Abstract
Objective
Evidence suggests that adherence to the Mediterranean (MedDiet) or MIND diet is neuroprotective but the association between these dietary patterns and cognition has not been evaluated in a nationally representative population of older US adults.
Design
Population-based cross-sectional study.
Participants/setting
Community-dwelling older adults from the Health and Retirement Study (n = 5,907).
Measurements
Adherence to dietary patterns was determined from food frequency questionnaires using a priori criteria to generate diet scores for MedDiet (range = 0–55) and MIND diet (range 0–15). Cognitive performance was measured using a composite test score of global cognitive function (range 0–27). Linear regression was used to compare cognitive performance across tertiles of dietary pattern. Logistic regression was used to examine the association between dietary patterns and clinically significant cognitive impairment. Models were adjusted for age, gender, race, educational attainment and other health and lifestyle covariates.
Results
Mean age of participants was 68 ± 10.8 years. Compared to those with low MedDiet score, participants with mid and high score were less likely to have poor cognitive performance (OR 0.85; 95% CI 0.71, 1.02: P = 0.08, and OR 0.65; 95% CI: 0.52, 0.81: P < 0.001, respectively) in fully adjusted models. Results for the MIND diet were similar. Higher score in each dietary pattern was independently associated with significantly better cognitive function (P < 0.001) in a dose-response manner (PTREND < 0.001).
Conclusion
In a large nationally representative population of older adults, greater adherence to the MedDiet and MIND diet was independently associated with better cognitive function and lower risk of cognitive impairment. Clinical trials are required to elucidate the role of dietary patterns in cognitive aging.
Keywords: dietary patterns, cognitive performance
INTRODUCTION
Dementia is a major cause of death and disability in older Americans1 and there is considerable interest in identifying lifestyle approaches, such as diet, for prevention of cognitive decline during aging2.
The Mediterranean diet (MedDiet), rich in fruit, vegetables, wholegrains, nuts, olive oil and fish, is proven to have vascular3 and anti-inflammatory4 benefits and may also be neuroprotective. Greater adherence to the MedDiet is associated with slower rate of cognitive decline5–6, reduced risk of cognitive impairment7–8 and dementia5,8 but findings are conflicting9–11 largely owing to significant heterogeneity between studies in terms of populations studied and methods used to assess diet and cognition. Studies from the US have limited generalizability due to a lack of representative study populations and multiple publications from the same cohorts. Additionally, most prospective studies have used population-specific median food intake thresholds to measure MedDiet adherence and this approach further limits the generalizability and comparability of findings, as similar scores reflect different eating patterns in different cohorts12. The MedDiet score13 is a different approach which uses absolute food intake targets derived from a Greek population and allows for more meaningful comparison between studies. Higher MedDiet score has been associated with slower rate of cognitive decline14–16 in a small number of studies that have used this dietary assessment method.
In summary, evidence to date is suggestive of a neuroprotective role for MedDiet but variation between studies makes it difficult to draw firm conclusions. Further investigation is needed to determine whether the MedDiet represents an optimal dietary pattern for protection against neurodegeneration in representative populations.
Another proposed neuroprotective dietary pattern, called MIND (Mediterranean-DASH diet Intervention for Neurodegeneration Delay), has been recently described16. The MIND diet is a modified version of MedDiet but incorporates additional foods based on current evidence in the diet-dementia field16. In one population-based study, the MIND score was more predictive of cognitive decline than the MedDiet score16 and higher MIND score was associated with reduced Alzheimer’s disease (AD)17. While these results in mostly older white females are encouraging, they require confirmation in other populations.
We aimed to determine the association between proposed neuroprotective dietary patterns characterized by the MedDiet and MIND scores, and objectively measured cognitive performance in a large sample of older adults from the nationally-representative population-based Health and Retirement Study (HRS).
METHODS
We used data from the HRS, a longitudinal, nationally representative survey in 30,000 community-dwelling adults aged > 50 years. The HRS commenced in 1992 to collect data on the antecedents and consequences of retirement in US adults and follows approximately 20,000 participants biennially. A detailed description of HRS has been published elsewhere18. The HRS was approved by the Health Sciences Institutional Review Board at the University of Michigan. All participants provided their consent on enrollment.
This present study is a cross-sectional analysis of participants from a core wave 12 survey (2014) who completed the HRS Health Care and Nutrition (HCNS) substudy (n = 8,035). The HCNS diet assessment was conducted between November 2013 and May 2014, and cognitive, demographic and covariate data were drawn from the core 2014 survey. We excluded respondents who required a by-proxy core 2014 interview and those with missing or incomplete cognitive data (n = 981). We also excluded those who reported extreme energy intakes outside of predefined levels (<800 or >8000 kcal/d for men and <600 or >6000 kcal/d for women) (n =291) and those who reported dementia or AD (n = 140) or stroke (n = 430), and those with missing covariates (n = 286). After exclusions, the final analytic sample was 5,907 participants.
Dietary assessment
Dietary intake was assessed using a validated 163-item semi-quantitative Harvard Food Frequency Questionnaire (FFQ)19,20. Adherence to MedDiet and MIND dietary patterns was assessed by calculating summary scores using predefined criteria13,16 (as shown in Supplementary Table S1 and S2). First, we selected FFQ food item(s) to create dietary components relevant for each dietary pattern. Next, we assigned individual scores for dietary components based on the frequency of recommended intake servings.
MedDiet score
MedDiet score13 comprises 11 dietary components corresponding to consumption frequency of foods consistent with the traditional MedDiet. Dietary components were scored 0–5 in agreement with predefined frequencies of serving for each point value and then summed to obtain a total score ranging from 0 to 55. Scores for dietary components consistent with the MedDiet (nonrefined grains, fruits, vegetables, potatoes, legumes, fish, olive oil) increase as consumption frequency increases and scores for food groups not characteristic of a MedDiet (red meat, poultry, full fat dairy products) decrease as consumption frequency increases. Alcohol intake was determined using frequency of alcoholic drinks daily (1 drink equivalent to 150mls; approximately 12g ethanol) and scored nonlinearly, with a score of 0 for no consumption or >4.5 drinks/day through to a maximum score of 5 for up to 2 drinks/day. Overall, higher MedDiet score indicates greater adherence to the traditional MedDiet.
MIND score
MIND score16 consists of 15 dietary components in which 10 are considered brain healthy food groups (green leafy vegetables, other vegetables, nuts, berries, beans, whole grains, seafood, poultry, olive oil, and wine) and five are considered unhealthy food groups (red meats, butter and stick margarine, cheese, pastries and sweets, and fried/fast food). Dietary components were scored 0, 0.5, or 1 depending on level of consumption. Olive oil use was scored 1 if intake ≥ 1 tbsp. daily and 0 otherwise. Scores for the 10 healthy components increased monotonically with higher consumption of reported servings, and scores were reversed for the five unhealthy components. Dietary component scores were then summed to obtain an overall score ranging from 0–15, where higher scores indicate greater adherence to the MIND diet.
Cognitive assessment
Cognitive performance was assessed by a global cognition score comprising three items: (1) immediate and delayed recall of 10 words from a word list randomly assigned for each participant (0–20 points), (2) backward counting (0–2 points), and, (3) serial seven subtraction (0–5 points)21. Possible scores ranged from 0 to 27, with higher scores indicating better overall cognitive function in domains of episodic memory, attention and working memory22. Clinically significant poor cognitive performance was defined as ≥1 SD below the mean global cognition score.
Covariates
Covariates of age, gender and race (white, black or other) were included. We also selected health and lifestyle covariates previously identified as potential modifiable risk factors for cognitive decline and dementia2: smoking, hypertension, diabetes, depression, low educational attainment, physical inactivity and obesity. Depressive symptoms were determined using a Center for Epidemiologic Studies Depression (CES-D8) short form score (score 0–8) with active depression symptoms defined as a CES-D8 cut point of ≥423. Low educational attainment was classified as completing less than high school education and physical inactivity was defined as engaging in vigorous activity less than twice weekly, as used in a previous HRS analysis24. Obesity was defined as a Body Mass Index (BMI) ≥30 kg/m2.
Statistical analysis
Participant characteristics were compared with tertiles of dietary pattern scores using descriptive statistical tests. Analysis of variance with Bonferroni post hoc comparison was used for continuous variables and chi-square test was used for categorical variables, with corresponding tests for linear trend. Pearson’s correlation coefficient was used to examine correlations for continuous variables. A multivariable general linear model was applied to investigate associations between dietary patterns (MedDiet and MIND score modelled in tertiles) and global cognition score. Participants in tertile 1 (lowest diet adherence) were the reference group for each analysis. Models were adjusted firstly for classic confounders age, gender, race and educational attainment (less than high school vs high school or more), and subsequently for potential mediators total wealth as a measure of socioeconomic status (total assets – total debt), hypertension (Yes/No), diabetes (Yes/No), current smoking (Yes/No), depression (CES-D8 ≥4), physical inactivity (Yes/No), obesity (BMI ≥30 vs BMI <30) and total energy intake (kcals/day). The risk of poor cognitive performance associated with adherence to each dietary pattern was estimated by using binary logistic regression analyses with corresponding odds ratios (OR) and 95% confidence intervals (CI), adjusted for covariates using the same approach described above. Sensitivity analyses were carried out after removal of individuals classified as demented on the global cognition score. In addition, analyses were repeated after applying a priori defined Greek cut-points to MedDiet tertiles (0–20, 21–35 and 36–55). Analyses were performed using SPSS version 22 (IBM SPSS, Chicago, IL).
RESULTS
The mean (SD) age of the 5, 907 participants was 68 ± 10.8 years at the core 2014 survey. Overall, 60% were women and 78% were white. Mean diet score was 27.6 ± 5.4 for MedDiet and 7.3 ± 1.8 for MIND, indicating moderate adherence for each dietary pattern. Average MedDiet score was similar to that reported in a Greek population 26.3 ± 3.213. As shown in Table 1, participants with highest MedDiet adherence were younger, more likely to be physically active and less likely to be hypertensive, diabetic or obese, with higher educational attainment and fewer reported depressive symptoms, compared with those with lowest adherence. Demographics were similar for MIND, but there was no observed difference in diabetes across tertiles of MIND score.
Table 1.
Participant Characteristics by Tertiles of MedDiet and MIND Diet Scores (n = 5,907)
| MedDiet Scorea | MIND Scoreb | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Tertile 1 (LOW;≤25) |
Tertile 2 (MID; 26–30) |
Tertile 3 (HIGH; >30–55) |
P for Trend |
Tertile 1 (LOW; ≤6.5) |
Tertile 2 (MID; >6.5–8.0) |
Tertile 3 (HIGH; >8.0–15.0) |
P for Trend |
|
|
|
||||||||
| n | 2110 | 2064 | 1733 | - | 2219 | 1825 | 1863 | - |
|
|
||||||||
| Age, mean (SD), y | 68.2 (10.6) | 67.8 (10.4) | 67.1 (10.7) | 0.001 | 68.5 (10.6) | 68.2 (10.6) | 66.5 (10.4) | <0.001 |
| Female, n (%) | 1261 (60) | 1215 (59) | 1072 (62) | 0.22 | 1235 (56) | 1067 (59) | 1246 (67) | <0.001 |
| Race, n (%) | ||||||||
| White | 1636 (78) | 1627 (79) | 1326 (77) | 1790 (80) | 1406 (77) | 1393 (75) | ||
| Black | 360 (17) | 301 (15) | 233 (13) | <0.001 | 299 (14) | 298 (16) | 297 (16) | <0.001 |
| Other | 114 (5) | 136 (7) | 174 (10) | 130 (6) | 121 (7) | 173 (9) | ||
| Energy intake, mean (SD), kcals | ||||||||
| Male | 1940 (862) | 1899 (826) | 2167 (881) | <0.001 | 1883 (801) | 2008 (889) | 2131 (899) | <0.001 |
| Female | 1641 (731) | 1693(762) | 2040 (815) | <0.001 | 1617 (708) | 1784 (821) | 1935 (798) | <0.001 |
| Education less than high school, n (%) | 397 (19) | 243 (12) | 195 (11) | <0.001 | 369 (17) | 270 (15) | 196 (11) | <0.001 |
| Current smoker, n (%) | 332 (16) | 207 (10) | 93 (5) | <0.001 | 355 (16) | 172 (9) | 105 (6) | <0.001 |
| Clinically obese, n (%) | 1029 (49) | 959 (47) | 673 (39) | <0.001 | 1034 (47) | 845 (46) | 782 (42) | 0.004 |
| Hypertension, n (%) | 1359 (64) | 1212 (59) | 933 (54) | <0.001 | 1384 (62) | 1103 (60) | 1017 (55) | <0.001 |
| Diabetes, n (%) | 538 (26) | 421 (20) | 332 (19) | <0.001 | 498 (22) | 413 (23) | 380 (20) | 0.13 |
| CES-D8 depression, n (%) | 598 (28) | 424 (21) | 312 (18) | <0.001 | 592 (27) | 392 (22) | 350 (19) | <0.001 |
| Physically inactive, n (%) | 1732 (82) | 1517 (74) | 1058 (61) | <0.001 | 1724 (80) | 1349 (74) | 1184 (64) | <0.001 |
| Diet components, mean (SD), serving/week | ||||||||
| Wholegrains | 4.9 (6.1) | 6.9 (6.6) | 9.0 (7.9) | <0.001 | 4.9 (5.8) | 6.5 (6.5) | 9.7 (8.1) | <0.001 |
| Vegetables | 9.8 (7.1) | 17.2 (10.4) | 26.8 (14.2) | <0.001 | 11.3 (8.5) | 16.1 (10.7) | 26.6 (14.5) | <0.001 |
| Fruit | 6.8 (6.1) | 10.3 (7.8) | 15.4 (10.8) | <0.001 | 6.6 (6.3) | 10.1(8.1) | 16.1(10.3) | <0.001 |
| Red meat | 5.8 (4.2) | 5.4 (4.0) | 4.2 (3.4) | <0.001 | 6.2 (4.4) | 5.0 (3.8) | 4.0 (3.2) | <0.001 |
| Fish | 0.5 (0.6) | 0.9 (0.9) | 1.4 (1.3) | <0.001 | 0.5 (0.6) | 0.8 (0.9) | 1.4 (1.4) | <0.001 |
| Nuts | 1.3 (2.5) | 2.1 (3.3) | 3.8 (4.9) | <0.001 | 1.1 (2.2) | 2.0 (3.3) | 4.2 (5.0) | <0.001 |
MedDiet = Mediterranean Diet; MIND =Mediterranean-DASH diet Intervention for Neurodegenerative Delay;
Possible range 0–55;
Possible range 0–15; CES-D8 = Center for Epidemiologic Studies Depression short form
Both diet scores were positively correlated (r = 0.68, P < 0.001) and showed a fair level of agreement in the population (Cohen’s kappa 0.36, P < 0.001).Weekly servings of wholegrains, vegetables, fruit, fish, nuts and olive oil increased linearly across tertiles for each dietary pattern (PTREND < 0.001) with individuals in the high tertile consuming between 2–3 times more than those in the low tertile. Conversely, weekly consumption of red meat decreased linearly across tertiles of diet score (PTREND < 0.001).
Table 2 shows unadjusted and adjusted global cognition score across tertiles of dietary patterns. Compared to participants with mid or low levels of adherence, those with high adherence to MedDiet or MIND had significantly better cognitive performance (P < 0.001 for both dietary patterns). In fully adjusted models, these associations were attenuated but individuals with highest diet adherence had significantly better cognitive scores (by 1.0 and 0.8 points for MedDiet and MIND respectively) than those with mid and low adherence and these associations showed a dose-response relationship (PTREND < 0.001).
Table 2.
Unadjusted and Adjusted Mean (SE) of Global Cognition Score by Tertile of MedDiet and MIND Diet Score (n = 5,907)
| MedDiet score | MIND diet score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| LOW | MID | HIGH |
P for Trend |
LOW | MID | HIGH |
P for Trend |
||
|
|
|||||||||
| n = 2110 | n = 2064 | n = 1733 | n = 2219 | n = 1825 | n = 1863 | ||||
|
|
|||||||||
| Global cognition scorea | Unadjusted | 14.5 (0.09) | 15.3 (0.09) | 16.0 (0.10) | <0.001 | 14.6 (0.09) | 15.2 (0.10) | 16.0 (0.10) | <0.001 |
| Model 1b | 14.7 (0.09) | 15.2 (0.09) | 15.9 (0.09) | <0.001 | 14.8 (0.08) | 15.2 (0.09) | 15.8 (0.09) | <0.001 | |
| Model 2c | 14.8 (0.09) | 15.2 (0.08) | 15.7 (0.10) | <0.001 | 14.9 (0.10) | 15.2 (0.09) | 15.6 (0.09) | <0.001 | |
Possible range 0–27.
Adjusted for gender, age, race (white, black, other), low education attainment (less than high school completed),
Model 1 adjusted for current smoking, total wealth (= assets − debt), obesity (BMI ≥30 kg/m2), hypertension, diabetes, physical inactivity; depression (CES-D8 ≥4) and total energy intake (kcals/d).
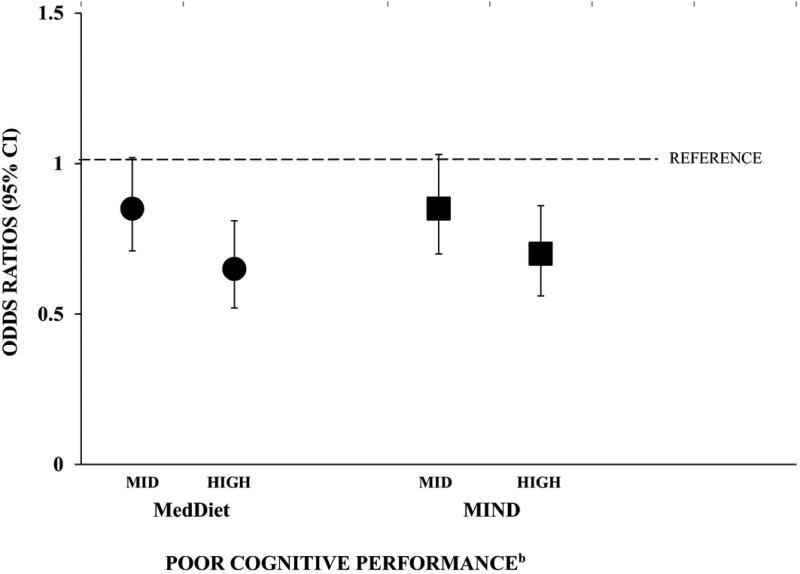
Impaired cognitive performance, defined as > 1SD (4.3 points) below the mean global cognitive score, was found in 831 (14%) participants. Figure 1 shows the adjusted likelihood of having poor cognitive performance with adherence to the dietary patterns. Compared to participants with low MedDiet score, those with mid score had 15 % lower odds of having poor cognitive performance (OR 0.85; 95% CI: 0.71, 1.02: P = 0.08). The association was significantly stronger for those with highest MedDiet score who had 35% lower odds of having poor cognitive performance compared to those with lowest score (OR 0.65; 95% CI: 0.52, 0.81: P < 0.001). Results were similar for individuals with mid and high MIND score (OR 0.85; 95% CI 0.70, 1.03: P =0.10 and OR 0.70; 95% CI: 0.56, 0.86: P = 0.001, respectively) when compared to those with low MIND score. In fully adjusted linear models, each 1 SD increase (5.4 units) in MedDiet was associated with 15% lower odds of poor cognitive performance (OR 0.85; 95% CI 0.78, 0.93, P < 0.001) and each 1 SD increase (1.8 units) in MIND diet was associated with 14% lower odds of poor cognitive performance (OR 0.86; 95% CI 0.79, 0.94, P < 0.001).
Figure 1.
Adjusteda Odds Ratios (95% CI) for Poor Cognitive Performance by Mid and High Tertiles Compared to Low Tertile (reference) of MedDiet and MIND Diet Scores
Analyses were repeated after removing participants with global cognition scores ≤ 6 (n = 143) but no notable changes were found in observed results. We also repeated the analyses using a priori defined cut-points for MedDiet tertiles derived from a Greek population13 and similar results were observed. In fully adjusted models, individuals in the highest Greek MedDiet tertile had 35% lower odds of cognitive impairment OR 0.65; 95% CI: 0.44, 0.98: P = 0.04) compared with those in the lowest Greek tertile.
DISCUSSION
In this large general population of community-dwelling older adults, neuroprotective dietary patterns characterized by MedDiet and MIND score were significantly associated with moderately better cognitive performance in a dose-response relationship. Individuals with the highest adherence to neuroprotective diets had a 30–35% lower risk of cognitive impairment defined as > 1SD or 4.3 points below the population mean global cognition score. While, the incidence of clinical cognitive impairment on the global cognition score was relatively low (14%) in this healthy population, our findings lend support to the hypothesis that diet modification may be an important public health strategy to protect against neurodegeneration during aging.
This study adds to the limited work done to investigate relations between dietary patterns and brain health. Although previous prospective studies examining associations between MedDiet and cognitive outcomes have largely reported contradictory findings, evidence is strengthened by recent results from the PREDIMED trial sub-study which demonstrated small but significant improvements in cognitive function in response to increasing MedDiet adherence26. To date, the effects of MIND on cognitive health have not been evaluated, however, greater adherence to MIND is linked with slower rates of cognitive decline16 and reduced risk of AD17. These studies have been conducted exclusively in one older, largely female, population from the Rush Memory and Aging Project and require replication in other cohorts. Our findings support a protective association of MIND on cognitive performance in a general population.
MedDiet and MIND have similar dietary profiles and recommend high intakes of plant foods, limited meat consumption, moderate intake of alcohol (wine in particular) and use of olive oil as a primary fat source. Unique to MIND are green vegetables and berries which are independently reported to offer protection against neurodegeneration12. In contrast, the MedDiet places greater emphasis on potatoes, fish and overall fruit and vegetable intake. Both dietary patterns are rich in antioxidants, monounsaturated and n-3 fatty acids and low in saturated fat. These individual nutrients have also been independently related to cognitive performance, for example, observational evidence has shown association between monounsaturated fat and n-3 fatty acids and a reduced risk of cognitive decline and dementia5, whereas increased saturated fat intake is shown to increase risk of cognitive decline and dementia27. However, the biological mechanisms for how dietary patterns exert neuroprotective effects are not clear. Several putative mechanisms for the MedDiet have been proposed28, and include beneficial impacts on neuronal cell signalling, vascular, antioxidant and anti-inflammatory biological pathways, but more comprehensive investigation is required. Furthermore, while the MedDiet and the new MIND diet have attracted most attention in the literature, they may not reflect an optimal dietary pattern for protection against neurodegeneration during aging.
Strengths of this study include its large sample size and community-based population of older adults which increases the external validity of findings. In addition, an extensively validated semi-quantitative FFQ was used to assess the dietary exposure. Furthermore, we generated dietary scores based on predefined absolute food intake thresholds and this approach increases the ability to meaningfully compare our findings with studies that employ a similar standardized dietary pattern methodology. A major limitation is the cross-sectional study design meaning we were unable to establish a causal relationship between dietary patterns and cognitive outcomes. In addition, dietary misclassification is possible as individuals may have changed their eating behavior as a result of cognitive impairment or other disease, although in our sensitivity models, removal of those with low cognitive scores did not alter the findings. As with all observational study, residual confounding is a possibility even though we adjusted the analyses for known diet-dementia confounders. Finally, the use of a summary cognition score allowed us to examine global cognitive function but not individual cognitive domains which may be differentially influenced by age and lifestyle factors.
In conclusion, this study shows that greater adherence to MedDiet and MIND dietary patterns are associated with better overall cognitive function in older adults and lower odds of cognitive impairment that could have important public health implications for preservation of cognition during aging. Given the limited evidence base and lack of clear dietary recommendations for cognitive health, further prospective population-based studies and clinical trials are required to elucidate the role of dietary patterns in cognitive aging and brain health.
Supplementary Material
Acknowledgments
Funding source: Dr. Yaffe is supported, in part, by a K24 Midcareer Investigator Award (AG031155) from the National Institute on Aging. Dr. McEvoy is supported by the Wellcome Trust Seed Award in Science 2016 (202097/Z/16/Z). Dr. Langa was supported by grants P30 AG053760 and P30 AG024824 from the National Institute on Aging (NIA). The Health and Retirement Study is funded by the NIA (U01 AG009740), and performed at the Institute for Social Research, University of Michigan.
Dr. Yaffe serves on Data Safety and Monitoring Boards for Takeda, Inc. and an NIH sponsored study, and she is a member of the Alzheimer's Association Medical and Scientific Advisory Council and a Senate member of the Council of the German Center for Neurodegenerative Diseases.
Sponsor's Role. The HRS was supported by a cooperative agreement (Grant U01 AG09740) between the National Institute on Aging and the University of Michigan. The National Institute on Aging (NIA) provided funding (U01 AG009740) for the 2013 HCNS, which was conducted by the Survey Research Center, at the Institute for Social Research, at the University of Michigan. The Sponsors had no role in the design, methods, analysis and preparation of this paper
Footnotes
Conflict of Interest. The authors have no relevant financial or personal conflicts to declare.
Author Contributions: CTM, KY: study design. CTM, KY, HG: analysis and data interpretation. CTM, KY, HG, KML: preparation of manuscript.
References
- 1.2016 Alzheimer’s disease facts and figures. [Accessed October 27 2016]; Available at: https://www.alz.org/documents_custom/2016-facts-and-figures.pdf.
- 2.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord Drug Targets. 2014;14:245–254. doi: 10.2174/1871530314666140922153350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 5.Lourida I, Soni M, Thompson-Coon J, et al. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24:479–489. doi: 10.1097/EDE.0b013e3182944410. [DOI] [PubMed] [Google Scholar]
- 6.van de Rest O, Berendsen AA, Haveman-Nies A, et al. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;13(6):154–168. doi: 10.3945/an.114.007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feart C, Samieri C, Barberger-Gateau P. Mediterranean diet and cognitive health: an update of available knowledge. Curr Opin Clin Nutr Metab Care. 2015;18:51–62. doi: 10.1097/MCO.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 8.Singh B, Parsaik AK, Mielke MM, et al. Association of mediterranean diet with mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;39:271–282. doi: 10.3233/JAD-130830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsson E, Karlström B, Kilander L, et al. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J Alzheimers Dis. 2015;43:109–119. doi: 10.3233/JAD-140867. [DOI] [PubMed] [Google Scholar]
- 10.Samieri C, Grodstein F, Rosner BA, et al. Mediterranean diet and cognitive function in older age. Epidemiology. 2013;24:490–499. doi: 10.1097/EDE.0b013e318294a065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vercambre MN, Grodstein F, Berr C, et al. Mediterranean diet and cognitive decline in women with cardiovascular disease or risk factors. J Acad Nutr Diet. 2012;112:816–823. doi: 10.1016/j.jand.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris MC. Nutrition and risk of dementia: Overview and methodological issues. Ann N Y Acad Sci. 2016;1367:31–37. doi: 10.1111/nyas.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panagiotakos DB, Pitsavos C, Arvaniti F, et al. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. 2007;44:335–340. doi: 10.1016/j.ypmed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Tangney CC, Kwasny MJ, Li H, et al. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. 2011;93:601–607. doi: 10.3945/ajcn.110.007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyama A, Houston DK, Simonsick EM, et al. Association between the Mediterranean diet and cognitive decline in a biracial population. J Gerontol A Biol Sci Med Sci. 2015;70:354–359. doi: 10.1093/gerona/glu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11:1015–1022. doi: 10.1016/j.jalz.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris MC, Tangney CC, Wang Y, et al. MIND diet associated with reduced incidence of Alzheimer's disease. Alzheimers Dement. 2015;11:1007–1014. doi: 10.1016/j.jalz.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonnega A, Faul JD, Ofstedal MB, et al. Cohort Profile: the Health and Retirement Study (HRS) Int J Epidemiol. 2014;43:576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 20.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 21.Herzog AR, Wallace RB. Measures of cognitive functioning in the AHEAD Study. J Gerontol B Psychol Sci Soc Sci. 1997;52:37–48. doi: 10.1093/geronb/52b.special_issue.37. [DOI] [PubMed] [Google Scholar]
- 22.Crimmins EM, Kim JK, Langa KM, et al. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66:i162–i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steffick D. Documentation of Affective Functioning Measures in the Health and Retirement Study. Ann Arbor, MI: University of Michigan; 2000. [Google Scholar]
- 24.Latham K, Peek CW. Self-rated health and morbidity onset among late midlife U.S. adults. J Gerontol B Psychol Sci Soc Sci. 2013;68:107–116. doi: 10.1093/geronb/gbs104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jessen F, Amariglio RE, Subjective Cognitive Decline Initiative (SCD-I) Working Group et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valls-Pedret C, Sala-Vila A, Serra-Mir M, et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern Med. 2015;175:1094–1103. doi: 10.1001/jamainternmed.2015.1668. [DOI] [PubMed] [Google Scholar]
- 27.Morris MC, Tangney CC. Dietary fat composition and dementia risk. Neurobiol Aging. 2014;35:S59–S64. doi: 10.1016/j.neurobiolaging.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knight A, Bryan J, Murphy K. Is the Mediterranean diet a feasible approach to preserving cognitive function and reducing risk of dementia for older adults in Western countries? New insights and future directions. Ageing Res Rev. 2016;25:85–101. doi: 10.1016/j.arr.2015.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.