Abstract
Purpose
Care bundles are recommended in patients at high risk for acute kidney injury (AKI), although they have not been proven to improve outcomes. We sought to establish the efficacy of an implementation of the Kidney Disease Improving Global Outcomes (KDIGO) guidelines to prevent cardiac surgery-associated AKI in high risk patients defined by renal biomarkers.
Methods
In this single-center trial, we examined the effect of a “KDIGO bundle” consisting of optimization of volume status and hemodynamics, avoidance of nephrotoxic drugs, and preventing hyperglycemia in high risk patients defined as urinary [TIMP-2]·[IGFBP7] > 0.3 undergoing cardiac surgery. The primary endpoint was the rate of AKI defined by KDIGO criteria within the first 72 h after surgery. Secondary endpoints included AKI severity, need for dialysis, length of stay, and major adverse kidney events (MAKE) at days 30, 60, and 90.
Results
AKI was significantly reduced with the intervention compared to controls [55.1 vs. 71.7%; ARR 16.6% (95 CI 5.5–27.9%); p = 0.004]. The implementation of the bundle resulted in significantly improved hemodynamic parameters at different time points (p < 0.05), less hyperglycemia (p < 0.001) and use of ACEi/ARBs (p < 0.001) compared to controls. Rates of moderate to severe AKI were also significantly reduced by the intervention compared to controls. There were no significant effects on other secondary outcomes.
Conclusion
An implementation of the KDIGO guidelines compared with standard care reduced the frequency and severity of AKI after cardiac surgery in high risk patients. Adequately powered multicenter trials are warranted to examine mortality and long-term renal outcomes.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-016-4670-3) contains supplementary material, which is available to authorized users.
Keywords: Acute kidney injury, KDIGO guidelines, Biomarkers, [TIMP-2]·[IGFBP7], Cardiac surgery, Major adverse kidney events
Introduction
Acute kidney injury (AKI) is a well-recognized complication following cardiac surgery and significantly affects morbidity and mortality [1]. Up to 30% of patients develop AKI after cardiac surgery, whereas severe AKI requiring dialysis is relatively rare [1]. Approximately 1% of all patients undergoing cardiac surgery develop a severe dialysis-dependent AKI, and this severity of AKI is associated with especially poor outcomes [1]. Although the mechanisms of AKI are not fully understood, injury to renal tubular epithelial cells is a universal aspect of the disease. Despite numerous clinical trials using several interventions [2], a reliable means to prevent AKI remains elusive.
In earlier studies, interventions for AKI were started after a change in kidney function, a surrogate for kidney damage, had occurred [3, 4]. However, no intervention has yet demonstrated an effect in treating AKI once it has occurred [3, 4]. In contrast to these studies, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for AKI recommend to initiate different supportive measures (volume management, maintenance of adequate blood pressure, and judicious avoidance of nephrotoxins) in patients at high risk for AKI [5], although it has not been proven that this approach reduces the occurrence or severity of AKI. High risk patients can be identified by renal biomarkers, but measuring biomarkers in all patients reduces the specificity of biomarkers. Therefore, it was suggested that renal biomarkers should be used in patients with a certain risk profile consisting of different risk factors and/or certain clinical conditions (e.g., sepsis or major surgery) [6, 7].
In contrast to the conventional biomarkers serum creatinine and urine output, which are markers of kidney function, new biomarkers allow a diagnosis to be made earlier and they allow kidney injury to be diagnosed even in the absence of concurrent or subsequent dysfunction [7]. A biomarker-based approach was first used in the EARLYARF trial but the applied biomarkers showed poor predictive performance [8]. The two new renal biomarkers, insulin-like growth factor-binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP-2), are involved in G1 cell cycle arrest and are able to identify patients at high risk for AKI [9]. We have previously shown that urinary [TIMP-2]·[IGFBP7] has excellent performance in predicting cardiac surgery-associated AKI (CSA-AKI) [10].
To investigate whether an implementation of a supportive care “bundle” in high risk patients for AKI as suggested by the KDIGO guidelines can reduce the occurrence and severity of CSA-AKI, we performed a single-center, clinical randomized controlled trial. Our goal was to acquire phase 2 equivalent data to support a larger multicenter trial to examine the effectiveness of a KDIGO bundle in reducing mortality and long-term renal dysfunction.
Methods
Participants
Eligible patients were adults at high risk for AKI who underwent cardiac surgery with the use of cardiopulmonary bypass (CPB) at the University of Muenster (Germany), between August 2014 and December 2015. High risk for AKI was defined as urinary [TIMP-2]·[IGFBP7] ≥ 0.3 (Nephrocheck® Test) 4 h after CPB.
Procedures
The surgical procedure and perioperative care were performed according to the standard of care for the center. All cardiac surgery patients received a central venous catheter and an arterial line. In the intraoperative period, patients with a low ejection fraction, known regional wall motion abnormalities, and/or valvular dysfunctions received a transesophageal echocardiography to monitor heart function during the operation (the probe was removed after surgery). [TIMP-2]·[IGFBP7] was measured in every patient undergoing on-pump cardiac surgery 4 h after CPB disconnection. According to recently published trials [9, 10], patients with an increased [TIMP-2]·[IGFBP7] are at high risk for the development of AKI and we therefore used this test to enrich the study population. Discarded urine was used to measure [TIMP-2]·[IGFBP7] and identify patients eligible for the study. Prior to patients being randomized into the study, the trial investigators obtained informed consent for participation in the study. Patients assigned to the control group received standard care including the specification to keep mean arterial pressure (MAP) >65 mmHg and central venous pressure (CVP) between 8 and 10 mmHg. Patients received angiotensin converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARBs) once the hemodynamic situation stabilized and hypertension occurred (according to the recommendations of the American College of Cardiology Foundation 2011). Patients assigned to the intervention group received a strictly controlled implementation of the KDIGO guidelines (“KDIGO CT surgery bundle”) consisting of the following measures: avoidance of nephrotoxic agents, discontinuation of ACEi and ARBs for the first 48 h after surgery, close monitoring of serum creatinine and urine output, avoidance of hyperglycemia for the first 72 h after surgery, consideration of alternatives to radiocontrast agents, close hemodynamic monitoring by using a PICCO catheter with an optimization of the volume status and hemodynamic parameters according to a prespecified algorithm (Supplementary Fig. S1).
Outcomes
The primary endpoint was the occurrence of AKI within the first 72 h after cardiac surgery. We defined AKI according to the KDIGO criteria (Supplementary Table S1). Secondary endpoints were severity of AKI within 72 h, the 30-, 60-, and 90-day all-cause mortality, need for renal replacement therapy (RRT) during index hospitalization and after 30, 60, and 90 days, persistent renal dysfunction (PRD) at days 30, 60, and 90 (defined as serum creatinine ≥0.5 mg/dl as compared to baseline), MAKEn (major adverse kidney events defined as combination of mortality, need for RRT, and PRD at day n; n = 30, 60, and 90, respectively), length of stay in the intensive care unit (ICU), and length of hospital stay.
We abstracted clinical variables from the medical record. The adherence to the hemodynamic protocol was documented every 3 h. Initiation of RRT was at the discretion of the ICU clinicians. Criteria for RRT were not included in the protocol.
Statistical analysis
We calculated a necessary sample size based on the primary endpoint, using nQuery Advisor version 7. According to previous trials, 80% of patients with a [TIMP-2]·[IGFBP7] ≥ 0.3 developed CSA-AKI [10]. The primary efficacy analysis was intended to show superiority of the validation strategy, applying a two-sided χ 2 test on significance level α = 0.05. As a result of a lack of reference values, we hypothesized to reduce the incidence of AKI to 65%. Resulting from these considerations and a power of 80%, the required sample size was calculated to be 138 evaluable patients per treatment group, i.e., 276 in total.
The primary efficacy analysis included all randomized patients (full analysis set) and was performed according to the intent-to-treat principle, i.e., all patients were analyzed according to their randomization. For the primary outcome and the secondary endpoint AKI severity all patients had complete data.
Categorical variables were summarized by frequency tables. Treatment groups were compared with the χ 2 test (or Fisher’s exact test if the respective frequency tables contained cells with expected counts <5). We estimated the odds ratio (OR) as well as the absolute risk reduction (ARR) between treatment groups, including 95% confidence intervals (CI). Normally distributed continuous variables were expressed as mean (standard deviation, SD), and were compared between groups with an unpaired Student’s t test. Continuous variables, which were not normally distributed, were analyzed using a nonparametric test (Mann–Whitney U test). Median values were compared between treatment groups using the Hodges–Lehmann estimator of location shift with associated 95% CI. In exploratory analyses two-sided p values were considered noticeable (“significant”) at p ≤ 0.05. Statistical analyses were performed using the SAS software (version 9.4 for Windows, SAS Institute Inc., Cary, NC, USA).
Results
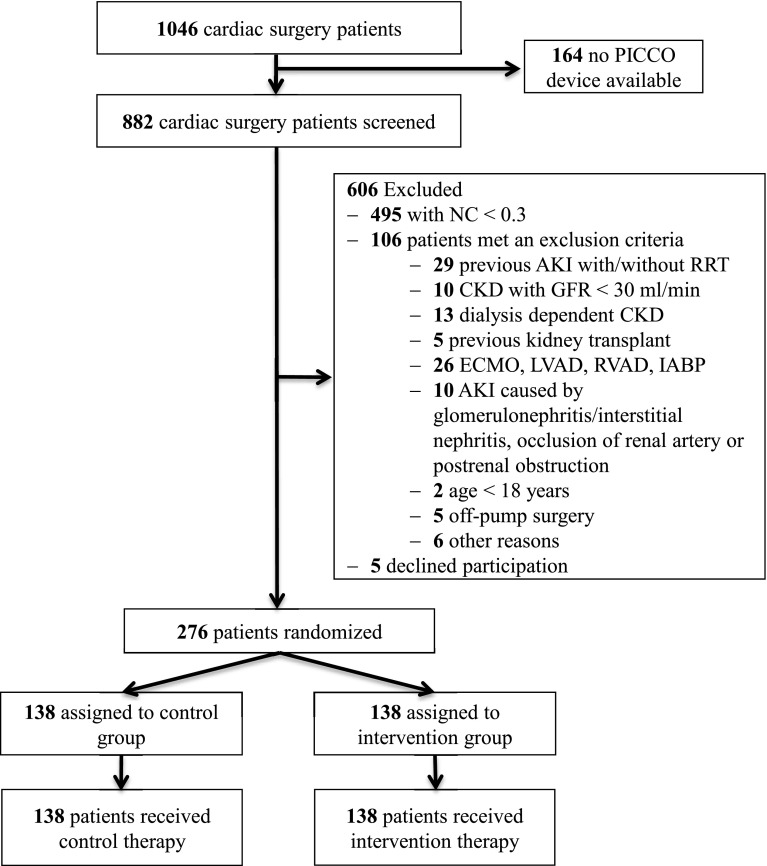
A total of 1046 patients underwent cardiac surgery during the recruitment period and 882 patients were properly screened because in 164 patients no PICCO device was available. Of these, 276 patients were enrolled and randomized to either the intervention group (n = 138) or the control group (n = 138) and included in the primary analysis (Fig. 1). All randomly assigned patients received their allocated treatment. Median urine [TIMP-2]·[IGFBP7] was 0.50 (Q1, Q3; 0.38, 0.87) in the intervention and 0.61 (Q1, Q3; 0.39, 1.09) in the control group (p = 0.149) (Table 1). The baseline and intraoperative characteristics did not differ between groups (Table 1). Clinical care outside the trial intervention was also similar (Table 1), including operative characteristics, volume administration, and the use of catecholamines. Use of catecholamines and administered volume at the time of randomization were similar in both groups (Supplementary Table S2). During the study period, significantly more patients in the intervention group [43 (31.2%)] received dobutamine compared to the sham group [13 (9.4%); p < 0.001] resulting in a significantly increased MAP and CVP at different time points (Table 2). Although the use of inotropes was significantly higher in the intervention group, the occurrence of atrial fibrillation within the first 12 h (Table 2) and the concentration of cardiac biomarkers was similar between the two groups (CK-MB: p = 0.229, troponin I: p = 0.140). In addition, the rate of hyperglycemia (p < 0.001) and use of ACEi and ARBs (p < 0.001) were significantly reduced in the intervention group compared to the control group (Table 2). However, the total administered volume was not different between the two groups (Table 2), but the patients in the intervention group received significantly less volume during the last 3 h of the intervention period (p = 0.024) (Supplementary Table S3).
Fig. 1.
Trial flowchart. Enrolled patients are randomized to study groups
Table 1.
Baseline and operative characteristics
| Control (n = 138) | Intervention (n = 138) | |
|---|---|---|
| Age, mean (±SD), (year) | 68.33 (11.6) | 68.40 (11.2) |
| Male sex, no. (%) | 105 (76.1) | 94 (68.1) |
| Weight, mean (±SD), kg | 86.4 (15.2) | 83.5 (15.1) |
| ASA grade, no. (%) | ||
| 1 | 3 (2.2) | 0 (0) |
| 2 | 11 (8.0) | 8 (5.8) |
| 3 | 99 (71.7) | 111 (80.4) |
| 4 | 25 (18.1) | 19 (13.8) |
| New York Heart Association classification, no. (%) | ||
| I | 9 (6.5) | 11 (8.0) |
| II | 65 (47.1) | 54 (39.1) |
| III | 52 (37.7) | 57 (41.3) |
| IV | 12 (8.7) | 16 (11.6) |
| SOFA score, mean (±SD) | 6.0 (2.2) | 5.9 (2.1) |
| APACHE score, mean (±SD) | 8.9 (3.9) | 8.5 (3.2) |
| EuroSCORE, median (Q1, Q3) | 5 (3, 8) | 6 (3, 8) |
| Preoperative creatinine, mean (±SD), mg/dl | 0.95 (0.29) | 0.95 (0.25) |
| eGFR, mean (±SD), ml/min/m2 | 94.4 (37.9) | 85.3 (26.7) |
| Comorbidities, no. (%) | ||
| Hypertension | 109 (79.0) | 116 (84.1) |
| Diabetes | 32 (23.2) | 35 (25.4) |
| IDDM | 15 (10.9) | 11 (8.0) |
| NIDDM | 17 (12.3) | 24 (17.4) |
| COPD | 11 (8.0) | 16 (11.6) |
| CKD | 11 (8.0) | 13 (9.4) |
| Previous heart surgery | 15 (10.9) | 13 (9.4) |
| Left ventricular ejection fraction <35% | 14 (10.3) | 9 (6.5) |
| Myocardial infarction | 48 (34.8) | 36 (26.1) |
| Atrial fibrillation | 35 (25.4) | 36 (26.1) |
| Medication, no. (%) | ||
| Aspirin | 84 (61.3) | 81 (58.7) |
| Clopidogrel | 15 (11.0) | 8 (5.8) |
| β-blockers | 91 (66.4) | 83 (60.1) |
| Statins | 77 (56.2) | 84 (60.9) |
| Diuretics | 66 (47.8) | 76 (55.1) |
| ACEi/ARBs | 64 (46.7) | 70 (50.7) |
| Intraoperative times, median (Q1, Q3), min | ||
| Aortic cross-clamp | 78 (58.0, 106.5) | 80 (60.0, 112.8) |
| Cardiopulmonary bypass | 117 (94.0, 155.0) | 124 (93.0, 159.0) |
| Procedure, no. (%) | ||
| CABG only | 64 (46.4) | 56 (40.6) |
| Valve only | 31 (22.5) | 35 (25.4) |
| Combined | 23 (16.7) | 21 (15.2) |
| Other | 20 (14.5) | 26 (18.8) |
| Intraoperative volume therapy, median (Q1, Q3), ml | ||
| Crystalloids | 2000 (2000, 3000) | 2500 (2000, 3000) |
| Colloids | 0 (0, 0) | 0 (0, 0) |
| Blood products | ||
| Erythrocyte concentrates | 0 (0, 0) | 0 (0, 300) |
| Thrombocyte concentrates | 0 (0, 0) | 0 (0, 0) |
| Fresh frozen plasma | 0 (0, 0) | 0 (0, 0) |
| Intraoperative medication, no. (%) | ||
| Norepinephrine | 136 (99.3) | 138 (100) |
| Epinephrine | 24 (17.4) | 27 (19.6) |
| Dobutamine | 17 (12.3) | 13 (9.5) |
| Vasopressin | 3 (2.2) | 1 (0.7) |
| Levosimendan | 10 (7.2) | 10 (7.2) |
| Baseline urine biomarkers, median (Q1, Q3) | ||
| Urine [TIMP-2]·[IGFBP7], ng/ml2/1000 | 0.61 (0.39, 1.09) | 0.50 (0.38, 0.87) |
ASA American Society of Anesthesiologists Physical Classification System, eGFR estimated glomerular filtration rate, IDDM insulin-dependent diabetes mellitus, NIDDM non-insulin-dependent diabetes mellitus, COPD chronic obstructive pulmonary disease, CKD chronic kidney disease, ACEi angiotensin-converting enzyme inhibitors, ARBs angiotensin II receptor blockers, CABG coronary artery bypass graft, SOFA sequential organ failure assessment, APACHE II acute physiology and chronic health evaluation, [TIMP-2]·[IGFBP7] tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein 7
Table 2.
Measures during the intervention period
| Control (n = 138) | Intervention (n = 138) | p value | |
|---|---|---|---|
| Patients with catecholamines during intervention period, no. (%) | |||
| Dobutamine | 13 (9.4) | 43 (31.2) | <0.001 |
| Epinephrine | 21 (15.2) | 29 (21.2) | 0.201 |
| Norepinephrine | 91 (65.9) | 94 (68.1) | 0.701 |
| Catecholamines during intervention period, median (Q1, Q3), µg/kg | |||
| Dobutamine | 1107.9 (407.6, 1387.6) | 1373.2 (960.7, 1700.0) | 0.093 |
| Epinephrine | 31.6 (9.3, 49.0) | 15.2 (5.5, 30.8) | 0.191 |
| Norepinephrine | 22.7 (7.3, 49.0) | 14.5 (5.2, 39.9) | 0.088 |
| Volume therapy during intervention period, median (Q1, Q3), ml | |||
| Total volume | 2745 (1968, 3625) | 2575 (1965, 3518) | 0.699 |
| Crystalloids | 2220 (1518, 3220) | 2220 (1720, 3220) | 0.470 |
| Colloids | 0 (0, 0) | 0 (0, 0) | 0.996 |
| Blood products | 0 (0, 0) | 0 (0, 0) | 0.561 |
| H2O | 250 (0, 613) | 200 (0, 400) | 0.057 |
| MAP, mean (±SD), mmHg | |||
| At randomization | 72 (11) | 73 (11) | 0.324 |
| 3 h | 72 (9) | 75 (10) | 0.017 |
| 6 h | 72 (9) | 73 (10) | 0.217 |
| 9 h | 71 (10) | 74 (9) | 0.007 |
| 12 h | 71 (11) | 75 (9) | 0.005 |
| CVP, mean (±SD), mmHg | |||
| At randomization | 9 (5) | 9 (4) | 0.956 |
| 3 h | 9 (4) | 10 (4) | 0.008 |
| 6 h | 9 (4) | 11 (5) | <0.001 |
| 9 h | 9 (4) | 10 (5) | 0.014 |
| 12 h | 10 (4) | 10 (4) | 0.137 |
| SvO2, mean (±SD), % | |||
| At randomization | 67 (9) | 67 (9) | 0.872 |
| 3 h | 66 (9) | 68 (9) | 0.180 |
| 6 h | 65 (9) | 69 (8) | <0.001 |
| 9 h | 65 (9) | 68 (10) | 0.010 |
| 12 h | 64 (9) | 68 (8) | <0.001 |
| Atrial fibrillation within 12 h, no. (%) | 15 (10.9) | 13 (9.4) | 0.690 |
| Hyperglycemiaa, no. (%) | 104 (75.4) | 70 (50.7) | <0.001 |
| ACEi/ARBsb, no. (%) | 42 (30.4) | 15 (10.9) | <0.001 |
| Nephrotoxic agentsc, no. (%) | 22 (15.9) | 18 (13.0) | 0.494 |
| Contrast agents | 19 (13.8) | 11 (8.0) | 0.122 |
| Vancomycin, gentamicin | 6 (4.3) | 9 (6.5) | 0.426 |
| Diureticsd, no. (%) | 113 (81.9) | 103 (74.6) | 0.144 |
| Infections, no./total no. (%) | 11 (8.0) | 9 (6.5) | 0.642 |
| Urine [TIMP-2]·[IGFBP7] at 12 h, ng/ml2/1000, median (Q1, Q3) | 0.84 (0.35, 1.57) | 0.58 (0.26, 1.20) | 0.045 |
| Relative change urine [TIMP-2]·[IGFBP7] 12 h vs. baseline, ng/ml2/1000, median (Q1, Q3) | 1.13 (0.52, 2.23) | 1.07 (0.38, 1.94) | 0.272 |
ACEi angiotensin converting enzyme inhibitors, ARBs angiotensin II receptor blockers, CVP central venous pressure, MAP mean arterial pressure, S v O 2 venous oxygen saturation, [TIMP-2]·[IGFBP7] tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein 7
aDefined as prolonged hyperglycemia (blood glucose level ≥150 mg/dl) >3 h within the first 72 h after cardiac surgery
bAngiotensin converting enzyme inhibitors and angiotensin II receptor blockers within 48 h after cardiac surgery
cWithin 72 h after cardiac surgery
dDiuretics within 72 h after cardiac surgery
The overall AKI incidence was 63.4% (175/276). Of the 276 study subjects, in 24 patients AKI was diagnosed by serum creatinine while in 143 patients, urine output criteria were met although the postoperative use of diuretics was similar in the two groups (Table 3). In eight patients, serum creatinine and urine output criteria were met. At 12 h after randomization, median urine [TIMP-2]·[IGFBP7] was 0.58 (Q1, Q3; 0.26, 1.20) in the intervention and 0.84 (Q1, Q3; 0.35, 1.57) in the control group (p = 0.045) (Table 2). Patients in the control group showed a non-significant change in [TIMP-2]·[IGFBP7] from 0.61 to 0.84 (p = 0.098) as compared to from 0.50 to 0.58 (p = 0.536) in the intervention group.
Table 3.
Primary and secondary outcomes
| Control (n = 138) | Intervention (n = 138) | p value | OR (intervention versus control) (95% CI) | RRRa (95% CI) | ARRb (95% CI) | |||
|---|---|---|---|---|---|---|---|---|
| Primary outcome | ||||||||
| AKI within 72 h, no./total no. (%) | 99/138 (71.7) | 76/138 (55.1) | 0.004 | 0.483 (0.293, 0.796) | 23.2% (7.8, 36.1%) | 16.6% (5.5, 27.9%) | ||
| Diagnosis based on, no. (%) | ||||||||
| Creatinine | 14 (14.1) | 10 (13.2) | ||||||
| Urine output | 81 (81.8) | 62 (81.6) | ||||||
| Both | 4 (4.0) | 4 (5.3) | ||||||
| Secondary outcomes | ||||||||
| AKI stage, no./total no. (%) | ||||||||
| 1 | 37/138 (26.8) | 35/138 (25.4) | 0.784 | 0.928 (0.542, 1.588) | 5.4% (–40.7, 36.4%) | 1.4% (–8.9, 11.8%) | ||
| Diagnosis based on, no. (%) | ||||||||
| Creatinine | 12 (32.4) | 9 (25.7) | ||||||
| Urine output | 23 (62.2) | 25 (71.4) | ||||||
| Both | 2 (5.4) | 1 (2.9) | ||||||
| 2 | 45/138 (32.6) | 30/138 (21.7) | 0.042 | 0.574 (0.335, 0.984) | 33.3% (0.8, 55.2%) | 10.9% (0.5, 21.3%) | ||
| Diagnosis based on, no. (%) | ||||||||
| Creatinine | 1 (2.2) | 1 (3.3) | ||||||
| Urine output | 42 (93.3) | 28 (93.3) | ||||||
| Both | 2 (4.4) | 1 (3.3) | ||||||
| 3 | 17/138 (12.3) | 11/138 (8.0) | 0.232 | 0.617 (0.278, 1.370) | 35.3% (–33.0, 68.5%) | 4.3% (–2.8, 11.5%) | ||
| Diagnosis based on, no. (%) | ||||||||
| Creatinine | 1 (5.9) | 0 (0) | ||||||
| Urine output | 16 (94.1) | 9 (81.8) | ||||||
| Both | 0 (0) | 2 (18.2) | ||||||
| Moderate/severe AKI, no./total no. ( %) | 62/138 (44.9) | 41/138 (29.7) | 0.009 | 0.518 (0.316, 0.851) | 33.9% (9.3, 51.8%) | 15.2% (4.0, 26.5%) | ||
| Requirement of RRT within 72 h, no./total no. (%) | 7/138 (5.1) | 10/138 (7.2) | 0.453 | 1.462 (0.540, 3.959) |
–42.9% (–264.5, 44.0%) | –2.2% (–7.8, 3.5%) | ||
| Requirement of RRT during hospital stay, no./total no. (%) | 9/138 (6.5) | 14/138 (10.1) | 0.276 | 1.618 (0.676, 3.874) | –55.6% (–247.4, 30.3%) | –3.6% (–10.1, 2.9%) |
||
| PRD on day 30, no./total no. (%) | 7/126 (5.6) | 14/129 (10.9) | 0.124 | 2.070 (0.806, 5.313) | –95.3% (–367.9, 18.4%) | –5.3% (–12.0, 1.4%) | ||
| PRD on day 60, no./total no. (%) | 6/125 (4.8) | 11/128 (8.6) | 0.228 | 1.865 (0.668, 5.207) | –79.0% (–369.3, 31.7%) | –3.8% (–9.9, 2.3%) | ||
| PRD on day 90, no./total no. (%) | 9/125 (7.2) | 9/126 (7.1) | 0.986 | 0.992 (0.380, 2.587) | 0.8% (–141.6, 59.3%) | 0.1% (–6.3, 6.4%) | ||
| Requirement of RRT on day 30, no./total no. (%) | 3/132 (2.3) | 4/131 (3.1) | 0.722 | 1.354 (0.297, 6.173) | –34.4% (–488.6, 69.3%) | –0.8% (–4.7, 5.4%) | ||
| Requirement of RRT on day 60, no./total no. (%) | 2/132 (1.5) | 4/130 (3.1) | 0.445 | 2.064 (0.371, 11.466) | –103.1% (–989.6, 62.2%) | –1.6% (–5.2, 2.1%) | ||
| Requirement of RRT on day 90, no./total no. (%) | 3/125 (2.4) | 1/126 (0.8) | 0.370 | 0.325 (0.033, 3.171) | 66.9% (–213.6, 96.5%) | 1.6% (–1.5, 4.7%) | ||
| 30-day all cause mortality, no./total no. (%) | 6/138 (4.4) | 7/138 (5.1) | 0.776 | 1.176 (0.385, 3.592) | –16.7% (–238.3, 59.8%) | –0.7% (–5.7, 4.3%) | ||
| 60-day all cause mortality, no./total no. (%) | 6/138 (4.4) | 8/138 (5.8) | 0.583 | 1.354 (0.457, 4.010) | –33.3% (–274.2, 52.5%) | –1.5% (–6.6, 3.7%) | ||
| 90-day all cause mortality, no./total no. (%) | 9/134 (6.7) | 11/137 (8.0) | 0.679 | 1.213 (0.486, 3.028) | –19.5% (–179.2, 48.8%) | –1.3% (–7.5, 4.9%) | ||
| MAKE30, no./total no. (%) | 13/132 (9.9) | 21/136 (15.4) | 0.169 | 1.672 (0.799, 3.495) | –56.8% (–200.0, 18.1%) | –5.6% (–13.5, 2.3%) | ||
| MAKE60, no./total no. (%) | 12/131 (9.2) | 19/136 (14.0) | 0.220 | 1.610 (0.748, 3.466) | –52.5% (–201.6, 22.9%) | –4.8% (–12.5, 2.8%) | ||
| MAKE90, no./total no. (%) | 18/134 (13.4) | 20/137 (14.6) | 0.782 | 1.102 (0.554, 2.189) | –8.7% (–96.2, 39.8%) | –1.2% (–9.4, 7.1%) | ||
| ICU stay, median (Q1, Q3), days | 2 (2, 5) | 3 (2, 5) | 0.392 | 0 (–1, 0) | ||||
| Hospital stay, median (Q1, Q3), days | 11 (8, 17) | 11 (8, 16) | 0.832 | 0 (–1, 1) | ||||
| Duration of ventilator support (Q1, Q3), h | 5 (1, 11) | 6 (2, 12) | 0.540 | 0 (–2, 1) | ||||
aRRR >0 indicates treatment effects in favor of the interventional treatment group
bARR >0 indicates treatment effects in favor of the interventional treatment group
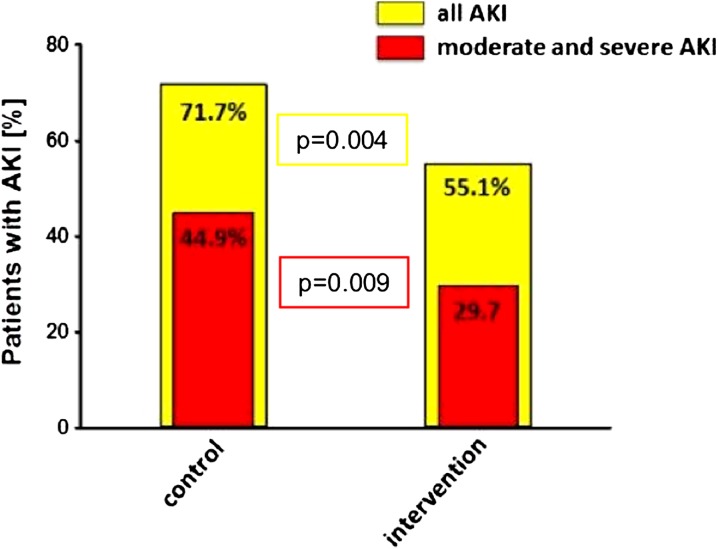
The primary outcome, occurrence of AKI within 72 h after cardiac surgery according to the KDIGO classification system, occurred in significantly fewer patients in the intervention group [76 (55.1%)] compared to the control [99 (71.7%)] group [p = 0.004; OR, 0.483 (95% CI, 0.293–0.796); ARR, 16.6% (95% CI, 5.5–27.9%)] (Table 3; Fig. 2). In the intervention group significantly lower rates of moderate and severe AKI were observed compared to the control group [41/138 (29.7%) vs 62/138 (44.9%); p = 0.009; OR, 0.518 (95% CI, 0.316–0.851); ARR, 15.2% (95% CI, 4.0–26.5%)] (Table 3). Patients with [TIMP-2]·[IGFBP7] > 2.0 at 4 h after cardiac surgery were at highest risk for developing AKI (81.3% in the intervention and 83.3% in the control group). There were no significant differences for any of the other secondary outcomes, including requirement of RRT during hospital stay, ICU and hospital stay as well as major adverse kidney events (MAKE) at day 30, 60, and 90 (Table 3).
Fig. 2.
Occurrence of cardiac surgery-associated AKI. Rate of CSA-AKI in control and intervention groups
Discussion
In this randomized clinical trial of patients undergoing cardiac surgery, the implementation of a bundle of supportive measures in high risk patients identified by elevated biomarker levels reduced the occurrence of AKI within 72 h compared to standard care.
The diagnosis of AKI relies on functional parameters that cannot be used for the early diagnosis of AKI. In contrast, AKI biomarkers may increase in response to a wide variety of insults and therefore they might be used to identify a status of kidney stress or an increased susceptibility to insults. Under such circumstances, biomarkers may allow one to implement preventive and protective measures well before clinical AKI becomes manifest according to well-established definitions [11]. In other fields of medicine, biomarkers allow an early start of the therapy as well as the initiation of molecularly targeted therapies [12–14]. As a result, experts in the field of critical care nephrology asked for an implementation of treatment strategies in high risk patients to prevent AKI [15, 16]. On the basis of the good performance of [TIMP-2]·[IGFBP7] in predicting AKI [9, 10] and the fact that [TIMP-2]·[IGFBP7] can be measured at the bedside, we decided to use these two AKI biomarkers to identify patients at high risk for CSA-AKI.
The pathophysiology of CSA-AKI is very complex and consists of renal ischemia, reperfusion, inflammation, oxidative stress, hemolysis, and toxins. Pharmacological and non-pharmacological preventive strategies have largely failed to reduce the occurrence of CSA-AKI in clinical trials [17, 18], although some treatments may be effective in specific patient populations [19]. As several signaling pathways are involved in the pathophysiology of CSA-AKI, we implemented a bundle of supportive measures which are recommended by the KDIGO guidelines. This multifactorial approach may improve renal perfusion [20] and reduces inflammation [21] and oxidative stress [21]. One would assume that these supportive bundles should be implemented in the whole cardiac surgery. However, some of the measures are not harmful whereas other measures might be harmful or even against other guidelines and should only be applied in high risk patients. Here we demonstrate that the implementation of the KDIGO guidelines resulted in more use of dobutamine, a reduced rate of hyperglycemia, and higher rate of discontinuing ACEi and ARBs. Although the increased use of dobutamine in the intervention group was associated with a significantly increased blood pressure at different time points, this difference and the small significant difference in fluids are probably not clinically relevant. In addition to the increased use of dobutamine, patients in the intervention group received a better hemodynamic monitoring resulting in the possibility of a more personalized medicine. The discontinuation of ACEi and ARBs might result in a change in serum creatinine not representing a structural AKI. However, patients in the intervention group showed lower tubular damage biomarker levels ([TIMP-2]·[IGFBP7]) at 12 h after randomization, supporting the hypothesis that the implementation of the bundle dampens tubular damage and protects from AKI. Hyperglycemia is a common complication after surgery and is reported to occur in 33.7–74% in non-diabetic patients after cardiac surgery [22]. By implementing the KDIGO guidelines, we were able to significantly reduce the rate of hyperglycemia in the intervention group. However, we do not know whether one measure or the combination of different measures is responsible for the reduced AKI rate in the study group.
Recently, Osawa et al. showed that a goal-directed therapy resulted in a significant reduction of the combined endpoint 30-day mortality and major complications but did not affect the occurrence of AKI [23]. However, patients included in this trial were not at high risk for AKI and the occurrence of AKI was far lower than in the PrevAKI trial. Our findings are in line with other studies, showing that a supportive bundle can reduce the occurrence of AKI [20, 24–26].
CSA-AKI is associated with an increased morbidity and mortality [1, 27]. One would assume that reducing the incidence of AKI has an impact on morbidity and mortality. Although we demonstrated in this trial that the implementation of the KDIGO guidelines significantly reduced the occurrence of AKI within 72 h after cardiac surgery, this intervention had no impact on secondary outcomes (ICU and hospital stay, MAKE at days 30, 60, and 90). We offer several possible reasons for these observed results. First, a follow-up duration of 90 days may be too short (e.g., in patients with relatively healthy kidneys and ample renal reserve before surgery). However, the association was robust at 90 days in prior observational studies [28]. A between-group difference during longer follow-up might be more likely if a benefit was not evident after such a short period. Second, mild to moderate AKI (as defined by the KDIGO classification system [5]) may not cause substantial chronic kidney disease (CKD). Multiple observational studies demonstrate a robust association between mild to moderate AKI and long-term declines in kidney function. However, patients who develop AKI (versus those who do not) in almost all observational studies are sicker and have more comorbidities, which also contribute to the development and progression of CKD. Third, the rate of complications was low and the study was not powered to show a difference. These factors may confound some of the observed associations between AKI and PRD [29]. Fourth, we used the KDIGO criteria to diagnose AKI and most AKIs were diagnosed on the basis of a decreased urine output. The association of oliguria with patient-centered outcomes has been shown in a general ICU population (where it is mainly shown for the higher AKI stages) [30], but not in a cardiac surgery population which could be explained by the relatively higher incidence of prerenal causes [31]. Further studies have to be performed to investigate whether an association of the oliguric component and long-term outcomes in cardiac surgery patients exists.
One strength of the PrevAKI trial is the generalizability of the data, because the number of exclusion criteria was limited. However, our active intervention was only used in selected patients with positive biomarker results.
The study is not without limitations. Hemodynamic optimization, glycemic control, and deferring ACEi/ARBs for the first 48 h after cardiac surgery may be already part of the postoperative management in other centers. However, it is known from other fields in medicine (e.g., sepsis) that the compliance to adhere to guidelines is low [32, 33]. According to the EuroSCORE, the 30-day mortality rate of patients in our institution is comparable with the treatment in other centers. Therefore, our data suggest that the adherence to guidelines can reduce the occurrence of CSA-AKI. Although a large difference in the AKI rate was detected, this was not a multicenter trial and, as with many single-center studies, the observed effect size is likely inflated. Another reason why larger trials are needed is that small trials cannot avoid small baseline differences. Therefore, an adequately powered multicenter trial is needed to confirm our results and establish a bundle of supportive measures to reduce the occurrence of CSA-AKI. In addition, this study was not blinded, which could contribute to measurement bias. Finally, we found a non-significant trend towards higher adverse kidney events (MAKE, requirement of RRT, and persistent renal dysfunction) in the intervention group early after surgery. Although the power analysis was performed for the primary endpoint AKI within 72 h after cardiac surgery, it might be possible that implementing this bundle of measures in cardiac surgery patients at high risk for AKI causes a deteriorated patient-centered outcome. Therefore, caution needs to be exercised by interpreting these results and future trials addressing this issue are required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflicts of interest
AZ has received unrestricted grant and lecture fees from Astute Medical as well as lecture fees from Fresenius and Braun. MM has received lecture fees from Astute Medical. The remaining authors declare that they have no conflicts of interest.
Funding/support
The trial is registered at http://apps.who.int/trialsearch/ (Identifier: DRKS00006139). The study was supported by the German Research Foundation (428/6-1 to AZ), the European Society of Intensive Care Medicine, the Innovative Medizinische Forschung (to MM), and an unrestricted research grant from Astute Medical.
Footnotes
The original version of this article was revised due to a retrospective Open Access order.
Take home message: An implementation of the KDIGO guidelines compared with standard care reduced the frequency and severity of cardiac surgery-associated AKI (CSA-AKI) in high risk patients identified by biomarkers. Future studies will be needed to address whether this approach has an impact on long-term outcomes.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-016-4670-3) contains supplementary material, which is available to authorized users.
An erratum to this article is available at https://doi.org/10.1007/s00134-017-4735-y.
References
- 1.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 2.Landoni G, Bove T, Szekely A, Comis M, Rodseth RN, Pasero D, Ponschab M, Mucchetti M, Bove T, Azzolini ML, Caramelli F, Paternoster G, Pala G, Cabrini L, Amitrano D, Borghi G, Capasso A, Cariello C, Carpanese A, Feltracco P, Gottin L, Lobreglio R, Mattioli L, Monaco F, Morgese F, Musu M, Pasin L, Pisano A, Roasio A, Russo G, Slaviero G, Villari N, Vittorio A, Zucchetti M, Guarracino F, Morelli A, De Santis V, Del Sarto PA, Corcione A, Ranieri M, Finco G, Zangrillo A, Bellomo R. Reducing mortality in acute kidney injury patients: systematic review and international web-based survey. J Cardiothorac Vasc Anesth. 2013;27:1384–1398. doi: 10.1053/j.jvca.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Bove T, Zangrillo A, Guarracino F, Alvaro G, Persi B, Maglioni E, Galdieri N, Comis M, Caramelli F, Pasero DC, Pala G, Renzini M, Conte M, Paternoster G, Martinez B, Pinelli F, Frontini M, Zucchetti MC, Pappalardo F, Amantea B, Camata A, Pisano A, Verdecchia C, Dal Checco E, Cariello C, Faita L, Baldassarri R, Scandroglio AM, Saleh O, Lembo R, Calabro MG, Bellomo R, Landoni G. Effect of fenoldopam on use of renal replacement therapy among patients with acute kidney injury after cardiac surgery: a randomized clinical trial. JAMA. 2014;312:2244–2253. doi: 10.1001/jama.2014.13573. [DOI] [PubMed] [Google Scholar]
- 4.Dardashti A, Ederoth P, Algotsson L, Bronden B, Grins E, Larsson M, Nozohoor S, Zinko G, Bjursten H. Erythropoietin and protection of renal function in cardiac surgery (the EPRICS Trial) Anesthesiology. 2014;121:582–590. doi: 10.1097/ALN.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 5. KDIGO AKI Work Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2(1):1–138
- 6.Chawla LS, Goldstein SL, Kellum JA, Ronco C. Renal angina: concept and development of pretest probability assessment in acute kidney injury. Crit Care. 2015;19:93. doi: 10.1186/s13054-015-0779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care. 2012;16:313. doi: 10.1186/cc11426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endre ZH, Walker RJ, Pickering JW, Shaw GM, Frampton CM, Henderson SJ, Hutchison R, Mehrtens JE, Robinson JM, Schollum JB, Westhuyzen J, Celi LA, McGinley RJ, Campbell IJ, George PM. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial) Kidney Int. 2010;77:1020–1030. doi: 10.1038/ki.2010.25. [DOI] [PubMed] [Google Scholar]
- 9.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmele T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, Gorlich D, Kellum JA, Zarbock A. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9:e93460. doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz N, Ronco C. Acute kidney stress—a useful term based on evolution in the understanding of acute kidney injury. Crit Care. 2016;20:23. doi: 10.1186/s13054-016-1184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong W, Yang X, Yan H, Zhang X, Su J, Chen Z, Liao R, Nie Q, Dong S, Zhou Q, Yang J, Tu H, Wu YL. Phase II study of biomarker-guided neoadjuvant treatment strategy for IIIA-N2 non-small cell lung cancer based on epidermal growth factor receptor mutation status. J Hematol Oncol. 2015;8:54. doi: 10.1186/s13045-015-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhardwaj A, Rehman SU, Mohammed AA, Gaggin HK, Barajas L, Barajas J, Moore SA, Sullivan D, Januzzi JL. Quality of life and chronic heart failure therapy guided by natriuretic peptides: results from the ProBNP outpatient tailored chronic heart failure therapy (PROTECT) study. Am Heart J. 2012;164(793–799):e791. doi: 10.1016/j.ahj.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Ronco C, McCullough PA, Chawla LS. Kidney attack versus heart attack: evolution of classification and diagnostic criteria. Lancet. 2013;382:939–940. doi: 10.1016/S0140-6736(13)61932-7. [DOI] [PubMed] [Google Scholar]
- 15.Ronco C. Acute kidney injury: from clinical to molecular diagnosis. Crit Care. 2016;20:201. doi: 10.1186/s13054-016-1373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstadt H, Boanta A, Gerss J, Meersch M. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315:2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 17.Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM, Investigators ET. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 18.Billings FT, Hendricks PA, Schildcrout JS, Shi Y, Petracek MR, Byrne JG, Brown NJ. High-dose perioperative atorvastatin and acute kidney injury following cardiac surgery: a randomized clinical trial. JAMA. 2016;315:877–888. doi: 10.1001/jama.2016.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Görlich D, Kellum JA, Meersch M. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 20.Coca SG, Garg AX, Swaminathan M, Garwood S, Hong K, Thiessen-Philbrook H, Passik C, Koyner JL, Parikh CR, TRIBE-AKI Consortium Preoperative angiotensin-converting enzyme inhibitors and angiotensin receptor blocker use and acute kidney injury in patients undergoing cardiac surgery. Nephrol Dial Transplant. 2013;28:2787–2799. doi: 10.1093/ndt/gft405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ingels C, Derese I, Wouters PJ, Van den Berghe G, Vanhorebeek I. Soluble RAGE and the RAGE ligands HMGB1 and S100A12 in critical illness: impact of glycemic control with insulin and relation with clinical outcome. Shock. 2015;43:109–116. doi: 10.1097/SHK.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 22.Garg R, Grover A, McGurk S, Rawn JD. Predictors of hyperglycemia after cardiac surgery in nondiabetic patients. J Thorac Cardiovasc Surg. 2013;145:1083–1087. doi: 10.1016/j.jtcvs.2012.07.089. [DOI] [PubMed] [Google Scholar]
- 23.Osawa EA, Rhodes A, Landoni G, Galas FR, Fukushima JT, Park CH, Almeida JP, Nakamura RE, Strabelli TM, Pileggi B, Leme AC, Fominskiy E, Sakr Y, Lima M, Franco RA, Chan RP, Piccioni MA, Mendes P, Menezes SR, Bruno T, Gaiotto FA, Lisboa LA, Dallan LA, Hueb AC, Pomerantzeff PM, Kalil Filho R, Jatene FB, Auler Junior JO, Hajjar LA. Effect of perioperative goal-directed hemodynamic resuscitation therapy on outcomes following cardiac surgery: a randomized clinical trial and systematic review. Crit Care Med. 2016;44:724–733. doi: 10.1097/CCM.0000000000001479. [DOI] [PubMed] [Google Scholar]
- 24.Schetz M, Vanhorebeek I, Wouters PJ, Wilmer A, Van den Berghe G. Tight blood glucose control is renoprotective in critically ill patients. J Am Soc Nephrol. 2008;19:571–578. doi: 10.1681/ASN.2006101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolhe NV, Staples D, Reilly T, Merrison D, McIntyre CW, Fluck RJ, Selby NM, Taal MW. Impact of compliance with a care bundle on acute kidney injury outcomes: a prospective observational study. PLoS One. 2015;10:e0132279. doi: 10.1371/journal.pone.0132279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolhe NV, Reilly T, Leung J, Fluck RJ, Swinscoe KE, Selby NM, Taal MW. A simple care bundle for use in acute kidney injury: a propensity score matched cohort study. Nephrol Dial Transplant. 2016;11:1846. doi: 10.1093/ndt/gfw087. [DOI] [PubMed] [Google Scholar]
- 27.Thakar CV, Kharat V, Blanck S, Leonard AC. Acute kidney injury after gastric bypass surgery. Clin J Am Soc Nephrol. 2007;2:426–430. doi: 10.2215/CJN.03961106. [DOI] [PubMed] [Google Scholar]
- 28.Koyner JL, Shaw AD, Chawla LS, Hoste EA, Bihorac A, Kashani K, Haase M, Shi J, Kellum JA, Sapphire I. Tissue inhibitor metalloproteinase-2 (TIMP-2) IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol. 2015;26:1747–1754. doi: 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rifkin DE, Coca SG, Kalantar-Zadeh K. Does AKI truly lead to CKD? J Am Soc Nephrol. 2012;23:979–984. doi: 10.1681/ASN.2011121185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26:2231–2238. doi: 10.1681/ASN.2014070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIlroy DR, Argenziano M, Farkas D, Umann T, Sladen RN. Incorporating oliguria into the diagnostic criteria for acute kidney injury after on-pump cardiac surgery: impact on incidence and outcomes. J Cardiothorac Vasc Anesth. 2013;27:1145–1152. doi: 10.1053/j.jvca.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Levy MM, Pronovost PJ, Dellinger RP, Townsend S, Resar RK, Clemmer TP, Ramsay G. Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome. Crit Care Med. 2004;32:S595–S597. doi: 10.1097/01.CCM.0000147016.53607.C4. [DOI] [PubMed] [Google Scholar]
- 33.Djurkovic S, Baracaldo JC, Guerra JA, Sartorius J, Haupt MT. A survey of clinicians addressing the approach to the management of severe sepsis and septic shock in the United States. J Crit Care. 2010;25(658):e651–e656. doi: 10.1016/j.jcrc.2010.04.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.