Abstract
Objective
The U.S. PrEP Demonstration Project (Demo) evaluated men-who-have-sex-with-men on pre-exposure prophylaxis (PrEP) post-marketing and found low seroconversion rates. The objective of this study is to examine hair levels as an adherence measure to PrEP.
Design
Using an “opt-in” design, participants of PrEP Demo were invited to enroll into a substudy where hair was collected quarterly.
Methods
Tenofovir concentrations were measured in hair by liquid-chromatography/tandem-mass-spectrometry. Hair levels consistent with ≥4 doses per week (protective in other studies) defined adequate adherence. Mixed effects multivariate logistic regression models examined factors associated with ≥4 doses/week. Separate mixed effects models evaluated the relationship between hair PrEP levels and changes in creatinine clearance (CrCl) over time.
Results
Overall, 58% of Demo participants enrolled into this opt-in study; reasons for non-participation included insufficient hair (61%) and concerns about hairstyle (27%). Hair and DBS levels consistent with ≥4 doses/week were highly concordant (84%). Hair levels showed adequate adherence in 87% of 875 person-visits (among 280 participants). Factors associated with adequate adherence in multivariate models were amphetamine use (aOR 2.59 (0.97–6.9, p 0.06), condomless receptive anal sex (aOR 2.28 (1.19–4.40, p 0.01), and stable housing (aOR 2.63 (1.03–6.67), p 0.04). Hair levels of tenofovir showed a monotonic relationship with decline in CrCl (p 0.01 for trend).
Conclusions
In this substudy of the PrEP Demonstration Project, hair and DBS levels were highly concordant and hair concentrations demonstrated adequate adherence 87% of the time, with stable housing and high-risk behavior associated with higher adherence. Daily PrEP drug-taking is associated with modest declines in CrCl.
Keywords: HIV prevention, pre-exposure prophylaxis, PrEP, pharmacologic measures, tenofovir/emtricitabine, hair concentrations, PrEP U.S. Demo Project, Enhancing PrEP in Community (EPIC), creatinine clearance
INTRODUCTION
After multiple clinical trials demonstrated its efficacy,[1–4] pre-exposure prophylaxis (PrEP) with oral tenofovir (TFV) disoproxil fumarate/emtricitabine (TDF/FTC) is now broadly recommended by the Centers for Disease Control and Prevention[5] and the World Health Organization[6] to prevent HIV acquisition. The measurement of PrEP drug levels in plasma (as an objective metric of adherence) proved critical to interpreting the effectiveness of PrEP in the clinical trials.[1, 7–9] However, plasma measures of TFV and FTC represent short-term exposure and are susceptible to “white-coat” (short term improvement in) adherence patterns.[10] Concentrations of TFV and/or FTC (or their metabolites) in dried blood spots (DBS)[11] and hair samples[12] measure longer-term adherence and exposure, are highly correlated,[13] are associated with both efficacy[14] and toxicities of PrEP,[15, 16] and have therefore been increasingly incorporated as adherence metrics into PrEP programs during scale-up. Since hair can be collected (even at home[17]) and stored at room temperature without biohazardous precautions, providing feasibility in the field, we sought to compare its utility as an alternative adherence measure to DBS in a large PrEP study.
The U.S. PrEP Demonstration Project (“PrEP Demo”) enrolled a large cohort of men-who-have-sex-with-men (MSM) and transgender women in three U.S. cities (Miami, San Francisco, Washington DC) for PrEP provision, collection of adherence measurements (including self-report and TFV-diphosphate (DP) concentrations in DBS) and testing for HIV and sexually transmitted infections (STIs).[18] The incidence of HIV infection in this study was very low (0.43 per 100 person-years) and self-reported adherence high (87.4% across all visits). The DBS measures in PrEP Demo showed that participants took ≥ 4 doses a week (consistent with protective levels[14]) over ~84% of visits. Factors associated with protective DBS levels in PrEP Demo were greater sexual risk (two condomless anal sex partners in the past 3 months) and stable housing, whereas being African American or enrollment at the Miami site were associated with lower DBS levels.[18]
Since modest but statistically significant declines in renal function with administration of daily TDF/FTC have been observed in the PrEP clinical trials and demonstration projects,[19–21] the association between drug exposure and decline in renal function is also of interest. In iPrEx OLE, we showed a monotonic relationship between number of doses of TDF/FTC taken per week as estimated by hair levels and percent decrease in creatinine clearance (CrCl).[15] In the full PrEP Demo cohort,[18] the mean creatinine increased from baseline to week 12 by 0.03 mg/dL (4.6%) (p<0.0001) and the mean CrCl decreased by 4.76 mL/min (3.0%) (p<0.0001). These changes remained stable through week 48 and greater declines in renal function were associated with higher DBS concentrations of PrEP drugs.[22, 23]
A subset of participants in PrEP Demo opted into the “Enhancing PrEP in Community” (EPIC) Hair Study, which collected quarterly hair samples for adherence monitoring. This analysis presents the results on the hair metrics in the EPIC Hair Study, examining in this large study concordance between DBS and hair levels, factors associated with higher concentrations of PrEP drugs in hair, and the relationship between drug exposure assessed by hair levels and renal function over time.
METHODS
Study population and procedures
PrEP Demo enrolled 557 HIV-negative MSM and transgender women from STI clinics in Miami and San Francisco and a community health center in Washington DC from October 2012 to January 2014.[18] Participants were provided TDF/FTC (300mg/200mg)-based PrEP for free over 48 weeks and seen at weeks 4, 12, 24, 36 and 48. Demographics collected at baseline included age, race, sexual orientation and gender identification, educational level, knowledge of and attitudes towards PrEP, and income. Instruments assessing self-reported adherence, depression, drug and alcohol use, current housing status and recent sexual behaviors were collected at each visit. Serum creatinine was measured quarterly and creatinine clearance (CrCl)[24] was estimated by the Cockcroft-Gault equation.[25] All Demo Project participants were approached at their 12 week visit to participate in the EPIC Hair Study. For those who opted into and consented for this separate study, hair samples were collected every 12 weeks. For those who did not opt into the EPIC Hair Study, reasons for not participating were collected.
Laboratory Procedures
Using previously described methods, 100 strands of hair were cut from the occipital scalp[12] from EPIC participants with small scissors. Of note, the procedure for cutting the hair takes two minutes or less. After storage and shipment at ambient temperature from the different sites to our University of California San Francisco (UCSF)-based “Hair Analytical Laboratory” (HAL), the proximal 1.5 centimeter of each hair sample (representing ~6 weeks exposure) was cut finely with scissors and 5 milligrams (mg) processed and analyzed using liquid-chromatography /tandem-mass-spectrometry (LC-MS/MS)[12]. The assays for measuring TFV and FTC in hair samples in the HAL have been peer-reviewed and approved by the Division of AIDS’ Clinical Pharmacology and Quality Assurance (CPQA) program.[26]
Statistical analysis
Concordance of PrEP drug levels in hair and DBS
The concordance of TFV-diphosphate in DBS and TFV in hair samples consistent with ≥ 4 doses/week of PrEP was tabulated after pooling study visits. The average number of tablets taken per week was estimated for each of the adherence measures and dichotomized into < 4 doses/week and ≥ 4 doses/week. The number of doses taken per week based on participants’ hair concentrations used “adherence benchmarks” established in a study called STRAND,[12] where directly observed TDF was given to HIV-uninfected volunteers at 2, 4 and 7 doses a week (with wash-out periods in-between) and hair levels for those dosing patterns calculated.[15] For DBS, dosing categories were based on a pharmacokinetic model[11] (recently confirmed with a directly observed dosing study)[27] of TFV-DP concentrations. The concordance between hair and DBS PrEP drug concentrations around the threshold of 4 doses/week was calculated.
Definition of adequate adherence
In iPrEx OLE, there were no HIV seroconversions among participants with DBS TFV-DP concentrations consistent with taking ≥4 doses per week (≥700 femtomole (fmol)/punch).[14] In EPIC, therefore, we defined adequate adherence as TFV levels in hair consistent with ≥4 doses per week (≥0.023 nanograms (ng)/milligram (mg) as defined in STRAND[12]).
Factors associated with adequate PrEP adherence
We used mixed effects multivariate logistic regression models to examine factors associated with protective TFV hair levels across all visits. We first tested a number of covariates - both baseline (age, race/ethnicity, site of enrollment, gender, knowledge of and attitudes towards PrEP) and time-varying (housing status, sexual behaviors, depression, drug and alcohol use) - in relationship to adequate adherence in univariate models. Any covariate that achieved a p-value of <0.10 in the univariate model was included in the final multivariate model.
Association between hair levels and declines in renal function
The percent change in creatinine or CrCl from the baseline value was estimated across all visits (mean ± 95% confidence interval (CI)) for each of four estimated dose per week categories by hair levels (<2 doses per week (<0.0096 ng/mg), 2–3 doses per week (0.0096 to <0.0206 ng/mg), 4–6 doses per week (0.0206 to <0.0370ng/mg), daily dosing (≥0.0370 ng/mg)) from separate mixed effects models with no covariates (intercept only) and random person effects. To obtain a p-value for trend across the categories of estimated doses per week, we assigned them integer scores of 1 to 4 and fit a model using all persons together with that score as the only predictor.
Ethical approvals
The study protocols for both PrEP Demo and the EPIC Hair Study were approved by Institutional Review Boards at all participating sites and participants provided written informed consent. Results of hair testing were not reported back to EPIC participants. Gilead Sciences donated study drug and had no input into the study.
RESULTS
Demographics of EPIC participants
Of the 507 participants in PrEP Demo approached to participate in the EPIC Hair Study, 294 (58%) enrolled, with 280 (55%) of those staying in the study and providing hair. The main reasons for not wanting to join the EPIC Hair Study (n=213) were related to concerns regarding hair collection (27% worried about hair style disruption; 61% concerned about not having enough hair; 2% worried collection could hurt) or not having enough time (9%). Only 1% of participants were concerned about hair levels revealing poor adherence to the study investigators.
Table 1 summarizes the baseline characteristics of the EPIC Hair Study participants (n=280). Demo participants who did not enroll in the EPIC hair study were significantly more likely to be from the Miami site (p <0.001) and have lower educational levels (p 0.026), were less likely to report condomless receptive anal sex (p 0.0012) or use recreational drugs (p 0.0013), and were less likely to be adherent to PrEP by both self-report (p 0.0007) and by DBS drug level data (p < 0.001). Among the enrollees who entered EPIC, hair data was available for a total of 875 person-visits for these 280 participants (mean three samples/ participant). The median age of participants was 34 (range 19–65) years; 99% were MSM; 71% reported condomless receptive anal sex in the past 3 months; and 60% reported recreational drug use (13% amphetamine) in the last 3 months. Among 875 person-visits with hair data, hair levels were ≥0.023 ng/mg, consistent with ≥4 doses/wk14,15, 87% of the time.
Table 1.
Baseline characteristics of EPIC Hair Substudy participants (N=280)
| Participant Characteristics (N=280) | N or Median (%, range or IQR) |
|---|---|
|
|
|
| Age at study entry | 34 (range 19–65) years |
|
|
|
| Gender | |
| Male | 276 (99%) |
| Transgender female | 4 (1%) |
|
|
|
| Site | |
| San Francisco | 201 (72%) |
| Miami | 22 (8%) |
| DC | 57 (20%) |
|
|
|
| Race/ethnicity | |
| White | 219 (78%) |
| Black | 15 (5%) |
| Latino | 65 (23%) |
| Other (Asian, Native, etc.) | 36 (13%) |
|
|
|
| Living situation (assessed at each of 875 person-visits) | |
| Living in house or apartment you rent or own | 243 (87%) |
| Living in house or apartment of friend or family | 37 (13%) |
|
|
|
| Receptive condomless anal intercourse (last 3 months) | 199 (71%) |
| Median episodes | 3 (range 0–115) |
|
|
|
| Any recreational drug use (last 3 months) | 217 (60%) |
|
|
|
| Amphetamine use (last 3 months) | 35 (13%) |
|
|
|
| Baseline creatinine (mg/dL) | 0.97 (IQR 0.83–1.10) |
|
|
|
| Baseline creatinine clearance (mL/min) | 129 (IQR 109–147) |
|
|
|
| “Good adherence” ≥ 4 doses/week (out of n= 875 person-visits) | 759 (87%) |
|
|
|
| Distribution of doses/week (out of 875 person-visits)1 | |
| <2 doses/week | 39 (4.5%) |
| 2–3 doses/week | 77 (8.8%) |
| 4–6 doses/week | 381 (43.5%) |
| 7 doses/week | 378 (43.2%) |
|
|
|
CrCl = creatinine clearance, calculated using the Cockcroft-Gault equation;
Based on hair levels (as estimated by STRAND study)
Concordance of ≥4 PrEP doses/week in Hair and DBS
We had DBS and hair concentrations at the same visits in 444 person-visits. The concordance of levels of TFV in hair and TFV-DP in DBS consistent with taking ≥4 PrEP doses/week in these visits was 84.3% (76.6% both ≥4 doses/week; 7.7% both <4 doses/week; 10.8% < 4 doses/week in hair but ≥4 doses/week in DBS; 4.9% ≥4 doses/week in hair but <4 doses/week in DBS).
Factors associated with adequate adherence
Several factors were examined in univariate analyses in relationship to hair TFV levels consistent with taking ≥4 doses/week. Table 2 shows the factors associated with adequate adherence in both univariate and multivariate analyses. In univariate analyses, increasing age, use of amphetamines in the last 3 months, not being from the Miami site, having a stable living situation over the past 3 months, and reporting condomless receptive anal sex over the past 3 months were all associated with adequate adherence (all p <0.10). Being African American was not associated with adherence in univariate analyses and not included in the final models. In the multivariate model, not being from the Miami site (p 0.22) and increasing age (per decade, p 0.14) no longer stayed significantly associated with hair levels consistent with ≥4 doses/week. Using amphetamines over the past 3 months maintained a trend towards a higher odds of adequate adherence (p 0.06). Condomless receptive anal sex over the past 3 months maintained a significant association with adherence (p 0.01). And finally, more unstable housing (living in a friend or family member’s abode instead of a house or apartments one rents or owns) maintained its association with a lower odds of achieving adequate adherence in multivariate models (p 0.04).
Table 2.
Correlates of TFV Concentrations Consistent with Protection in Hair Samples
| Characteristic | OR (95% CI), p-value | aOR (95% CI), p-value |
|---|---|---|
|
|
|
|
| Age (per decade) | 1.42 (1.02–2.00), p 0.04 | 1.30 (0.92–1.83), p 0.14 |
|
|
|
|
| African American vs. other | 0.83 (0.18–3.80), p 0.81 | |
|
|
|
|
| Depressed (PHQ2 scale) | 0.84 (0.40–1.72), p 0.63 | |
|
|
|
|
| Site (versus San Francisco) | ||
| Miami | 0.31 (0.09–1.05), p 0.06 | 0.45 (0.13–1.60), p 0.22 |
| Washington DC | 1.39 (0.60–3.20), p 0.44 | 1.53 (0.64–3.6), p 0.34 |
|
|
|
|
| Receptive condomless anal intercourse (yes vs no, last 3 months) | 1.99 (1.11–3.60), p 0.021 | 2.28 (1.19–4.40), p 0.01 |
|
|
|
|
| Living situation (unstable vs not, last 3 months) | 0.36 (0.14–0.89), p 0.026 | 0.38 (0.15–0.97), p 0.04 |
|
|
|
|
| Using amphetamines (yes vs. no, last 3 months) | 3.09 (1.18–8.10), p 0.022 | 2.59 (0.97–6.9), p 0.06 |
|
|
|
|
Relationship between hair concentrations of TFV and changes in creatinine clearance
Another study within PrEP Demo examines the factors associated with changes in creatinine clearance over time[22, 23], so our analysis was restricted to examining the relationship between dosing patterns as assessed by hair levels among EPIC Hair Study participants and mean % changes in creatinine or creatinine clearance across all visits.
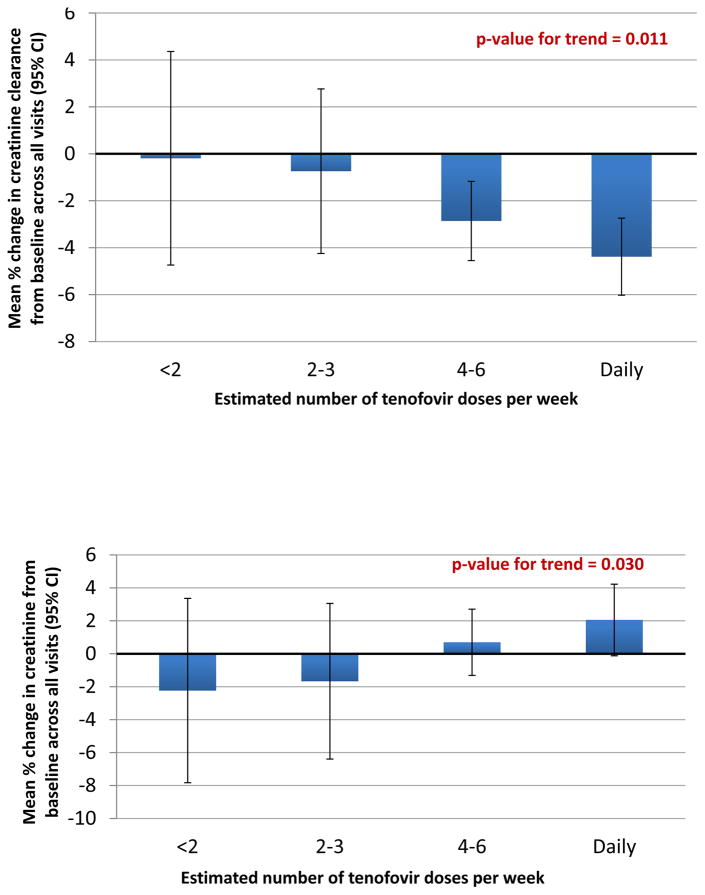
Among the 875 person-visits with hair data, 859 had concomitant creatinine measurements. Approximately 4.4% (38/859) of these person-visits had hair levels consistent with <2 pills per week; 8.6% (n=74) had levels consistent with 2–3 pills per week; 44% (n=378) had levels consistent with 4–6 pills per week; and 43% (n=369) had levels consistent with daily dosing. There was a monotonic relationship between increasing use of PrEP and both mean percent decrease in CrCl from baseline (Figure 1) and mean percent increase in creatinine from baseline (Figure 2) across all visits (p 0.011 and 0.030 for trend, respectively). For participants in the EPIC Hair Study, the percentage of visits where CrCl fell to ≤60ml/min or ≤70ml/min was low (0% and 0.3%, respectively).
Figure 1.
Mean % changes in creatinine clearance (top panel) and creatinine (bottom panel) in the EPIC study by doses per week as estimated by hair TFV concentrations
DISCUSSION
This is one of the largest studies to examine hair levels of TFV/FTC as an adherence measure among MSM on PrEP. Long-term adherence metrics in the setting of PrEP use have the potential to identify those in need of adherence intervention to increase the effectiveness of PrEP. Hair collection has some feasibility advantages in the field since no phlebotomy or cold chain is required and hair samples can be self-collected.[17] In this substudy of the Demo Project, we demonstrated that DBS and hair concentrations of PrEP drugs were highly concordant (84%) and that high-risk behavior (condomless receptive anal sex and amphetamine use), and a more stable living situation were associated with higher levels of adherence to PrEP drugs as adjudicated by hair levels. Furthermore, we found a greater decline in renal function (and greater increases in creatinine) from baseline with higher hair levels of PrEP drugs.
Our findings of relatively high adherence levels in the EPIC Hair Study by hair concentrations (87% of person-visits had hair levels consistent with ≥4 doses per week) were very similar to the adherence assessment provided by DBS measures in the overall PrEP Demo cohort (84% of person-visits with DBS levels consistent with≥4 doses per week).[18] Although objective adherence measures showed low adherence to study product in the placebo-controlled trials of TDF/FTC, when the effectiveness of PrEP to prevent HIV acquisition was unknown, adherence to PrEP in open-label studies and demonstration projects, after PrEP was proven effective and approved by a variety of regulatory bodies around the world,[5, 6] has been high,[14, 18, 28, 29] especially among MSM at high risk. PrEP Demo reported similar factors associated with higher levels of adherence as measured by DBS concentrations as we report in the EPIC Hair Study, namely higher-risk behaviors (e.g. condomless anal sex), stable housing, and older age (trend in both studies)[18].
Long-term pharmacologic measures not only assess behavior (e.g. adherence or drug-taking), but also serve as direct measures of pharmacokinetic exposure, which can be useful when monitoring for the known toxicities of TDF/FTC-based therapy, specifically renal toxicity[15] and declines in bone mineral density (BMD).[16] In iPrEx OLE, hair concentrations of TFV predicted renal decline[15]; in the randomized iPrEx trial, higher concentrations of intracellular TFV-DP in peripheral blood mononuclear cells (PBMCs) were associated with greater declines in CrCl[19] or BMD[16]; and in the PrEP Demo project both DBS concentrations of TFV-DP and hair levels of TFV and FTC were associated with declines in CrCl and increases in creatinine over time.[23] These associations between PrEP drug exposure and renal decline stress the need for vigilant attention to monitoring CrCl regularly to ensure safety; indeed, patients with high levels of adherence to daily dosing may require more frequent monitoring.[15]
Studies are often choosing either hair monitoring, DBS monitoring, or both to examine patterns of adherence to PrEP drugs as PrEP rolls out across the globe. The choice of one measure over another is often made based on feasibility in the field. Like hair, DBS are easy to collect, but require phlebotomy, a cold chain, and biohazardous precautions. DBS concentrations of TFV-DP and FTC-triphosphate (TP) can provide information on both longer-term and shorter-term patterns of adherence,[11] as can the segmental analyses of hair samples.[30] Hair collection has varying levels of acceptability to participants for sampling, however, depending on study design. In this “opt-in” study, acceptability was only 58%. Of note, many participants who declined to participate were from the Miami site where hair is often worn very short (although collection from even very short hair yields testable samples). Although 9% participants perceived hair collection would take too much time, the procedure takes less than 2 minutes, which can be counseled to field staff and participants to mitigate this widely-held misperception.
By contrast, in a study of young MSM where hair collection was “opt-out” (Adolescent Trials Network (ATN) studies 110 and 113), the hair collection rate was >95% (personal communication, manuscript under review[31]). In other studies where hair collection is incorporated early in the protocol and the field staff trained that hair can be collected from very short hair, takes very little time, and does not disrupt hair styles, acceptability has been very high.[32–34] New data that self-collected hair yields similar concentration data to hair collected in the field[17] may enhance the acceptability of this measure. Of importance in this study was that only 1% of participants were concerned about providing a sample that could reveal their adherence level and other studies have suggested that provision of drug levels could improve subsequent drug-taking.[35, 36] However, both DBS and hair require expensive liquid chromatography/ tandem mass spectrometry equipment for analyses and are performed in high-level research laboratories in the U.S. Lower-cost methods that can be applied to real-world implementation in the field and to assess drug taking using point-of-care technology will be the next advances in the field.
In sum, we demonstrate the utility of hair measures for adherence monitoring within a PrEP demonstration project among MSM and transgender women in the U.S., finding hair and DBS PrEP drug levels to be concordant, and confirming findings from the larger cohort that high-risk behavior likely encourages adherence to PrEP. As in the parent study, higher levels of PrEP use are associated with greater declines in renal function. Since current US PrEP guidelines are not recommending anything less than daily dosing to achieve maximal PrEP efficacy, older patients with higher adherence patterns may require more frequent monitoring of creatinine clearance to maximize safety in this population. Both DBS and hair measures can provide objective assessment of adherence and indicate high exposure levels associated with risk of toxicities, providing studies with choices on adherence metrics based on their setting, participant preference, and design. The provision of PrEP to at-risk individuals worldwide is integral to ending the HIV epidemic, and approaches to maximize efficacy and minimize adverse effects will aid in effective roll-out.
Acknowledgments
The Demo Project was supported by grant UM1AI069496 from the National Institute for Allergies and Infectious Diseases (NIAID). The Enhancing PrEP in Community (EPIC) study was supported by NIMH/NIH (R01 MH095628, P.I. Liu). The hair assays for this study were supported by NIAID/NIH (2R01 AI098472, P.I. Gandhi). Statistical support was partially provided by UCSF CTSI grant#UL1 TR000004. This work was also supported the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12 HD052163 [P.I. C. Brindis, N. Adler] to support C. Koss) and the National Institute of Mental Health (T32 MH19105-28 to support P. Murnane). We thank the participants of the Demo Project and the dedicated study staff. We also thank Nhi Phung, Dr. Hideaki Okochi and Josh Lacanienta from the UCSF HAL. We acknowledge and are grateful to Hao Zhang MD (program officer at NIAID for 2R01 AI098472) for his invaluable support and scientific input.
Footnotes
Meeting at which this work was presented:
Conference on Retroviruses and Opportunistic Infections, February 13–16, 2017; Seattle, WA; Abstract 978
Conflicts of Interest
Gilead Sciences donated FTC/TDF for participants in the study and paid for the DBS assays, but provided no other financial support and did not contribute to data interpretation or manuscript development.
References
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England journal of medicine. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control (CDC) [Accessed August 1, 2016];Preexposure Prophylaxis for the Prevention of HIV in the United States: A Clinical Practice Guideline. 2014 http://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf.
- 6.World Health Organization (WHO) [Accessed August 1, 2016];Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015 Sep 30; http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1. [PubMed]
- 7.Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66(3):340–348. doi: 10.1097/QAI.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. The New England journal of medicine. 2012;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. The New England journal of medicine. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials. 2008;9(4):238–246. doi: 10.1310/hct0904-238. [DOI] [PubMed] [Google Scholar]
- 11.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29(2):384–390. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu AY, Yang Q, Huang Y, Bacchetti P, Anderson PL, Jin C, et al. Strong Relationship between Oral Dose and Tenofovir Hair Levels in a Randomized Trial: Hair as a Potential Adherence Measure for Pre-Exposure Prophylaxis (PrEP) PLoS One. 2014;9(1):e83736. doi: 10.1371/journal.pone.0083736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandhi M, Glidden DV, Liu A, Anderson PL, Horng H, Defechereux P, et al. Strong Correlation Between Concentrations of Tenofovir (TFV) Emtricitabine (FTC) in Hair and TFV Diphosphate and FTC Triphosphate in Dried Blood Spots in the iPrEx Open Label Extension: Implications for Pre-exposure Prophylaxis Adherence Monitoring. J Infect Dis. 2015;212(9):1402–1406. doi: 10.1093/infdis/jiv239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi M, Glidden DV, Mayer K, Schechter M, Buchbinder S, Grinsztejn B, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. Lancet HIV. 2016;3(11):e521–e528. doi: 10.1016/S2352-3018(16)30153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulligan K, Glidden DV, Anderson PL, Liu A, McMahan V, Gonzales P, et al. Effects of Emtricitabine/Tenofovir on Bone Mineral Density in HIV-Negative Persons in a Randomized, Double-Blind, Placebo-Controlled Trial. Clin Infect Dis. 2015;61(4):572–580. doi: 10.1093/cid/civ324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saberi P, Neilands TB, Ming K, Johnson MO, Kuncze K, Koss CA, et al. Strong Correlation between Concentrations of Antiretrovirals in Home-Collected and Study-Collected Hair Samples: Implications for Adherence Monitoring. J Acquir Immune Defic Syndr. 2017 doi: 10.1097/QAI.0000000000001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med. 2016;176(1):75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon MM, Lama JR, Glidden DV, Mulligan K, McMahan V, Liu AY, et al. Changes in renal function associated with oral emtricitabine/tenofovir disoproxil fumarate use for HIV pre-exposure prophylaxis. AIDS. 2014;28(6):851–859. doi: 10.1097/QAD.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mugwanya KK, Wyatt C, Celum C, Donnell D, Mugo NR, Tappero J, et al. Changes in glomerular kidney function among HIV-1-uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis: a randomized clinical trial. JAMA Intern Med. 2015;175(2):246–254. doi: 10.1001/jamainternmed.2014.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Gvetadze RJ, et al. Renal function of participants in the Bangkok tenofovir study--Thailand, 2005–2012. Clin Infect Dis. 2014;59(5):716–724. doi: 10.1093/cid/ciu355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu A, Vittinghoff E, anderson P, Cohen SE, Doblecki-Lewis S, Bacon O, et al. Changes in Renal Function Associated with TDF/FTC PrEP Use in the US Demo Project. Conference of Retroviruses and Opportunistic Infections (CROI); 2016 February 23–26; Boston, MA. 2016. abstract 867. [Google Scholar]
- 23.Liu ETA. Changes in Renal Function Associated with Daily Tenofovir Disoproxil Fumarate/Emtricitabine for HIV Pre-Exposure Prophylaxis Use in the United States Demonstration Project. 2017 paper under review. [Google Scholar]
- 24.Centers for Disease Control (CDC) Preexposure Prophylaxis for the Prevention of HIV in the United States: A Clinical Practice Guideline. 2014 http://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf.
- 25.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 26.DiFrancesco R, Tooley K, Rosenkranz SL, Siminski S, Taylor CR, Pande P, et al. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin Pharmacol Ther. 2013;93(6):479–482. doi: 10.1038/clpt.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson P, Liu A, Castillo-Mancilla J, Seifert S, McHugh C, Wagner T, et al. TFV-DP in Dried Blood Spots (DBS) Following Directly Observed Therapy: DOT-DBS Study. Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2017. [Google Scholar]
- 28.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baeten JM. Making an Impact With Preexposure Prophylaxis for Prevention of HIV Infection. J Infect Dis. 2016;214(12):1787–1789. doi: 10.1093/infdis/jiw224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbosa J, Faria J, Carvalho F, Pedro M, Queiros O, Moreira R, et al. Hair as an alternative matrix in bioanalysis. Bioanalysis. 2013;5(8):895–914. doi: 10.4155/bio.13.50. [DOI] [PubMed] [Google Scholar]
- 31.Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, Horng H, et al. Comparison of measures of adherence to HIV pre-exposure prophylaxis (PrEP) among adolescent and young men who have sex with men in the United States. 2017 doi: 10.1093/cid/cix755. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hickey MD, Salmen CR, Tessler RA, Omollo D, Bacchetti P, Magerenge R, et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr. 2014;66(3):311–315. doi: 10.1097/QAI.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasitsuebsai W, Kerr SJ, Truong KH, Ananworanich J, Do VC, Nguyen LV, et al. Using Lopinavir Concentrations in Hair Samples to Assess Treatment Outcomes on Second-Line Regimens Among Asian Children. AIDS Res Hum Retroviruses. 2015;31(10):1009–1014. doi: 10.1089/aid.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koss C, Natureeba P, Mwesigwa J, Cohan D, Nzarubara B, Bacchetti P, et al. Hair Concentrations of Antiretrovirals Predict Viral Suppression in HIV-Infected Pregnant and Breastfeeding Ugandan Women. AIDS. 2015;29(7):825–830. doi: 10.1097/QAD.0000000000000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montgomery ET, Mensch B, Musara P, Hartmann M, Woeber K, Etima J, et al. Misreporting of Product Adherence in the MTN-003/VOICE Trial for HIV Prevention in Africa: Participants’ Explanations for Dishonesty. AIDS Behav. 2017;21(2):481–491. doi: 10.1007/s10461-016-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Straten A, Montgomery ET, Musara P, Etima J, Naidoo S, Laborde N, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS. 2015;29(16):2161–2171. doi: 10.1097/QAD.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]