Abstract
During malaria infection, a small proportion of erythrocytic asexual stages undergo sexual differentiation. Male and female gametocytes ingested in the blood meal initiate the sexual development of malaria parasites in the mosquito midgut. During blood feeding on a host, a mosquito ingests, in addition to mature gametocytes, host immune factors present in the blood, as well as large excess of erythrocytic asexual stages. In the current study we addressed the impact of the presence of large excess of asexual stages, hitherto not known or even suspected to influence, on the infectivity of gametocytes in the mosquito. Asexual stages resulted in a dose-dependent inhibition of infectiousness of gametocytes, and some of this could be explained by the presumed effect of hemozoin and other unknown asexual-stage components on the mosquito immune system, affecting survival and maturation of parasites in the mosquito midgut. Interactions between asexual and sexual stages, maturity and ratio of male and female gametocytes, host immune factors and mosquito innate immune factors are some of the variables that determine the infectiousness of gametocytes in the mosquitoes and ultimately malaria transmission success. Understanding of determinants affecting malaria transmission will be critical to approaches directly targeting the transmission process for malaria elimination.
Keywords: Malaria Transmission, Mosquitoes, Zygote, Ookinete, Oocyst
Introduction
Nearly half the world’s population lives in areas endemic for malaria caused by four species of Plasmodium. Globally, they account for nearly 214 million infections and ~half a million deaths, and > 90% of all malaria infections result from two Plasmodium species, i.e. Plasmodium falciparum and P. vivax (WHO). Efforts to control and prevent malaria are continuously challenged by ever emerging resistance to anti-malarial drugs and no effective vaccines are available for mass deployment. Historically, severe malaria including malarial deaths have been associated with P. falciparum, there is growing realization that infection caused by P. vivax can also lead to similar severe complications.
A minimum requirement for successful malaria transmission includes two mosquito bites, one injects sporozoites leading to establishment of infection in the host and the other delivers circulating male and female intra-erythrocytic gametocytes in the ingested blood meal that initiate the complex cycle of propagating the transmission cycle through the mosquito vector. Sexual differentiation is central to malaria transmission via mosquito vector. A small fraction of asexually replicating erythrocytic parasites undergo commitment to sexual differentiation. These sexually committed non-dividing stages then undergo a development process resulting in the formation of mature transmission competent sexual stages (male and female gametocytes). Upon ingestion by the mosquitoes, gametocytes undergo a series of sequential development leading to completion of sporogony (formation of sporozoites) (Beier, 1998; Sinden et al., 1996). The sporogonic developmental path includes: (1) gametogenesis and exflagellation leading to emergence of free female and male gametes from erythrocytic female and male gametocytes in the midgut lumen (within 15–20 minutes post ingestion of gametocytes in the blood meal); (2) fertilization between female and male gametes forming zygotes (within 30 minutes post blood meal); (3) meiotic nuclear division of zygote and transformation into motile mature ookinetes (20–24 hours post blood meal); (4) traversal of peritrophic membrane and invasion of midgut epithelial cell wall (within 24–30 hours post blood meal); (5) development of ookinetes into oocysts (7–10 days post blood meal); and (6) sporozoite production in the oocyst, egress in the hemolymph and invasion of salivary glands (14–20 days post blood meal). (Baton and Ranford-Cartwright, 2005). The infectiousness of a vertebrate host to mosquito vector has been examined by looking at oocysts in the mosquito midgut and sporozoites in the salivary gland. While absolutely critical for transmission, studies have suggested that gametocyte density alone is not a sensitive parameter that impacts overall infectiousness to mosquitoes (Graves et al., 1988; Jeffery and Eyles, 1955; Muirhead-Thomson, 1954; THOMSON, 1957). Studies employing feeding of mosquitoes on the blood from infected people or on cultured parasites have revealed that gametocyte density may have a greater impact on the proportion of infected mosquitoes rather than the actual oocyst burden (reviewed in (Churcher et al., 2012; Taylor and Read, 1997b). During natural infection, numerous factors, including environmental, parasitological, vector and host immunity have been shown to influence overall transmission success (reviewed in (Paul et al., 2004; Talman et al., 2004).
Even though gametocytes arise from sexual differentiation of erythrocytic asexual stages (Baton and Ranford-Cartwright, 2005; Dixon et al., 2008; Lobo and Kumar, 1998; Taylor and Read, 1997a), the asexual stages, while responsible for all the malarial pathology do not directly participate in mosquito infection, and are generally present in large excess- as much as 50-fold higher in density and ~10-fold higher in prevalence as compared to gametocytes (reviewed in (McKenzie and Bossert, 1998; Taylor and Read, 1997a). While there is no question that compared to asexual stages, P. falciparum gametocytes detected microscopically are not as abundant, more recent approaches based on RT-PCR detection of gametocyte transcripts have helped to identify the presence of sub-microscopic gametocytes (Bousema and Drakeley, 2011). Further complications in estimating gametocyte burden arise from the fact that the immature stages of P. falciparum gametocytes are believed to be sequestered in the bone marrow and any method relying on estimating gametocyte burden based on detection in the blood is most certainly going to be an underrepresentation (Aguilar et al., 2014). Whether the presence of relatively higher biomass of erythrocytic asexual stage components affects the infectivity outcome of gametocytes has never been systematically evaluated in mosquito transmission studies. Because of too many variables associated with field studies, we chose to investigate the impact of asexual erythrocytic stages on the infectiousness of gametocytes to mosquitoes using culture derived sexual stage parasites. Identification of determinants affecting transmission potential of gametocytes is expected to have significant impact on the approaches designed to monitor the success of malaria elimination efforts largely relying on the success of transmission reduction.
Materials and Methods
Parasites and mosquito infection
P. falciparum (NF54) asexual and sexual stages were maintained in culture using O+ human red blood cells (RBC) and normal human serum (NHS) (Interstate Blood Bank, Memphis, TN, USA) (Ifediba and Vanderberg, 1981; Ponnudurai et al., 1989; Trager and Jensen, 1976; Trager and Jensen, 1997) and used for mosquito infection by membrane feeding technique. The blood meal for mosquito infection consisted of human red blood cells, normal human serum and erythrocytic sexual stages. Mature stage V gametocytes were diluted to desired final gametocytemia (0.075 to 0.6%) using O+ normal human serum and O+ human RBCs to a final 50% hematocrit and immediately fed to female Anopheles mosquitoes (4–5 day old adults, starved for 5–6 hours) using parafilm covered water jacketed glass feeders maintained at 37°C using a circulating water bath. Various species of Anopheles tested included An. gambiae (Keele strain), An. stephensi and An. freeborni. For infection with a mix of gametocytes and asexual stages, blood meals were prepared as above included, in addition to gametocytes, varying concentrations (0.3%, 0.6% and 1.2%) of culture-derived erythrocytic asexual stages. Similarly, mosquitoes were fed on gametocyte blood meals that contained varying concentrations of purified hemozoin. Mosquitoes were allowed to feed for 20 minutes, followed by removal of any unfed mosquitoes. Blood fed mosquitoes were maintained in incubators at 26–27°C and ~80% relative humidity for 8–10 days on 10% dextrose dipped cotton balls. Midguts from individual mosquitoes were stained with 0.1% mercurochrome and number of oocysts enumerated microscopically.
Purification of hemozoin
Parasite derived hemozoin (malaria pigment) was purified by SDS-urea method (Chen et al., 2001). Briefly, cultured asexual stage parasites were treated with 0.15% saponin to lyse red blood cells and freed parasites stored at −80°C. Frozen parasite pellets were resuspended in 2% SDS and sonicated to completely lyse parasites. The pellet obtained after centrifugation (20,000 rpm, 20 min, 25°C) was extracted with 2% SDS, 3–5 times or until the supernatant was clear and colorless. The pellet was then incubated (overnight at 37°C) with 2 mg/ml proteinase K in 10 mM Tris buffer, pH 8.0 containing 0.5% SDS and 1 mM CaCl2, followed by three washes using 2% SDS as above. Finally, the pellet was treated (3–4 hours at room temperature) with 6M urea using a tube rotator. After an additional 3 washes with 2% SDS, the purified hemozoin pellet was washed 8–10 times using deionized water and stored at −20°C until further use. The concentration of hemozoin was determined using hemin as standard. Hemin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in DMSO to prepare a 6.5 mg/ml solution (10 mM concentration). The stock solution of hemin was serially diluted in 2% SDS and 10 mM NaOH to yield (0.1 to 1000 μM standards). Purified hemozoin after sonication was likewise diluted in 2% SDS and 10 mM NaOH and the absorbance was recorded at 400 nm. The concentration of hemozoin was determined based on hemin extinction coefficient of 105.
Immunofluorescence detection of P. falciparum stages in the mosquito midgut
Two different approaches were used to detect parasites undergoing development in the mosquito midgut. Antibody against Pfs25 protein expressed in zygotes and ookinetes (Lobo and Kumar, 1998) was used for immunofluorescent detection of mosquito stage parasites. Initially, blood-fed midguts were dissected in PBS and gently homogenized in 20 μl of the 3% acetic acid to lyse the red blood cells. The midgut contents were washed once with PBS and resuspended in 10 μl PBS for spotting (2 μl) in 8-well slides. Air dried spots were fixed with pre-chilled methanol (−20°C) for 30 minutes, blocked with 5% milk in PBS (1 hour, room temperature) and followed by incubation with mouse anti-Pfs25 antibody in a moist chamber at room temperature for 30 minutes. Following 3–5 washes in PBS, FITC-conjugated goat anti-mouse Ig (Southern Biotech, Birmingham, AL, USA) was added and the slides incubated for 30 minutes in the dark. Following PBS washing, slides were dried, mounted and examined using fluorescent microscope (Chege and Beier, 1994; Zollner et al., 2005). Multiple fields from 2–3 replicate spots were examined for enumeration of fluorescent parasites designated as zygote (round), retort (immature form of ookinete) and ookinete (elongated) and the numbers averaged and extrapolated to the total number of parasites per midgut. The counts were used to estimate proportion of each stage at different time periods. In order to evaluate parasites at time points, later than 24 hours, we adapted the procedure established for murine malaria parasite P. berghei (Han et al., 2000).
Briefly, mosquito midguts were dissected in 1% paraformaldehyde in PBS and cut longitudinally one minute later using a sharp scalpel blade. After one more minute continued incubation in 1% paraformaldehyde, the midgut epithelium was peeled away from blood bolus and incubated for 1 hour in 4% paraformaldehyde (50 μl in a 96 well plate). The midguts were carefully washed 2 times with PBS, incubated with PBST (PBS + 0.1% Tween 20) for 2 minutes followed by 3 washes with PBS. Midguts were incubated in the blocking solution (10% goat serum, 0.2% BSA in PBST) for 1 hour. Washed midguts were incubated with anti-Pfs25 antibody for 1 hour at room temperature, washed with PBS and further incubated with FITC-conjugated goat anti-mouse IgG for 1 hour in the dark. After washing, midguts were transferred to a glass slide on a drop of fluorescent mounting medium with DAPI (Abcam, Cambridge, MA, USA), and mounted using a coverslip and sealed with clear nail polish for examination using fluorescence microscope. Midguts at 28 hour time point were examined to detect ookinetes during the process of traversing through midgut epithelium.
Data analysis
GraphPad Prism software (version 5.0) was used to analyze statistical significance of data.
Results
Successful development of Plasmodium in the mosquito vector involves a well-orchestrated sequence of events. A quantitative assessment of parasite stages developing within the mosquito midgut (zygotes, ookinetes and oocysts) can provide a stepwise approach to evaluate transmission potential of gametocytes under different infection situations.
Relationship between gametocyte density and oocyst development in different anopheline species
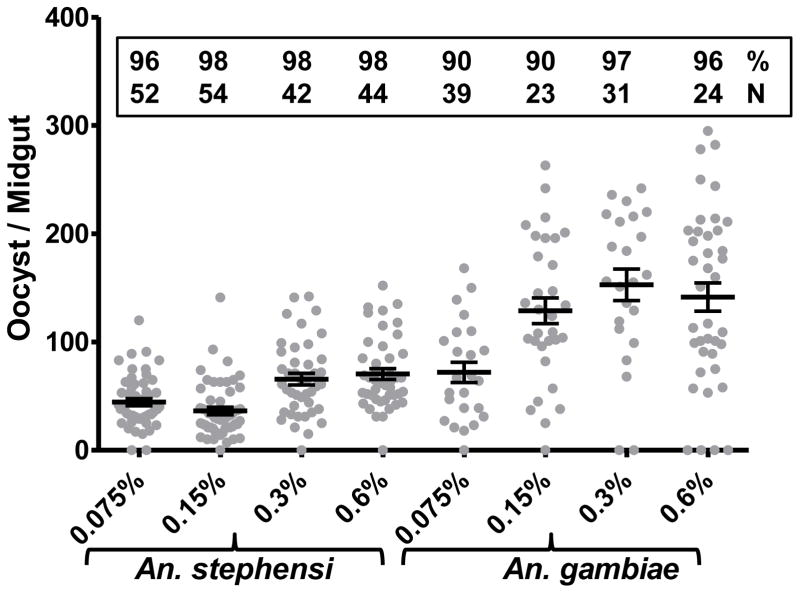
We first evaluated mosquito infectivity as a function of varying gametocyte densities in the blood meal. Fig. 1 shows results (pooled from two independent mosquito feeding assays) on oocyst development in two different species of mosquitoes given a blood meal with varying final percentages of gametocytes. Individual mosquito midguts were examined for the presence of oocysts. As shown, there was a positive correlation between gametocyte density and oocyst number, plateauing at ~0.3% final gametocyte density. However, the two mosquito species differed in terms of oocyst numbers: higher oocyst numbers in An. gambiae than An. stephensi. For all subsequent studies we fixed gametocyte density at 0.3% in the blood meal.
Figure 1. Mosquito infection as a function of gametocyte density.
An. stephensi and An. gambiae mosquitoes were given a blood meal consisting of indicated (X-axis) final gametocytemia (0.075% to 0.6%). Eight to 10 days post blood feed individual mosquitoes were examined for the presence of oocysts in the midgut (Y-axis). Results were pooled for two independent repeat feeds and the number of oocysts per midgut are shown for each gametocyte concentration group of infected mosquitoes. The horizontal lines represent the mean ± SEM. Numbers inside the box represent percent of infected mosquitoes out of indicated total number (N) dissected.
Evaluation of the effect of erythrocytic asexual stages on the infectivity of gametocyte in different species of anophelines
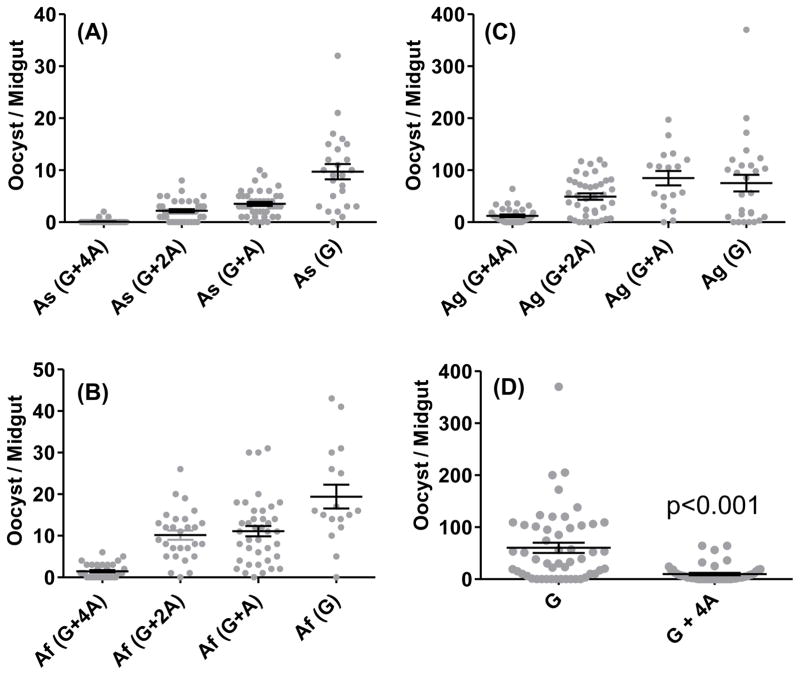
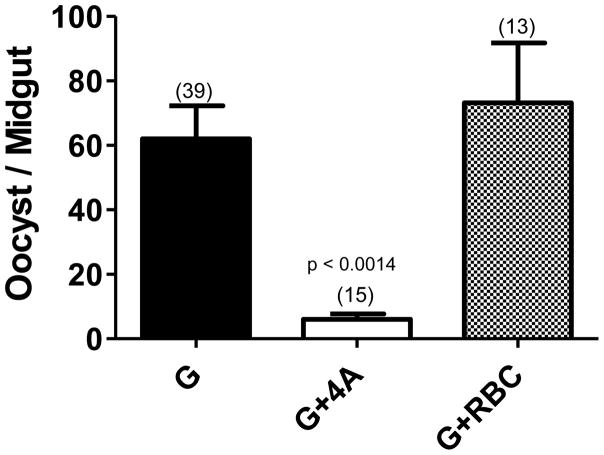
In order to systematically evaluate the impact of asexual stage parasite components on the outcome of initial sporogonic development, we employed three different species of Anopheles mosquitoes and combinations of varying proportions of erythrocytic mature gametocytes and erythrocytic asexual stages. As shown in Fig. 2 [panel A for An. stephensi (As), panel B for An. freeborni (Af) and panel C for An. gambiae (Ag)], there were anopheline species-specific differences in the mean oocyst numbers when given a blood meal containing 0.3% gametocytes only [As(G) versus Af(G) versus Ag(G) data points]. However, when increasing amounts of mixed asexual stages of P. falciparum [0.3% (G+A), 0.6% (G+2A), 1.2% (G+4A)] were combined with 0.3% gametocytes, the infectiousness of gametocytes was significantly compromised in asexual stage density-dependent manner in all the three species of anophelines. Panel D shows results pooled for four independent feeds for G versus G+4A groups in An. gambiae. The oocyst numbers in G+4A group were significantly lower (84%) as compared to G alone group (Mann Whitney, p<0.0001). In order to establish the specificity of asexual stage-mediated inhibition of gametocyte infectivity, we tested the effect of conditioned uninfected red blood cells also maintained in culture at 1.2% asexual stages equivalent total cell density. As shown in Fig. 3, culture-conditioned uninfected red blood cells did not affect infectivity of gametocyte whereas, red blood cells infected with asexual stages caused ~90% inhibition (p<0.0014).
Figure 2. Mosquito infection as a function of varying asexual (A) to gametocyte (G) ratio.
An. stephensi (As) (panel A), An. freeborni (Af) (panel B) and An. gambiae (Ag) (panel C) mosquitoes were given a blood meal consisting of fixed 0.3% gametocytes (G) alone or combined with varying concentrations of asexual parasites: 0.3% asexual combination group represented as G+A; 0.6% asexual combination group as G+2A; and 1.2% asexual combination as G+4A along the X-axis. The number of oocysts per mosquito midgut are shown for each group. The horizontal lines represent the mean ± SEM. Panel D shows comparative results pooled for four independent feeds for G versus G+4A groups in An. gambiae. The oocyst numbers in G+4A group were significantly lower (84%) as compared to G alone group (Mann Whitney, p<0.0001).
Figure 3. Inhibition of mosquito infection is observed in the presence of asexual parasites and not conditioned uninfected red blood cells (RBC).
An. gambiae mosquitoes were given a blood meal consisting of either 0.3% gametocytes (G) or 0.3% gametocytes combined with 1.2% asexual parasites (G+4A) or 0.3% gametocytes combined with uninfected red blood cells maintained like asexual cultures (G+RBC). The oocyst burden is shown as mean ± SEM. Numbers within parenthesis indicate total number of mosquitoes dissected. Mean oocyst number in G+4A groups was 90% lower as compared to G alone group and the difference was statistically significant (p<0.0014).
Hemozoin negatively impacts infectivity of gametocytes in the mosquitoes
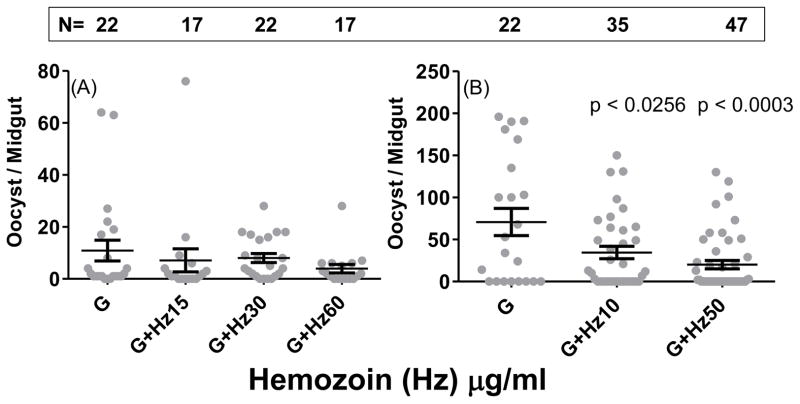
Seeking to understand the mechanism of inhibitory activity of asexual stages, we reasoned that the excess of hemozoin stored in the digestive vacuoles of the parasites and released during normal growth cycle may mediate some of the inhibition of infectivity of gametocytes to mosquitoes via activation of mosquito anti-parasite defense mechanisms. We evaluated the effect of varying concentrations of purified natural hemozoin in gametocyte - mosquito infectivity experiments. Fig. 4 shows hemozoin dose-dependent inhibition of oocyst development in the mosquitoes infected with 0.3% gametocytes in the blood meal.
Figure 4. Exogenous hemozoin in the blood meal suppresses mosquito infection.
An. gambiae mosquitoes were given a blood meal consisting of either 0.3% gametocytes (G) or 0.3% gametocytes combined with indicated concentrations of two different preparations of purified hemozoin (Hz) in independent experiments (A and B). The number of oocysts per mosquito midgut is shown for each group. The horizontal lines represent mean ± SEM. Numbers within the box indicate total number (N) of mosquitoes dissected. Statistical significance is indicated by p values.
Quantitative analysis of early sporogonic development of P. falciparum
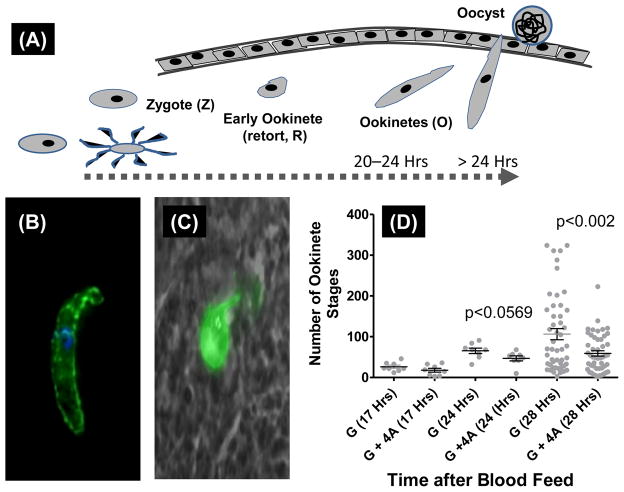
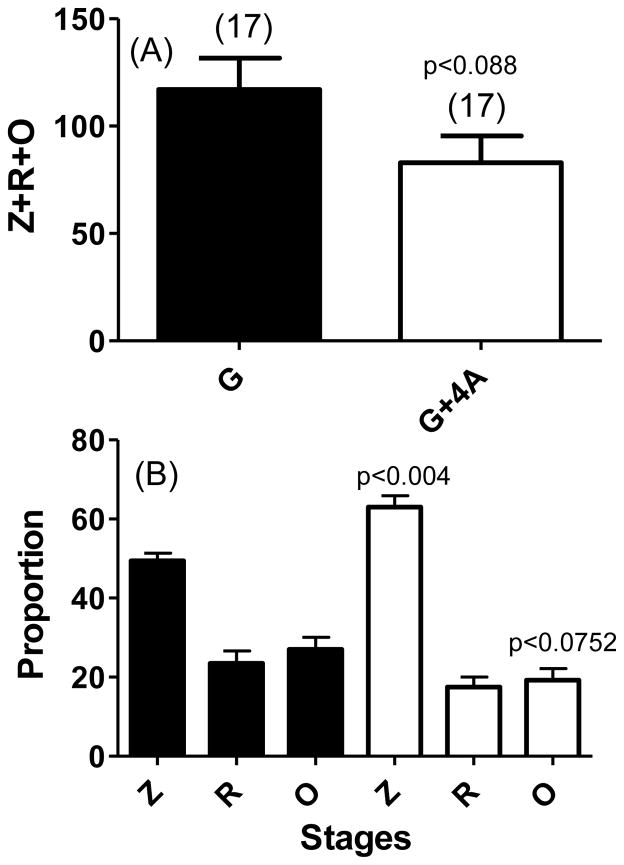
In all the above studies, we investigated oocyst stages on days 8–10 after feeding mosquitoes on a mixture of erythrocytic sexual and asexual stages as the outcome. We reasoned that activation of mechanisms that target early stage development and survival of zygotes (within 30 minutes of ingestion) and ookinetes (20–30 hours) will yield similar inhibition of oocyst stages. Mosquitoes were allowed to ingest blood meals containing 0.3 % gametocytes (G) only or blood meal that included a mix of 0.3% gametocytes and 1.2% asexual stages (G+4A). Mosquito midguts were harvested at different time points (<20 hours and >24 hours) and midgut contents were analyzed for the presence of zygotes, immature forms of developing ookinetes, and mature ookinetes by immunofluorescence microscopy (schematically represented in Fig. 6A). We also chose a time point that would allow us to capture ookinetes during traversal through the midgut epithelial layer. Total number of mosquito midgut stages (undeveloped female gametes/zygotes + immature retort forms of ookinetes and fully mature ookinetes) were not significantly different between G and G+4A groups (Fig. 5A). When further analyzed for relative proportions of each stage in the midguts from the two groups (G versus G+4A), a significant proportion of the parasites in G+4A groups remained arrested in pre-ookinete stages and this was reflected in reduced numbers of mature ookinetes with the difference approaching statistical significant values (p<0.0752) (Fig. 5B). Lastly, analysis of ookinetes at 24 and 28 hours after blood meal allowed us to compare mature ookinetes (Fig. 6B) during traversal through the midgut epithelial barrier (Fig. 6C). Quantification of these data indicated statistically significant effect on the reduced number of ookinetes traversing the midgut wall [Fig. 6D, G (28 Hrs) versus G+4A (28 Hrs) data points].
Figure 6. Comparison of mosquito midgut developmental stages of P. falciparum, prior to and during traversal through midgut epithelium.
Panel (A) shows the time scale of various morphological developmental stages: zygotes (Z), immature forms of developing ookinetes known as retort forms (R) and mature ookinetes (O) during the 20–24 hours of ingestion of blood meal. Between 24 and 28 hours blood meal ingestion, mature ookinetes begin to traverse the midgut epithelium which then go on to develop into oocyst. Panel (B) shows typical mature midgut ookinete. Panel (C) shows a representation of ookinete during traversal through midgut epithelium. Panel (D) shows quantitation of various parasite stages at 17, 24 and 28 hours post ingestion of blood meal comprised of either 0.3% gametocytes (G) or 0.3% gametocytes + 1.2% asexual stages (G+4A). Statistical significance of difference between the two groups at 24 and 28 hour time points are indicated by p values.
Figure 5. Quantitation of early post-fertilization midgut development stages of P. falciparum.
An. gambiae mosquitoes were given a blood meal consisting of either 0.3% gametocytes (G, black bars) or 0.3% gametocytes combined with 1.2% asexual parasites (G+4A, white bars). Twenty hours post blood meal, individual mosquito midguts were dissected and treated with 3% acetic acid prior to spotting on multi-well IFA slides in duplicate wells. Methanol-fixed dried spots were treated with anti-Pfs25 monoclonal antibody followed by FITC conjugated anti-mouse IgG to visualize undeveloped female gametes and/or zygotes (Z), immature retort forms of ookinetes (R) and mature ookinetes (O). The data is expressed as Z+R+O in the two groups (G and G+4A) of mosquitoes (panel A) or relative proportion of each stage (panel B). Statistical significance (p values) of differences between G and G+4A groups are indicated for total parasites (Z+R+O) (panel A) and proportion of Z and O (panel B).
Discussion
Ingestion of mature gametocytes by female anopheline mosquitoes initiates the sexual development cycle leading to formation of sporozoites required for further transmission. However, during blood feeding on a host, a mosquito ingests, in addition to mature gametocytes, many other components such as anti-malaria drugs and host immune factors present in the blood, as well as large excess of erythrocytic asexual and immature gametocytes. However, the latter forms are biologically inapt for malaria transmission. Not only completion of all the developmental steps is critical for transmission success, the various mosquito stages of the parasite need to defend against the components of the host adaptive immune system ingested in the blood meal and avoid being destroyed by the innate immunity mechanisms of the mosquito itself. Studies have shown exquisite susceptibility of motile ookinete stage parasites to complement-like proteins produced by the mosquito innate immune cells (Molina-Cruz et al., 2015; Ramphul et al., 2015). Oocysts are likewise also vulnerable to mosquito defense mechanism (Smith and Barillas-Mury, 2016).
In the current study we wished to experimentally address the impact of the presence of large excess of erythrocytic asexual stages, hitherto not known or even suspected to influence, on the infectivity of gametocytes in the mosquito vector. We first titrated infectivity of gametocyte by varying percentage of mature gametocytes in the blood meal. Our studies revealed a positive association between gametocyte density and mosquito oocyst numbers in two different species of Anopheles vectors with 0.3% final gametocytemia as the optimum density for both species of mosquitoes tested. In all the future studies we fixed gametocyte concentration at 0.3% and varied other components. A similar positive association between gametocyte density and mosquito infection has been reported in many previous published studies using murine and human malaria parasites (both lab adapted and field isolates) in different anopheline species (Carter and Graves, 1988; Da et al., 2015; Dawes et al., 2009; Drakeley et al., 1999; Poudel et al., 2008; Sinden et al., 2007). Apart from the actual number of gametocytes, maturity and sex ratios of the gametocytes and the presence of natural immune factors can also influence the outcome of infection in the mosquitoes.
We next rationalized that during natural transmission, a mosquito feeding on an infected individual will ingest not only gametocytes but also erythrocytic asexual stages, often present at much higher proportion. While asexual stages do not initiate mosquito infection in the mosquitoes, we hypothesized that the presence of large excess of various components of the asexual stages in the ingested blood meal might interact with mosquito immune system and indirectly influence survival of mosquito midgut stages of the parasite. Indeed, our studies demonstrated a dose-dependent inhibition of oocyst number when the blood meal also contained increasing proportions (1:1, 1:2 and 1:4) of asexual stages. Such inhibition was seen only when asexual parasite infected red blood cells were combined with gametocytes and uninfected erythrocytes maintained in culture for similar duration did not show any inhibitory function. This was a rather unexpected outcome because asexual parasites themselves are not direct players in initiating infection in the mosquitoes. Previous in vivo studies have demonstrated cytokine-mediated inactivation of gametocyte and loss of mosquito infection by cytokines and other host factors elicited during blood stage infection and malaria paroxysm (Naotunne et al., 1991; Naotunne et al., 1993). Likewise, studies using an in vivo mouse malaria model identified suppressive role of poorly defined cytotoxic immune humoral factors elicited during rising asexual stage infection, indirectly linking asexual parasitemia with modulation of gametocyte infectiousness in the mosquitoes (Sinden, 1991; Sinden et al., 1996). However, the ex vivo mosquito infection studies reported here address the direct role of asexual parasite components affecting infectiousness of gametocyte.
Further extension of our investigation led to identification of hemozoin as one of the components mediating such inhibition. Hemozoin is the by-product of hemoglobin digestion in the parasite and has been shown to accumulate in the digestive vacuoles of the parasite in extremely high concentrations (100–400 mM ferriprotoporphyrin IX equivalent) (Ginsburg et al., 1998). Another potential source for natural delivery of hemozoin to mosquitoes will be leucocytes ingested in the whole blood meal. Leucocytes acquire hemozoin through phagocytic uptake of dying parasites and free hemozoin released in the circulation. Ingestion of hemozoin by anopheline mosquitoes has been shown to increase nitric oxide production which in turn may limit the development of the parasite in the mosquito midgut (Akman-Anderson et al., 2007; Simões et al., 2015). Thus components derived from asexual stages of the malaria parasites can influence the outcome of parasite development potentially via activation of the mosquito immune system. In a previous study, feeding hemoglobin to mosquitoes was shown to inhibit infectiousness of P. yoelii nigeriensis gametocytes in An. stephensi (Motard et al., 1990). We also propose that hemozoin may not be the only inhibitory component and further studies will be required to characterize additional molecular components that activate mosquito immune pathways modulating infectivity of gametocytes in the mosquitoes.
Reduced functionality of midgut developmental stages of the parasite or compromised survival of ookinetes during their journey through the midgut wall may account for significant reductions in the oocyst numbers in the presence of components of asexual stage parasites. Immunofluorescence staining of developing zygotes and ookinetes in the mosquito midgut was employed to quantify these early stages in an effort to dissect the stage affected by the presence of asexual stages. Initially we quantified zygotes, immature ookinetes known as ‘retort forms’ and mature ookinetes in the midguts of mosquitoes at 20 hour time point, and we did not see any significant difference in the total number. Focusing on the proportion of each stage, our analysis did reveal that significant numbers of zygotes failed to complete their development into mature ookinetes. This clearly indicated that the initial stages of gametogenesis (formation of male and female gametes and fertilization between gametes were not hampered in the presence of large excess of asexual parasites during mosquito infection by gametocytes. Results presented also suggest that either the process of ookinete maturation or their survival is likely to be the targets of mechanisms triggered by asexual parasites in the mosquito midgut. Indeed, studies looking at ookinetes at a time point when they are expected to traverse the midgut wall supported the forgoing interpretation. We saw a significant reduction in the number of mature ookinetes undergoing traversal through the midgut wall. These studies do not rule out further reduction resulting from targeting of oocysts directly by mechanisms triggered by the presence of asexual stage parasite components.
The studies presented here provide a new dimension to the biological complexity of the malaria transmission process. Dynamic interactions between densities of asexual and sexual stages, their relative proportions in the blood meal, differences in the gametocyte maturity, differences in the ratio of male and female gametocyte, presence of drugs, vertebrate host immune factors and mosquito innate immune factors are some of the variables that determine the overall infectiousness of gametocytes in the mosquitoes and ultimately malaria transmission success.
Acknowledgments
I wish to acknowledge discussions with my former colleagues during the initial stages of the work supported by the technical staff of the “Parasite and Mosquito Core” of the Johns Hopkins Malaria Research Institute. I wish to also acknowledge NIAID intramural malaria program for kind supply of An. freeborni mosquitoes for reported studies. Partial financial support from NIH grant NIH RO1 AI47089 and AI127544 is also acknowledged.
Abbreviations
- RBC
Red blood cells
- NHS
normal human serum
- SDS
sodium dodecyl sulfate
- FITC
fluorescein isothiocyanate
Footnotes
Author contribution
N. Kumar designed research, supervised and performed assays, analyzed data and wrote the manuscript.
Conflict of interest
The author declares no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar R, Magallon-Tejada A, Achtman AH, Moraleda C, Joice R, Cisteró P, Li Wai Suen CSN, Nhabomba A, Macete E, Mueller I, Marti M, Alonso PL, Menéndez C, Schofield L, Mayor A. Molecular evidence for the localization of Plasmodium falciparum immature gametocytes in bone marrow. Blood. 2014;123:959–966. doi: 10.1182/blood-2013-08-520767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akman-Anderson L, Olivier M, Luckhart S. Induction of nitric oxide synthase and activation of signaling proteins in Anopheles mosquitoes by the malaria pigment, hemozoin. Infection and immunity. 2007;75:4012–4019. doi: 10.1128/IAI.00645-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baton LA, Ranford-Cartwright LC. Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends in parasitology. 2005;21:573–580. doi: 10.1016/j.pt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Beier JC. Malaria parasite development in mosquitoes. Annual review of entomology. 1998;43:519–543. doi: 10.1146/annurev.ento.43.1.519. [DOI] [PubMed] [Google Scholar]
- Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clinical microbiology reviews. 2011;24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R, Graves P. Gametocytes. Malaria: principles and practice of malariology. 1988;1:253–306. [Google Scholar]
- Chege G, Beier JC. Immunodetection of Plasmodium falciparum zygotes and ookinetes in Anopheles blood meals. Journal of the American Mosquito Control Association. 1994;10:419–422. [PubMed] [Google Scholar]
- Chen MM, Shi L, Sullivan DJ., Jr Haemoproteus and Schistosoma synthesize heme polymers similar to Plasmodium hemozoin and β-hematin. Molecular and Biochemical Parasitology. 2001;113:1–8. doi: 10.1016/s0166-6851(00)00365-0. [DOI] [PubMed] [Google Scholar]
- Churcher TS, Blagborough AM, Delves M, Ramakrishnan C, Kapulu MC, Williams AR, Biswas S, Da DF, Cohuet A, Sinden RE. Measuring the blockade of malaria transmission – An analysis of the Standard Membrane Feeding Assay. International Journal for Parasitology. 2012;42:1037–1044. doi: 10.1016/j.ijpara.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Da DF, Churcher TS, Yerbanga RS, Yaméogo B, Sangaré I, Ouedraogo JB, Sinden RE, Blagborough AM, Cohuet A. Experimental study of the relationship between Plasmodium gametocyte density and infection success in mosquitoes; implications for the evaluation of malaria transmission-reducing interventions. Experimental parasitology. 2015;149:74–83. doi: 10.1016/j.exppara.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Dawes EJ, Zhuang S, Sinden RE, Basáñez MG. The temporal dynamics of Plasmodium density through the sporogonic cycle within Anopheles mosquitoes. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103:1197–1198. doi: 10.1016/j.trstmh.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Dixon MW, Thompson J, Gardiner DL, Trenholme KR. Sex in Plasmodium: a sign of commitment. Trends in parasitology. 2008;24:168–175. doi: 10.1016/j.pt.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Drakeley C, Secka I, Correa S, Greenwood B, Targett G. Host haematological factors influencing the transmission of Plasmodium falciparum gametocytes to Anopheles gambiae ss mosquitoes. Tropical Medicine & International Health. 1999;4:131–138. doi: 10.1046/j.1365-3156.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg H, Famin O, Zhang J, Krugliak M. Inhibition of glutathione-dependent degradation of heme by chloroquine and amodiaquine as a possible basis for their antimalarial mode of action. Biochemical pharmacology. 1998;56:1305–1313. doi: 10.1016/s0006-2952(98)00184-1. [DOI] [PubMed] [Google Scholar]
- Graves P, Burkot T, Carter R, Cattani J, Lagog M, Parker J, Brabin B, Gibson F, Bradley D, Alpers M. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua New Guinea. Parasitology. 1988;96:251–263. doi: 10.1017/s003118200005825x. [DOI] [PubMed] [Google Scholar]
- Han YS, Thompson J, Kafatos FC, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. The EMBO journal. 2000;19:6030–6040. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifediba T, Vanderberg J. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature. 1981;294:364–366. doi: 10.1038/294364a0. [DOI] [PubMed] [Google Scholar]
- Jeffery G, Eyles D. Infectivity to mosquitoes of Plasmodium falciparum as related to gametocyte density and duration of infection. Am J Trop Med Hyg. 1955;4:781–789. doi: 10.4269/ajtmh.1955.4.781. [DOI] [PubMed] [Google Scholar]
- Lobo C, Kumar N. Sexual differentiation and development in the malaria parasite. Parasitology Today. 1998;14:146–150. doi: 10.1016/s0169-4758(97)01210-6. [DOI] [PubMed] [Google Scholar]
- McKenzie FE, Bossert WH. The Optimal Production of Gametocytes by Plasmodium falciparum. Journal of Theoretical Biology. 1998;193:419–428. doi: 10.1006/jtbi.1998.0710. [DOI] [PubMed] [Google Scholar]
- Molina-Cruz A, Canepa GE, Kamath N, Pavlovic NV, Mu J, Ramphul UN, Ramirez JL, Barillas-Mury C. Plasmodium evasion of mosquito immunity and global malaria transmission: The lock-and-key theory. Proceedings of the National Academy of Sciences. 2015;112:15178–15183. doi: 10.1073/pnas.1520426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motard A, Baccam D, Landau I. Temporary loss of Plasmodium gametocytes infectivity during schizogony. Annales de parasitologie humaine et comparée. 1990;65:218–220. [Google Scholar]
- Muirhead-Thomson R. Low gametocyte thresholds of infection of Anopheles with Plasmodium falciparum; a significant factor in malaria epidemiology. Br Med J. 1954;1:68–70. doi: 10.1136/bmj.1.4853.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naotunne T, Karunaweera N, Del Giudice G, Kularatne M, Grau G, Carter R, Mendis K. Cytokines kill malaria parasites during infection crisis: extracellular complementary factors are essential. J Exp Med. 1991;173:523–529. doi: 10.1084/jem.173.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naotunne T, Karunaweera N, Mendis K, Carter R. Cytokine-mediated inactivation of malarial gametocytes is dependent on the presence of white blood cells and involves reactive nitrogen intermediates. Immunol. 1993;78:555–562. [PMC free article] [PubMed] [Google Scholar]
- Paul R, Diallo M, Brey P. Mosquitoes and transmission of malaria parasites - not just vectors. Malaria Journal. 2004;3:39. doi: 10.1186/1475-2875-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnudurai T, Lensen A, Van Gemert G, Bensink M, Bolmer M, Meuwissen JT. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology. 1989;98:165–173. doi: 10.1017/s0031182000062065. [DOI] [PubMed] [Google Scholar]
- Poudel SS, Newman RA, Vaughan JA. Rodent Plasmodium: population dynamics of early sporogony within Anopheles stephensi mosquitoes. Journal of Parasitology. 2008;94:999–1008. doi: 10.1645/GE-1407.1. [DOI] [PubMed] [Google Scholar]
- Ramphul UN, Garver LS, Molina-Cruz A, Canepa GE, Barillas-Mury C. Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proceedings of the National Academy of Sciences. 2015;112:1273–1280. doi: 10.1073/pnas.1423586112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões ML, Gonçalves L, Silveira H. Hemozoin activates the innate immune system and reduces Plasmodium berghei infection in Anopheles gambiae. Parasites & vectors. 2015;8:12. doi: 10.1186/s13071-014-0619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden R. Asexual blood stages of malaria modulate gametocyte infectivity to the mosquito vector–possible implications for control strategies. Parasitology. 1991;103:191–196. doi: 10.1017/s0031182000059473. [DOI] [PubMed] [Google Scholar]
- Sinden R, Butcher G, Billker O, Fleck S. Regulation of infectivity of Plasmodium to the mosquito vector. Advances in parasitology. 1996;38:53–117. doi: 10.1016/s0065-308x(08)60033-0. [DOI] [PubMed] [Google Scholar]
- Sinden RE, Dawes EJ, Alavi Y, Waldock J, Finney O, Mendoza J, Butcher GA, Andrews L, Hill AV, Gilbert SC. Progression of Plasmodium berghei through Anopheles stephensi is density-dependent. PLoS Pathog. 2007;3:e195. doi: 10.1371/journal.ppat.0030195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Barillas-Mury C. Plasmodium Oocysts: Overlooked Targets of Mosquito Immunity. Trends in Parasitology. 2016;32:979–990. doi: 10.1016/j.pt.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Talman A, Domarle O, McKenzie F, Ariey F, Robert V. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar J. 2004;3:24. doi: 10.1186/1475-2875-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Read A. Why so few transmission stages? Reproductive restraint by malaria parasites. Parasitol Today. 1997a;13:135–140. doi: 10.1016/s0169-4758(97)89810-9. [DOI] [PubMed] [Google Scholar]
- Taylor LH, Read AF. Why so few transmission stages? Reproductive restraint by malaria parasites. Parasitology Today. 1997b;13:135–140. doi: 10.1016/s0169-4758(97)89810-9. [DOI] [PubMed] [Google Scholar]
- THOMSON R. The malarial infectivity of an African village population to mosquitoes (Anopheles gambiae). A random xeno-diagnostic survey. American Journal of Tropical Medicine and Hygiene. 1957;6:971–979. doi: 10.4269/ajtmh.1957.6.971. [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Continuous culture of Plasmodium falciparum: its impact on malaria research. Int J Parasitol. 1997:27. doi: 10.1016/s0020-7519(97)00080-5. [DOI] [PubMed] [Google Scholar]
- WHO, WHO. World Malaria Report 2015. World Health Organization; Geneva: 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en. [Google Scholar]
- Zollner GE, Ponsa N, Coleman RE, Sattabongkot J, Vaughan JA. Evaluation of procedures to determine absolute density of Plasmodium vivax ookinetes. Journal of Parasitology. 2005;91:453–457. doi: 10.1645/GE-391R. [DOI] [PubMed] [Google Scholar]