Abstract
Objectives
Nigella sativa (black seed or black cumin), which belongs to the Ranunculacea family, is an annual herb with many pharmacological properties. Among its many active constituents, thymoquinone (TQ) is the most abundant constituent of the volatile oil of Nigella sativa (N. sativa) seeds, and it is the constituent to which most properties of this herb are attributed.
Methods
PubMed-Medline, Scopus, and Web of Science databases were searched to identify randomized control trials (RCTs) investigating the therapeutic effects of N. sativa and/or TQ. In this review, we investigated the clinical uses of N. sativa and TQ in the prevention and the treatment of different diseases and morbidity conditions in humans.
Results
Black seed and TQ are shown to possess multiple useful effects for the treatment of patients with several diseases, such as inflammatory and auto-immune disorders, as well as metabolic syndrome. Also, other advantages, including antimicrobial, anti-nociceptive and anti-epileptic properties, have been documented. The side effects of this herbal medicine appear not to be serious, so it can be applied in clinical trials because of its many advantages.
Conclusion
Some effects of N. sativa, such as its hypoglycemic, hypolipidemic and bronchodilatory effects, have been sufficiently studied and are sufficiently understood to allow for the next phase of clinical trials or drug developments. However, most of its other effects and applications require further clinical and animal studies.
Keywords: black seed, clinical trials, diseases, Nigella sativa, safety, thymoquinone
1. Introduction
Nigella sativa (black seed or black cumin), which belongs to the Ranunculacea family, is an annual herb with many pharmacological properties [1]. The use of N. sativa (NS) seeds and oil in traditional remedies goes back more than 2000 years, and the herb is described as ‘the Melanthion’ by Hippocrates and Discroides [2]. Black seeds and their oil have a long history of folklore usage in the Indian and the Arabian civilizations as food and medicine and have been commonly used as treatment for a variety of health conditions pertaining to the respiratory system, digestive tract, kidney and liver functions, cardiovascular system, and immune system support, as well as for general well-being [3, 4]. NS contains many active components, such as thymoquinone (TQ), alkaloids (nigellicines and nigelledine), saponins (alpha-hederin), flavonoids, proteins, fatty acids, and many others, that have positive effects in the treatment of patients with different diseases [5, 6]. TQ is the most abundant constituent in the volatile oil of NS seeds, and most of the herb’s properties are attributed to it.
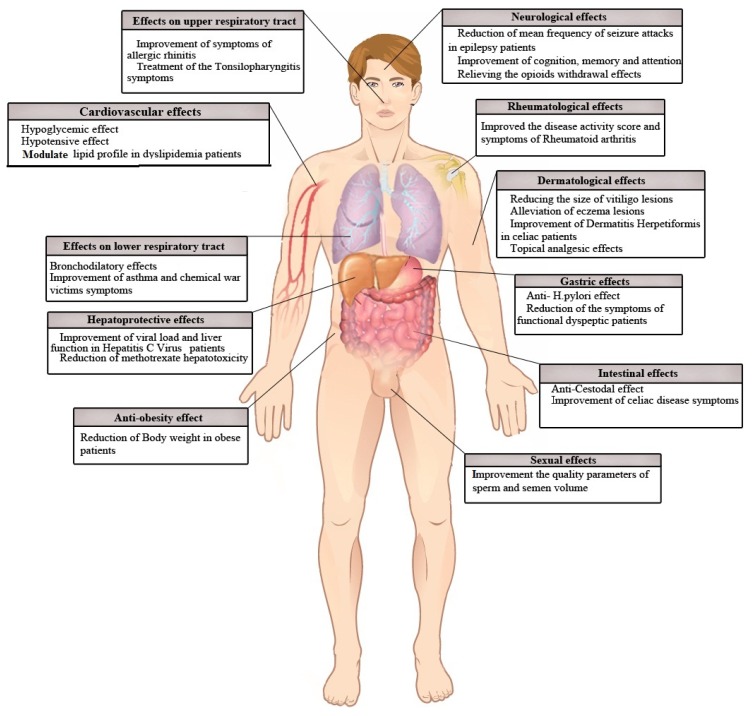
Cell culture studies and animal models have indicated several therapeutic potentials, such as anti-cancer [5, 7–9], antimicrobial [10, 11], analgesic [12, 13], antipyretic [14], contraceptive and anti-fertility, anti-oxytocic [3], anti-tussive [15], anti-inflammatory [12, 16], and anti-oxidant [17–19] potentials, for black seed and its active component TQ. NS or TQ anticancer activity has been demonstrated for blood, breast, colon, pancreatic, liver, lung, fibrosarcoma, prostate, and cervix cancer cell lines and in animal models of lung, kidney, skin, colon, and breast cancer [7]. Black seed’s antimicrobial effects include those on gram-negative and gram-positive bacteria, viruses, parasites, Schistosoma, and fungi pathogens [10]. NS was also found to be able to relieve the symptoms of or cure patients with several diseases, such as hypertension, dyslipidemia, metabolic syndrome, diabetes [5, 20, 21], asthma [3], convulsion [22–24], and natural and chemical toxicities [25, 26]. Additionally, a suggestion was made that NS and TQ utilization could prevent many disorders [6], including neurobehavioral [27], kidney [28, 29], and liver [4] disorders. For these reasons, in this review, we investigated the clinical uses of NS and TQ in the prevention and treatment of different diseases and morbidity conditions in humans. Fig. 1.
Figure 1.
Schematic description for the effects of Nigella sativa in different parts of the human body.
2. Methods
PubMed-Medline, Scopus, and Web of Science databases were searched to identify randomized control trials (RCTs) investigating the therapeutic effects of NS and/or TQ. We reviewed the existing literature published until April 2016 by using the following keywords: “Nigella sativa”, “black cumin”, “black seeds”, “thymoquinone”, and “patient”, “clinic” or “clinical trial”. All studies assessing the effects of NS or TQ, topical use or oral intake, on human health conditions were included.
3. Results
3.1. Safety
Administration of NS oil (5 mL/day) to healthy volunteers for 8 weeks was reported not to have any notable liver, kidney, or gastrointestinal side effects [30, 31]. NS oil intake (equivalent to oil obtained from 0.7 g of seeds) for 40 days showed reasonable kidney and liver safety in patients with type 2 diabetes mellitus (DM); neither did it alter the platelet or the total leukocyte count [32]. Another study performed on 39 centrally obese men demonstrated that intake of NS seeds (3 g/day for 3 months) had no detectable side effects [33]. Qidwai et al reported that the administration of NS seeds (2 g/day for 6 weeks) did not affect the serum alanine aminotransferase (ALT) or the serum creatinine (Cr) levels in adults [34]. Furthermore, NS at doses of 1, 2 and 3 g/day for 3 months did not adversely affect either the renal or the hepatic functions of diabetic patients [35]. Another study revealed that NS seed powder intake for 40 days caused no significant change in total leukocyte and platelet count [36].
Treatment with NS tea (5 g/day) added to the usual oral anti-diabetic drugs, diet, and exercise for 6 months showed significant decreases in aspartate aminotransferase (AST), ALT, serum total, direct and indirect bilirubin, serum Cr, and blood urea levels in type 2 diabetic patients, as well as improved liver and kidney functions [37]. The intake of NS extract (200 or 400 mg/day) for 2 months was also reported to cause no observable complications in patients with mild hypertension [38].
No adverse effects were reported in applications of NS oil twice daily for six months to the lesions of vitiligo patients [39]. Intake of NS oil (0.05 mL/kg/day) in osteopenic postmenopausal women for three months showed no beneficial effects; however, no adverse side effects were produced, either [40]. In another study conducted on Iranian infertile men in order to assess the impacts of NS oil (5 mL/day) on abnormal semen quality, no side effects were observed [41]. Whereas no severe side effects were reported in administration of NS oil (5 mL/day) to functional dyspeptic patients, some mild adverse impacts were observed, including nausea, bloating, and burning sensation [42]. On the other hand, some cases of epigastric pain and hypoglycemia were reported as adverse effects when NS oil capsules were used to treat patients with hepatitis C virus (HCV) [43]. Dirjomuljono et al demonstrated the safety of NS extract (1,080 mg/day) when used to treat patients with acute tonsillopharyngitis [44]. In another study that investigated the anti-cestodal effects of NS powdered seeds (40 mg/kg body weight) on children infected with cestodes, no serious side effects were reported [45].
Treatment of seasonal allergic rhinitis patients with NS seeds (250 mg/day) for two weeks has been reported not to cause any adverse effects [46]. However, some patients with allergic rhinitis when treated using nasal drops of NS oil showed nasal dryness [47]. Dogar et al confirmed that side effects produced by treatment of children with acute lymphoblastic leukemia (ALL), aged between 2 – 18 years, with NS powder (40 mg/kg in two equal doses for 3 months), along with conventional therapy, were remarkably less than they were for L-asparaginase and conventional therapy (note: conventional therapy includes daunorubicin, vincristine, and prednisolone). Thus, one can conclude that NS powder, as an anti-cancer agent, is a beneficial substitute for L-asparaginase in the treatment of patients with ALL [48].
Nevertheless, in a patient suffering from DM, along with coronary artery disease and hypertension, acute renal failure due to the use of NS tablets (2,000 – 2,500 mg/day) was reported to have occurred 6 days after the start of treatment [49]. Bamosa suggested that this adverse effect could not have been related to NS because other clinical trials with many more human subjects had demonstrated the safety of NS in higher doses over longer periods of intake and that the adverse effect could probably be attributed to contamination of the tablets with other products [50]. Ibraheim also reported that only the total oil showed significant increases in the AST and the ALT levels while both the oil and the crushed seeds showed significant increases in the γ-GT and the alkaline phosphatase (ALP) activities [51]. A case of systemic and contact bullous drug eruption as erythematous plaques with vesicles and bullous lesions because of topical application and digestion of NS oil was reported [52]. In addition, another report showed that the use of NS at 5 g/day inhibited CYP2D6- and CYP3A4-mediated metabolism of dextromethorphan in healthy human volunteers, showing that it may interact with other CYP2D6 and CYP3A4 substrates [53].
Taken together, NS has been established as a safe herbal product. Nevertheless, according to the mentioned studies, some adverse effects, including nausea, bloating, and burning sensation, have been reported after administration of NS oil in functional dyspeptic patients, and a slight increase in liver and kidney enzymatic markers has been shown following consumption of NS oil and crushed seeds.
3.2. Metabolic syndrome
Metabolic syndrome is a cluster of cardio-metabolic conditions that include obesity, insulin resistance, atherogenic dyslipidemia, and high blood pressure (BP) [54] and is a major contributor to the development of diabetes [55]. A RCT done on 60 patients with metabolic syndrome showed that NS oil (5 mL/day) used in combination with atorvastatin and metformin could decrease fasting blood sugar (FBS), LDL, and TC significantly after six weeks of use, but had no significant effect on body mass index (BMI) or waist circumference (WC) [56].
Another study by Amin et al evaluated the effect of black seeds (1.5 g/day) alone and concurrent with turmeric (900 mg and 1.5 g/day, respectively) on 250 patients with metabolic syndrome. The results show that NS and turmeric alone improved BMI, WC and BF% after 4 weeks, compared to baseline. Combination therapy improved all parameters including BMI, BF%, WC, hip circumference (HC), BP, total cholesterol (TC), triglyceride (TG), and C-reactive protein (CRP) except HDL-cholesterol with lower FBG and LDL-cholesterol as compared to placebo after four weeks. After 8 weeks, NS reduced lipids and FBG, whereas turmeric decreased LDL-cholesterol and CRP. Combination therapy showed an improvement in all parameters and reduced BF%, FBG, cholesterol, TG, LDL-cholesterol, CRP and increased HDL-cholesterol [57].
In another study, premenopausal women underwent the following protocol: 12-week administration of NS-powder capsules (1,600 mg/day), a 2-week washout period, and 12-week administration of a placebo, and vise versa. The authors of the study concluded that NS could help to control weight gain, the lipid profile, and the blood glucose and hormonal levels [58]. According to a clinical trial by Najmi et al, NS (500 mg/day) after 8 weeks was able to improve the efficacy of the therapeutic protocol (metformin + atorvastatin + aspirin) in patients with metabolic syndrome and poor glycemic control so that NS-treated patients indicated significant improvements in their FBG, postprandial blood glucose (PPBG), haemoglobin A1c (HbA1c), and LDL-C levels [59].
The effects of NS and TQ on different components of metabolic syndrome and cardiovascular disease risk factors, including high BP, obesity, dyslipidemia, and high blood glucose, have been established [21]. The clinical uses of NS and TQ in the prevention and treatment of these factors will be discussed in subsequent subsections.
3.3. Hypertension
Hypertension (HT) is a lifestyle-related disease, and dietary modifications are effective for its management and prevention. In a study performed by Dehkordi and Kamkhah, which evaluated the anti-hypertensive effect of NS, the intake of NS extract (200 or 400 mg/day) for 2 months was reported to decrease both the systolic and the diastolic BP in patients with mild HT as compared with the baseline and the placebo values [38]. Another study by Huseini et al on healthy volunteers who received NS oil (5 mL/day) for two months revealed the hypotensive effect of NS, with the systolic and the diastolic BP being lowered significantly as compared with both the placebo and the baseline values [31].
3.4. Obesity
Obesity, defined as excess fat mass, increases the risks for multiple metabolic diseases, such as type 2 diabetes, cardiovascular disease, and several types of cancer [60]. Some RCTs demonstrated that NS oil in combination with a low-calorie diet decreased weight in obese women in comparison to the placebo level [61, 62]. It also caused the superoxide dismutase (SOD) level to be elevated [61] and the TG and the LDL-C levels to be decreased [62].
Another study was performed on 39 centrally obese men to evaluate the effect of NS on body weight, WC, and some biochemical parameters [33]. The results of that study showed that intake of NS seeds (3 g/day for 3 months) caused a very significant reduction in body weight and WC, but insignificant reductions in the serum free testosterone level, as well as the systolic and the diastolic BP, and an insignificant increase in the adiponectin level. The reduction of serum free testosterone in the control group, who received two capsules of 750 mg flour twice daily, was more than it was in the treatment group, thus indicating that NS can inhibit the decrease in the serum free testosterone level.
3.5. Dyslipidemia
Dyslipidemia is defined as the derangements of one or more of the lipoproteins in blood, such as elevated TC, LDL-C, and/or TG, or low levels of HDL-C alone [63]. Moeen-ud-din et al reported that the intake of 2 teaspoons of NS seeds for 6 weeks by hyperlipidemic patients decreased their LDL-C (P-value < 0.001) and increased their HDL-C (P-value < 0.01) as significantly as niacin [64]. The results of another RCT indicated that compared to mineral oil, administration of NS oil (5 mL/day) to healthy volunteers for 8 weeks induced significant decreases in the fasting blood cholesterol, LDL, TG, glucose, and HbA1C levels [30]. Administration of NS seeds (two spoons/day) to male and female hyperlipidemic patients for 4 weeks significantly (P-value < 0.001) increased HDL-C and decreased body weight [65].
Menopausal women are one of the high risk groups for developing dyslipidemia. In a study by Ibrahim et al, the administration of powdered NS seeds (1 g/day) over a two-month intervention was found to decrease significantly the TG, LDL-C and TC levels, but to increase the HDL-C level [66]. However, one month after cessation of treatment, the lipid profiles in the NS-treated group tended to change towards the pretreatment levels. Another clinical trial done for a similar duration showed that a supplement of powdered NS seeds (1 g/day) could improve some biochemical parameters, including the lipid profile and blood glucose, in menopausal women, but had no significant effect on their body weight [67].
According to a World Health Organization (WHO) report, coronary heart disease is a major cause of mortality in the world [68]. The results of a study which examined the effects of NS on the lipid profiles in patients with stable coronary artery disease indicated that intake of NS powder (500 mg/day) for 6 months concurrent with statin (10 – 20 mg/day) both decreased the serum levels of TG, VLDL, LDL, and TC and elevated the HDL level significantly whereas statin alone decreased neither the TG, VLDL, LDL nor TC level [69].
In healthy female volunteers, administration of both crushed seeds and the formulated total oil of NS caused significant reductions in the prolactin, glucose, triglyceride, and cholesterol levels. Additionally, administration of the crushed seeds caused a significant increase in the white blood cell count (WBC) and the hemoglobin level whereas administration of NS oil only increased the hemoglobin level significantly [51]. In another study, Bamosa et al suggested that the administration of NS at 2 g/day for 2 weeks decreased the blood levels of both glucose and cholesterol in healthy volunteers [70].
A large RCT compared the effects of simvastatin (10 mg/day) alone and concurrent with NS seeds (500 mg/day) and garlic oil (0.625 mg/day) on the lipid profile in 258 patients with hyperlipidemia over an 8-week treatment [71]. In that study, a comparison of mean values between the two treatment groups indicated a highly significant difference (P = < 0.01) for cholesterol, TG, non-HDL, and LDL reductions and a significant difference (P = 0.03) for HDL elevation [71]. Sabzghabaee et al reported that the TC, LDL, and TG serum levels in hypercholesterolemic patients who took NS at 2 g/day for 4 weeks decreased significantly [72]. The uses of NS extract at 200 and 400 mg/day for 2 months were also reported to have decreased both the total and the LDL cholesterol in patients with mild HT as compared with the baseline [38].
Of course, some controversies exist in the clinical trial results. Qidwai et al reported that NS seeds (2 g/day) administration had not affected the BMI, waist-hip ratio, BP, FBS or serum lipids in adults after 6 weeks of use [34]. Unlike pervasive evidence for the effects of NS use on the lipid profile, the administration of powdered NS seeds was reported to have had no significant effect on BP, serum lipid levels, blood sugar, or body weight in adults [73]. According to some meta-analyses, overall, the use of NS was shown to reduce the plasma levels of TC, LDL-C and TG, but its effect on HDL-C was not significant [74, 75]. Whereas the use of NS seed oil was observed to have greater effects on the serum TC and the LDL-C levels, versus the use of seed powder, elevation of the HDL-C levels was found only after supplementation with NS seed powder [75].
3.6. Diabetes
DM is characterized by chronic elevation of blood glucose, which is a central factor in the production of reactive oxygen species (ROS) that, in turn, promote cellular damage and contribute to the development and progression of diabetic complications [76]. In a report by Heshmati et al, although the administration of NS oil at 3 g/day for 12 weeks was stated to have caused insignificant decreases in the BMI, insulin level, and insulin resistance, as well as an increase in the HDL-C level, in patients with type 2 diabetes, the FBS, TG, LDL-C, and HbA1c levels were observed to have been lowered significantly in the intervention group as compared to the placebo group [77].
Another clinical trial showed that NS oil intake (equivalent to oil obtained from 0.7 g of seeds) for 40 days caused a significant reduction of FBS and a significant rise of insulin in type 2 DM patients [32]. In still another study, the addition of NS oil at 5 mL/day to atorvastatin at 10 mg/day and metformin at 1 g/day was shown to have induced significant reductions in the TC, LDL-C, and FBS levels after 6 weeks of use in patients with insulin resistance [78]. Furthermore, according to a study performed by Hadi et al on 43 type 2 diabetic patients, after an 8-week treatment, the administration of NS oil extract at 1 g/day decreased the serum levels of TG, LDL-C, and TC, as well as the LDL-C to HDL-C ratio, significantly in comparison to the placebo [79]. Moreover, treatment with NS tea at 5 g/day added to the usual oral anti-diabetic drugs, diet, and exercise for 6 months led to significant decreases in the FBG, PPBG, and HbA1c level in type 2 diabetic patients [37]. Another clinical trial showed that the administration of NS at a dose of 2 g/day over a 12-week treatment caused significant decreases in the FBG, 2hPG, and HbA1c levels without any significant change in body weight [35]. In that study, insulin resistance, calculated by using a homeostatic model assessment, was also reduced while β-cell function was increased significantly. However, a dose of 1 g/day caused insignificant changes, and no further increments in the beneficial responses were observed with a dose of 3 g/day, indicating that 2 g/day was the optimum dose. Bilal et al reported the highly significant decreases in the FBG, TC, LDL-C and TG levels and increases in the HDL-C and the insulin levels were observed in patients with type 2 diabetes after 40 days of NS seed powder intake [36]. However, in that study, all mentioned values, except the HDL level, significantly reversed again after 40 days of placebo intake. Table 1.
Table 1.
Effects of Nigella sativa supplementation on patients with diabetes
| Study design | Population | Part of the herb, dose, and duration of treatment | Result | References |
|---|---|---|---|---|
| Randomized, double-blind, placebo-controlled trial | 72 Type 2 DM patients (men and women aged 30 – 60 years old) | NS oil (3 g/day)12 weeks | ↓ BMI, insulin level and insulin resistance as well as HDL-C as compared with baseline, ↓ FBS, TG, LDL-C and HbA1c as compared to the placebo group | [77] |
|
| ||||
| - | 41 Type 2 DM patients (men and women aged 30 – 60 years old) | NS oil (equivalent to oil obtained from 0.7 g of seeds)40 days | ↓ FBS, ↑ insulin, but reversed after 40 days of placebo administration | [36] |
|
| ||||
| Prospective study | 60 patients with insulin resistance (men and women) | NS oil (5 mL/day) + atorvastatin (10 mg/day) and metformin (1 g/day)6 weeks | ↓ TC, LDL-C and FBS | [78] |
|
| ||||
| Randomized, double blind, placebo-controlled clinical trial | 43 Type 2 DM patients (men and women) | NS oil extract (1 g/day)8 weeks | ↓ TG, LDL-C, TC, and LDL-C to HDL-C ratios as compared to the placebo | [79] |
|
| ||||
| - | 41 type 2 DM patients (men and women) | NS tea (5 g/day) + usual oral anti-diabetic drugs, diet, and exercises6 months | ↓ FBG, PPBG and HbA1c | [37] |
|
| ||||
| - | 94 type 2 DM patients (men and women) | NS seeds 2 g/day or 3 g/day12 weeks | ↓ FBG, 2hPG, and HbA1c, ↓ Insulin resistance, ↑ β-cell function; no significant change in body weight | [35] |
| NS seeds 1 g/day,12 weeks | Insignificant changes | [35] | ||
|
| ||||
| - | 41 type 2 DM patients (men and women aged 30 – 60 years old) | NS seed powder, 40 days | ↓ FBG, TC, LDL-C and TG, ↑ HDL-C and insulin; all mentioned values except HDL significantly reversed again after 40 days of placebo intake | [36] |
DM, Diabetes mellitus; NS, Nigella sativa; BMI, Body mass index; HDL-C, High density lipoprotein-cholesterol; TG, Triglyceride; LDL-C, Low density lipoprotein-cholesterol; HbA1c, Haemoglobin A1c; FBS, Fasting blood sugar; TC, Total cholesterol; FBG, Fasting blood glucose; PPBG, Postprandial blood glucose; 2hPG, 2 hours postprandial glucose.
3.7. Nervous system
Akhondian et al demonstrated that treatment of intractable pediatric seizures with water extract of black seed (40 mg/kg/8 h) versus placebo, as an adjuvant therapy to anti-epileptic drugs, led to a significant reduction in the mean frequency of seizures [80]. A similar clinical trial done by the same author had similar outcomes, although TQ (1 mg/kg) was administered as an add-on therapy instead of water extract of black seed [81]. On the other hand, another study conducted by Shawki et al had a different result; after administration of 40 – 80 mg/kg/day of black seed oil as an adjuvant therapy for 4 weeks, no beneficial effects on the frequency and severity of seizures in intractable epileptic children were observed [82].
In a placebo-controlled clinical trial addressing memory and cognition, healthy elderly volunteers took a 500-mg NS capsule twice a day over a period of 9 weeks [83]. At the end of that period, through special tests, the authors observed improved cognition, memory, and attention. Similarly, another CT performed on healthy adolescent males aged 14 to 17 years established the modulatory effects on cognition, mood, and anxiety of NS taken in the form of a 500-mg NS capsule once a day for four weeks [84].
Sangi et al introduced NS administration as a effective non-opiate treatment for opioid dependence [85]. They found that NS treatment offers some advantages in contrast with opiate treatments, such as relieving the withdrawal effects, maintaining the physiological parameters, and improving appetite. Table 2.
Table 2.
Clinical effects of Nigella sativa supplementation on the nervous system
| Study design | Population | Part of the herb, dose, and duration of treatment | Result | References |
|---|---|---|---|---|
| Double-blinded crossover clinical trial | 23 epileptic children | Water extract of black seed (40 mg/kg/8 h), 10 weeks | Significant reduction of mean frequency of seizures | [80] |
| Pilot, double-blinded crossover clinical trial | 22 epileptic children | Thymoquinone (1 mg/kg), 10 weeks | Significant reduction of frequency of seizures | [81] |
| Prospective, randomized, single-blinded, controlled, crossover pilot study | 30 intractable epileptic patients | 40 – 80 mg/kg/day of black seed oil, 10 weeks | No beneficial effects in the frequency and severity of seizures | [82] |
| Placebo-controlled clinical trial | 40 healthy elderly volunteers | 500 mg NS seed capsule twice a day, 9 weeks | Improvement of cognition, memory, and attention | [83] |
| Placebo-controlled clinical trial | 48 healthy adolescent males | 500 mg NS capsule once daily, 4 weeks | Enhancements of mood, anxiety, and cognition | [84] |
| - | 35 male opiate addicts | 500 mg NS daily, 12 weeks | Significant reduction of withdrawal effects | [85] |
| Randomized, triple-blind, active, and placebo-controlled clinical trial | 52 women with cyclic mastalgia | Topical 600 mg NS oil twice a day, 2 months | Significant improvement of the pain scores | [86] |
NS, Nigella sativa.
3.8. Analgesic effects
Huseini et al showed that as compared with the topical administration of diclofenac, topical administration of NS oil had significant therapeutic effects on patients with cyclic mastalgia without any adverse effects [86]. In that study, 600 mg of NS oil (in the first treatment group) and 20 mg of topical diclofenac (in the second treatment group) were applied to the painful area twice daily for two months.
3.9. Dermatology
Vitiligo is an autoimmune skin disease occurring due to the destruction of skin melanocytes that produce skin pigment. A RCT comparing the efficacy of applying NS oil and fish oil to the lesions of vitiligo twice a day for six months indicated that the former is more effective than the latter in reducing the size of the lesions [39].
Hand eczema is a pruritic papulovesicular dermatitis severely influencing the patient’s quality of life. Yousefi et al compared the effects of NS ointment, betamethasone, and eucerin on the severity of hand eczema and the patients’ quality of life [87]. In that study, for new cases of hand eczema in patients between 18 and 60 years of age the mentioned drugs were applied twice daily for 4 weeks. Through particular measures, the authors concluded that NS may be as effective as betamethasone in enhancing quality of life and alleviating the severity of eczema and that both were more effective than eucerin.
In contrast, for a comparison of the therapeutic effects of NS oil ointment and aplacebo on patients with atopic dermatitis (eczema), 20 patients were asked to apply NS oil on one arm and a placebo on the other every day for 4 weeks [88]. The authors of that study reported that no meaningful difference could be found in terms of the parameters measured, e.g., severity, pruritis, transepidermal water loss, and skin hydration, between the treatment with NS oil ointment and with a placebo.
Arsenical keratosis manifests itself in both the palms of the hands and the soles of the feet due to chronic arsenic consumption caused by drinking contaminated water. Taking capsules of NS oil (500 mg) and vitamin E (200 mg) for eight weeks has been shown to reduce the body’s arsenic load, thereby contributing to an overall alleviation of the symptoms in patients with this disease [89].
3.10. Infectious diseases
Infection with HCV often leads to chronic hepatitis, which, in turn, results in liver cirrhosis and hepatocellular carcinomas. A study conducted in Egypt on HCV patients demonstrated that ethanolic extracts of NS and Zingiber officinale (Z. officinale), alone and together, had beneficial effects on HCV patients; i.e., their liver function was improved and the viral load was decreased [90]. In that study, a mixture of these extracts was observed to be more effective than each one alone. Patients included in that study were treated with capsules containing 500 mg of NS and/or Z. officinale twice daily for one month. In a similar study, HCV patients received capsules of NS oil (450 mg) three times a day over a 3-month period. That treatment led to the same results reported in Ref. 90, i.e., decreased viral load and improved liver function [43].
Onifade et al confirmed that treatment of a sero-positive human immunodeficiency virus (HIV) infected man with NS concoction (10 mL twice/day for six months) resulted in the reduction of the viral load to an undetectable level in 3 months, an elevation of the CD4 count, an alleviation of the symptoms, and a sustained sero-reversion [91]. Similarly, another study conducted by the same author on a sero-positive HIV infected woman revealed the efficacy of NS and honey therapy (10 mL thrice/day for 1 year) for sustained sero-reversion [92]. These effects can be ascribed to the probable virucidal activity of NS [91].
The helicobacter pylori (H. pylori) bacterium can cause many diseases, such as peptic ulcers and gastric cancer. Infection with H. pylori has a high prevalence worldwide. Salem et al stated that in a four-week course, the efficacy of NS powder (2 g/day) administered together with omeprazole to eradicate an H. pylori infection in non-ulcer dyspeptic patients was relatively the same as that of triple therapy, although 1 g/day or 3 g/day of NS powder given together with omeprazole was not as effective, indicating that the optimal dose of NS was 2 g/day [93]. (Triple therapy includes clarithromycin, amoxicillin, and omeprazole.)
In a study conducted on children who were infected with cestodes, the efficacy of single oral administration of NS powdered seeds and ethanolic extract (40 mg/kg body weight) was proven to reduce the percentage of fecal eggs per gram, which means NS has an anti-cestodal effect [45]. Furthermore, in a RCT in which 100 infected women were included, the therapeutic effects of black seed capsules (500 mg twice daily) used together with clotrimazole vaginal cream on C. albicans vaginitis were compared with those of placebo capsules (500 mg twice daily) used in combination with the same vaginal cream [94]. In that study, after a 7-day treatment, the black seed capsules used with clotrimazole vaginal cream were more impressive in reducing the symptoms of the disease, such as vaginal itching, discharge, irritation, vulvovaginal redness and inflammation [94].
3.11. Reproductive system
Kolahdooz et al proved that treatment of infertile Iranian men with 2.5 mL of NS oil twice a day for two months, in contrast with a placebo treatment in the same manner, could enhance sperm parameters, including sperm count, motility and morphology, semen volume, pH, and its round cells [41]. Other beneficial effects of NS on Leydig cells, reproductive organs, and sexual hormones in infertile men have also been confirmed [95].
3.12. Respiratory system
As for lower respiratory tract illnesses (LRTI), Boskabady et al investigated the antiasthmatic effects of NS boiled extract (50 and 100 mg/kg), theophylline (5 mg/kg), and salbutamol (200 μg) [96]. In that study, 15 asthmatic patients were recruited, and each patient received one of these 4 treatments in random order at intervals of 48 hours for 2 weeks. The results of that study demonstrated that in spite of the fact that both doses of NS boiled extract showed bronchodilatory effects, their efficacies for pulmonary function test (PFT) elevation were less than those of theophylline and salbutamol. The results also revealed the prophylactic effects of NS boiled extract on adult asthmatic patients. In another study, over a 3-month period, the daily intake of 15 mL/kg of 0.1% NS boiled extract led to more impressive improvements in the PFT parameters and alleviations of the symptoms of asthma than the intake of the placebo solution did [97]. Ahmad et al conducted a study on 5- to15-year-old LRTI patients with wheezing and investigated the beneficial impacts of the standard treatment alone and the standard treatment combined with the use of NS oil [98]. The authors of that study concluded that the standard treatment when administered with NS oil (0.1 mg/kg for 14 days) had more beneficial effects in reducing the pulmonary index and improving the peak expiratory flow rate [98]. In another study, daily administration of 0.375 mL/kg of 50% NS boiled water extract, as compared with a placebo solution to victims of chemical warfare, as compared with a placebo solution, for two months effected meaningful improvements in the PFT measures and the respiratory symptoms, indicating that the use of NS had a prophylactic impact on victims of chemical warfare [99].
Allergic rhinitis (AR) is an inflammatory response of the nasal mucosa to natural allergens and is characterized by sneezing, rhinorrhea, nasal congestion, and itching [47, 100]. Moreover, a study comparing the therapeutic effects of NS seeds (250 mg/day) and montelukast (10 mg/day) on patients with seasonal allergic rhinitis over a course of two weeks illustrated that both improved the daytime and ophthalmic symptoms considerably, although NS was more efficient in alleviating the nighttime symptoms [46]. Similarly, Nikakhlagh et al showed the positive effects of NS oil capsule consumption over four weeks on the symptoms of allergic rhinitis, i.e., nasal mucosal congestion, nasal itching, runny nose, sneezing attacks, turbinate hypertrophy, and mucosal pallor [100]. Like the previous studies, that of Alsamarai et al demonstrated that nasal drops of NS oil, in comparison with nasal drops of ordinary food oil, could significantly improve the symptoms of AR patients, as well as their ability to tolerate exposure to allergens [47]. In that study, each drop comprised 15 mL of oil, and the patients applied 2 drops nasally (one in each nostril) 3 times daily for 6 weeks. In an additional study of AR patients who underwent a month of allergen-specific immunotherapy and then received treatment with NS seeds (2 g/d) during the next month, in contrast with the AR patients who only received immunotherapy for two months, the former showed more progress in their immune status, e.g., PMN functions and CD8 counts [101]. That study showed that NS administration contributed to a more effective immunotherapy. According to a study by Oysu et al, some of the nasal symptoms of geriatric patients, for example, nasal dryness, obstruction, and crusting, can be significantly improved by using NS oil [102].
As for patients with tonsillopharyngitis, the results of a study by Dirjomuljono et al indicated that NSPN capsules containing 360 mg of NS and 50 mg of Phyllanthus niruri extracts, as compared with a placebo, if given three times a day for 7 days to patients with acute tonsillopharyngitis, could significantly alleviate the symptoms of the disease due to their anti-inflammatory and immuno-modulatory effects [44]. In another study, which was undertaken on patients with inhalation allergy, the beneficial impacts of NS oil on the immune status of those patients, including enhancement of the NK cell count and percentage of lymphocytes, were confirmed [103].
3.13. Skeletal system
Rheumatoid arthritis (RA) is a chronic systemic disease characterized by inflammation of the joints, degeneration of collagen fibers in mesenchymal tissues, and atrophy of bones. Although the etiology is not well understood, autoimmunity seems to play a role in the etiology of RA. A study on 40 female RA patients who received placebo capsules twice a day in the first month and 500-mg NS oil capsules twice a day in the next month observed that the use of NS oil capsules notably improved the disease’s activity score and alleviated its symptoms, which could be attributed to the modulatory effect of NS on the immune system [104].
A clinical trial conducted on osteopenic postmenopausal women showed that the intake of NS oil (0.05 cc/kg/d) for three months had no considerable effects on bone turnover markers [40]. Furthermore, in another clinical trial, in which 15 osteoporotic postmenopausal women were included, no beneficial effects of consuming NS extract (0.05 mL/kg/d for three months) on bone turnover were observed [105].
3.14. Gastrointestinal system
Celiac disease (CD) is characterized by increased sensitivity to gluten and by inflammation and destruction of the small intestine mucosa due to an autoimmune mechanism. In a study by Osman et al, the prescription of a gluten free diet (GFD) together with the consumption of a NS oil capsule (450 mg) twice daily as dietary supplement for one year was more effective than a GFD alone in the treatment of patients with iron deficiency anemia associated with refractory CD; i.e., the hematological and immunological indices and the duodenal histology recovery were improved [106]. Dermatitis herpetiformis (DH) is an autoimmune skin disease caused by CD and is characterized by itchiness, a burning sensation, and chronic dermatitis. Similar to the former CT, adding NS oil capsules to a GFD for a period of 6 months was found to enhance the efficacy of a GFD in the treatment of the disease [107]. Furthermore, Mohtashami et al. reported that administration of a honey-based formulation of NS oil (5 mL NS oil/day), in comparison with a placebo, for 8 weeks could significantly improve the symptoms, such as dyspepsia severity, and decrease the rate of H. pylori infection in functional dyspeptic patients [42].
3.15. Anti-toxicity effects
Leukemia is a tumoral growth of WBCs in the bone marrow and is caused by a malignant neoplasm of hematopoietic stem cells. In ALL, which is the most common childhood malignancy [108], increased numbers of lymphoblasts are present in the circulating blood and in different tissues and organs [48]. A study by Hagag et al reported that NS oil (80 mg/kg/day) administered for one week after each methotrexate treatment could reduce hepatotoxicity and improve the survival rate in ALL children undergoing that treatment [108]. Table 3.
Table 3.
Effects of Nigella sativa supplementation on patients with some diseases of the respiratory system
| Disease | Study design | Population | Part of the herb, dose, and duration of treatment | Result | References |
|---|---|---|---|---|---|
| Lower respiratory tract illnesses | - | 15 asthmatic patients | NS boiled extract(50 and 100 mg/kg) | NS was less effective than theophylline and salbutamol, on pulmonary function tests | [96] |
| Controlled clinical trial | 29 asthmatic adults | 15 mL/kg/day of 0.1% NS boiled extract, 3 months | Prophylactic effects of NS on asthmatic patients | [97] | |
| Prospective, randomized, open study | 84 patients of wheeze associated LRTI | NS oil (0.1 mg/kg), 14 days | More beneficial effects in reducing the pulmonary index and improving the peak expiratory flow rate | [98] | |
| Randomized, double-blind, placebo-controlled trial | 40 chemical war victims | 0.375 mL/kg/day of 50 g NS boiled water extract, 2 months | Prophylactic effect of NS on chemical war victims | [99] | |
|
| |||||
| Allergic rhinitis | Single-blind comparative clinical trial | 47 untreated adult patients with seasonal allergic rhinitis | NS seeds (250 mg/day), 2 weeks | Decreasing daytime, ophthalmic, and nighttime symptoms | [46] |
| Double-blind clinical trial | 68 patients with allergic rhinitis | Nasal drops of NS oil (each drop: 15 mL oil), 6 weeks | Notable symptomatic improvement of patients | [47] | |
|
| |||||
| Tonsillopharyngitis | Comparative, parallel, randomized, double-blind, placebo-controlled study | 186 acute tonsillopharyngitis patients | NSPN capsule (360 mg NS and 50 mg Phyllanthus niruri extracts), thrice/day, 7 days | Treatment of the symptoms of the disease | [44] |
NS, Nigella sativa.
4. Discussion
This review article summarized different studies on the clinical uses of NS and TQ in the prevention and the treatment of different diseases. Results indicated that NS has beneficial effects when used in the therapies for various diseases, including cardiovascular, nervous system, skin, infectious, reproductive system, respiratory system, skeletal system, and gastrointestinal diseases. Taken together, the role of NS in the treatment of different diseases is discussed in the following sentences.
Dyslipidemia plays an important role in the genesis of cardiovascular diseases (CVDs) [71], and hypercholesterolemia is the most important risk factor for atherosclerosis [72]. Lipid abnormalities are accountable for 56% of patients with heart disease and 18% of those with an infarction; further, it is associated with one third of deaths worldwide. In this article, we reviewed 19 clinical trials that reported the ameliorative effect of NS on the lipid profile. This finding shows that the use of NS supplements can improve the lipid profile and prevent CVDs both in healthy people and hyperlipidemic patients. The exact mechanisms of the lipid-modifying effects of NS are not known, but might be associated with the inhibition of intestinal cholesterol absorption, decreased hepatic cholesterol synthesis, and up-regulation of LDL receptors [74].
On the other hand, dyslipidemia is an important risk factor responsible for cardiovascular disease in patients with diabetes [109], so alleviation/elimination of lipid abnormalities is important in the prevention of the complications of diabetes [79]. Thus, keeping the lipid profile of diabetic patients in the normal range can improve their health status, and NS intake, in combination with anti-diabetics and statins or fibrates, can help diabetic patients to control both dyslipidemia and blood sugar. We also reported the results of 13 clinical trials that presented data on the hypoglycemic effect of NS, 8 of which included patients with insulin resistance. The results showed that the use of black seed could decrease the HbA1c (5 studies) and the PPBG (2 studies) levels significantly.
Obesity is typically associated with increased risk factors of CVDs. Therefore, a therapeutic approach that aims to control body weight and the metabolic profile might be effective in preventing CVDs [62]. The results of this review demonstrate that the use of NS may have a weight-lose effect in obese men and women. Because of the lipid-modifying, hypoglycemic, and weight-lowering effects of black seed, its use in the treatment of patients with metabolic syndrome may be beneficial [21].
According to some studies reviewed, NS may be a useful herb for improving sexual function because of its inhibitory effect on prolactin [51] and excitatory effect on testosterone [33]. In addition, spermatogenesis can be stimulated by NS [95]. Consequently, it presents a good option for use in the treatment of infertile men. However, more studies are needed due to the lack of an adequate number of studies proving this effect.
TQ has an antioxidant role, improves the body’s defense system, induces apoptosis, and controls the Akt pathway [7]. Immune system modulation is one of the most important properties of NS, and a number of studies have been done in order to prove this significant effect. In this article, we reviewed five studies that directly showed the immuno-modulatory effect of NS [39, 44, 104, 106, 107]. In addition, other investigations have demonstrated that NS may be an optimum choice for treating patients with allergy-related diseases, such as asthma, atopic eczema and allergic rhinitis [110].
Moreover, the anti-nociceptive effect of NS has been widely investigated in animal models, and a few studies have evaluated that effect in humans. In this paper, the anti-microbial activities of NS against bacteria, viruses, and parasites have also been highlighted. The conclusion is that it can be used in the treatment of infectious diseases [111].
The existing drugs for some diseases produce adverse side effects, do not lead to complete recovery, or are sometimes expensive. Thus, herbal medicines, such as NS, can be useful alternatives; however, before such herbal medicines are used extensively, many RCTs should be conducted in order to evaluate and confirm the effects of herbal medicines [46, 48, 108]. One obvious example is sero-reversion in HIV-infected patients. Highly active anti-retroviral therapy does not cause sero-reversion in HIV-infected patients. Onifade et al proved that NS contributes to sustained sero-reversion in patients with HIV infection [91, 92].
Some authorities, despite achieving positive outcomes for the features of NS, suggest further investigations with larger samples and groups, diverse doses of NS, and longer periods of study [42, 83–85, 93, 97–99]. After the beneficial effects of NS have been confirmed, it can be used in treatment protocols. In contrast, some authors have reached results that conflict with the traditional beliefs about NS. Actually, the evidence for the positive effects of NS in the treatment of osteopenic postmenopausal women and intractable epileptic children is conflicting [40, 82, 105]. Therefore, more studies should be planned in these situations.
5. Conclusion
In conclusion, the use of black seeds and their active constituent TQ has been shown to have multiple useful effects in the treatments of patients with several diseases, such as inflammatory and auto-immune disorders, as well as metabolic syndrome. In this study, we also reviewed other advantages of NS, e.g., its antimicrobial properties, anti-nociceptive and anti-epileptic impacts, etc. We found that the side effects of this herbal medicine did not appear serious, so it can be applied in clinical trials because most of its major effects have been shown to be beneficial. Some properties of NS, such as its hypoglycemic, hypolipidemic, and bronchodilatory properties, are sufficiently understood so that NS can be used for subsequent phases of clinical trials or for drug development. However, most of the other effects and applications of NS require further clinical and animal studies.
Acknowledgment
The authors thank Mashhad University of Medical Sciences, Mashhad, Iran.
Footnotes
This paper meets the requirements of KS X ISO 9706, ISO 9706-1994 and ANSI/NISO Z39.48-1992 (Permanence of Paper).
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Ziaee T, Moharreri N, Hosseinzadeh H. Review of pharmacological and toxicological effects of Nigella sativa and its active constituents. J Med Plants. 2012;2(42):16–42. [Google Scholar]
- 2.Darakhshan S, Bidmeshki Pour A, Hosseinzadeh Colagar A, Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharmacol Res. 2015;95–96:138–58. doi: 10.1016/j.phrs.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3(5):337–52. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mollazadeh H, Hosseinzadeh H. The protective effect of Nigella sativa against liver injury: a review. Iran J Basic Med Sci. 2014;17(12):958–66. [PMC free article] [PubMed] [Google Scholar]
- 5.Shafiq H, Ahmad A, Masud T, Kaleem M. Cardio-protective and anti-cancer therapeutic potential of Nigella sativa. Iran J Basic Med Sci. 2014;17(12):967–79. [PMC free article] [PubMed] [Google Scholar]
- 6.Butt MS, Sultan MT. Nigella sativa: reduces the risk of various maladies. Crit Rev Food Sci Nutr. 2010;50(7):654–65. doi: 10.1080/10408390902768797. [DOI] [PubMed] [Google Scholar]
- 7.Khan MA, Chen HC, Tania M, Zhang DZ. Anticancer activities of Nigella sativa (black cumin) Afr J Tradit Complement Altern Med. 2011;8(5):226–32. doi: 10.4314/ajtcam.v8i5S.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee S, Padhye S, Azmi A, Wang Z, Philip PA, Kucuk O, et al. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr Cancer. 2010;62(7):938–46. doi: 10.1080/01635581.2010.509832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abukhader MM. Thymoquinone in the clinical treatment of cancer: fact or fiction? Pharmacogn Rev. 2013;7(14):117–20. doi: 10.4103/0973-7847.120509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forouzanfar F, Bazzaz BSF, Hosseinzadeh H. Black cumin (Nigella sativa) and its constituent (thymoquinone): a review on antimicrobial effects. Iran J Basic Med Sci. 2014;17(12):929–38. [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseinzadeh H, Fazly Bazzaz BS, Haghi MM. Antibacterial activity of total extracts and essential oil of Nigella sativa L. seeds in mice. Pharmacologyonline. 2007;2:429–35. [Google Scholar]
- 12.Amin B, Hosseinzadeh H. Black Cumin (Nigella sativa) and its active constituent, thymoquinone: an overview on the analgesic and anti-inflammatory effects. Planta Med. 2015;82(1–2):8–16. doi: 10.1055/s-0035-1557838. [DOI] [PubMed] [Google Scholar]
- 13.Amin B, Taheri MM, Hosseinzadeh H. Effects of intraperitoneal thymoquinone on chronic neuropathic pain in rats. Planta Med. 2014;80(15):1269–77. doi: 10.1055/s-0034-1383062. [DOI] [PubMed] [Google Scholar]
- 14.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17(4):299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 15.Hosseinzadeh H, Eskandari M, Ziaee T. Antitussive effect of thymoquinone, a constituent of Nigella sativa seeds, in guinea pigs. Pharmacologyonline. 2008;2:480–4. [Google Scholar]
- 16.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5(13–14):1749–70. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Hosseinzadeh H, Taiari S, Nassiri-Asl M. Effect of thymoquinone, a constituent of Nigella sativa L., on ischemia-reperfusion in rat skeletal muscle. Naunyn-Schmiedebergs Arch Pharmacol. 2012;385(5):503–8. doi: 10.1007/s00210-012-0726-2. [DOI] [PubMed] [Google Scholar]
- 18.Hosseinzadeh H, Moghim FF, Mansouri S, Mansouri T. Effect of Nigella sativa seed extracts on ischemia-reperfusion in rat skeletal muscle. Pharmacologyonline. 2007;2:326–35. [Google Scholar]
- 19.Hosseinzadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14(9):621–7. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Shabana A, El-Menyar A, Asim M, Al-Azzeh H, Al Thani H. Cardiovascular benefits of black cumin (Nigella sativa) Cardiovasc Toxicol. 2013;13(1):9–21. doi: 10.1007/s12012-012-9181-z. [DOI] [PubMed] [Google Scholar]
- 21.Razavi B, Hosseinzadeh H. A review of the effects of Nigella sativa L. and its constituent, thymoquinone, in metabolic syndrome. J Endocrinol Invest. 2014;37(11):1031–40. doi: 10.1007/s40618-014-0150-1. [DOI] [PubMed] [Google Scholar]
- 22.Parvardeh S, Nassiri-Asl M, Mansouri SMT, Hosseinzadeh H. Study on the anticonvulsant activity of thymoquinone, the major constituent of Nigella sativa L. seeds, through intracerebroventricular injection. J Med Plants. 2005;4:45–52. [Google Scholar]
- 23.Hosseinzadeh H, Parvardeh S, Nassiri-Asl M, Mansouri MT. Intracerebroventricular administration of thymoquinone, the major constituent of Nigella sativa seeds, suppresses epileptic seizures in rats. Med Sci Monit. 2005;11(4):106–10. [PubMed] [Google Scholar]
- 24.Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11(1):56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 25.Pourbakhsh H, Taghiabadi E, Abnous K, Hariri AT, Hosseini SM, Hosseinzadeh H. Effect of Nigella sativa fixed oil on ethanol toxicity in rats. Iran J Basic Med Sci. 2014;17(12):1020–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Mehri S, Shahi M, Razavi BM, Hassani FV, Hosseinzadeh H. Neuroprotective effect of thymoquinone in acrylamideinduced neurotoxicity in wistar rats. Iran J Basic Med Sci. 2014;17(12):1007–11. [PMC free article] [PubMed] [Google Scholar]
- 27.Javidi S, Razavi BM, Hosseinzadeh H. A review of neuropharmacology effects of Nigella sativa and its main component, thymoquinone. Phytother Res. 2016;30(8):1219–29. doi: 10.1002/ptr.5634. [DOI] [PubMed] [Google Scholar]
- 28.Havakhah S, Sadeghnia HR, Hajzadeh MA, Roshan NM, Shafiee S, Hosseinzadeh H, et al. Effect of Nigella sativa on ischemia-reperfusion induced rat kidney damage. Iran J Basic Med Sci. 2014;17(12):986–92. [PMC free article] [PubMed] [Google Scholar]
- 29.Hosseinzadeh H, Montahaei R. Protective effect of Nigella sativa L. extracts and thymoquinone, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. Pharmacologyonline. 2007;1:176–89. [Google Scholar]
- 30.Amini M, Huseini HF, Mohtashami R, Ghamarchehre MA. Hypolipidemic effects of Nigella sativa L. seeds oil in healthy volunteers: a randomized, double-blind, placebo-controlled clinical trial. J Med Plants. 2011;10:133–8. doi: 10.1002/ptr.4944. [DOI] [PubMed] [Google Scholar]
- 31.Huseini HF, Amini M, Mohtashami R, Ghamarchehre ME, Sadeqhi Z, Kianbakht S, et al. Blood pressure lowering effect of Nigella sativa L. seed oil in healthy volunteers: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. 2013;27(12):1849–53. doi: 10.1002/ptr.4944. [DOI] [PubMed] [Google Scholar]
- 32.Bilal A, Masud T, Uppal AM, Naveed AK. Effects of Nigella sativa oil on some blood parameters in type 2 diabetes mellitus patients. Asian J Chem. 2009;21(7):5373–81. [Google Scholar]
- 33.Datau EA, Wardhana, Surachmanto EE, Pandelaki K, Langi JA, Fias Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med Indones. 2010;42(3):130–4. [PubMed] [Google Scholar]
- 34.Qidwai W, Hamza HB, Qureshi R, Gilani A. Effectiveness, safety, and tolerability of powdered Nigella sativa (kalonji) seed in capsules on serum lipid levels, blood sugar, blood pressure, and body weight in adults: results of a randomized, double-blind controlled trial. J Altern Complement Med. 2009;15(6):639–44. doi: 10.1089/acm.2008.0367. [DOI] [PubMed] [Google Scholar]
- 35.Bamosa AO, Kaatabi H, Lebda FM, Elq AM, Al-Sultan A. Effect of Nigella Sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010;54(4):344–54. [PubMed] [Google Scholar]
- 36.Bilal A, Masud T, Uppal AM. BS5-5 Black seed (Nigella sativa) regulates glucose, insulin level and lipid profile in patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;79:19–20. doi: 10.1016/S0168-8227(08)70696-X. [DOI] [Google Scholar]
- 37.El-Shamy KA, Mosa MMA, El-Nabarawy SK, El-Qattan M. Effect of Nigella sativa tea in type 2-diabetic patients as regards glucose homeostasis, liver and kidney functions. J Appl Sci Res. 2011;7(12):2524–34. [Google Scholar]
- 38.Dehkordi FR, Kamkhah AF. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol. 2008;22(4):447–52. doi: 10.1111/j.1472-8206.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 39.Ghorbanibirgani A, Khalili A, Rokhafrooz D. Comparing Nigella sativa oil and fish oil in treatment of vitiligo. Iran Red Crescent Med J. 2014;16(6):e4515. doi: 10.5812/ircmj.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valizadeh N, Zakeri HR, Shafiee A, Sarkheil P, Heshmat R, Larijani B. The effect of Nigella sativa extract on biochemical bone markers in osteopenic postmenopausal women. Iran J Endocrinol Metab. 2009;10(6):571–80. [Google Scholar]
- 41.Kolahdooz M, Nasri S, Modarres SZ, Kianbakht S, Huseini HF. Effects of Nigella sativa L. seed oil on abnormal semen quality in infertile men: a randomized, double-blind, placebo-controlled clinical trial. Phytomedicine. 2014;21(6):901–5. doi: 10.1016/j.phymed.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Mohtashami R, Huseini HF, Heydari M, Amini M, Sadeqhi Z, Ghaznavi H, et al. Efficacy and safety of honey based formulation of Nigella sativa seed oil in functional dyspepsia: a double blind randomized controlled clinical trial. J Ethnopharmacol. 2015;175:147–52. doi: 10.1016/j.jep.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 43.Barakat EMF, El Wakeel LM, Hagag RS. Effects of Nigella sativa on outcome of hepatitis C in Egypt. World J Gastroenterol. 2013;19(16):2529–36. doi: 10.3748/wjg.v19.i16.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dirjomuljono M, Kristyono I, Tjandrawinata RR, Nofiarny D. Symptomatic treatment of acute tonsillo-pharyngitis patients with a combination of Nigella sativa and Phyllanthus niruri extract. Int J Clin Pharmacol Ther. 2008;46(6):295–306. doi: 10.5414/CPP46295. [DOI] [PubMed] [Google Scholar]
- 45.Akhtar MS, Riffat S. Field trial of Saussurea lappa roots against nematodes and Nigella sativa seeds against cestodes in children. J Pak Med Assoc. 1991;41(8):185–7. [PubMed] [Google Scholar]
- 46.Ansari MA, Ansari NA, Junejo SA. Montelukast versus Nigella sativa for management of seasonal allergic rhinitis: a single blind comparative clinical trial. Pak J Med Sci. 2010;26(2):249–54. [Google Scholar]
- 47.Alsamarai AM, Abdulsatar M, Ahmed Alobaidi AH. Evaluation of topical black seed oil in the treatment of allergic rhinitis. Antiinflamm Antiallergy Agents Med Chem. 2014;13(1):75–82. doi: 10.2174/18715230113129990014. [DOI] [PubMed] [Google Scholar]
- 48.Dogar MZUH, Adi H, Akhtar MS, Sheikh MA. Preliminary assessment of efficacy of Nigella sativa seeds in acute lymphoblastic leukemia in local children. Pharmacologyonline. 2009;2:769–77. [Google Scholar]
- 49.Arslan E, Sayin S, Demirbas S, Cakar M, Somak NG, Yesilkaya S, et al. A case study report of acute renal failure associated with Nigella sativa in a diabetic patient. J Integr Med. 2013;11(1):64–6. doi: 10.3736/jintegrmed2013010. [DOI] [PubMed] [Google Scholar]
- 50.Bamosa A. Nigella sativa is a safe herbal product. J Integr Med. 2014;12(1):66. doi: 10.1016/S2095-4964(14)60007-8. [DOI] [PubMed] [Google Scholar]
- 51.Ibraheim ZZ. Effect of Nigella sativa seeds and total oil on some blood parameters in female volunteers. Saudi Pharm J. 2002;10:54–9. [Google Scholar]
- 52.Gelot P, Bara-Passot C, Gimenez-Arnau E, Beneton N, Maillard H, Celerier P. [Bullous drug eruption with Nigella sativa oil]. Ann Dermatol Venereol. 2012;139(4):287–91. doi: 10.1016/j.annder.2012.01.025. French. [DOI] [PubMed] [Google Scholar]
- 53.Al-Jenoobi FI, Al-Thukair AA, Abbas FA, Ansari MJ, Alkharfy KM, Al-Mohizea AM, et al. Effect of black seed on dextromethorphan O- and N-demethylation in human liver microsomes and healthy human subjects. Drug Metab Lett. 2010;4(1):51–5. doi: 10.2174/187231210790980435. [DOI] [PubMed] [Google Scholar]
- 54.Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47(3):181–90. doi: 10.1016/j.dld.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 55.Kim B, Feldman EL. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp Mol Med. 2015;47:e149. doi: 10.1038/emm.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Najmi A, Haque SF, Naseeruddin M, Khan RA. Effect of Nigella sativa oil on various clinical and biochemical parameters of metabolic syndrome. Int J Diabetes Metab. 2008;16:85–7. doi: 10.4103/0973-3930.41980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amin F, Islam N, Anila N, Gilani AH. Clinical efficacy of the co-administration of Turmeric and Black seeds (Kalongi) in metabolic syndrome - a double blind randomized controlled trial- TAK-MetS trial. Complement Ther Med. 2015;23(2):165–74. doi: 10.1016/j.ctim.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Latiff LA, Parhizkar S, Dollah MA, Tajuddin Syed Hassan S. Alternative supplement for enhancement of reproductive health and metabolic profile among perimenopausal women: a novel role of Nigella sativa. Iran J Basic Med Sci. 2014;17(12):980–5. [PMC free article] [PubMed] [Google Scholar]
- 59.Najmi A, Nasiruddin M, Khan RA, Haque SA. Therapeutic effect of Nigella sativa in patients of poor glycemic control. Asian J Pharm Clin Res. 2012;5:224–8. [Google Scholar]
- 60.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34(1):1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Namazi N, Mahdavi R, Alizadeh M, Farajnia S. Oxidative stress responses to Nigella sativa oil concurrent with a low-calorie diet in obese women: a randomized, double-blind controlled clinical trial. Phytother Res. 2015;29(11):1722–8. doi: 10.1002/ptr.5417. [DOI] [PubMed] [Google Scholar]
- 62.Mahdavi R, Namazi N, Alizadeh M, Farajnia S. Effects of Nigella sativa oil with a low-calorie diet on cardiometabolic risk factors in obese women: a randomized controlled clinical trial. Food Funct. 2015;6(6):2041–8. doi: 10.1039/C5FO00316D. [DOI] [PubMed] [Google Scholar]
- 63.Ni WQ, Liu XL, Zhuo ZP, Yuan XL, Song JP, Chi HS, et al. Serum lipids and associated factors of dyslipidemia in the adult population in Shenzhen. Lipids Health Dis. 2015;14:71. doi: 10.1186/s12944-015-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moeen-ud-din H, Murad S, Fatima A. Placebo controlled study on comparison of effects of Nigella sativa and nicotinic acid along with low fat diet and physical exercise on LDL-cholesterol and HDL-cholesterol. Pak J Med Health Sci. 2014;8:306–9. [Google Scholar]
- 65.Fatima A, Shad MN, Asrar A, Murad S. Effects of Nigella sativa on HDL-c & body weight. Pak J Med Health Sci. 2014;8(1):122–4. [Google Scholar]
- 66.Ibrahim RM, Hamdan NS, Mahmud R, Imam MU, Saini SM, Rashid SN, et al. A randomised controlled trial on hypolipidemic effects of Nigella Sativa seeds powder in menopausal women. J Transl Med. 2014;12:82. doi: 10.1186/1479-5876-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ibrahim RM, Hamdan NS, Ismail M, Saini SM, Abd Rashid SN, Abd Latiff L, et al. Protective effects of Nigella sativa on metabolic syndrome in menopausal women. Adv Pharm Bull. 2014;4(1):29–33. doi: 10.5681/apb.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mathews MJ, Liebenberg L, Mathews EH. The mechanism by which moderate alcohol consumption influences coronary heart disease. Nutr J. 2015;14:33. doi: 10.1186/s12937-015-0011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tasawar Z, Siraj Z, Ahmad N, Mushtaq H. The effects of Nigella sativa (Kalonji) on lipid profile in patients with stable coronary artery disease in Multan, Pakistan. Pak J Nut. 2011;10:162–7. doi: 10.3923/pjn.2011.162.167. [DOI] [Google Scholar]
- 70.Bamosa AO, Basil A, Sowayan AA, Sowayan SA. Effect of oral ingestion of Nigella sativa seeds on some blood parameters. Saudi Pharm J. 2007;2:126–9. [Google Scholar]
- 71.Ahmad Alobaidi AH. Effect of Nigella sativa and Allium sativum coadminstered with simvastatin in dyslipidemia patients: a prospective, randomized, double-blind trial. Antiinflamm Antiallergy Agents Med Chem. 2014;13(1):68–74. doi: 10.2174/18715230113129990013. [DOI] [PubMed] [Google Scholar]
- 72.Sabzghabaee AM, Dianatkhah M, Sarrafzadegan N, Asgary S, Ghannadi A. Clinical evaluation of Nigella sativa seeds for the treatment of hyperlipidemia: a randomized, placebo controlled clinical trial. Med Arch. 2012;66(3):198–200. doi: 10.5455/medarh.2012.66.198-200. [DOI] [PubMed] [Google Scholar]
- 73.Roufogalis B, Sekhon B. No statistically significant effects of powdered Nigella sativa (kalonji) seed on serum lipid levels, blood sugar, blood pressure or body weight in adults. Focus Altern Complement Ther. 2009;14(4):292–4. doi: 10.1211/fact.14.4.0008. [DOI] [Google Scholar]
- 74.Asgary S, Sahebkar A, Goli-malekabadi N. Ameliorative effects of Nigella sativa on dyslipidemia. J Endocrinol Invest. 2015;38(10):1039–46. doi: 10.1007/s40618-015-0337-0. [DOI] [PubMed] [Google Scholar]
- 75.Sahebkar A, Beccuti G, Simental-Mendia LE, Nobili V, Bo S. Nigella sativa (black seed) effects on plasma lipid concentrations in humans: a systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol Res. 2016;106:37–50. doi: 10.1016/j.phrs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 76.Kaatabi H, Bamosa AO, Badar A, Al-Elq A, Abou-Hozaifa B, Lebda F, et al. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS One. 2015;10(2):e0113486. doi: 10.1371/journal.pone.0113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heshmati J, Namazi N, Memarzadeh MR, Taghizadeh M, Kolandooz F. Nigella sativa oil affects glucose metabolism and lipid concentrations in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Food Res Int. 2015;70:87–93. doi: 10.1016/j.foodres.2015.01.030. [DOI] [Google Scholar]
- 78.Najmi A, Nasiruddin M, Khan R, Haque SF. Effect of Nigella sativa oil on various clinical and biochemical parameters of insulin resistance syndrome. Int J Diabetes Dev Ctries. 2008;28(1):11–4. doi: 10.4103/0973-3930.41980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hadi S, Mirmiran P, Hosseinpour-Niazi S, Hedayati M, Azizi F. Effect of Nigella sativa oil extract on lipid profiles in type 2 diabetic patients: a randomized, double blind, placebo-controlled clinical trial. Iran J Endocrinol Metab. 2015;16(6):411–8. [Google Scholar]
- 80.Akhondian J, Parsa A, Rakhshande H. The effect of Nigella sativa L. (black cumin seed) on intractable pediatric seizures. Med Sci Monit. 2007;13(12):CR555–9. [PubMed] [Google Scholar]
- 81.Akhondian J, Kianifar H, Raoofziaee M, Moayedpour A, Toosi MB, Khajedaluee M. The effect of thymoquinone on intractable pediatric seizures (pilot study) Epilepsy Res. 2011;93(1):39–43. doi: 10.1016/j.eplepsyres.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 82.Shawki M, El Wakeel LM, Shatla R, El-Saeed G, Ibrahim S, Badary O. The clinical outcome of adjuvant therapy with black seed oil on intractable paediatric seizures: a pilot study. Epileptic Disord. 2013;15(3):295–301. doi: 10.1684/epd.2013.0602. [DOI] [PubMed] [Google Scholar]
- 83.Bin Sayeed MS, Asaduzzaman M, Morshed H, Hossain MM, Kadir MF, Rahman MR. The effect of Nigella sativa Linn. seed on memory, attention and cognition in healthy human volunteers. J Ethnopharmacol. 2013;148(3):780–6. doi: 10.1016/j.jep.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Bin Sayeed MS, Shams T, Fahim Hossain S, Rahman MR, Mostofa A, Fahim Kadir M, et al. Nigella sativa L. seeds modulate mood, anxiety and cognition in healthy adolescent males. J Ethnopharmacol. 2014;152(1):156–62. doi: 10.1016/j.jep.2013.12.050. [DOI] [PubMed] [Google Scholar]
- 85.Sangi S, Ahmed SP, Channa MA, Ashfaq M, Mastoi SM. A new and novel treatment of opioid dependence: Nigella sativa 500 mg. J Ayub Med Coll Abbottabad. 2008;20(2):118–24. [PubMed] [Google Scholar]
- 86.Huseini HF, Kianbakht S, Mirshamsi MH, Zarch AB. Effectiveness of topical Nigella sativa seed oil in the treatment of cyclic mastalgia: a randomized, triple-blind, active, and placebo-controlled clinical trial. Planta Med. 2016;82(4):285–8. doi: 10.1055/s-0035-1558208. [DOI] [PubMed] [Google Scholar]
- 87.Yousefi M, Barikbin B, Kamalinejad M, Abolhasani E, Ebadi A, Younespour S, et al. Comparison of therapeutic effect of topical Nigella with betamethasone and eucerin in hand eczema. J Eur Acad Dermatol Venereol. 2013;27(12):1498–504. doi: 10.1111/jdv.12033. [DOI] [PubMed] [Google Scholar]
- 88.Stern T, Bayerl C. Black seed oil ointment - a new approach for the treatment of atopic dermatitis? Aktuelle Derm. 2002;28(3):74–9. doi: 10.1055/s-2002-25234. [DOI] [Google Scholar]
- 89.Bashar T, Misbahuddin M, Hossain MA. A double-blind, randomize, placebo-control trial to evaluate the effect of Nigella sativa on palmer arsenical keratosis patients. Bangladesh J Pharmacol. 2014;9:15–21. doi: 10.3329/bjp.v9i1.17167. [DOI] [Google Scholar]
- 90.Abdel-Moneim A, Morsy BM, Mahmoud AM, Abo-Seif MA, Zanaty MI. Beneficial theraputic effects of Nigella sativa and/or Zingiber officinale in HCV patients in Egypt. Excli J. 2013;12:943–55. [PMC free article] [PubMed] [Google Scholar]
- 91.Onifade AA, Jewell AP, Adedeji WA. Nigella sativa concoction induced sustained seroreversion in HIV patient. Afr J Tradit Complement Altern Med. 2013;10(5):332–5. [PMC free article] [PubMed] [Google Scholar]
- 92.Onifade AA, Jewell AP, Okesina AB. Seronegative conversion of an HIV positive subject treated with Nigella sativa and honey. Afr J Infect Dis. 2015;9(2):47–50. [Google Scholar]
- 93.Salem EM, Yar T, Bamosa AO, Al-Quorain A, Yasawy MI, Alsulaiman RM, et al. Comparative study of Nigella Sativa and triple therapy in eradication of Helicobacter Pylori in patients with non-ulcer dyspepsia. Saudi J Gastroenterol. 2010;16(3):207–14. doi: 10.4103/1319-3767.65201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fard FA, Zahrani ST, Bagheban AA, Mojab F. Therapeutic effects of Nigella Sativa Linn (Black Cumin) on Candida albicans vaginitis. Arch Clin Infect Dis. 2015;10(1):e22991. [Google Scholar]
- 95.Mandavi R, Heshmati J, Namazi N. Effects of black seeds (Nigella sativa) on male infertility: a systematic review. J Herb Med. 2015;5(3):133–9. doi: 10.1016/j.hermed.2015.03.002. [DOI] [Google Scholar]
- 96.Boskabady MH, Mohsenpoor N, Takaloo L. Antiasthmatic effect of Nigella sativa in airways of asthmatic patients. Phytomedicine. 2010;17(10):707–13. doi: 10.1016/j.phymed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 97.Boskabady MH, Javan H, Sajady M, Rakhshandeh H. The possible prophylactic effect of Nigella sativa seed extract in asthmatic patients. Fundam Clin Pharmacol. 2007;21(5):559–66. doi: 10.1111/j.1472-8206.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 98.Ahmad J, Khan RA, Malik MA. A study of Nigella sativa oil in the management of wheeze associated lower respiratory tract illness in children. Afr J Pharm Pharmacol. 2009;3(5):248–51. [Google Scholar]
- 99.Boskabady MH, Farhadi J. The possible prophylactic effect of Nigella sativa seed aqueous extract on respiratory symptoms and pulmonary function tests on chemical war victims: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2008;14(9):1137–44. doi: 10.1089/acm.2008.0049. [DOI] [PubMed] [Google Scholar]
- 100.Nikakhlagh S, Rahim F, Aryani FH, Syahpoush A, Brougerdnya MG, Saki N. Herbal treatment of allergic rhinitis: the use of Nigella sativa. Am J Otolaryngol. 2011;32(5):402–7. doi: 10.1016/j.amjoto.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 101.Isik H, Cevikbas A, Gurer US, Kiran B, Uresin Y, Rayaman P, et al. Potential adjuvant effects of Nigella sativa seeds to improve specific immunotherapy in allergic rhinitis patients. Med Princ Pract. 2010;19(3):206–11. doi: 10.1159/000285289. [DOI] [PubMed] [Google Scholar]
- 102.Oysu C, Tosun A, Yilmaz HB, Sahin-Yilmaz A, Korkmaz D, Karaaslan A. Topical Nigella sativa for nasal symptoms in elderly. Auris Nasus Larynx. 2014;41(3):269–72. doi: 10.1016/j.anl.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Deurer A, Schleicher P, Kalus U, Pruβ A, Kiesewetter H. Effect of black cumin oil on the immune status of patients with inhalation energy. Biol Med. 2002;31:75–8. [Google Scholar]
- 104.Gheita TA, Kenawy SA. Effectiveness of Nigella sativa oil in the management of rheumatoid arthritis patients: a placebo controlled study. Phytother Res. 2012;26(8):1246–8. doi: 10.1002/ptr.3679. [DOI] [PubMed] [Google Scholar]
- 105.Valizadeh N, Zakeri HR, Amin Asnafi G, Shafiee A, Sarkhail P, Heshmat R, et al. Impact of black seed (Nigella sativa) extract on bone turnover markers in postmenopausal women with osteoporosis. Daru. 2010;1:20–5. [Google Scholar]
- 106.Osman MT, Taha BI, Al-Duboni G, Muhamed LA. Immunomodulatory effect of Nigella sativa oil treatment in iron deficiency anemia caused by refractory coeliac disease. Res J Pharm Biol Chem Sci. 2012;3:887–95. [Google Scholar]
- 107.Osman MT, Kutty MK. Immunotherapeutic application of Nigella sativa oil in management of dermatitis herpetiformis associated with refractory coeliac disease. Res J Pharm Biol Chem Sci. 2013;4:1181–6. [Google Scholar]
- 108.Hagag AA, AbdElaal AM, Elfaragy MS, Hassan SM, Elzamarany EA. Therapeutic value of black seed oil in methotrexate hepatotoxicity in Egyptian children with acute lymphoblastic leukemia. Infect Disord Drug Targets. 2015;15(1):64–71. doi: 10.2174/1871526515666150320161440. [DOI] [PubMed] [Google Scholar]
- 109.Qidwai W, Ashfaq T. Effect of dietary supplementation of black seed (N. Sativa L.) on lipid profile of patients suffering from diabetes. Antiinflamm Antiallergy Agents Med Chem. 2014;13(1):3–8. doi: 10.2174/18715230113129990020. [DOI] [PubMed] [Google Scholar]
- 110.Kalus U, Pruss A, Bystron J, Jurecka M, Smekalova A, Lichius JJ, et al. Effect of Nigella sativa (black seed) on subjective feeling in patients with allergic diseases. Phytother Res. 2003;17(10):1209–14. doi: 10.1002/ptr.1356. [DOI] [PubMed] [Google Scholar]
- 111.Esmail F, Farhad Rahmani N, Mehrtash M, Fatemeh Sadat M, Jalilvand M. The effects of 8-week Nigella sativa supplementation and aerobic training on lipid profile and VO2 max in sedentary overweight females. Int J Prev Med. 2014;5(2):210–6. [PMC free article] [PubMed] [Google Scholar]