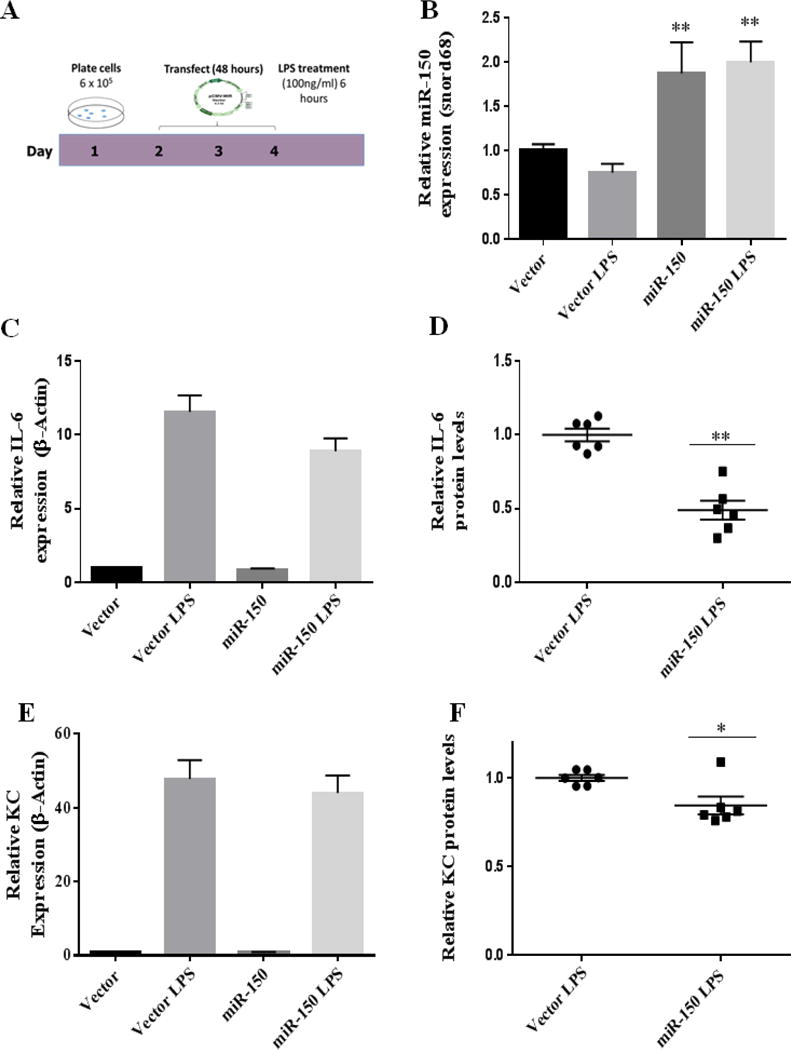
A.) Schematic of time course of YAMCs transfection experiment: YAMCs were transiently transfected with a miR-150 plasmid, 48 hours later the cells were treated for 6 hour with LPS (100ng/ml). B.) Expression of miR-150 following transfection and LPS challenge. Values were calculated using a ΔΔCT method and normalized to cells containing the empty vector. Snord68 was used as an endogenous control. RNA isolated from YAMCs was used to examine IL-6 (C) and KC (E) expression. Values were calculated using a ΔΔCT method and normalized to cells containing the empty vector. β-actin was used as an endogenous control. Secreted IL-6 (D) and KC (F) was examined by ELISA; results were normalized to vector LPS (n = 3 individual experiments performed in duplicate). *p < 0.05, **p < 0.01 by One-Way ANOVA or student’s t-test compared to vector or vector LPS.
Discussion
In this study, we examined whether ethanol and burn injury modulates microRNA expression and biogenesis. We observed decreased expression in mRNA and protein levels of microRNA biogenesis components (Drosha and Ago-2) day one following ethanol and burn injury in IECs. This was accompanied with a reduction in miR-150 expression in IECs day one following ethanol and burn injury. Using an in vitro overexpression approach, our findings further suggest that the decrease in miR-150 could potentially contribute to increased intestinal inflammation. These data suggest that altered microRNA expression and biogenesis components following ethanol and burn injury could result in increased intestinal inflammation.
Aberrant microRNA expression has been observed in patients with sepsis (32, 33, 39); which is a major adverse effect following ethanol and burn injury (8, 9). Vasilescu et al confirmed by qRT-PCR that miR-150 levels are significantly diminished in plasma from sepsis patients. Furthermore, they found that levels of TNF-α, IL-10 and IL-18 were elevated in these patients. These inflammatory mediators are predicted targets of miR-150. The group proposed using the plasma level ratio of miR-150 and IL-18 as a predictor of sepsis severity (33). microRNAs are promiscuous; each microRNA has the ability to regulate hundreds of different genes based on the complementary binding of the microRNA to its target (27). Therefore, it is quite possible and likely true that miR-150 have targets in addition to the targets listed here. Furthermore, we cannot discount that disruption in microRNA biogenesis likely reduces expression of many microRNAs; therefore, more microRNAs maybe in play in the pathology associated with ethanol and burn injury.
One major observation following ethanol and burn injury is increased levels of pro-inflammatory mediators (IL-6, KC, IL-18) day one following injury, which are normalized to sham values day three following injury(18, 40). These elevated pro-inflammatory mediators within the intestine bed are shown to contribute to intestine tissue damage and barrier disruption following ethanol and burn injury (13, 16). These studies correlate well with the present data as we observed a decrease in miR-150 which accompanied altered microRNA biogenesis day one following the combined insult of ethanol and burn injury. Furthermore, microRNAs and microRNA biogenesis expression is normalized to sham values day three following injury. Interestingly, miR-150 which had reduced expression day one following injury; has been shown to have an inverse relationships between their expression and levels of inflammatory mediators (32–34, 38, 39, 41, 42). These findings are in agreement with our in vitro study. We observed that overexpression of miR-150 significantly reduces levels of IL-6 (50%) and KC (20%) following stimulation with LPS. Therefore, reduced miR-150 levels observed following ethanol and burn injury likely contributes to the increased levels of pro-inflammatory mediators. One limitation of the current study is that the in vitro transfection of miR-150 was done in colonocytes and the in vivo studies were carried out in small intestinal epithelial cells. While there is a possibility that small and large intestinal epithelial cells respond differently to a stimulus such as LPS, a recent study from our laboratory suggests a similar trend in inflammation in both the small and large intestines (colon) except that the inflammatory response after injury was relatively of a higher magnitude in the large intestine (40). Based on this observation, we believe that colonocytes could be used for in vitro studies to address the role of miR-150. Together these observations suggests that overexpression of miR-150 can cause a decrease in colonocytes release of IL-6 and KC. While, these findings remain to be confirmed in epithelial cells from the small intestine, it is likely that small intestinal epithelial cells will follow a similar trend.
MicroRNAs regulate key components of the intestinal barrier function including the expression of tight junction proteins, inflammatory mediators, apoptosis and proliferation (29, 31, 34, 38, 43–46). Environmental factors (e.g. injury, ethanol, and disease) can impair microRNA expression and biogenesis, which may adversely affect the components of the intestinal barrier (31, 43, 47–50). McKenna et al. utilized Dicer1loxP/loxP, Villin–Cre mutant mice which lack the obligatory microRNA processing enzyme in the small and large intestinal epithelium. The ablation of Dicer-1 altered intestinal morphology and number of goblet cells. Similarly, Dicer-1 deficiency increased number of apoptotic cells and intestinal inflammation, while decreasing differentiation. Ablation of Dicer-1 led to tight junction mislocalization (Claudin-7) and disruption of protein levels (Claudin-4) which coincided with diminished barrier integrity (51). These data clearly show the indispensable role that microRNAs play in intestinal barrier maintenance. Gaulke et al have shown that environmental factors (SIV infection) also can impact microRNA biogenesis components (Dicer-1 and Argonaute-2) contributing to SIV mediated enteropathy (47). Furthermore, impairment of microRNA biogenesis and increased levels of microRNA degradation machinery resulted in decreased microRNA expression. Although the present study did not observe any changes in Dicer-1 expression, these studies illustrate the importance of microRNAs and their processing enzymes for normal intestine function. Therefore, the reduction of Drosha and Argonaute-2 following the combined insult is likely to influence normal intestinal homeostasis. Although, microRNA biogenesis has been linked to microRNA expression levels, we cannot discount the effects of microRNA degradation in reduced expression of microRNAs.
Taken together, these data suggest that ethanol and burn injury decreases the expression of microRNA biogenesis components and miR-150 in IECs. Such a decrease in miR-150 can lead to an increase in intestinal inflammation including increased IL-6 and KC which in turn can contribute to intestinal barrier disruption observed after ethanol and burn injury. Future studies will examine whether restoration of miR-150 or microRNA biogenesis improves gut barrier function following ethanol and burn injury.
Highlights.
Ethanol and burn injury decreases microRNA biogenesis components Drosha and Argonaute-2 in small intestine.
Ethanol and burn injury results in diminished expression of miR-150 in the small intestine.
In Vitro overexpression of miR-150 in young adult mouse colonocytes suppresses IL-6 and KC suggesting that microRNA-150 may have a role in inflammation observed following ethanol and burn injury.
Acknowledgments
This study was supported by NIH R01AA015731 (MAC), T32AA013527 (MAC) and F31 AA024367 (AMH).
Abbreviations
- Ago-2
Argonaute-2
- IECs
Intestinal epithelial cells
- LPS
lipopolysaccharide
- miR
microRNA
- miRISC
miRNA induced silencing complex
- PBS
Phosphate-buffered saline
- YAMCs
young adult mouse colonocytes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burn incidence and treatment in the United States: 2013 fact sheet. 2013 [Google Scholar]
- 2.Jones JD, Barber B, Engrav L, Heimbach D. Alcohol use and burn injury. The Journal of Burn Care & Rehabilitation. 1991;12:148–152. doi: 10.1097/00004630-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Haum A, Perbix W, Hack HJ, Stark GB, Spilker G, Doehn M. Alcohol and drug abuse in burn injuries. Burns : Journal of the International Society for Burn Injuries. 1995;21:194–199. doi: 10.1016/0305-4179(95)80008-c. [DOI] [PubMed] [Google Scholar]
- 4.McGill V, Kowal-Vern A, Fisher SG, Kahn S, Gamelli RL. The impact of substance use on mortality and morbidity from thermal injury. The Journal of Trauma. 1995;38:931–934. doi: 10.1097/00005373-199506000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Hammer AM, Rendon JL, Choudhry MA. Intestine immune homeostasis after alcohol and burn injury. Shock (Augusta, Ga.) 2015;43:540–548. doi: 10.1097/SHK.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammer AM, Khan OM, Morris NL, Li X, Movtchan V, Cannon AR, Choudhry MA. The effects of alcohol intoxication and burn injury on the expression of claudins and mucins in the small and large intestines. Shock (Augusta, Ga.) 2015 doi: 10.1097/SHK.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maier RV. Ethanol abuse and the trauma patient. Surgical Infections. 2001;2:133–41. doi: 10.1089/109629601750469456. ; discussion 141–4. [DOI] [PubMed] [Google Scholar]
- 8.Silver GM, Albright JM, Schermer CR, Halerz M, Conrad P, Ackerman PD, Lau L, Emanuele MA, Kovacs EJ, Gamelli RL. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. Journal of Burn Care & Research : Official Publication of the American Burn Association. 2008;29:784–789. doi: 10.1097/BCR.0b013e31818481bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messingham KA, Faunce DE, Kovacs EJ. Alcohol, injury, and cellular immunity. Alcohol (Fayetteville, N.Y.) 2002;28:137–149. doi: 10.1016/s0741-8329(02)00278-1. [DOI] [PubMed] [Google Scholar]
- 10.Gosain A, Gamelli RL. Role of the gastrointestinal tract in burn sepsis. The Journal of Burn Care & Rehabilitation. 2005;26:85–91. doi: 10.1097/01.bcr.0000150212.21651.79. [DOI] [PubMed] [Google Scholar]
- 11.Costantini TW, Loomis WH, Putnam JG, Drusinsky D, Deree J, Choi S, Wolf P, Baird A, Eliceiri B, Bansal V, Coimbra R. Burn-induced gut barrier injury is attenuated by phosphodiesterase inhibition: Effects on tight junction structural proteins. Shock (Augusta, Ga.) 2009;31:416–422. doi: 10.1097/SHK.0b013e3181863080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. The Journal of Burn Care & Rehabilitation. 2005;26:383–391. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- 13.Zahs A, Bird MD, Ramirez L, Choudhry MA, Kovacs EJ. Anti-IL-6 antibody treatment but not IL-6 knockout improves intestinal barrier function and reduces inflammation after binge ethanol exposure and burn injury. Shock (Augusta, Ga.) 2013;39:373–379. doi: 10.1097/SHK.0b013e318289d6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rendon JL, Li X, Akhtar S, Choudhry MA. Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock (Augusta, Ga.) 2013;39:11–18. doi: 10.1097/SHK.0b013e3182749f96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: A cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol (Fayetteville, N.Y.) 2004;33:199–208. doi: 10.1016/j.alcohol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Akhtar S, Choudhry MA. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochimica Et Biophysica Acta. 2012;1822:196–203. doi: 10.1016/j.bbadis.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2012;303:G1356–64. doi: 10.1152/ajpgi.00526.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Akhtar S, Kovacs EJ, Gamelli RL, Choudhry MA. Inflammatory response in multiple organs in a mouse model of acute alcohol intoxication and burn injury. Journal of Burn Care & Research : Official Publication of the American Burn Association. 2011;32:489–497. doi: 10.1097/BCR.0b013e3182223c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahs A, Bird MD, Ramirez L, Turner JR, Choudhry MA, Kovacs EJ. Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2012;303:G705–12. doi: 10.1152/ajpgi.00157.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akhtar S, Choudhry MA. Gut inflammation in response to injury: Potential target for therapeutic intervention. Recent Patents on Anti-Infective Drug Discovery. 2011;6:206–215. doi: 10.2174/157489111796887837. [DOI] [PubMed] [Google Scholar]
- 21.Akhtar S, Li X, Kovacs EJ, Gamelli RL, Choudhry MA. Interleukin-18 delays neutrophil apoptosis following alcohol intoxication and burn injury. Molecular Medicine (Cambridge, Mass.) 2011;17:88–94. doi: 10.2119/molmed.2010.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippai D, Bala S, Catalano D, Kodys K, Szabo G. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcoholism, Clinical and Experimental Research. 2014;38:2217–2224. doi: 10.1111/acer.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi C, Liang Y, Yang J, Xia Y, Chen H, Han H, Yang Y, Wu W, Gao R, Qin H. MicroRNA-21 knockout improve the survival rate in DSS induced fatal colitis through protecting against inflammation and tissue injury. PloS One. 2013;8:e66814. doi: 10.1371/journal.pone.0066814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finnegan EF, Pasquinelli AE. MicroRNA biogenesis: Regulating the regulators. Critical Reviews in Biochemistry and Molecular Biology. 2013;48:51–68. doi: 10.3109/10409238.2012.738643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He L, Hannon GJ. MicroRNAs: Small RNAs with a big role in gene regulation. Nature Reviews. Genetics. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 26.Ha M, Kim VN. Regulation of microRNA biogenesis. Nature Reviews. Molecular Cell Biology. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 27.Du T, Zamore PD. Beginning to understand microRNA function. Cell Research. 2007;17:661–663. doi: 10.1038/cr.2007.67. [DOI] [PubMed] [Google Scholar]
- 28.Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and function. Thrombosis and Haemostasis. 2012;107:605–610. doi: 10.1160/TH11-12-0836. [DOI] [PubMed] [Google Scholar]
- 29.Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323–1333. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biton M, Levin A, Slyper M, Alkalay I, Horwitz E, Mor H, Kredo-Russo S, Avnit-Sagi T, Cojocaru G, Zreik F, Bentwich Z, Poy MN, Artis D, Walker MD, Hornstein E, Pikarsky E, Ben-Neriah Y. Epithelial microRNAs regulate gut mucosal immunity via epithelium-T cell crosstalk. Nature Immunology. 2011;12:239–246. doi: 10.1038/ni.1994. [DOI] [PubMed] [Google Scholar]
- 31.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcoholism, Clinical and Experimental Research. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 32.How CK, Hou SK, Shih HC, Huang MS, Chiou SH, Lee CH, Juan CC. Expression profile of MicroRNAs in gram-negative bacterial sepsis. Shock (Augusta, Ga.) 2015;43:121–127. doi: 10.1097/SHK.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 33.Vasilescu C, Rossi S, Shimizu M, Tudor S, Veronese A, Ferracin M, Nicoloso MS, Barbarotto E, Popa M, Stanciulea O, Fernandez MH, Tulbure D, Bueso-Ramos CE, Negrini M, Calin GA. MicroRNA fingerprints identify miR-150 as a plasma prognostic marker in patients with sepsis. PloS One. 2009;4:e7405. doi: 10.1371/journal.pone.0007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z, Ye P, Wang S, Wu J, Sun Y, Zhang A, Ren L, Cheng C, Huang X, Wang K, Deng P, Wu C, Yue Z, Xia J. MicroRNA-150 protects the heart from injury by inhibiting monocyte accumulation in a mouse model of acute myocardial infarction. Circulation. Cardiovascular Genetics. 2015;8:11–20. doi: 10.1161/CIRCGENETICS.114.000598. [DOI] [PubMed] [Google Scholar]
- 35.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nature Protocols. 2007;2:2307–2311. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- 36.Martinez NJ, Gregory RI. Argonaute2 expression is post-transcriptionally coupled to microRNA abundance. RNA (New York, N.Y.) 2013;19:605–612. doi: 10.1261/rna.036434.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Qin YW, Brewer G, Jing Q. MicroRNA degradation and turnover: Regulating the regulators. Wiley Interdisciplinary Reviews. RNA. 2012;3:593–600. doi: 10.1002/wrna.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manoharan P, Basford JE, Pilcher-Roberts R, Neumann J, Hui DY, Lingrel JB. Reduced levels of microRNAs miR-124a and miR-150 are associated with increased proinflammatory mediator expression in kruppel-like factor 2 (KLF2)-deficient macrophages. The Journal of Biological Chemistry. 2014;289:31638–31646. doi: 10.1074/jbc.M114.579763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roderburg C, Luedde M, Vargas Cardenas D, Vucur M, Scholten D, Frey N, Koch A, Trautwein C, Tacke F, Luedde T. Circulating microRNA-150 serum levels predict survival in patients with critical illness and sepsis. PloS One. 2013;8:e54612. doi: 10.1371/journal.pone.0054612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris NL, Li X, Earley ZM, Choudhry MA. Regional variation in expression of pro-inflammatory mediators in the intestine following a combined insult of alcohol and burn injury. Alcohol (Fayetteville, N.Y.) 2015;49:507–511. doi: 10.1016/j.alcohol.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong D, Piao YS, Yamashita S, Oshima H, Oguma K, Fushida S, Fujimura T, Minamoto T, Seno H, Yamada Y, Satou K, Ushijima T, Ishikawa TO, Oshima M. Inflammation-induced repression of tumor suppressor miR-7 in gastric tumor cells. Oncogene. 2012;31:3949–3960. doi: 10.1038/onc.2011.558. [DOI] [PubMed] [Google Scholar]
- 42.Zhao XD, Lu YY, Guo H, Xie HH, He LJ, Shen GF, Zhou JF, Li T, Hu SJ, Zhou L, Han YN, Liang SL, Wang X, Wu KC, Shi YQ, Nie YZ, Fan DM. MicroRNA-7/NF-kappaB signaling regulatory feedback circuit regulates gastric carcinogenesis. The Journal of Cell Biology. 2015;210:613–627. doi: 10.1083/jcb.201501073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775–784. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi J, Qiao Y, Wang P, Li S, Zhao W, Gao C. microRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-kappaB1 in murine macrophages. FEBS Letters. 2012;586:1201–1207. doi: 10.1016/j.febslet.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Shen J, Cheng J, Fan X. MicroRNA-21 regulates intestinal epithelial tight junction permeability. Cell Biochemistry and Function. 2015;33:235–240. doi: 10.1002/cbf.3109. [DOI] [PubMed] [Google Scholar]
- 46.Balakrishnan A, Stearns AT, Park PJ, Dreyfuss JM, Ashley SW, Rhoads DB, Tavakkolizadeh A. Upregulation of proapoptotic microRNA mir-125a after massive small bowel resection in rats. Annals of Surgery. 2012;255:747–753. doi: 10.1097/SLA.0b013e31824b485a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaulke CA, Porter M, Han YH, Sankaran-Walters S, Grishina I, George MD, Dang AT, Ding SW, Jiang G, Korf I, Dandekar S. Intestinal epithelial barrier disruption through altered mucosal microRNA expression in human immunodeficiency virus and simian immunodeficiency virus infections. Journal of Virology. 2014;88:6268–6280. doi: 10.1128/JVI.00097-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nata T, Fujiya M, Ueno N, Moriichi K, Konishi H, Tanabe H, Ohtake T, Ikuta K, Kohgo Y. MicroRNA-146b improves intestinal injury in mouse colitis by activating nuclear factor-kappaB and improving epithelial barrier function. The Journal of Gene Medicine. 2013;15:249–260. doi: 10.1002/jgm.2717. [DOI] [PubMed] [Google Scholar]
- 49.Chassin C, Hempel C, Stockinger S, Dupont A, Kubler JF, Wedemeyer J, Vandewalle A, Hornef MW. MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Molecular Medicine. 2012;4:1308–1319. doi: 10.1002/emmm.201201298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scharl M, Rogler G. Microbial sensing by the intestinal epithelium in the pathogenesis of inflammatory bowel disease. International Journal of Inflammation. 2010;2010:671258. doi: 10.4061/2010/671258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKenna LB, Schug J, Vourekas A, McKenna JB, Bramswig NC, Friedman JR, Kaestner KH. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 2010;139:1654–64, 1664.e1. doi: 10.1053/j.gastro.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


