Abstract
The mechanisms of Leishmania resistance to antimonials have been primarily determined in experimentally derived Leishmania strains. However, their participation in the susceptibility phenotype in field isolates has not been conclusively established. Being an intracellular parasite, the activity of antileishmanials is dependent on internalization of drugs into host cells and effective delivery to the intracellular compartments inhabited by the parasite. In this study we quantified and comparatively analyzed the gene expression of nine molecules involved in mechanisms of xenobiotic detoxification and Leishmania resistance to antimonial drugs in resistant and susceptible laboratory derived and clinical L.(Viannia) panamensis strains (n=19). In addition, we explored the impact of Leishmania susceptibility to antimonials on the expression of macrophage gene products having putative functions in transport, accumulation and metabolism of antimonials. As previously shown for other Leishmania species, a trend of increased abcc3 and lower aqp-1 expression was observed in the laboratory derived Sb-resistant L.(V.) panamensis line. However, this was not found in clinical strains, in which the expression of abca2 was significantly higher in resistant strains as both, promastigotes and intracellular amastigotes. The effect of drug susceptibility on host cell gene expression was evaluated on primary human macrophages from patients with cutaneous leishmaniasis (n=17) infected ex-vivo with the matched L.(V.) panamensis strains isolated at diagnosis, and in THP-1 cells infected with clinical strains (n=6) and laboratory adapted L.(V.) panamensis lines. Four molecules, abcb1 (p-gp), abcb6, aqp-9 and mt2a were differentially modulated by drug resistant and susceptible parasites, and among these, a consistent and significantly increased expression of the xenobiotic scavenging molecule mt2a was observed in macrophages infected with Sb-susceptible L. (V.) panamensis. Our results substantiate that different mechanisms of drug resistance operate in laboratory adapted and clinical Leishmania strains, and provide evidence that parasite-mediated modulation of host cell gene expression of molecules involved in drug transport and metabolism could contribute to the mechanisms of drug resistance and susceptibility in Leishmania.
Keywords: ABC transporter, aquaporin, meglumine antimoniate, Leishmania Viannia panamensis, macrophage
Introduction
Antimonial drugs, Glucantime® and Pentostam®, continue to be the first line treatment option for dermal leishmaniasis in the Americas. However, documented treatment failure rates between 20% and 74% [1-5], in some cases attributed to parasite drug resistance [6], together with the toxicity and extended length of treatment regimens, threaten the usefulness of these drugs. Investigations on laboratory derived drug resistant Leishmania strains have revealed mechanisms of experimental resistance to antimonials based on parasite detoxification, sequestration, efflux, and altered activation of pentavalent antimonials (SbV) to the bioactive trivalent form (SbIII) [7-12]. These functions have been associated with the over-expression of ATP-Binding Cassette (ABC) membrane transporters and metabolic enzymes primarily through mechanisms of gene amplification in SbIII-resistant Leishmania [13, 14]. However, the relationship between the expression of these genes and the susceptibility phenotype in clinical strains has been elusive and findings inconclusive [15-17].
The evaluation of in vitro screening systems to assess drug susceptibility of intracellular Leishmania has shown that the use of different host cells such as primary murine or human macrophages and cell lines (eg. THP-1, U-937, J774) results in differences in EC50 and EC90 values for a range of antileishmanial drugs analyzed in the same Leishmania strain [18]. This provides evidence of a central role of host cells in the antileishmanial effect of chemotherapeutics.
Our group and others have shown that the interaction between Leishmania and the human host extends beyond modulation of immunological functions to determinants of pharmacological responses [19-24]. L. (V.) panamensis infection of primary human macrophages and exposure to meglumine antimoniate (MA) or miltefosine modulate the expression of host cell ABC transporters and Solute Liquid Carriers (SLC), metabolic enzymes and scavenging molecules, differentially regulating the antileishmanial effect of these drugs [19, 20]. Sb-resistant L donovani strains have been shown to induce expression of macrophage ABC transporters P-glycoprotein (P-gp), Multidrug resistance associated protein–1 (MRP-1) [21] and repression of macrophage gamma-glutamylcysteine synthetase (γ-GCS) [24], resulting respectively in reduced antimony accumulation and limited reduction to SbIII in cells infected with Sb-resistant parasites. These findings lend support to the concept that drug resistance in Leishmania can be conferred by a multiplicity of redundant mechanisms dependent both on the parasite and the host. Thus, in addition to traditional intrinsic or acquired mechanisms of Leishmania drug resistance, the capacity of the parasite to modulate drug transport, metabolism and detoxification within its host cell, together with variations that occur between individuals, converge into what is defined as the drug susceptibility phenotype.
In this study we quantified and comparatively analyzed the gene expression of molecules involved in mechanisms of antimonial drug resistance in Leishmania [7, 10-12, 25], using laboratory derived and clinical strains of L (V.) panamensis categorized as resistant or susceptible to MA as intracellular amastigotes. Furthermore we explored the impact of parasite drug susceptibility on the modulation of macrophage gene products potentially involved in transport, accumulation and metabolism of antimonials, as putative mechanisms contributing to the susceptibility profile of this intracellular parasite. Screening of gene expression profiles in the host cell and the parasite provides evidence of the host-pathogen relationships that contribute to determine the susceptibility phenotype of Leishmania to antimonial drugs.
Materials and Methods
Ethics statement
This study was approved and monitored by the Institutional Review Board for Ethical Conduct of Research Involving Human Subjects of the Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM) with approval code CIEIH 1209, in accordance with national and international guidelines. All individuals voluntarily participated in the study. Written informed consent was obtained from each participant.
Study Design
This study sought to examine the relationship between Leishmania susceptibility to antimonial drugs and the expression of Leishmania and macrophage drug-response related genes. To achieve this, gene expression profiling of molecules with reported evidence of association with xenobiotic detoxification, Sb drug transport or metabolism was evaluated in Sb sensitive and resistant promastigotes of laboratory-derived and clinical strains of L. (V.) panamensis, followed by evaluation of differentially expressed genes in intracellular amastigotes. The modulatory influence of infection with Sb-susceptible and resistant L. (V.) panamensis on host cell gene expression was evaluated in THP-1 monocytic cells and primary human macrophages.
Reagents and chemicals
Additive-free meglumine antimoniate (MA) Walter Reed 214975AK; lot no. BLO918690-278-1A1W601) was kindly provided by the Walter Reed Army Institute, Silver Spring, MD. Phorbol-12-myristate 13-acetate (PMA) was obtained from Sigma -Aldrich.
Patients and samples
Seventeen adult patients, 18 to 60 years of age, with parasitological confirmation of active cutaneous leishmaniasis (CL) under 6 months of disease evolution, and from which Leishmania isolates were available, participated in this study. Peripheral blood samples were obtained prior to initiation of treatment for isolation of mononuclear cells. Leishmania strains were isolated at diagnosis from all patients, and typed by reactivity to a panel of monoclonal antibodies and isoenzyme electrophoresis [26]. Parasite strains and mononuclear cells were stored, respectively, in liquid nitrogen and at -80°C until use.
Leishmania strains and EC50
Clinical strains and laboratory derived L(V.) panamensis lines (Sb-susceptible L. (V.) panamensis; MHOM/COL/03/3594/LUC001 [L.p.001] and Sb-resistant; MHOM/COL/86/1166-1000.1 [Lp.1000.1]) were kept at 25°C in RPMI supplemented with 10% heat-inactivated FBS. The Sb-resistant L.p.1000.1 line was previously selected as promastigotes by in vitro drug pressure to SbIII [25] and maintained under drug pressure in culture (1000μM SbIII). For evaluation of gene expression profiles in promastigotes L (V.) panamensis clinical strains obtained from CIDEIM Biobank were selected based on the susceptibility phenotype as intracellular amastigotes defined as highly Sb-susceptible (reduction of parasite burden ≥ 78%; n=9) and highly Sb-resistant (reduction of parasite burden ≤ 5%; n=10) (Table 1), and independent of the outcome of treatment of patients from whom the strains were isolated. A subgroup of these strains (n=3/group) was selected for analyses of the modulatory effects on THP-1 cell gene expression. Selection of clinical strains presenting with extreme phenotypes was based on the comparability of these to the phenotypes of laboratory-adapted lines L.p.001 and Lp.1000.1, for which the reduction of parasite burden was, on average of four independent experiments, 83% and 10%, respectively.
Table 1.
Susceptibility profiles of Leishmania (V.) panamensis clinical strains.
| Sb-Resistant Strains | Susceptible Strains | ||
|---|---|---|---|
| Code | % reduction of parasite burden* | Code | % reduction of parasite burden |
| MHOM/CO/84/2198 | 0% | MHOM/CO/85/2496 | 96% |
| MHOM/CO/84/2168 | 0% | MHOM/CO/86/2476 | 88% |
| MHOM/CO/85/2452 | 0% | MHOM/CO/85/2363 | 80% |
| MHOM/CO/84/2169 | 0% | MHOM/CO/85/2348 | 78% |
| MHOM/CO/85/2472 | 3% | MHOM/CO/85/2420 | 88% |
| MHOM/CO/84/2183 | 0% | MHOM/CO/85/2423 | 98% |
| MHOM/CO/85/1131 | 0% | MHOM/CO/85/2277 | 95% |
| MHOM/CO/06/5033 | 5% | MHOM/CO/05/3951 | 93% |
| MHOM/CO/03/3783 | 0% | MHOM/CO/06/5035 | 97% |
| MHOM/CO/04/3832 | 0% | ||
Percentage reduction of parasite burden was established by comparing against infected macrophages without drug exposure.
Drug susceptibility was evaluated by in vitro survival of intracellular amastigotes to MA. Briefly, PMA-differentiated U-937 macrophages were exposed to L.V. panamensis promastigotes for 2h, washed with PBS and infection allowed to proceed for additional 24h. Infected macrophages were then exposed to 32 μg/ml MA (selected based on the Cmax achieved in CL patients undergoing treatment with Glucantime®) for 72h, with one change of drug-containing medium at 48h. Susceptibility of intracellular parasites was measured by evaluation of the percent parasite survival after exposure to MA, compared to the infected control without drug exposure [27]. Leishmania strains were defined as Sb-susceptible and Sb-resistant when % survival after drug exposure was <30% and >70%, respectively [27].
Cell Culture, differentiation and infection
The human monocytic cell line THP1 was maintained at 1 × 106 cells/ml in RPMI 1640 (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 μg/ml streptomycin, 100 U/ml penicillin at 37°C and 5% CO2. Cultured cells were differentiated into macrophages using 250ng/ml PMA for 3 hours and then seeded in 6-well plates at a density of 1 × 106 cells/well. Primary human macrophages were differentiated from monocytes in peripheral blood mononuclear cells (PBMCs) from CL patients. PBMCs were collected by centrifugation over a Ficoll-Hypaque gradient (Sigma-Aldrich). Differentiation of macrophages was achieved by adherence to cell culture plastic ware in serum-free RPMI for 2h, followed by culture for 7 days in RPMI supplemented with 20% FBS at 37°C and 5% CO2. THP-1 and primary macrophages were infected with stationary phase promastigotes opsonized with heat inactivated human AB+ serum, at a 10:1 Leishmania-macrophage ratio for 2h [28], washed twice with PBS and incubated for 24h at 34°C with 5% CO2.
RNA extraction and gene expression
Total RNA was extracted from logarithmic phase promastigotes with the RNA isolation system (Promega), and from THP-1 and primary macrophages using TRIzol reagent (Invitrogen, USA) followed by organic extraction. Promastigote and primary macrophage RNA were treated with DNAse I, and the latter cleaned with RNeasyMini kit columns (Qiagen). RNAs were stored at -80°C until use. RNA was reverse transcribed with High capacity cDNA reverse transcription kit (Applied Biosystems) using ≤ 2μg of RNA per sample and controlling for the same RNA quantity for all samples in each independent experimental batch. Based on the total RNA input for cDNA synthesis, resulting parasite and macrophage cDNA was diluted with nuclease free water free in 1:5 and 1:2 proportions, respectively, and used for analysis of gene expression by quantitative reverse transcription real-time PCR (qRT-PCR). TaqMan® probes used for evaluation of human macrophage gene expression were: abcb1 (p-gp, (Probe No.Hs00184500_m1), abcb6 (Hs01039213_m1), abcc1 (mrp-1,Hs01561510_m1), abcc2 (Hs00166123_m1), slc7a11 (Hs00204928_m1), aqp-9 (Hs01035887_m1), mt2a (Hs01591333_g1), and gapdh (Hs99999905_m1). Primers for amplification of Leishmania abca2, abca3, abcc2, abcc3, abcg4, abcg6, aqp1, sams, sahh and β-tubulin were designed using Primer Blast NCBI software (Supplementary Table 1), and used for evaluation of gene expression in promastigotes and intracellular amastigotes. For the later, primers were also evaluated against cDNA from uninfected macrophages to test for cross-reactivity with host cell molecules. The efficiency of the qPCR reactions was ≥ 90% and ≤ 110% for all primer sets tested against promastigote DNA. Efficiency of ABCC3 and ABCA2 primer sets against intracellular amastigotes was 86% and 70% respectively. Amplification of parasite gene transcripts was performed using SybrGreen PCR Master Mix (Applied Biosystems). Reverse transcription and qPCR no-template controls were included in each run. Modulation of macrophage gene expression was estimated by relative quantification calculated by ΔΔCt method normalized to GAPDH and controlled against gene expression of uninfected and unexposed macrophages. Basal gene expression in promastigotes and intracellular amastigotes was estimated by quantification relative to a standard curve constructed from five 1:4 serial dilutions of cDNA from 1×106 parasites, all for which the dynamic range of dilution values was determined to be 1 to 2-8. Parasite gene expression was normalized to β-tubulin. Calculations were made in Excel, Windows Office 2010.
Statistical analysis and data management
Gene expression analyses were conducted blindly with respect to the susceptibility phenotype of the Leishmania strains. Codes were revealed once the full data set was obtained. D'Agostino and Pearson omnibus test was applied to determine parametric or non-parametric distribution of quantitative data. For parametric data, Student's T-Test and One-way ANOVA with Tukey's post-test were employed for analysis of variance between two, or more than two groups, respectively. Mann Whitney U-Test and Kruskall-Wallis with Dunn's post-test were employed for analysis of non-parametric data. Correlation analysis of transporter gene expression was assessed by Pearson's test. Statistical significance was defined as p<0.05. Data were analyzed using Prism 5 software (GraphPad Software, Inc., La Jolla, CA).
Results
Expression of abca2 is significantly higher in Sb-resistant L. (V.) panamensis clinical strains and is positively correlated with expression of other ABC transporters
Gene expression of nine molecules with reported evidence of association with xenobiotic detoxification, Sb drug transport or metabolism (Supplemental Table 2) was evaluated in promastigotes of laboratory derived Sb-susceptible (L.p.001) and Sb-resistant (Lp.1000.1) control lines, and promastigotes of 19 clinical strains isolated from individuals with dermal leishmaniasis caused by L. (V.) panamensis defined as Sb-resistant (n=10) or Sb-susceptible (n=9) (Table 1). Among the evaluated genes, higher expression of abcc3 and lower expression of Aquaporin-1 (aqp-1) were observed in Sb-resistant Lp.1000.1 compared to the drug sensitive line L.p.001 (Figure 1A), and this was concordant with what was previously shown for other experimentally selected strains of different Leishmania species [7, 29]. In contrast, expression of abcc3 and aqp-1 was not significantly different among Sb-resistant and susceptible clinical strains of L (V.) panamensis (Figure 1B). The expression of abca2, abca3 and abcc3 was higher than that of abcc2, abcg4, abcg6, aqp-1,sahh, and sams in both Sb-resistant and susceptible clinical strains (Figure 1B). As promastigotes, Sb-resistant strains expressed significantly more abca2 and S-adenosylhomocysteine hydrolase (sahh) compared to Sb-susceptible strains (Figure 1B). Expression of abca2 was evaluated in intracellular amastigotes, and no differences were observed among Sb-resistant (n=3) and susceptible (n=3) clinical strains (Figure 1C). However, exposure of infected THP-1 cells to MA significantly induced expression of abca2 in Sb-resistant but not in Sb-susceptible L.V. panamensis intracellular amastigotes (Figure 1C). Expression of abca2 was significantly and positively correlated with expression of abca3, abcc3, abcg4 and abcg6 in logarithmic phase promastigotes (Figure 2).
Figure 1. Gene expression profiles of L. (V.) panamensis clinical isolates.
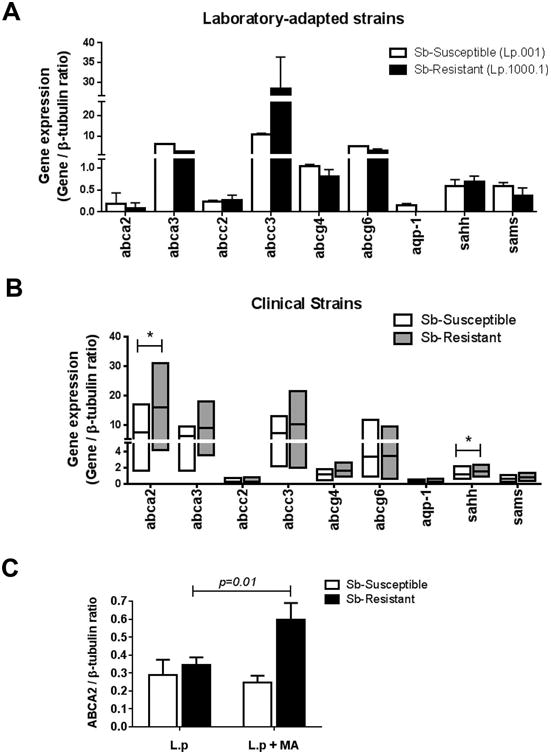
Gene expression of antimony-response related genes in promastigotes of laboratory derived L.(V.) panamensis Sb-resistant (L.p.1000.1) and Sb-susceptible (L.p.001) strains (A) and Sb-resistant (n=10) and Sb-susceptible (n=9) clinical L. (V.) panamensis strains (B). Gene expression of abca2 in L.(V.) panamensis clinical strains as intracellular amastigotes (C). Data are presented as the mean value ± SD of two (A) or three (C) independent biological replicas, and median and range of individual expression data for clinical strains (B). (*) p <0.05.
Figure 2. Expression of abca2 is positively correlated to expression of abcg4, abcg6, abca3 and abcc3 in promastigotes of L. (V.) panamensis clinical strains.
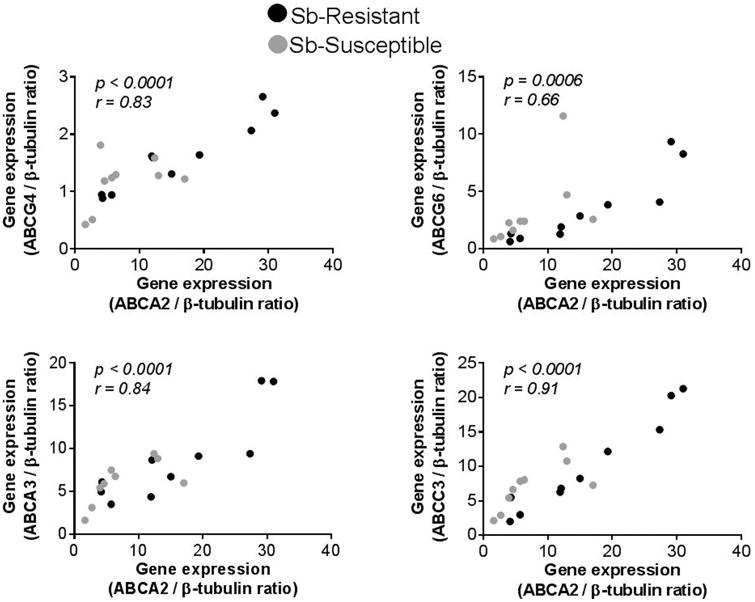
Correlation analysis of abca2 gene expression and abcg4, abcg6, abca3 and abcc3 in logarithmic phase promastigotes of L. (V.) panamensis clinical strains [n=19; Sb-resistant (n=9, Black circles) and Sb-susceptible (n=10, Grey circles)]. Statistical correlation was assessed by the Pearson's correlation test and significance established when p<0.05.
Expression of abcb6, aqp-9 and mt2a is differential in macrophages infected with Sb-resistant and susceptible L. (V.) panamensis
Differential expression of macrophage ABC transporters and enzymes involved in glutathione metabolism has been reported during infections with Sb-resistant and susceptible L. donovani [21, 24]. Whether these mechanisms operate in other Leishmania species is unknown. The expression of drug-response molecules which were previously shown to be altered during ex vivo L. (V.) panamensis infections [19] was evaluated in human macrophages infected with Sb-resistant and susceptible L. (V.) panamensis. In contrast to findings in studies of L. donovani, expression of abcb1 (p-gp) and abcc1 (mrp-1) in THP-1 cells was not significantly different during infections with the Sb-resistant or susceptible laboratory derived lines of L. (V.) panamensis in the presence or absence of MA (Figure 3A). Likewise, no differences in the expression of abcc2 and slc7a11 were observed in cells infected with these strains. Infection with Sb-susceptible L.p.001 induced expression of abcb6 in THP-1 cells and was significantly higher than in cells infected with the Sb-resistant line L.p1000.1. However, this difference was not observed when infected cells were exposed to MA (Figure 3A). Significantly higher expression of metallothionein-2a (mt2a) and aqp-9 occurred in macrophages infected with the L.p.001 line and exposed to MA, compared to cells infected with Sb-resistant L.p.1000.1 (Figure 3A). These results show that L. (V.) panamensis strains having different drug susceptibilities can differentially modulate the expression of drug-response genes in THP-1 cells.
Figure 3. Sb-susceptible and Sb-resistant L. (V.) panamensis differentially modulate host cell gene expression in THP-1 cells.
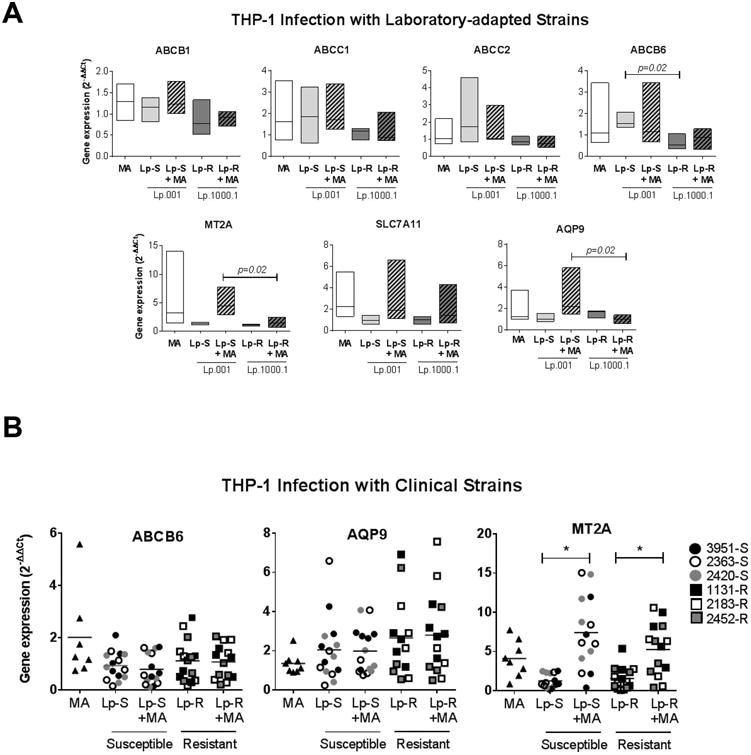
THP-1 macrophages were infected for 24 h with Sb-susceptible (Lp-S; Lp.001) or Sb-resistant (Lp-R; Lp. 1000.1) laboratory derived L. (V.) panamensis lines (A) or clinical strains (n=3/group; strain codes denoted in figure key) (B). Cells were also exposed to 32μg/ml MA for 24h (MA), or infected for 24h followed by 24h of drug exposure (Lp-S + MA or Lp-R + MA). Gene expression data are presented as fold change values relative to uninfected and untreated cells. Floating bars represent the range and median values (A) and scatterplots show individual data and median values (B) of at least four independent experiments. Statistical significance established by One-way ANOVA with Tukey's post-test; (*) p<0.05.
Based on the results obtained with laboratory derived lines, we evaluated the effect of infection with clinical strains of different susceptibilities to MA (n=3/ group) on the expression of macrophage genes abcb6, aqp-9 and mt2a. In contrasts to the results with laboratory derived lines, no differences in expression were elicited by infection of THP-1 cells with susceptible or resistant clinical strains of L. (V.) panamensis (Figure 3B), indicating that laboratory derived lines and clinical strains differentially modulate host cell responses.
To explore the contribution of the host cell system to the results, we developed an experimental model aiming to recreate more accurately the natural infection. Primary macrophages derived from PBMCs from patients with active CL were infected ex vivo with the Leishmania strain isolated from the original lesion at diagnosis. All strains were identified as L. (V.) panamensis and susceptibility profiles determined as described in materials and methods. As previously shown [19], exposure of primary macrophages to MA significantly induced the expression of abcb6, mt2a and slc7a11 (Figure 4). In contrast to infection with Sb-resistant strains, Sb-susceptible L. (V.) panamensis followed by drug exposure resulted in significant induction of mt2a gene expression and repression of abcb1 (Figure 4). No differences were observed in expression of abcb6, abcc1, aqp-9 and slc7a11.
Figure 4. MT2A is induced upon infection with Sb-susceptible L. (V.) panamensis in primary human macrophages.
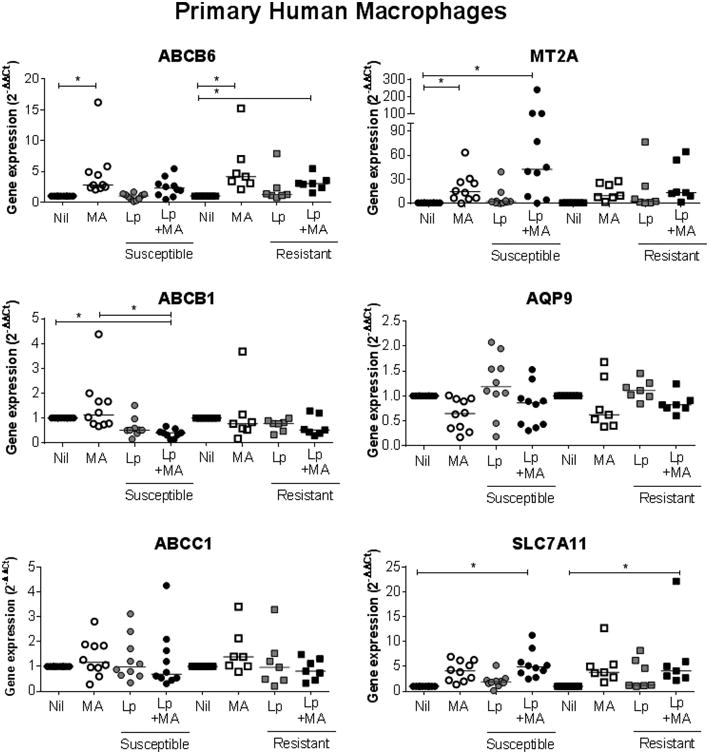
The expression of drug-response genes was evaluated in primary macrophages derived from PBMCs from CL patients (n=17). Cells were infected for 24h with the strain isolated from each participant at diagnosis (Sb-susceptible, n = 10, [circles] and Sb-resistant, n = 7, [squares]), exposed to 32 μg/mL MA for 24h (white symbols), or infected for 24h followed by 24h drug exposure (black symbols). Gene expression data are presented as fold change values relative to uninfected and untreated cells. Horizontal lines denote median values of individual expression data from each strain-macrophage pair. Assessment of statistical significance was done by Kruskall-Wallis with Dunn's post test. (*)p<0.05.
Discussion
In the context of infections with intracellular pathogens, microbial drug susceptibility results from complex interactions: 1) the ability of the pathogen to circumvent the antimicrobial effect of drugs by events such as molecular changes in the drug target, drug efflux or sequestration and drug metabolism/inactivation, 2) the capacity of the pathogen to modulate its host cell resulting in changes in drug exposure and in immune determinants that may favor microbial survival (or elimination), and 3) the inter-individual and within host-cell variability in drug transport and metabolism that may account for alterations in intracellular drug concentrations and distribution. However, drug susceptibility in Leishmania has been almost exclusively investigated in relation with mechanisms of parasite drug resistance based drug transport (influx/efflux), sequestration and metabolism/inactivation [30].
In this study we conducted a systematic assessment of the gene expression of molecules previously shown to be involved in mechanisms of drug resistance in Leishmania promastigotes, in a comparative analysis of the expression profiles in laboratory derived and clinical strains of L. (V.) panamensis categorized by in vitro evaluation as intracellular amastigotes, as resistant or susceptible to meglumine antimoniate. Research on laboratory derived Leishmania strains has revealed mechanisms of resistance to antimonial drugs that include gene amplification and modulation of the drug detoxifying machinery constituted by ABC transporters, aquaporins and enzymes involved in trypanothione metabolism [8-10, 31, 32]. In agreement with previous reports, increased expression of abcc3 and repression of aqp-1 were also found in our laboratory derived Sb-resistant L. (V.) panamensis line compared to the susceptible strain. However, these differences in gene expression were absent from drug resistant and susceptible clinical strains, providing further evidence that the mechanisms of antimonial drug resistance selected by in vitro drug pressure on the promastigote stage do not occur among Sb-resistant clinical strains of L. (Viannia) and L. (Leishmania) species [15-17]. This could be potentially attributable to a selection bias introduced by the drug concentrations at which parasites are exposed in vivo as intracellular amastigotes (antimony Cmax in human plasma ∼ 20 – 40 μg/mL [33, 34]) vs. in vitro as promastigotes (using selection concentrations > 10 times higher than plasma Cmax [17, 35]), or by the population heterogeneity or polyclonality of clinical strains favouring heterogeneous mechanisms of drug resistance [16]. Indeed, it has been shown that selection of in vitro drug resistance in Leishmania as intracellular amastigotes to two different antileishmanials, pentavalent antimonials and paromomycin, does not necessarily confer resistance to the drug as promastigotes [17, 36]. Together, cumulative evidence supports the interpretation that methods of selection and the life stage in which selective pressure is exerted, result in different mechanisms of drug resistance.
Among clinical strains, expression of abca2 was significantly higher in Sb-resistant compared to Sb-susceptible clinical strains of L. (V.) panamensis, both as promastigotes and intracellular amastigotes. In L. tropica, overexpression of ABCA2 has been shown to reduce intracellular accumulation of glycerophospholipids, increase the exocytic activity and decrease infectivity of macrophages; however, it did not confer drug resistance to amphothericin B or alkyl-lysophospholipids [37]. Whether increased gene expression of abca2 directly contributes to antimonial drug resistance remains to be determined.
The genomic structure of Leishmania is characterized by polycistronic gene arrangements. Consequently, transcriptional regulation of gene expression often affects the transcription of multiple genes within specific gene families. This study revealed a positive correlation between expression of abca2 and abca3, abcc3, abcg4 and abcg6. Among these, only abca2 and abca3 are found within the same chromosome but not as tandemly arranged genes. Therefore, the positive correlation of gene expression could suggest co-regulation independently of polycistronic gene expression [38] and a multi-gene contribution to antimonial drug susceptibility phenotype.
The intraphagosomal nature of Leishmania within its mammalian host implies that the activity of antileishmanials is dependent on drug internalization into host cells and the phagolysosomal compartment. Reduction of the pro-drug SbV to the bioactive SbIII has been shown to occur both in Leishmania amastigotes and host cells, but not promastigotes [10, 39, 40]. Thus, alterations in host cell drug uptake and metabolism could directly impact exposure of the intracellular parasite to the drug, parasite survival and the efficacy of treatments. Leishmania modulate immune cell functions, which in turn promote parasite internalization and survival and this is largely accomplished by rapid modulation of host cell signaling [41]. It is therefore plausible that Leishmania modulate host cell functions involved in drug transport and metabolism thereby providing a mechanism of protection against antileishmanial drugs. Indeed, studies have shown that drug resistant L. donovani induce expression of ABC transporters MRP-1and P-gp in macrophages [23] and repress γ-GCS [24], promoting efflux of drug from host cells and limited intracellular reduction to SbIII. Although no differences in mrp-1 and p-gp expression were observed THP-1 cells infected with in L. (V.) panamensis, p-gp expression was downregulated during infection of primary human macrophages with Sb-susceptible but not Sb-resistant L. (V.) panamensis (Figure 4), consistent with observations in L. donovani infections, potentially resulting in decreased drug efflux from infected cells.
Interestingly, increased expression of metallothionein 2A was consistently observed in THP-1 cells and primary macrophages infected with Sb-susceptible L. (V.) panamensis compared to infections with drug resistant strains. In mammalian cells, mt2a expression is induced by MA [19] and the closely related metalloid AsIII [42]. Metallothionein (MT) null mice are more sensitive to arsenic induced nephrotoxicity and hepatotoxicity [43, 44], suggesting that MTs play a role in detoxifying/scavenging arsenic and potentially antimonials. MTs are localized in the cytoplasm and in lysosomes of mammalian cells [45], and thus can transport bound molecules to intracellular compartments such as phagolysosomes. Furthemore, MTs are rich in cysteine residues and this amino acid facilitates SbV to SbIII reduction [39]. We have previously shown that mt2a expression in primary human macrophages is inversely correlated with intracellular survival of L. (V.) panamensis after SbV exposure [19]. Hence, down-regulation of mt2a expression by infection with Sb-resistant L. (V.) panamensis could favor intracellular parasite survival limiting intracellular drug accumulation, efficient transport to the phagolysosome or reduction to SbIII. These findings prompt functional validation of the identified putative mechanisms of resistance.
Conclusions
Results from this study further support the growing body of evidence that different mechanisms of drug resistance operate in laboratory selected and naturally or therapeutically selected clinical strains of Leishmania. Furthermore, we provide evidence that parasite-mediated modulation of host cell gene expression of molecules involved in drug transport and metabolism could constitute mechanisms of resistance. Participation of host cells in the antileishmanial effect of drugs opens new opportunities for development of host-directed co-therapies.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of the CIDEIM BioBank personnel for management and typing of Leishmania strains and the CIDEIM Biostatistics and Data Management Unit for fruitful discussions on data analysis methods. We also thank CIDEIM Clinical Units in Tumaco and Cali for enrollment and recruitment of study participants, and we especially wish to acknowledge the collaboration of the patients who participated in this study. This work was conducted in partial fulfillment of the requirements for the MSc degree in Biomedical Sciences of Universidad del Valle to MCB.
Financial Support: This work was supported by the US National Institutes of Health (NIH) Grant R01AI104823 and (https://www.niaid.nih.gov/), US NIH International Fogarty Center Global Infectious Disease Research Training Program, Award Number D43 TW006589 (https://www.fic.nih.gov/), and COLCIENCIAS grant 222965843177, contract No.007-2015. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. AW was supported by a Wilbur G. Downs fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Navin TR, et al. Placebo-controlled clinical trial of sodium stibogluconate (Pentostam) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J Infect Dis. 1992;165(3):528–34. doi: 10.1093/infdis/165.3.528. [DOI] [PubMed] [Google Scholar]
- 2.Romero GA, et al. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. Am J Trop Med Hyg. 2001;65(5):456–65. doi: 10.4269/ajtmh.2001.65.456. [DOI] [PubMed] [Google Scholar]
- 3.Soto J, et al. Short report: efficacy of pentavalent antimony for treatment of colombian cutaneous leishmaniasis. Am J Trop Med Hyg. 2005;72(4):421–2. [PubMed] [Google Scholar]
- 4.Llanos-Cuentas A, et al. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin Infect Dis. 2008;46(2):223–31. doi: 10.1086/524042. [DOI] [PubMed] [Google Scholar]
- 5.Castro MDM, et al. Risk factors for therapeutic failure to meglumine antimoniate and miltefosine in adults and children with cutaneous leishmaniasis in Colombia: A cohort study. PLoS Negl Trop Dis. 2017;11(4):e0005515. doi: 10.1371/journal.pntd.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojas R, et al. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J Infect Dis. 2006;193(10):1375–83. doi: 10.1086/503371. [DOI] [PubMed] [Google Scholar]
- 7.Gourbal B, et al. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J Biol Chem. 2004;279(30):31010–7. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, et al. Leishmania major LmACR2 is a pentavalent antimony reductase that confers sensitivity to the drug pentostam. J Biol Chem. 2004;279(36):37445–51. doi: 10.1074/jbc.M404383200. [DOI] [PubMed] [Google Scholar]
- 9.Denton H, McGregor JC, Coombs GH. Reduction of anti-leishmanial pentavalent antimonial drugs by a parasite-specific thiol-dependent reductase, TDR1. Biochem J. 2004;381(Pt 2):405–12. doi: 10.1042/BJ20040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaked-Mishan P, et al. Novel Intracellular SbV reducing activity correlates with antimony susceptibility in Leishmania donovani. J Biol Chem. 2001;276(6):3971–6. doi: 10.1074/jbc.M005423200. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee A, et al. Role of ABC transporter MRPA, gamma-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J Antimicrob Chemother. 2007;59(2):204–11. doi: 10.1093/jac/dkl494. [DOI] [PubMed] [Google Scholar]
- 12.Mukhopadhyay R, et al. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc Natl Acad Sci U S A. 1996;93(19):10383–7. doi: 10.1073/pnas.93.19.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haimeur A, Ouellette M. Gene amplification in Leishmania tarentolae selected for resistance to sodium stibogluconate. Antimicrob Agents Chemother. 1998;42(7):1689–94. doi: 10.1128/aac.42.7.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ubeda JM, et al. Modulation of gene expression in drug resistant Leishmania is associated with gene amplification, gene deletion and chromosome aneuploidy. Genome Biol. 2008;9(7):R115. doi: 10.1186/gb-2008-9-7-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres DC, et al. Targeted gene expression profiling in Leishmania braziliensis and Leishmania guyanensis parasites isolated from Brazilian patients with different antimonial treatment outcomes. Infect Genet Evol. 2010;10(6):727–33. doi: 10.1016/j.meegid.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Decuypere S, et al. Molecular mechanisms of drug resistance in natural Leishmania populations vary with genetic background. PLoS Negl Trop Dis. 2012;6(2):e1514. doi: 10.1371/journal.pntd.0001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyeneche-Patino DA, et al. Antimony resistance and trypanothione in experimentally selected and clinical strains of Leishmania panamensis. Antimicrob Agents Chemother. 2008;52(12):4503–6. doi: 10.1128/AAC.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifert K, Escobar P, Croft SL. In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J Antimicrob Chemother. 65(3):508–11. doi: 10.1093/jac/dkp500. [DOI] [PubMed] [Google Scholar]
- 19.Gomez MA, et al. Leishmania panamensis infection and antimonial drugs modulate expression of macrophage drug transporters and metabolizing enzymes: impact on intracellular parasite survival. J Antimicrob Chemother. 2014;69(1):139–49. doi: 10.1093/jac/dkt334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohmen LC, et al. Functional Validation of ABCA3 as a Miltefosine Transporter in Human Macrophages: IMPACT ON INTRACELLULAR SURVIVAL OF LEISHMANIA (VIANNIA) PANAMENSIS. J Biol Chem. 2016;291(18):9638–47. doi: 10.1074/jbc.M115.688168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mookerjee Basu J, et al. Inhibition of ABC transporters abolishes antimony resistance in Leishmania Infection. Antimicrob Agents Chemother. 2008;52(3):1080–93. doi: 10.1128/AAC.01196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee B, et al. Antimony-Resistant Leishmania donovani Exploits miR-466i To Deactivate Host MyD88 for Regulating IL-10/IL-12 Levels during Early Hours of Infection. J Immunol. 2015;195(6):2731–42. doi: 10.4049/jimmunol.1402585. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee B, et al. Antimony-resistant but not antimony-sensitive Leishmania donovani up-regulates host IL-10 to overexpress multidrug-resistant protein 1. Proc Natl Acad Sci U S A. 2013;110(7):E575–82. doi: 10.1073/pnas.1213839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter KC, et al. Resistance of Leishmania donovani to sodium stibogluconate is related to the expression of host and parasite gamma-glutamylcysteine synthetase. Antimicrob Agents Chemother. 2006;50(1):88–95. doi: 10.1128/AAC.50.1.88-95.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker J, et al. Discovery of factors linked to antimony resistance in Leishmania panamensis through differential proteome analysis. Mol Biochem Parasitol. 2012;183(2):166–76. doi: 10.1016/j.molbiopara.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Saravia NG, et al. Heterogeneity, geographic distribution, and pathogenicity of serodemes of Leishmania viannia in Colombia. Am J Trop Med Hyg. 2002;66(6):738–44. doi: 10.4269/ajtmh.2002.66.738. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez O, et al. Novel approach to in vitro drug susceptibility assessment of clinical strains of Leishmania spp. J Clin Microbiol. 2012;50(7):2207–11. doi: 10.1128/JCM.00216-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robledo S, et al. Human monocyte infection by Leishmania (Viannia) panamensis. Role of complement receptors and correlation of susceptibility in vitro with clinical phenotype. J Immunol. 1994;152(3):1265–76. [PubMed] [Google Scholar]
- 29.Haimeur A, et al. Amplification of the ABC transporter gene PGPA and increased trypanothione levels in potassium antimonyl tartrate (SbIII) resistant Leishmania tarentolae. Mol Biochem Parasitol. 2000;108(1):131–5. doi: 10.1016/s0166-6851(00)00187-0. [DOI] [PubMed] [Google Scholar]
- 30.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19(1):111–26. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leprohon P, Legare D, Ouellette M. Intracellular localization of the ABCC proteins of Leishmania and their role in resistance to antimonials. Antimicrob Agents Chemother. 2009;53(6):2646–9. doi: 10.1128/AAC.01474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legare D, et al. The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase. J Biol Chem. 2001;276(28):26301–7. doi: 10.1074/jbc.M102351200. [DOI] [PubMed] [Google Scholar]
- 33.Cruz A, et al. Pharmacokinetics of antimony in children treated for leishmaniasis with meglumine antimoniate. J Infect Dis. 2007;195(4):602–8. doi: 10.1086/510860. [DOI] [PubMed] [Google Scholar]
- 34.Chulay JD, Fleckenstein L, Smith DH. Pharmacokinetics of antimony during treatment of visceral leishmaniasis with sodium stibogluconate or meglumine antimoniate. Trans R Soc Trop Med Hyg. 1988;82(1):69–72. [PubMed] [Google Scholar]
- 35.Brochu C, et al. Antimony uptake systems in the protozoan parasite Leishmania and accumulation differences in antimony-resistant parasites. Antimicrob Agents Chemother. 2003;47(10):3073–9. doi: 10.1128/AAC.47.10.3073-3079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendrickx S, et al. Experimental induction of paromomycin resistance in antimony-resistant strains of L. donovani: outcome dependent on in vitro selection protocol. PLoS Negl Trop Dis. 2012;6(5):e1664. doi: 10.1371/journal.pntd.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araujo-Santos JM, et al. The overexpression of an intracellular ABCA-like transporter alters phospholipid trafficking in Leishmania. Biochem Biophys Res Commun. 2005;330(1):349–55. doi: 10.1016/j.bbrc.2005.02.176. [DOI] [PubMed] [Google Scholar]
- 38.Ouellette M, Papadopoulou B. Coordinated gene expression by post-transcriptional regulons in African trypanosomes. J Biol. 2009;8(11):100. doi: 10.1186/jbiol203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira Cdos S, et al. Thiol-induced reduction of antimony(V) into antimony(III): a comparative study with trypanothione, cysteinyl-glycine, cysteine and glutathione. Biometals. 2003;16(3):441–6. doi: 10.1023/a:1022823605068. [DOI] [PubMed] [Google Scholar]
- 40.Hansen C, et al. Reduction of Sb(V) in a human macrophage cell line measured by HPLC-ICP-MS. Biol Trace Elem Res. 2011;144(1-3):234–43. doi: 10.1007/s12011-011-9079-9. [DOI] [PubMed] [Google Scholar]
- 41.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18(2):293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albores A, et al. Arsenic induces and enhances rat hepatic metallothionein production in vivo. Chem Biol Interact. 1992;85(2-3):127–40. doi: 10.1016/0009-2797(92)90057-r. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, et al. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci. 2000;55(2):460–7. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, et al. Chronic combined exposure to cadmium and arsenic exacerbates nephrotoxicity, particularly in metallothionein-I/II null mice. Toxicology. 2000;147(3):157–66. doi: 10.1016/s0300-483x(00)00194-3. [DOI] [PubMed] [Google Scholar]
- 45.Sabolic I, et al. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals. 2010;23(5):897–926. doi: 10.1007/s10534-010-9351-z. [DOI] [PubMed] [Google Scholar]
- 46.Leprohon P, et al. Modulation of Leishmania ABC protein gene expression through life stages and among drug-resistant parasites. Eukaryot Cell. 2006;5(10):1713–25. doi: 10.1128/EC.00152-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauvage V, et al. The role of ATP-binding cassette (ABC) proteins in protozoan parasites. Mol Biochem Parasitol. 2009;167(2):81–94. doi: 10.1016/j.molbiopara.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Castanys-Munoz E, et al. A novel ATP-binding cassette transporter from Leishmania is involved in transport of phosphatidylcholine analogues and resistance to alkyl-phospholipids. Mol Microbiol. 2007;64(5):1141–53. doi: 10.1111/j.1365-2958.2007.05653.x. [DOI] [PubMed] [Google Scholar]
- 49.Campos-Salinas J, et al. A new ATP-binding cassette protein is involved in intracellular haem trafficking in Leishmania. Mol Microbiol. 2011;79(6):1430–44. doi: 10.1111/j.1365-2958.2010.07531.x. [DOI] [PubMed] [Google Scholar]
- 50.Decuypere S, et al. Gene expression analysis of the mechanism of natural Sb(V) resistance in Leishmania donovani isolates from Nepal. Antimicrob Agents Chemother. 2005;49(11):4616–21. doi: 10.1128/AAC.49.11.4616-4621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandal S, et al. Assessing aquaglyceroporin gene status and expression profile in antimony-susceptible and -resistant clinical isolates of Leishmania donovani from India. J Antimicrob Chemother. 2010;65(3):496–507. doi: 10.1093/jac/dkp468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


