Abstract
Aims
1) To identify trajectories of cannabis use across adolescence, 2) to measure the influence of cannabis use characteristics on functional connectivity of the nucleus accumbens (NAcc), and 3) to assess whether patterns of functional connectivity related to cannabis use are associated with psychosocial functioning 2 years later.
Design
The Pitt Mother & Child Project (PMCP) is a prospective, longitudinal study of male youth at high risk for psychopathology based on family income and gender.
Setting
Participants were recruited between age 6–17 months from the Women, Infants, and Children Nutritional Supplement program (WIC) in the Pittsburgh, Pennsylvania area.
Participants
N=158 PMCP young men contributed fMRI and substance use data at age 20.
Measurements
Latent class growth analysis was used to determine trajectories of cannabis use frequency from age 14–19. Psychophysiological interaction (PPI) analysis was used to measure functional connectivity between the NAcc and prefrontal cortex (PFC). Adolescent cannabis use trajectory, recent frequency of use, and age of initiation were considered as developmental factors. We also tested whether functional connectivity was associated with depressive symptoms, anhedonia, and educational attainment at age 22.
Findings
We identified three distinct trajectories of adolescent cannabis use, characterized by stable high, escalating, or stable low use. Cannabis use trajectory group had a significant effect on NAcc functional connectivity to the medial PFC (F=11.32, Z=4.04, pFWE-corr=.000). The escalating trajectory group displayed a pattern of negative NAcc-mPFC connectivity that was linked to higher levels of depressive symptoms (r=−.17, p=.041), anhedonia (r=−.19, p=.028), and lower educational attainment (t=−2.77, p=.006) at age 22.
Conclusions
Pattern of cannabis use frequency across adolescence in US youth could have consequences for mood symptoms and educational attainment in early adulthood via altered function in neural reward circuitry.
Introduction
Cannabis use is a critical and growing public health problem among adolescents and young adults. In the United States, adolescent cannabis use has increased since the mid-2000s and is consistently higher than nicotine use, with 44.5% of 12th graders reporting lifetime use of cannabis in 2016, and 6.0% reporting daily use in the last 30 days (1). Although a majority of adolescents consider cannabis benign (2), adolescent use is associated with poor educational attainment (3) and mental health problems in adulthood (4). Risk for use, use-related problems, and poor psychosocial outcomes are particularly high among male adolescents who live in urban, low-SES settings (5). However, the mechanisms linking adolescent cannabis use to poor psychosocial functioning in early adulthood remain poorly understood.
Adolescent cannabis use has been linked with a constellation of negative outcomes that share motivational impairments, including depression (4), anhedonia – difficulty with motivation for or enjoyment of rewarding experiences (6), and low academic achievement, including poor school performance and early school drop-out (3). All of these factors can have profound negative impacts on adult functioning in vocational and social domains (3, 7), suggesting that altered motivational processing may be a core feature of cannabis abuse that can lead to a variety of problematic outcomes (8).
One potential mechanism for the effects of adolescent cannabis use on motivation and later psychosocial functioning is via alterations in neural reward circuitry, which is consistently implicated in the initiation and maintenance of substance use, and the transition to addiction (for review, please see 9). Critical regions in this circuit include the striatum, the PFC, including the anterior cingulate cortex (ACC), and the thalamus (9–11), which collectively integrate information about reward, motivation, and long-term goals to guide adaptive behavior (12). Previous literature has reported altered morphology and neural response to reward in these regions among adolescent and adult cannabis users (13).
Cannabis exposure is hypothesized to impact brain structure and function via downregulation and desensitization of neural endocannabinoid receptors (14), which are densely expressed throughout the cortex and basal ganglia (15). These receptors regulate neuronal migration, axonal pathfinding, and the generation of glial cells, indicating that the endocannabinoid system has an important influence on synaptic pruning and myelination (16). Therefore, cannabis exposure may influence reward circuit function by altering the connectivity of key regions. Congruently, several studies have reported altered connectivity in association with cannabis use, including aberrant resting-state functional connectivity (rsFC) of the default mode, insula, and lateral visual networks among cannabis users (17, 18), reduced interhemispheric rsFC among cannabis dependent adolescents (19), and heightened rsFC of the medial frontal gyrus in association with cannabis use among high risk youth (20). Furthermore, increased functional connectivity between the PFC and occipitoparietal cortex has been reported in association with increased cognitive control demands among heavy cannabis users relative to controls (21), and cannabis dependent individuals display heightened functional connectivity between the PFC and basal ganglia during successful inhibition, relative to non-dependent cannabis users (22).
Functional connectivity of the NAcc, the locus of initial drug response and a hub of the reward circuit (9), could be influenced by use over time (23). Indeed, acute cannabis exposure has been found to decrease rsFC between the NAcc and PFC, ACC, striatum, and thalamus (24), and reduced rsFC between the NAcc and PFC has been reported among patients with schizophrenia and cannabis use disorder relative to controls (25). In particular, one previous report demonstrated that regular cannabis users display greater functional connectivity between the NAcc and the ACC, striatum, and cerebellum to cannabis cues relative to neutral cues (26). However, to our knowledge no previous studies have examined functional connectivity of these regions in relation to non-drug reward or among young adults with various patterns of cannabis use over time.
Because the onset and peak of cannabis use occur during adolescence when widespread neurodevelopmental changes in reward systems are ongoing, adolescence represents a vulnerable window for drug effects (27). Cannabis use follows a clear developmental pattern: initiation typically occurs by the mid-teens (28) and peaks before age 25 (28), and use disorders are most prevalent between ages 15–20 (29). Animal studies have demonstrated that cannabis exposure in adolescence is associated with more severe and persistent behavioral changes relative to adult exposure, particularly decreased reward motivation and greater depressive-like behaviors (30), but neural and psychosocial effects in human adolescents are not well understood. In particular, the influence of cannabis use on NAcc connectivity could be moderated by 1) trajectories of exposure (consistent vs. escalating vs. decreasing use) across development; 2) recent frequency of use; and/or 3) developmental timing of use (i.e. early- vs. late-onset use).
Distinct trajectories of cannabis use could have different consequences for the development of neural circuitry and implications for functioning. For example, sharply increasing exposure across adolescence could have pernicious effects on reward circuitry because of challenges to adaptation during a window of rapid development (31). Indeed, there is evidence to suggest that an escalating pattern of cannabis use in adolescence is as predictive of clinically significant substance use in adulthood as early-onset use (32) and may be even more strongly associated with problem behavior than a pattern of persistently high use (33). Conversely, recent studies have indicated that some cannabis-related cognitive changes can reverse after abstinence in long-term users, suggesting that patterns of use over time might not be as impairing as level of recent use (34).
Alternatively, early initiation and consistent high-frequency use could confer particularly strong risk because of the duration and intensity of exposure at a vulnerable period in development. Congruently, several studies have demonstrated that earlier-onset cannabis use (i.e., beginning by age 16) is associated with more significant abnormalities in PFC function (27), worse neuropsychological functioning (35), educational problems (3), and later drug dependence (36). Another consideration is that many cannabis users also use alcohol (37), and cannabis effects may be attenuated when accounting for alcohol use (38).
We examined adolescent pattern of cannabis use, reward circuit function, and psychosocial adjustment in a high-risk sample: low-income young men from an urban setting (5) who have been assessed longitudinally since infancy as part of the Pitt Mother & Child Project (39). Specifically, our aims were to 1) determine distinct trajectories of cannabis use across adolescence, 2) measure the influence of cannabis use trajectory, recent frequency of use, and age of initiation on functional connectivity between the NAcc and PFC, and 3) to assess whether patterns of functional connectivity are associated with affective functioning and/or educational attainment 2 years later. Based on prior evidence that regular cannabis users display greater NAcc connectivity to cannabis-related stimuli (26), we hypothesized that a stable, high-frequency pattern of use across adolescence, greater recent cannabis use, and early initiation would be associated with reduced functional connectivity in response to non-drug-related monetary reward. Furthermore, we hypothesized that trajectory of use across adolescence—because of developmental vulnerability—would have predictive power for reward circuit function even above the contributions of current use. Finally, we hypothesized that cannabis-related alterations in reward circuitry at age 20 would predict higher depressive symptoms, higher anhedonia, and lower educational attainment at age 22.
Methods
Design
The Pitt Mother & Child Project (PMCP) is a prospective, longitudinal study of risk and resiliency among low-income male participants recruited from the Pittsburgh, Pennsylvania area (39). This study was initiated in 1991 and a total of N=310 families were recruited from Women, Infant, and Children Nutritional Supplement Program (WIC) Clinics when participants were 6–17 months old. Participants completed in-person assessments at age 1.5, 2, 3.5, 5, 5.5, 6, 8, 10, 11, 12, 15, 17, 20, and 22. This study has the strength of allowing for concurrent examination of the relative influence of adolescent trajectory of cannabis use, recent frequency of use, and age of onset. Because subjects were recruited in infancy and followed prospectively, it was not possible to control the number of participants that would ultimately go on to engage in cannabis use. Rather, we took a data-driven approach to determine the different trajectories of cannabis use that were present among PMCP participants, and to use these trajectory groups as the basis for our functional connectivity analyses.
Participants
Participants were n=158 PMCP participants who completed an fMRI scan and substance use measures at age 20. All subjects were eligible to participate in the imaging study unless they reported any standard MRI contraindications. Procedures were approved by the University of Pittsburgh Institutional Review Board. All participants provided written informed consent to study procedures and were compensated for their participation.
Measures
Cannabis Use
Cannabis use characteristics were assessed at age 20 using the Lifetime History of Drug Use and Drug Consumption (40), a psychometrically sound, semi-structured interview. This was the first visit at which participants were asked about their frequency and quantity of use across development. For each year since their first use, participants reported their average days/month of cannabis use, average quantity, maximum quantity, and maximum number of days using their maximum quantity of cannabis. Retrospective report of cannabis use is valid (41), and may sometimes be more accurate than concurrent report because adolescents may under-report their current use because of concerns about legal consequences (42). The current analyses focused on average frequency of use for each year and age of initiation.
Alcohol Use
Annual alcohol use frequency (days/month) and quantity (average number of drinks/occasion) for each year since participants’ first alcohol use were assessed at age 20 using the Lifetime Drinking History (43). The product of alcohol use frequency and quantity was calculated for each year and average alcohol exposure from age 10 to 19 was included as a covariate in all functional connectivity analyses.
Age 22 Psychosocial Outcomes
Depression
Participants completed the Beck Depression Inventory (BDI; 44) at age 20 and 22, a widely used, 21-item self-report questionnaire that had adequate reliability in this sample (α=.89). BDI scores did not change significantly from age 20 to 22 (F(302,1)=.803, p=.371).
Anhedonia
At age 22 only, participants completed the Snaith-Hamilton Pleasure Scale (45), a psychometrically sound, 14-item questionnaire appropriate for measuring anhedonia in typical and clinical samples. Reliability was adequate in this sample (α=.851).
Educational Attainment
Participants reported their highest level of school completed on a 13-point scale (“below grade 9” to “completion of graduate degree”) (46). For the current analyses, participants were classified as having either obtained “high school diploma (or GED) or less” or “some higher education or more” (39.9% at age 20; 52% at 22). Educational attainment increased from age 20 to age 22 (X2=57.85, p=.00).
Functional Connectivity Analysis
fMRI Acquisition Procedure
Participants (n=186) underwent functional imaging on a Siemens TIM Trio 3T scanner using a gradient echo planar imaging (EPI) sequence with 34 axial slices (3.1mm thick; TR/TE=2000/28ms, FOV=20cm, matrix=64×64), as reported previously (47–55). A reference EPI scan was acquired and inspected for artifacts and signal. Twenty-eight participants were excluded because of intoxication (n=4), psychosis (n=1), <75% response rate (n=13), poor coverage (n=8), or unusable data (n=2), resulting in the final sample of n=158 included in the current analyses. All participants had <2mm mean movement in any direction.
The fMRI paradigm was an 8-min, 24-trial, slow event-related card-guessing game that assesses response to anticipation and receipt of monetary reward (see 49 for task details). Participants guessed whether a card was high or low, anticipated win or loss, and received feedback and monetary outcome. Trials were presented in a fixed, pseudorandom order, with participants unaware of fixed outcomes. This task robustly activates reward circuitry, including the NAcc (see Supplemental Figure 1 for task main effects).
fMRI Functional Connectivity Analysis
We conducted preprocessing and analysis using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Imaging data were realigned, unwarped, co-registered to segmented structural images, spatially normalized into standard stereotactic space (Montreal Neurological Institute) using a 12-parameter affine model, and smoothed with a 6mm FWHM Gaussian filter. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. Functional connectivity analyses of win anticipation > loss anticipation and win outcome > loss outcome were conducted using psychophysiological interaction (gPPI) (for description of PPI, please see 56). These contrasts were chosen because PPI analyses require the contrast of 2 active task conditions (rather than 1 active condition and 1 baseline condition) (56). The bilateral NAcc, defined anatomically using WFU PickAtlas (v. 3.0.4), was the seed region because of its critical role in reward processing (12) and association with substance use (10). Functional connectivity was examined between the NAcc and a region of interest including the PFC and ACC, defined anatomically using WFU PickAtlas (v. 3.0.4) to include Brodmann Areas 9–12, 24–25, 32–33, and 44–47 (see supplemental Figure 2).
Data Analyses
Aim 1: Developmental Trajectory of Adolescent Cannabis Use
Trajectory group analyses used frequency of cannabis use from age 14–19 years. This range covers a large portion of adolescence, includes the typical age of initiation (28), and included past-year use because assessments occurred on or around participants’ 20th birthdays. We used a latent class growth analyses in MPlus, which identifies clusters of people in common developmental pathways (58, 59). Models with 1–4 groups were tested (Supplementary Table 1). We followed recommendations that the smallest group contain at least 5% of the sample (60).
Aim 2: Influence of Cannabis Use Characteristics on Age 20 Functional Connectivity
One-way ANOVA was used to test the effect of trajectory group on NAcc functional connectivity. Clusters demonstrating significant effects of trajectory group were used as a mask for post-hoc pairwise t-tests. Regression was used to test the effect of recent frequency of cannabis use and age of initiation on NAcc functional connectivity. Daily nicotine use, antisocial personality disorder (present/absent), lifetime alcohol exposure, and SES were covariates because of their influence on reward circuitry (61) or association with trajectory group. Functional connectivity with the PFC was tested at p<.005 (57) and correction for multiple comparisons used a cluster-level FWE-corrected p<.05 threshold (62). This threshold was selected to balance risk for Type I and Type II error, based on current recommendations in the field (57). Carter et al. (57) calculated power curves to illustrate sample sizes necessary to detect moderate effects at various statistical thresholds. Based on their calculations, a threshold of p<.001 would be likely to obscure meaningful effects in on our current sample size, whereas a threshold of p<.005 would allow us to detect a moderate effect.
Aim 3: Relation between Age 20 Functional Connectivity and Age 22 Psychosocial Outcomes
Pearson bivariate correlation analyses tested whether functional connectivity related to adolescent cannabis use was associated with depression, anhedonia, or educational attainment at age 22.
Results
Developmental Trajectory of Adolescent Cannabis Use
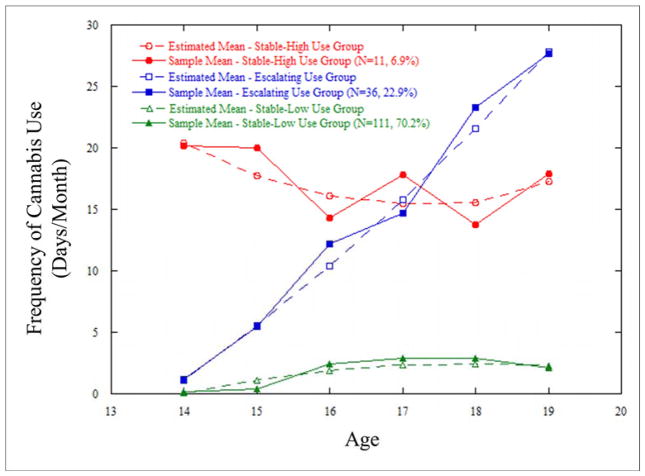
Trajectory analyses yielded a 3-group model (Supplementary Table 1). The stable-high group (6.9%; n=11) contained participants with an early age of initiation who had consistent, high-frequency use. The escalating group (22.9%; n=36) contained participants who exhibited increasing frequency of use and typical age of onset. The stable-low group (70.2%; n=111) contained participants who reported either no (n=33) or infrequent (n=78) cannabis use. (Figure 1; Table 1).
Figure 1.
Developmental Trajectories of Cannabis Use across Adolescence in Young Men with Low Socioeconomic Status
Table 1.
Sample Characteristics and Key Variables, by Trajectory Groupa
| Total Sample (N=158) | Group 1 Stable-High Use (N=11, 7%) |
Group 2 Escalating Use (N=36, 22.8%) |
Group 3 Stable-Low Use (N=111, 70.3%) |
Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Characteristics (Age 20) | |||||||||||
|
| |||||||||||
| N | % | N | % | N | % | N | % | X2 | df | p | |
|
| |||||||||||
| Race | |||||||||||
| European American | 87 | 55 | 4 | 36 | 23 | 64 | 60 | 54 | 5.17 | 4 | ns |
| African American | 58 | 37 | 7 | 64 | 10 | 28 | 41 | 37 | |||
| Other | 13 | 8 | 0 | 0 | 3 | 8 | 10 | 9 | |||
| M | SD | M | SD | M | SD | M | SD | F | df | p | |
|
| |||||||||||
| Age | 20.09 | .26 | 20.09 | .35 | 20.07 | .24 | 20.10 | .26 | .102 | 2, 155 | ns |
|
| |||||||||||
| SESb | .199 | .691 | .781(2,3) | .632 | .128(1) | .635 | .165(1) | .693 | 4.41 | 2, 154 | .014 |
|
| |||||||||||
| Task Reaction Time (ms) | 1087.32 | 336.01 | 1180.25 | 265.74 | 1009.31 | 313.29 | 1103.00 | 348.22 | 1.30 | 2, 127 | ns |
|
| |||||||||||
| Substance Use and Mental Health Variables (Age 20) | |||||||||||
|
| |||||||||||
| N | % | N | % | N | % | N | % | X2 | df | p | |
|
| |||||||||||
| Cannabis Abusec | 29 | 18 | 4(3) | 36 | 18(3) | 50 | 7(1,2) | 6 | 37.19 | 2, 155 | .000 |
|
| |||||||||||
| Cannabis Dependencec | 17 | 11 | 4(3) | 36 | 11(3) | 31 | 2(1,2) | 2 | 31.48 | 2, 155 | .000 |
|
| |||||||||||
| Antisocial Personality Disorderc | 12 | 8 | 3(3) | 27 | 7(3) | 19 | 2(1,2) | 2 | 18.58 | 2, 154 | .000 |
|
| |||||||||||
| Other Substance Abuse/Dependencec | 5 | 3 | 0 | 0 | 3 | 8 | 2 | 2 | 4.17 | 2, 155 | ns |
|
| |||||||||||
| Daily Nicotine Use | 46 | 29 | 7(3) | 64 | 16(3) | 44 | 23(1,2) | 21 | 14.24 | 2, 155 | .001 |
|
| |||||||||||
| Alcohol Abuse/Dependence c | 22 | 14 | 3 | 27 | 10(3) | 28 | 9(2) | 8 | 10.53 | 2, 155 | .005 |
|
| |||||||||||
| M | SD | M | SD | M | SD | M | SD | F | df | p | |
|
| |||||||||||
| Current Frequency of Cannabis Use (Average Days/Month) | 9.5 | 12.2 | 17.5(3) | 14.3 | 23.6(3) | 9.2 | 3.3(1,2) | 6.8 | 92 | 2, 153 | .000 |
|
| |||||||||||
| Age of First Significant Cannabis Use | 15.74 | 2.03 | 13.31(2,3) | .90 | 15.47(1) | 1.57 | 16.29(1) | 2.08 | 12.80 | 2, 110 | .000 |
|
| |||||||||||
| Average Annual Lifetime Alcohol Exposure (mean Frequency*Quantity for each year) | 31.21 | 69.64 | 28.7(3) | 29.6 | 18.6(3) | 29.3 | 7.5(1,2) | 12.7 | 9.32 | 2, 153 | .000 |
|
| |||||||||||
| Frequency of Other Illicit Drug Used | .11 | .6 | 0 | 0 | .31 | .91 | .05 | .48 | 2.44 | 2, 141 | ns |
|
| |||||||||||
| N | % | N | % | N | % | N | % | F | df | p | |
|
| |||||||||||
| Mood Disorderc,e | 18 | 11.4 | 2 | 18.2 | 5 | 13.9 | 11 | 9.9 | .48 | 2, 155 | ns |
|
| |||||||||||
| Anxiety Disorderc,f | 20 | 12.7 | 4(3) | 36.4 | 5 | 13.9 | 11(1) | 9.9 | 3.27 | 2,155 | .041 |
|
| |||||||||||
| ADHDg | 11 | 7.0 | 0 | 0 | 3 | 8.3 | 8 | 7.2 | .42 | 2, 137 | ns |
|
| |||||||||||
| ODDg | 9 | 5.7 | 2 | 18.2 | 3 | 8.3 | 4 | 3.6 | 2.55 | 2, 137 | ns |
|
| |||||||||||
| CDg | 16 | 10.1 | 2 | 18.2 | 6 | 16.7 | 8 | 7.2 | 1.79 | 2, 137 | ns |
|
| |||||||||||
| Psychosocial Outcomes (Age 22) | |||||||||||
|
| |||||||||||
| M | SD | M | SD | M | SD | M | SD | F | df | p | |
|
| |||||||||||
| Depressive Symptomsh | 4.59 | 5.59 | 5.70 | 5.52 | 6.31 | 6.86 | 3.95 | 5.07 | 2.44 | 2, 143 | ns |
|
| |||||||||||
| Anhedoniai | 1.65 | 2.30 | 1.56 | 1.67 | 1.77 | 2.51 | 1.61 | 2.29 | .06 | 2, 138 | ns |
|
| |||||||||||
| N | % | N | % | N | % | N | % | X2 | df | p | |
|
| |||||||||||
| Educational Attainment (Greater than High School)j | 78 | 52 | 2(3) | 18.2 | 9(3) | 25 | 67(1,2) | 60.4 | 19.66 | 2 | .000 |
Note:
Groups are based on results of developmental trajectory models for change in frequency of cannabis use from age 14–19 (with age 19 representing the past year); superscript numbers in parentheses indicate group(s) from which each group differed.
Defined as mean neighborhood risk (ages 1.5–15) (72).
Diagnoses determined based on the SCID at age 20.
Mean frequency of use at age 20 for ecstasy, hallucinogens, inhalants, opioids, sedatives, stimulants, and cocaine/crack assessed with the Lifetime History of Drug Use; average frequency of use for each substance was less than 1 day/month.
Mood Disorders includes major depressive disorder, bipolar disorder, and dysthymia.
Anxiety Disorders includes panic disorder, social phobia, specific phobia, obsessive compulsive disorder, post-traumatic stress disorder, and generalized anxiety disorder.
attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), and conduct disorder (CD) were assessed using the K-SADS at age 17 and data from this assessment was available for n=140 participants in the current sample.
Depressive symptoms measured using the Beck Depression Inventory (BDI; 44).
Anhedonia measured using the Snaith-Hamilton Pleasure Scale (SHAPS; 45).
Educational attainment was defined as a dichotomous variable, computed as “high school diploma (or GED) or less” or “some higher education or more”.
Influence of Cannabis Use Characteristics on Age 20 Functional Connectivity
Trajectory
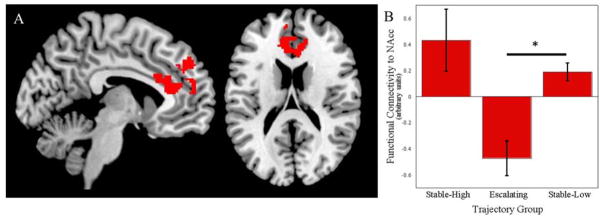
Consistent with previous reports (22), groups did not vary significantly in their BOLD activation, but differed significantly in functional connectivity between the bilateral NAcc and the medial PFC to win outcome > loss outcome (Table 2). The escalating group displayed negatively correlated activation of the NAcc and mPFC, whereas activity in these regions was positively correlated in both the stable-low and stable-high groups. Post-hoc pairwise t-tests revealed that functional connectivity differed significantly between the escalating and stable-low groups (k=509, pFWE-corr=.002, t=4.57, Z=4.41, peak MNI: −6, 32, 16). The difference between the escalating and stable-high trajectory groups (k=145, puncorrected=.014, t=4.40, peak MNI: 0, 48, 38) did not meet the FWE-corrected significance threshold (see Figure 2).
Table 2.
Effect of Trajectory of Adolescent Cannabis Use on Age 20 Nucleus Accumbens Functional Connectivity, and Association with Age 22 Psychosocial Outcomes
| Cluster-Level Statistics | Peak-Level Statistics | Age 22 Outcome Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | k | pFWE-corr | F | Z | MNI | Depressive Symptoms (n=144) | Anhedonia (n=139) | Educational Attainment (n=148) | ||
| x | y | z | ||||||||
| mPFC | 617 | .000 | 11.32 | 4.04 | −6 | 32 | 16 | r=−.17* | r=−.19* | t=−2.77** |
Note:
p<.05
p<.01.
Depressive symptoms were measured using the Beck Depression Inventory at age 22 (BDI; 44). Anhedonia was measured using the Snaith-Hamilton Pleasure Scale at age 22 (SHAPS; 45). Assocation between functional connectivity and age 22 depressive symptoms and anhedonia was assessed using pearson bivariate correlations. Educational attainment was defined as a dichotomous variable, computed as “high school diploma (or GED) or less” or “some higher education or more”. Therefore, a t-test was used to assess the relationship between this variable and functional connectivity.
Figure 2.
Trajectory of Adolescent Cannabis Use Predicts Functional Connectivity of the Nucleus Accumbens in Response to Monetary Reward at Age 20 Controlling for Lifetime Alcohol Use.
Note. Functional connectivity within the medial prefrontal cortex (mPFC; A) differed for three trajectory groups (B). Bars in (B) represent mean functional connectivity for each trajectory group; error bars represent standard error of the mean. Group-effect results were used as a mask for post hoc tests comparing pairs of groups, which yielded a significant difference between the escalating and stable-low trajectory groups We note that using a statistical threshold of p<.001 for the overall group analysis yielded 3 smaller clusters within the current mPFC cluster (k=59, puncorrected=.013, F=11.32, Z=4.04, peak MNI: 6, 32, 16; k=39, puncorrected=.037, F=10.08, Z=3.78, peak MNI: 10, 30, 22; k=69, puncorrected=.008, F=10.06, Z=3.78, peak MNI: −6, 44, 36).
Recent frequency of cannabis use and age of initiation did not predict functional connectivity
Relation between Age 20 Functional Connectivity and Age 22 Psychosocial Outcomes
Negative functional connectivity between the NAcc and the region of the mPFC that differentiated the cannabis use trajectory groups at age 20 was associated with higher depression, higher anhedonia, and lower educational attainment at age 22 (Table 2).
Discussion
For young men from urban, low-SES backgrounds, trajectory of cannabis use across adolescence was related to differences in NAcc functional connectivity in response to receiving monetary reward at age 20, even after controlling for average annual alcohol use. Functional connectivity, in turn, was associated with higher depressive symptoms and anhedonia, and lower educational attainment at age 22. Most notably, an escalating pattern of cannabis use across adolescence—rather than stable, high-frequency use—was associated with negative functional coupling between the NAcc and mPFC.
According to the incentive sensitization model, repeated substance use leads to a sensitization of the reward system to drug-related stimuli relative to natural rewards (63, 64) and circuit-level changes in the mesocortiolimbic system give rise to attentional and decision-making biases that promote drug-seeking behavior (65). Congruently, recent studies have reported that cannabis use is linked to stronger functional NAcc-ACC connectivity to cannabis cues (26), but blunted NAcc response to monetary cues (66), and the current study reports negative NAcc-mPFC connectivity to monetary reward among those with an escalating pattern of adolescent cannabis use. Future studies should include both drug-related and non-drug-related rewards to evaluate whether individuals with an escalating pattern of cannabis use may display a shift away from natural rewards and toward drug rewards (63). This could also contribute to symptoms of depression and anhedonia, as well as impaired educational performance.
Contrary to our expectations, the escalating trajectory group showed a distinct pattern of negative functional connectivity between the NAcc and mPFC, relative to both the stable-low and stable-high groups. The stable-high group was characterized by lower SES, higher rates of antisocial personality disorder, and earlier age of cannabis use initiation. Both acute and chronic stress exposure have substantial impacts on endocannabinoid system functioning (67). Because this group has been exposed to a high level of stress, it is possible that cannabis exposure may have a different impact on neural development in these participants and may even buffer against neurotoxic effects of chronic stress. Future research is necessary to assess whether neural effects of cannabis exposure are moderated by early life or chronic stress. Alternatively, the brain may undergo neuroadaption in the context of early-onset, consistent use, which may attenuate exposure effects. Although early-onset substance use is a well-established risk factor for later substance dependence and poor psychosocial development, evidence suggests that an escalating trajectory of use is similarly harmful (32, 33, 68). Given the small size of our stable-high use group, future research is necessary to explore differential effects of stable-high versus escalating cannabis exposure and characteristics that distinguish individuals with each pattern.
Our findings that altered functional connectivity between the NAcc and PFC is associated with poor psychosocial outcomes supports the hypothesis that disrupted neural circuit function may result in poor regulation of motivational systems, including difficulty enhancing positive affect, pursuing goals, or focusing on future reward. The observed association between NAcc-mPFC functional connectivity and sub-threshold symptomatology has important implications, and the long-term consequences to SES, health, and social functioning could be dramatic. Among young men from urban, low-SES backgrounds, educational attainment may be a particularly salient marker of later status, including upward social mobility (69).
Our sample includes low-income males from an urban community, a particularly high-risk population (5). However, this focus prevented us from examining gender differences or effects in women, young people from higher SES, or young adults living in rural or suburban communities. Additionally, our high-risk, prospective design precluded the ability to recruit subjects based on patterns of use. Rather, we took a data-driven approach to determine the different trajectories of cannabis use that were present within this sample. The small size of the resulting stable-high trajectory group may have limited our power to detect significant relationships with this group of participants. Future research could use larger samples or utilize additional risk criteria for sample inclusion, such as parental use of substances, mental health issues, or high levels of infant negative emotionality, to maximize the number of infants showing cannabis use in adolescence.
Finally, because neural response to reward was measured at age 20, it is impossible to disentangle premorbid patterns of neural function from effects of cannabis use. Individuals at high risk for substance abuse have been found to display differences in neural structure, function, and connectivity in the absence of personal substance use (70). Therefore, aberrant patterns of neural response to reward among cannabis users are likely to result from a combination of predisposing neural abnormalities and exposure effects that interact across development. Abnormalities in neural reward circuitry may also be influenced by early life stress (49) and/or neurotoxic effects of co-occurring alcohol exposure (71).
In conclusion, our findings suggest that different trajectories of adolescent cannabis use are associated with distinct patterns neural reward circuit function in early adulthood, with escalating use appearing to confer higher risk for impaired motivation, including higher depressive symptoms, anhedonia, and poor educational attainment. Alterations in reward circuit function represent a potential mechanism by which cannabis users experience psychosocial consequences that could be pernicious for adult functioning.
Supplementary Material
Acknowledgments
The authors thank the young men and mothers who have participated in the Pitt Mother & Child Project, Marigrace Ambrosia for assistance with figures and formatting, and the staff members who assisted with data collection.
Footnotes
Conflict of Interest Declaration: Financial support for this research was provided by National Institutes of Health grant R01 DA026222 (MPIs: DSS and EEF). Ms. Lichenstein has received funding from the NSF (GRFP DGE-1247842). Drs. Shaw and Forbes have received funding from the NIH, NARSAD, and the Klingenstein Third Generation Foundation. All authors declare no potential conflicts of interest.
References
- 1.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2016: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2017. [Google Scholar]
- 2.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, Miech RA. Monitoring the Future national survey results on drug use, 1975–2013: Volume I, Secondary school students. Ann Arbor: Institute for Social Research, The University of Michigan; 2014. [Google Scholar]
- 3.Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95(11):1621–30. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- 4.Marmorstein NR, Iacono WG. Explaining associations between cannabis use disorders in adolescence and later major depression: a test of the psychosocial failure model. Addictive behaviors. 2011;36(7):773–6. doi: 10.1016/j.addbeh.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2011: Volume II. Ann Arbor: Institute for Social Research, The University of Michigan: University of Michigan; 2012. [Google Scholar]
- 6.Bloomfield MA, Morgan CJ, Kapur S, Curran HV, Howes OD. The link between dopamine function and apathy in cannabis users: an [18F]-DOPA PET imaging study. Psychopharmacology (Berl) 2014;231(11):2251–9. doi: 10.1007/s00213-014-3523-4. [DOI] [PubMed] [Google Scholar]
- 7.Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry. 2002;59(3):225–31. doi: 10.1001/archpsyc.59.3.225. [DOI] [PubMed] [Google Scholar]
- 8.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescent: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160(6):1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature neuroscience. 2005;8(11):1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 11.Galvan A. Neural systems underlying reward and approach behaviors in childhood and adolescence. Curr Top Behav Neurosci. 2014;16:167–88. doi: 10.1007/7854_2013_240. [DOI] [PubMed] [Google Scholar]
- 12.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batalla A, Bhattacharyya S, Yucel M, Fusar-Poli P, Crippa JA, Nogue S, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PloS one. 2013;8(2):e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubino T, Parolaro D. The Impact of Exposure to Cannabinoids in Adolescence: Insights from Animal Models. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(5):1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubman DI, Cheetham A, Yucel M. Cannabis and adolescent brain development. Pharmacology & therapeutics. 2015;148:1–16. doi: 10.1016/j.pharmthera.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Pujol J, Blanco-Hinojo L, Batalla A, Lopez-Sola M, Harrison BJ, Soriano-Mas C, et al. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. Journal of psychiatric research. 2014;51:68–78. doi: 10.1016/j.jpsychires.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Kelly C, Castellanos FX, Tomaselli O, Lisdahl K, Tamm L, Jernigan T, et al. Distinct effects of childhood ADHD and cannabis use on brain functional architecture in young adults. NeuroImage Clinical. 2017;13:188–200. doi: 10.1016/j.nicl.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, et al. Altered resting-state connectivity in adolescent cannabis users. The American journal of drug and alcohol abuse. 2013;39(6):372–81. doi: 10.3109/00952990.2013.848213. [DOI] [PubMed] [Google Scholar]
- 20.Houck JM, Bryan AD, Feldstein Ewing SW. Functional connectivity and cannabis use in high-risk adolescents. The American journal of drug and alcohol abuse. 2013;39(6):414–23. doi: 10.3109/00952990.2013.837914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding IH, Solowij N, Harrison BJ, Takagi M, Lorenzetti V, Lubman DI, et al. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(8):1923–33. doi: 10.1038/npp.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filbey F, Yezhuvath U. Functional connectivity in inhibitory control networks and severity of cannabis use disorder. The American journal of drug and alcohol abuse. 2013;39(6):382–91. doi: 10.3109/00952990.2013.841710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernst M, Torrisi S, Balderston N, Grillon C, Hale EA. FMRI functional connectivity applied to adolescent neurodevelopment. Annu Rev Clin Psychol. 2015;11:361–77. doi: 10.1146/annurev-clinpsy-032814-112753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramaekers JG, van Wel JH, Spronk D, Franke B, Kenis G, Toennes SW, et al. Cannabis and cocaine decrease cognitive impulse control and functional corticostriatal connectivity in drug users with low activity DBH genotypes. Brain Imaging Behav. 2016;10(4):1254–63. doi: 10.1007/s11682-015-9488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer AS, Whitfield-Gabrieli S, Roth RM, Brunette MF, Green AI. Impaired functional connectivity of brain reward circuitry in patients with schizophrenia and cannabis use disorder: Effects of cannabis and THC. Schizophrenia research. 2014;158(1–3):176–82. doi: 10.1016/j.schres.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filbey FM, Dunlop J. Differential reward network functional connectivity in cannabis dependent and non-dependent users. Drug and alcohol dependence. 2014;140:101–11. doi: 10.1016/j.drugalcdep.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Frontiers in psychiatry. 2013;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SAMHSA. Results from the 2013 National Survey on Drug Use and Health: National findings. Rockville, MD: 2013. NSDUH Series H-48, DHHS Publication No. SMA 14–4863. [Google Scholar]
- 29.Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychological medicine. 2006;36(10):1447–60. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- 30.Bambico FR, Nguyen NT, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiology of disease. 2010;37(3):641–55. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn CM, Wilson W, Swartzwelder S. Enduring effects of adolescent drug exposure. Biol Psychiatry. 2013;74(7):480–1. doi: 10.1016/j.biopsych.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson SE, Van Ryzin MJ, Dishion TJ. Alcohol, marijuana, and tobacco use trajectories from age 12 to 24 years: demographic correlates and young adult substance use problems. Dev Psychopathol. 2015;27(1):253–77. doi: 10.1017/S0954579414000650. [DOI] [PubMed] [Google Scholar]
- 33.Passarotti AM, Crane NA, Hedeker D, Mermelstein RJ. Longitudinal trajectories of marijuana use from adolescence to young adulthood. Addict Behav. 2015;45:301–8. doi: 10.1016/j.addbeh.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970–6. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, et al. Cannabis use before age 15 and subsequent executive functioning. The British journal of psychiatry : the journal of mental science. 2011;198(6):442–7. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- 36.Chen CY, Storr CL, Anthony JC. Early-onset drug use and risk for drug dependence problems. Addictive behaviors. 2009;34(3):319–22. doi: 10.1016/j.addbeh.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCurley C, Snyder HN. Co-occurrence of substance use behaviors in youth. Juvenile Justice Bulletin. 2008 [Google Scholar]
- 38.Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(4):1505–12. doi: 10.1523/JNEUROSCI.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaw DS, Hyde LW, Brennan LM. Early predictors of boys’ antisocial trajectories. Development and psychopathology. 2012;24(3):871–88. doi: 10.1017/S0954579412000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skinner H. Development and validation of a lifetime alcohol consumption assessment procedure. Toronto: Addiction Research Foundation; 1982. Substudy No. 1248. [Google Scholar]
- 41.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154–62. doi: 10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- 42.Ensminger ME, Juon HS, Green KM. Consistency between adolescent reports and adult retrospective reports of adolescent marijuana use: explanations of inconsistent reporting among an African American population. Drug Alcohol Depend. 2007;89(1):13–23. doi: 10.1016/j.drugalcdep.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skinner H. Development and validation of a lifetime alcohol consumption assessment procedure. Toronto: 1982. [Google Scholar]
- 44.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 45.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry. 1995;167(1):99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- 46.Conger RD, Conger KJ. Resilience in Midwestern families: Selected findings from the first decade of a prospective, longitudinal study. Journal of Marriage and Family. 2002;64:361–73. [Google Scholar]
- 47.Hasler BP, Sitnick SL, Shaw DS, Forbes EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Res. 2013 doi: 10.1016/j.pscychresns.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan JK, Shaw DS, Forbes EE. Maternal depression and warmth during childhood predict age 20 neural response to reward. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(1):108–17. e1. doi: 10.1016/j.jaac.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casement MD, Shaw DS, Sitnick SL, Musselman SC, Forbes EE. Life stress in adolescence predicts early adult reward-related brain function and alcohol dependence. Social cognitive and affective neuroscience. 2015;10(3):416–23. doi: 10.1093/scan/nsu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choe DE, Shaw DS, Forbes EE. Maladaptive social information processing in childhood predicts young men’s atypical amygdala reactivity to threat. Journal of child psychology and psychiatry, and allied disciplines. 2015;56(5):549–57. doi: 10.1111/jcpp.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgan JK, Shaw DS, Forbes EE. Fearfulness moderates the link between childhood social withdrawal and adolescent reward response. Social cognitive and affective neuroscience. 2015;10(6):761–8. doi: 10.1093/scan/nsu113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilliam M, Forbes EE, Gianaros PJ, Erickson KI, Brennan LM, Shaw DS. Maternal depression in childhood and aggression in young adulthood: evidence for mediation by offspring amygdala-hippocampal volume ratio. Journal of child psychology and psychiatry, and allied disciplines. 2015;56(10):1083–91. doi: 10.1111/jcpp.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan JK, Shaw DS, Olino TM, Musselman SC, Kurapati NT, Forbes EE. History of Depression and Frontostriatal Connectivity During Reward Processing in Late Adolescent Boys. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2016;45(1):59–68. doi: 10.1080/15374416.2015.1030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattson WI, Hyde LW, Shaw DS, Forbes EE, Monk CS. Clinical neuroprediction: Amygdala reactivity predicts depressive symptoms 2 years later. Social cognitive and affective neuroscience. 2016;11(6):892–8. doi: 10.1093/scan/nsw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hyde LW, Shaw DS, Murray L, Gard A, Hariri AR, Forbes EE. Dissecting the role of amygdala reactivity in antisocial behavior in a sample of young, low-income, urban men. Clinical psychological science : a journal of the Association for Psychological Science. 2016;4(3):527–44. doi: 10.1177/2167702615614511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Social cognitive and affective neuroscience. 2012;7(5):604–9. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carter CS, Lesh TA, Barch DM. Thresholds, power, and sample sizes in clinical neuroimaging. Biological Psychiatry: CNNI. 2016;1(2):99–100. doi: 10.1016/j.bpsc.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Muthén B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of Quantitative Methodology for the Social Sciences. Newbury Park, CA: Sage Publications; 2004. pp. 345–68. [Google Scholar]
- 59.Muthén L, Muthén B. MPlus user’s guide: Version 5.2. Los Angeles, CA: Muthén & Muthén; 2008. [Google Scholar]
- 60.Nagin DS, Odgers CL. Group-Based Trajectory Modeling (Nearly) Two Decades Later. J Quant Criminol. 2010;26(4):445–53. doi: 10.1007/s10940-010-9113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruijnzeel AW, Alexander JC, Perez PD, Bauzo-Rodriguez R, Hall G, Klausner R, et al. Acute Nicotine Administration Increases BOLD fMRI Signal in Brain Regions Involved in Reward Signaling and Compulsive Drug Intake in Rats. Int J Neuropsychopharmacol. 2014;18(2) doi: 10.1093/ijnp/pyu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chumbley JR, Friston KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. NeuroImage. 2009;44(1):62–70. doi: 10.1016/j.neuroimage.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 63.Filbey FM. Weeding Through Marijuana’s Effects on the Brain. JAMA psychiatry. 2016;73(8):773–4. doi: 10.1001/jamapsychiatry.2016.1133. [DOI] [PubMed] [Google Scholar]
- 64.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 65.Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neuroscience and biobehavioral reviews. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, et al. Association of Marijuana Use With Blunted Nucleus Accumbens Response to Reward Anticipation. JAMA psychiatry. 2016;73(8):838–44. doi: 10.1001/jamapsychiatry.2016.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morena M, Patel S, Bains JS, Hill MN. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brook JS, Zhang C, Brook DW. Antisocial behavior at age 37: developmental trajectories of marijuana use extending from adolescence to adulthood. Am J Addict. 2011;20(6):509–15. doi: 10.1111/j.1521-0391.2011.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forrest LF, Hodgson S, Parker L, Pearce MS. The influence of childhood IQ and education on social mobility in the Newcastle Thousand Families birth cohort. BMC public health. 2011;11:895. doi: 10.1186/1471-2458-11-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tessner KD, Hill SY. Neural circuitry associated with risk for alcohol use disorders. Neuropsychol Rev. 2010;20(1):1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ewing SWF, Sakhardande A, Blakemore SJ. The effect of alcohol consumption on the adolescent brain: A systematic review of MRI and fMRI studies of alcohol-using youth. Neuroimage-Clinical. 2014;5:420–37. doi: 10.1016/j.nicl.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vanderbilt-Adriance E, Shaw DS. Conceptualizing and re-evaluating resilience across levels of risk, time, and domains of competence. Clin Child Fam Psychol Rev. 2008;11(1–2):30. doi: 10.1007/s10567-008-0031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.