Abstract
Treatment of acute and chronic wounds is one of the primary challenges faced by doctors. Bioderived materials have significant potential clinical value in tissue injury treatment and defect reconstruction. Various strategies, including drug loading, addition of metallic element(s), cross-linking and combining two or more distinct types of materials with complementary features, have been used to synthesize more suitable materials for wound healing. In this review, we describe the recent developments made in the processing of bioderived materials employed for cutaneous wound healing, including newly developed materials such as keratin and soy protein. The focus was on the key properties of the bioderived materials that have shown great promise in improving wound healing, restoration and reconstruction. With their good biocompatibility, nontoxic catabolites, microinflammation characteristics, as well as their ability to induce tissue regeneration and reparation, the bioderived materials have great potential for skin tissue repair.
Keywords: wound healing, tissue-engineered skin, bioderived materials, acellular matrix
Introduction
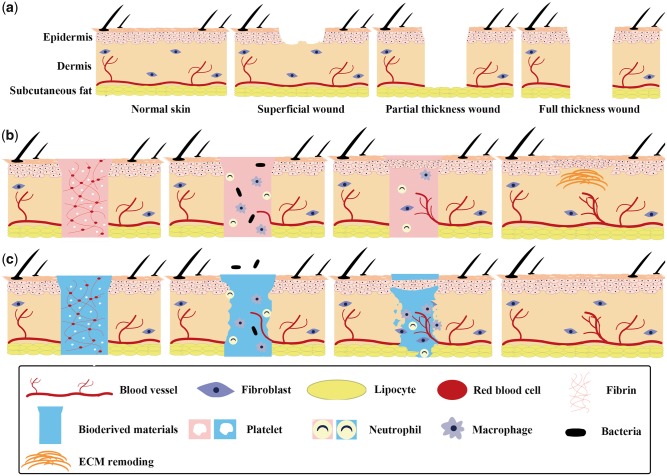
The skin, which is composed of the epidermis, the dermis and subcutaneous tissue (Fig. 1a), plays a critical role in ensuring human health, for it protects tissues and organs from physical, mechanical, chemical and microbial damage [1]. Wounds of the skin can be classified as superficial, partial-thickness and full-thickness wounds (Fig. 1a) based on their thickness [2]. Although most dermal wounds are healed by a natural healing process (Fig. 1b), the healing process may be limited or may fail to regenerate and reconstruct fully functional skin tissue under acute or severe conditions, as in the case of partial-thickness and full-thickness wounds [3]. Therefore, skin wound healing, especially under acute or severe conditions, is a common challenge encountered by plastic and reconstructive surgeons. The use of bioderived materials, which are prepared from specially treated naturally occurring tissues, has become an attractive approach for treating such wounds because of the ability of these materials to inhibit bacterial growth, stimulate cell growth and angiogenesis, and enhance wound healing [3–6]. Figure 1c shows a diagrammatic sketch of wound healing using bioderived materials.
Figure 1.
Diagrammatic sketch of wounds and wound healing. (a) classification of skin wounds based on depth. (b) schematic illustration of classical healing process of full-thickness wounds. (c) schematic illustration showing healing of full-thickness wounds using bioderived materials
However, their clinical significance is limited because of their unsatisfactory mechanical properties, biodegradability and reproducibility. In this regard, various strategies have been developed in recent years to address these problems and synthesize more suitable materials for wound healing. These advanced strategies include drug loading, adding elements (e.g. gold, silver and zinc, among others), cross-linking and combining two or more diverse types of materials with complementary features. This article aims to review the most relevant recent developments in the synthesis of bioderived materials for cutaneous wound healing, including newly reported materials such as keratin and soy protein. The key properties of the bioderived materials that have shown great promise in improving wound healing, restoration and reconstruction are discussed.
Scaffolds from natural polymers
The use of natural polymers, including natural polysaccharide polymers (e.g. cellulose, chitosan and hyaluronic acid, among others) and natural proteins (e.g. silk fibroin, collagen and fibrin glue, among others) (see Fig. 2), is an important direction in the development of tissue engineering scaffolds, because these materials exhibit high cellular affinity, and their use does not result in chronic inflammation, immunological reactions, or toxicity [7].
Figure 2.
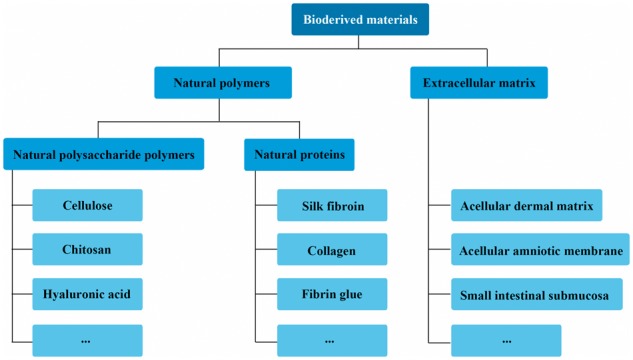
Classification of bioderived materials based on chemical composition of scaffolds
Natural polysaccharide polymers
Cellulose
Cellulose, which is one of the most abundant natural polysaccharides, has attracted considerable interest because of its high durability and low inflammatory response. Further, bacterial cellulose (BC), which is generated through the bacterial fermentation of glucose, is being explored extensively. Its advantages, such as its high wetting strength, elasticity and permeability as well as its ability to accelerate wound healing and reduce scar tissue, make it highly suited for the healing of skin wounds. It exhibits linear elastic behaviour for elongations greater than 35%, with its tensile properties being similar to that of the porcine carotid artery [8]. Different nanoscale structures can have different effects on skin wound healing. In general, BC films with larger pores and a looser and rougher structure result in better cell migration, a higher recovery rate and weaker inflammatory response [9].
However, BC is synthesized by bacteria and exhibits a certain degree of immunogenicity and low antibacterial activity, which limit its applicability. Its antibacterial activity can be improved by loading it with a suitable drug or by adding various elements to it (e.g. silver and copper), while its immunogenicity can be reduced through chemical modification [10, 11]. Another shortcoming may be the difficulty in forming BC in the desired geometries. Bottan et al. could obtain nanofibers with the desired geometrical characteristics through guided assembly-based biolithography (GAB) and were thus able to effectively control the cellular activities that are fundamental to skin wound healing [12].
Chitosan
Chitosan (CS), a deacetylated form of chitin whose structure is similar to that of glycosaminoglycans (GAG), has promising prospects in wound repair [13–16]. Upon depolymerization caused by enzymes such as glucosaminidases, lipases and lysozyme, chitosan yields bioactive chito-oligosaccharides, which show superior antimicrobial properties, and N-acetyl-b-d-glucosamine (NAG), which can promote hyaluronic acid synthesis and fibroblast proliferation and facilitate ordered collagen deposition at the wound site [13, 17].
Several factors affect wound healing, including the molecular weight of the CS sample used, the degree of deacetylation, which is the molar ratio of the d-glucosamine units to the sum of the NAG and d-glucosamine units, and the physiochemical modifications made [18, 19]. In general, the higher the molecular weight and degree of deacetylation of the CS sample used, the higher the wound-healing rate will be [20].
CS is a basic polysaccharide and although the free amino groups of CS can be protonated under acidic conditions, thus making it water soluble, its applicability remained limited because of its poor solubility under the pH conditions of the body and because of its loose cationic nature [21, 22]. Strategies to address these problems include the modification of CS by combining it with metal (oxide) nanoparticles (such as those of ZnO and Ag, see Table 1). The CS derivatives reported in the literature in the last few years are N-succinyl-chitosan, Oleoyl CS, quaternised CS, O-carboxymethyl CS and N,N,N-trimethyl-chitosan [23–29]. However, impurities such as the myosin residues generated during the extraction of CS can lead to an inflammatory reaction and/or the formation of microabscesses after transplantation [30]. Accordingly, high-purity CS should be used in skin tissue engineering.
Table 1.
Studies (2012–17) on repairing skin tissue using natural polysaccharide polymers
| Material | Fabrication method | Biological in vitro results | Biological in vivo results | Reference |
|---|---|---|---|---|
| Bacterial cellulose | Guided assembly-based biolithography | HDF seeded on BC for 12 h; immunostaining and wide-field microscopy: higher cell density was measured and specific cell alignment was detected on structured BC, in contrast to flat control substrates. | BC dressing on artificial wounds in live mouse model for 21 days: structured BC was not degraded or invaded by host cells; better fibroblast infiltration and new collagen deposition in structured BC with levels of vascularization and inflammation comparable to those elicited at interface with autologous full skin graft. | [12] |
| Vaccarin solution impregnation |
|
BC dressing on artificial wounds in live mouse model for 14 days: new neovascularization, stratified squamous epithelium, dense new-born subcutaneous tissue, collagen fibre and hyperplastic fibrous connective tissue were observed in BC- and BC–Vac-membrane-treated groups. | [41] | |
| ZnO nanoparticle suspension impregnation | Samples placed over agar plates containing lawn of selected bacterial strains and incubated at 37 °C for 24 h; antimicrobial assay: BC–ZnO nanocomposites showed activity against gram-negative bacterial strains, including Escherichia coli (90%), Citrobacter freundii (90.9%) and Pseudomonas aeruginosa (87.4%) and gram-positive bacterial strain Staphylococcus aureus (94.3%). | BC–ZnO nanocomposites on artificial wounds in live mouse model for 15 days: healthy granulated tissue, regenerated sebaceous glands and new blood vessels and epithelium in regeneration were observed in BC–ZnO-nanocomposite-treated group; in contrast, ulceration and necrotic tissues were observed in negative group. | [42] | |
| Chitosan | Introducing succinyl groups into glucosamine units of CS N-terminal |
|
NSC powder or CS powder on artificial wounds in live rabbit model for 14 days: rate of macroscopic wound healing: NSC > CS > control; NSC-treated wounds showed better-organized superficial epithelium and were nearly completely repaired, with more fibroblasts, neovascularization and collagen tissues, as well as clearer and orderly boundary layer between epidermis and dermis. | [23] |
| Mixing with hexagonal nanoparticles | Cytotoxicity assay: peripheral blood mononuclear cells, keratinocytes, or fibroblasts seeded on CS for 24 h; MTT assay: both keratinocytes and fibroblasts exhibit normal or moderately enhanced growth on CS films containing hexagonal nanoparticles. | — | [40] | |
| Hyaluronic acid | Anti-TNF-α conjugation | TNF-α capture: conjugate or antibody applied on surface of collagen gel for 15, 30, 60 and 90 min; ELISA analysis: both (anti-TNF-α)–HA and anti-TNF-α result in capturing nearly 90% of TNF-α in collagen gel within 90 min, with slower capture by (anti-TNF-α)–HA than by anti-TNF-α over first 60 min. | Anti-TNF-α in PBS, (anti-TNF-α)–HA on artificial burn wounds in adult Sprague–Dawley rat models for 24 h: at 24 h, more antibodies are present than would be expected in first 600 mm of wound. Below 100 mm from wound surface is cell-dense area that corresponds to first peak of antibody accumulation at both 1 and 24 h. | [43] |
| Mixing with PFC | — |
|
[44] |
HDF, human dermal fibroblasts; Vac, vaccarin; NSC, CS scaffold after introduction of succinyl groups into the glucosamine units of the N-terminal; ELISA, enzyme-linked immunosorbent assay; PFC, platelet-derived factor concentrate.
Hyaluronic acid
Hyaluronic acid (HA), which is distributed widely in tissues such as the skin, cardiac valves and the umbilical cord, is an anionic glycosaminoglycan with good hydrophilicity, viscoelasticity and lubrication [31–33]. As an essential component of the vertebrate extracellular matrix (ECM), HA is not only free of immunogenicity but also provides a suitable growth environment for cells and regulates cell adhesion, migration, proliferation and differentiation [34]. It can reduce graft contracture during skin healing by stimulating the production of more CD44 receptors, which advance the local enzymolysis of HA and promote wound surface vascularization. After the addition of HA during skin healing, the expressions of collagen I and III increase, while the ratio of the two collagen types decreases [35]. Further, in contrast to high-molecular-weight HA, low-molecular-weight HA promotes angiogenesis and granulated tissue formation [36].
The use of HA for scaffolds, however, is restricted because it readily dissolves and biodegrades in the body. Thus, the modification of HA using various cross-linking methods, such chemical or photoinduced cross-linking, is usually necessary. The use of cross-linked HA networks in rats, dogs and horses has yielded positive results, especially with respect to the healing rate [37–39].
In the last few years (2012–2017), many research groups have studied the possibility of using natural polysaccharide polymers that have been modified with bioactive substances or nanoparticles to improve their cell viability and antibacterial activity and strengthen their effect on tissue formation for skin tissue repair (Table 1). Further, CS films with hexagonal silver nanoparticles that generate heat and exhibit enhanced drug delivery properties when illuminated by infrared light have been synthesized (Table 1) [40]. These materials were found to be suitable for healing dermal wounds because they not only facilitated cell proliferation and mitigated bacterial infections but also promoted the delivery of small molecules into the cells.
Natural proteins
Silk fibroin
Silk fibroin (SF) is a natural macromolecular protein consisting of both light and heavy chains, which are linked by a single disulphide bond, in a 1:1 ratio [45]. SF is suitable for developing wound-healing materials because of its desirable properties, including its high biodegradability, ease of chemical modification, good oxygen and water vapor permeabilities, and ability to provide a moist environment for cells [46, 47]. In addition, SF scaffolds with nanosized fibres are better suited for cell adhesion and the spreading of collagen I [47].
The molecular weight of the SF sample used may affect wound healing [46]. In general, SF with a narrow molecular-weight distribution heals faster with more re-epithelization and results in smaller residual scars and fewer infections and foreign body reactions than does SF with a wider molecular-weight distribution.
However, tailoring the mechanical properties of SF to the desired level remains a challenge for researchers because most of the biomaterials developed using SF solutions are weak and brittle, unlike native silk fibres [48].
Fibrin glue
Fibrinogen extracted by methods such as ammonium sulfate precipitation, polyethylene glycol precipitation, or ethanol precipitation is treated with thrombin to fabricate fibrin glue (FG), whose structure and mechanical strength can be regulated by varying the degree of cross-linking [49, 50]. FG exhibits various desirable biological properties and is a promising choice for tissue engineering scaffolds as well as a bioactive substance carrier [51, 52].
FG can improve haemostasis, reduce bacterial infections, help fibroblasts in the surrounding tissue to move through the wound, and increase the rate of keratinocyte spreading and replication [53]. Evidence shows that FG expresses the basal keratin-14 gene and the collagen I gene and forms a fully autologous skin construct with a continuous epidermal–dermal junction similar in structure to native skin in vivo [54]. FG is being used commercially and has been found to be superior in all aspects (including with respect to exudation, coloration, oedema and cosmetic appearance) as compared to platelet-rich plasma during clinical evaluations [53, 55]. However, the shortcomings of FG with respect to wound healing include its high cost, difficulty in storage, long preparation time and the potential risk of transfusion-transmitted diseases [53].
Collagen
Collagens, which are a family of fibrous proteins consisting of α-chains with a triple-helical structure, are the major constituents of the healthy dermal ECM and play a primary role in regulating tissue remodelling during wound healing [56–58]. Collagen can provide cell-binding sites such as those for integrin receptors to regulate cell adhesion, migration and other cellular processes, including proliferation and differentiation in the early stage [59]. It also offers several other advantages. For instance, it can be readily isolated and purified, exhibits low toxicity, and its mechanical, chemical and immunological properties are well documented [60, 61].
However, the journey from bench to bedside is a long one. Hence, more attention should be paid not only to the construction of scaffolds with optimized structures and properties but also to the development of collagen-like materials that mimic natural collagen in terms of their properties [62].
Several other natural polysaccharide polymers and natural proteins, such as starch, pectin, the carbohydrate polymers produced by bacteria and fungi, alginate, keratin, wool, gelatin and soy protein, can be used for skin wound healing [21, 63–68]. Recent relevant studies (2012–2017) on natural proteins for skin tissue repair are listed in Table 2. However, despite their excellent biocompatibility, the suitability of natural proteins for use in tissue engineering applications will increase only if their mechanical properties, biodegradability and reproducibility can be improved to match the application requirements.
Table 2.
Studies (2012–17) on repairing skin tissue using natural proteins
| Material | Fabrication method | Biological in vitro results | Biological in vivo results | Reference |
|---|---|---|---|---|
| Silk fibroin | Mixing with SSD | NHEK and NHEF separately seeded on SF–SSDX for 1 h; microscope: a significantly (P < 0.05) higher number of NHEK adhered to SF than to SF–SSDX. Initial spreading of NHEK adhering to SF–SSDX decreased gradually when SSD concentrations were increased. The case for NHEF was the same. | SF–SSDX on artificial wounds in live rat model for 14 days: sizes of wounds treated with SF/AgS 1.0 and Acticoat were much smaller than those in other groups, and rate of closure of wound treated with SF–SSDX was significantly higher (P < 0.05) than that in control group. | [69] |
| Immobilization with Cys-KR12 |
|
— | [70] | |
| Fibrin glue | Cryoprecipitation and cryocentrifugation | — | FG grafting on artificial wounds in live pig model for 14 days: number of new microvessels was significantly higher (P < 0.05) in FG group than in control group at day 3. Intensity of inflammation was significantly lower in FG group than in control group at day 7. | [71] |
| Collagen | Incorporation with gold nanoparticles | Cytotoxicity assay: L929 cells incubated in CS–AuX extract for 24 h; MTT cell viability assay: cell viability for all scaffold extracts was higher than 90%. Cell attachment assay: L929 fibroblasts seeded on CS–AuX for 24 h; SEM: fibroblasts on scaffolds gained their natural spindle-like shape with outstretched pseudopods spreading over the surface. | CS–AuX on artificial wounds in live rat model for 14 days: milder inflammatory reaction and higher neovascularization were observed with CS–AuX than in other groups; better wound closure was observed with CS–X, CS–AuX and MatriDerm than in untreated control. | [72] |
| Modification with CBD-E7 peptide | — | Collagen/CBD-E7 peptide on artificial wounds in live porcine model for 28 days: significantly more MSCs were retained on CBD-E7 collagen scaffold than on pristine collagen scaffold at day 3 post-surgery; significantly rapid wound healing in collagen/CBD-E7 peptide group at days 14, 21 and 28 than in other groups; significantly higher capillary density in collagen/CBD-E7 peptide group than in other groups. | [73] |
SSD, C10H9AgN4O2S; NHEK, normal human epidermal keratinocyte; NHEF, normal human epidermal fibroblast; HaCaT, human keratinocytes; LPS, lipopolysaccharides; Raw264.7, murine monocytes; CS–AuX, collagen-containing gold nanoparticles; MSCs, mesenchymal stem cells.
Scaffolds from extracellular matrix
Cutaneous wound healing is a complex process requiring the integration of biological and molecular events that include cell migration and proliferation, ECM deposition, angiogenesis and remodelling [74]. ECM scaffolds can consist of a diverse range of molecules (e.g. collagen, fibrin, elastin, glycosaminoglycans and growth factors, among others) that mediate structural and/or biological properties [75]. They find wide use in tissue engineering owing to their inherent advantages such as their excellent biocompatibility, desirable biological activity, and ability to promote tissue regeneration. As a result, they have recently become the focus of growing interest [7, 75, 76]. In addition, they can release growth factors in a highly spatiotemporally controlled manner and can modulate their intracellular signalling [59]. A number of studies have shown that ECM scaffolds from one type of tissue can provide abundant growth signals for the cells of another type of tissue [77, 78]. In the field of skin tissue engineering, the acellular dermal matrix (ADM), acellular amniotic membrane (AAM) and small intestinal submucosa (SIS) have been studied extensively.
The ADM is the remaining ECM of the dermal and basement membranes once the cellular components (the epidermis and the cells of the dermis) have been removed and can provide the mechanical support lacking at the wound site, improve the quality of wound healing, increase the survival rate of the epidermal membrane, and prevent wound contraction and scar formation [79]. Dermal grafts obtained from human subjects have been used to treat burns and full-thickness skin defects [80, 81]. However, their applicability is limited by their high cost, the limited availability of cadaver skin, and the risk of disease transmission [82]. Thus, ADMs derived from the skins of pigs, goats, fish and ostriches have been used; these have yielded satisfactory results with respect to wound healing [83–85]. The stiffness of the ADM may affect wound healing. In general, epidermis treated with a softer ADM derived from younger mice exhibits better collagen density than that treat with ADM derived from older mice. In addition, the orientation and stiffness of the new dermal tissue grown closer to the normal tissue are also favourable [86].
The AAM, which is composed of collagen, elastin, laminin, fibronectin and growth factors, is an attractive biomaterial for use in wound healing, as it helps reduce pain and wound dehydration, promotes epithelialization, prevents scarring and exhibits anti-inflammatory, antimicrobial and antifibroblastic effects [87–89]. AAMs prepared by the chemical detergent-enzymatic extraction method have been shown to preserve the tissue matrix and the reticular structure well and thus have promise for use as membranes for skin wound healing [90].
SIS, an ECM material derived from swine, is more advantageous for promoting angiogenesis, cell growth and differentiation and tissue regeneration because of its innate growth factors (transforming growth factor-beta, basic fibroblast growth factor, vascular endothelial growth factor and epidermal growth factor, among others) [91]. It also exhibits several advantages, such as immunogenicity, good cellular compatibility and anisotropy and therefore shows promise for use in the repair of soft tissue [92–95]. In addition, seeding MSCs on SIS can promote appendage formation with less scarring [96].
Recent relevant studies (2012–2017) on ECM for skin tissue repair are listed in Table 3.
Table 3.
Studies (2012–17) on repairing skin tissue using extracellular matrix
| Material | Fabrication method | Biological in vitro results | Biological in vivo results | Reference |
|---|---|---|---|---|
| Acellular dermal matrix | Seeding with BMSCs | BMSCs seeded on ADM for 14 days; confocal microscopy: proliferation indexes of MSC in ADM scaffolds on days 1, 4, 7 and 14 were 0.18 ± 0.07%, 0.32 ± 0.04%, 0.45 ± 0.11% and 100 ± 0.09%, respectively. | ADM on artificial wounds in live mice model for 21 days: dermal differentiation, epithelial maturation, skin appendage regeneration and neovascularization of wound treated with BMSC-seeded ADM scaffolds were better than those in control group. | [97] |
| PHD-2 siRNA solution impregnation | — | ADM on artificial wounds in live mice model for 14 days: cellularity and vascularity of wound treated with PHD-2 siRNA–ADM scaffolds were better than those in control group. | [98] | |
| Acellular amniotic membrane | Seeding with ADMSCs | ADMSCs seeded on AAM for 7 days; HE staining: spindle-like ADMSCs grew well at day 3; ADMSCs fused into patches on surface of AAM and turned into multiple layers at day 7. | AAM on artificial wounds in live nude mice model for 28 days: wound-healing rate and number of epidermal layers in ADMSC–AAM seeding group were significantly higher than those in hAM and AAM groups (P < 0.05); typical hair follicle structure appeared in ADMSC–AAM seeding group. | [99] |
| Small intestinal submucosa | Seeding with BMSCs | BMSCs seeded on SIS for 21 days; MTT assay and HE staining: BMSCs grow and proliferate well on SIS scaffolds. | SIS on artificial wounds in live rat model for 7 weeks: epithelization in decellularized SIS group was faster than in native SIS; skin appendage-like structures were observed only in decellularized SIS group at day 28. | [100] |
BMSCs, bone marrow mesenchymal stem cells; PHD2, prolyl hydroxylase domain-2; ADMSCs, adipose-derived mesenchymal stem cells.
Scaffolds from composite biomaterials
Tissues and organs are complex and orderly wholes composed of many different components. The ideal scaffold must exhibit certain properties, particularly high biocompatibility, bioactivity and mechanical strength.
Recent research has focused on combining two or more different materials with complementary features using physical or chemical methods to fabricate scaffold materials. These composites exhibit improved performance as dermal wound-healing materials (Table 4). Composites developed using several natural polymers can yield scaffolds similar to the ECM [101]. In addition, composites developed from synthetic polymers and bioderived materials can endow materials with various desirable properties such as high bioactivity, biodegradability and mechanical strength (Fig. 3) [102–104].
Table 4.
Studies (2012–17) on repairing skin tissue using composite biomaterials
| Classification | Material | Fabrication method | Biological in vitro results | Biological in vivo results | Reference |
|---|---|---|---|---|---|
| Natural polymers–natural polymers | Gelatin–HA | Electrospinning and cross-linking | — | Gelatin–HA on artificial wounds in live rat model for 14 days: more epidermis and fewer inflammatory cells were found in GE/HA nanofiber and ChitoHeal gel groups than in antiseptic gauze group. | [105] |
| Natural polymers–ECM | Decellularized peritoneum– HA–EGF | 1. Coating decellularized peritoneum with sodium hyaluronate 2. Soaking in EGF solution | NIH3T3 cells cultured in culture medium containing EGF for 96 h; MTT assay: cell viability increased as EGF concentrations increased. | Scaffolds on artificial wounds in live rabbit model for 20 days: decellularized peritoneum–HA–EGF group recovered best among all groups, with wound-healing rates of 87.41% after 20 days post-surgery; thicker epidermis and dermis layers were observed in decellularized peritoneum–HA–EGF group than in decellularized peritoneum group. | [106] |
| Natural polymers–synthetic polymers | PCL–CA–CS–collagen | 1. Co-electrospinning of PCL and CA 2. Alternately soaking in CS and collagen solutions every 20 min |
|
Fibroblast-seeded scaffolds on artificial wounds in live rat model for 7 days: the CS/collagen coatings in scaffolds had positive effect on neovascularization and led to increased wound-healing rate; fibroblast-seeded PCL–CA–CS–collagen promoted complete re-epithelialization and regeneration of skin appendages; regenerated skin with fibroblast-seeded PCL–CA–CS–collagen covering exhibited smooth surface and loose collagen fibre arrangement similar to that of normal skin. | [107] |
| Castor-oil-based polymer–CS–ZnO | 1. Mixing castor oil with CS–ZnO nanoparticles 2. Reacting with HDI 3. Crosslinking using GLA |
|
Castor-oil-based polymer–CS–ZnO on artificial wounds in live rat model for 14 days: castor-oil-based polymer–CS–ZnO group healed much faster with better re-epithelialization and collagen deposition than did castor oil group and gauze group. | [108] |
EGF, epidermal growth factor; PCL, polycaprolactone; CA, cellulose acetate; NHDFs, normal human dermal fibroblast; HDI, hexamethylene diisocyanate; GLA, glutaraldehyde.
Figure 3.
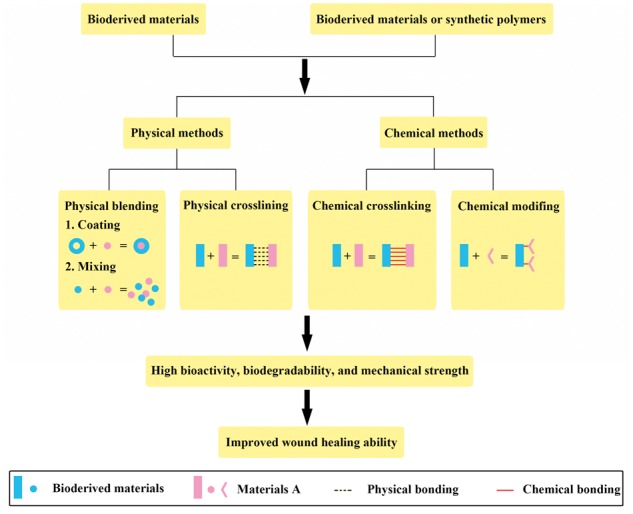
Classification of methods for fabricating composite scaffolds
Challenges and perspectives
Owing to their unique properties, bioderived materials have significant clinical value in facilitating and accelerating wound healing and inducing skin regeneration in full-thickness wounds. However, their potential for use in dermal wound healing would increase further if their mechanical properties, biodegradability and reproducibility could be improved to fit the application requirements better. These limitations may be addressed through cross-linking, combining two or more different types of materials with complementary features, or by developing other novel strategies in the future.
Acknowledgments
The authors would like to thank the National Key Research and Development Program of China (2017YFC1104702) and the National Nature Science Foundation of China (31600792, 31771065).
Funding
This work was supported by the National Key Research and Development Program of China (grant number 2017YFC1104702); and the National Natural Science Foundation of China (grant numbers 31600792, 31771065).
Conflict of interest statement. None declared.
References
- 1. Bi H, Jin Y.. Current progress of skin tissue engineering: Seed cells, bioscaffolds, and construction strategies. Burns Trauma 2013;1:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patrulea V, Ostafe V, Borchard G. et al. Chitosan as a starting material for wound healing applications. Eur J Pharm Biopharm 2015;97:417–26. [DOI] [PubMed] [Google Scholar]
- 3. Chandika P, Ko SC, Jung WK.. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int J Biol Macromol 2015;77:24–35. [DOI] [PubMed] [Google Scholar]
- 4. Kim H, Kong WH, Seong KY. et al. Hyaluronate-epidermal growth factor conjugate for skin wound healing and regeneration. Biomacromolecules 2016;17:3694–705. [DOI] [PubMed] [Google Scholar]
- 5. Li Y, Han Y, Wang X. et al. Multifunctional hydrogels prepared by dual ion cross-linking for chronic wound healing. ACS Appl Mater Interfaces 2017;9:16054–62. [DOI] [PubMed] [Google Scholar]
- 6. Wang J, Windbergs M.. Functional electrospun fibers for the treatment of human skin wounds. Eur J Pharm Biopharm 2017; pii: S0939-6411(17)30628-8. doi: 10.1016/j.ejpb.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 7. Mano JF, Silva GA, Azevedo HS. et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface R Soc 2007;4:999–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bäckdahl H, Helenius G, Bodin A. et al. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006;27:2141–9. [DOI] [PubMed] [Google Scholar]
- 9. Li Y, Wang S, Huang R. et al. Evaluation of the effect of the structure of bacterial cellulose on full thickness skin wound repair on a microfluidic chip. Biomacromolecules 2015;16:780–9. [DOI] [PubMed] [Google Scholar]
- 10. Kucińska-Lipka J, Gubanska I, Janik H.. Bacterial cellulose in the field of wound healing and regenerative medicine of skin: recent trends and future prospectives. Polymer Bull 2015;72:2399–419. [Google Scholar]
- 11. Wen X, Zheng Y, Wu J. et al. In vitro and in vivo investigation of bacterial cellulose dressing containing uniform silver sulfadiazine nanoparticles for burn wound healing. Progress Nat Sci Mater Int 2015;25:197–203. [Google Scholar]
- 12. Bottan S, Robotti F, Jayathissa P. et al. Surface-structured bacterial cellulose with Guided Assembly-Based Biolithography (GAB). Acs Nano 2015;9:206.. [DOI] [PubMed] [Google Scholar]
- 13. LogithKumar R, KeshavNarayan A, Dhivya S. et al. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr Polym 2016; 20151:172–88. doi: 10.1016/j.carbpol.2016.05.049. Epub 2016 May 18. [DOI] [PubMed] [Google Scholar]
- 14. Sandeep K, Maiti SK, Naveen K. et al. Effect of medical grade chitosan powder in full thickness skin wound healing in rat model. Adv Anim Vet Sci 2014;2:270–6. [Google Scholar]
- 15. Ji C, Shi J.. Thermal-crosslinked porous chitosan scaffolds for soft tissue engineering applications. Mater Sci Eng C Mater Biol Appl 2013;33:3780–5. [DOI] [PubMed] [Google Scholar]
- 16. Hilmi AB, Halim AS, Hassan A. et al. In vitro characterization of a chitosan skin regenerating template as a scaffold for cells cultivation. Springerplus 2013;2:79.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charernsriwilaiwat N, Opanasopit P, Rojanarata T. et al. Lysozyme-loaded, electrospun chitosan-based nanofiber mats for wound healing. Int J Pharm 2012;427:379–84. [DOI] [PubMed] [Google Scholar]
- 18. Dai T, Tanaka M, Huang YY. et al. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti-Infect Ther 2011;9:857–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Croisier F, Jérôme C.. Chitosan-based biomaterials for tissue engineering. Eur Polym J 2013;49:780–92. [Google Scholar]
- 20. Alsarra IA. Chitosan topical gel formulation in the management of burn wounds. Int J Biol Macromol 2009;45:16–21. [DOI] [PubMed] [Google Scholar]
- 21. Ahmed S, Ikram S.. Chitosan based scaffolds and their applications in wound healing. Achiev Life Sci 2016;10:27–37. [Google Scholar]
- 22. Bui V, Park D, Lee YC.. Chitosan combined with ZnO, TiO2 and Ag nanoparticles for antimicrobial wound healing applications: a mini review of the research trends. Polymers 2017;9:21.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang F, Lv L, Lu F. et al. Preparation and characterization of N-chitosan as a wound healing accelerator. Int J Biol Macromol 2016;93:1295–303. [DOI] [PubMed] [Google Scholar]
- 24. Datta S, Rameshbabu AP, Bankoti et al. Oleoyl chitosan based nanofiber mats impregnated with amniotic membrane derived stem cells for accelerated full-thickness excisional wound healing. ACS Biomater. Sci. Eng 2017; doi: 10.1021/acsbiomaterials.7b00189. [DOI] [PubMed] [Google Scholar]
- 25. Sajomsang W, Gonil P, Saesoo S. et al. Antifungal property of quaternized chitosan and its derivatives. Int J Biol Macromol 2012;50:263–9. [DOI] [PubMed] [Google Scholar]
- 26. Wei L, Li Q, Tan W. et al. Synthesis, characterization, and the antioxidant activity of double quaternized chitosan derivatives. Molecule 2017;22:pii: E501. doi: 10.3390/molecules22030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruihua H, Bingchao Y, Zheng D. et al. Preparation and characterization of a quaternized chitosan. J Mater Sci 2011;47:845–51. [Google Scholar]
- 28. Park SH, Kim EH, Lee HJ. et al. Wound healing effect of visible light-curable chitosan with encapsulated EGF. Macromol Res 2016;24:336–41. [Google Scholar]
- 29. Zhou Z, Yan D, Cheng X. et al. Biomaterials based on N,N,N-trimethyl chitosan fibers in wound dressing applications. Int J Biol Macromol 2016;89:471–6. [DOI] [PubMed] [Google Scholar]
- 30. Yang JL, Xie HG, Yu WT. et al. Progress in the research on chitosan for tissue engineering. J Funct Mater 2013;44:1521–5. [Google Scholar]
- 31. Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol 2002;21:25–9. [DOI] [PubMed] [Google Scholar]
- 32. Fakhari A, Berkland C.. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater 2013;9:7081–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sze JH, Brownlie JC, Love CA.. Biotechnological production of hyaluronic acid: a mini review. 3 Biotec 2016;6:67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Almond A. Hyaluronan. Cell Mol Life Sci 2007;64:1591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao JY, Chai JK, Song HF. et al. Influence of hyaluronic acid on wound healing using composite porcine acellular dermal matrix grafts and autologous skin in rabbits. Int Wound J 2013;10:562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Longinotti C. The use of hyaluronic acid based dressings to treat burns: a review. Burns Trauma 2014;2:162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang G, Prestwich GD, Mann BK.. Thiolated carboxymethyl-hyaluronic-Acid-based biomaterials enhance wound healing in rats, dogs, and horses. ISRN Veterinary Sci 2011;2011:851593.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hadley HS, Stanley BJ, Fritz MC. et al. Effects of a cross-linked hyaluronic acid based gel on the healing of open wounds in dogs. Veterinary Surg VS 2013;42:161–9. [DOI] [PubMed] [Google Scholar]
- 39. Hemshekhar M, Thushara RM, Chandranayaka S. et al. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int J Biol Macromol 2016;86:917–28. [DOI] [PubMed] [Google Scholar]
- 40. Levi-Polyachenko N, Jacob R, Day C. et al. Chitosan wound dressing with hexagonal silver nanoparticles for hyperthermia and enhanced delivery of small molecules. Colloids Surfaces B Biointerfaces 2016;142:315–24. [DOI] [PubMed] [Google Scholar]
- 41. Qiu Y, Qiu L, Cui J. et al. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater Sci Eng C Mater Biol Appl 2016;59:303–9. [DOI] [PubMed] [Google Scholar]
- 42. Khalid A, Khan R, Ul-Islam M. et al. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr Polym 2017;164:214–21. [DOI] [PubMed] [Google Scholar]
- 43. Friedrich EE, Washburn NR.. Transport patterns of anti-TNF-alpha in burn wounds: therapeutic implications of hyaluronic acid conjugation. Biomaterials 2017;114:10–22. [DOI] [PubMed] [Google Scholar]
- 44. Mashiko T, Minabe T, Yamakawa T. et al. Platelet-derived factor concentrates with hyaluronic acid scaffolds for treatment of deep burn wounds. Plastic Reconstruct Surg Global Open 2016;4:e1089.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kasoju N, Bora U.. Silk fibroin in tissue engineering. Adv Healthcare Mater 2012;1:393–412. [DOI] [PubMed] [Google Scholar]
- 46. Kim MK, Yoo KY, Kwon KJ. et al. Powdered wound dressing materials made from wild silkworm antheraea pernyi silk fibroin on full-skin thickness burn wounds on rats. Maxillofacial Plastic Reconstruct Surg 2014;36:111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Min BM, Lee G, Kim SH. et al. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004;25:1289–97. [DOI] [PubMed] [Google Scholar]
- 48. Kundu B, Rajkhowa R, Kundu SC. et al. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev 2013;65:457–70. [DOI] [PubMed] [Google Scholar]
- 49. Luo JC, Yang ZM.. Preparation of fibrin glue and application in tissue engineering. Journal of Biomed Eng Res 2004;3:187–90. [Google Scholar]
- 50. Grasman JM, Page RL, Dominko T. et al. Crosslinking strategies facilitate tunable structural properties of fibrin microthreads. Acta Biomater 2012;8:4020–30. [DOI] [PubMed] [Google Scholar]
- 51. Oseni AO, Butler PE, Seifalian AM.. Rapid production of autologous fibrin hydrogels for cellular encapsulation in organ regeneration. Methods Mol Biol 2013;1001:145–52. [DOI] [PubMed] [Google Scholar]
- 52. Layman H, Li X, Nagar E. et al. Enhanced angiogenic efficacy through controlled and sustained delivery of FGF-2 and G-CSF from fibrin hydrogels containing ionic-albumin microspheres. J Biomater Sci Polym Ed 2012;23:185–206. [DOI] [PubMed] [Google Scholar]
- 53. Currie LJ, Sharpe JR, Martin R.. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plastic Reconstruct Surg 2001;108:1713–26. [DOI] [PubMed] [Google Scholar]
- 54. Mazlyzam AL, Aminuddin BS, Fuzina NH. et al. Reconstruction of living bilayer human skin equivalent utilizing human fibrin as a scaffold. Burns 2007;33:355–63. [DOI] [PubMed] [Google Scholar]
- 55. Hermeto LC, Rd R, Pádua SB. et al. Comparative study between fibrin glue and platelet rich plasma in dogs skin grafts. Acta Cirurgica Brasileira 2012;27:789. [DOI] [PubMed] [Google Scholar]
- 56. Inwoo B, Kiyoshi O, Asami Y. et al. Biochemical properties of acid-soluble collagens extracted from the skins of underutilised fishes. Food Chem 2008;108:49–54. [Google Scholar]
- 57. Myllyharju J, Kivirikko KI.. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet Tig 2004;20:33–43. [DOI] [PubMed] [Google Scholar]
- 58. Cen LLW, Cui L, Zhang W. et al. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr Res 2008; 63:492–6. [DOI] [PubMed] [Google Scholar]
- 59. Briquez PS, Hubbell JA, Martino MM.. Extracellular matrix-inspired growth factor delivery systems for skin wound healing. Adv Wound Care 2015;4:479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Duan X, Sheardown H.. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: mechanical properties and corneal epithelial cell interactions. Biomaterials 2006;27:4608–17. [DOI] [PubMed] [Google Scholar]
- 61. Ju YM, Yu B, Koob TJ. et al. A novel porous collagen scaffold around an implantable biosensor for improving biocompatibility. I. In vitro/in vivo stability of the scaffold and in vitro sensitivity of the glucose sensor with scaffold. J Biomed Mater Res A 2008;87:136–46. [DOI] [PubMed] [Google Scholar]
- 62. Dong C, Lv Y.. Application of collagen scaffold in tissue engineering: recent advances and new perspectives. Polymers 2016;8:42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dongargaonkar AA, Bowlin GL, Yang H.. Electrospun blends of gelatin and gelatin-dendrimer conjugates as a wound-dressing and drug-delivery platform. Biomacromolecules 2013;14:4038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O'Meara S, Martyn-St James M. Alginate dressings for venous leg ulcers. Cochrane Database Syst Rev 2013;(4):CD010182. doi: 10.1002/14651858.CD010182.pub2. [DOI] [PubMed] [Google Scholar]
- 65. Dumville JC, O'Meara S, Deshpande S. et al. Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev 2012;CD009110. [DOI] [PubMed] [Google Scholar]
- 66. Bhardwaj N, Sow WT, Devi D. et al. Silk fibroin-keratin based 3D scaffolds as a dermal substitute for skin tissue engineering. Integr Biol Quant Biosci Nano Macro 2015;7:53–63. [DOI] [PubMed] [Google Scholar]
- 67. Shevchenko RV, Santin M.. Pre-clinical evaluation of soybean-based wound dressings and dermal substitute formulations in pig healing and non-healing in vivo models. Burns Trauma 2014;2:187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pallavicini P, Arciola CR, Bertoglio F. et al. Silver nanoparticles synthesized and coated with pectin: An ideal compromise for anti-bacterial and anti-biofilm action combined with wound-healing properties. J Colloid Interface Sci 2017;498:271–81. [DOI] [PubMed] [Google Scholar]
- 69. Jeong L, Kim MH, Jung JY. et al. Effect of silk fibroin nanofibers containing silver sulfadiazine on wound healing. Int J Nanomed 2014;9:5277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Song DW, Kim SH, Kim HH. et al. Multi-biofunction of antimicrobial peptide-immobilized silk fibroin nanofiber membrane: implications for wound healing. Acta Biomater 2016;39:146–55. [DOI] [PubMed] [Google Scholar]
- 71. Dejyong K, Kaewamatawong T, Brikshavana P. et al. Efficacy of bubaline fibrin glue on full-thickness pinch and punch skin grafting in a pig. J Biomater Appl 2017;31:1215–24. [DOI] [PubMed] [Google Scholar]
- 72. Akturk O, Kismet K, Yasti AC. et al. Collagen/gold nanoparticle nanocomposites: a potential skin wound healing biomaterial. J Biomater Appl 2016;31:283–301. [DOI] [PubMed] [Google Scholar]
- 73. Wang H, Yan X, Shen L. et al. Acceleration of wound healing in acute full-thickness skin wounds using a collagen-binding peptide with an affinity for MSCs. Burns Trauma 2014;2:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nie C, Yang D, Morris SF.. Local delivery of adipose-derived stem cells via acellular dermal matrix as a scaffold: a new promising strategy to accelerate wound healing. Med Hypotheses 2009;72:679–82. [DOI] [PubMed] [Google Scholar]
- 75. Badylak SF, Freytes DO, Gilbert TW.. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater 2009;5:1–13. [DOI] [PubMed] [Google Scholar]
- 76. Andree B, Bar A, Haverich A. et al. Small intestinal submucosa segments as matrix for tissue engineering: review. Tissue Eng B Rev 2013;19:279–91. [DOI] [PubMed] [Google Scholar]
- 77. Sánchez-Palencia DM, D′Amore A, González-Mancera A. et al. Effects of fabrication on the mechanics, microstructure and micromechanical environment of small intestinal submucosa scaffolds for vascular tissue engineering. J Biomech 2014;47:2766–73. [DOI] [PubMed] [Google Scholar]
- 78. Celik O, Esrefoglu M, Hascalik S. et al. Use of porcine small intestinal submucosa to reconstruct an ovarian defect. Int J Gynaecol Obstetrics Off Organ Int Federation Gynaecol Obstetrics 2009;106:218–22. [DOI] [PubMed] [Google Scholar]
- 79. Wainwright DJ, Bury SB.. Acellular dermal matrix in the management of the burn patient. Aesthetic Surg J/Am Soc Aesthetic Plastic Surg 2011;31:13S–23S. [DOI] [PubMed] [Google Scholar]
- 80. Park JY, Lee TG, Kim JY. et al. Acellular dermal matrix to treat full thickness skin defects: follow-up subjective and objective skin quality assessments. Arch Craniofacial Surg 2014;15:14.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Levin F, Turbin RE, Langer PD.. Acellular human dermal matrix as a skin substitute for reconstruction of large periocular cutaneous defects. Ophthalmic Plastic Reconstruct Surg 2011;27:44–7. [DOI] [PubMed] [Google Scholar]
- 82. Liu Z, Zhou Q, Zhu J. et al. Using genipin-crosslinked acellular porcine corneal stroma for cosmetic corneal lens implants. Biomaterials 2012;33:7336–46. [DOI] [PubMed] [Google Scholar]
- 83. Farahani FK, Fattahian H, Kajbafzade AM.. Experimental study on ostrich acellular dermal matrix in repair of full-thickness wounds of guinea pig. J Nara Med Assoc 2015;50:63–73. [Google Scholar]
- 84. Zhang Z, Lv L, Mamat M. et al. Xenogenic (porcine) acellular dermal matrix is useful for the wound healing of severely damaged extremities. Exp Therapeutic Med 2014;7:621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Baldursson BT, Kjartansson H, Konradsdottir F. et al. Healing rate and autoimmune safety of full-thickness wounds treated with fish skin acellular dermal matrix versus porcine small-intestine submucosa: a noninferiority study. Int J Lower Extremity Wounds 2015;14:37–43. [DOI] [PubMed] [Google Scholar]
- 86. Han X, Liu H, Chen M. et al. Acellular dermal matrix from one-day-old mouse skin on adult scarless cutaneous wound repair by second harmonic generation microscopic imaging. RSC Adv 2016;6:71852–62. [Google Scholar]
- 87. Wilshaw SP, Kearney JN, Fisher J et al. Production of an acellular amniotic membrane matrix for use in tissue engineering. Tissue Eng 2006;12:2117–29. [DOI] [PubMed]
- 88. Chen YJ, Chung MC, Jane Yao CC. et al. The effects of acellular amniotic membrane matrix on osteogenic differentiation and ERK1/2 signaling in human dental apical papilla cells. Biomaterials 2012;33:455–63. [DOI] [PubMed] [Google Scholar]
- 89. Wilshaw SP, Kearney J, Fisher J. et al. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng A 2008;14:463–72. [DOI] [PubMed] [Google Scholar]
- 90. Luo JC, Li XQ, Yang ZM.. Preparation of human acellular amniotic membrane and its cytocompatibility and biocompatibility. Chin Reconstructive Surg 2004; 18:108. [PubMed] [Google Scholar]
- 91. Fan MR, Gong M, Da LC. et al. Tissue engineered esophagus scaffold constructed with porcine small intestinal submucosa and synthetic polymers. Biomed Mater 2014;9:015012.. [DOI] [PubMed] [Google Scholar]
- 92. Tan B, Wei RQ, Tan MY. et al. Tissue engineered esophagus by mesenchymal stem cell seeding for esophageal repair in a canine model. J Surg Res 2013;182:40–8. [DOI] [PubMed] [Google Scholar]
- 93. Luo JC, Chen W, Chen XH. et al. A multi-step method for preparation of porcine small intestinal submucosa (SIS). Biomaterials 2011;32:706–13. [DOI] [PubMed] [Google Scholar]
- 94. Wang W, Zhang X, Chao NN. et al. Preparation and characterization of pro-angiogenic gel derived from small intestinal submucosa. Acta Biomater 2016;29:135–48. [DOI] [PubMed] [Google Scholar]
- 95. Kim MS, Hong KD, Shin HW. et al. Preparation of porcine small intestinal submucosa sponge and their application as a wound dressing in full-thickness skin defect of rat. Int J Biol Macromol 2005;36:54–60. [DOI] [PubMed] [Google Scholar]
- 96. Song YZ, Cui HX, Wang ZX. et al. Preparation and biocompatibility of human acellular amniotic membrane matrix. J Clin Rehabil Tissue Eng Res 2008;12:51–5. [Google Scholar]
- 97. Wang Q, Jin Y, Deng X. et al. Second-harmonic generation microscopy for assessment of mesenchymal stem cell-seeded acellular dermal matrix in wound-healing. Biomaterials 2015;53:659–68. [DOI] [PubMed] [Google Scholar]
- 98. Vandegrift MT, Szpalski C, Knobel D. et al. Acellular dermal matrix-based gene therapy augments graft incorporation. J Surg Res 2015;195:360–7. [DOI] [PubMed] [Google Scholar]
- 99. Minjuan W, Jun X, Shiyun S. et al. Hair follicle morphogenesis in the treatment of mouse full-thickness skin defects using composite human acellular amniotic membrane and adipose derived mesenchymal stem cells. Stem Cells Int 2016;2016:8281235.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Parmaksiz M, Elcin AE, Elcin YM.. Decellularization of bovine small intestinal submucosa and its use for the healing of a critical-sized full-thickness skin defect, alone and in combination with stem cells, in a small rodent model. J Tissue Eng Regenerative Med 2015;49:337–9. [DOI] [PubMed] [Google Scholar]
- 101. Chang KH, Liao HT, Chen JP.. Preparation and characterization of gelatin/hyaluronic acid cryogels for adipose tissue engineering: in vitro and in vivo studies. Acta Biomater 2013;9:9012–26. [DOI] [PubMed] [Google Scholar]
- 102. Badrossamay MR, Balachandran K, Capulli AK. et al. Engineering hybrid polymer-protein super-aligned nanofibers via rotary jet spinning. Biomaterials 2014;35:3188–97. [DOI] [PubMed] [Google Scholar]
- 103. He X, Zhai Z, Wang Y. et al. New method for coupling collagen on biodegradable polyurethane for biomedical application. J Appl Polym Sci 2012;126:E354–E61. [Google Scholar]
- 104. Xu D, Wu K, Zhang Q. et al. Synthesis and biocompatibility of anionic polyurethane nanoparticles coated with adsorbed chitosan. Polymer 2010;51:1926–33. [Google Scholar]
- 105. Ebrahimi-Hosseinzadeh B, Pedram M, Hatamian-Zarmi A. et al. In vivo evaluation of gelatin/hyaluronic acid nanofiber as Burn-wound healing and its comparison with ChitoHeal gel. Fibers Polym 2016;17:820–6. [Google Scholar]
- 106. Su Z, Ma H, Wu Z. et al. Enhancement of skin wound healing with decellularized scaffolds loaded with hyaluronic acid and epidermal growth factor. Mater Sci Eng C 2014;44:440–8. [DOI] [PubMed] [Google Scholar]
- 107. Huang R, Li W, Lv X. et al. Biomimetic LBL structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing. Biomaterials 2015;53:58–75. [DOI] [PubMed] [Google Scholar]
- 108. Diez-Pascual AM, Diez-Vicente AL.. Wound healing bionanocomposites based on castor oil polymeric films reinforced with chitosan-modified ZnO nanoparticles. Biomacromolecules 2015;16:2631–44. [DOI] [PubMed] [Google Scholar]