Summary
Background
Many HIV-infected individuals present with advanced HIV disease. These patients are at high risk of death after antiretroviral therapy (ART) initiation, but risk factors for death in these patients are unclear.
Methods
We used data from a multi-site randomized trial comparing empiric versus preventive TB therapy in HIV-infected adults initiating ART with CD4 counts <50 cells/mm3 to evaluate risk factors for death within 48 weeks after ART initiation. Cox proportional hazards models were fit to evaluate characteristics present at baseline and at 4 weeks after ART initiation, including the week 4 CD4 cell response and new opportunistic infections (OIs).
Findings
Of 850 enrolled, the median pre-ART CD4 count was 18 cells/mm3 and 67 (7.9%) died. Baseline risk factors for death included lymphadenopathy, lower CD4 count, lower serum albumin, high white blood cell (WBC) count, elevated neutrophil percent, and lower hemoglobin. Among 746 participants with data at week 4, the median changes in CD4 count and viral load for those who died (n=43) vs. survived were 26 vs. 56 cells/mm3 and −2.7 vs. −2.7 log10 copies/mL, respectively. Each 20 cell/mm3 lower change in week 4 CD4 count was associated with a 20% increased risk of post week-4 mortality (adj. HR 1.20, 1.01–1.42, p=.038).
Interpretation
Evidence of active infection and sub-optimal immunologic response during the first month of ART are associated with death in the first year after ART initiation in those with advanced HIV disease taking TB preventative therapy. Strategies to reduce early mortality in this population warrant further investigation.
Introduction
Provision of antiretroviral therapy (ART) to HIV-infected individuals has saved millions of lives in resource-limited settings (1). However, CD4+ T-cell counts remain low among individuals presenting for care in many areas (2), and up to 17% of adults die in the first year after ART initiation (3). Risk factors for death in the initial year after starting ART (i.e., early mortality) include a low CD4 count, low body mass index (BMI), and anemia, with a CD4 count <50 cells/mm3 conferring particularly high risk (3).
World Health Organization (WHO) guidelines urge providers to screen HIV-infected patients for TB-associated symptoms and to start asymptomatic individuals on ART and isoniazid preventive therapy (IPT), which reduces TB-associated morbidity in HIV-infected individuals (4). To date, studies evaluating risk factors for early mortality have generally evaluated patients at all stages of HIV disease who receive care in settings where WHO recommendations on TB screening and IPT use are not implemented (3). These studies may not generalize to settings where TB screening and IPT are systematically performed, which is an increasingly important knowledge gap as efforts to improve implementation of these guidelines increases.
Studies evaluating risk factors for early mortality in large, diverse populations with advanced HIV are needed. Identifying factors associated with early mortality may enable formulation of therapeutic or diagnostic interventions. It is also important to understand how patients who die respond or fail to respond to ART. Because most who suffer early mortality die before responses to ART are assessed, responses and events early on ART in these individuals remain largely unknown. Early ART adherence has been shown to be high in those suffering early adverse treatment outcomes, and one study evaluating patients initiating ART with advanced HIV and pulmonary TB found that sub-optimal CD4 recovery by week 4 was strongly associated with an increased risk of early mortality (5, 6). Opportunistic illnesses that emerge very early after ART initiation also confer an increased risk of early mortality, although most studies have focused on TB, which may be less common as implementation of TB screening and IPT improves (7, 8).
We used data from a large, multi-site international randomized trial of empiric TB therapy in HIV-infected adults initiating ART with CD4 counts <50 cells/mm3 to examine pre-ART risk factors for early mortality and to evaluate the associations between early (week 4) ART responses and emergent OIs and the risk of death between 4 and 48 weeks after ART initiation.
Methods
The study was approved by local ethics committees at the study sites.
Study design
We conducted a secondary analysis of a clinical trial to evaluate the association between baseline (pre-ART) characteristics and early mortality after ART initiation. Early mortality was defined as death within 48 weeks after ART initiation (henceforth referred to as early mortality). Baseline risk factors included demographics and pre-ART clinical characteristics including symptoms and WHO stage 3 and 4 OIs. In addition, we evaluated the association between early ART response, defined as the change in viral load and CD4 count from baseline (pre-ART) to week 4 after ART initiation, and death occurring between 4 and 48 weeks after ART initiation (i.e., post-week 4 mortality). WHO stage 3 and 4 OIs newly diagnosed by week 4 were also included as risk factors in this analysis.
Study setting
AIDS Clinical Trials Group Study 5274 (A5274) is an international clinical trial that randomized ART-naïve, HIV-infected adults age 13 years or older with CD4 counts <50 cells/mm3 1:1 in an open label design to a strategy of ART + four drug empiric TB therapy (Empiric arm) or ART+ IPT (IPT arm). As reported elsewhere, empiric TB therapy did not improve 24- or 48-week survival (9). The study took place at 18 sites in 10 countries, with 78% of participants living in sub-Saharan Africa (9). All sites reported a TB incidence >100/100,000 person years and had ART programs with documented high early mortality rates (>10–20 per 100 person years) among outpatients. Sites generally followed guidelines for ART initiation within their country programs.
Study subjects
Potential trial participants were referred to study clinics from local ART clinics. At the study clinics, potential participants were screened for TB using a standard TB symptoms screen (9). Any positive screen required further workup as per local standard of care. All sites had capacity to perform AFB smear, chest radiograph, ultrasound, mycobacterial culture, and the Xpert MTB/RIF® assay. Use of specific tests was left up to site providers until June 2013, when the Data Safety Monitoring Board requested that sputum Xpert MTB/RIF be performed prior to enrollment. Persons suspected of having TB, or for whom screening procedures identified confirmed or probable TB, were excluded. Participants without symptoms on screening or who had no microbiologic or clinical diagnosis of TB despite symptoms were eligible. Additional inclusion criteria included having liver function tests ≤2.5× the upper limit of normal, creatinine clearance ≥30mL/min, and Karnofsky Performance Score ≥30. Exclusion criteria included use of single dose nevirapine in the preceding 2 years, receipt of TB treatment or IPT within 96 and 48 weeks prior to study entry, respectively, and a history of or household exposure to multidrug resistant (MDR)-TB (see appendix for full eligibility criteria).
Study treatments and data collection
All participants received efavirenz-containing ART with either study-provided tenofovir/emtricitibine (Truvada®) or locally available nucleoside reverse transcriptase inhibitors. Participants in the Empiric arm received fixed-dose combination rifampin/isoniazid/ethambutol/ pyrazinamide for 8 weeks, followed by fixed-dose combination rifampin/isoniazid for 16 weeks, beginning within 7 days after ART initiation. Participants in the IPT arm received 300mg of isoniazid daily for 24 weeks, beginning within 7 days of ART. All participants received pyridoxine. Participants attended study visits at screening, enrollment, and weeks 1, 2, 4, 8, 12, 16, 20, 24, 36 and 48. Blood was collected for CD4 and HIV-1 RNA at entry, week 4 and week 24. Those diagnosed with TB received TB drug resistance testing and standard TB treatment. Causes of death and WHO stage 3 and 4 diagnoses were externally adjudicated by an independent physician panel.
Statistical analysis
We considered two main analyses: time to death within 48 weeks after ART initiation (for analyses of baseline risk factors for early mortality), and time to death between weeks 4 and 48 after ART initiation (for analyses of week-4 CD4 response, virologic response, and newly diagnosed OIs on ART and mortality). All deaths after week 4 were included in both analyses and the baseline risk factor analysis also included 9 deaths occurring between baseline and week 4. Participants who withdrew consent (n=8) or had an unknown vital status (n=2) were censored at the time of their last visit. Plots of survival curves were produced using the Kaplan-Meier method, with variables either dichotomized around the median value or at clinically meaningful cut-points. Risk related to baseline CD4 count was expressed in 10 cells/mm3 increments due to limited variability of CD4 counts in this highly advanced HIV population. Cox proportional hazards models and 95% confidence intervals (CIs) were used to estimate hazard ratios. Proportional hazards assumptions were assessed using plots of the observed score process for each covariate against simulated score processes under proportional hazards (10, 11). For the baseline analysis, adjusted hazard ratios are based on multivariable models that include the covariate of interest, and the pre-specified covariates of age, sex, BMI, hemoglobin, CD4 count, and log 10 HIV-1 RNA. Since there were relatively few events (66) in the analysis, to avoid overfitting we used this approach rather than including all covariates of interest in a single model. For the post-week-4 mortality analysis, a single model was fit with the covariates above and 4-week CD4 change, 4-week viral load change, viral suppression (HIV-1 RNA < 400 copies/mL), and any new opportunistic infections from baseline to week 4 (yes/no). This analysis was pre-specified to test the association of mortality and 4-week CD4 change, adjusted for viral load and other key covariates. Trial study arm was not associated with survival (9) and was not included as a covariate.
Because there were few missing data at baseline, all eligible participants with hemoglobin, CD4 count, and viral load data were included in the analysis of baseline risk factors using complete case analysis (n=837). For the post-week-4 mortality analysis, participants who were alive but missing CD4 counts or viral load at week 4 or baseline hemoglobin, CD4 count, or viral load were initially excluded (n=94). However, a multiple imputation (MI) analysis was performed to incorporate participants initially excluded for missing these data. Fully conditional specification with predictive mean matching imputation was performed as it is more robust to departures from multivariate normality (12) (13). The imputation model included all variables in the post-week-4 analysis model, the log of follow up time from week 4 to 48, survival status, and albumin, absolute neutrophil count, neutrophil percent, and white blood cell count. A total of 100 imputations were performed; results were combined using Rubin’s method (14). The validity of the multiple imputation analysis is based on the missing at random assumption; that the missing data depends on the observed data included in the model.
Those missing week-4 data because they had died or were lost to follow-up prior to week 4 were not included in the post-week-4 analyses. We noted a very high mortality rate in participants who were alive at week 4 but missed their week-4 visit. We evaluated the possible effects of missing data at week 4 in these participants by 1) repeating the analysis with multiple imputation after excluding these individuals and 2) performing sensitivity analysis varying assumptions about these participants’ 4-week CD4 and viral load responses. Ten imputations were performed per sensitivity analysis. Change in CD4 count at week 4 was expressed in increments of 20 cells/mm3 due to relatively high variability of this parameter. Analyses were performed with SAS Version 9.4 using procedures PHREG for the mortality analyses, LIFETEST and SGPLOT for Kaplan-Meier plots, and MI and MIANALYZE for the multiple imputation analysis. This study is registered in ClincalTrial.gov, trial number NCT01380080.
Study oversight
The trial was funded by the National Institutes of Allergy and Infectious Diseases (NIAID) through the AIDS Clinical Trials Group and was overseen by an NIAID-appointed Data Safety Monitoring Board.
Results
From October 31, 2011 until June 9, 2014, a total of 851 were randomized in A5274 (Supplemental Figure 1)(9). Suspected or confirmed active illness including TB was a common reason for exclusion (Supplemental Figure 1). One participant was found to be ineligible after enrollment and was discontinued at day 10 for having a screening CD4 count ≥ 50 cells/mm3. 443 enrolled participants had a chest x-ray done during screening.
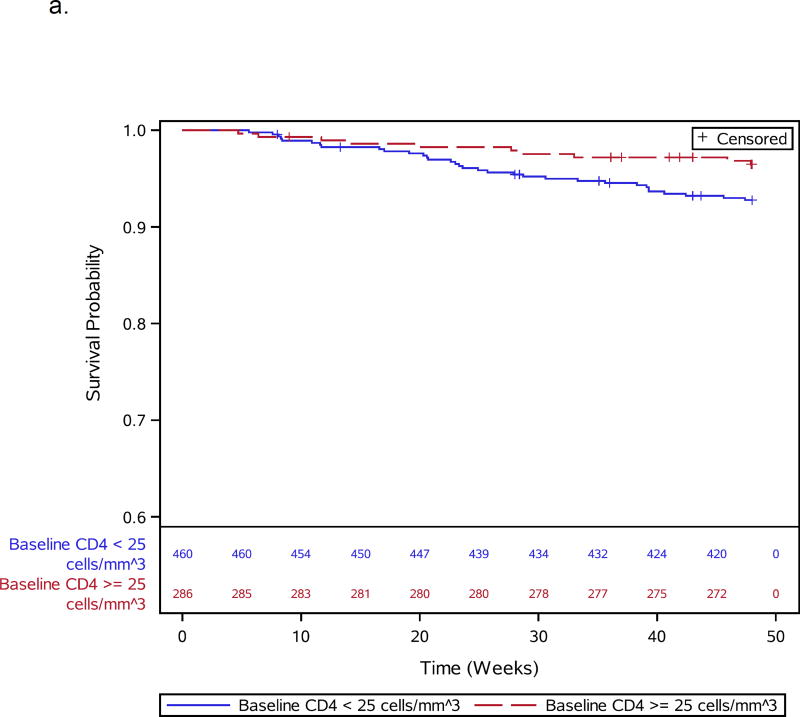
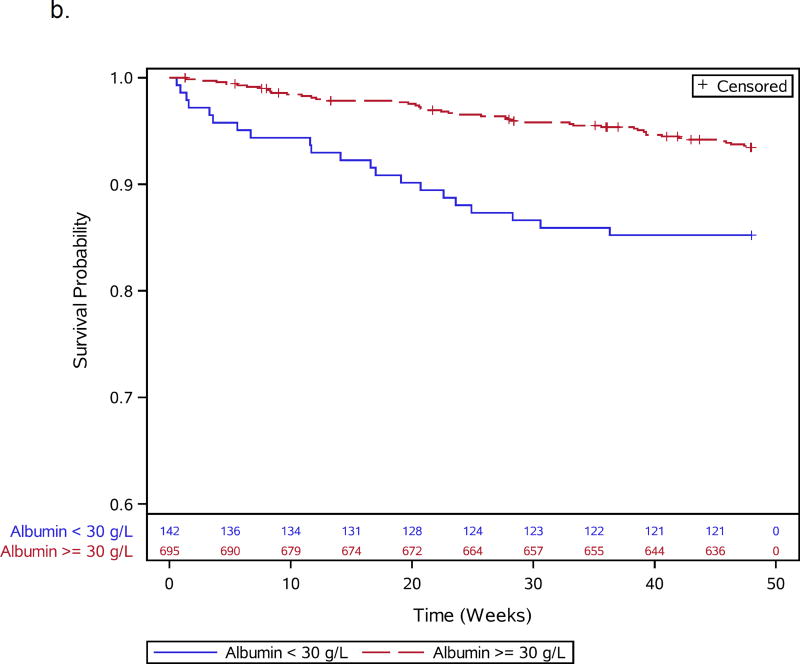
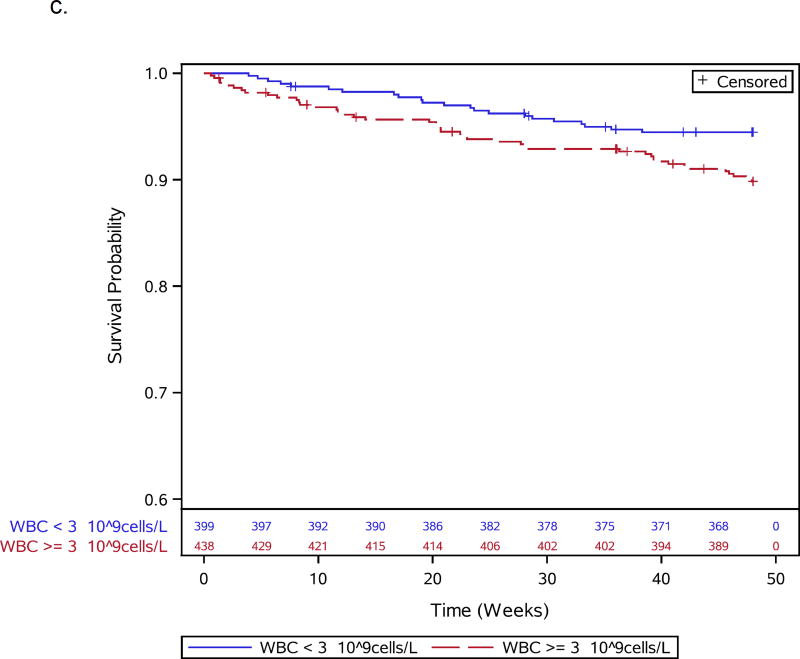
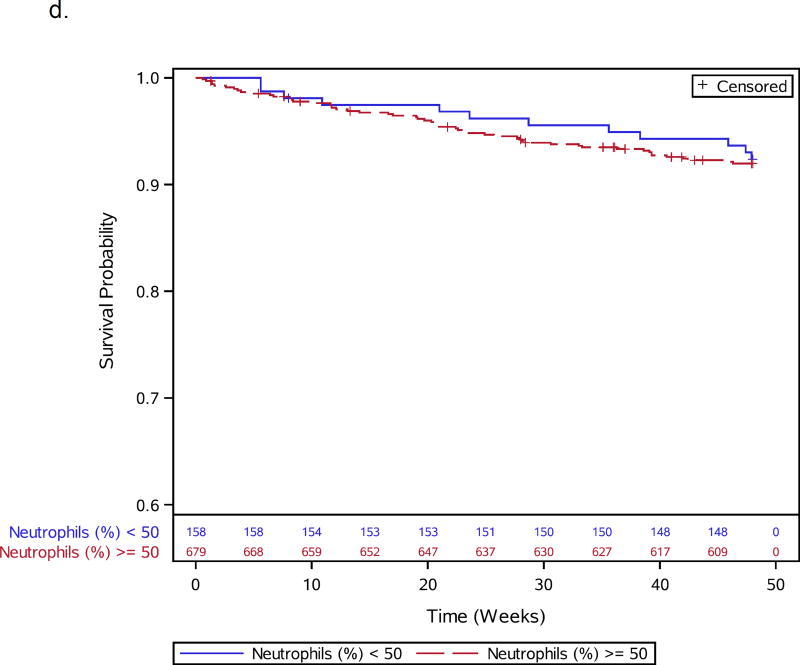
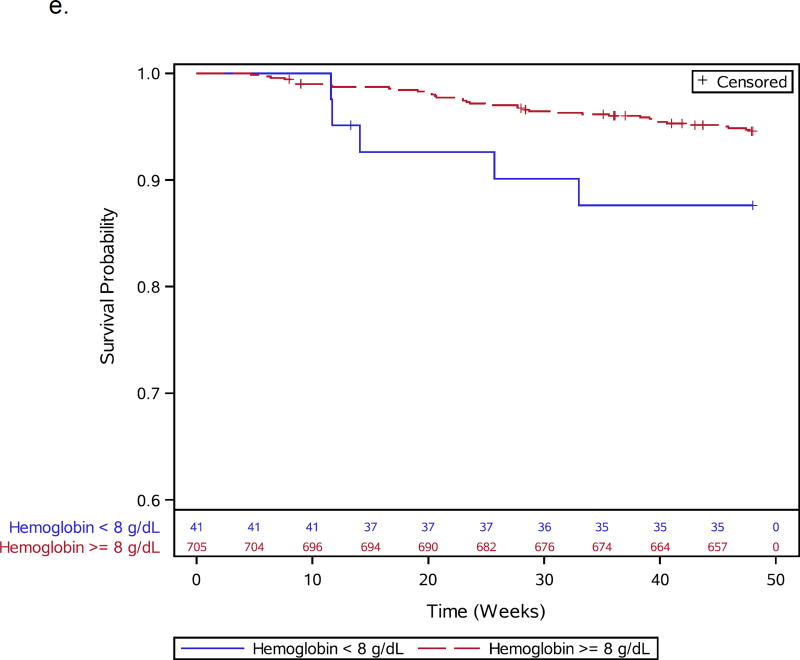
Thirteen participants, including 1 death, were missing CD4 count (n=3), viral load (n=6), or hemoglobin (n=4) values and were excluded from baseline analyses, leaving 837 participants for further evaluation (Supplemental Figure 1). During 48 weeks of follow-up, 66 of 837 (7.9%) participants died. Participant characteristics for the 66 deaths and 771 survivors included in the baseline analysis are shown in Table 1. Specific OIs present at baseline are given in Supplemental Table 1. Table 2 summarizes all 66 adjudicated causes of death. Most participants (809) were given co-trimoxazole prior to entry. Forty-seven events, including 7 TB-related deaths, were confirmed as HIV/AIDS-related, 8 were non-HIV-related, 1 was due to renal failure in the IPT arm attributed to tenofovir); there was insufficient information for another 10 deaths. In addition to increased age, pre-ART risk factors independently associated with early mortality included slightly lower CD4 count, lymphadenopathy, lower hemoglobin, decreased albumin, higher WBC, and higher neutrophil percent (Table 1; Figure 1a–e). WHO stage 3 or 4 conditions at baseline were not associated with early mortality (Table 1).
Table 1.
Baseline Characteristics and Early Mortality in Adults Initiating Antiretroviral Therapy with Advanced HIV Disease
| Death by Week 48 | |||||
|---|---|---|---|---|---|
| Characteristic | Yes (N=66) |
No (N=771) |
Adjusted HR (95% CI)1,2 |
N (no. events) |
|
| Age (years) | Median (IQR) | 38.5 (31.0, 47.0) | 35 (30, 41) | 0.95 (0.93–0.98) | 837 (66) |
| BMI (kg/m^2) | Median (IQR) | 19.2 (17.6, 21.4) | 20.3 (18.3, 22.7) | 837 (66) | |
| 18.5–25 vs. > 25 | 1.75 (0.61–4.99) | ||||
| < 18.5 vs. > 25 | 1.64 (0.55–4.96) | ||||
| Hemoglobin (g/dL) | Median (IQR) | 10.6 (8.8, 11.8) | 11.3 (10.1, 12.6) | 1.33 (1.17–1.51) | 837 (66) |
| Absolute CD4 count | Median (IQR) | 14.5 (7.0, 19.0) | 19 (9, 33) | 1.46 (1.20–1.78)3 | 837 (66) |
| log 10 HIV-1 RNA/mL | Median (IQR) | 5.4 (4.9, 5.9) | 5.3 (4.9, 5.7) | 0.86 (0.60–1.24) | 837 (66) |
| Sex | N (%) | 837 (66) | |||
| Male | 36 (55%) | 410 (53%) | Reference | ||
| Female | 30 (45%) | 361 (47%) | 1.02 (0.61–1.72) | ||
| Albumin (g/L) | Median (IQR) | 33.0 (28.0, 36.7) | 37.0 (32.1, 41.0) | 1.08 (1.03–1.13) | 834 (66) |
| Absolute neutrophil count (10^9 cells/L) | Median (IQR) | 1.9 (1.2, 3.2) | 1.6 (1.1, 2.3) | 0.80 (0.72–0.88) | 837 (66) |
| Neutrophils (%) | Median (IQR) | 57.5 (43.6, 73.4) | 53.7 (44.2, 63.7) | 0.98 (0.97–1.00) | 827 (66) |
| Serum creatinine (mg/dL) | Median (IQR) | 0.8 (0.6, 0.9) | 0.7 (0.6, 0.9) | 0.99 (0.96–1.01) | 784 (65) |
| White blood cell count (10^9 cells/L) | Median (IQR) | 3.5 (2.5, 4.8) | 3.0 (2.3, 4.0) | 0.80 (0.73–0.89) | 837 (66) |
| CD4 to CD8 ratio (× 100) | Median (IQR) | 3.5 (1.5, 5.1) | 4.2 (2.1, 7.1) | 1.01 (0.95–1.07) | 836 (66) |
| Signs and symptoms (yes vs no): | |||||
| Unknown | 2 (3%) | 6 (1%) | 829 (64) | ||
| Cough lasting 2 or more weeks | 6 (9%) | 131 (17%) | 0.454 (0.19–1.06) | 829 (64) | |
| Headache in past 30 days | 9 (14%) | 101 (13%) | 1.01 (0.49–2.04) | 829 (64) | |
| Fever > 38 C | 1 (1.5%) | 48 (6%) | 0.20 (0.03–1.45) | 829 (64) | |
| Night sweats in past 2 weeks | 8 (12%) | 67 (9%) | 1.28 (0.60–2.71) | 829 (64) | |
| Unintentional weight loss past 30 days | 37 (56%) | 369 (48%) | 1.28 (0.77–2.13) | 829 (64) | |
| Enlarged axillary/cervical lymph nodes | 11 (17%) | 67 (9%) | 2.15 (1.11–4.17) | 829 (64) | |
| Any symptom | 42 (64%) | 475 (62%) | 1.00 (0.59–1.71) | 829 (64) | |
| Any diagnosed WHO stage 3 or 4 condition | 29 (44%) | 287 (37%) | 1.05 (0.63–1.73) | 837 (66) | |
Hazard ratios for continuous variables are given per unit decrease, unless otherwise noted
Hazard ratios adjusted for age, sex, BMI (categorical), hemoglobin, CD4 count, and log 10 HIV−1 RNA/mL
Hazard ratio is per 10 unit decrease
Table 2.
Primary Causes of Early Mortality in in Adults Initiating Antiretroviral Therapy with Advanced HIV Disease
| Treatment Strategy | |||
|---|---|---|---|
| Empiric (N=30) |
IPT (N=36) |
Total (N=66) |
|
| Primary Cause of Death HIV infection or HIV-related diagnosis | 23 (77%) | 24 (67%) | 47 (71%) |
| Pulmonary or extra pulmonary tuberculosis | 3 | 4 | 7 |
| Pulmonary – respiratory disease/failure | 4 | 3 | 6 |
| Bacterial Sepsis | 1 | 3 | 4 |
| Cryptococcal meningitis | 2 | 2 | 4 |
| Hematologic disease (other than clotting) | 2 | 2 | 4 |
| Cardiovascular shock | 2 | 1 | 3 |
| Kaposi Sarcoma | 3 | 0 | 3 |
| Bacterial pneumonia – probable | 1 | 1 | 2 |
| Pneumocystis carinii pneumonia | 1 | 0 | 1 |
| Other | 4 | 8 | 12 |
| Non-HIV diagnosis | 2 (7%) | 6 (17%) | 8 (12%) |
| Toxicity | 0 (0%) | 1 (3%) | 1 (2%) |
| No information available | 5 (17%) | 5 (14%) | 10 (15%) |
Figures 1.
a–e: Survival curves for baseline laboratory risk factors for early mortality after ART initiation identified by multivariable analysis. Pre-ART values of 1.a) CD4 count (above or below 25 cells/mm3, 1.b) albumin (dichotomized at 30 gm/L), 1.c) white blood cell count (dichotomized at median), and 1.d) neutrophil percentage (dichotomized at median) and 1.e) hemoglobin (dichotomized at 8 gm/dL) are shown.
Seven-hundred and forty-six of 850 (88%) participants, including 43 who died, had complete baseline and week-4 data available for the complete case analysis of early response and survival (Supplemental Figure 1). Nine participants who died and 1 who was lost to follow-up before week 4 were excluded from week-4 analyses. Participants initially excluded from the week-4 assessment appeared slightly sicker in terms of lower hemoglobin and BMI and somewhat greater prevalence of WHO grade 3 or 4 conditions than those included (Supplemental Table 2). Table 3 presents early virologic and immunologic responses stratified by post-week 4 mortality on ART for both the complete case and multiple imputation analyses, which included 840 participants (850 randomized minus 10 who died or were lost prior to week 4) and 58 deaths. In complete case analysis, the median 4-week changes in CD4 count and viral load for deaths vs non-deaths after week 4 were 26 vs 56 cells/mm3 and −2.7 vs −2.7 log10 copies/mL, respectively (Table 3). While the median change in viral load was the same among those who died and those who survived, the distributions were different, as evidenced by the 3rd quartile of change in viral load being smaller in those who died compared to those who survived (−0.4 vs. −2.4 log10 copies/mL). Lower virologic responses may have been related to lower early adherence, since the median number of weeks participants were completely adherent to their prescribed HIV medications over the first 3 weeks were 2.8 weeks among those who survived (n=758) and 2.5 weeks among those who died (n=48; p=0.02). Sub-optimal early CD4 count change and early virologic responses were independently associated with early mortality after adjusting for age, sex, BMI, hemoglobin, baseline CD4, baseline viral load, and newly diagnosed WHO stage 3 or 4 conditions (Table 3).
Table 3.
Characteristics by Death Status Among Participants in Post Week-4 Mortality Analysis
| Death by Week 48 | CC Analysis | MI Analysis3 | |||
|---|---|---|---|---|---|
| Characteristic | Yes (N=43) |
No (N=703) |
Adjusted HR (95% CI)1,2 |
Adjusted HR (95% CI)1,2 |
|
| Age (years) | Mean | 38.3 | 36.5 | 0.96 (0.93–1.00) | 0.96 (0.93–0.99) |
| Median (IQR) | 37 (31, 47) | 36 (30, 41) | |||
| BMI (kg/m^2) | Median (IQR) | 19.1 (17.5, 21.4) | 20.4 (18.3, 22.9) | ||
| 18.5–25 vs. > 25 | 5.19 (0.69–39.31) | 1.66 (0.56–4.92) | |||
| < 18.5 vs. > 25 | 4.57 (0.58–36.00) | 1.23 (0.39–3.91) | |||
| Hemoglobin (g/dL) | Median (IQR) | 10.6 (8.6, 11.9) | 11.3 (10.1, 12.6) | 1.32 (1.12–1.55) | 1.32 (1.15–1.51) |
| Absolute CD4 Count | Median (IQR) | 16 (9, 24) | 19 (9, 32) | 1.30 (1.05–1.60)4 | 1.43 (1.17–1.75)4 |
| Log 10 HIV-1 RNA/mL | Median (IQR) | 5.1 (4.7, 5.8) | 5.3 (4.9, 5.7) | 0.62 (0.38–1.02) | 0.59 (0.37–0.93) |
| Sex | Male | 22 (51%) | 372 (53%) | Reference | Reference |
| Female | 21 (49%) | 331 (47%) | 1.48 (0.75–2.91) | 1.20 (0.67–2.15) | |
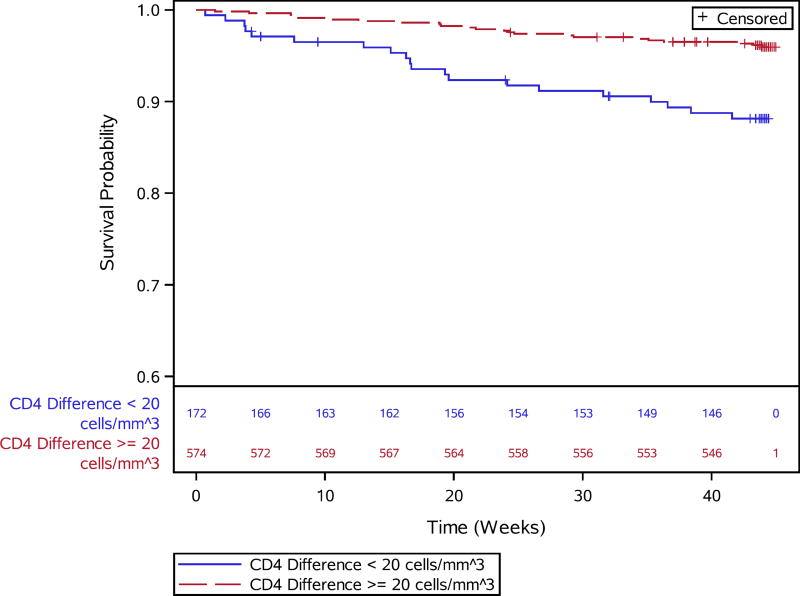
| Change in CD4 Count from Week 0 to Week 4 | Median (IQR) | 26 (3, 43) | 56 (22, 96) | 1.25 (1.06–1.47)5 | 1.20 (1.01–1.42)5 |
| Change in log 10 HIV-1 RNA from Week 0 to Week 4 | Median (IQR) | −2.7 (−3.1, −0.4) | −2.7 (−3.0, −2.4) | 0.50 (0.35–0.74) | 0.53 (0.37–0.76) |
| HIV-1 RNA < 400 at Week 4 | Yes | 20 (47%) | 335 (48%) | Reference | Reference |
| No | 23 (53%) | 368 (52%) | 0.45 (0.17–1.18) | 0.63 (0.26–1.55) | |
| New WHO Clinical Stage 3 or 4 Conditions by Week 4 | Yes | 6 (14.0%) | 37 (5.3%) | 2.76 (1.11–6.88) | 2.03 (0.87–4.72) |
| No | 37 (86.0%) | 666 (94.7%) | Reference | Reference | |
Hazard ratios for continuous variables are given per unit decrease, unless otherwise noted
Hazard ratios adjusted for all variables included in this table
Additional variables in imputation model: albumin, absolute neutrophil count, neutrophils (%), and white blood cell count
Hazard ratio is per 10 unit decrease
Hazard ratio is per 20 unit decrease
Results for complete case and multiple imputation analyses were consistent with respect to early immune recovery associations (Table 3). In the multiple imputation analysis, each 20 unit lower change in week 4 CD4 count was associated with a 20% increased risk of death (adj. HR 1.20, 1.01–1.42; Figure 2). Overall, 43 of 746 (5.7%) were newly diagnosed with an OI at week 4 (Table 3). Specific newly diagnosed OIs are listed in Supplemental Table 3. Newly diagnosed WHO stage 3 or 4 conditions were associated with an increased risk of post-week-4 mortality in the complete-case analysis but the hazard ratio crossed 1 in the multiple imputation analysis (adj. HR 2.03, 0.87–4.72).
Figure 2.
Survival curves for post-week 4 mortality and week 4 CD4 cell count change above or below 20 cells/mm3 from baseline.
Twenty-four participants alive at week 4 were missing week 4 CD4 and viral load data due to a missed visit. Of these 24 participants, 11 (46%) died prior to week 48. In a separate analysis using multiple imputation but excluding these 24 participants, the association of mortality with CD4 and viral load change remained the same (Supplemental Table 4). Sensitivity analyses where imputed CD4 change and viral load change were shifted by fixed amounts in this group of participants produced similar results under a variety of scenarios (Supplemental Table 5).
Discussion
In this study, 7.9% of HIV-infected adults initiating ART with CD4 counts <50 cells/mm3 died during 48 weeks of follow-up. Pre-ART factors associated with death were lymphadenopathy, lower hemoglobin, lower albumin, slightly lower CD4 count, higher WBC count and higher neutrophil percent. Lower CD4 increases in the initial 4 weeks of therapy were also independently associated with an increased risk of subsequent mortality despite virologic suppression. Taken together, these data suggest that early detection and treatment of OIs prior to ART initiation and rapid early immune recovery are key to survival on ART in advanced HIV disease.
Evaluating risk factors for mortality on ART in a group of individuals with advanced HIV disease revealed several findings that differ from previous studies. For example, a lower pre-ART CD4 count is consistently a strong risk factor for early mortality in a population where a broad range of baseline CD4 counts are represented (3). In this study, however, baseline CD4 counts were significantly lower in those who died, but the absolute difference was small and not clinically useful. Similarly, several studies have documented an association between BMI and survival on ART (15, 16). However, in this population albumin levels, a marker of macronutrient status, and not BMI itself (measured as a continuous or dichotomous variable), were associated with death. This may suggest a role for nutritional interventions in HIV-infected patients presenting for HIV care, as recommended by WHO (17). However, decreased hepatic albumin synthetic function may also relate to the presence of active infections, which appeared to play a prominent role in treatment outcomes in this population. Of note, higher pre-ART WBC counts and neutrophil percentages, both risk factors for death in this study, were plausibly due to co-infections that were present but undiagnosed at baseline. Consistent with this, leukocytosis was an independent risk factor for mortality in a South African cohort with a median CD4 count of 106 cells/mm3 (18), and neutrophilia has previously been associated with TB-associated mortality in hospitalized patients in South Africa (19). Notably, in the present study many of these deaths were not attributed to TB. These data indicate that diagnostic and treatment strategies capable of addressing a diverse array of coinfections in populations with advanced HIV are needed at the time of ART initiation and soon thereafter.
The magnitude of immunologic response after several months of ART is associated with longer-term survival in HIV-infected individuals (20, 21), but less is known about the relationship between early CD4 recovery and early mortality. One study among HIV-infected adults with pulmonary TB in Botswana (median pre-ART CD4 count of 61 cells/mm3) examined the association between the week-4 CD4 count change and death within 24 weeks after ART initiation (6). As in the present report, patients who died had a clinically meaningful and significantly lower change in the CD4 cell count from baseline to week 4 (median difference of 49 cells/mm3) compared to survivors (6). Our study findings confirm this association in a diverse patient population with highly advanced HIV disease without known TB at the time of ART initiation. Notably, in both studies the median virologic responses at week 4 after ART were similar among those who subsequently died or survived, although some of those who died (i.e., those in the lowest virologic response quartile) had minimal virologic response (6). These data suggest that virologic suppression, while a necessary first step in ART response, does not necessarily ensure clinical recovery in advanced HIV. Substantial early CD4 reconstitution appears crucial. The data also suggest that in patients with advanced HIV infection integrase inhibitor-based ART, which has been associated with more rapid virologic and immunologic responses (22, 23), may prove beneficial. Further controlled study in cohorts with advanced HIV is needed.
Our study has several limitations. Early CD4 count recovery is largely due to redistribution of T cells from lymphatic tissue (24), and data on factors that might impact immune recovery, such as the extent of lymph node fibrosis and immune activation prior to ART initiation (25, 26), were unavailable. Multiple studies have evaluated factors associated with longer-term immune recovery on ART (26–28), whereas few have focused on determinants of early immune restoration. Nonetheless, our data is consistent with findings from Uganda, where incident TB after ART initiation was associated with sub-optimal immune recovery during ART (29). Second, while the large patient population was diverse with respect to region, patients were followed in clinical trial settings and had a relatively low mortality rate. Thus, the results may not be generalizable to sites where close follow-up is not performed or to sites that do not screen for TB or administer IPT. Because most deaths were AIDS related, however, the relationship between CD4 recovery and mortality would likely be stronger in settings where the incidence of OIs was higher. Third, some participants died prior to week 4, limiting our ability to analyze CD4 changes in these individuals. Exclusion of these participants, who may have very poor immune recovery, could have resulted in our underestimating the strength of the association between early CD4 recovery and death. Finally, the prognostic and therapeutic value of CD4 counts are unclear in an era where ART is recommended for all HIV-infected individuals. Yet, our data suggest that patients with sub-optimal levels of immune recovery may plausibly benefit from more intensive evaluation and diagnostics. The predictive utility of this approach needs evaluation.
WHO now recommends ART for all HIV-infected individuals regardless of CD4 count (30), and optimal lab testing and evaluation prior to ART initiation are debated (31). In the present study, despite close observation in a trial and despite use of anti-tubercular drugs, approximately 1 in 14 starting ART with advanced HIV did not survive the first year of therapy. The associations between mortality, slow immune response and newly diagnosed OIs underscores the need for careful clinical evaluation of patients with very advanced HIV prior to and soon after ART initiation. Further research evaluating how this can be accomplished in an era of decentralization and differentiated HIV care is needed.
Supplementary Material
Acknowledgments
We thank Dr. Richard Chaisson for his very helpful review of the manuscript. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701 and grant numbers U01AI069497 (AG), R01AI080417 (AG), and UM1AI069465 (Amita Gupta). JB was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services (contract number HH-SN272200800014C). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The team gratefully acknowledges the external event reviewers: Drs. J. Varma, C. Vinnard, and D. Murdoch.
References
- 1.UNAIDS. How AIDS changed everything: MDG 6: 15 YEARS, 15 LESSONS OF HOPE FROM THE AIDS RESPONSE. Fact Sheet 2015 [Google Scholar]
- 2.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis. 2015;60:1120–1127. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Nadkarni G, Yang WT, Chandrasekhar A, Gupte N, Bisson GP, Hosseinipour M, Gummadi N. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One. 2011;6:e28691. doi: 10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Organization, W. H., editor. WHO policy on collaborative TB/HIV activities: Guidelines for national programmes and other stakeholders. 2012 [PubMed]
- 5.Steele KT, Steenhoff AP, Newcomb CW, Rantleru T, Nthobatsang R, Lesetedi G, Bellamy SL, Nachega JB, Gross R, Bisson GP. Early mortality and AIDS progression despite high initial antiretroviral therapy adherence and virologic suppression in Botswana. PLoS One. 2011;6:e20010. doi: 10.1371/journal.pone.0020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravimohan S, Tamuhla N, Steenhoff AP, Letlhogile R, Nfanyana K, Bellamy SL, MacGregor RR, Gross R, Weissman D, Bisson GP. Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet Infect Dis. 2015;15:429–438. doi: 10.1016/S1473-3099(15)70008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn SD, Edwards DJ, Kranzer K, Vogt M, Bekker LG, Wood R. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS. 2009;23:1875–1880. doi: 10.1097/qad.0b013e32832e05c8. [DOI] [PubMed] [Google Scholar]
- 8.Bassett IV, Wang B, Chetty S, Giddy J, Losina E, Mazibuko M, Bearnot B, Allen J, Walensky RP, Freedberg KA. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis. 2010;51:823–829. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosseinipour MC, Bisson GP, Miyahara S, Sun X, Moses A, Riviere C, Kirui FK, Badal-Faesen S, Lagat D, Nyirenda M, Naidoo K, Hakim J, Mugyenyi P, Henostroza G, Leger PD, Lama JR, Mohapi L, Alave J, Mave V, Veloso VG, Pillay S, Kumarasamy N, Bao J, Hogg E, Jones L, Zolopa A, Kumwenda J, Gupta A, Adult, A. C. T. G. A. S. T. Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet. 2016;387:1198–1209. doi: 10.1016/S0140-6736(16)00546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin DY. MULCOX2: a general computer program for the Cox regression analysis of multivariate failure time data. Computer methods and programs in biomedicine. 1993;40:279–293. doi: 10.1016/0169-2607(93)90013-b. [DOI] [PubMed] [Google Scholar]
- 11.Lin DY, Wei LJ, Ying Z. Model-checking techniques based on cumulative residuals. Biometrics. 2002;58:1–12. doi: 10.1111/j.0006-341x.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Buuren S, Brand J, Groothuis-Oudshoorn C, Rubin D. Fully conditional specification in multivariate imputation. Journal of Statistical Computation and Simulation. 2006;76:1049–1064. [Google Scholar]
- 13.Van Buuren S. Flexible Imuptation of Missing Data. CRC Press; 2012. pp. 68–74. [Google Scholar]
- 14.Rubin DB. Multiple imputation for nonresponse in surveys. Wiley-Interscience; Hoboken, N.J.: 2004. [Google Scholar]
- 15.Paton NI, Sangeetha S, Earnest A, Bellamy R. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV medicine. 2006;7:323–330. doi: 10.1111/j.1468-1293.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 16.Johannessen A, Naman E, Ngowi BJ, Sandvik L, Matee MI, Aglen HE, Gundersen SG, Bruun JN. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infect Dis. 2008;8:52. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. World Health Organization, F. a. A. O. o. t. U. N., editor. Nutritional care and support for people living with HIV/AIDS: A training course. 2009
- 18.Komati S, Shaw PA, Stubbs N, Mathibedi MJ, Malan L, Sangweni P, Metcalf JA, Masur H, Hassim S. Tuberculosis risk factors and mortality for HIV-infected persons receiving antiretroviral therapy in South Africa. AIDS. 2010;24:1849–1855. doi: 10.1097/QAD.0b013e32833a2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe DM, Bandara AK, Packe GE, Barker RD, Wilkinson RJ, Griffiths CJ, Martineau AR. Neutrophilia independently predicts death in tuberculosis. Eur Respir J. 2013;42:1752–1757. doi: 10.1183/09031936.00140913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chene G, Sterne JA, May M, Costagliola D, Ledergerber B, Phillips AN, Dabis F, Lundgren J, D'Arminio Monforte A, de Wolf F, Hogg R, Reiss P, Justice A, Leport C, Staszewski S, Gill J, Fatkenheuer G, Egger ME. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 21.May M, Sterne JA, Sabin C, Costagliola D, Justice AC, Thiebaut R, Gill J, Phillips A, Reiss P, Hogg R, Ledergerber B, D'Arminio Monforte A, Schmeisser N, Staszewski S, Egger M, Antiretroviral Therapy Cohort, C. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21:1185–1197. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markowitz M, Nguyen BY, Gotuzzo E, Mendo F, Ratanasuwan W, Kovacs C, Prada G, Morales-Ramirez JO, Crumpacker CS, Isaacs RD, Gilde LR, Wan H, Miller MD, Wenning LA, Teppler H, Protocol 004 Part, I. I. S. T. Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46:125–133. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 23.Serrano-Villar S, Zhou Y, Rodgers AJ, Moreno S. Different impact of raltegravir versus efavirenz on CD4/CD8 ratio recovery in HIV-infected patients. The Journal of antimicrobial chemotherapy. 2017;72:235–239. doi: 10.1093/jac/dkw375. [DOI] [PubMed] [Google Scholar]
- 24.Bucy RP, Hockett RD, Derdeyn CA, Saag MS, Squires K, Sillers M, Mitsuyasu RT, Kilby JM. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest. 1999;103:1391–1398. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schacker TW, Reilly C, Beilman GJ, Taylor J, Skarda D, Krason D, Larson M, Haase AT. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART-associated change in peripheral CD4 cell count. AIDS. 2005;19:2169–2171. doi: 10.1097/01.aids.0000194801.51422.03. [DOI] [PubMed] [Google Scholar]
- 26.Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, Gripshover B, Salata RA, Taege A, Lisgaris M, McComsey GA, Kirchner E, Baum J, Shive C, Asaad R, Kalayjian RC, Sieg SF, Rodriguez B. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 28.Marchetti G, Bellistri GM, Borghi E, Tincati C, Ferramosca S, La Francesca M, Morace G, Gori A, Monforte AD. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 29.Hermans SM, Kiragga AN, Schaefer P, Kambugu A, Hoepelman AI, Manabe YC. Incident tuberculosis during antiretroviral therapy contributes to suboptimal immune reconstitution in a large urban HIV clinic in sub-Saharan Africa. PLoS One. 2010;5:e10527. doi: 10.1371/journal.pone.0010527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Organization, W. H. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. World Health Organization; Geneva: 2015. [PubMed] [Google Scholar]
- 31.Rosen S, Fox MP, Larson BA, Sow PS, Ehrenkranz PD, Venter F, Manabe YC, Kaplan J, Models for Accelerating Treatment Initiation Technical, C. Accelerating the Uptake and Timing of Antiretroviral Therapy Initiation in Sub-Saharan Africa: An Operations Research Agenda. PLoS Med. 2016;13:e1002106. doi: 10.1371/journal.pmed.1002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.