Abstract
The present work focuses firstly on the evaluation of the effect of laccase on enzymatic hydrolysis of hazelnut husk which is one of the most abundant lignocellulosic agricultural residues generated in Turkey. In this respect, the co-enzymatic treatment of hazelnut husk by cellulase and laccase, without a conventional pretreatment step is evaluated. Using 2.75 FPU/g substrate (40 g/L substrate) and a ratio of 131 laccase U/FPU achieved the highest reducing sugars concentration. Gas chromatography mass spectrometry confirmed that the hydrolysate was composed of glucose, xylose, mannose, arabinose and galactose. The inclusion of laccase in the enzyme mixture [carboxymethyl cellulase (CMCase) and β-glucosidase] increased the final glucose content of the reducing sugars from 20 to 50%. Therefore, a very significant increase in glucose content of the final reducing sugars concentration was obtained by laccase addition. Furthermore, the production of cellulases and laccase by Pycnoporus sanguineus DSM 3024 using hazelnut husk as substrate was also investigated. Among the hazelnut husk concentrations tested (1.5, 6, 12, 18 g/L), the highest CMCase concentration was obtained using 12 g/L husk concentration on the 10th day of fermentation. Besides CMCase, P. sanguineus DSM 3024 produced β-glucosidase and laccase using hazelnut husk as carbon source. In addition to CMCase and β-glucosidase, the highest laccase activity measured was 2240 ± 98 U/L (8.89 ± 0.39 U/mg). To the best of our knowledge, this is the first study to report hazelnut husk hydrolysis in the absence of pretreatment procedures.
Keywords: Hazelnut husk, Lignocellulose, Hydrolysis, Cellulase, Laccase, P. sanguineus
Introduction
Lignocellulosic biomass, the most abundant renewable carbon source on earth, has attracted considerable interest for the production of second generation biofuels and value added chemicals (Boonmee 2012; Chaturverdi and Verma 2013; Weiss et al. 2013; Kumar et al. 2016; Guerriero et al. 2016; Haldar et al. 2016). Agricultural and forestry residues (Ghobadian et al. 2008; Hashem et al. 2013), industrial and municipal wastes, and energy crops are considered as lignocellulosic materials and their vast availability helps to reduce the production cost of biofuels and value added chemicals (Sharma et al. 2002; Mohanram et al. 2013). It is predicted that a major part of future energy supply could be generated using discarded lignocellulosic biomass (corn stover, wheat straw, rice husk, etc.) (Nanda et al. 2014).
The first step for the production of fermentable sugars from lignocellulosic biomass is the disintegration of the lignin–cellulose–hemicellulose complex followed by the hydrolysis of the cellulosic and hemicellulosic parts of this complex to produce fermentable sugars. Generally, physico-chemical methods are employed in order to hydrolyze lignocellulosic biomass to fermentable sugars (Sindhu et al. 2011; Chaturverdi and Verma 2013; Rico et al. 2014; Nieves et al. 2016; Tozluoğlu 2016). However, the main drawback of these processes is the production of undesirable byproducts such as furfural and 5-hydroxymethyl-2-furaldehyde that are toxic for microorganisms and reduce conversion of sugars to biofuels and other value added products (Mohanram et al. 2013). For this reason, enzymatic hydrolysis, which eliminates this drawback, is considered as a replacement for physico-chemical pretreatment methods (Sindhu et al. 2016).
Much research is focused on the hydrolysis of lignocellulosic biomass using a variety of enzymes, mostly cellulases and/or other auxiliary enzyme mixtures, to produce fermentable sugars (Camarero et al. 2007; Sathitsuksanoh et al. 2012; Fontes and Gilbert 2010; Woolridge 2014; Nanda et al. 2014; Khare et al. 2015; Alvarez et al. 2016; Kumar et al. 2016; Pal et al. 2016; Haldar et al. 2016). Prior to enzymatic conversion of lignocellulosic raw material to fermentable sugars, a pretreatment step is applied to solubilize the hemicellulose fraction, to modify the lignin fraction and to increase the accessibility of enzymes (Kumar et al. 2016). Pretreatment options vary from early technologies such as liquid hot water or hydrothermal treatment to newer technologies like co-solvent based lignocellulosic extraction. As these operations require additional equipment and energy, researchers have focused instead on using multi-enzymatic systems involving both hydrolytic and oxidative transformations; in other words, treatment with both cellulase and ligninolytic enzymes such as laccase in order to overcome these impediments (Moreno et al. 2016). The pretreatment step to prepare lignocellulosic biomass for efficient hydrolysis has a major impact on the economic feasibility of fermentable sugar production. Replacement of the pretreatment options which require additional equipment with enzymatic pretreatment that can occur simultaneously with enzymatic hydrolysis can be an advantageous approach to reduce capital investment and improve overall economic feasibility.
Therefore, in this work, we evaluate the use of cellulase and laccase simultaneously to hydrolyze hazelnut husk which is the most abundant lignocellulosic agricultural residue generated in Turkey. A co-objective of this paper is to illustrate the potentiality of hazelnut husk not just as a lignocellulosic feedstock for fermentable sugar production but also as a substrate (carbon source) for the production of lignocellulolytic enzyme mixture components [carboxymethyl cellulase (CMCase), β-glucosidase and laccase]. This potentiality paves the way toward consolidated bioprocessing of lignocellulosic hazelnut husk.
After industrial processing of hazelnut, a large amount of hazelnut husk (approximately 200,000 tons/year) is generated and it is either burned or discarded in the field. Disposing of hazelnut husk in this way is wasting valuable biomass whose potential utilization as feedstock for biofuel production is highlighted before (Guney 2013), since hazelnut husk is composed of 55.1% holocellulose, 35.1% lignin and 8.22% ash (Çöpür et al. 2007).
Çöpür et al. (2013) evaluated the usage of methods such as treatments with NaBH4, NaOH, H2O2 and H2SO4 for delignification and xylan solubilization of hazelnut husk as examples of various pretreatment approaches. The pretreated samples having the highest glucan to lignin ratio were then hydrolyzed enzymatically using cellulase and cellobiase (Novozyme 188). Since such pretreatment steps have different drawbacks as mentioned earlier, in this work, we investigated the production of fermentable sugars by the co-enzymatic hydrolysis of hazelnut husk without any separate physico-chemical pretreatment steps. To the best of our knowledge, this is the first study to report on hazelnut husk hydrolysis in the absence of pretreatment procedures.
Additionally, since lignocellulosic biomass can be utilized as a raw material for the production of enzymes required for the co-enzymatic hydrolysis, the potentiality of hazelnut husk as a substrate (carbon source) for enzyme production has been also investigated. Quiroz-Castañeda et al. (2011) studied the production of enzymes by using oak and cedar sawdust, rice husk, corn stubble, wheat straw and jatropha seed husk as raw materials. Similarly, Birhanlı and Yeşilada (2013) have utilized lignocellulosic wastes such as walnut shells, corncob, pulverized apricot seed shells, and pulverized bulrush for laccase production. Additionally, Sommer et al. (2004) and Akpınar and Urek (2014) used agricultural residues to produce cellulases, xylanases and laccases from white rot fungi. Pycnoporus sanguineus belongs to the white rot fungal group of Basidiomycetes and it has an ability to grow on lignocellulosic substrates to produce cellulases, xylanases and laccases. Guney (2013) also suggested that conversion technologies should be investigated to evaluate hazelnut husk as a promising source of biomass. The evaluation of hazelnut husk as a carbon source for the production of enzyme mixtures has not been investigated. Hence, the results obtained in this work will also help to develop bioprocesses using hazelnut husk both as a lignocellulosic feedstock for fermentable sugar production and a substrate for lignocellulolytic enzyme production possibly paving the way towards consolidated bioprocessing.
Materials and methods
Materials and fungus strain
Hazelnut husk was supplied by the growers in northern region of Turkey. Chemicals used in this work were supplied by Merck AG (Darmstadt, Germany) and Sigma Chem. Ltd. (USA). Laccase (Novozyme 51003; 348.89 U/mL, 9.36 mg/mL protein) and cellulase [Novozyme 188; 57.36 U/mL (CMCase), 148.80 U/mL (β-glucosidase) and 2.25 U/mL [filter paper activity (FPase)], 44.04 mg/mL protein] were kindly supplied from the representative of Novozyme in Turkey. The fungal strain P. sanguineus DSM 3024 was obtained from the German Collections of Microorganisms and Cell Cultures (Leibniz Institute DSMZ), Braunschweig, Germany.
Hazelnut husks were milled for size reduction by a manually operated kitchen grinder. Final sample size was approximately 5 × 5 mm square particles and 5 mm strips after grinding. Ground samples were washed twice with tap water and once with distilled water and then were cloth filtered. The solid samples were dried at 60 °C overnight until constant weight was achieved and were used as substrate for further studies.
Enzymatic hydrolysis of hazelnut husks
The hydrolysis of hazelnut husks was performed by two enzymatic methods: (1) the sample was hydrolyzed by using cellulase only (Novozyme 188) and (2) the sample was co-enzymatically hydrolyzed by using cellulase (Novozyme 188) and laccase (Novozyme 51003), simultaneously. Since commercial enzymes contain additives and these additives interact with the reagents used in DNS method so, the enzymes were dialyzed before the hydrolysis process against appropriate buffers. The amount of hazelnut husk (as feedstock) was varied from 4 to 80 g/L (4, 12, 20, 40 and 80 g/L) with a constant amount of cellulase (100 μL) in each experiment to achieve the desired enzyme loadings (22.5, 7.5, 4.5, 2.75 and 1.125 FPU/g substrate, respectively). For the co-enzymatic hydrolysis reactions, a volumetrically equivalent (1:1 v/v for cellulase to laccase) amount of laccase (which yields an activity of 29.66 U/mL) was also added to the reaction medium. On a separate set of experiments, various cellulase to laccase loading ratios were tested (31, 131, 232 and 310 laccase U/FPU) in order to elucidate the effect of enzyme loadings ratio on co-enzymatic hydrolysis.
Temperature and pH were kept constant at 45 °C and pH 4.5 as these are the reported optimal and stable conditions for Novozyme 188 (Samaratunga et al. 2015). All the reactions were run up to 4 h by which time total reducing sugars concentrations in the hydrolysates had stabilized. These parameters were maintained constant throughout all experiments. After hydrolysis, the solid part was removed and the supernatant was further analyzed. Sugar concentration was expressed as g/L, gram of entity per liter of total reaction mixture. Experiments were performed in triplicate and the mean values were used in all data shown.
Analytical methods used for determination of reducing sugars and total sugar
Reducing sugars in the hydrolysate were determined by the DNS method (Miller 1959) at 540 nm with d-glucose as the standard and by high-pressure liquid chromatography (HPLC, Agilent Technologies USA) with a refractive index detector, according to the method described by Khaw and Ariff (2009). The total sugar concentration was determined by using the method described by Taylor (1995).
Determination of enzyme activity
CMCase, FPase, β-glucosidase and laccase activities were determined using the methods proposed by Ghose (1987), Gilna and Khaleel (2011) and Garcia et al. (2006), respectively. One unit (CMCase U) of CMCase activity is defined as the amount of enzyme required to liberate 1 µmol of glucose from CMC/min at 45 °C and pH 4.5. One unit (FPU) of FPase activity is defined as the amount of enzyme required to liberate the equivalent of 1 μmol of reducing sugars/min at 45 °C and pH 4.5. One unit (β-glucosidase U) of β-glucosidase activity was expressed as the amount of enzyme required to release 1 µmol of para-nitrophenol/min at 45 °C and pH 4.5. One unit (laccase U) of laccase activity was defined as the amount of enzyme required to oxidize 1 µmol of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)/min at 45 °C and pH 4.5. Protein concentrations of laccase and cellulases were determined using the Bradford assay with BSA as a standard (Bradford 1976). The selected temperature and pH values for the enzyme activity assays were the same as the enzymatic hydrolysis conditions.
Fermentation medium, mycelia suspension preparation and enzyme production
The composition of the fermentation medium used included the following: 1.5, 6, 12, 18 g/L hazelnut husk, 10 g/L yeast extract, and 10 g/L malt extract at pH 7.0. Mycelia suspension was prepared by the method described by Teoh and Mashitah (2010). Mycelia suspension (10 mL) was added to 90 mL fermentation medium in 250 mL Erlenmeyer flasks. After the culture was incubated in an incubator shaker at 30 °C and 180 rpm for 10 days, the culture fluid was harvested and centrifuged at 4000 rpm for 20 min. The supernatant was concentrated with ultrafiltration using Amicon Ultra-15 Centrifugal Filter Unit with Ultracel-10 membrane (Millipore) tubes. The concentrated supernatant was used to measure the activities of cellulase and laccase enzymes.
Results and discussion
Enzymatic hydrolysis of hazelnut husk by simultaneous treatment of cellulase and laccase
In the present work, we investigated the enzymatic hydrolysis of hazelnut husk using cellulase only and co-enzymatic hydrolysis using cellulase with laccase simultaneously to evaluate how the combination of enzymes performs lignocellulosic biomass degradation. Enzymatic hydrolysis of hazelnut husk was performed in acetate buffer (100 mM, pH 4.5) by using two commercial enzyme preparations Novozyme 188 (cellobiase-rich cellulase from Aspergillus niger) and Novozyme 51003 (a commercial laccase from Myceliophthora thermophila). The main difficulty in using a commercial enzyme is the lack of characterization of the enzymes in the mixture (Dien et al. 2008; Khaw and Ariff 2009; Pryor and Nahar 2010). In order to clarify this point, we also determined the FPU, β-glucosidase and CMCase activities in Novozyme 188. For the removal of enzyme additives, Novozyme 188 was dialyzed against 100 mM acetate buffer at pH 4.5. Enzyme activities of CMCase, β-glucosidase and FPase found in Novozyme 188 were 57.36 CMCase U/mL, 148.8 β-glucosidase U/mL and 2.25 FPU/mL, respectively.
In the literature, it is noted that the key parameters for optimal efficiency of enzymatic hydrolysis processes are optimal enzyme and substrate loadings. Andersen et al. (2008) proposed that at low enzyme loading synergy studies, cellulases and endoglucanases are the most important enzymes for enzymatic hydrolysis of cellulose. It is also noted in the literature that in such studies, β-glucosidase is added in excess quantities without any regards to optimal ratio (Van Dyk and Pletschke 2012). According to these explanations, we used Novozyme 188 as the cellulase enzyme since the endoglucanase content in Novozyme 188 is higher compared to other enzymes in the preparation. According to Sun and Cheng (2002), cellulase loadings of 7–33 FPU/g substrate is generally used to investigate the synergistic relationship between enzymes. Thus, in this work, enzyme loadings between 1.125 and 22.5 FPU/g substrate (by varying substrate concentration while keeping enzyme concentration constant) were used in the hydrolysis reactions.
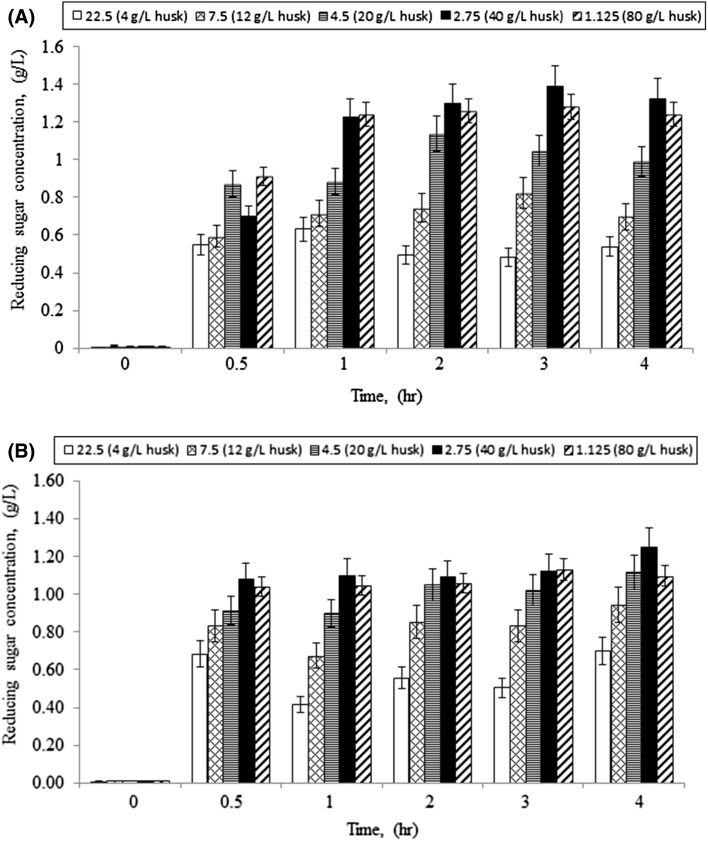
Figure 1a and b show the time-dependent hydrolysis profiles of hazelnut husk by cellulase-only and cellulase–laccase co-enzymatic reactions, respectively. Different substrate loadings ranging from 1.125 FPU/g substrate (80 g/L husk) to 22.5 FPU/g substrate (4 g/L husk) were achieved by using a constant cellulase concentration in all experiments with appropriate substrate concentrations. In all experiments, a volumetrically equivalent (1:1 v/v) amount of laccase (29.66 U/mL) was used. As seen from Fig. 1a, b, the hydrolysis of hazelnut husk followed a typical trend of cellulose hydrolysis both in the presence and absence of laccase. In the initial logarithmic phase, reducing sugars were released rapidly, and then rate of sugar production declined as the reaction proceeded. Reducing sugars concentration increased steadily with increasing time and tended to reach its final value of 1.32 ± 0.11 g/L after 4 h when cellulase was used alone. We obtained the highest reducing sugars concentration by using 2.75 FPU/g substrate (40 g/L substrate) enzyme loading which was lower than generally reported enzyme loading values (Nanda et al. 2014). Increasing substrate concentration from 40 to 80 g/L caused a slight decrease in final reducing sugars concentration. As a result of simultaneous laccase and cellulase treatment, the reducing sugars concentration remained comparable, although with a slight decrease from 1.32 ± 0.11 to 1.25 ± 0.06 g/L as seen in Fig. 1. This observation can be due to the mechanism whereby laccase present in the system degrades lignin and some of the cellulase enzymes un-specifically adsorb onto the degraded lignin thus decreasing overall hydrolytic efficiency.
Fig. 1.
Time profile of hazelnut husk hydrolysis a using cellulase and b using cellulase and laccase together at different enzyme loadings (FPU/g substrate, condition: S 0 = 4–80 g/L, 45 °C, pH 4.5)
Teoh and Don (2011) investigated the hydrolysis of pressed pericarp fibers (PPFs) using cellulases as CMCase, FPase, β-glucosidase and laccase simultaneously. They obtained a sugar content of 0.111 g/L using 70% (v/v) palm oil mill effluent solid and 60 g/L non-sterilized PPFs. Based on the solid lignocellulosic biomass, our result of 1.25 ± 0.06 g/L from 40 g/L hazelnut husk was higher than that of obtained by Teoh and Don (2011). Compared to the results obtained by Khaw and Ariff (2009), a similar reaction time profile was observed for hazelnut husk hydrolysis.
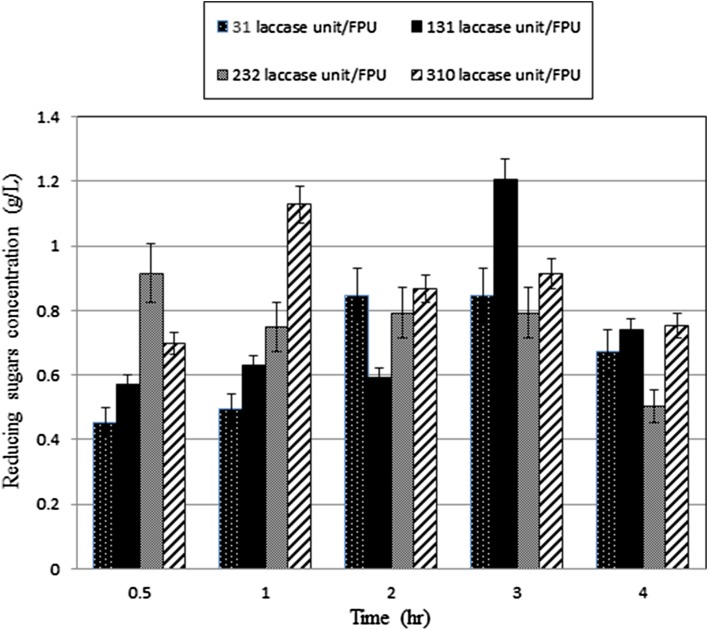
Another important aspect of co-enzymatic systems is the ratio of the enzymes used. In order to investigate this issue, laccase to cellulase ratios of 31, 131, 232 and 310 laccase U/FPU were used. Figure 2 shows the effect of laccase loadings on hazelnut husk hydrolysis. Maximum reducing sugars concentration was achieved with a ratio of 131 laccase U/FPU. For higher laccase loadings, reducing sugars concentration decreased. Maximum reducing sugars concentration of 1.21 ± 0.06 was obtained with 131 laccase U/FPU (1 mL laccase/mL cellulase). The results indicated that the ratio of laccase to cellulase at 131 laccase U/FPU enhance reducing sugars production since laccase could oxidize the aromatic ring of lignin and promote the formation of micropores on hazelnut husk to enhance the accessibility of cellulose. However, higher laccase loadings may lead to inhibitory effects of oxidizing products which results in the poor utilization of substrate (Gamble et al. 2000).
Fig. 2.
Effect of laccase loading on hazelnut husk hydrolysis
Determination of reducing sugars composition in hazelnut husk hydrolysate
An important step to control the bioconversion of lignocellulose to reducing sugars is the determination of monosaccharides including hexoses and pentoses in the complex hydrolysate medium (Andersen et al. 2008). In the present work, reducing sugars produced from hazelnut husk by cellulase-only and cellulase–laccase co-enzymatic treatments were determined using HPLC (Li et al. 2013). Since the highest reducing sugars concentration was obtained at 2.75 FPU/g substrate, monosaccharide composition of hazelnut husk hydrolysate was determined by using the supernatant obtained with 2.75 FPU/g substrate (40 g/L hazelnut husk). As illustrated in Table 1, the monosaccharides in the hazelnut husk hydrolysates are rhamnose, xylose, arabinose, mannose, glucose and galactose.
Table 1.
% Composition of reducing sugars (retention time of reducing sugars in hazelnut husk analysed by ZORBAX carbohydrate analysis column [rhamnose (3.68), xylose (4.23), arabinose (4.54), mannose (4.94), glucose (5.23), galactose (5.51)]) in hazelnut husk hydrolysate at the end of hydrolysis in the presence and absence of laccase at 2.75 FPU/g substrate enzyme loading
| Glucose (%) | Xylose (%) | Arabinose (%) | Galactose (%) | Mannose (%) | Rhamnose (%) | |
|---|---|---|---|---|---|---|
| Without laccase | < 10 | 36.54 | < 10 | 44.89 | 11.27 | < 10 |
| With laccase | 50.17 | 14.07 | < 10 | < 10 | 30.56 | < 10 |
When hazelnut husk was treated with cellulase and laccase simultaneously, glucose content of the total reducing sugars concentration reached its maximum of 50.17% (0.627 g/L) at the end of the hydrolysis period (Table 1). This result shows that laccase addition results in a very significant increase in glucose content of the final reducing sugars concentration since the glucose content of the cellulase-only hydrolysis is less than 10% of the final reducing sugars concentration. Similar results were obtained by Moreno et al. (2016) for the combination of alkali extraction and laccase treatment. Using a bacterial laccase together with alkali extraction, they enhanced enzymatic hydrolysis and concentrations of both glucose and xylose were increased by 21 and 30%, respectively (Moreno et al. 2016). When the sugar compositions were compared, the monosaccharides (arabinose, mannose, glucose and xylose) found in enzymatically hydrolyzed hazelnut husk were similar to those of the hazelnut husk hydrolyzed with acid (Ceylan and Ünal 2015). Besides acid hydrolysis, Çöpür et al. (2013) investigated the effect of different pretreatment techniques on enzymatic hydrolysis of hazelnut husk. Regarding the presence of glucose and xylose, predominant mono sugars in hydrolysates, results obtained in the present work are similar with the results reported by Çöpür et al. (2013) and Ceylan and Ünal (2015). However, glucose concentration (0.627 g/L) obtained in this work was one fifth of the glucose concentration (3.13 g/L) reported by Çöpür et al. (2013). The significance of the results obtained in this work is the fact that co-enzymatic hydrolysis of hazelnut husk by using cellulase and laccase simultaneously without a separate pretreatment process boosts the glucose content of the final reducing sugars concentration.
The reason behind this observation may be that when laccase is not present and lignin fractions are not degraded to expose the cellulose fraction, cellulase enzymes act predominantly on the hemicellulosic fractions of the lignocellulosic substrate and release the monosaccharides that make up that hemicellulosic part. On the other hand, when laccase is present and more of the cellulose is exposed and accessible to the cellulase enzymes, these enzymes can hydrolase the cellulose releasing more glucose compared to other monosaccharides. There is a very complex web of interactions between the kinetics of adsorption and hydrolysis of the cellulosic, hemicellulosic and lignin parts of the substrate and the released poly- and monosaccharides which needs to be studies in greater detail to be able to elucidate the reaction mechanisms that are taking place.
Evaluation of hazelnut husk as a raw material for cultivation of P. sanguineus DSM 3024 to produce mixed enzymes
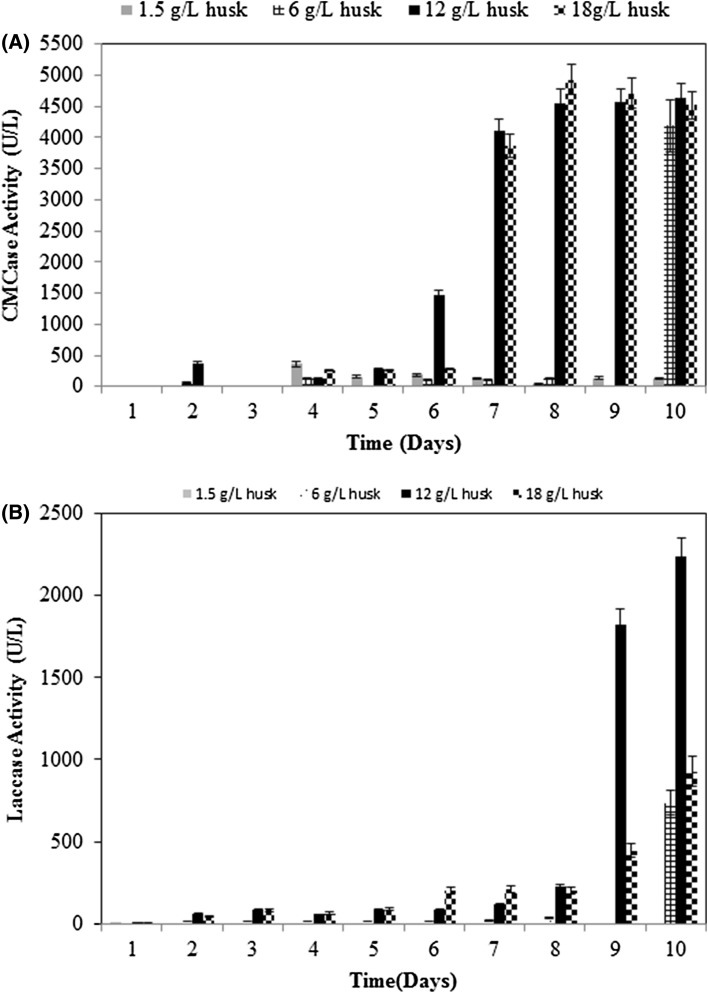
In the present work, hazelnut husk was also evaluated as a raw material (carbon source) to produce lignocellulolytic enzymes (cellulases and laccase) from P. sanguineus DSM 3024. By the actions of its nonspecific extracellular enzymes, the white rot fungus P. sanguineus has a unique ability to completely hydrolyze lignin as well as polysaccharides found in lignocellulosic material (Vikineswary et al. 2006; Teoh and Mashitah 2010). Figure 3 demonstrates the time course profile of enzyme production by P. sanguineus, using hazelnut husk as the carbon source. β-Glucosidase activity, 216 ± 8 U/L (8.57 ± 0.32 U/mg) obtained from P. sanguineus by using hazelnut husk was 21-fold lower than CMCase activity. We did not detect any FPase activity in the fermentation medium. In addition to CMCase and β-glucosidase, the highest laccase activity, 2240 ± 98 U/L (8.89 ± 0.39 U/mg, Fig. 3b), was obtained using 12 g/L husk concentration on the 10th day of fermentation. The results showed that P. sanguineus produced laccase and cellulase enzyme mixture that is capable of degrading cellulose and lignin found in hazelnut husk. Quiroz-Castañeda et al. (2011) studied the production of cellulolytic enzymes by P. sanguineus using lignocellulosic materials of different origin and composition. CMCase activities (IU/mg) were 2.73 for wheat straw, 0.72 for oak sawdust, 0.65 for wheat straw, 0.26 for corn stubble, 0.25 for jatropha seed husk, and 0.24 for rice husk. When the CMCase activity obtained in this work is compared with the results obtained by Quiroz-Castañeda et al. (2011), hazelnut husk can be considered a good inducer for the production of CMCase. In other words, hazelnut husk can be used as raw material for cellulase and laccase production. Moreover, depending on fermentation period, enzyme mixtures containing different percentages of laccase, CMCase and β-glucosidase can be obtained.
Fig. 3.
CMCase (a) and laccase (b) activity in liquid culture of Pycnoporus sanguineus DMSZ 3024 using hazelnut husk as a carbon source
Conclusions
In the present work, firstly, the potentiality of co-enzymatic hydrolysis of hazelnut husk without a separate pretreatment step; and secondly, the production of lignocellulolytic enzymes using hazelnut husk as a raw material were investigated. When cellulase is used alone for the hydrolysis of hazelnut husk the glucose content of the resulting reducing sugars concentration was found to be around 20%. The addition of laccase to the enzyme mixture resulted in the possible delignification of the lignocellulosic substrate increasing the accessibility of the cellulose fractions and thus, the glucose content of the final reducing sugars concentration reached about 50%. Through these findings, we have shown the hydrolysis of hazelnut husk by enzyme mixtures without using any pretreatment processes. Even though reducing sugars concentration obtained in this work did not quite reach the values reported for hydrolysis using pretreatment processes, all of these values can potentially be increased to effective levels by scale-up and further optimization studies.
Furthermore, with the utilization of hazelnut husk as a carbon source by P. sanguineus, we showed that hazelnut husk may have a promising future as a feed stock for the production of valuable biochemical products. The results obtained in this work will help to develop bioprocesses to generate value added chemicals and pave the way towards consolidated bioprocessing.
Acknowledgements
This work was supported by The Scientific and Technological Council of Turkey (TUBITAK-MAG) by the Project No. 113M053.
Compliance with ethical standards
Conflict of interest
No conflict of interest was declared.
References
- Akpınar M, Urek RO. Extracellular ligninolytic enzymes production by Pleurotus eryngii on agroindustrial wastes. Prep Biochem Biotechnol. 2014;44:772–781. doi: 10.1080/10826068.2013.867870. [DOI] [PubMed] [Google Scholar]
- Alvarez C, Reyes-Sosa FM, Diez B. Enzymatic hydrolysis of biomass from wood. Microb Biotechnol. 2016;9:149–156. doi: 10.1111/1751-7915.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen N, Johansen KS, Michelsen M, Stenby EH, Krogh KB, Olsson L. Hydrolysis of cellulose using mono-component enzymes shows synergy during hydrolysis of phosphoric acid swollen cellulose (PASC), but competition on Avicel. Enzym Microb Technol. 2008;42:362–370. doi: 10.1016/j.enzmictec.2007.11.018. [DOI] [Google Scholar]
- Birhanlı E, Yeşilada Ö. The utilization of lignocellulosic waste for laccase production under semi-solid and submerged fermentation conditions. Turk J Biol. 2013;37:450–456. doi: 10.3906/biy-1211-25. [DOI] [Google Scholar]
- Boonmee A. Hydrolysis of various thai agricultural biomasses using the crude enzyme from Aspergillus aculeatus Iızuka Fr60 isolated from soil. Braz J Microbiol. 2012;43:456–466. doi: 10.1590/S1517-83822012000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camarero S, Ibarra D, Martínez ÁT, Romero J, Gutiérrez A, del Río JC. Paper pulp delignification using laccase and natural mediators. Enzyme Microb Techno. 2007;40(5):1264–1271. doi: 10.1016/j.enzmictec.2006.09.016. [DOI] [Google Scholar]
- Ceylan S, Ünal S. The saccharification of hazelnut husks to produce bioethanol. Energy Source A. 2015;37:972–979. doi: 10.1080/15567036.2011.601794. [DOI] [Google Scholar]
- Chaturverdi V, Verma P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech. 2013;3:415–431. doi: 10.1007/s13205-013-0167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çöpür Y, Tozluoğlu A, Özkan M. Evaluating pretreatment techniques for converting hazelnut husks to bioethanol. Bioresour Technol. 2013;129:182–190. doi: 10.1016/j.biortech.2012.11.058. [DOI] [PubMed] [Google Scholar]
- Çöpür Y, Güler C, Akgül M, Taşçıoğlu C. Some chemical properties of hazelnut husk and its suitability for particleboard production. Build Environ. 2007;47:2568–2572. doi: 10.1016/j.buildenv.2006.07.011. [DOI] [Google Scholar]
- Dien BS, Ximenes EA, O’Bryan BJ, Moniruzzaman M, Li XL, Balan V, Dale B, Cotta MA. Enzyme characterization for hydrolysis of APEX and liquid hot-water pretreated distillers’ grains and their conversion to ethanol. Bioresour Technol. 2008;99:5216–5225. doi: 10.1016/j.biortech.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Fontes CM, Gilbert HJ. Cellulosomes: highly efficient nanomachines designed to de-construct plant cell wall complex carbohydrates. Annu Rev Biochem. 2010;79:655–681. doi: 10.1146/annurev-biochem-091208-085603. [DOI] [PubMed] [Google Scholar]
- Gamble GR, Snook ME, Henriksson G, Akin DE. Phenolic constituents in flax bast tissue and inhibition of cellulase and pectinase. Biotechnol Lett. 2000;22:741–746. doi: 10.1023/A:1005608304142. [DOI] [Google Scholar]
- Garcia TA, Santiago MF, Ulhoa CJ. Properties of laccases produced by Pycnoporus sanguineus induced by 2,5-xylidine. Biotechnol Lett. 2006;28:633–636. doi: 10.1007/s10529-006-0026-3. [DOI] [PubMed] [Google Scholar]
- Ghobadian B, Rahimi H, Hashjin TT, Khatamifar M. Production of bioethanol and sunflower methyl ester and investigation of fuel blend properties. J Agric Sci Technol. 2008;10:225–232. [Google Scholar]
- Ghose TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59:257–268. [Google Scholar]
- Gilna VV, Khaleel KM. Cellulase enzyme activity of Aspergillus fumigatus from mangrove soil on lignocellulosic substrate. Recent Res Sci Technol. 2011;3:132–134. [Google Scholar]
- Guerriero G, Hausman J-F, Strauss J, Ertan H, Siddiqui KS. Lignocellulosic biomass: biosynthesis, degradation, and industrial utilization. Eng Life Sci. 2016;16:1–16. doi: 10.1002/elsc.201400196. [DOI] [Google Scholar]
- Guney MS. Utilization of hazelnut husk as biomass. Sustain Energy Technol Assess. 2013;4:72–77. [Google Scholar]
- Haldar H, Se D, Gayen K. A review on the production of fermentable sugars from lignocellulosic biomass through conventional and enzymatic route—a comparison. Int J Green Energy. 2016;13:972–979. doi: 10.1080/15435075.2016.1181075. [DOI] [Google Scholar]
- Hashem M, Ali EH, Abdel-Basset R. Recycling rice straw into biofuel “Ethanol” by Saccharomyces cerevisiae and Pichia guilliermondii. J Agric Sci Technol. 2013;15:709–721. [Google Scholar]
- Khare SK, Pandey A, Larroche C. Current perspectives in enzymatic saccharification of lignocellulosic biomass. Biochem Eng J. 2015;102:38–44. doi: 10.1016/j.bej.2015.02.033. [DOI] [Google Scholar]
- Khaw TS, Ariff AB. Optimization of enzymatic saccharification of palm mill effluent solid and oil palm fruit fibre to fermentable sugar. J Trop Agric Food Sci. 2009;37:85–94. [Google Scholar]
- Kumar R, Meisam T, Karimi K, Horvath IS. Recent updates on lignocellulosic biomass derived ethanol—a review. Biofuel Res J. 2016;9:347–356. doi: 10.18331/BRJ2016.3.1.4. [DOI] [Google Scholar]
- Li H, Long C, Zhou J. Rapid analysis of mono-saccharides and oligo-saccharides in hydrolysates of lignocellulosic biomass by HPLC. Biotechnol Lett. 2013;35:1405–1409. doi: 10.1007/s10529-013-1224-4. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Mohanram S, Amat D, Choudhary J, Arora A, Nain L. Novel perspectives for evolving enzyme cocktails for lignocellulose hydrolysis in biorefineries. Sustain Chem Process. 2013;1:1–15. doi: 10.1186/2043-7129-1-15. [DOI] [Google Scholar]
- Moreno AD, Ibarra D, Mialon A, Ballesteros M. A bacterial laccase for enhancing saccharification and ethanol fermentation of stream-pretreated biomass. Fermentation. 2016;11:1–15. [Google Scholar]
- Nanda S, Dalai AK, Kozinski JA. Butanol and ethanol production from lignocellulosic feedstock: biomass pretreatment and bioconversion. Energy Sci Eng. 2014;2:138–148. doi: 10.1002/ese3.41. [DOI] [Google Scholar]
- Nieves DC, Ruiz HA, de Carnedas LZ, Alvarez GM, Aguilar CN, Ilyina A, Hernandez JLM. Enzymatic hydrolysis of chemically pretreated mango stem bark residues at high solid loading. Ind Crops Prod. 2016;83:500–508. doi: 10.1016/j.indcrop.2015.12.079. [DOI] [Google Scholar]
- Pal S, Joy S, Trimukhe KD, Kumbhar PS, Varma AJ, Padmanabhan S. Pretreatment and enzymatic process modification strategies to improve efficiency of sugar production from sugarcane bagasse. 3 Biotech. 2016;6:1–14. doi: 10.1007/s13205-016-0446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor SW, Nahar N. Deficiency of cellulose activity measurements for enzyme evaluation. Appl Biochem Biotechnol. 2010;162:1737–1750. doi: 10.1007/s12010-010-8955-7. [DOI] [PubMed] [Google Scholar]
- Quiroz-Castañeda RE, Pérez-Mejía N, Martínez-Anaya C, Acosta-Urdapilleta L, Folch-Mallol J. Evaluation of different lignocellulosic substrates for the production of cellulases and xylanases by the basidiomycete fungi Bjerkandera adusta and Pycnoporus sanguineus. Biodegradation. 2011;22:565–572. doi: 10.1007/s10532-010-9428-y. [DOI] [PubMed] [Google Scholar]
- Rico A, Rencoret J, del Río JC, Martínez AT, Gutiérrez A. Pretreatment with laccase and a phenolic mediator degrades lignin and enhances saccharification of Eucalyptus feedstock. Biotechnol Biofuels. 2014;7:1–14. doi: 10.1186/1754-6834-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaratunga A, Kudina O, Nahar N, et al. Modeling the effect of pH and temperature for cellulases immobilized on enzymogel nanoparticles. Appl Biochem Biotechnol. 2015;176(4):1114–1130. doi: 10.1007/s12010-015-1633-z. [DOI] [PubMed] [Google Scholar]
- Sathitsuksanoh N, George A, Zhang YHP. New lignocellulose pretreatments using cellulose solvents: a review. J Chem Technol Biotechnol. 2012;88:169–180. doi: 10.1002/jctb.3959. [DOI] [Google Scholar]
- Sharma SK, Kaira KL, Grewal HS. Enzymatic saccharification of pretreated sunflower stalks. Biomass Bioenergy. 2002;23:237–243. doi: 10.1016/S0961-9534(02)00050-8. [DOI] [Google Scholar]
- Sindhu R, Kuttiraja M, Binod P, Janu KU, Sukumaran RK, Pandey A. Dilute acid pretreatment and enzymatic saccharification of sugarcane tops for bioethanol production. Bioresour Technol. 2011;102:10915–10921. doi: 10.1016/j.biortech.2011.09.066. [DOI] [PubMed] [Google Scholar]
- Sindhu R, Binod P, Pandey A. Biological pretreatment of lignocellulosic biomass—an overview. Bioresour Technol. 2016;199:76–82. doi: 10.1016/j.biortech.2015.08.030. [DOI] [PubMed] [Google Scholar]
- Sommer P, Georgieva T, Ahring BK. Potential for using thermophilic anaerobic bacteria for bioethanol production from hemicellulose. Biochem Soc Trans. 2004;32:283–289. doi: 10.1042/bst0320283. [DOI] [PubMed] [Google Scholar]
- Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83:1–11. doi: 10.1016/S0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Taylor KACC. A modification of the phenol/sulfuric acid assay for total carbohydrates giving more comparable absorbances. Appl Biochem Biotechnol. 1995;53:207–214. doi: 10.1007/BF02783496. [DOI] [Google Scholar]
- Teoh YP, Don MM. Kinetic model for the hydrolysis of sterilized palm press fibre. Chem Eng Sci. 2011;66:3523–3530. doi: 10.1016/j.ces.2011.04.011. [DOI] [Google Scholar]
- Teoh YP, Mashitah MD. Cellulase production by Pycnoporus sanguineus on oil palm residues through pretreatment and optimization study. J Appl Sci. 2010;10:1036–1043. doi: 10.3923/jas.2010.1036.1043. [DOI] [Google Scholar]
- Tozluoğlu A. The effects of boron derivatives as pretreatment chemicals for biodegradation of sunflower stalks. Maderas Cienc Tecnol. 2016;18(4):663–676. [Google Scholar]
- Van Dyk JS, Pletschke BI. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—factors affecting enzymes, conversion and synergy. Biotechnol Adv. 2012;30:1458–1480. doi: 10.1016/j.biotechadv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Vikineswary S, Abdullah N, Renuvathani M, Sekaran M, Pandey A, Jones EBG. Productivity of laccase in solid substrate fermentation of selected agro-residues by Pycnoporus sanguineus. Bioresour Technol. 2006;97:171–177. doi: 10.1016/j.biortech.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Weiss ND, Börjesson J, Pedersen LS, Meyer AS. Enzymatic lignocellulose hydrolysis: improved cellulase productivity by insoluble solids recycling. Biotechnol Biofuels. 2013;6:1–14. doi: 10.1186/1754-6834-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolridge EM. Mixed enzyme systems for delignification of lignocellulosic biomass. Catalysts. 2014;4:1–35. doi: 10.3390/catal4010001. [DOI] [Google Scholar]