Abstract
Purpose
To report the clinical course of 6 patients with refractory neurotrophic corneal ulcers that were treated with topical insulin drops.
Methods
Retrospective chart review of patients who had neurotrophic corneal ulcers or epithelial defects refractory to standard medical and surgical treatment. Insulin drops, prepared by mixing regular insulin in artificial tears with a polyethylene glycol and propylene glycol base at a concentration of 1 unit per mL, were prescribed 2–3 times daily.
Results
Six patients, 2 to 73 years of age, developed neurotrophic corneal ulcers refractory to a range of medical and surgical treatments, including bandage contact lens, amniotic membrane grafting and permanent tarsorrhaphy. Each patient was started on topical insulin drops with complete corneal re-epithelialization within 7 to 25 days.
Conclusion
Topical insulin may be a simple and effective treatment for refractory neurotrophic corneal ulcers. Further study is needed to determine the clinical efficacy and side effect profile of insulin drops.
Introduction
Neurotrophic keratopathy is a degenerative disease of the corneal epithelium secondary to impaired corneal innervation by the trigeminal nerve. Standard treatment involves aggressive lubrication of the corneal surface, therapeutic contact lenses, amniotic membrane grafts and tarsorrhaphy. Refractory neurotrophic corneal ulcers occur when treatment response is incomplete and are potentially blinding. Insulin is a widely available, relatively safe, and familiar medication that has been shown to improve corneal epithelial healing in vitro and in diabetic animal models. However, clinical experience with topical insulin in patients with non-healing corneal wounds is minimal. The purpose of this study is to present 6 patients with refractory neurotrophic corneal ulcers that were treated with topical insulin.
Methods and Materials
A retrospective chart review of 6 patients at the University of Wisconsin was conducted with approval from the university’s Institutional Review Board. All patients were prescribed insulin drops as compassionate use for the treatment of neurotrophic corneal ulcers after standard treatment had failed. The risks, benefits, and alternatives of the treatment were discussed with all patients and/or their parents, and they verbally consented to the off-label use of insulin.
Unless otherwise noted, the drops were prepared by injecting regular insulin into a new bottle of artificial tears with a polyethylene glycol and propylene glycol base at a concentration of 1 unit per mL. Drops were prepared by pharmacy, the patients’ providers, or the patients themselves with detailed written instructions. This was done with sterile technique. The drops were refrigerated and used up to 1 month after preparation.
Results
Case 1
A 2-year-old girl with a history of an excised teratoma involving the left orbit resulting in proptosis and lagophthalmos presented with a large corneal ulcer of the left eye. The ulcer measured 7 × 4 mm on presentation (Figure 1A). Corneal sensation was absent in the affected eye. After 5 months of aggressive treatment, including permanent lateral tarsorrhaphy and inferior rectus recession to encourage Bell’s phenomenon, the ulcer remained. The patient was started on topical insulin drops 3 times daily. After 14 days of treatment, the ulcer had healed completely (Figure 1B). The insulin was tapered to once daily. At 18 month follow-up, the corneal epithelium remained intact.
Figure 1.
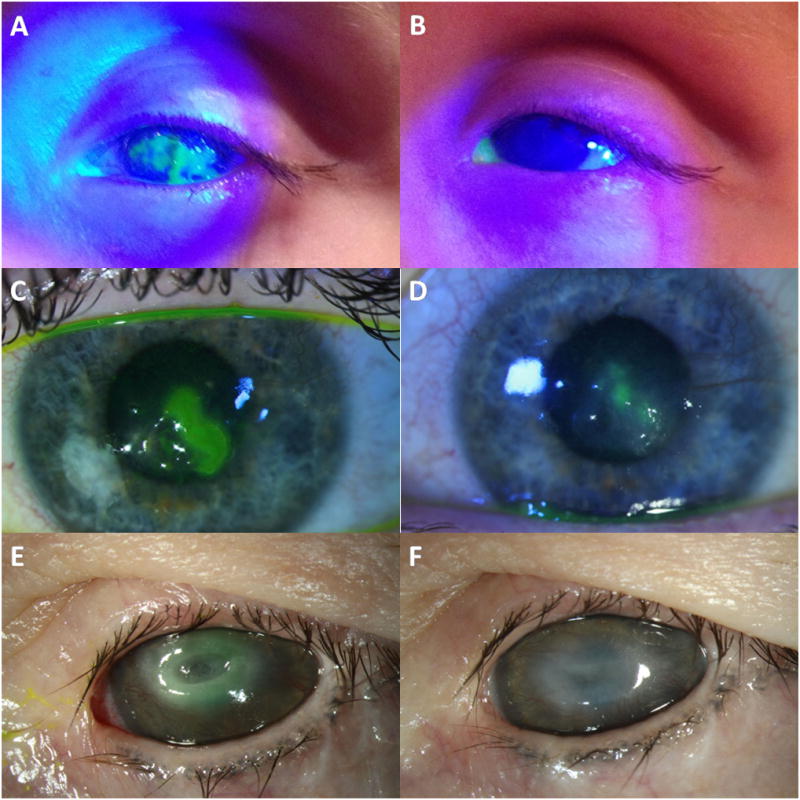
Three Patients Treated With Topical Insulin. A 2-year-old girl with proptosis and lagophthalmos from an orbital teratoma presented with a neurotrophic corneal ulcer refractory to lubrication and permanent lateral tarsorrhaphy (A). Insulin eye drops were initiated with resolution of the ulcer after 14 days (B). A 24-year-old woman with neurotrophic keratopathy secondary to herpes zoster keratitis presents with a corneal ulcer refractory to lubrication and use of scleral contact lens (C). Insulin drops were initiated with resolution of the ulcer after 25 days (D). A 47-year-old woman with neurotrophic keratopathy secondary to cranial nerve injury presents with near descemetocele in the central cornea of the left eye (E). Insulin drops were initiated with resolution of the ulcer after 7 days (F).
Case 2
A 2-year-old boy with a history of aniridia and surgically controlled bilateral congenital glaucoma presented with a corneal epithelial defect measuring 5 × 6.5 mm in the left eye. Formal corneal sensation testing was not performed; however, the patient’s cornea was presumed to be hypesthetic as he was asymptomatic. The patient was treated with antibiotic ointment and amniotic membrane grafting twice. After 5 months with persistent epithelial defect, insulin drops were started 3 times daily. After 13 days of treatment, the corneal epithelium had healed completely and the drop was tapered to twice daily. The patient has had no recurrent epithelial defect 1 year since starting the treatment.
Case 3
A 24-year-old woman with a history of herpes zoster keratoconjunctivitis and neurotrophic keratopathy in the left eye presented with a persistent epithelial defect and mild ulceration. She developed the defect, which measured 3 × 2 mm, despite daily use of a scleral contact lens (Figure 1C). Insulin drops were started twice daily and at 2 week follow-up, the defect improved to 1 × 1 mm. After another 2 weeks, the epithelial defect had resolved, with some residual epithelial irregularity (Figure 1D). The patient used the insulin drops a total of 25 days; they were discontinued and follow-up was transferred when she moved out of state.
Case 4
A 47-year-old woman with a history of neurotrophic keratopathy in both eyes secondary to cranial nerve injury and a large corneal scar in the left eye from a previous ulcer, presents with a near descemetocele in the central cornea of the left eye. The area of thinning measured 1.5 × 2.5 mm on presentation (Figure 1E). The patient had previously had a surgical tarsorrhaphy in the left eye. She was diabetic on lispro insulin and was started on insulin drops (2 Units of lispro in 10 mL of artificial tears) 2–3 times daily. After 7 days of treatment, the ulcer had healed completely with thickened epithelium over the previous descemetocele (Figure 1F). The drops were used a total of 3 weeks and were discontinued secondary to patient preference.
Case 5
A 71-year-old woman presents with a non-healing, asymptomatic 4 × 4 mm epithelial defect following vitrectomy. She was treated with lubrication, bandage contact lens and amniotic membrane graft with minimal improvement over the course of 4 months. Insulin drops were started twice and after 14 days of treatment, the epithelial defect had resolved. The patient was tapered to insulin drops once daily, then self-discontinued the drops after several months. At 1 year follow-up, the epithelium remained intact with no recurrent defect.
Case 6
A 73-year-old woman with a history of herpes zoster neurotrophic keratopathy presents with non-healing epithelial defect of the left eye. She was treated with amniotic membrane three times, temporary tarsorrhaphy, and chronic topical steroid therapy. After two months, the defect persisted at 1.5 × 2.5 mm, and the patient was started on insulin drops 3 times daily. After 8 days of treatment, the epithelial defect had resolved. Four months after initial presentation, the patient developed a large infectious ulcer and crystalline keratopathy. She was continued on insulin drops during this time with the epithelial defect slowly improving. Three months after development of the crystalline keratopathy, the patient had a flare of zoster keratitis and the insulin drops were discontinued.
Discussion
We present 6 patients who developed neurotrophic corneal ulcers or epithelial defects that were refractory to a range of standard medical and surgical treatments. The addition of topical insulin resulted in rapid and complete corneal re-epithelialization ranging from 7 to 25 days following initiation of treatment. One patient developed crystalline keratopathy while on the treatment, though this was likely secondary to chronic topical steroid use. No other local or systemic side effects were noted, including change in corneal vascularization or opacity.
Topical insulin has been found to improve healing of decubitus ulcers1 and experimentally-induced superficial skin wounds in diabetic and non-diabetic individuals2. The effect of topical insulin on corneal wound healing has been well-studied in rodent models. Notably, in diabetic rats, topical insulin improves corneal sensation and improves wound healing after corneal abrasions3. Experience with insulin in corneal wound healing in humans is limited to two case series. A 1945 study reported improved healing of corneal ulcers after systemic administration of insulin4. A 2013 retrospective study looked at 5 patients who developed corneal epithelial defects during vitreoretinal surgery who were treated with topical insulin drops and reported faster re-epithelialization compared to 10 patients who were treated with lubrication5. There have been no previous studies of topical insulin use in patients with neurotrophic corneal defects.
The mechanism of insulin in promoting cornea wound healing in our patients remains speculative, but data suggest that restoration of corneal nerves and/or improved epithelial cell migration may play key roles. In diabetic mice, topical insulin has been shown to slow the loss of sub-basal plexus corneal nerves6. Furthermore, the addition of insulin promoted cell migration and closure of artificial wounds in cultured sheets of corneal epithelial cells in an in vitro model of corneal epithelial wound healing7.
Insulin-like growth factor-1 (IGF-1) has been shown to be an important modulator of corneal wound healing. In several pre-clinical studies, IGF-1 was shown to act synergistically with substance-P to promote corneal epithelium wound healing8. In two case series, patients with neurotrophic corneal epithelial defects treated with a topical combination of substance P-derived peptides and either IGF-1 or IGF-1-derived peptides underwent complete epithelial resurfacing within 4 weeks, at response rates of 89%9 and 73%10.
Topical insulin may be a simple and effective treatment for refractory neurotrophic corneal ulcers. Our case series is limited by the heterogeneity in the patient presentations, differences in treatment frequency and duration, and lack of a comparative control. Further study is needed to determine the clinical efficacy and side effect profile of topical insulin in corneal wound healing.
Acknowledgments
Source of Funding:
This work was supported in part by an unrestricted grant from the Research to Prevent Blindness, Inc. to the UW Madison Department of Ophthalmology and Visual Sciences, and in part by a National Eye Institute Vision Research Core grant (P30 EY016665) to the UW Madison Department of Ophthalmology and Visual Sciences.
Footnotes
Conflicts of Interest
Dr. Michael Struck holds the patent for use of topical insulin to treat corneal epithelial disease. The remaining authors have no conflicts of interest to disclose
References
- 1.Van Ort SR, Gerber RM. Topical application of insulin in the treatment of decubitus ulcers: a pilot study. Nurs Res. 1976;25:9–12. [PubMed] [Google Scholar]
- 2.Greenway SE, Filler LE, Greenway FL. Topical insulin in wound healing: a randomised, double-blind, placebo-controlled trial. J Wound Care. 1999;8:526–528. doi: 10.12968/jowc.1999.8.10.26217. [DOI] [PubMed] [Google Scholar]
- 3.Zagon IS, Klocek MS, Sassani JW, et al. Use of topical insulin to normalize corneal epithelial healing in diabetes mellitus. Arch Ophthalmol. 2007;125:1082–1088. doi: 10.1001/archopht.125.8.1082. [DOI] [PubMed] [Google Scholar]
- 4.Aynsley TR. The use of insulin in the treatment of corneal ulcers. Br J Ophthalmol. 1945;29:361–363. doi: 10.1136/bjo.29.7.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastion ML, Ling KP. Topical insulin for healing of diabetic epithelial defects?: A retrospective review of corneal debridement during vitreoretinal surgery in Malaysian patients. Med J Malaysia. 2013;68:208–16. [PubMed] [Google Scholar]
- 6.Chen DK, Frizzi KE, Guernsey LS, et al. Repeated monitoring of corneal nerves by confocal microscopy as an index of peripheral neuropathy in type-1 diabetic rodents and the effects of topical insulin. J Peripher Nerv Syst. 2013;18:306–315. doi: 10.1111/jns5.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanley LJ, McCaig CD, Forrester JV, et al. Insulin, not leptin, promotes in vitro cell migration to heal monolayer wounds in human corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:1088–1094. doi: 10.1167/iovs.03-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida T, Yanai R. Advances in treatment for neurotrophic keratopathy. Curr Opin Ophthalmol. 2009;20:276–281. doi: 10.1097/icu.0b013e32832b758f. [DOI] [PubMed] [Google Scholar]
- 9.Nishida T, Chikama T, Morishige N, Yanai R, Yamada N, Saito J. Persistent epithelial defects due to neurotrophic keratopathy treated with a substance p-derived peptide and insulin-like growth factor 1. Jpn J Ophthalmol. 2007;51(6):442–447. doi: 10.1007/s10384-007-0480-z. [DOI] [PubMed] [Google Scholar]
- 10.Yamada N, Matsuda R, Morishige N, Yanai R, Chikama T, Nishida T, et al. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 2008;92(7):896–900. doi: 10.1136/bjo.2007.130013. [DOI] [PubMed] [Google Scholar]


