Abstract
Objective
Tesamorelin reduces visceral adipose tissue (VAT) in HIV. We investigated whether reductions in VAT with tesamorelin are associated with changes in alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Design and Methods
We utilized data from two multicenter Phase III trials of tesamorelin among 806 HIV-infected patients with abdominal obesity. These studies showed that the majority of patients treated with tesamorelin are “responders,” defined a priori by the FDA as achieving ≥ 8% reduction in VAT. In the current analysis, we sought to examine the impact of VAT reduction on ALT and AST among patients participating in the Phase III trials with baseline elevated ALT or AST. Within this group, we compared changes in ALT and AST in VAT responders versus nonresponders after 26 weeks of treatment, and then assessed the effects of drug discontinuation on these endpoints over a subsequent 26-week period.
Results
At baseline, VAT was positively associated with ALT (P = 0.01). In subjects assigned to tesamorelin with baseline ALT or AST > 30 U/L, VAT responders experienced greater reductions in ALT (−8.9 ± 22.6 vs. 1.4 ± 34.7 U/L, P = 0.004) and AST (−3.8 ± 12.9 vs. 0.4 ± 22.4 U/L, P = 0.04) compared to nonresponders over 26 weeks. This improvement among VAT responders persisted over 52 weeks even in those switched to placebo despite a partial re-accumulation of VAT.
Conclusions
A clinically significant VAT reduction with tesamorelin was associated with improved liver enzymes among HIV-infected patients with abdominal obesity and elevated baseline transaminases.
Keywords: transaminases, liver, intra-abdominal fat, tesamorelin, HIV
Introduction
Twenty percent of individuals with HIV have abnormal liver tests, compared to 10% among the general U.S. population [1]. In a report of HIV-infected subjects, nonalcoholic fatty liver disease (NAFLD) was the most common condition to be associated with elevated liver enzymes (30%), followed by excessive alcohol use (13%), chronic hepatitis B (9%), and chronic hepatitis C (5%) [1]. Cross-sectional studies have identified abdominal obesity as a risk factor for increased hepatic transaminases in HIV-infected [2] and uninfected individuals alike [2, 3]. The Fat Redistribution and Metabolic Change in HIV Infection (FRAM) study found that, for every doubling of visceral adipose tissue (VAT), alanine aminotransferase (ALT) rose by 8–10% among HIV-infected subjects [2]. Despite the baseline association between increased VAT and liver enzymes, the longitudinal implication of a selective reduction in VAT on hepatic transaminases has not been well explored. Specifically, does a clinically significant decrease in VAT in the treatment of abdominal obesity in HIV result in improvements in ALT and aspartate aminotransferase (AST)?
Tesamorelin is a synthetic growth hormone-releasing hormone (GHRH) analogue that is approved for the treatment of abdominal adiposity in HIV. In two Phase III clinical trials of HIV-infected patients with abdominal obesity, tesamorelin selectively reduced VAT area by 15% over 26 weeks without altering subcutaneous adipose tissue (SAT) or body mass index (BMI) [4–6]. In approving each study design, the U.S. Food and Drug Administration (FDA) a priori defined VAT reduction ≥ 8% as a clinically significant change. In the combined Phase IIl trials, 69% of subjects receiving tesamorelin achieved VAT reduction ≥ 8%, compared to 33% of those receiving placebo (P < 0.001) [7]. Based on these data, the FDA approved tesamorelin for the treatment of HIV-associated abdominal fat accumulation in 2010. Despite its clinical efficacy, the subcutaneous route of administration of this medication has limited its more widespread use.
Given the increased prevalence of liver dysfunction among HIV-infected patients, particularly those with increased abdominal adiposity, we sought to characterize the clinical impact of a tesamorelin-mediated VAT reduction on ALT and AST among the large subset of Phase III trial participants who had elevated ALT or AST at baseline. Using the FDA definition, we compared changes in liver enzymes among tesamorelin responders with VAT reduction ≥ 8% to nonresponders with VAT reduction < 8% over 26 weeks. We also examined the durability of changes in hepatic transaminases among initial VAT responders 26 weeks after tesamorelin discontinuation.
Subjects and Methods
In this study, we utilized data from two similarly designed multicenter Phase III clinical registration trials of tesamorelin (Theratechnologies Inc., Montreal, Canada) in HIV-infected patients with abdominal obesity [4–6, 8].
Study design
Eligible participants were HIV-infected men and women, aged 18 to 65 years, with abdominal fat accumulation (defined in men as waist circumference ≥ 95 cm and waist-hip ratio ≥ 0.94, and in women as waist circumference ≥ 94 cm and waist-hip ratio ≥ 0.88 [9]) who had CD4 cell count > 100 cells/mm3 and HIV viral load < 10,000 copies/mL on stable ART for ≥ 8 weeks. Exclusion criteria included a known history of diabetes mellitus requiring medication, fasting glucose ≥ 150 mg/dL, or any history of malignancy. Hepatic transaminases did not serve as a basis for trial inclusion or exclusion.
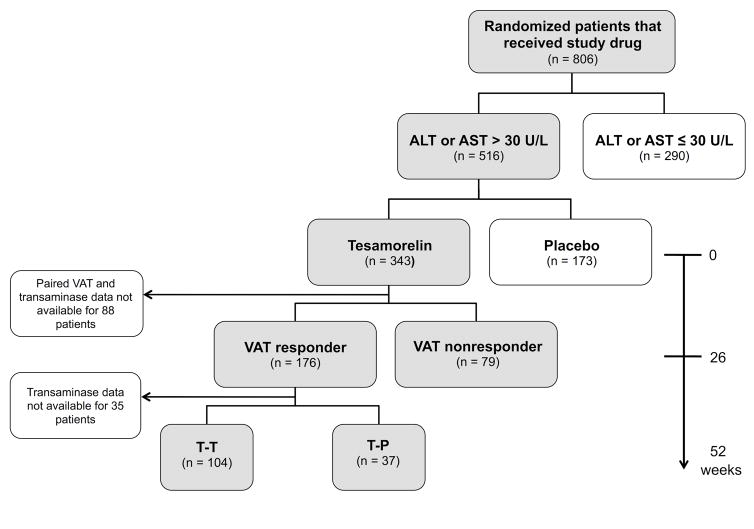
Both Phase III clinical trials consisted of a 26-week main study and a subsequent 26-week extension phase. In the main study, subjects were randomized in a 2:1 ratio to receive tesamorelin (2 mg) or placebo subcutaneously daily for 26 weeks. In the extension phase, individuals assigned to placebo were switched to tesamorelin (P-T), whereas those originally assigned to tesamorelin were re-randomized to continue tesamorelin (T-T) or to start placebo (T-P). The studies were approved by the Institutional Review Boards at their respective study sites, and all subjects provided written informed consent to participate. The current analysis includes patients with elevated baseline transaminases who were initially randomized to tesamorelin, and who were classified as responders or nonresponders based on their magnitude of VAT reduction in response to treatment.
Study procedures
The study protocol for each Phase III trial has been described previously [4–6, 8]. Eligible subjects underwent a baseline visit, which included a history and physical exam, a single-slice abdominal computed tomography (CT) scan to determine VAT and SAT area, and a whole-body dual energy x-ray absorptiometry (DXA) scan to determine trunk and extremity fat. Information on viral hepatitis status, ART regimen, and use of testosterone and/or lipid-lowering therapy was collected based on participant self-report. Measurement of fasting ALT and AST also was performed using standard methodology. CT scan, DXA, and ALT and AST were reassessed at 26 and 52 weeks.
Statistical analysis
Observed case analyses were performed using all available data as outlined in Figure 1. Subjects were divided into those with evidence of increased hepatic transaminases based on ALT and/or AST > 30 U/L and those without elevations in these markers. Clinical attributes were compared among randomized subjects with elevated versus normal transaminases using one-way analysis of variance (ANOVA) for continuous variables and Fisher’s exact or Chi square test for categorical variables. We next identified factors independently associated with natural log-transformed ALT or AST at baseline using stepwise multivariable regression. Candidate variables were selected based on their clinical relevance and association with liver enzymes in univariate analyses (P < 0.05). In a bidirectional stepwise procedure, variables with P < 0.05 entered the model, whereas variables with P ≥ 0.15 were removed.
Figure 1. Analysis schema.
T-T, tesamorelin responders at week 26 assigned to continue tesamorelin for an additional 26 weeks. T-P, tesamorelin responders at week 26 switched to placebo for 26 weeks.
In our responder analysis, we sought to examine whether, among individuals with elevated baseline ALT or AST, VAT responders would have a greater reduction in ALT and AST compared to nonresponders after 26 weeks of tesamorelin treatment (Figure 1). We focused on subjects with elevated hepatic transaminases since we expected that a decline in ALT or AST would be most clinically relevant among this group, and given the high prevalence of this laboratory abnormality among patients with HIV and abdominal obesity [1, 2]. A cutoff of ALT or AST > 30 U/L was selected in accordance with prior reports [1, 10].
Clinical characteristics were compared between VAT responders and nonresponders with baseline ALT or AST > 30 U/L using one-way ANOVA for continuous variables and Fisher’s exact or Chi square test for categorical variables. Changes in ALT or AST by responder status were assessed using multivariable models that controlled for baseline ALT or AST, clinical trial (i.e., whether data were collected in the first or second Phase III trial), and viral hepatitis. Responder × hepatitis and responder × trial interactions were also tested. To determine whether a relationship between VAT responder status and change in transaminases was unique to tesamorelin, a similar analysis was performed among placebo-treated participants dichotomized by VAT reduction ≥ 8% or < 8% over 26 weeks.
Secondarily, we compared the proportion of tesamorelin-treated VAT responders and nonresponders with elevated baseline transaminases who had normalization of ALT or AST (≤ 30 U/L) at week 26 using Fisher’s exact test. We additionally constructed a logistic regression model to calculate the odds ratio (OR) with 95% confidence interval (CI) for resolution of elevated ALT or AST at week 26 among responders versus nonresponders after adjustment for baseline ALT or AST, clinical trial, and viral hepatitis. Within-group changes in ALT and AST over 26 weeks also were assessed by responder status.
We next sought to characterize the durability of changes in liver enzymes among initial VAT responders with baseline ALT or AST > 30 U/L following re-randomization to tesamorelin (T-T) or placebo (T-P) during the extension phase (Figure 1). Changes in ALT, AST, and VAT from baseline to week 52 were compared between treatment groups using multivariable models that controlled for baseline value, change from baseline to week 26, and clinical trial. ALT and AST also were compared within treatment groups.
Values are reported as mean ± standard deviation (SD). A critical value of P < 0.05 was used to designate statistical significance. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Baseline characteristics of randomized subjects
Of 806 randomized participants who received study medication, 516 (64%) had ALT or AST > 30 U/L at study entry (Figure 1). Demographic, immunologic, and metabolic characteristics of the study subjects in relation to baseline ALT or AST elevation are reported in Table 1. Elevated transaminases were associated with male sex (P < 0.0001), race (P = 0.0003), viral hepatitis (P = 0.006), testosterone use (P < 0.0001), lipid-lowering therapy (P = 0.03), and ART regimen (P = 0.04). Individuals with increased ALT or AST had greater VAT (191 ± 83 vs. 167 ± 83 cm2, P < 0.0001), lower abdominal SAT (214 ± 112 vs. 263 ± 136 cm2, P < 0.0001), and lower limb fat (6.6 ± 3.9 vs. 8.7 ± 5.1 kg, P < 0.0001). In contrast, there was no difference in BMI (28.9 ± 3.9 vs. 29.2 ± 4.8 kg/m2, P = 0.44) between groups.
Table 1.
Baseline characteristics of randomized subjects with elevated or normal transaminases
| Variable | ALT or AST > 30 U/L (n = 516) | ALT or AST ≤ 30 U/L (n = 290) | P-value |
|---|---|---|---|
| Demographic | |||
| Age (y) | 47.6 ± 7.4 | 47.6 ± 7.6 | 0.98 |
| Male % | 92 | 72 | < 0.0001 |
| Race % | 0.0003 | ||
| White | 80 | 69 | |
| Black | 10 | 19 | |
| Asian | 1 | 1 | |
| Hispanic | 8 | 10 | |
| Other | 2 | 1 | |
| Hepatitis C % | 10 | 7 | 0.09 |
| Any viral hepatitis % | 25 | 17 | 0.006 |
| Testosterone % | 24 | 13 | < 0.0001 |
| Lipid-lowering therapy % | 48 | 40 | 0.03 |
| Immunologic | |||
| CD4 (cells/mm3) | 611 ± 303 | 579 ± 264 | 0.13 |
| Undetectable viral load % | 77 | 77 | 0.33 |
| ART % | 0.04 | ||
| NRTI alone | 5 | 4 | |
| NRTI + NNRTI | 35 | 30 | |
| NRTI + PI | 42 | 52 | |
| NRTI + NNRTI + PI | 11 | 7 | |
| Other | 7 | 6 | |
| Metabolic | |||
| BMI (kg/m2) | 28.9 ± 3.9 | 29.2 ± 4.8 | 0.44 |
| VAT (cm2) | 191 ± 83 | 167 ± 83 | < 0.0001 |
| SAT (cm2) | 214 ± 112 | 263 ± 136 | < 0.0001 |
| Fat in limbs (kg) | 6.6 ± 3.9 | 8.7 ± 5.1 | < 0.0001 |
Mean ± SD
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; BMI, body mass index; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
Results of stepwise multivariable regression models relating clinical variables to ALT and AST at baseline are shown in Table 2. VAT remained significantly related to ALT (P = 0.01) when controlling for sex, viral hepatitis, testosterone use, and limb fat. VAT was not associated with AST in a multivariable model.
Table 2.
Multivariable models relating clinical characteristics to liver transaminases at baseline by stepwise approach
| ln (ALT) (n = 762) | ln (AST) (n = 762) | |||
|---|---|---|---|---|
| Parameter | β-coefficient | P-value | β-coefficient | P-value |
| Male sex | 0.29 | < 0.0001 | 0.10 | 0.02 |
| Race | ||||
| Any viral hepatitis | 0.15 | 0.0006 | 0.16 | < 0.0001 |
| Testosterone use | 0.11 | 0.02 | 0.067 | 0.06 |
| Lipid-lowering tx | ||||
| VAT (cm2) | 0.00057 | 0.01 | ||
| SAT (cm2) | ||||
| Limb fat (kg) | −0.010 | 0.03 | −0.012 | 0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue.
Candidate variables are listed in the leftmost column. Variables were included in the model if P < 0.05, and were removed if P ≥ 0.15.
Tesamorelin responder analysis
Among participants with baseline ALT or AST > 30 U/L randomized to tesamorelin, there were 177 (69%) VAT responders and 80 (31%) VAT nonresponders after 26 weeks. Baseline clinical characteristics by responder status are shown in Supplemental Table 1. There was no difference in age, sex, race, CD4 count, viral load, or ART regimen between groups. Responders and nonresponders also had comparable baseline ALT (50 ± 20 vs. 53 ± 23 U/L, P = 0.31), AST (37 ± 13 vs. 39 ± 15 U/L, P = 0.46), BMI (28.7 ± 3.6 vs. 29.6 ± 4.6 kg/m2, P = 0.09), and VAT (190 ± 83 vs. 202 ± 77 cm2, P = 0.27). Viral hepatitis was less common among VAT responders than nonresponders (21% vs. 38%, P = 0.009).
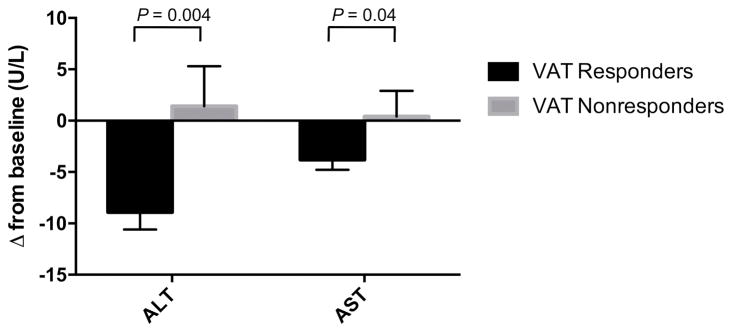
Liver enzymes at the conclusion of the main study were available for analysis in 176 responders and 79 nonresponders with elevated baseline ALT or AST (Figure 1). Results of the responder analysis are shown in Figure 2 and Supplemental Table 2. Twenty-six weeks after randomization to tesamorelin, ALT decreased by 8.9 ± 22.6 U/L among VAT responders, whereas it increased by 1.4 ± 34.7 U/L among VAT nonresponders (responders vs. nonresponders, P = 0.004). Similarly, VAT responders experienced a 3.8 ± 12.9 U/L reduction in AST compared to a 0.4 ± 22.4 U/L increase among VAT nonresponders (responders vs. nonresponders, P = 0.04). The relationship between responder status and change in ALT or AST among tesamorelin-treated patients was not modified by viral hepatitis status or clinical trial. Thus, VAT responders with and without viral hepatitis had comparable declines in liver enzymes. Within-group comparisons of change in transaminases at week 26 compared to baseline were significant among VAT responders (week 26 vs. 0, ALT P < 0.001, AST P < 0.001), but not among VAT nonresponders (week 26 vs. 0, ALT P = 0.71, AST P = 0.87).
Figure 2. Changes in ALT and AST among tesamorelin VAT responders and nonresponders with baseline ALT or AST > 30 U/L at week 26.
P-values are based on comparisons of responders and nonresponders adjusting for baseline ALT or AST, clinical trial, and viral hepatitis status. Responder × hepatitis and responder × trial interactions were not significant. Error bars represent the standard error of the mean.
Among tesamorelin-treated subjects with elevated baseline transaminases, 35% of VAT responders compared to 18% of VAT nonresponders had normalization of ALT at week 26 (responders vs. nonresponders, P = 0.007). The odds of resolution of elevated ALT were 2.5 times higher (OR 95% CI 1.2, 5.3) among responders compared to nonresponders. In contrast, there was no difference in the frequency by which AST normalized at week 26 between VAT responders and nonresponders (responders vs. nonresponders, 52% vs. 41%, P = 0.10; OR 1.5, 95% CI 0.8, 2.8).
Among placebo-treated subjects, VAT reduction ≥ 8% was not associated with a concurrent decline in ALT (VAT ≥ 8% vs. < 8%, −6.5 ± 21.1 vs. −4.8 ± 21.1 U/L, P = 0.75) or AST (VAT ≥ 8% vs. < 8%, −2.1 ± 21.5 vs. −2.9 ± 15.5 U/L, P = 0.33), which is in contrast to tesamorelin-treated patients.
Extension phase analysis
Of the 177 tesamorelin VAT responders with elevated baseline transaminases, 141 (80%) had data available for analysis following completion of the 26-week extension phase. Among those individuals, 104 were randomized to continue tesamorelin (T-T) and 37 were reassigned to placebo (T-P) (Figure 1).
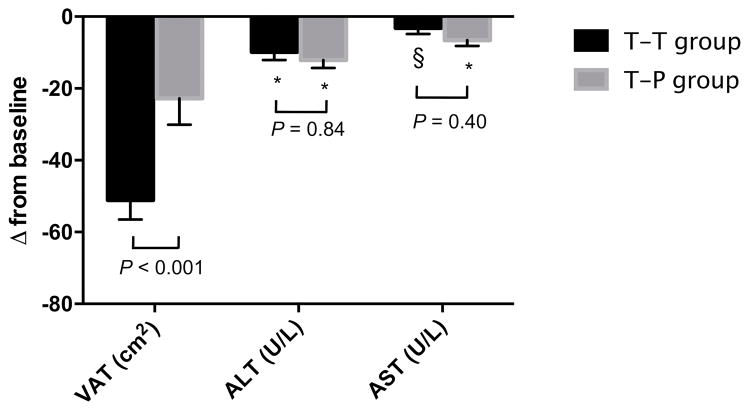
Results of the extension phase analysis are shown in Figure 3. There was a significant reduction in ALT over 52 weeks within both T-T (week 52 vs. 0, −9.8 ± 23.8 U/L, P < 0.001) and T-P groups (week 52 vs. 0, −12.1 ± 13.5 U/L, P < 0.001). The magnitude of this decline over 52 weeks did not differ between groups (T-T vs. T-P, P = 0.84). Moreover, ALT did not return toward baseline in the T-P group during the 26-week period on placebo (week 52 vs. 26, −2.4 ± 13.7 U/L, P = 0.24). Change in AST from baseline to week 52 followed a similar pattern such that reductions were sustained in both T-T (week 52 vs. 0, −3.2 ± 16.5, P = 0.03) and T-P groups (week 52 vs. 0, −6.6 ± 9.4 U/L, P < 0.001), and did not differ between groups (T-T vs. T-P, P = 0.40). In contrast to ALT and AST, VAT re-accumulated among tesamorelin VAT responders who were reassigned to placebo (week 52 vs. 26, 26 ± 35 cm2, P < 0.001), and consequently the decline in VAT from baseline to week 52 was less pronounced in the T-P than the T-T group (T-T vs. T-P, −51 ± 55 vs. −23 ± 44 cm2, P < 0.001).
Figure 3. Changes among initial tesamorelin VAT responders with baseline ALT or AST > 30 U/L following re-randomization to tesamorelin (T-T) or placebo (T-P) at week 52.
P-values shown reflect comparisons of T-T and T-P groups controlling for baseline value, change from baseline to week 26, and clinical trial. Results of within-group comparisons of liver enzymes between week 52 and baseline are designated as * P < 0.001, § P < 0.05. Error bars represent the standard error of the mean.
Discussion
The purpose of this study was to gain further insights into the effects of VAT reduction on the liver by applying clinically significant, FDA-defined thresholds of VAT response to Phase III clinical trial data. This study demonstrates that selective reduction of VAT in response to tesamorelin is associated with improved ALT and AST among HIV-infected individuals with abdominal obesity and elevated liver enzymes. Specifically, ALT decreased by 9 U/L (18%) and AST decreased by 4 U/L (10%) over 26 weeks among VAT responders with no change in ALT or AST in nonresponders. This improvement persisted at 52 weeks, even among those re-randomized to placebo despite a partial return to baseline in VAT.
The prevalence of high ALT or AST in our cohort was 64%, which far exceeds HIV and general population norms of 10–20% [1]. This finding likely relates to the requirement of increased waist circumference and waist-hip ratio for study inclusion, as these are known risk factors for increased ALT [3]. In view of the high burden of elevated liver enzymes in HIV-infected patients with abdominal obesity [1, 2] as well as the high prevalence of increased waist circumference in the setting of HIV [11], strategies to reduce these markers may be of special clinical relevance to this population. Viral hepatitis and use of testosterone or lipid-lowering therapy also were associated with hepatic transaminase elevations among individuals with HIV.
Serum ALT and AST are standard laboratory screening tests that may indicate hepatocellular damage. ALT is considered to be a more specific marker of hepatocyte injury because it is almost exclusively expressed in the liver, whereas AST is also present in tissues including the heart, skeletal muscle, and kidneys [12]. Along with visceral obesity [2], increased ALT has been associated with other cardiometabolic risk factors including insulin resistance, hypertension, and dyslipidemia [13].
To our knowledge, this is one of the first longitudinal interventional studies to associate a relatively selective reduction in VAT with improved ALT and AST. Of note, these relationships were demonstrated only among tesamorelin-treated subjects, but not among the small group of placebo-treated patients who experienced reductions in VAT. Though we did not measure liver fat in the current study, the improvement in ALT and AST among VAT responders compared to nonresponders may reflect regression of hepatic steatosis within this group. VAT is intimately linked to hepatic physiology based on its physical connection to the liver through the portal venous system. Augmentation of GH also may directly reduce liver fat, as GH reduces hepatic de novo lipogenesis in HIV [14]. We previously have shown a decrease in liver fat in association with VAT reduction among 50 HIV-infected patients with abdominal obesity randomized to tesamorelin versus placebo for 26 weeks [15]. The current study examines a much larger sample of men and women, and only includes subjects with elevated ALT and AST in whom a reduction in liver enzymes may be most clinically relevant. The mean baseline ALT in this responder analysis was 51 U/L compared to 20 U/L in our previous report [15]. The current study also uniquely includes an extension phase such that the durability of changes in ALT and AST following the discontinuation of tesamorelin can be assessed. Aside from a reduction in liver fat, the decline in ALT and AST among tesamorelin responders may reflect improved hepatic inflammation or fibrosis, but further studies are needed to investigate this important question through assessment of histologic indices.
The sustained decrease in hepatic transaminases despite a partial return to baseline in VAT is a novel finding in this study. This observation may reflect a delayed hepatic response to VAT re-accumulation following tesamorelin discontinuation, and thus studies of longer duration are needed to further characterize these changes. Nonetheless, the continued improvement in ALT and AST among VAT responders for at least 6 months following tesamorelin discontinuation is potentially important information for clinicians using this drug.
In the current study, the longitudinal association between VAT and liver enzymes was not modified by viral hepatitis status. Thus, the decline in hepatic transaminases that accompanied a tesamorelin-mediated VAT reduction was comparable among individuals with and without viral hepatitis. This finding extends the results of our cross-sectional analysis, which showed a baseline correlation between VAT and ALT that was independent of viral hepatitis status. Similarly, in another cross-sectional report, the FRAM study demonstrated no interaction between VAT and HCV status in relation to ALT among HIV-infected subjects [2]. Our longitudinal analysis highlights the intriguing possibility that strategies to reduce VAT may be of unique benefit to HIV-infected individuals with concomitant HCV and abdominal adiposity. Further studies are needed to relate tesamorelin-mediated changes in VAT to histologic outcomes among viral hepatitis patients.
The strengths of this study include its large cohort of over 800 HIV-infected men and women and its longitudinal interventional design in which we assess changes in ALT and AST among individuals with abdominal obesity. One limitation of our analysis is that, from these two large trials, no detailed measures of alcohol use were available. However, since heavy alcohol use may be expected to mask the relationship between changes in VAT and liver enzymes, the robust nature of our findings is noteworthy. The large number of subjects in these trials also precluded the assessment of viral hepatitis serologies such that hepatitis status was based on participant self-report. Another limitation of the current study is our use of ALT and AST rather than more specific radiographic or histologic measures of hepatic disease. Nonetheless, our analyses showing potent effects on ALT and AST now provide increased rationale for such studies.
In conclusion, we show for the first time that a selective, clinically significant reduction in VAT of ≥ 8% is associated with improved ALT and AST among tesamorelin-treated individuals with elevations in these markers at baseline. This decrease in ALT and AST occurred irrespective of viral hepatitis status, and outlasted the reduction in VAT among initial tesamorelin responders who were reassigned to placebo. Through its longitudinal interventional design, this study suggests potential clinical benefits of VAT reduction on the liver among HIV-infected patients.
Supplementary Material
Acknowledgments
Source of Funding: J.C.M. and C.M. are employed by Theratechnologies. J.F. is a consultant for Theratechnologies, and is on speaker bureaus for ViiV Healthcare, Gilead Sciences, and Merck. J.M. is a consultant for Theratechnologies. T.L.S. has served on an advisory board for Theratechnologies, and has received research funding from Kowa Pharmaceuticals and Novo Nordisk. S.K.G. has served as consultant to Theratechnologies, Navidea Biopharmaceuticals, Bristol-Myers Squibb, Novo Nordisk, Merck, and Gilead Sciences, and has received research funding from Theratechnologies, Kowa Pharmaceuticals, Navidea Biopharmaceuticals, Gilead Sciences, and Immunex. Funding sources were Theratechnologies Inc., NIH T32 DK007028 (L.T.F.), and NIH P30 DK040561 (S.K.G.).
We would like to thank the participants of this study as well as the clinical trial site staff. We also appreciate the contribution of Olivier Briand of Excelsus Statistics to the statistical analysis.
The study was supported by Theratechnologies Inc., NIH T32 DK007028 (L.T.F.), and NIH P30 DK040561 (S.K.G.). Theratechnologies was not involved in analysis design, drafting of the manuscript, nor the decision to submit this work for publication.
Footnotes
Conflicts of Interest: L.T.F., N.C., M.N.F., and J.W. have nothing to disclose.
Trial registration: NCT00123253, NCT00435136, NCT00608023
L.T.F., T.L.S., and S.K.G. contributed to analysis design, interpretation of results, and drafting the initial manuscript. J.C.M., J.F., and C.M. contributed to data collection. J.M. contributed to the statistical analysis. All authors edited and provided substantial input to the manuscript.
This work received an oral presentation at the Endocrine Society annual meeting, Orlando, FL, April 1–4, 2017.
This analysis was based on clinical trials with registration numbers NCT00123253, NCT00435136, and NCT 00608023.
Disclosure summary: L.T.F., N.C., M.N.F., and J.W. have nothing to disclose. J.C.M. and C.M. are employed by Theratechnologies. J.F. is a consultant for Theratechnologies, and is on speaker bureaus for ViiV Healthcare, Gilead Sciences, and Merck. J.M. is a consultant for Theratechnologies. T.L.S. has served on an advisory board for Theratechnologies, and has received research funding from Kowa Pharmaceuticals and Novo Nordisk. S.K.G. has served as a consultant to Theratechnologies, Navidea Biopharmaceuticals, Bristol-Myers Squibb, Novo Nordisk, Merck, and Gilead Sciences, and has received research funding from Theratechnologies, Kowa Pharmaceuticals, Navidea Biopharmaceuticals, Gilead Sciences, and Immunex.
References
- 1.Crum-Cianflone N, Collins G, Medina S, Asher D, Campin R, Bavaro M, et al. Prevalence and factors associated with liver enzyme abnormalities among human immunodeficiency virus-infected persons. Clin Gastroenterol Hepatol. 2010;8:183–191. doi: 10.1016/j.cgh.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tien PC, Kotler DP, Overton ET, Lewis CE, Rimland D, Bacchetti P, et al. Regional adipose tissue and elevations in serum aminotransferases in HIV-infected individuals. J Acquir Immune Defic Syndr. 2008;48:169–176. doi: 10.1097/QAI.0b013e3181685700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stranges S, Dorn JM, Muti P, Freudenheim JL, Farinaro E, Russell M, et al. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology. 2004;39:754–763. doi: 10.1002/hep.20149. [DOI] [PubMed] [Google Scholar]
- 4.Falutz J, Mamputu JC, Potvin D, Moyle G, Soulban G, Loughrey H, et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab. 2010;95:4291–4304. doi: 10.1210/jc.2010-0490. [DOI] [PubMed] [Google Scholar]
- 5.Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med. 2007;357:2359–2370. doi: 10.1056/NEJMoa072375. [DOI] [PubMed] [Google Scholar]
- 6.Falutz J, Potvin D, Mamputu JC, Assaad H, Zoltowska M, Michaud SE, et al. Effects of tesamorelin, a growth hormone-releasing factor, in HIV-infected patients with abdominal fat accumulation: a randomized placebo-controlled trial with a safety extension. J Acquir Immune Defic Syndr. 2010;53:311–322. doi: 10.1097/QAI.0b013e3181cbdaff. [DOI] [PubMed] [Google Scholar]
- 7.Stanley TL, Falutz J, Marsolais C, Morin J, Soulban G, Mamputu JC, et al. Reduction in visceral adiposity is associated with an improved metabolic profile in HIV-infected patients receiving tesamorelin. Clin Infect Dis. 2012;54:1642–1651. doi: 10.1093/cid/cis251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falutz J, Allas S, Mamputu JC, Potvin D, Kotler D, Somero M, et al. Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. AIDS. 2008;22:1719–1728. doi: 10.1097/QAD.0b013e32830a5058. [DOI] [PubMed] [Google Scholar]
- 9.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Despres JP. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. American Journal of Clinical Nutrition. 1996;64:685–693. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 10.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 11.Lake JE, Stanley T, Apovian C, Bhasin S, Brown TT, Capeau J, et al. Practical Review of Recognition and Management of Obesity and Lipohypertrophy in HIV Infection. Clin Infect Dis. 2017 doi: 10.1093/cid/cix178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giboney PT. Mildly elevated liver transaminase levels in the asymptomatic patient. Am Fam Physician. 2005;71:1105–1110. [PubMed] [Google Scholar]
- 13.Porter SA, Pedley A, Massaro JM, Vasan RS, Hoffmann U, Fox CS. Aminotransferase levels are associated with cardiometabolic risk above and beyond visceral fat and insulin resistance: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013;33:139–146. doi: 10.1161/ATVBAHA.112.300075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz JM, Mulligan K, Lee J, Lo JC, Wen M, Noor MA, et al. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab. 2002;87:942. doi: 10.1210/jcem.87.2.8391. [DOI] [PubMed] [Google Scholar]
- 15.Stanley TL, Feldpausch MN, Oh J, Branch KL, Lee H, Torriani M, et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA. 2014;312:380–389. doi: 10.1001/jama.2014.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.