Abstract
Background
Hyperalgesia that develops following nerve ligation corresponds temporally and in magnitude with the number of thalamic mast cells located contralateral to the ligature. We tested the possibility that mast cells modulate nociception centrally, similar to their role in the periphery.
Methods
We examined the central effect of two hyperalgesic compounds that also induce mast cell degranulation and stabilized mast cells using cromolyn.
Results
Thermal hyperalgesia (tail flick) induced by nerve growth factor (NGF, a neurotrophic compound) and mechanical hyperalgesia (von Frey) induced by dynorphin A (1–17) (opioid compound) each correlated with the percent of thalamic mast cells that were degranulated. Degranulation of these mast cells by the central injection of compound 48/80, devoid of neurotrophic or opioid activity, was sufficient to recapitulate thermal hyperalgesia. Stabilization of mast cells by central injections of cromolyn produced no analgesic effect on baseline tail flick or von Frey fiber sensitivity, but inhibited thermal hyperalgesia produced by compound 48/80 and tactile hyperalgesia induced by dynorphin and by Freund’s complete adjuvant. Finally, chemical nociception produced by the direct activation of nociceptors by formalin (phase I) was not inhibited by centrally injected cromolyn whereas chemical nociception dependent on central sensitization (formalin-phase II and acetic acid-induced abdominal stretches) was.
Conclusions
These convergent lines of evidence suggest that degranulation of centrally located mast cells sensitizes central nociceptive pathways leading to hyperalgesia and tonic chemical sensitivity.
1. Background
In the periphery, mast cells are located in close proximity to primary afferent C-fibers that transmit nociception, i.e., nerve activity related to pain sensation. They are not involved in the mediation of pain signals but they support increased nociceptive sensitivity (Chatterjea et al., 2012) and can sensitize surrounding fibers (Heron and Dubayle, 2013) and cause hyperalgesia, i.e., enhanced sensitivity to pain. In contrast, mast cell stabilization attenuates hyperalgesia (Oliveira et al., 2011; Parada et al., 2001; Woolf et al., 1996; Yasuda et al., 2013; Zuo et al., 2003). Chemicals released from activated nociceptors, in turn, increase mast cell activity (Matsuda et al., 1989) creating a positive feedback loop that has been proposed to support the transition from acute to chronic and to neuropathic pain (Austin and Moalem-Taylor, 2010).
In the parenchyma of the mouse brain, mast cells are almost exclusively located in the thalamus and are less abundant on dura and pia (Florenzano and Bentivoglio, 2000; Silverman et al., 2000). They can be numerous in the leptomeninges but this area is highly variable (Rats: Dropp et al 1972, Dropp et al 1976, Florenzano & Bentivoglio et al 2000, Goldschmidt et al 1984, Mice: Yang et al 1999). Mast cells are sensitive to stress (Esposito et al 2001) such that acute immobilization or even simple handling can cause mast cells to degranulate, including those in the thalamus (Persinger et al 1980, Theoharides et al 1995). Stress-induced mast cell degranulation may be due to corticotropin-releasing factor (CRF) and its related proteins, urocortin I, urocortin II or urocortin III (Paus et al 2006, Theoharides et al 2004), which activate CRF1 or CRF2 receptors on mast cells (Theoharides et al 1995, McEvoy et al 2001, Singh et al 1999).
The role of mast cells in the central nervous system (CNS) is unclear and cannot be extrapolated from that in the periphery as their vesicular content depends on the tissue in which they reside (Galli, 1990; Kitamura, 1989; Zhuang et al., 1999). However, mediators released locally from central mast cells are known to alter permeability of the blood-brain barrier (Esposito et al., 2001; Zhuang et al., 1996) and can further increase populations of mast cells by chemotactic activity (Halova et al., 2012). Based on their distribution in the thalamus, including the somatosensory thalamic nuclei, these mast cells are especially well positioned to influence key relay stations for nociception in a fashion similar to those abutting nerves in the periphery (Kovacs et al., 2006).
The question arises whether activation of centrally located mast cells influences nociception, similar to their effect in the periphery, and the impact of such an action. To test this hypothesis and to determine the modalities affected, we explored the relationship between central mast cells and behavioral nociceptive response used to reflect nociception in mice. We measured changes in nociception relative to the incidence of mast cell degranulation in various models of hyperalgesia. We studied the correlation between the incidence of mast cell degranulation in the thalamus and the degree of hyperalgesia (i.e., enhanced nociceptive responses) induced by central injections of two distinctly different hyperalgesic compounds whose common effect is to induce mast cell degranulation. The first was nerve growth factor (NGF) (Horigome et al., 1993), a compound that also interacts with the TrkA receptor (Hao et al., 2000), and the second was dynorphin A (1–17) (Sugiyama and Furuta, 1984), a compound that also interacts with the kappa opioid receptor (Laughlin et al., 2001). We then determined whether central injection of compound 48/80, a mast cell degranulator devoid of neurotrophic or opioid activity, was able to recapitulate and cromolyn, a mast cell stabilizer, to inhibit these hyperalgesic responses. To determine whether cromolyn merely inhibits neuronal activity directly, we compared the effect of cromolyn on thermal and tactile behavioral nociceptive responses to that on hyperalgesia, which is dependent on sensitization. We also compared the selectivity of cromolyn’s effect on tonic chemical nociceptive activity supported by central sensitization to its effect on phasic chemical pathways where it is not. Although each manipulation alone is not exclusively selective for mast cells, these lines of investigation converge to provide evidence suggesting that thermal and tactile hyperalgesia and tonic chemical nociception are linked to central mast cell activity. In contrast, baseline thermal and mechanical nociceptive responses and phasic chemical nociceptive responses, whose magnitude do not appear to depend on central sensitization, do not appear to depend on central mast cell activity.
2. Methods
2.1 Animals and housing
Adult male or female Swiss Webster mice weighing 20–25 g (Harlan Sprague Dawley, INC; Indianapolis, IN) were housed four (males) or five (females) per cage and allowed to acclimate for at least one week prior to use. Mice had free access to food and water, and were housed in a room with a constant temperature of 23°C on a 12-h light–dark cycle. Females were used except where indicated in figure legends as our preliminary studies suggest that the activity of their mast cells corresponds better temporally and in magnitude with nociceptive responses than those in males (Taiwo et al., 2005). Genetically mast cell-deficient mice were not used as they would not allow us to differentiate between effects produced by deficiencies in central mast cells from those produced by an absence of peripheral mast cells. All procedures were approved and performed according to the guidelines of the International Association for the Study of Pain (IASP), the University of Minnesota Animal Care and Use Committee, and the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (DHEW Publication NIH 78-23, revised 1995).
2.2 Drugs and chemicals
All chemicals and drugs were purchased from Sigma-Aldrich Corporation (Saint Louis, MO) and dissolved in saline. We compared the sensitivity of hyperalgesia to cromolyn in models of hyperalgesia caused by the central injection of compounds (NGF and dynorphin) with known abilities to induce, amongst a variety of effects, mast cell degranulation. Dynorphin A (1–17) (dynorphin) was injected intrathecally (i.t.) daily for 3 days at a dose of 3 nmol and a volume of 10 μl and the mice were tested for tactile sensitivity in the von Frey fiber assay 24 h after the last injection. Although dynorphin has been reported to induce hyperalgesia in mice after a single injection (Laughlin et al., 2001), repeated injections were necessary in our experiments, consistent with the variability reported in mice on personal communication with the authors who originally described that effect. NGF or cytochrome C were delivered intracerebroventricularly (i.c.v.) at 1 μg/10 μl (while mice were under isoflurane anesthesia) and tested in the von Frey fiber assay 48 h later. Cytochrome C was used as a control protein (Hao et al., 2000) as it is a similar size as NGF but is non-neuroactive. Compound 48/80 was injected i.t. at 1 μg/10 μl and tested in the tail flick assay 24 h later. The mast cell stabilizing sodium salt of cromolyn (Mazzari et al., 1996) was injected i.t. at a dose of 50 μg/20 μl 15–60 min before other drugs or behavioral testing, as stated in the figures.
2.3 Drug delivery
These studies required central injection of drugs to influence the target tissue to differentiate the effect of these drugs on central verses peripheral mast cells. The central injection of these compounds minimizes the possibility that their effects result from degranulation of peripheral mast cells. To avoid the use of i.c.v. injections that require anesthesia and cause sufficient stress to temporarily influence pain sensitivity, and to avoid tissue damage associated with i.c.v. injections, we used intrathecal injections for all centrally injected compounds except NGF, whose effects persist for several days, allowing recovery from the stress of injection. Injection of all other compounds into the CNS was accomplished using intrathecal injections delivered at the L5–L6 intravertebral space using a 30-gauge, 0.5 inch disposable needle on a 50 μL Luer tip Hamilton syringe in lightly restrained, un-anesthetized mice (Hylden and Wilcox, 1980). Drugs injected i.t. were dissolved in relatively large volumes that are known to distribute supraspinally (reviewed by Fairbanks, 2003). Consistent with this, our preliminary studies of the intrathecal injection of a dye indicate that a 20 μl-volume distributes to the ventricles within 5 min whereas injections of 2 μl i.t. remain in the spinal cord area. This is consistent with the report that 5 μl is the upper limit that can be injected without appreciable and rapid redistribution to the basal cisterns of the mouse brain (Rieselbach et al., 1962).
2.4 Freund’s complete adjuvant
To determine whether tactile mechanical sensitivity produced by other models of hyperalgesia was also sensitive to the inhibitory effect of cromolyn injected centrally just as they are to cromolyn injected peripherally, 20 μl of 50% Freund’s complete adjuvant (FCA) or saline was delivered intradermally (i.d.) into the plantar surface of both hind limbs and hyperalgesia measured by the application of von Frey fibers before and 24 h after injection.
2.5 Von Frey fiber assay
Mechanical sensitivity to von Frey fibers (# 3.22=0.16 g, # 3.84=0.6 g or # 4.17=1.4 g) was measured daily for at least 3 days to establish a baseline. After injection, mice were placed on a wire mesh grate under a glass 6-ounce (177 ml) custard cup, which prevents escape but allows movement of all four limbs and head. The von Frey fiber was applied ten times to the plantar surface of each hind paw, to the point of bending. A positive response was defined as a sudden, brisk lifting, shaking or licking of the paw. Typically 2–3 medium-sized fibers were initially used 15 min apart (Fang et al., 2003) to produce responses that include a spectrum from allodynic (light touch perceived as nociceptive) to hyperalgesic (enhanced nociception). We then used the fiber size that provided responses of sufficient magnitude to be inhibited by pharmacologic manipulations but also small enough to be potentiated. The number of positive responses/paw to the #3.84 von Frey fiber out of 10 was totaled and used as the mechanical sensitivity in experiments evaluating the sensitivity of responses to cromolyn.
2.6 Formalin assay
Response to the injection of formalin result in two waves of behavioral responses: phase I is generally described as phasic and neurogenic, resulting from direct activation of chemical nociceptors, whereas tonic Phase II responses result from the central sensitization of nociceptive pathways leading to motor responses (Coderre and Melzack, 1992). To determine the role of central mast cells in models of chemical pain that allowed us to differentiate between responses brought about by direct activation of nociceptors (phase I of the formalin assay) compared to responses produced by sensitization of nociceptive pathways (phase II of the formalin assay and acetic acid-induced behaviors) we also used the formalin assay and the acetic acid-induced abdominal stretch assay (Coderre and Melzack, 1992; Altier and Stewart, 1999; Shibata et al., 1989). These studies assessed whether responses were sensitive to the inhibitory effect of cromolyn injected centrally just as they are to cromolyn injected peripherally (formalin phase II: Prada et al., 2001; acetic acid-induced abdominal stretches: Ribeiro et al., 2000). During the formalin assay, mice were placed individually into plastic cylinders at least 30 min before the injection of formalin. Phase I is generally described as phasic and neurogenic, resulting from direct activation of chemical nociceptors, whereas tonic Phase II responses result from the central sensitization of nociceptive pathways leading to motor responses (Coderre and Melzack, 1992). Each mouse was injected subcutaneously (s.c.) in the dorsal surface of the left rear paw with 20 μl of 5% formalin in 0.85% NaCI using a 30-gauge needle. The mouse was immediately returned to the cylinder and the number of behaviors (when the mouse shook, licked or bit the injected foot) was monitored over 5-min intervals during phase I (0–15 min) and phase II (15–50 min). The two phases are mediated by distinctly different pathways, as indicated by their sensitivity to distinct activation of stress-induced antinociceptive pathways (Altier and Stewart, 1999) and analgesic drugs. For example, all formalin-induced responses are centrally mediated, as indicated by the ability of opioid compounds to inhibit both Phase I and Phase II responses. However, Phase I responses are relatively more sensitive to desensitization of primary afferent C-fibers by capsaicin whereas phase II responses are relatively more sensitive to non-steroidal anti-inflammatory drugs such as aspirin (Shibata et al., 1989).
2.7 Abdominal stretch assay
Mice were injected intraperitoneally (i.p.) with 0.3 ml of 1% acetic acid and placed immediately in a large glass cylinder containing approximately 2 cm of bedding. The number of abdominal stretches in a 10-min interval was counted beginning 5 min after injection (Fang et al., 2003). The abdominal stretch assay is widely believed to reflect a mild chemical nociceptive response that is used to screen for non-narcotic analgesics as it involves centrally mediated behaviors in response to the peripheral release of compounds, such as prostaglandins (Collier et al., 1968).
2.8 Tail flick assay
Animals were manually restrained and the tail submerged to a distance of 1 cm from the base of the tail in a water bath maintained at 53±1°C (Fang et al., 2003). The withdrawal latency was defined as the time required for the animal to withdraw its tail from the water and is widely used to reflect thermal nociception. To avoid tissue damage, cut-off times of 15 sec were utilized.
2.9 Tissue collection, staining and counting of the mast cells
Mice were euthanized at the same time of day to avoid any influence of circadian rhythm. Mice were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) prior to transcardial perfusion with 15 ml of ice-cold phosphate-buffered saline (PBS, pH 7.4) followed by 35 ml of ice-cold PBS containing 4% formaldehyde (pH 6.9). After perfusion, brains were rapidly removed and stored in the same fixative solution overnight and thereafter in PBS solution containing 30% sucrose for an additional 2 days at 4°C. The diencephalon from the anterior commissure to the rostral midbrain was cut at 40 μm in the coronal plane using a freezing sliding microtome. Tissue slices were mounted on gelatin-coated slides and stained with 0.125% acidified (pH: 2–2.5) aqueous toluidine blue O solution (JT Baker Chemical, Phillipsburg, NJ) for 30 min. After staining, sections were dehydrated in increasing alcohol series, dipped in xylene, and coverslipped using DPX solidifying mountant (Sigma). Cells were mapped based on The Mouse Brain Stereotaxic Coordinates (Paxinos and Franklin, 2001).
Toluidine blue stain attaches to glycosaminoglycans in mast cell granules allowing mast cells to be identified by their metachromatic, purple, cytoplasmatic granules, in contrast to the neurons and surrounding tissue, which are stained light blue. The degree of degranulation is commonly used to reflect activation of mast cells. Granulated mast cells are dark, the metachromatically stained granules packed densely in the cells with no individually isolated granules detectable (Fig. 1). Degranulated mast cells are pinker and paler in appearance, their individual granules are distinctly separated or completely absent. In case of ongoing degranulation, toluidine blue-stained granules are found in close vicinity to the cell. We have found a high correspondence (95%) between the findings by different observers using this approach. Because the numbers of mast cells in rodents vary widely among sections and are distributed in clusters near the vasculature, serial reconstruction of the whole thalamic area is necessary to account for all cells (Coggeshall and Lekan, 1996; Guillery and Herrup, 1997). Mast cells with visible nuclei in the focal plane were counted and the Abercrombie correction factor was used to minimize the possibility of double counting of cells in consecutive tissue sections (Abercrombie, 1946).
Figure 1.
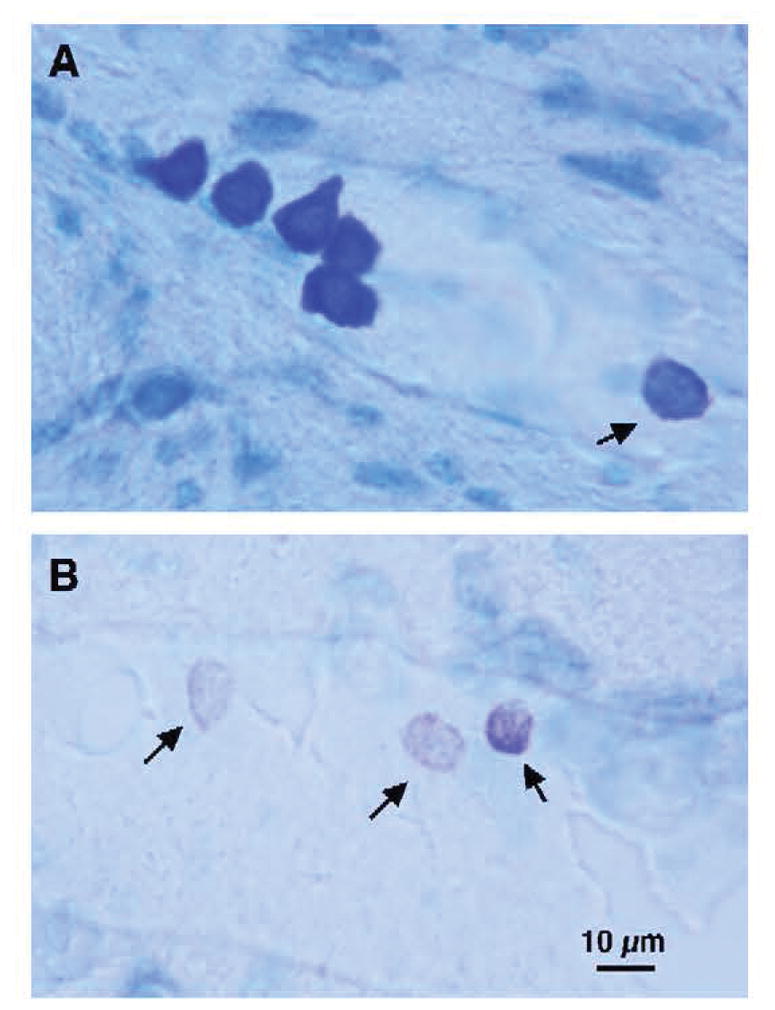
Characteristic mast cells stained with toluidine blue (dark bluish black) in the murine thalamus. (A) The five mast cells on the left illustrate granulated mast cells containing such a high density of granules that each is only individually visible at high magnification. On the right, the lighter cell (small arrow) exemplifies a partially degranulated mast cell with only a few intact and scattered granules remaining. (B) Three partially degranulated mast cells (small arrows) are stippled (pinkish-violet) on a background of pale blue parenchymal cells. Magnification indicated by the bar in panel B.
2.10 Data analysis
Mean values (± S.E.M.) are presented throughout the figures. Statistical analysis of the results was performed using a two-tailed Student’s unpaired t test between two groups or a one-way ANOVA followed by post hoc Tukey’s test for comparison between multiple groups tested at the same time, as indicated. We also determined the degree of correlation between the behavioral and mast cell parameters of mice using the Pearson’s correlation coefficient (r). In all cases, a difference was considered significant if the probability that it occurred because of chance alone was less than 5% (P<0.05).
3. Results
3.1 Does degranulation of toluidine blue-stained thalamic cells correlate with nociceptive behaviors?
Dynorphin 1–17 and von Frey Fiber Sensitivity
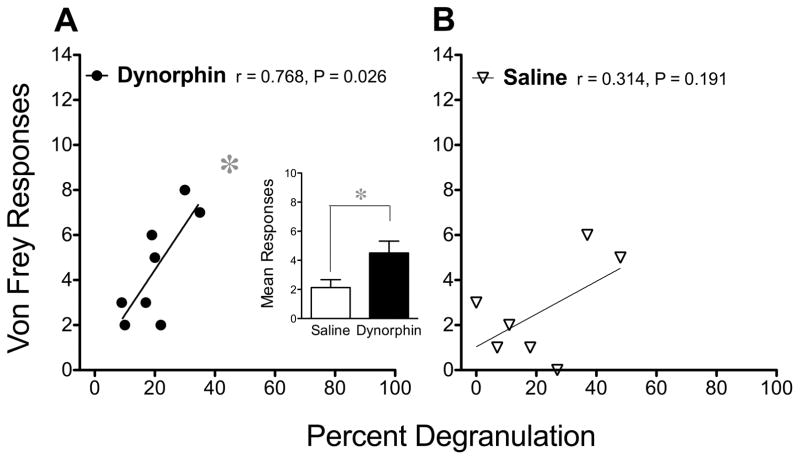
Degranulation of mast cells can be produced by many positively charged peptides (reviewed by Mousli et al., 1990), including the endogenously occurring opioid peptide dynorphin (Sugiyama and Furuta, 1984). This compound not only induces mast cell degranulation, it also enhances tactile nociceptive sensitivity when injected i.t. in mice (Laughlin et al., 2001). Consistent with this, we observed an increase in the mean number of behavioral responses to a von Frey fiber, 24 h after the last of three daily injections of dynorphin A (1–17) (3 nmol/10 μl i.t.) when compared to the sensitivity to von Frey fibers after saline (Fig. 2 insert). When killed immediately after behavioral testing, von Frey fiber responses correlated positively with the percent of mast cells that were found to be degranulated in the thalamus of mice injected with dynorphin (Fig. 2A). This correlation suggests that the greater the degree of mast cell activity, the greater the sensitivity of mice to tactile mechanical nociception. Comparable values from saline-injected controls formed no significant correlation (Fig. 2B). In addition, no statistically significant correlations between the number of mast cells and nociceptive behaviors were found.
Figure 2.
Correlation between the percent of mast cells that are degranulated and von Frey fiber sensitivity after dynorphin A (1–17). (A) Dynorphin (n=8) or (B) saline (n=7) was injected daily for 3 days (3 nmol/10 μl i.t.) in male and female mice and von Frey fiber sensitivity measured 24 h after the last injection. The insert depicts the mean (±SEM) number of behavioral responses to a #3.84 von Frey fiber in each group (males and females housed and tested separately). A significant difference between these means was determined using an unpaired Student’s t-test. The relationship between thalamic mast cell degranulation and von Very fiber responses in the main graphs were studied by calculating correlation coefficients (r) and the probability that each correlation is significant (P), as indicated, after injections of dynorphin or saline. Throughout the figures, Pearson’s correlation coefficients, Student’s t-tests and analysis of variance (ANOVA) each used a cutoff of P < 0.05 as statistically significant, as indicated by an asterisk.
Nerve growth factor (NGF) and Tail Flick Latencies
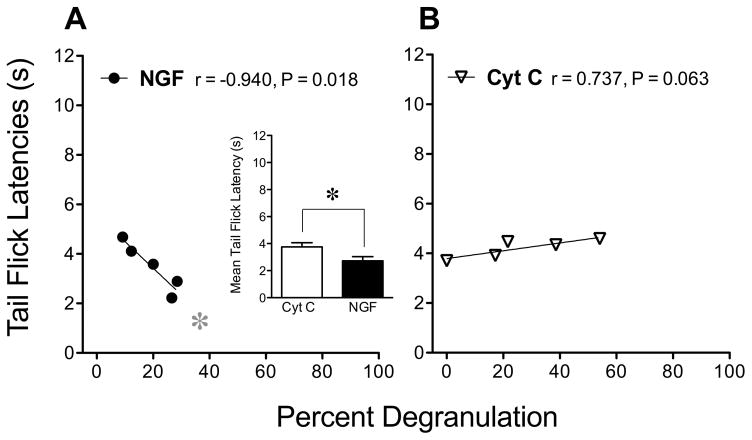
NGF can also activate mast cells (Horigome et al., 1993) and mast cells can synthesize and release NGF (Leon et al., 1994). Because NGF has been shown to cause thermal hyperalgesia, we studied the effect of centrally injected NGF on the degranulation of mast cells in the thalamus relative to tail flick latencies after injection of this protein. Consistent with previous studies (Hao et al., 2000), i.c.v. injection of NGF (1 μg/10 μl) appeared to induce thermal hyperalgesia 48 h later, as indicated by decreases in the mean tail flick latencies measured at 53°C when compared to those after injection of cytochrome C, the inactive control protein (Fig. 3 insert). In the group injected with NGF, tail flick latencies correlated negatively with the percent of thalamic mast cells that were degranulated (Fig. 3A) such that the greater the incidence of degranulation, the lower the tail flick threshold. Tail flick latencies after injection of cytochrome C did not correlate with thermal sensitivity (Fig. 3B). In addition, no statistically significant correlations between the number of mast cells and nociceptive behaviors were found.
Figure 3.
Correlations between the percent of mast cells that are degranulated and decreases in tail flick latency induced by NGF. (A) NGF (n=5) or (B) cytochrome C (n=5) was injected (1 μg/10 μl, i.c.v. in male mice) and 48 h later thermal nociception measured using the tail flick assay at 53°C. The insert depicts the mean (±SEM) tail flick latencies in the corresponding groups of mice. Significant differences in these means were determined using an unpaired Student’s t-test and was indicated by an asterisk. The relationships between mast cell degranulation and tail flick latencies were studied by calculating correlation coefficients (r) and the probability that the correlation is significant (P).
3.2 Does Compound 48/80 induce hyperalgesia?
Effect of Compounds 48/80 on Tail Flick Latencies
Administration of compound 48/80, a mixed polymer, is widely used for non-IgE-dependent stimulation of mast cells. We used this polymer to determine whether compounds that are able to induce the degranulation of mast cells but are devoid of either neurotrophic or kappa-opioid receptor activity, are also capable of inducing hyperalgesia when injected centrally. We found that compound 48/80 (1 μg/10 μl), injected intrathecally 60 min after an intrathecal injection of saline, decreased tail flick latencies 24 h later when compared to their baseline control values (Fig. 4), suggesting thermal hyperalgesia, while those injected with saline did not (data not shown). Saline was injected as a control for the effect of cromolyn, as described in the next section.
Figure 4.
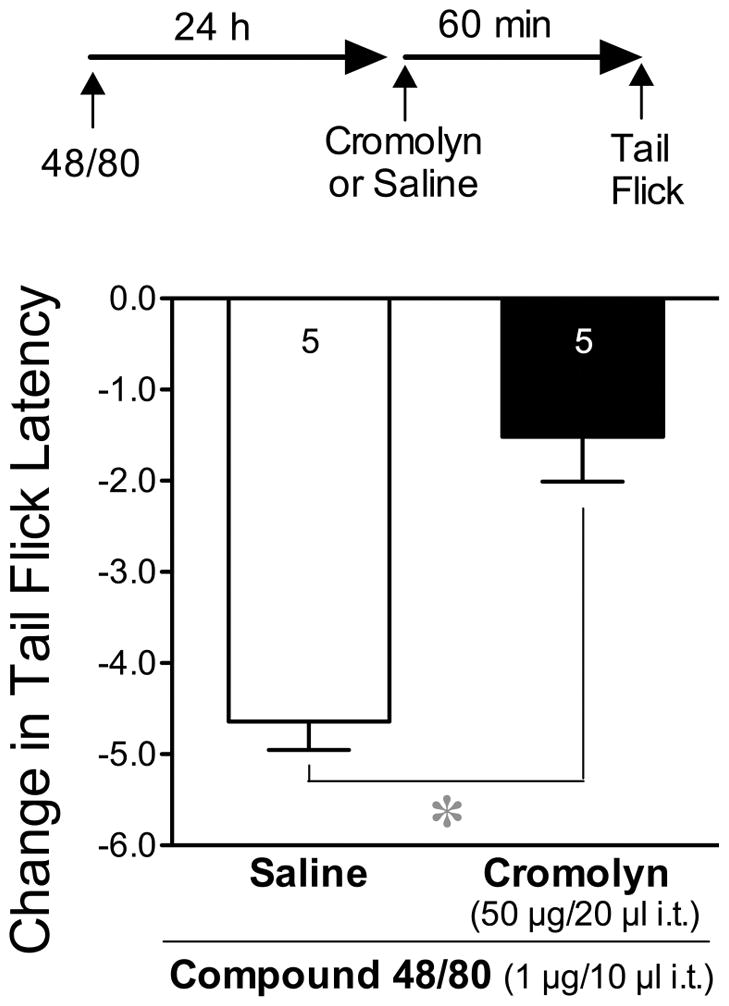
Thermal hyperalgesia induced by compound 48/80 and inhibited by cromolyn. Data represent the mean (±SEM) decrease in tail flick latencies (53°C), when compared to their baseline values before injection, 24 h after i.t. injections of compound 48/80 (1 μg/10 μl) and 60 min after an i.t. injection of saline or cromolyn (50 μg/20 μl). Baseline values of groups taken prior to injections did not differ from each other. The number of mice per group throughout the remaining figures is indicated at the base of the columns or in the key of each figure. Significant differences in the mean decreases in tail flick latencies were determined by comparing the two treatment groups using an unpaired Student’s t-test.
3.3 Does cromolyn inhibit hyperalgesia?
Effect of Cromolyn on Compound 48/80-induced Decreases in Tail Flick Latencies
Cromolyn was used to determine whether compounds that are capable of stabilizing murine mast cells (Mazzari et al., 1996) prevent the apparent hyperalgesic effects produced by diverse compounds whose actions include an ability to induce mast cell degranulation. Pretreatment of mice with cromolyn (50 μg/20 μl i.t) 60 min prior to compound 48/80 (1 μg/10μl i.t.) prevented the decrease in tail flick latencies, consistent with inhibition of the thermal hyperalgesic effect of compound 48/80 (Fig. 4).
Cromolyn on Dynorphin-induced Increases in von Frey Fiber Sensitivity
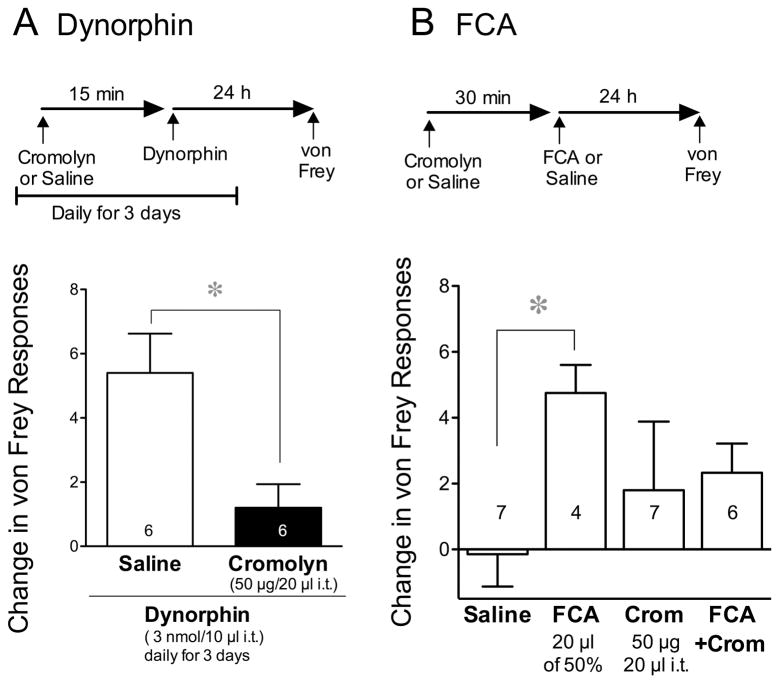
To determine whether the increase in von Frey fiber sensitivity induced by dynorphin not only correlates with mast cell degranulation, but also depends upon degranulation for its activity, we injected cromolyn (50 μg/20 μl i.t.) or saline prior to each daily injection of dynorphin (3 nmoles/10 μl i.t.). Daily injections of dynorphin 15 min after saline increased the number of von Frey responses 24 h following the last injection, consistent with mechanical hyperalgesia. However, this effect of dynorphin was prevented by cromolyn (50 μg/20 μl, i.t.), delivered prior to each injection of dynorphin (Fig. 5A). There was no effect of this dose and route of cromolyn on baseline von Frey fiber sensitivity in the absence of dynorphin, suggesting that the effect of cromolyn was selectively anti-hyperalgesic rather than antinociceptive.
Figure 5.
Effect of cromolyn on mechanical hyperalgesia induced by dynorphin A (1–17) and by Freund’s complete adjuvant (FCA). (A) Data reflect the mean (±SEM) change in number of #3.84 von Frey fiber responses 24 h after the last of three daily injections of dynorphin (3 nmol/10 μl i.t.), each given 15 min after an i.t. injection of saline or cromolyn (50 μg/20 μl). Significant differences were determined using a Student’s t-test. (B) Data reflect the mean change in tactile sensitivity to a von Frey fiber 24 h after an intradermal injection of saline or FCA into the plantar surface of both hind limbs (20 μl of 50%). Prior to each injection, baseline values of groups depicted in each panel did not differ from each other. Saline or cromolyn (Crom, 50 μg/20 μl) was injected i.t. 30 min before FCA or saline, as indicated, and again 30 min before nociceptive testing. The effect of these treatments on mechanical sensitivity was determined using one-way ANOVA followed by Tukey’s post hoc analysis.
Cromolyn on FCA-induced Increases in von Frey Fiber Sensitivity
In light of its effect on dynorphin-induced von Frey fiber sensitivity, we explored the effect of cromolyn on the increase in tactile sensitivity induced by FCA, a model of inflammatory pain whose effect on central mast cells is not known. Twenty-four h after injection into both hind paws, FCA (20 μl of 50% i.d.) increased responses to a von Frey fiber (#3.84). Injection of saline 30 min prior to FCA and again 30 min prior to testing for von Frey fiber sensitivity failed to inhibit FCA-induced increases in von Frey fiber sensitivity compared to those after saline (Fig. 5B). However, pretreatment with cromolyn prevented the development of the hyperalgesic effect of FCA when compared to the effect of saline plus FCA.
Cromolyn on Formalin-induced Behaviors
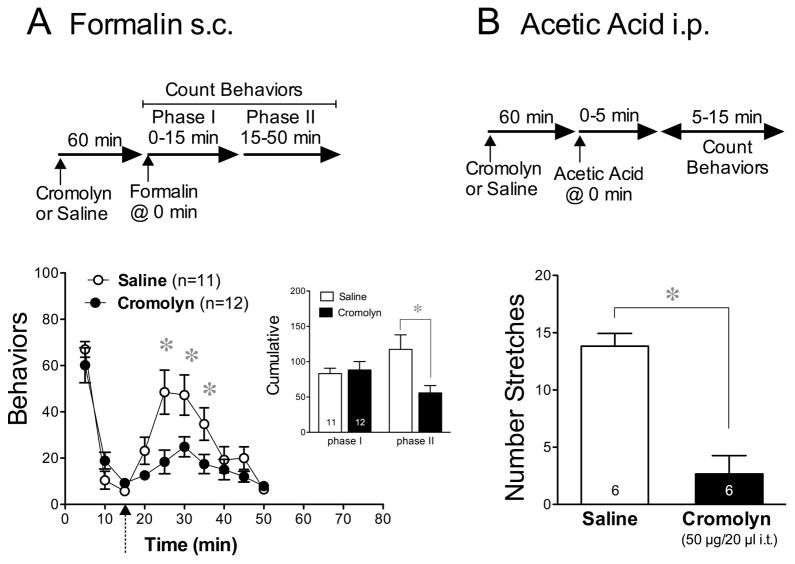
We then determined whether the inhibitory effect of cromolyn is restricted to mechanical and thermal models of hyperalgesia or whether other modalities of nociception are susceptible. Using the formalin model of chemical pain allowed us to compare the effect of cromolyn on chemical nociceptive responses that are produced by direct activation of primary afferent fibers (phase I, 0–5 min) to those resulting from central sensitization of distinct nociceptive pathways (phase II, 5–15 min). The mean cumulative behaviors observed during these two phases are summarized in the insert of Fig. 6A.
Figure 6.
Effect of cromolyn on chemical nociception induced by formalin and by acetic acid. (A) Time-course of the mean number of nociceptive behaviors in response to formalin (20 μl of 5% s.c.) injected into one hind paw 15 min after an i.t. injection of either cromolyn (50 μg/20 μl) or saline. The insert depicts the mean (±SEM) cumulative number of behavioral responses induced in these groups during phase I compared to phase II. The division between the two phases is indicated on the main graph by the dotted arrow. (B) The mean (±SEM) number of abdominal stretches induced by a 0.3 ml injection of 1% acetic acid delivered i.p. 1 h after an i.t. injection of cromolyn (50 μg/20 μl) or saline. Throughout the panels, differences between treatment groups and between values at each time-interval were determined using an unpaired Student’s t-test.
Pretreatment with cromolyn (50 μg/20 μl, i.t.) 15 min before injection of formalin attenuated the flinching and licking behaviors during phase II but not phase I of the formalin assay (Fig. 6A). When separate groups of mice were pretreated with cromolyn at the same dose (50 μg) but in a smaller 5-μl volume, to restrict the distribution of cromolyn to the area of the spinal cord, behavioral responses to formalin were not reduced in spite of the higher concentration of the injected drug in the spinal area (data not shown). This suggests that the influence of cromolyn on formalin when injected in the 20-μl volume came about by its action in the brain rather than in the spinal cord area. It also indicates that the action of cromolyn in the spinal cord was insufficient to inhibit formalin-induced nociceptive activity.
Cromolyn on Acetic Acid-induced Behaviors
To confirm the inhibitory effect of cromolyn on chemical nociceptive pathways that are dependent on tonic sensitization, we also tested the effect of cromolyn on acetic acid-induced abdominal stretching behaviors. Using this assay, the i.p. injection of a noxious compound elicits a characteristic behavioral response that can be quantified. Intrathecal injection of cromolyn (50 μg/20 μl i.t.) 60 min before an i.p. injection of 1% acetic acid inhibited the mean number of abdominal stretches compared to those induced by the acid after pretreatment with vehicle (Fig. 6B).
4. Conclusions
The present study provides several lines of evidence supporting the possibility that mast cells located in the CNS contribute to increased sensitivity of various nociceptive modalities, similar to the well-known sensitizing effect of mast cells on nociceptors in the periphery.
Our first line of evidence is the strong correlational data between the incidence of mast cell degranulation in the CNS and both thermal (tail flick) and mechanical (von Frey) hyperalgesia, correlations that were not statistically significant in their respective controls. This approach is similar to that used to study peripheral mast cells in a population of patients with irritable bowel syndrome in which the number of activated mast cells within 5 μm of nerves in the colonic mucosa correlated strongly with the persistence and severity of their abdominal pain (Barbara et al., 2004). In our study, decreases in the tail flick latency indicate that thermal hyperalgesia was induced by NGF, a neurotrophic compound (Hao et al., 2000) that also activates mast cells (Horigome et al., 1993) and increases in von Frey fiber sensitivity indicate that tactile mechanical hyperalgesia was induced by dynorphin, a kappa opioid compound (Laughlin et al., 2001) that also degranulates mast cells (Sugiyama and Furuta, 1984). The correlations confirm the presence of these compounds in the brain and verify their ability to degranulate mast cells at the doses used.
These correlational data raise the possibility that hyperalgesia is linked to the degranulation of mast cells in the CNS, similar to the contribution of peripheral mast cells to hyperalgesia. However, correlations alone do not establish whether thalamic mast cells increase nociceptive sensitivity or, whether activation of nociceptive pathways degranulates thalamic mast cells. To explore whether degranulation of central mast cells is sufficient to cause hyperalgesia, our second line of inquiry was to establish compound 48/80’s effect on nociception by its action of tail flick latencies. In spite of the fact that compound 48/80, a mast cell degranulating agent, lacked any neurotrophic or kappa-opioid receptor activity, when injected i.t., it decreased tail flick latencies, indicating a decreased thermal nociceptive threshold. This is consistent with its effect peripherally in mice where topical application of compound 48/80 causes thermal hyperalgesia (Chatterjea et al., 2012). When injected i.p., compound 48/80 also excites meningeal nociceptors (Levy et al., 2007), increases C-Fos expression in spinal dorsal horn neurons (Levy et al., 2012), and causes cutaneous and delayed tactile hypersensitivity, all in a cromolyn-sensitive fashion (Levy et al., 2012), indicating that the effects of compound 48/80 are mediated by mast cell activity.
The third line of investigation, to determine the effect of compounds that stabilize mast cells on hyperalgesia, led us to examine the effect of centrally injected cromolyn on models of hyperalgesia. In spite of its lack of antinociceptive efficacy on thermal nociception (tail flick), central injections of cromolyn attenuated the thermal hyperalgesia produced by compound 48/80 i.t., consistent with the peripheral effect of cromolyn on compound 48/80 injected i.p. (Levy et al., 2012). Cromolyn also prevented tactile hyperalgesia induced by dynorphin and by FCA, in spite of its lack of an antinociceptive effect on tactile nociceptive responses (von Frey fiber) in control mice. Peripheral effects of cromolyn (i.p.) in rats also suppress both thermal and mechanical hyperalgesia induced by nerve injury (Zuo et al., 2003). These results are consistent with depletion of peripheral mast cell granular content (via chronic systemic administration of compound 48/80) that attenuates mechanical sensitivity induced by FCA in rats (Woolf et al., 1996).
The fourth line of investigation was to determine whether the role of mast cells in chemical pain by direct activation of nociceptors differs from that in chemical pain induced by sensitization of nociceptive pathways. Cromolyn inhibited tonic behavioral responses following an injection of formalin (phase II), and abdominal stretch behaviors produced by an injection of acetic acid, both believed to result from sensitization of nociceptive pathways. However, in these same mice cromolyn was without effect on phase I formalin-induced responses thought to reflect a transient and direct activation of nociceptors. These findings closely mimic the spectrum of effects of cromolyn on peripheral mast cells as depletion of peritoneal mast cell populations (through multiple and increasing doses of compound 48/80 i.p.) also decreases acetic acid-induced behaviors in mice (Ribeiro et al., 2000) and reduces formalin-evoked flinching during phase II (Parada et al., 2001).
When injected peripherally, it is unlikely that cromolyn inhibits mast cells in the CNS due to poor penetration of the blood brain barrier (Norris, 1996). Similarly, when injected centrally, the effects of cromolyn are not likely the result of peripheral actions as the dose used was small and delivery both i.c.v. and i.t. are highly reliable. Although spinal mast cells may be affected by i.t. injections, based on the volume injected, the long time-course of effects examined, and the location of mast cells more numerous in the thalamus than in the spinal cord, mast cells in the brain appear to be the most probable site of action. In support of this was the hyperalgesia produced by i.c.v. injections of NGF and the corresponding degranulation of mast cells in the thalamus. Although dynorphin was injected i.t., the volume delivered would not have remained exclusively in the spinal cord and the behavioral effects produced also correlated well with the degranulation of mast cells in the thalamus, an important area of sensory neurotransmission. Importantly, intrathecal injection of cromolyn in a small volume failed to prevent formalin-induced behaviors while the same dose in a larger volume did. This indicates that the action of cromolyn in the spinal cord is insufficient to inhibit nociceptive activity and suggests that the influence of cromolyn in a 20 μl-volume was probably due to its action in the brain. In summary, our data indicate that centrally located mast cells play a role in the development of hyperalgesia and suggest that the primary site of their action is in the brain.
In mice, mast cells are selectively distributed in the thalamus and not in the remaining parenchyma of the CNS (Florenzano and Bentivoglio, 2000; Silverman et al., 2000). The ventral posterolateral (VPL) and ventral posteromedial (VPM) thalamic nuclei are somatotopically organized such that innervation from the body forms a homunculus within these two nuclei. The more densely innervated the body part, the larger the area on the homunculus. Degranulation of a single mast cell located in the periphery would be expected to impact only a small and localized area of the body. However, a single mast cell located in somatosensory areas of the thalamus is poised to influence input from a much larger area of the body. Based on this organization, the relative influence of mast cells along pain pathways might be greater in the CNS than in the periphery. Furthermore, their effect might be especially large in the relatively smaller areas of the homunculus that receive input from larger, but less densely-innervated regions of the body.
The population of central mast cells is not static. Repeated activation of mast cells is known to recruit additional cells, enlarging the existing population (Esposito et al., 2001; Halova et al., 2012; Lindsberg et al., 2010; Zhuang et al., 1996). If thalamic mast cells are, in fact, pro-nociceptive, one might speculate that recruitment of additional mast cells to the CNS would exacerbate hyperalgesia. Consistent with this, unilateral spinal nerve ligation leads to an asymmetrical distribution of mast cells in the thalamus of mice and the intensity of tactile mechanical hyperalgesia induced by the ligation corresponds temporally and in magnitude with the relative incidence of thalamic mast cells contralateral to the ligature (Taiwo et al., 2005).
We speculate that mast cells residing along nociceptive pathways in the thalamus contribute to the development of hyperalgesia, just as they do in the periphery. However, in addition to the degranulation of mast cells, compound 48/80 also has a direct excitatory action on enteric neurons and visceral afferents in guinea pigs (Schemann et al., 2012), NGF interacts with TrkA receptors (Hao et al., 2000) and dynorphin binds kappa-opioid receptors (Chavkin and Goldstein, 1981; Zhu et al., 1997), among others (Laughlin et al., 2001). In a similar fashion, cromolyn produces direct effects on neuronal activity other than its well-characterized stabilization of mast cells (Mazzari et al., 1996). Thus, it is prudent to consider the possibility that, in spite of their common link to mast cells, their effects on nociceptive activity may be unrelated to mast cells. However, the fact that cromolyn failed to influence tail flick latecies or von Frey fiber sensitivity in the absence of a hyperalgesic manipulation indicates that cromolyn has no inherent antinociceptive activity, as one might have expected if it acted on nociceptive neurons directly. Consistent with this, the two distinct chemical nociceptive pathways activated by formalin (reflected by phase I and II behaviors), were differentially sensitive to cromolyn, inhibiting phase II responses but not phase I. This selectivity of action argues against a simple inhibitory effect of cromolyn and together suggest that cromolyn prevents sensitization of nociceptive pathways by inhibiting mast cell activity rather than neuronal pathways themselves.
Even histologic documentation of mast cell degranulation by each hyperalgesic compound and of mast cell stabilization by cromolyn would not provide cause and effect proof that their effects on nociception are brought about by their effects on mast cells. However, the strong correlations between hyperalgesic responses and degranulation of central mast cells together with the recapitulation of hyperalgesia by compound 48/80, the inhibition of these behaviors by cromolyn, and the inhibition of tonic but not phasic chemically-induced behaviors together provide four convergent lines of evidence supporting the possibility that mast cells in the CNS contribute to sensitization of central nociceptive pathways leading to hyperalgesia.
This study is clinically relevant as stress is linked to exacerbation of pain in many chronic painful conditions (Theoharides et al 1995). Mast cells are not only a target for corticotropin-releasing factor (CRF) (Theoharides et al 1995, McEvoy et al 2001, Singh et al 1999), leading to mast cell degranulation, they are also a source of CRF and urocortins (Kempuraj et al 2004). This suggests an autocrine function that may sustain their activity centrally. If mast cells in the CNS play a role in hyperalgesia, degranulation in response to CRF would be expected to enhance pain sensitivity. Increases in mast cell activity and number in response to repeated stress-induced degranulation might not only enhance pain, as suggested by spinal nerve ligation studies (Taiwo et al., 2005), but also provide a new target for therapeutic interventions. Consistent with the possibility of a link between stress, CRF and hyperalgesia, we previously found that swim stress-induced musculoskeletal hyperalgesia in mice is brought about, in part, by CRF2 receptor activity (Abdelhamid et al., 2013), a type that is expressed on mast cells (Theoharides et al 1995, McEvoy et al 2001, Singh et al 1999).
In summary, degranulation of mast cells in the CNS appears to be sufficient to induce hyperalgesia. Inhibition of mast cell activity selectively inhibits the effect of hyperalgesic manipulations but has no effect on thermal or tactile nociception. This, together with the inhibition of phase II formalin- and acetic acid-induced behaviors, but not formalin’s phase I behaviors, by centrally injected cromolyn provide multiple converging lines of evidence that support the possibility that degranulation of centrally located mast cells plays a role in the modulation of nociception, similar to their effect in the periphery. Until compounds are available that induce or inhibit degranulation more selectively, our data do not prove the hypothesis that mast cells in the brain contribute to the hyperalgesia. However, because the dominant explanatory variable is an effect on mast cells, these data merit further study to address this possibility.
Significance.
Hyperalgesia induced by spinal nerve ligation corresponds temporally and in magnitude with degranulation of thalamic mast cells. Here we provide evidence that hyperalgesia induced by NGF, formalin and dynorphin also may depend on mast cell degranulation in the CNS whereas cromolyn, a mast cell stabilizer, blocks these effects in mice.
Acknowledgments
Funding source:
This work was funded by NIH Grant AR056092 (to A.A.L.) from the National Institutes of Arthritis and Musculoskeletal and Skin Diseases.
This work was supported by a grant from NIH from the National Institutes on Arthritis and Musculoskeletal and Skin Diseases [AR056092]. The authors thank Dr. Cholawat Pacharinshak and Paul Overback for their help with the behavioral testing, Paul R. Larson and Robert C. Larson for the excellent technical help during the tissue preparation and Myra G. Nunez for the preparation of the figures.
Footnotes
No conflicts of interest.
Author contributions
As senior author, A.A.L. directed the study and edited the manuscript. K.J.K. did the animal testing, part of the tissue preparation, histological evaluation and participated in the writing of sections of the manuscript. CLK verified the data entry, formatted the graphs, composed, and prepared the final manuscript for publication. All authors discussed the results and commented on the manuscript.
References
- Abdelhamid RE, Kovacs KJ, Pasley JD, Nunez MG, Larson AA. Forced swim-induced musculoskeletal hyperalgesia is mediated by CRF2 receptors but not by TRPV1 receptors. Neuropharmacology. 2013;72:29–37. doi: 10.1016/j.neuropharm.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Altier N, Stewart J. Minireview: The Role of Dopamine in the Nucleus Accumbens in Analgesia. Life Sci. 1999;65:2269–2287. doi: 10.1016/s0024-3205(99)00298-2. [DOI] [PubMed] [Google Scholar]
- Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229:26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Chatterjea D, Wetzel A, Mack M, Engblom C, Allen J, Mora-Solano C, Paredes L, Balsells E, Martinov T. Mast cell degranulation mediates compound 48/80-induced hyperalgesia in mice. Biochem Biophys Res Commun. 2012;425:237–243. doi: 10.1016/j.bbrc.2012.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Goldstein A. Specific receptor for the opioid peptide dynorphin: structure--activity relationships. Proc Natl Acad Sci U S A. 1981;78:6543–6547. doi: 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre Melzack 1992
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol. 1968;32:295–210. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dropp JJ. Mast cells in the central nervous system of several rodents. Anat Rec. 1972;174:227–37. doi: 10.1002/ar.1091740207. [DOI] [PubMed] [Google Scholar]
- Dropp JJ. Mast cells in mammalian brain. Acta Anat (Basel) 1976;94:1–21. doi: 10.1159/000144540. [DOI] [PubMed] [Google Scholar]
- Esposito P, Gheorghe D, Kandere K, Pang X, Connolly R, Jacobson S, Theoharides TC. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- Fairbanks C. Spinal delivery of analgesics in experimental models of pain and analgesia. Advanced Drug Delivery Reviews. 2003;55:1007–1041. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Fang M, Kovacs KJ, Fisher LL, Larson AA. Thrombin inhibits NMDA-mediated nociceptive activity in the mouse: possible mediation by endothelin. J Physiol. 2003;549:903–917. doi: 10.1113/jphysiol.2002.036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florenzano F, Bentivoglio M. Degranulation, density, and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J Comp Neurol. 2000;424:651–669. [PubMed] [Google Scholar]
- Galli SJ. New insights into “the riddle of the mast cells”: microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990;62:5–33. [PubMed] [Google Scholar]
- Goldschmidt RC, Hough LB, Glick SD, Padawer J. Mast cells in rat thalamus: nuclear localization, sex difference and left-right asymmetry. Brain Res. 1984;323:209–17. doi: 10.1016/0006-8993(84)90291-9. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Herrup K. Quantification without pontification: choosing a method for counting objects in sectioned tissues. J Comp Neurol. 1997;386:2–7. doi: 10.1002/(sici)1096-9861(19970915)386:1<2::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hao J, Ebendal T, Xu X, Wiesenfeld-Hallin Z, Eriksdotter Jonhagen M. Intracerebroventricular infusion of nerve growth factor induces pain- like response in rats. Neurosci Lett. 2000;286:208–212. doi: 10.1016/s0304-3940(00)01107-1. [DOI] [PubMed] [Google Scholar]
- Halova I, Draberova L, Draber P. Mast cell chemotaxis - chemoattractants and signaling pathways. Front Immunol. 2012;3:119. doi: 10.3389/fimmu.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron A, Dubayle D. A focus on mast cells and pain. J Neuroimmunol. 2013;264:1–7. doi: 10.1016/j.jneuroim.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Horigome K, Pryor JC, Bullock ED, Johnson EM., Jr Mediator release from mast cells by nerve growth factor. Neurotrophin specificity and receptor mediation. J Biol Chem. 1993;268:14881–14887. [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Kempuraj D, Papadopoulou NG, Lytinas M, Huang M, Kandere-Grzybowska K, Madhappan B, Boucher W, Christodoulou S, Athanassiou A, Theoharides TC. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinology. 2004;145:43–8. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Hernadi I, Wilhelm M. Mast cells modulate maintained neuronal activity in the thalamus in vivo. J Neuroimmunol. 2006;171:1–7. doi: 10.1016/j.jneuroim.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Laughlin TM, Larson AA, Wilcox GL. Mechanisms of induction of persistent nociception by dynorphin. J Pharmacol Exp Ther. 2001;299:6–11. [PubMed] [Google Scholar]
- Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Kainz V, Burstein R, Strassman AM. Mast cell degranulation distinctly activates trigemino-cervical and lumbosacral pain pathways and elicits widespread tactile pain hypersensitivity. Brain Behav Immun. 2012;26:311–317. doi: 10.1016/j.bbi.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab. 2010;30:689–702. doi: 10.1038/jcbfm.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Kawakita K, Kiso Y, Nakano T, Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989;142:927–931. [PubMed] [Google Scholar]
- Mazzari S, Canella R, Petrelli L, Marcolongo G, Leon A. N-(2-hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by down-modulating mast cell activation. Eur J Pharmacol. 1996;300:227–236. doi: 10.1016/0014-2999(96)00015-5. [DOI] [PubMed] [Google Scholar]
- McEvoy AN, Bresnihan B, FitzGerald O, Murphy EP. Corticotropin-releasing hormone signaling in synovial tissue from patients with early inflammatory arthritis is mediated by the type 1 alpha corticotropin-releasing hormone receptor. Arthritis Rheum. 2001;44:1761–7. doi: 10.1002/1529-0131(200108)44:8<1761::AID-ART311>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Mousli M, Bueb JL, Bronner C, Rouot B, Landry Y. Trends Pharmacol Sci. 1990;11:358–362. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- Norris AA. Pharmacology of sodium cromoglycate. Clin Exp Allergy. 1996;26(Suppl 4):5–7. doi: 10.1111/j.1365-2222.1996.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Oliveira SM, Drewes CC, Silva CR, Trevisan G, Boschen SL, Moreira CG, de Almeida Cabrini D, Da Cunha C, Ferreira J. Involvement of mast cells in a mouse model of postoperative pain. Eur J Pharmacol. 2011;672:88–95. doi: 10.1016/j.ejphar.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Parada CA, Tambeli CH, Cunha FQ, Ferreira SH. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience. 2001;102:937–944. doi: 10.1016/s0306-4522(00)00523-6. [DOI] [PubMed] [Google Scholar]
- Paus R, Theoharides TC, Arck PC. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006;27:32–9. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KJB. The Mouse Brain Stereotaxic Coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- Persinger MA. Handling factors not body marking influence thalamic mast cell numbers in the preweaned albino rat. Behav Neural Biol. 1980;30:448–59. doi: 10.1016/s0163-1047(80)91283-2. [DOI] [PubMed] [Google Scholar]
- Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato AB, Poole S, Ferreira SH, Cunha FQ. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol. 2000;387:111–118. doi: 10.1016/s0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- Rieselbach RE, Di Chiro G, Freireich EJ, Rall DP. Subarachnoid distribution of drugs after lumbar injection. New Engl J Med. 1962;267:1273–1278. doi: 10.1056/NEJM196212202672502. [DOI] [PubMed] [Google Scholar]
- Schemann M, Kugler EM, Buhner S, Eastwood C, Donovan J, Jiang W, Grundy D. The mast cell degranulator compound 48/80 directly activates neurons. PLoS One. 2012;7:e52104. doi: 10.1371/journal.pone.0052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Ohkubo T, Takahashi H. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38:347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: A link to neurogenic skin disorders. Brain Behav Immun. 1999;13:225–39. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci. 2000;20:401–408. doi: 10.1523/JNEUROSCI.20-01-00401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama K, Furuta H. Histamine release induced by dynorphin-(1–13) from rat mast cells. Jpn J Pharmacol. 1984;35:247–252. doi: 10.1254/jjp.35.247. [DOI] [PubMed] [Google Scholar]
- Taiwo OB, Kovacs KJ, Sun Y, Larson AA. Unilateral spinal nerve ligation leads to an asymmetrical distribution of mast cells in the thalamus of female but not male mice. Pain. 2005;114:131–140. doi: 10.1016/j.pain.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Spanos C, Pang X, Alferes L, Ligris K, Letourneau R, Rozniecki JJ, Webster E, Chrousos GP. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–50. doi: 10.1210/endo.136.12.7588332. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–8. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma QP, Allchorne A, Poole S. Peripheral cell types contributing to the hyperalgesic action of nerve growth factor in inflammation. J Neurosci. 1996;16:2716–2723. doi: 10.1523/JNEUROSCI.16-08-02716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chien C, Lu K. Morphological, immunohistochemical and quantitative studies of murine brain mast cells after mating. Brain Res. 1999;846:30–9. doi: 10.1016/s0006-8993(99)01935-6. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Kido K, Ohtani N, Masaki E. Mast cell stabilization promotes antinociceptive effects in a mouse model of postoperative pain. J Pain Res. 2013;6:161–166. doi: 10.2147/JPR.S41527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Luo LY, Li JG, Chen C, Liu-Chen LY. Activation of the cloned human kappa opioid receptor by agonists enhances [35S]GTPgammaS binding to membranes: determination of potencies and efficacies of ligands. J Pharmacol Exp Ther. 1997;282:676–684. [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Brain mast cell degranulation regulates blood-brain barrier. J Neurobiol. 1996;31:393–403. doi: 10.1002/(SICI)1097-4695(199612)31:4<393::AID-NEU1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Distribution and local differentiation of mast cells in the parenchyma of the forebrain. J Comp Neurol. 1999;408:477–488. doi: 10.1002/(sici)1096-9861(19990614)408:4<477::aid-cne3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Perkins NM, Tracey DJ, Geczy CL. Inflammation and hyperalgesia induced by nerve injury in the rat: a key role of mast cells. Pain. 2003;105:467–479. doi: 10.1016/S0304-3959(03)00261-6. [DOI] [PubMed] [Google Scholar]