Abstract
Unicentric Castleman’s disease (UCD) is a rare disorder of unknown etiology characterized by localized lymphoid tissue proliferation and interfollicular hypervascularity. A 33-year-old Caucasian female presented with vague abdominal discomfort and pain with pressure. Ultrasound and computed tomography detected a large peripancreatic mass. Robotic-assisted resection of the mass along with en bloc dissection of the encased left adrenal gland was done. Frozen section examination confirmed UCD hyaline vascular variant in a retroperitoneal accessory spleen. Preoperative diagnosis of UCD is difficult due to its lack of specific symptoms and its cytologic similarity to reactive lymphadenopathy and other lymphoproliferative disorders. Surgical resection is standard treatment and provides the pathological specimen required for diagnostic confirmation. Here, robotic-assisted laparoscopy allowed visualization, mobilization, precise resection and extraction of the mass from a difficult to access retroperitoneal region.
INTRODUCTION
Castleman’s disease (CD) is a rare disorder characterized by benign angiofollicular lymphoid hyperplasia. Its incidence is believed to be 0.001–0.05% [1]. Definitive diagnosis can only be obtained with surgical pathology [2]. Standard treatment is resection with good prognosis [3]. We report a rare case of CD arising from a retroperitoneal accessory spleen treated by robotic-assisted laparoscopy.
CASE REPORT
A 33-year-old Caucasian female with an unremarkable medical history and vague abdominal discomfort presented with a retroperitoneal peripancreatic mass on ultrasound. A triple-phase intravenous contrast computed tomography (CT) confirmed an inhomogeneous lesion (>7 cm) displacing the stomach and distal pancreas (Fig. 1). Endoscopic ultrasound-guided fine needle aspiration biopsy (EUS-FNA) showed polymorphic lymphocytes and penetrating capillaries. Two months later, a second EUS-FNA revealed the mass had increased in size; the biopsy was negative for malignancy. Resection was indicated due to the patient’s young age and the difficulty of obtaining definitive diagnosis.
Figure 1:
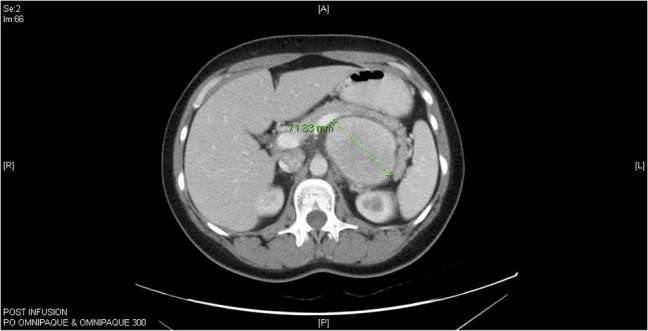
Computed tomography (CT) abdomen horizontal section of Castleman’s disease in accessory spleen.
Exploratory laparoscopy was conducted with the da Vinci Surgical Robot (Intuitive Surgical, Sunnyvale, CA): the 12 mm camera trocar was placed in the left periumbilical region along the midaxillary line. Three 8 mm operative robotic trocars were inserted, one in the left subcostal region, one in the right subcostal region and one in the right periumbilical region along the midaxillary line. Finally, a 12 mm assistant trocar was placed between the camera and left subcostal trocars.
The gastrocolic ligament was opened to enter the lesser sac, exposing the mass bound by the spleen on the left, the abdominal aorta on the right, the splenic vessels and distal pancreas superiorly, and the left renal vessels inferiorly. Mobilization of the transverse colon and splenic flexure downwards and the distal pancreas and spleen upwards allowed visualization of the capsulated and well-circumscribed retroperitoneal mass (Fig. 2). The left adrenal gland was completely encased and had to be dissected en bloc alongside resection of the lesion. The mass had to be carefully detached from the splenic vessels superiorly and the renal vessels inferiorly as these structures were tenaciously attached to it. The specimen was extracted through a 5 cm periumbilical incision and sent for frozen section examination. Operative time was 140 min with minimal blood loss. The patient was discharged postoperative Day 2 without complications.
Figure 2:
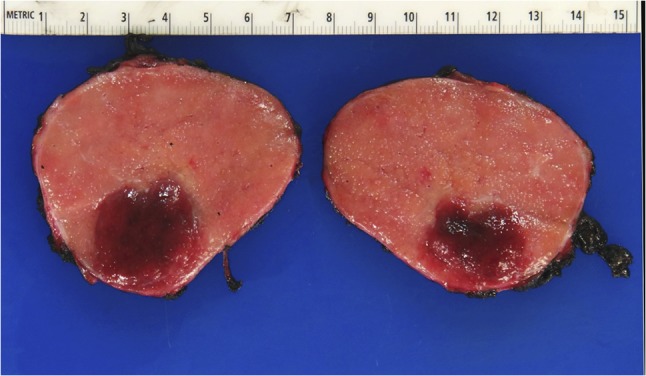
Gross pathology of Castleman’s disease arising from accessory spleen.
Histological examination (Fig. 3) diagnosed an accessory spleen with unicentric Castleman’s disease (UCD) hyaline vascular variant (7.7 × 6.0 × 5.8 cm3) without pathologic changes to the attached lymph node (2.2 × 2.3 × 2.0 cm3) or the encased adrenal gland (5.5 × 1.5 × 0.8 cm3). A blood panel revealed no evidence of leukemia or lymphoma. The patient was seen for follow-up 1 week postsurgery and reported no pain or discomfort. She will continue to be monitored with physical examinations, scans and serum tumor markers.
Figure 3:
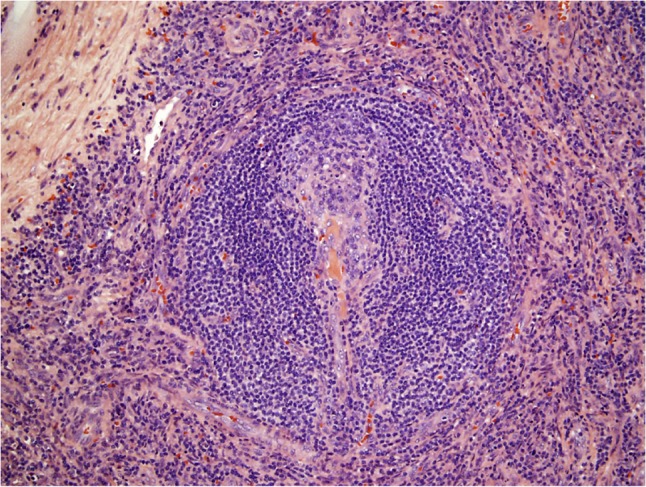
Histological pathology of Castleman’s disease, hyaline vascular variant. Involuted follicular germinal center penetrated by hyalinized small vessels and surrounded by concentric ‘onion rings’ of mantle zone lymphocytes.
DISCUSSION
In 1956, Benjamin Castleman first described the localized form of CD with hyaline vascular histopathology as a solitary, benign enlargement involving hyperplastic lymph nodes [4]. CD can present as UCD or multicentric CD (MCD). Histologically, there are three variants: hyaline vascular, plasma cell and mixed [3]. Although etiology is unknown, immunodeficiency, aberrant autoimmunity and chronic inflammation may contribute to CD pathophysiology. Inflammatory mediator interleukin 6 (IL-6) may play a pivotal role by inducing lymphocyte differentiation and proliferation and stimulating angiogenesis [2, 3, 5].
MCD is a systemic disease with symptoms of fever, weight loss, fatigue, peripheral lymphadenopathy and hepatosplenomegaly [3, 5]. It is associated with human herpes virus 8 (HHV-8) and human immunodeficiency virus (HIV). HHV-8 is thought to manufacture a viral homolog of IL-6 which activates IL-6 signaling pathways and induces endothelial cells to produce IL-6 [5]. Although it is unnecessary to test an individual with UCD for HIV, testing should be done if early-stage MCD is suspected. MCD is also associated with malignancies such as Kaposi’s sarcoma, lymphomas and POEMS syndrome [3, 5]. Due to MCD’s systemic pathology, surgery is not recommended; instead, immunotherapy, chemotherapy and radiotherapy are used [3].
UCD is not associated with infections or malignancies. It is more common than MCD; 72% of CD cases are UCD hyaline vascular variant [3]. UCD distributes in the thorax (30%), neck (23%), abdomen (20%), retroperitoneum (17%), axilla (5%), groin (3%) and pelvis (2%) [6]. Here, we present UCD hyaline vascular variant arising in an accessory spleen. An accessory spleen is a congenital development of splenic tissue away from the spleen occurring in 16% of the population [7]. Our patient’s lesion was discovered accidentally when she came in for an ultrasound after complaining of abdominal pain with pressure. The mass’s heterogeneous composition was most likely due to its substantial size as small UCD tumors are homogeneous [3]. EUS-FNA revealed interfollicular hypervascularity and polymorphous proliferation of small lymphocytes, histiocytes, eosinophils and plasma cells, suggestive of UCD mixed with splenic tissue. It should be emphasized that confirmation of UCD is possible only upon postoperative examination. Imaging can suggest UCD and be used to rule out MCD. However, UCD resembles other tumors on imaging. Preoperative cytology can indicate UCD but cannot exclude reactive lymphadenopathy and other lymphoproliferative disorders. Final pathology confirmed features indicative of UCD hyaline vascular variant on a background of splenic tissue: involuted follicular germinal centers predominated by dendritic cells, concentric rings of mantle zone lymphocytes and penetrating hyalinized small vessels. Following resection, a precautionary leukemia/lymphoma panel was performed and yielded negative results.
The robotic-assisted surgical approach offers 3D imaging and fine motion scaling, tremor elimination and wristed movements with 7 degrees of freedom for dexterity and ergonomy [8]. Postsurgically, patients have shorter length of stay, less pain, decreased opioid consumption and attenuated inflammatory response compared to open procedures [2, 9, 10]. Here, the mass, located in a hard to access region surrounded by delicate vasculature, was precisely resected and extracted with minimal blood loss and no perioperative complications.
UCD should be considered when a mass is symptomatic or when lymphoproliferative features are identified. Diagnosis can only be confirmed with postoperative pathology. Surgical resection is curative and can be safely performed with robotic-assisted procedures.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Mantas D, Damaskos C, Dailiani P, Samarkos M, Korkolopoulou P. Castleman’s disease of the spleen. Acta Chir Belg 2016;117:203–8. [DOI] [PubMed] [Google Scholar]
- 2. Bracale U, Pacelli F, Milone M, Bracale UM, Sodo M, Merola G, et al. Laparoscopic treatment of abdominal unicentric Castleman’s disease: a case report and literature review. BMC Surg 2017;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casper C. The aetiology and management of Castleman disease at 50 years: translating pathophysiology to patient care. Br J Haematol 2005;129:3–17. [DOI] [PubMed] [Google Scholar]
- 4. Castleman B, Iverson I, Menendez VP. Localized lymph node hyperplasia resembling thymoma. Cancer 1956;9:822–30. [DOI] [PubMed] [Google Scholar]
- 5. El-Osta HE, Kurzrock R. Castleman’s disease: from basic mechanisms to molecular therapeutics. Oncologist 2011;16:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman’s disease: a systematic review of 404 published cases. Ann Surg 2012;255:677–84. [DOI] [PubMed] [Google Scholar]
- 7. Mortelé KJ, Mortelé B, Silverman SG. CT features of the accessory spleen. Am J Roentgenol 2004;183:1653–7. [DOI] [PubMed] [Google Scholar]
- 8. Zenoni S, Arnoletti JP, De la Fuente S. Minimally invasive approach for patients requiring pancreatoduodenectomy. JAMA Surg 2013;148:1154–7. [DOI] [PubMed] [Google Scholar]
- 9. Cohn DE, Castellon-Larios K, Huffman L, Salani R, Fowler JM, Copeland LJ, et al. A prospective, comparative study for the evaluation of postoperative pain and quality of recovery in patients undergoing robotic versus open hysterectomy for staging of endometrial cancer. J Minim Invasive Gynecol 2016;23:429–34. [DOI] [PubMed] [Google Scholar]
- 10. Okholm C, Goetze JP, Svendsen LB, Achiam MP. Inflammatory response in laparoscopic vs. open surgery for gastric cancer. Scand J Gastroenterol 2014;49:1027–34. [DOI] [PubMed] [Google Scholar]


