Abstract
Purpose
The aim of this study was to determine if zona pellucida thickness variation (ZPTV) is associated with implantation and if this relationship changes with use of assisted hatching (AH).
Methods
Day 3 embryos from single or double embryo transfers (DETs) performed between 2014 and 2016 were included. ZPTV was assessed by examining photographs taken before transfer using an automated image processing platform to segment the zona pellucida (ZP) with an active contour technique. One hundred points were obtained of ZP thickness (ZPT) of each embryo to calculate ZPTV ([maximum ZPT–mean ZPT]/mean ZPT). Logistic regression was used to calculate the odds ratio (OR) and 95% confidence intervals (CI) of implantation by tertile of ZPTV. Maternal age and AH were adjusted for a priori. Other cycle and embryo characteristics were adjusted for if they altered the continuous effect estimate by >10%.
Results
There was no statistically significant association between ZPTV and implantation across tertiles although embryos with greater ZPTV showed a trend of decreased implantation (Tertile 2 (T2) versus Tertile 1 (T1), OR = 0.80, CI = 0.50–1.28; Tertile 3 (T3) versus Tertile 1 (T3), OR = 0.75, CI = 0.47–1.20). While similar nonsignificant trends for the association between ZPTV and implantation were observed across tertiles after stratification of embryos hatched or not, embryos with the greatest ZPTV had slightly higher odds for implantation when AH was utilized (T3 vs. T1: with AH, OR = 0.89, CI = 0.49–1.62; without AH, OR = 0.61, 0.29–1.27).
Conclusion
ZPTV was not associated with implantation after day 3 transfer. This finding did not vary by use of AH.
Keywords: Zona pellucida thickness, Zona pellucida thickness variation, Assisted hatching, Cleavage stage, Implantation rate, Zona hardening
Introduction
Hatching of the embryo from the zona pellucida (ZP) is a critical step for implantation [1, 2]. The ZP begins to form during early folliculogenesis and is comprised, in the human, of four glycoprotein layers (ZP1–ZP4), which are secreted by oocytes and granulosa cells and are involved in support of the ova [3, 4]. The ZP is involved in induction of the acrosomal reaction and promotes sperm-egg fusion. After fertilization, a measurable hardening of the ZP takes place that is thought to be important for (1) polyspermy blockage, (2) protection of the developing embryo, and (3) oviductal transport [3, 4].
During preimplantation development, the ZP undergoes gradual thinning that, at least after blastulation, is presumably due to increasing pressure, which has been postulated to involve a “stretching mechanism” [5] and embryonic production of glycoproteins to reduce the thickness of the zona in preparation for blastocyst hatching [6]. Interestingly, some embryos have more uniform zonae, while others have localized regions of thinness [6, 7]. Cohen et al. (1989) was the first to describe zona pellucida thickness variation (ZPTV) after analyzing videos of cleavage stage embryos and found that those embryos with >25% ZPTV were more likely to implant [6]. More recent studies have also investigated ZPTV of cleavage stage embryos and have similarly reported higher implantation rates with ZPTV >20 and >25% [8, 9]. Moreover, Gabrielsen et al. (2001) found that ZPTV assessment was additive in the morphological grading and selection of poor quality cleavage stage embryos that led to clinical pregnancy [10]. Although these studies consistently found that greater ZPTV was associated with either implantation or clinical pregnancy, the analyses were performed with crude measurements of the ZP at only 3–4 points around the embryo and sample sizes were small, ranging from 86 to 255 embryos.
In addition to investigations on ZPTV, studies have also focused on developing the method of “assisted hatching” (AH) in an effort to bypass the proposed hardening of the ZP [6], thereby facilitating implantation. Assisted hatching is the process by which the ZP of a preimplantation embryo is artificially opened in an attempt to facilitate the natural “hatching” of the blastocyst in preparation for implantation. Initial studies revealed that embryos with a ZP thickness (ZPT) > 15 μm had higher implantation rates when AH was applied [11]. Furthermore, poor prognosis patients with elevated basal FSH were noted to have embryos with thin zona “that were difficult to pierce” and for which implantation was improved by AH [11, 12]. Subsequent human and animal studies also reported that a greater ZP thickness (ZPT) was associated with lower implantation rates [13, 14]. However, others failed to find such a significant association between ZPT and implantation [2, 9]. Notably, a 2010 study randomly assigned embryos from women aged <38 years with a ZP >13 μm to AH or no AH and found no statistical difference in implantation rate, clinical pregnancy rate or live birth rate [15]. However, this study did not investigate the relationship between ZPTV and clinical outcome.
Despite the high use of AH in IVF (in the USA, utilization is reported as over 50% in fresh autologous IVF/ICSI day 3 embryo transfers [16]), the evidence in support of its benefit is weak at best. Furthermore, the procedure is associated with negative side effects including an increased risk of monozygotic and monochorionic twinning, leading to increased risks of fetal and maternal morbidity [17–21]. While an earlier meta-analysis suggested greater pregnancy rates in patients with repeat implantation failure [22, 23] who used AH, a recent analysis of over 750,000 cycles found no such benefit either in the general patient population or in a subanalysis restricted to repeat implantation failure patients [16]. Moreover, more recent studies [24, 25] found no evidence for improvement in likelihood of live birth following transfer of hatched embryos. A Cochrane review that included 31 studies reached the same conclusion [22]. However, again, none of these studies investigated the relationship between ZPTV and use of AH on clinical outcome.
This study was designed to test the hypothesis that ZPTV is an independent predictor of implantation and, given the relative lack of evidence in support of using AH in clinical practice, to determine whether AH improved implantation rate based on the degree of ZPTV. Additionally, the use of a more precise and detailed measurement of ZPTV allowed a more rigorous investigation of these proposed relationships.
Materials and methods
Institutional review board approval
This study was approved by the Institutional Review Board of Partner’s Healthcare, protocol #2014P002738.
Patient selection
This is a retrospective cohort study that included all autologous fresh day 3 embryo transfer cycles involving either single embryo transfers (SET) or double embryo transfers (DET) that were performed in women aged 24–44 years between March 2014 and April 2016 at Brigham and Women’s Hospital in Boston. The majority of embryos were from the first cycle for each patient; in a few cases, embryos came from a second or third cycle. Embryos were created by either in vitro fertilization or intracytoplasmic sperm injection (ICSI), and none were subjected to blastomere biopsy prior to transfer.
Ovarian stimulation protocols
Controlled ovarian hyperstimulation protocols using gonadotropin releasing hormone (GnRH) agonist, GnRH antagonists, or a poor responder protocol using very low GnRH agonist were utilized as previously described [26]. Follicular and serum estradiol (E2) monitoring was performed per standard protocol. After the two lead follicles were 17–18 mm in mean diameter, patients received the ovulatory trigger. Oocyte retrieval was performed 36 h after the trigger was administered.
Laboratory protocols
Gametes and embryos were incubated at a temperature of 37 °C in an atmosphere of 5% O2, 5% CO2, and 90% N2 [26]. Standard insemination or ICSI was performed within 4 to 6 h after retrieval. Assessment of fertilization was performed 16–18 h after insemination or ICSI, and zygotes having two pronuclei were cultured in 25 μl microdrops of Global medium (IVF Online, Guelph, Ontario, Canada) with 5% human serum albumin [26]. Day 3 embryo morphology was determined between hours 66 and 69 post-insemination/ICSI. The embryo quality defined as embryo score was based on blastomere number, degree of fragmentation, and extent of asymmetry [27]. Embryos were assigned a fragmentation score of 0, 1–9, 10–25, 26–50, or >50% and a symmetry score of perfect, moderate asymmetry, or severe asymmetry. All the embryos were then given a numerical score (1–18) using an internally generated grading system based on the above parameters.
Embryo transfer and luteal progesterone support
Embryo transfer was performed on day 3 under ultrasound guidance using a Wallace catheter (Marlow/Cooper Surgical, Shelton, CT, USA). Luteal progesterone supplementation was begun 2 days after retrieval with vaginal progesterone (8% Crinone; Watson Pharmaceuticals, Parsippany, NJ, USA) or 1 day after retrieval with intramuscular progesterone (50 mg dose) (locally compounded at one of two pharmacies: Village Fertility, Waltham, MA 02451 or Freedom Fertility, Byfield, MA 01922) and continued until 10 weeks in patients who became pregnant.
Assisted hatching
All hatching procedures were performed within 2 h of transfer by using a laser (Lykos, Hamilton Thorne, Beverley, MA) device to create an opening of approximately 20 μm. The location of hatching was chosen by embryologists to avoid direct contact with any blastomeres. Quality assurance standards required all embryologists performing AH to undergo training by the same lab director and quarterly competency assessments to ensure consistency in performance.
Consistent with the clinical protocols at our center, AH was performed on patients age ≥ 35 years undergoing IVF, or if they had failed at least one previous cycle (defined by no evidence of implantation) in our program. For ICSI cycles, AH was performed on patient’s age ≥ 38 years, or if they had failed at least one previous cycle in our program.
Data collection
The following variables were collected from our standard clinical electronic records for each cycle: Woman’s age at cycle start, body mass index (BMI), all infertility diagnoses, day 3 FSH, cycle attempt number, peak estradiol, number of oocytes retrieved, embryo score, and number of embryos transferred.
Clinical outcomes
Clinical outcomes were assessed per embryo transferred. The study population who failed to have a successful pregnancy was defined as: (1) not pregnant (negative serum hCG (<3 mIU/mL), (2) biochemical pregnancy (serum hCG ≥ 3 mIU/mL but with no intra- or extra-uterine sac visualized on transvaginal ultrasound), or (3) ectopic pregnancy (defined by extra-uterine pregnancy visualized on ultrasound). Failed implantation was defined as a negative serum hCG, a biochemical pregnancy, or an ectopic pregnancy. All clinical pregnancies (i.e., those with an intrauterine sac) were followed with primary outcomes defined by ultrasound visualization as: Sac = intrauterine sac at ≥5 weeks, Fetus 7+ = viable fetus at ≥7 weeks with positive fetal heart beat, and Fetus 24+ = viable fetus at ≥24 weeks with positive fetal heart beat (i.e., beyond the 2nd trimester).
Measurement of ZPTV
Immediately before transfer, all embryos were imaged in a random orientation that allowed a clear 2-D image of the embryos with blastomeres in focus using a Watec analog camera on an inverted microscope using Nomarski differential interference contrast microscopy (Olympus IX73P1F) with ×60 magnification. The images were archived using a Canon Selphy CP910 printer. For image processing, the printed images were scanned using an HP Epson Perfection V200 Photo scanner. Image processing software was developed in MATLAB to segment the ZP from the scanned images by using an active contour method [28, 29]. The software was programmed to exclude any artifact and clearly delineate the ZP. In the initial validation work of our software, measurements of the ZPTV were done multiple times to insure the same ZPTV was calculated for each embryo.
The software calculated the smoothed derivative of the original image I (x,y) as:
| 1 |
where G x(x, y) and G y(x, y) are the smoothed derivative of the image in x and y directions:
| 2 |
| 3 |
The summation in Equations 2 and 3 are over all pixels, and σ is the width of the smoothing Gaussian filter. Calculation of the normalized gradient image N(x, y) = G(x, y)/ max(G), where max(G) is the maximum value of G(x, y) was performed next. The inner and outer boundaries of the ZP were determined by a closed contour Γ, which minimizes the energy functional:
| 4 |
where . The first integral is over the length of the contour, and the second integral is over the area enclosed by the contour. For segmenting, the outer edge of the ZP, a negative value of β in Eq. 4, was chosen, and for segmenting the inner edge, a positive value was chosen. As an initial guess, segmentation started with a circle close to the outer edge of the ZP to minimize the energy in Eq. 4 to obtain the actual edge. The shape of the outer edge was used as the initial guess to segment the inner edge of the ZP. If necessary, surrounding artifacts, debris, and spermatozoa were removed from the image.
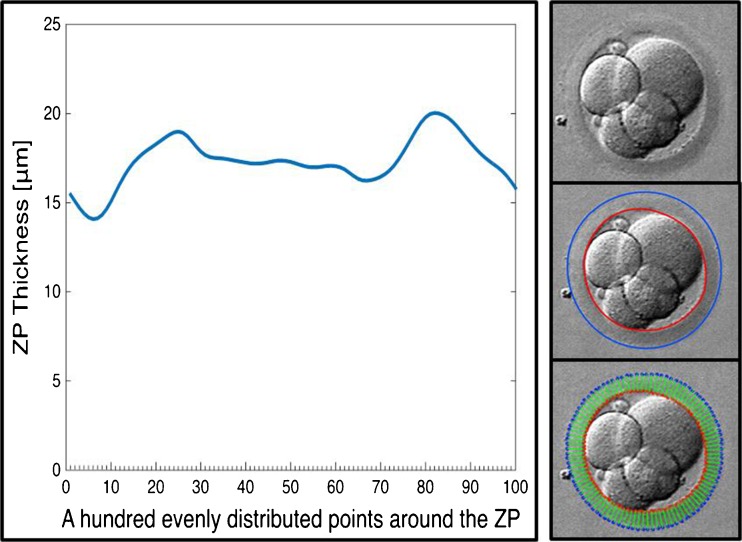
For the segmenting the ZP, the thickness D was measured for hundred equidistant points along the outer and inner edges. This was used to calculate the average thickness, ZPT, ZPTV, of the ZP as:
| 5 |
| 6 |
where ZPTmax is the maximum thickness of the zona pellucida (Fig. 1).
Fig. 1.
Segmention of the zona pellucida (ZP) with hundred equidistant points along the outer and inner edges to calculate average thickness, ZPT, and ZPTV
Statistical analysis
For the embryos with known developmental fate (n = 768), multivariable logistic regression was performed to calculate the odds ratio (OR) of implantation and 95% confidence intervals (CI) and two-sided Wald p values (p value) using generalized estimating equations to account for the correlation between multiple cycles from the same patient. ZPTV was assessed in tertiles (T) based on ZPTV distribution among all 768 embryos (T1 ≤15.0%, T2 >15.0 to <20.8%, T3 ≥20.8%) and also continuously for every 5% increase in ZPTV. Tertiles were chosen to allow sufficient power to provide numbers in each group as to not force an arbitrary dichotomy. Age and AH were adjusted for a priori, while other cycle characteristics (including day 3 FSH mIU/L, infertility diagnoses of ovulatory dysfunction or decreased ovarian reserve, cycle attempt number, and body mass index (BMI) and embryo characteristics (defined by a numerical scoring system of 1–18, in decreasing order of quality) were adjusted for when they changed the continuous effect estimate by at least 10%. Other cycle characteristics such as ICSI and the infertility diagnoses of endometriosis and male factor were tested and not found to alter the effect estimate by at least 10% and were not adjusted for [30]. To investigate whether the association between ZPTV and implantation differed between embryos with and without AH, analyses stratified embryos by AH or no AH and tested for interaction using likelihood ratio tests. Analyses were performed using Statistical Analysis Software version 9.3 (SAS Institute, Inc., Cary, NC.)
Results
Characteristics of embryos
The initial cohort included 957 embryos, 307 transferred singly, and 650 transferred in pairs. There were 172 embryos from DETs for which only one embryo implanted that were excluded from the implantation analyses, given that embryo-specific implantation data were unknown. Similarly, DETs, where a vanishing twin was noted, were excluded from the dataset. Seventeen embryos were unable to be analyzed due to an inability to assess the ZPTV accurately (5 from SETs and 12 from DETs). Failure to assess the ZPTV occurred if either the embryo was too close to the border of the photographic image or the two embryos in a DET were photographed too close together preventing accurate assessment of the ZP with our software. If one embryo from a DET was excluded, the other was also excluded. The final study cohort analyzed to quantify the relation between ZPTV and implantation comprised 768 embryos from 461 women (302 from SETs and 466 from DETs), all of which had known cycle outcomes.
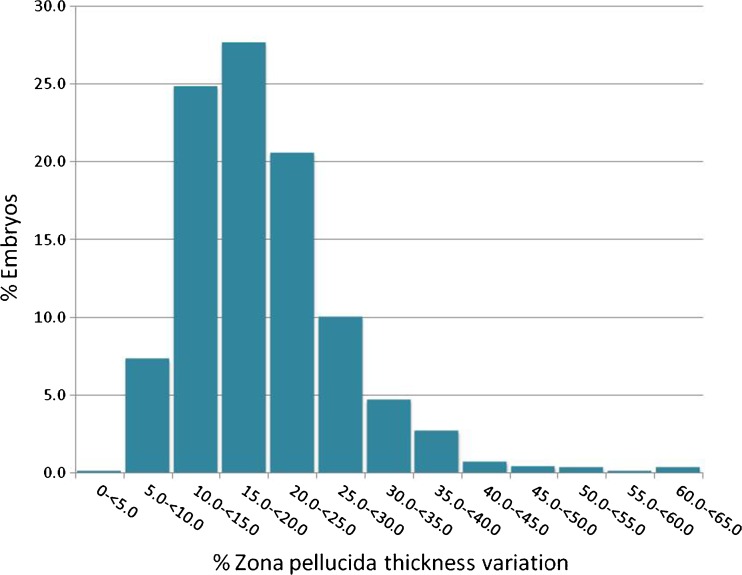
Mean ZPTV was 19.38 ± 7.98% (mean ± standard deviation), and median ZPTV was also 19.38 with a range of values between 3.99 and 64.30% (Fig. 2). Tertile cut points (33rd percentile) were: T1 <15.0% (n = 251), T2 >15.0% to <20.8% (n = 247), T3 ≥ 20.8% (n = 270). Among the tertiles, woman’s age at cycle start, infertility diagnosis, day 3 FSH, previous cycle attempt number, number of embryos transferred, and embryo numerical score were similar (Table 1).
Fig. 2.
Distribution of zona pellucida thickness variation (ZPTV) in embryos analyzed (n = 768)
Table 1.
Demographics and characteristics of IVF cycles for embryos (n = 768) stratified by zona pellucida thickness variation (ZPTV)
| ZPTV Tertile 1a
ZPTV ≤15.0% (n = 251) |
ZPTV Tertile 2 ZPTV >15.0 to <20.8% (n = 247) |
ZPTV Tertile 3 ZPTV ≥20.8% (n = 270) |
|
|---|---|---|---|
| Age (years) | 36.7 ± 3.9b | 36.4 ± 3.9 | 36.3 ± 4.2 |
| BMI (kg/m2) | |||
| <30 ≥30 |
199 (79.3)c
52 (20.7) |
187 (75.7) 60 (24.3) |
212 (78.8) 57 (21.2) |
| Infertility diagnosis | |||
| PCOS Male factor Other female factor Unexplained |
23 (9.2) 59 (23.5) 140 (55.8) 67 (26.7) |
25 (10.1) 62 (25.1) 140 (56.7) 69 (27.9) |
33(12.2) 64 (23.7) 129 (47.8) 83 (30.7) |
| Day 3 FSH (mIU/L) | 8.6 ± 3.2 | 8.9 ± 4.3 | 9.2 ± 4.5 |
| Previous cycle attempt number | 0.8 ± 1.2 | 1.0 ± 1.3 | 0.8 ± 1.1 |
| Number of embryos transferred | 1.6 ± 0.5 | 1.6 ± 0.5 | 1.6 ± 0.5 |
| % embryos with AH | 169 (67.3) | 166 (67.2) | 173 (64.1) |
| % embryos from ICSI | 145 (57.8) | 152 (61.5) | 146 (54.1) |
| Embryo score | 8.7 ± 6.1 | 8.76 ± 6.0 | 8.43 ± 6.2 |
ICSI intracytoplasmic sperm injection, BMI body mass index, AH assisted hatching, PCOS polycystic ovary syndrome, FSH follicle-stimulating hormone
aTertile were defined at the 33% cut point
b n ± SD c n (%)
Association between ZPTV and implantation
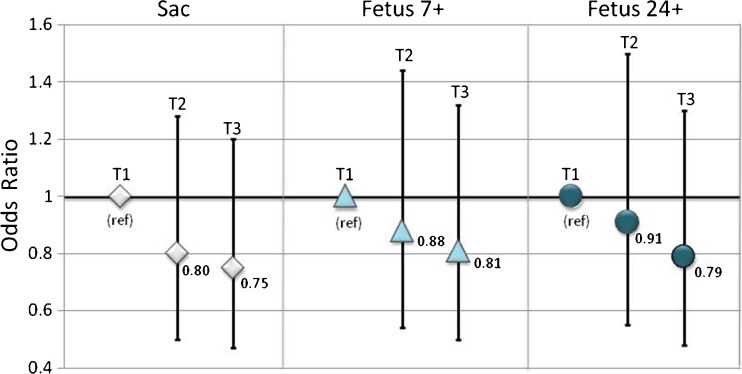
A nonsignificant trend of implantation was observed for greater ZPTV tertile (T2 vs. T1: OR = 0.80; CI = 0.50–1.28 vs. OR: 1.00 (ref); T3 vs T1: OR = 0.75; CI = 0.47–1.20 vs 1.00 (ref) (Table 2, Fig. 3). Similar associations were observed for clinical pregnancy progression as measured by presence of a fetal heart beat at ~7 weeks and continuation of pregnancy with a viable fetus ≥24 weeks; however, none of these associations were statistically significant at p value <0.05 (Table 2, Fig. 3). The linear relationship between ZPTV and implantation was quantified for every 5% increase in ZPTV. There was a nonsignificant trend, demonstrating 9% lower odds of presence of a gestational sac (OR 0.91, CI 0.81–1.03), a 7% lower odds of a fetal heart beat with at least 7 weeks of gestation (OR 0.93, CI 0.82–1.05), and an 8% lower odds of continuation of pregnancy with a viable fetus at ≥24 weeks of gestation (OR 0.92, CI 0.81–1.05).
Table 2.
Association between zona pellucida thickness variation (ZPTV) and cycle outcome
| ZPTV Tertile 1 ≤ 15.0% (n = 251) |
ZPTV Tertile 2 >15.0 to <20.8% (n = 247) |
ZPTV Tertile 3 ≥20.8% (n = 270) |
Continuous ZPTV % (for every 5% unit increase) |
|
|---|---|---|---|---|
| Sac | 56 (22.3)a | 48 (19.5) | 52 (19.3) | |
| Age and AH adjusted | 1.00 (ref) | 0.84 (0.55–1.31)b | 0.83 (0.54–1.29) | 0.93 (0.84–1.04) |
| Multivariable adjusted | 1.00 (ref) | 0.80 (0.50–1.28) | 0.75 (0.47–1.20) | 0.91 (0.81–1.03) |
| Fetus 7+ | 51 (20.3) | 47 (19.0) | 50 (18.5) | |
| Age and AH adjusted | 1.00 (ref) | 0.92 (0.59–1.45) | 0.89 (0.57–1.40) | 0.95 (0.85–1.05) |
| Multivariable adjusted | 1.00 (ref) | 0.88 (0.54–1.44) | 0.81 (0.50–1.32) | 0.93 (0.82–1.05) |
| Fetus 24+ | 48 (19.1) | 45 (18.2) | 46 (17.0) | |
| Age and AH adjusted | 1.00 (ref) | 0.94 (0.59–1.49) | 0.87 (0.54–1.39) | 0.94 (0.84–1.06) |
| Multivariable adjusted | 1.00 (ref) | 0.91 (0.55–1.50) | 0.79 (0.48–1.30) | 0.92 (0.81–1.05) |
Logistic regression models adjusted a priori for: age and assisted hatching (AH), multivariable model additionally adjusted for embryo score, Day 3 FSH, ovulatory infertility, diminished ovarian reserve, BMI, IVF cycle number
AH assisted hatching, Sac intrauterine sac at ≥5 weeks, Fetus 7+ viable fetus at ≥7 weeks with positive fetal heart beat, Fetus 24+ viable fetus at ≥24 weeks with positive fetal heart beat (i.e., beyond the 2nd trimester)
a n (%)
bOdds ratio, 95% confidence interval
Fig. 3.
Association between zona pellucida thickness variation (ZPTV) and implantation. Bar graph height is placed at OR values and bars indicate 95% confidence intervals. Tertile 1 (T1): ZPTV ≤15.0%; Tertile 2 (T2): ZPTV >15.0 to <20.8%; Tertile 3 (T3): ZPTV ≥20.8%; Sac: intrauterine sac at ≥5 weeks, Fetus 7+: viable fetus at ≥7 weeks with positive fetal heart beat, Fetus 24+: viable fetus at ≥24 weeks with positive fetal heart beat (i.e., beyond the 2nd trimester)
Exploration of ZPTV and implantation heterogeneity by AH
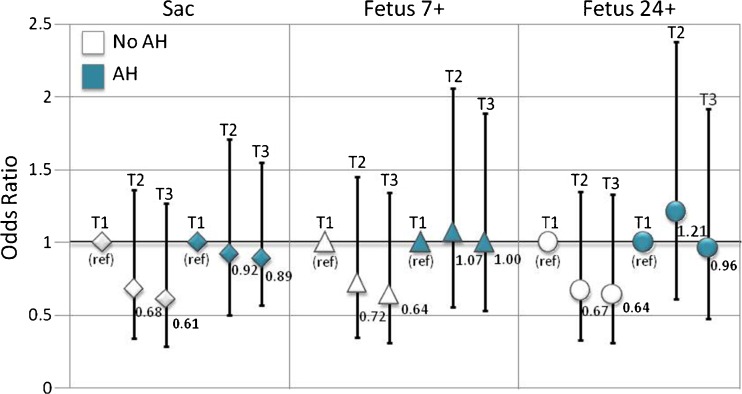
The association between ZPTV and implantation was not found to vary by AH usage (p value, test for interaction: Sac p value = 0.51; Fetus 7+ p value = 0.51, and Fetus 24+ p value = 0.54). There was a suggestion of increased implantation with use of AH, relative to without AH use, across all tertiles of ZPTV for all three measures of clinical outcome assessed (Table 3; Fig. 4), but none of these reached statistical significance.
Table 3.
Association between zona pellucida thickness variation (ZPTV) and cycle outcome stratified by assisted hatching (AH)
| ZPTV Tertile 1 ≤ 15.0% (n = 251) |
ZPTV Tertile 2 >15.0 to <20.8% (n = 247) |
ZPTV Tertile 3 ≥ 20.8% (n = 270) |
Continuous ZPTV % (for every 5% unit increase) |
P value interactiona | |
|---|---|---|---|---|---|
| Sac | 56 (22.3)b | 48 (19.5) | 52 (19.3) | ||
| No assisted hatching used | 29 (35.4) | 22 (27.2) | 26 (26.8) | 0.84 (0.69–1.02) | 0.51 |
| 1.00 (ref) | 0.68 (0.34–1.36)c | 0.61 (0.29–1.27) | |||
| Assisted hatching used | 27(16.0) | 26 (15.8) | 26 (15.0) | 0.97 (0.84–1.12) | |
| 1.00 (ref) | 0.92 (0.50–1.71) | 0.89 (0.49–1.62) | |||
| Fetus 7+ | 51 (20.3) | 47 (19.0) | 50 (18.5) | ||
| No assisted hatching used | 28 (34.2) | 22 (27.2) | 26 (26.8) | 0.85 (0.70–1.04) | 0.51 |
| 1.00 (ref) | 0.72 (0.35–1.45) | 0.64 (0.31–1.34) | |||
| Assisted hatching used | 23 (13.6) | 25 (15.1) | 24 (13.9) | 0.99 (0.85–1.15) | |
| 1.00 (ref) | 1.07 (0.56–2.06) | 1.00 (0.53–1.89) | |||
| Fetus 24+ | 48 (19.1) | 45 (18.2) | 46 (17.0) | ||
| No assisted hatching used | 28 (34.1) | 21 (25.9) | 26 (26.8) | 0.84 (0.69–1.03) | 0.54 |
| 1.00 (ref) | 0.67 (0.33–1.35) | 0.64 (0.31–1.33) | |||
| Assisted hatching used | 20 (11.8) | 24 (14.5) | 20 (11.6) | 0.99 (0.84–1.16) | |
| 1.00 (ref) | 1.21 (0.61–2.38) | 0.96 (0.48–1.92) |
Logistic regression models adjusted a prior for: age and assisted hatching (AH), multivariable model additionally adjusted for embryo quality, day 3 FSH, ovulatory infertility, diminished ovarian reserve, BMI, cycle number
Sac: intrauterine sac at ≥5 weeks, fetus 7+: viable fetus at ≥7 weeks with positive fetal heart beat, fetus 24+: viable fetus at ≥24 weeks with positive fetal heart beat (i.e., beyond the 2nd trimester)
aP-interaction calculated with Likelihood ratio test
b n (%)
cOdds ratio, 95% confidence interval
Fig. 4.
Association between ZPTV and implantation by tertile and stratified by use of assisted hatching (AH). Bar graph height is placed at OR values and bars indicate 95% confidence intervals. Tertile 1 (T1): ZPTV ≤15.0%; Tertile 2 (T2): ZPTV >15.0 to <20.8%; Tertile 3 (T3): ZPTV ≥20.8%; Sac: intrauterine sac at ≥5 weeks, Fetus 7+: viable fetus at ≥7 weeks with positive fetal heart beat, Fetus 24+: viable fetus at ≥24 weeks with positive fetal heart beat (i.e., beyond the 2nd trimester)
Discussion
Our rigorous measurement of thickness of the ZP and assessment of ZPTV using a novel imaging processing platform found no association between degree of variation in the ZP and implantation. Furthermore, when we stratified these embryos by whether or not AH was performed, we found that the association between ZPTV and implantation was not modified by this practice.
Our results stand in contrast to the belief that the overall thickness and variation in thickness of the ZP is an important feature associated with successful implantation of the cleavage stage embryo. Interestingly, our more thorough investigation of the ZPTV found that it lacked a statistically significant association with implantation rate, although a trend was seen towards decreased implantation rates with greater ZPTV (Table 2, Fig. 3). In contrast, past literature has reported improved implantation in embryos with a ZPTV >20–25% [6, 8–10]. Our discrepant results can likely be attributed to our 100 point examination and segmentation of the ZP, which may have better characterized ZPTV with a more accurate reflection of variation. In addition, we analyzed a much larger sample size than used in previous studies and relied only on known implantation data for our analysis.
Previous investigators have studied the use of AH in embryos with ZPT > 13–15 μm [11, 15]. Development and use of AH were first described as potentially overcoming a “hardened” ZP in poor prognosis patients with elevated FSH or a “thickened” ZP > 15 μm [11]. We are the first to investigate if AH altered the association between ZPTV and implantation rate. Similar to Hagemann et al. (2010) who found that AH did not improve implantation rate in embryos with ZP > 13 μm, we found that the effect of ZPTV on implantation rate did not vary when AH was utilized [15]. Although we found across all tertiles of ZPTV that those embryos with AH trended towards a higher implantation rate than those without AH, this was not statistically significant (Fig. 4). We cannot exclude the possibility that a type 2 error might be a factor in our results (accepting no difference when one actually exists) given that when we stratified by AH among our tertiles, our numbers decreased in size (Table 3). Our study was not designed to specifically answer whether AH improved implantation rate based on a specific ZPTV, and future prospective studies with patients randomized to groups either with or without AH are needed to rigorously exclude the impact of ZPTV on utility of AH. The trends demonstrated in our data indicate that AH might be beneficial for some embryos, but at this time, the identifying factor is unknown.
Our study has several strengths. Firstly, we have analyzed a large numbers of embryos for ZPTV (n = 768), more than any other study to our knowledge investigating this embryo characteristic. Secondly, we utilized a new algorithm to ensure accurate depiction of ZPTV. Previous studies have been subject to inter-observer variability regarding where to measure ZPT. In contrast, our novel approach, which captured 100 measurements at uniform distances around the circumference of the ZP, therefore provides a considerable improvement over previous work. Thirdly, previous studies analyzed embryos transferred in groups of up to three or more, which were not linked to known developmental fate [6, 8–10]. In contrast, our study is, to our knowledge, the first to include only embryos with linked and definitively known implantation fate.
Despite the above strengths, our study is not without limitations. Firstly, woman’s age at cycle start is a component of the algorithm that determines use of AH as standard of care, and while we adjusted for many known confounding factors, there may be residual confounding by patient indication for IVF or for AH. Secondly, we included women who contributed multiple cycles, and therefore, the data are not independent but are weighted towards those who contributed the most cycles, and by definition, that means those who did not achieve a live birth in their initial cycle(s) or by those who conceived in their first cycle and returned for another cycle; nevertheless, we did control for this potential bias by using generalized estimating equations to account for the correlation between multiple cycles from the same patient. Thirdly, we excluded embryos due to their unknown implantation fate. While this exclusion allowed us to identify the ZPTV of the embryo and associate it with its implantation potential, it omitted analysis of DET cycles with a singleton gestation (18.3% of all embryos in our dataset). Lastly, our ZPTV visual analysis was done using a two-dimensional approach, whereas the embryo is a three-dimensional structure. In the future, further studies assessing ZPTV would preferably be done by assessing the whole ZP, or at a minimum assessing multiple planes through repeated two-dimensional images. In addition, our study regarding the association between AH, ZPTV, and implantation would ideally be followed up with a randomized controlled trial in which women are randomized to having AH or not. By randomizing embryos in a prospective fashion to AH or not, we could then confirm if there exists a specific cut point of ZPTV for which AH improved implantation.
Conclusion
We found that ZPTV did not affect implantation, nor did AH alter this relationship. Further studies are needed to clarify the relationships among ZPTV, implantation, and AH, in which our novel approach is applied, ideally with 3-D imaging. Investigation of less studied characteristics of day 3 embryos such as the ZPTV is vital as we continually try to improve our ability to hone noninvasive approaches for assessment of developmental competency. Indeed, for patients who are unable to develop blastocysts in culture, and for older patients who have decreased ovarian response, cleavage stage embryo transfer might be the patient’s best and only option [31].
Compliance with ethical standards
Conflict of interest
The authors declare they have no conflict of interest.
Funding
This study was partially funded by the New England Fertility Society REI Fellow Research grant supported by Ferring Pharmaceuticals, Inc.
References
- 1.Cohen J. Assisted hatching of embryos. J In Vitro Fert Embryo Trans. 1991;8:179–190. doi: 10.1007/BF01130802. [DOI] [PubMed] [Google Scholar]
- 2.Balakier H, Sojecki A, Motamedi G, et al. Is the zona pellucida thickness of human embryos influenced by women’s age and hormonal levels? Fertil Steril. 2012;98:77–83. doi: 10.1016/j.fertnstert.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Sinowatz F, Topfer-Peterson E, Kolle S, et al. Functional morphology of the zona pellucida. Anat Histol Embryol. 2001;30:257–263. doi: 10.1046/j.1439-0264.2001.00337.x. [DOI] [PubMed] [Google Scholar]
- 4.Lefievre L, Conner SJ, Salpekar A, Olufowobi O, Ashton P, Pavlovic B, Lenton W, Afnan M, Brewis IA, Monk M, et al. Four zona pellucida glycoproteins are expressed in the human. Hum Reprod. 2004;19:1580–1586. doi: 10.1093/humrep/deh301. [DOI] [PubMed] [Google Scholar]
- 5.Pelletier C, Keefe DL, Trimarchi JR. Noninvasive polarized light microscopy quantitatively distinguishes the multilaminar structure of the zona pellucida of living human eggs and embryos. Fertil Steril. 2004;81(Suppl 1):850–856. doi: 10.1016/j.fertnstert.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J, Inge KL, Suzman M, Wiker SR, Wright G. Videocinematography of fresh and cryopreserved embryos: a retrospective analysis of embryonic morphology and implantation. Fertil Steril. 1989;51:820–827. doi: 10.1016/S0015-0282(16)60673-8. [DOI] [PubMed] [Google Scholar]
- 7.De Vos A, Van Steirteghem A. Zona hardening, zona drilling and assisted hatching: new achievements in assisted reproduction. Cells Tissues Organs. 2000;166:220–227. doi: 10.1159/000016734. [DOI] [PubMed] [Google Scholar]
- 8.Palmstierna M, Murkes D, Csemiczky G, Andersson O. Zona pellucida thickness variation and occurrence of visible mononucleated blastomeres in pre-embryos are associated with a high pregnancy rate in IVF treatment. J Assist Reprod Genet. 1998;15:70–75. doi: 10.1007/BF02766828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun YP, Xu Y, Cao T, Su YC, Guo YH. Zona pellucida thickness and clinical pregnancy outcome following in vitro fertilization. Int J Gynecol Obstet. 2005;89:258–262. doi: 10.1016/j.ijgo.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Gabrielsen A, Lindenberg S, Petersen K. The impact of zona pellucida thickness variation of human embryos on pregnancy outcome in relation to suboptimal embryo development. A prospective randomized controlled study. Hum Reprod. 2001;16:2166–2170. doi: 10.1093/humrep/16.10.2166. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J, Alikani M, Trowbridge J, Rosenwaks Z. Implantation enhancement by selective assisted hatching using zona drilling of human embryos with poor prognosis. Hum Reprod. 1992;7:685–691. doi: 10.1093/oxfordjournals.humrep.a137720. [DOI] [PubMed] [Google Scholar]
- 12.Loret De Mola JR, Garside WT, Bucci J, Tureck RW, Heyner S. Analysis of the human zona pellucida during culture: correlation with diagnosis and the preovulatory hormonal environment. J Assist Reprod Genet. 1997;14:322–326. doi: 10.1007/BF02765837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garside WT, Loret De Mola JR, Bucci JA, Tureck RW, Heyner S. Sequential analysis of zona thickness during in vitro culture of human zygotes: correlation with embryo quality, age, and implantation. Mol Reprod Dev. 1997;47:99–104. doi: 10.1002/(SICI)1098-2795(199705)47:1<99::AID-MRD13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 14.Marco-Jimenez F, Naturil-Alfonso C, Jimenez-Trigos E, Lavara R, Vicente JS. Influence of zona pellucida thickness on fertilization, embryo implantation and birth. Anim Reprod Sci. 2012;132:96–100. doi: 10.1016/j.anireprosci.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Hagemann AR, Lazendorf SE, Jungheim ES, et al. A prospective, randomized, double-blinded study of assisted hatching in women younger than 38 years undergoing in vitro fertilization. Fertil Steril. 2010;93:586–591. doi: 10.1016/j.fertnstert.2009.01.116. [DOI] [PubMed] [Google Scholar]
- 16.Kissin DM, Kawwass JF, Monsour M, Boulet SL, Session DR, Jamieson DJ, National ART. Surveillance System (NASS) group. Assisted hatching: trends and pregnancy outcomes, United States, 2000-2010. Fertil Steril. 2014;102:795–801. doi: 10.1016/j.fertnstert.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanter JR, Boulet SL, Kawwass JF, Jamieson DJ, Kissin DM. Trends and correlates of monozygotic twinning after single embryo transfer. Obstet Gynecol. 2015;125:111–117. doi: 10.1097/AOG.0000000000000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hershlag A, Paine T, Cooper GW, et al. Monozygotic twinning associated with mechanical assisted hatching. Fertil Steril. 1999;71:144–146. doi: 10.1016/S0015-0282(98)00402-6. [DOI] [PubMed] [Google Scholar]
- 19.Luke B, Brown MB, Wantman E, et al. Factors associated with monozygosity in assisted reproductive technology pregnancies and the risk of recurrence linked cycles. Fertil Steril. 2014;101:683–689. doi: 10.1016/j.fertnstert.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiewe MC, Whitney JB, Anderson RE. Potential risk of monochorionic dizygotic twin blastocyst formation associated with early laser dissection of group culture embryos. Fertil Steril. 2015;103:417–421. doi: 10.1016/j.fertnstert.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Skiadas CC, Missmer SA, Benson CB, Gee RE, Racowsky C. Risk factors associated with pregnancies containing a monochorionic pair following assisted reproductive technologies. Hum Reprod. 2008;23:1366–1371. doi: 10.1093/humrep/den045. [DOI] [PubMed] [Google Scholar]
- 22.Carney SK, Das S, Blake D, Farquhar C, Seif MM, Nelson L. Assisted hatching on assisted conception in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). Cochrane Database Syst Rev. 2012;12:CD001894. [DOI] [PMC free article] [PubMed]
- 23.Martins WP, Rocha IA, Ferriani RA, Nastri CO. Assisted hatching human embryos: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2011;17:438–453. doi: 10.1093/humupd/dmr012. [DOI] [PubMed] [Google Scholar]
- 24.Shi W, Hongwei T, Zhang W, Li N, Li M, Li W, et al. A prospective randomized controlled study of laser-assisted hatching on the outcome of first fresh IVF-ET cycle in advanced age women. Reprod Sci. 2016;23:1397–1401. doi: 10.1177/1933719116641764. [DOI] [PubMed] [Google Scholar]
- 25.Butts SF, Owen C, Mainiqi M, Senapati S, Seifer DB, Dokras A. Assisted hatching and intracytoplasmic sperm injection are not associated with improved outcomes in assisted reproduction cycles for diminished ovarian reserve: an analysis of cycles in the United States from 2004 to 2011. Fertil Steril. 2013;102:1041–1047. doi: 10.1016/j.fertnstert.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machtinger R, Bormann CL, Ginsburg ES, Racowsky C. Is the presence of a non-cleaved embryo on day 3 associated with poorer quality of the remaining embryos in the cohort? J Assist Reprod Genet. 2015;32:677–683. doi: 10.1007/s10815-015-0455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Racowsky C, Combelles CMH, Nureddin A, Pan Y, Finn A, Miles L, Gale S, O’Leary T, Jackson KV. Day 3 and day 5 morphological predictors of embryo viability. Reprod BioMed Online. 2003;6:323–331. doi: 10.1016/S1472-6483(10)61852-4. [DOI] [PubMed] [Google Scholar]
- 28.Farhadifar R, Needleman D. Automated segmentation of the first mitotic spindle in differential interference contrast microscopy images of C. elegans embryos. Methods Mol Biol. 2014;1136:41–45. doi: 10.1007/978-1-4939-0329-0_3. [DOI] [PubMed] [Google Scholar]
- 29.Kass M, Witkin A, Terzopoulos D. Snakes: active contour models. Int J Comput Vis. 1988;1:321. doi: 10.1007/BF00133570. [DOI] [Google Scholar]
- 30.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349. doi: 10.2105/AJPH.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldman RH, Kaser DJ, Missmer SA, Srouji SS, Farland LV, Racowsky C. Building a model to increase live birth rate through patient-specific optimization of embryo transfer day. J Assist Reprod Genet. 2016;33:1525–1532. doi: 10.1007/s10815-016-0803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]