Abstract
Increasing numbers of women in the US are getting too little sleep. Inadequate sleep has been associated with impaired metabolic function and endocrine disruption. Sister Study cohort participants (n=50,884), completed baseline and follow-up questionnaires on sleep patterns. Incident breast cancers estrogen receptor (ER) status of the tumor were ascertained from questionnaires and medical records. Cox regression was used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (95% CIs). Analyses of sleep characteristics reported at the first follow-up interview included only participants who were breast cancer-free at time of follow-up interview. Over ~7 years of follow-up, 2,736 breast cancer cases (invasive and ductal carcinoma in situ) were diagnosed. There was little evidence that usual sleep duration or other sleep characteristics were associated with breast cancer. However, relative to those with no difficulty sleeping, women who reported having difficulty sleeping ≥ 4 nights a week were at an increased risk of overall (HR=1.32, 95% CI: 1.09–1.61) and postmenopausal breast cancer (HR=1.51, 95% CI 1.24–1.85). Risk of ER+ invasive cancer was elevated for women who reported having a light or television on in the room while sleeping (HR=1.20, 95% CI: 0.97–1.47) or who typically got less sleep than they needed to feel their best (HR=1.21, 95% CI: 0.98–1.50). In our study, most sleep characteristics, including sleep duration, were not associated with an increased risk although higher risk was observed for some markers of inadequate or poor quality sleep.
Introduction
Sleep quality is important for an individual’s physical and mental health and poor sleep has been associated with numerous chronic diseases1. The impact of sleep disruption and short sleep is particularly relevant as the modern work force is trending towards more variable work schedules2 as well as increasing exposure to electronic screen light in the evenings and to artificial light at night which may be impacting sleep patterns and reducing sleep quality.
Inadequate or interrupted sleep and the resulting disruptions in circadian rhythm have been associated with numerous health outcomes, including obesity and metabolic disorders,3 as well as cancer.4 In rats, constant light exposure increases susceptibility to the development of mammary tumors.5 The mechanisms underlying this effect are unknown. Exposure to artificial light at night may suppress melatonin, leading to increases in circulating estrogen levels and modulation of estrogen signaling pathways.6, 7 Melatonin may also act as a tumor suppressor, therefore lower melatonin levels have the potential to increase risk.7, 8 Shortened sleep duration, which may be considered a proxy for darkness, is also associated with decreased immune9 and metabolic function10, 11 in addition to endocrine disruption.12
Prior research on self-reported usual sleep duration and breast cancer risk has been meta-analyzed leading to the conclusion that there is little to no association.8, 13 However, most of the prior studies have been limited to just a single self-reported measure of sleep duration and with only a few studies considering additional relevant factors such as questions regarding sleep quality and/or disruptions14–17, use of medications as sleep aids14, 17, or timing of sleep (night versus day). Additionally, few studies have considered tumor hormone receptor subtype which is important as risk factors may vary by hormone receptor subtype status.18
In this prospective study, we evaluated the association between sleep characteristics and exposure to artificial nocturnal light and breast cancer risk with a focus on potential etiologic heterogeneity by estrogen-receptor (ER) status of the tumor.
Materials and Methods
Study population
The Sister Study is a prospective cohort study (n=50,884) designed to evaluate environmental and genetic risk factors for breast cancer development. Women without a personal history of breast cancer were recruited between 2003–2009 using a multi-media campaign and a network of breast cancer advocates and professionals. Eligibility criteria included being between the ages 35–74, living in the United States or Puerto Rico, and having a sister who had been diagnosed with breast cancer.19 The Sister Study was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences. All study participants provided written informed consent. In this analysis, we used data from the Sister Study Data Release 5.01, which included cases diagnosed up to August 31st, 2015.
At study baseline, participants completed an extensive computer-assisted telephone interview, in which they reported information on their demographics, medical and family history, and lifestyle factors including sleep patterns and characteristics (Figure I). During the study follow-up period, participants completed detailed questionnaires (every two to three years) and annual health updates on current risk factor information and to notify the study of changes in health. In 2012–2014, the detailed follow-up questionnaire included additional information on sleep patterns. The participation rate for the sleep follow-up questionnaire was 84%. Study participants who completed the follow-up questionnaire were slightly more likely to be non-Hispanic white, but other demographic and lifestyle factors were similar to those of the baseline study population.
Figure I.
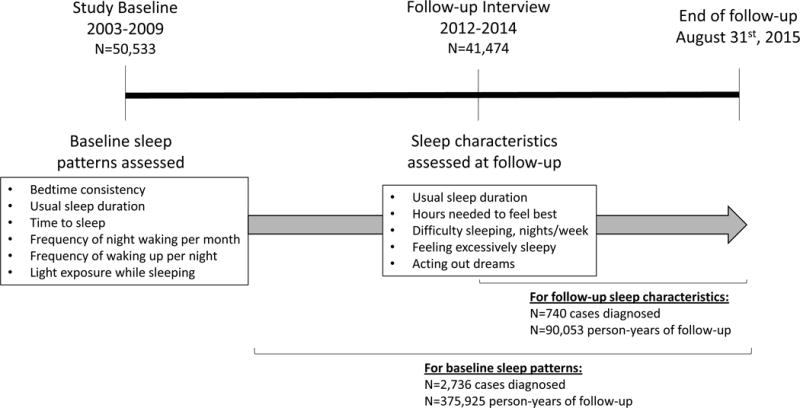
Timeline for sleep data collection and follow-up, NIEHS Sister Study.
Outcome assessment
Incident breast cancer cases, including both ductal carcinoma in situ (DCIS) and invasive disease, were ascertained via annual health updates and detailed follow-up questionnaires. Women reporting breast cancer were asked for consent to obtain their medical records to confirm the diagnosis and obtain additional information on tumor characteristics including extent of disease (invasive or in situ), stage and estrogen receptor (ER+ versus ER−) status. For this data release, 81.1% of medical records had been successfully obtained. When medical record information was not available, self-reported information was used based on very high agreement (https://sisterstudy.niehs.nih.gov/English/brca-validation.htm).
Exposure and covariate assessment
As part of the baseline questionnaire, women were asked detailed questions about their sleep patterns, including whether they sleep during the daytime or nighttime, their usual sleep duration, the length of time it takes to go to sleep, any types of light present while sleeping, frequency of waking up at night, frequency of naps and sleep medication use. During follow-up, more questions on sleep were asked, including how much sleep the individual needs to feel their best and their usual sleep duration in the year prior to follow-up. From these questions, we classified the participants as getting as much or more sleep than they felt they needed or less sleep than they felt that they needed. Participants also reported if they had difficulty sleeping, felt excessively sleepy during the day, or acted out their dreams (physical enactment of dreams while sleeping). Study questionnaires are available on the Sister Study Website (www.sisterstudy.niehs.nih.gov).
From the baseline study interview, information on demographics (race/ethnicity, education, income, marital status), reproductive history, lifestyle factors such as smoking and alcohol consumption, and use of exogenous hormones was obtained. Menopausal status (premenopausal versus postmenopausal) was determined from questionnaire data and updated throughout follow-up. Height and weight at baseline were measured in a home visit by a trained examiner and used to calculate body mass index (BMI, kg/m2).
Statistical Analysis
We excluded the small proportion of women in the cohort who were diagnosed with invasive or DCIS breast cancer prior to completion of the baseline interview (n=76), were currently shift workers (n=269) or who were blind (n=6). We excluded current shift workers as they would likely have disrupted sleep patterns because of their shift work but the sample size was too small to stratify on shift working status. The final analytic sample size was n=50,533 (99%).
Descriptive statistics were compared for study participants stratified by usual sleep duration as assessed at baseline. To evaluate the association between baseline sleep characteristics and breast cancer risk, adjusted Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI). The time scale for the Cox model was age with person-time accruing from age at study enrollment to age at breast cancer diagnosis or age of last follow-up.
The analyses of sleep characteristics assessed at the follow-up interview were limited to women who were breast cancer-free when they completed the follow-up interview (n=41,474). For these variables, the time scale of the Cox model was left-censored at age of follow-up interview, meaning that study participants entered the Cox model at age of follow-up interview and remained in the model until their age of breast cancer diagnosis, censoring event, or end of follow-up, whichever occurred first.
We also evaluated the association between sleep characteristics and breast tumor estrogen receptor status (ER+, ER−), extent (invasive, DCIS) and menopausal status at diagnosis (premenopausal, postmenopausal). For ER-specific analyses, cases without the outcome of interest were censored at the time of diagnosis. The analyses of ER subtype were limited to invasive tumors only, as ER status information is less complete for in situ cases. For the sleep characteristics assessed at the time of follow-up interview, we were unable to consider associations with ER− tumors as there were few subsequent ER− cases. To evaluate differences by ER status, we conducted a case-case analysis comparing ER+ to ER− breast cancer. When considering premenopausal breast cancer as an outcome, women who became postmenopausal during the follow-up period were censored at age of menopause. The women who reached menopause without developing breast cancer then contributed person-time to the postmenopausal-specific analyses.
The proportional hazards assumption was assessed using an interaction term with survival time in the regression model and with log-log survival plots. We found no evidence of time-variation in the HR associations.
Sleep medication use in the prior six weeks (yes, no) and body mass index at baseline were assessed as potential effect measure modifiers for the relationship between the different sleep characteristics and breast cancer. We also considered whether the associations between measures of sleep quality and breast cancer risk were modified by sleep duration. A cross-product term and likelihood ratio test was used to evaluate interactions on the multiplicative scale.
We also considered whether changes in sleep duration over time were associated with breast cancer risk by evaluating whether sleep duration stayed the same, increased or decreased from baseline to follow-up. We used categorical variables (≤6, 7–8, ≥9 hours), so an individual was characterized as having increased or decreased sleep if their reported sleep duration at the follow-up interview fell into a different category.
Confounders were identified using the prior literature and a directed acyclic graph.20 Multivariable-adjusted models included covariates assessed at baseline, including race (non-Hispanic white, other), education (≤high school or equivalent, some college, 4-year degree or higher), household income (<49,999, 50,000–99,999, 100,000+), marital status (never married, legally married or living as married, separated or divorced or widowed), hormone replacement therapy use (none, estrogen only, progesterone only or combination therapy), use of oral contraceptives (ever, never), alcohol consumption (never, former, current drinker ≤ 1 drink/day, current drinker >1 drink/day), age at menarche (continuous), parity (nulliparous or 1, 2–3, ≥4), age at first birth (<21 years, 21–24, 25–28, 29–31, ≥32), age at menopause (premenopausal, <40, 40–50, 51–55, >55 years based on enrollment information), pack-years of smoking (never smoker, smoker <20 pack-years, smoker ≥20 pack-years) and metabolic equivalent hours of physical activity per week (<27.0, 27.0–44.3, 44.4–67.1, >67.2). Missing data on adjustment covariates was minimal (<3% total) and thus, we did a complete case analysis. We also conducted a sensitivity analysis in which BMI was included as a confounder in the adjustment set.
To address the possibility of reverse causality, we conducted sensitivity analyses limiting to cases diagnosed after the first two years of study follow-up after baseline. For the analyses investigating the association between sleep characteristics at follow-up and breast cancer, we excluded all cases diagnosed prior to the follow-up interview. Additionally, we conducted an analysis evaluating the time to diagnosis by sleep characteristics ascertained at the follow-up survey.
To assess the validity of self-reported sleep characteristics over time, we conducted some additional analyses to evaluate the similarity of sleep characteristics reported at baseline and at follow-up in women who were not diagnosed with breast cancer. First, we determined the correlation between continuous measures and calculated a weighted kappa (≤6, 7–8, ≥9 hours) for sleep duration at baseline and follow-up. Second, we evaluated the average sleep duration at baseline by whether the participant reported getting enough sleep at follow-up. We also evaluated whether those who reported sleep disruption at baseline (frequency of night waking per month and times waking up per night) were more likely to report difficulty sleeping at follow-up.
Two-sided tests were used with a p value of 0.05 to evaluate statistical significance. All analyses were performed using SAS version 9.3 software (SAS Institute, Inc., Cary, NC).
Results
From baseline to the end of follow-up, 2,736 breast cancers were diagnosed during 375,925 person-years of follow-up (mean=7.4, standard deviation (SD)=2.0). On average, women reported getting about 7 hours of sleep per night at baseline (mean=7.1, SD=1.1). Almost a quarter of the study population reported getting insufficient sleep (≤6 hours) on average, whereas only 7% got 9 or more hours of sleep (Table I). Women who reported ≤6 hours sleep at baseline were more likely to be non-white and to be of a lower socioeconomic status, with both lower educational attainment and lower annual household income. They were also less likely to be married or living as married. Long sleepers (≥9 hours) were slightly older, were more likely to consume more than one alcoholic drink per day and more likely to use postmenopausal hormones.
Table I.
Study population characteristics by usual sleep duration at baseline, Sister Study.
| Usual Sleep Duration at Baseline
|
||||||||
|---|---|---|---|---|---|---|---|---|
| ≤ 6 hours (N=12,064) | 7 hours (N=17,737) | 8 hours (N=17,140) | ≥9 hours (N=3,513) | |||||
|
|
||||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Age at baseline | 55.5 | (8.7) | 55.3 | (8.8) | 55.9 | (9.2) | 56.7 | (9.6) |
| BMI at baseline | 29.0 | (6.8) | 27.6 | (6.1) | 27.2 | (5.9) | 27.6 | (6.2) |
| MET hours/week physical activity | 50.6 | (32.6) | 50.5 | (30.5) | 51.2 | (31.2) | 49.0 | (31.8) |
| Age at menarche | 12.6 | (1.6) | 12.6 | (1.5) | 12.7 | (1.5) | 12.8 | (1.6) |
| Paritya | 2.4 | (1.1) | 2.4 | (1.1) | 2.4 | (1.1) | 2.4 | (1.1) |
| Age at menopauseb | 48.6 | (6.9) | 49.4 | (6.2) | 49.6 | (6.2) | 49.5 | (6.5) |
| Pack-yearsc | 15.9 | (16.4) | 13.7 | (14.4) | 14.4 | (15.1) | 16.8 | (16.9) |
|
|
||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | |
|
|
||||||||
| Non-white race | 3,407 | (28.3) | 2,531 | (14.3) | 1,897 | (11.1) | 393 | (11.2) |
| Education | ||||||||
| High school degree or less | 2,233 | (18.5) | 2,451 | (13.8) | 2,486 | (14.5) | 552 | (15.7) |
| Some college | 4,533 | (37.6) | 5,821 | (32.8) | 5,501 | (32.1) | 1,162 | (33.1) |
| College degree or higher | 5,295 | (43.9) | 9,465 | (53.4) | 9,152 | (53.4) | 1,799 | (51.2) |
| Income | ||||||||
| < 49,999 | 3,681 | (31.8) | 3,905 | (22.9) | 3,900 | (23.7) | 960 | (28.6) |
| 50,000–99,999 | 4,692 | (40.5) | 7,076 | (41.5) | 6,658 | (40.4) | 1,345 | (40.0) |
| 100,000+ | 3,211 | (27.7) | 6,092 | (35.7) | 5,909 | (35.9) | 1,055 | (31.4) |
| Marital status | ||||||||
| Never married | 833 | (6.9) | 945 | (5.3) | 816 | (4.8) | 137 | (3.9) |
| Married, living as married | 8,123 | (67.4) | 13,405 | (75.6) | 13,400 | (78.2) | 2,784 | (79.3) |
| Separated, divorced, widowed | 3,105 | (25.7) | 3,384 | (19.1) | 2,924 | (17.1) | 592 | (16.9) |
| Postmenopausal hormone useb | ||||||||
| None | 3,591 | (43.6) | 4,818 | (41.4) | 4,324 | (37.9) | 858 | (35.3) |
| Estrogen only | 2,350 | (28.5) | 3,023 | (26.0) | 3,137 | (27.5) | 720 | (29.6) |
| Progesterone or combination therapy | 2,294 | (27.9) | 3,805 | (32.7) | 3,962 | (34.7) | 855 | (35.1) |
| Birth control pills | ||||||||
| Ever | 10,035 | (83.3) | 14,988 | (84.5) | 14,410 | (84.1) | 2,921 | (83.2) |
| Alcohol consumption | ||||||||
| Never | 595 | (4.9) | 579 | (3.3) | 626 | (3.7) | 132 | (3.8) |
| Former | 2,214 | (18.4) | 2,481 | (14.0) | 2,396 | (14.0) | 570 | (16.3) |
| Current drinker, <= 1 drink/day | 8,213 | (68.2) | 12,826 | (72.4) | 11,873 | (69.4) | 2,290 | (65.3) |
| Current drinker, >1 drink day | 1,018 | (8.5) | 1,833 | (10.3) | 2,220 | (13.0) | 516 | (14.7) |
Among parous, N=41,253
Among postmenopausal at baseline, N=33,086
Among smokers, N=22,117
Sleep characteristics assessed at baseline, including usual sleep duration, time it takes to fall asleep and waking at night, were not consistently associated with an increased risk of overall breast cancer (Table II). There was some evidence that sleeping ≤6 hours was associated with ER− breast cancer (HR=1.26, 95% CI: 0.92–1.73) but not ER+ (HR=0.87, 95% CI: 0.76–1.00) relative to sleeping 7 hours a night. Confidence intervals for these associations were wide and a case-case comparison of the association between getting 6 or fewer hours and ER+ versus ER− breast cancer was not statistically significant (p=0.2). There were no associations observed with other baseline sleep characteristics including time to sleep, waking at night or sleep medication use. Additionally, no associations were observed with increasing frequency of sleep medication use and frequency of napping (data not shown).
Table II.
Sleep characteristics and breast cancer risk by ER tumor subtype, NIEHS Sister Study.
| Overall | ER+ invasive | ER− invasive | ||||||
|---|---|---|---|---|---|---|---|---|
| Sleep Measures – Baseline Survey | Person-years N=375,925 | All breast cancer N=2,736 | Age-adjusted HR (95% CI) | Adjusted HRa (95% CI)1 | ER+ N=1,574 | Adjusted HRa (95% CI)1 | ER− N=281 | Adjusted HRa (95% CI)1 |
| Bedtime consistency | ||||||||
| Consistent bedtime | 318,467 | 2,325 | 1 (referent) | 1 (referent) | 1,341 | 1 (referent) | 238 | 1 (referent) |
| Inconsistent (night) | 51,535 | 367 | 0.94 (0.84, 1.05) | 0.92 (0.82, 1.03) | 206 | 0.92 (0.79, 1.08) | 39 | 0.91 (0.63, 1.31) |
| Inconsistent and irregular (night and day) | 4,434 | 39 | 1.20 (0.87, 1.64) | 1.25 (0.90, 1.74) | 25 | 1.43 (0.93, 2.18) | 2 | NE |
| Usual sleep duration | ||||||||
| <6 hours | 87,472 | 626 | 0.94 (0.85, 1.04) | 0.96 (0.86, 1.06) | 321 | 0.87 (0.76, 1.00) | 80 | 1.26 (0.92, 1.73) |
| 7 hours | 133,141 | 1,008 | 1 (referent) | 1 (referent) | 593 | 1 (referent) | 92 | 1 (referent) |
| 8 hours | 129,057 | 895 | 0.9 (0.82, 0.98) | 0.90 (0.82, 0.99) | 534 | 0.90 (0.79, 1.01) | 90 | 1.05 (0.78, 1.42) |
| 9+ hours | 25,729 | 201 | 1.00 (0.86, 1.17) | 1.03 (0.88, 1.20) | 121 | 1.06 (0.87, 1.29) | 18 | 0.94 (0.54, 1.64) |
| Time it takes to fall asleep | ||||||||
| < 15 min | 167,636 | 1,249 | 1.07 (0.98, 1.16) | 1.05 (0.97, 1.15) | 730 | 1.06 (0.95, 1.19) | 123 | 1.02 (0.78, 1.34) |
| 15 – 30 min | 141,602 | 992 | 1 (referent) | 1 (referent) | 574 | 1 (referent) | 99 | 1 (referent) |
| 30 min–1 hour | 44,605 | 339 | 1.09 (0.96, 1.23) | 1.10 (0.97, 1.25) | 193 | 1.09 (0.92, 1.30) | 41 | 1.24 (0.84, 1.81) |
| 1 hour + | 21,471 | 152 | 0.99 (0.84, 1.18) | 0.98 (0.81, 1.17) | 73 | 0.85 (0.65, 1.10) | 18 | 1.02 (0.59, 1.77) |
| Sleep medication use in prior six weeks | ||||||||
| None | 285,228 | 2,091 | 1 (referent) | 1 (referent) | 1,190 | 1 (referent) | 219 | 1 (referent) |
| Yes | 90,591 | 644 | 0.95 (0.87, 1.04) | 0.97 (0.88, 1.06) | 383 | 0.99 (0.88, 1.12) | 62 | 0.9 (0.67, 1.21) |
| Frequency of waking up at night | ||||||||
| Most or every night | 234,799 | 1,809 | 1.09 (0.92, 1.29) | 1.05 (0.88, 1.24) | 1,050 | 1.07 (0.85, 1.35) | 191 | 1.18 (0.68, 2.04) |
| ≥ once a week | 93,708 | 612 | 0.97 (0.81, 1.16) | 0.92 (0.76, 1.10) | 347 | 0.94 (0.73, 1.20) | 62 | 1.00 (0.56, 1.79) |
| 1–3 days a month | 24,736 | 163 | 1.02 (0.81, 1.27) | 0.98 (0.78, 1.23) | 95 | 0.99 (0.73, 1.34) | 11 | 0.72 (0.33, 1.59) |
| < 1 a month | 22,396 | 151 | 1 (referent) | 1 (referent) | 81 | 1 (referent) | 17 | 1 (referent) |
| Times wake up per night | ||||||||
| Never | 8,359 | 50 | 1 (referent) | 1 (referent) | 30 | 1 (referent) | 7 | 1 (referent) |
| 1 | 216,804 | 1,538 | 1.17 (0.88, 1.56) | 1.08 (0.81, 1.44) | 896 | 0.97 (0.67, 1.40) | 149 | 1.06 (0.43, 2.59) |
| 2 | 97,253 | 743 | 1.23 (0.92, 1.65) | 1.14 (0.85, 1.53) | 425 | 1.00 (0.69, 1.45) | 85 | 1.32 (0.53, 3.26) |
| 3+ | 52,965 | 400 | 1.21 (0.90, 1.63) | 1.13 (0.83, 1.53) | 221 | 0.95 (0.64, 1.39) | 39 | 1.06 (0.41, 2.72) |
Adjusted for race, education, income, marital status, postmenopausal hormone use, use or oral contraceptives, alcohol consumption, age at menarche, parity, age at first birth, age at menopause, pack years of smoking, physical activity.
Among women completing the first follow-up questionnaire, usual sleep duration in the year prior to follow-up was similarly not associated with breast cancer (Table III). However, reporting having difficulty sleeping ≥ 4 nights per week was associated with an increased risk of overall breast cancer (HR=1.32, 95% CI: 1.09–1.61) compared to having no difficulty sleeping. Elevated HRs for ER+ breast cancer were observed for women who reported needing ≥9 hours of sleep to feel their best relative to needing 7–8 hours (HR=1.29, 95% CI: 0.98–1.70) and for women who were getting less sleep than they felt they needed (HR=1.21, 95% CI: 0.98–1.50). Feeling excessively sleepy during the daytime was not associated with a higher risk of breast cancer. No association was observed with changes in sleep duration over time (data not shown). Elevated HRs for measures of difficulty sleeping were also evident in analyses restricted to postmenopausal (but not premenopausal) breast cancer (Table IV). For example, difficulty sleeping on a regular basis was associated with a higher risk of postmenopausal breast cancer (HR=1.21, 95% CI:1.03–1.43); especially for difficulty sleeping on more than 4 days a week (HR=1.51, 95% CI 1.24–1.85).
Table III.
Sleep characteristics and breast cancer risk by ER tumor subtype, NIEHS Sister Study.
| Overall breast cancer | ER+ | |||||
|---|---|---|---|---|---|---|
| Sleep Measures - Follow-up Survey | Person-years N=90,053 | All breast cancer N=740 | Age-adjusted HR (95% CI) | Adjusted HR (95% CI)a | ER+ invasive N=400 | Adjusted HR (95% CI)a |
| Hours of sleep needed to feel best | ||||||
| ≤ 6 hours | 9,264 | 67 | 0.86 (0.67, 1.12) | 0.89 (0.68, 1.17) | 35 | 0.95 (0.65, 1.38) |
| 7–8 hours | 67,391 | 557 | 1 (referent) | 1 (referent) | 291 | 1 (referent) |
| 9+ hours | 12,550 | 110 | 1.05 (0.85, 1.29) | 1.05 (0.84, 1.30) | 71 | 1.29 (0.98, 1.7) |
| Sleep duration (past year) | ||||||
| ≤ 6 hours | 31,285 | 263 | 1.05 (0.90, 1.23) | 1.07 (0.91, 1.26) | 132 | 0.97 (0.77, 1.22) |
| 7–8 hours | 52,069 | 418 | 1 (referent) | 1 (referent) | 233 | 1 (referent) |
| 9+ hours | 5,625 | 50 | 1.01 (0.74, 1.37) | 1.01 (0.74, 1.39) | 31 | 1.04 (0.68, 1.57) |
| Difference between hours of sleep needed and reported sleep duration | ||||||
| Equal or more sleep | 44,484 | 364 | 1 (referent) | 1 (referent) | 190 | 1 (referent) |
| Got less sleep | 44,427 | 367 | 1.07 (0.93, 1.25) | 1.10 (0.94, 1.28) | 206 | 1.21 (0.98, 1.5) |
| Difficulty sleeping on a regular basis | ||||||
| No | 48,283 | 387 | 1 (referent) | 1 (referent) | 212 | 1 (referent) |
| Yes | 41,095 | 349 | 1.07 (0.93, 1.24) | 1.11 (0.95, 1.29) | 187 | 1.08 (0.88, 1.33) |
| Nights/week have difficulty sleeping | ||||||
| No difficulty | 48,283 | 387 | 1 (referent) | 1 (referent) | 212 | 1 (referent) |
| <1 per week | 6,337 | 44 | 0.89 (0.65, 1.22) | 0.93 (0.67, 1.28) | 19 | 0.80 (0.50, 1.28) |
| ≥ 1 per week | 34,273 | 302 | 1.11 (0.95, 1.30) | 1.15 (0.98, 1.35) | 165 | 1.13 (0.91, 1.41) |
| Few days a week (≤4) | 18,527 | 141 | 0.97 (0.80, 1.18) | 1.01 (0.83, 1.24) | 76 | 0.98 (0.74, 1.29) |
| Most days (>4) | 15,746 | 161 | 1.28 (1.06, 1.55) | 1.32 (1.09, 1.61) | 89 | 1.32 (1.01, 1.72) |
| Ever feel excessively sleepy during the day | ||||||
| No | 53,356 | 443 | 1 (referent) | 1 (referent) | 243 | 1 (referent) |
| Yes | 35,961 | 291 | 0.98 (0.84, 1.14) | 0.98 (0.84, 1.15) | 155 | 0.97 (0.78, 1.20) |
| Acting out dreams | ||||||
| No | 80,235 | 651 | 1 (referent) | 1 (referent) | 353 | 1 (referent) |
| Yes | 9,157 | 86 | 1.21 (0.96, 1.52) | 1.17 (0.92, 1.48) | 46 | 1.19 (0.86, 1.65) |
Adjusted for race, education, income, marital status, postmenopausal hormone use, use or oral contraceptives, alcohol consumption, age at menarche, parity, age at first birth, age at menopause, pack years of smoking, physical activity.
Table IV.
Sleep characteristics and breast cancer, by menopausal status at diagnosis, NIEHS Sister Study.
| Premenopausal | Postmenopausal | |||
|---|---|---|---|---|
| Sleep Measures - Follow-up survey | Cases (N=104) | Adjusted HR (95% CI)a | Cases (N=634) | Adjusted HR (95% CI)b |
| Hours of sleep needed to feel best | ||||
| ≤ 6 hours | 9 | 1.19 (0.57, 2.49) | 58 | 0.86 (0.64, 1.15) |
| 7–8 hours | 76 | 1 (referent) | 479 | 1 (referent) |
| 9+ hours | 18 | 1.00 (0.58, 1.72) | 92 | 1.05 (0.83, 1.33) |
| Sleep duration (past year) | ||||
| ≤ 6 hours | 35 | 0.93 (0.61, 1.43) | 226 | 1.09 (0.92, 1.31) |
| 7–8 hours | 64 | 1 (referent) | 354 | 1 (referent) |
| 9+ hours | 4 | NE | 46 | 1.04 (0.75, 1.45) |
| Difference between hours of sleep needed and reported sleep duration | ||||
| Equal or more sleep | 43 | 1 (referent) | 321 | 1 (referent) |
| Got less sleep | 60 | 0.93 (0.62, 1.40) | 305 | 1.12 (0.95, 1.33) |
| Difficulty sleeping on a regular basis | ||||
| No | 71 | 1 (referent) | 315 | 1 (referent) |
| Yes | 33 | 0.65 (0.42, 1.00) | 315 | 1.21 (1.03, 1.43) |
| Nights/month have difficulty sleeping | ||||
| <1 per week | 75 | 1 (referent) | 355 | 1 (referent) |
| ≥ 1 per week | 29 | 0.71 (0.46, 1.12) | 272 | 1.26 (1.06, 1.49) |
| Few days a week (≤4) | 19 | 0.89 (0.53, 1.50) | 122 | 1.06 (0.86, 1.32) |
| Most days (>4) | 10 | 0.52 (0.26, 1.05) | 150 | 1.51 (1.24, 1.85) |
| Ever feel excessively sleepy during the day | ||||
| No | 62 | 1 (referent) | 380 | 1 (referent) |
| Yes | 41 | 0.74 (0.48, 1.12) | 249 | 1.02 (0.86, 1.21) |
| Acting out dreams | ||||
| No | 91 | 1 (referent) | 558 | 1 (referent) |
| Yes | 13 | 0.96 (0.52, 1.77) | 73 | 1.21 (0.93, 1.56) |
Adjusted for race, education, income, marital status, postmenopausal hormone use, use or oral contraceptives, alcohol consumption, age at menarche, parity, age at first birth, age at menopause, pack years of smoking, physical activity.
Adjusted for race, education, income, marital status, postmenopausal hormone use, use or oral contraceptives, alcohol consumption, age at menarche, parity, age at first birth, pack years of smoking, physical activity.
Variables related to exposure to nighttime light are shown in Table V. Compared to women who had no light on while sleeping or who slept with a mask on, sleeping with artificial light on in the room (defined as a light or television) was associated with an elevated HR of ER+ tumors (HR=1.20, 95% CI 0.97, 1.47), but not ER− tumors (HR=0.97, 95% CI: 0.61–1.55) (Table V). Similarly, slightly elevated HRs for ER+ tumors were observed for sleeping with artificial light coming from outside the room (HR=1.11, 95% CI 0.96–1.30), although results were not statistically significant.
Table V.
Light at night exposure and breast cancer risk by ER subtype, NIEHS Sister Study.
| Overall breast cancer | ER+ Invasive | ER− Invasive | ||||||
|---|---|---|---|---|---|---|---|---|
| Light at Night – Baseline Survey | Person-years N=375,925 | All breast cancer N=2,736 | Age-adjusted HR (95% CI) | Adjusted HR (95% CI) a | ER+ N=1,574 | Adjusted HR (95% CI) a | ER− N=281 | Adjusted HR (95% CI) a |
| Types of light while sleeping | ||||||||
| No light | 65,315 | 486 | 1 (referent) | 1 (referent) | 264 | 1 (referent) | 53 | 1 (referent) |
| Daylight | 10,283 | 65 | 0.87 (0.67, 1.13) | 0.87 (0.66, 1.15) | 41 | 1.05 (0.74, 1.5) | 8 | 1.04 (0.49, 2.21) |
| Light/television in room | 43,826 | 336 | 1.07 (0.93, 1.23) | 1.09 (0.93, 1.26) | 178 | 1.20 (0.97, 1.47) | 39 | 0.97 (0.61, 1.55) |
| Light outside the room | 127,897 | 936 | 0.99 (0.89, 1.10) | 1.01 (0.90, 1.13) | 543 | 1.11 (0.96, 1.3) | 101 | 1.01 (0.71, 1.43) |
| Nightlight | 246,940 | 1762 | 0.96 (0.87, 1.07) | 0.97 (0.87, 1.08) | 1,028 | 1.07 (0.93, 1.23) | 178 | 0.91 (0.66, 1.25) |
| When awakened at night, turns on light? | ||||||||
| Never wakes up or doesn’t turn on the light when | 331,131 | 2393 | 1 (referent) | 1 (referent) | 1,378 | 1 (referent) | 250 | 1 (referent) |
| Turns on light | 41,537 | 320 | 1.06 (0.95, 1.20) | 1.07 (0.95, 1.21) | 183 | 1.07 (0.91, 1.26) | 28 | 0.89 (0.59, 1.34) |
| Light already on | 2,673 | 18 | 0.89 (0.56, 1.41) | 0.86 (0.52, 1.40) | 11 | 0.88 (0.46, 1.70) | 2 | NE |
Adjusted for race, education, income, marital status, postmenopausal hormone use, use or oral contraceptives, alcohol consumption, age at menarche, parity, age at first birth, age at menopause, pack years of smoking, physical activity.
Including BMI in the models did not notably change the estimates of association (data not shown) nor did it act as an effect measure modifier of associations. Results were similar by breast tumor stage and for invasive disease versus ductal carcinoma in situ (DCIS) (data not shown). Self-reported use of any sleep medication and sleep duration did not modify the associations between other sleep characteristics and breast cancer risk (data not shown).
Excluding cases diagnosed in the first two years of study follow-up did not alter results or study conclusions (data not shown). Similarly, time from follow-up survey to diagnosis did not notably vary by follow-up sleep characteristics. For example, time to diagnosis was similar when comparing those who reported no difficulty sleeping on a regular basis (mean=2.2 years, range 0.1–3.5 years) and those who did (mean=2.2 years, range 0.1–3.5 years). This finding was consistent across other follow-up sleep characteristics.
There was moderate correlation between usual sleep duration at baseline and at follow-up (continuous correlation, r=0.52; categorical weighted kappa, k=0.38). Study participants who got enough sleep to feel their best at follow-up reported a median baseline usual sleep duration of 8 hours, whereas women who were not getting enough sleep reported a median of 7 hours of sleep per night. There was also evidence to support that women who indicated they had more disrupted sleep at baseline (determined as increasing frequency of night waking per month and times waking up per night) were also more likely to say they had difficulty sleeping at the follow-up visit. For example, compared to those who reported never waking up at night, those who reported waking up 3+ times per night at baseline were more likely to report having difficulty sleeping at the follow-up survey (67% vs 23%).
Discussion
In this large prospective study of women at risk for breast cancer, most sleep characteristics, including usual sleep duration, were not associated with an increased risk of breast cancer. However, we did observe that frequently having difficulty of sleeping or sleeping with a television or light on in the room were associated with an increased risk of breast cancer. Not getting enough sleep to feel one’s best was also associated with breast cancer risk. These sleep characteristics may be indicators of diminished sleep quality. The findings from this study suggest that although most sleep characteristics were not strongly related to breast cancer risk, improved measures of sleep quality may be needed to better understand the role, if any, sleep disturbance has in the etiology of breast cancer.
Few studies have considered markers of sleep quality and other sleep characteristics in association with breast cancer. Two prior studies did not observe an association using self-reported sleep quality on a 4-point scale (poor, fairly poor, fairly good, good).15, 16 In the Women’s Health Initiative, no association was observed for having insomnia, reporting restless sleep or for a composite score of sleep disturbances.17 Sleep disturbance metrics were not related to breast cancer risk in a case-control study in Australia.14 Our finding, of elevated risk associated with difficulty sleeping, particularly in postmenopausal women, contrasts with these prior studies. We also noted an elevated risk for ER+ breast cancer associated with a personalized determination of getting less sleep than participants needed. The association in our study that was most pronounced was in women who had difficulty sleeping most days of the week and may reflect more chronic sleep disruption.
We also observed elevated HRs with exposure to artificial light at night, especially for ER+ breast tumors. Although not statistically significant, the point estimates are consistent with other studies that have largely shown positive associations between artificial light at night exposure and breast cancer risk21–25 and are in line with a meta-analysis reporting a 17% increase in overall breast cancer risk for artificial light exposure.8 We were limited by not having additional information about artificial light in the evenings prior to bedtime or on duration of light exposure during sleep.
One candidate mechanism underlying the association between circadian disruption and breast cancer involves altered estrogen signaling from reduced melatonin production.12 In a meta-analysis, reduced levels of melatonin was found to be associated with an increased risk of breast cancer.8 Circadian disruption may also impact processes important for normal cellular function such as cell-cycle regulation, DNA repair and apoptosis by alterations in the expression of the CLOCK gene.12, 26 Another possible mechanism is through altered immune function and metabolism.26 Inadequate or interrupted sleep has been associated with diabetes and obesity,27 chronic inflammation12 and cardiovascular disease.28 Decreased sleep may impact obesity via influencing hormone levels such as leptin and ghrelin, which are important in controlling appetite and energy expenditure11. In this cohort we have also previously reported sleep duration to be associated with patterns of less favorable eating behaviors such as snacking,29 suggesting that inadequate sleep may also be a marker of poor health behaviors.
We found that the associations with exposure to artificial light at night were more evident for estrogen receptor-positive breast cancer. Circadian disruption reduces melatonin production, which in turn increases circulating estrogen levels, and would be hypothesized to increase the risk of estrogen receptor-positive disease.12 Thus, these findings are consistent with the idea that the biologic mechanism of these associations may be related to estrogen signaling pathways.
Previous studies have reported a mix of positive, negative and null results for the association between sleep duration and breast cancer risk, but meta-analyses have found little evidence of heterogeneity,8, 13, 15–17, 30–39 and have largely concluded null associations.8, 13, 40 Some studies have shown variability in sleep duration with ER status with a decreased risk for ER+ breast cancer with short sleep and an increased risk for ER− breast cancer with short sleep.17, 35, 38 Although we saw suggestive differences in risk estimates for ER+ and ER− breast cancer, these estimates were not appreciably different. These studies and ours have relied on self-reported usual sleep duration, which is subject to misclassification,41 which may account for differences across studies.
Addressing the role of BMI in the sleep and breast cancer relationship is challenging. Obesity is a risk factor for postmenopausal breast cancer42 and is associated with sleep quality43 and duration.44 Given that BMI and baseline sleep characteristics were measured at the same time, we cannot determine the temporal order. Therefore, it is possible that BMI could be on the casual pathway between sleep duration and breast cancer or vice versa. Therefore, we chose to not adjust for BMI in our main analysis as adjusting for a mediator may induce bias.45 However, to address the possibility that BMI is influencing sleep patterns, we considered a sensitivity analysis where BMI was included as a covariate in the model and our results were largely unchanged. Stratifying on BMI did also not show evidence that BMI was an important effect measure modifier of this relationship.
Our study included an extensive questionnaire on sleep characteristics and patterns, including on sleep duration and quality as well as exposure to artificial light at night, with data collected at two separate time points prior to breast cancer diagnosis. This allowed us to consider a wide array of possible sleep-related factors that could be relevant for breast cancer and to evaluate sleep medication use. The Sister Study had a small number of current shift workers, which may reflect the study population’s demographic composition. As such, we excluded current shift workers from our study population as shift work both alters sleep schedules and may independently influence breast cancer risk,46 although the latter has recently been called into question.47 We attempted to address the possibility of reverse causation by excluding cases that occurred during the first two years of follow-up and the associations with the baseline sleep characteristics remained the same. This approach was not possible for the characteristics only assessed at follow-up interview due to the smaller number of cases diagnosed after the second interview. Therefore, we evaluated whether time to diagnosis varied by follow-up sleep characteristics and found no evidence to support that reverse causation was impacting our results. However, we cannot rule out the possibility that an undiagnosed breast tumor or other unknown factors (such as underlying comorbidities) may be disrupting sleep and thus impacting breast cancer risk.
Some limitations should be considered in the interpretation of our results. Prior studies have shown misclassification for self-reported sleep duration,41 which may extend to other sleep characteristics.
However, comparison of responses across our baseline and follow-up survey, showed reasonable consistency in reporting sleep duration and disrupted sleep, supporting the use of self-reported data. It is also possible that difficulty sleeping at night and artificial light at night exposure may be more memorable and easily reported than determining the usual hours of sleep. Unfortunately, most sleep tracking devices are not very reliable at measuring sleep disruption,48 and thus future technological advances may be necessary to ensure that sleep tracking devices or smartphones can be used to adequately measure sleep disruptions. Although we saw no increase in risk for any sleep medication use or increasing frequency of sleep medication use in the last six weeks, we did not consider more detailed information on specific types of medications or historic medication use; frequent use of hypnotics has been previously found to be modestly related to breast cancer risk49.
We had the benefit of a large sample size which allowed us to consider ER status, although we were limited in our ability to study ER− breast cancer or include other tumor subtype markers such as the progesterone receptor or HER2.
In conclusion, we found little evidence to support that either short or long usual sleep duration as well as other sleep characteristics including time it takes to fall asleep, napping patterns, and recent sleep medication use were associated with breast cancer risk. However, our findings do suggest that experiencing difficulty sleeping and being exposed to artificial light at night are associated with an increased risk of breast cancer. These associations were most evident for women with postmenopausal breast cancer or ER+ tumors, which are the most common types of breast cancer in the United States. These findings support the need for future studies to consider the role of sleep quality and sleep disruptions in breast cancer risk.
Novelty and Significance.
This study is innovative in its consideration of extensive and novel sleep characteristics at two separate time points. Markers of inadequate sleep, such as difficulty sleeping, exposure to artificial nocturnal light and not getting enough sleep to feel your best, but not usual sleep duration or other sleep characteristics, were associated with breast cancer risk.
Acknowledgments
This study was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES044005).
Footnotes
The authors report no conflicts of interest.
References
- 1.Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–61. doi: 10.2147/NSS.S134864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa G. Shift work and occupational medicine: an overview. Occup Med. 2003;53:83–8. doi: 10.1093/occmed/kqg045. [DOI] [PubMed] [Google Scholar]
- 3.Gangwisch J. Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obesity reviews. 2009;10:37–45. doi: 10.1111/j.1467-789X.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens RL, Gold KA, Gozal D, Peppard PE, Jun JC, Lippman SM, Malhotra A. Sleep and Breathing … and Cancer? Cancer Prev Res. 2016;9:821–7. doi: 10.1158/1940-6207.CAPR-16-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah PN, Mhatre MC, Kothari LS. Effect of melatonin on mammary carcinogenesis in intact and pinealectomized rats in varying photoperiods. Cancer Res. 1984;44:3403–7. [PubMed] [Google Scholar]
- 6.Schernhammer ES, Rosner B, Willett WC, Laden F, Colditz GA, Hankinson SE. Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol Biomarkers Prev. 2004;13:936–43. [PubMed] [Google Scholar]
- 7.Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16:254–8. doi: 10.1097/01.ede.0000152525.21924.54. [DOI] [PubMed] [Google Scholar]
- 8.Yang WS, Deng Q, Fan WY, Wang WY, Wang X. Light exposure at night, sleep duration, melatonin, and breast cancer: a dose-response analysis of observational studies. Eur J Cancer Prev. 2014;23:269–76. doi: 10.1097/CEJ.0000000000000030. [DOI] [PubMed] [Google Scholar]
- 9.Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. The Journal of clinical endocrinology and metabolism. 2003;88:26–30. doi: 10.1210/jc.2002-020827. [DOI] [PubMed] [Google Scholar]
- 10.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:3003200. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9:70013–3. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17:273–84. doi: 10.1016/j.smrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Qin Y, Zhou Y, Zhang X, Wei X, He J. Sleep duration and breast cancer risk: a meta-analysis of observational studies. Int J Cancer. 2014;134:1166–73. doi: 10.1002/ijc.28452. [DOI] [PubMed] [Google Scholar]
- 14.Girschik J, Fritschi L, Erren TC, Heyworth J. Quantitative exposure metrics for sleep disturbance and their association with breast cancer risk. Cancer Causes Control. 2013;24:919–28. doi: 10.1007/s10552-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 15.Girschik J, Heyworth J, Fritschi L. Self-reported sleep duration, sleep quality, and breast cancer risk in a population-based case-control study. American journal of epidemiology. 2013;177:316–27. doi: 10.1093/aje/kws422. [DOI] [PubMed] [Google Scholar]
- 16.Verkasalo PK, Lillberg K, Stevens RG, Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 2005;65:9595–600. doi: 10.1158/0008-5472.CAN-05-2138. [DOI] [PubMed] [Google Scholar]
- 17.Vogtmann E, Levitan EB, Hale L, Shikany JM, Shah NA, Endeshaw Y, Lewis CE, Manson JE, Chlebowski RT. Association between sleep and breast cancer incidence among postmenopausal women in the Women’s Health Initiative. Sleep. 2013;36:1437–44. doi: 10.5665/sleep.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, Gaudet M, Schmidt MK, Broeks A, Cox A, Fasching PA, Hein R, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. Journal of the National Cancer Institute. 2011;103:250–63. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandler DP. Sister Study Methods Paper. Submitted. [Google Scholar]
- 20.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 21.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. Journal of the National Cancer Institute. 2001;93:1557–62. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 22.O’Leary ES, Schoenfeld ER, Stevens RG, Kabat GC, Henderson K, Grimson R, Gammon MD, Leske MC. Shift work, light at night, and breast cancer on Long Island, New York. American journal of epidemiology. 2006;164:358–66. doi: 10.1093/aje/kwj211. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Zheng T, Holford TR, Boyle P, Zhang Y, Dai M. Light at night and breast cancer risk: results from a population-based case–control study in Connecticut, USA. Cancer Causes & Control. 2010;21:2281–5. doi: 10.1007/s10552-010-9653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloog I, Haim A, Stevens RG, Barchana M, Portnov BA. Light at night co‐distributes with incident breast but not lung cancer in the female population of Israel. Chronobiology international. 2008;25:65–81. doi: 10.1080/07420520801921572. [DOI] [PubMed] [Google Scholar]
- 25.Bauer SE, Wagner SE, Burch J, Bayakly R, Vena JE. A case-referent study: light at night and breast cancer risk in Georgia. International journal of health geographics. 2013;12:1. doi: 10.1186/1476-072X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J Clin. 2014;64:207–18. doi: 10.3322/caac.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nature reviews Endocrinology. 2009;5:253–61. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, DeRoo LA, Sandler DP. Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr. 2011;14:889–95. doi: 10.1017/S136898001000296X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinheiro SP, Schernhammer ES, Tworoger SS, Michels KB. A prospective study on habitual duration of sleep and incidence of breast cancer in a large cohort of women. Cancer Res. 2006;66:5521–5. doi: 10.1158/0008-5472.CAN-05-4652. [DOI] [PubMed] [Google Scholar]
- 31.McElroy JA, Newcomb PA, Titus-Ernstoff L, Trentham-Dietz A, Hampton JM, Egan KM. Duration of sleep and breast cancer risk in a large population-based case-control study. J Sleep Res. 2006;15:241–9. doi: 10.1111/j.1365-2869.2006.00523.x. [DOI] [PubMed] [Google Scholar]
- 32.Wu AH, Wang R, Koh WP, Stanczyk FZ, Lee HP, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis. 2008;29:1244–8. doi: 10.1093/carcin/bgn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kakizaki M, Kuriyama S, Sone T, Ohmori-Matsuda K, Hozawa A, Nakaya N, Fukudo S, Tsuji I. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99:1502–5. doi: 10.1038/sj.bjc.6604684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu AH, Stanczyk FZ, Wang R, Koh WP, Yuan JM, Yu MC. Sleep duration, spot urinary 6-sulfatoxymelatonin levels and risk of breast cancer among Chinese women in Singapore. Int J Cancer. 2013;132:891–6. doi: 10.1002/ijc.27653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian X, Brinton LA, Schairer C, Matthews CE. Sleep duration and breast cancer risk in the Breast Cancer Detection Demonstration Project follow-up cohort. Br J Cancer. 2015;112:567–71. doi: 10.1038/bjc.2014.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P, Ren FM, Lin Y, Su FX, Jia WH, Su XF, Tang LY, Ren ZF. Night-shift work, sleep duration, daytime napping, and breast cancer risk. Sleep Med. 2015;16:462–8. doi: 10.1016/j.sleep.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Gu F, Xiao Q, Chu LW, Yu K, Matthews CE, Hsing AW, Caporaso NE. Sleep Duration and Cancer in the NIH-AARP Diet and Health Study Cohort. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao Q, Signorello LB, Brinton LA, Cohen SS, Blot WJ, Matthews CE. Sleep duration and breast cancer risk among black and white women. Sleep Med. 2016;20:25–9. doi: 10.1016/j.sleep.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurley S, Goldberg D, Bernstein L, Reynolds P. Sleep duration and cancer risk in women. Cancer Causes Control. 2015;26:1037–45. doi: 10.1007/s10552-015-0579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erren TC, Morfeld P, Foster RG, Reiter RJ, Gross JV, Westermann IK. Sleep and cancer: Synthesis of experimental data and meta-analyses of cancer incidence among some 1,500,000 study individuals in 13 countries. Chronobiol Int. 2016;33:325–50. doi: 10.3109/07420528.2016.1149486. [DOI] [PubMed] [Google Scholar]
- 41.Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22:462–8. doi: 10.2188/jea.JE20120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White AJ, Nichols HB, Bradshaw PT, Sandler DP. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer. 2015;121:3700–8. doi: 10.1002/cncr.29552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin H-M, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. The Journal of Clinical Endocrinology & Metabolism. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 44.Cappuccio FP, Taggart FM, Kandala N, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. SLEEP-NEW YORK THEN WESTCHESTER- 2008;31:619. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, Poole C. Illustrating bias due to conditioning on a collider. International journal of epidemiology. 2010;39:417–20. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia Y, Lu Y, Wu K, Lin Q, Shen W, Zhu M, Huang S, Chen J. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 2013;37:197–206. doi: 10.1016/j.canep.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Travis RC, Balkwill A, Fensom GK, Appleby PN, Reeves GK, Wang XS, Roddam AW, Gathani T, Peto R, Green J, Key TJ, Beral V. Night Shift Work and Breast Cancer Incidence: Three Prospective Studies and Meta-analysis of Published Studies. Journal of the National Cancer Institute. 2016;108 doi: 10.1093/jnci/djw169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolla BP, Mansukhani S, Mansukhani MP. Consumer sleep tracking devices: a review of mechanisms, validity and utility. Expert review of medical devices. 2016;13:497–506. doi: 10.1586/17434440.2016.1171708. [DOI] [PubMed] [Google Scholar]
- 49.Hartz A, Ross JJ. Cohort study of the association of hypnotic use with mortality in postmenopausal women. BMJ Open. 2012;2:2012–001413. doi: 10.1136/bmjopen-2012-001413. [DOI] [PMC free article] [PubMed] [Google Scholar]


