Abstract
Objective:
The aim was to compare the visual, refractive, topographic and biomechanical outcomes in patients with progressive keratoconus treated with either conventional or accelerated crosslinking at one year follow up.
Methods:
It is a prospective, non-randomised interventional study of 76 patients who underwent conventional (CXL; 3mW/cm2 for 30 minutes) or accelerated cross linking (KXL; 30mW/cm2 for 4 minutes) for progressive keratoconus. Baseline and postoperative visual acuity, manifest refraction, corneal topography, pachymetry, endothelial cell density and biomechanical parameters of corneal hysteresis and corneal resistance factor were evaluated and compared.
Results:
The 2 groups were comparable in terms of uncorrected and best corrected visual acuity and spherical equivalent. Both groups showed no significant increase in K1, K2 and Kmean from baseline at 12 months. There was also no difference between the CXL and KXL group for postoperative corneal topography as well as central and minimal pachymetry up to 12 months. There was a significant increase in both corneal hysteresis (0.62mm Hg, P=0.04) and corneal resistance factor (0.91mm Hg, P=0.003) in the KXL group at 12 months but not in the CXL group. There was no significant endothelial cell loss throughout follow up in both the groups.
Conclusion:
We have established comparability of the 2 protocols in stabilizing the progression of keratoconus. Our findings also suggested an added biomechanical advantage of accelerated crosslinking at 1 year follow up.
Keywords: Cross linking, Keratoconus, Corneal biomechanics, Collagen, Topographic, Biomechanical outcomes
1. INTRODUCTION
Collagen crosslinking is an established treatment for keratoconus and other ectatic corneal disorders, with proven efficacy in slowing or halting disease progression [1-3]. In the procedure, the induction of cross-links between intrastromal collagen fibrils by photosensitizer riboflavin and ultraviolet A (UV-A) irradiation confers added corneal rigidity and strength, thereby stabilizing the ectatic process. Since the first report of crosslinking in the treatment of progressive keratoconus in 2003 [4], there has been extensive research on expanded indications and various modifications of the procedure evolved from the original “Dresden protocol” for conventional crosslinking, including accelerated protocols with increased irradiance over shorter duration, treatment through an intact epithelium (“epi-on”), as well as combination therapy with intracorneal ring segments and refractive surgery across different crosslinking platforms [2, 5].
Of interest, accelerated or high-fluence protocols present a promising alternative to the time-consuming conventional crosslinking. The potential advantages include reduced exposure time, better patient compliance and lower infection risk. According to Bunsen-Roscoe’s law of reciprocity, an increased intensity of UV-A irradiation coupled with reduced exposure time theoretically delivers a total energy dose to the tissue equivalent to that in conventional treatment, with similar biological effect [6, 7]. Ex-vivo experiments on porcine corneas have yielded similar outcomes on biomechanical properties with high energy and short irradiation time settings compared to standard protocol [6]. However, others have reported reduced treatment efficiency, postulated to be a result of intrastromal oxygen diffusion capacity and increased oxygen consumption associated with higher irradiances [7], and limited biomechanical strengthening beyond irradiance of 50 mW/cm2 and exposure time of less than 2 minutes [8] in animal tissue. There has been no significant difference in corneal stiffness between human eyes crosslinked with high and low intensity protocols ex-vivo [9]. Several clinical studies have suggested that the effectiveness of accelerated crosslinking is comparable to conventional treatment with similar safety profiles [10-19]. However, the lack of a uniform protocol and differing research methodologies have made comparisons between these studies difficult and more evidence is needed to confirm the efficacy of accelerated crosslinking in spite of its purported advantages over standard protocol.
This paper aims to compare the visual, refractive, topographic and biomechanical outcomes in patients with progressive keratoconus who were treated with either conventional or accelerated crosslinking.
2. MATERIALS AND METHODS
Two prospective interventional studies of patients who underwent conventional and accelerated cross linking for progressive keratoconus were conducted consecutively in Singapore National Eye Centre, Singapore from 2008 to 2015. The studies were approved by the SingHealth Centralised Institutional Review Board (CIRB) and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all the participants.
The studies were registered with ClinicalTrials.gov: NCT 01123057, NCT02638376.
An initial detailed screening of all potential study subjects was first performed to determine suitability for crosslinking. Seventy six patients with 76 eyes, who were at least 18 years of age with documented progressive keratoconus based on topographic (increase of 1.0D or more in the steepest keratometry), pachymetric (reduction in minimal corneal thickness of 5% or more), visual acuity and refractive changes (increase in cylinder of more than 1.00D or spherical equivalent of more than 0.50D) over at least 6 months, were included in the studies. Eyes with corneal pachymetry of less than 400µm at the thinnest point, endothelial cell density of less than 2000 cells/cm2, corneal scarring, nystagmus or any motility disorders which prevented a steady gaze during examination and imaging, and other significant ocular disease were excluded. Patients who have a history of autoimmune disorders or were pregnant or breastfeeding at the time of recruitment were also excluded. All patients would have discontinued their rigid gas permeable (RGP) contact lens wear for at least 3 days before the screening visit.
All subjects underwent a complete ophthalmic examination which included uncorrected (UCVA) and best corrected visual acuity (BCVA) based on the logMAR chart, manifest refraction, slit lamp and dilated fundoscopy. Corneal topography was performed using the Pentacam conventional Scheimpflug system (Oculus Optikgerate GmbH, Munchholzhauser str.29, 35582 Wetlar, Germany). The values representing the flat, steep and mean keratometry (K1, K2 and Kmean) were recorded. The central and minimal pachymetric measurements were also derived from the Pentacam system. The endothelial cell density (ECD) was assessed using a non-contact specular microscope, Konan Noncon Robo SP8000 (Konan Medical, Hyogo, Japan). The biomechanical parameters of corneal hysteresis and corneal resistance factor were measured using a dynamic bidirectional applanation device (Ocular Response Analyzer, Reichert Ophthalmic Instruments, Depew, NY, USA). These investigations were performed at baseline and on follow up.
All crosslinking procedures were performed by three surgeons (LL, JM, CC) under topical anaesthesia and sterile conditions in the operating theatre. In conventional crosslinking (CXL), corneal pachymetry was first performed before the procedure. The corneal epithelium was then partially removed from a 9.0 mm treatment zone using a smooth spatula. One drop of isotonic riboflavin 0.1% with dextran 20% solution (MedioCROSS D, Medio-Haus-Medizinprodukte GmbH, Kiel, Germany) were instilled every 2 minutes for 30 minutes (15 drops). Thereafter, the corneal thickness measurement was repeated and if less than 400µm, 2 drops of hypotonic riboflavin 0.1% solution (MedioCROSS H, Medio-Haus-Medizinprodukte GmbH, Kiel, Germany) was instilled every 10 to 15 seconds until the corneal thickness was at least 400 µm. The patient’s eye was then positioned under the UV illumination device (UV-X, Peschke Meditrade GmbH, Huenenberg, Switzerland), taking care to ensure that the beam diameter was within the treatment zone avoiding the limbal area and a 5cm distance between beam aperture and eye. The riboflavin solution was then instilled every 2 minutes during the illumination process at an irradiance of 3mW/cm2 for 30 minutes (total energy: 5.4J/cm2). In accelerated crosslinking (KXL), 1 drop of dextran-free riboflavin 0.1% solution (VibeX Rapid, Avedro, Inc., Waltham, Massachusetts, MA, USA) was instilled every 2 minutes for 10 minutes after epithelial removal. The eye was then rinsed thoroughly with balanced salt solution and aligned under the UV illumination system (KXL, Avedro, Inc., Waltham, Massachusetts, MA, USA), following which irradiation was conducted at 30 mW/cm2 continuously for 4 minutes (total energy: 7.2J/cm2). Key differences between the 2 protocols have been summarised in Table 1. For both protocols, a bandage contact lens was applied post-procedure and the patients were started on topical antibiotics (moxifloxacin hydrochloride 0.5%) and steroids (prednisolone acetate 0.12%), which were tapered at 1 month after complete epithelial healing. All patients were reviewed at 1 day, 1 week, 1 month, 3 months, 6 months and 12 months after the procedure.
Table 1.
Conventional and accelerated crosslinking protocols.
| Conventional Crosslinking | Protocol | Accelerated Crosslinking |
|---|---|---|
| Yes | Removal of epithelium | Yes |
| Isotonic riboflavin 0.1% with dextran 20% solution | Riboflavin solution | Dextran-free riboflavin 0.1% solution |
| Every 2 mins for 30mins | Duration of soak | Every 2mins for 10mins |
| UV-X, Peschke Meditrade GmbH, Huenenberg, Switzerland | UV illumination device | KXL, Avedro, Inc., Waltham, Massachusetts, MA, USA |
| 3mW/cm2 for 30 minutes (total energy: 5.4J/cm2). | Illumination protocol | 30 mW/cm2 continuously for 4 minutes (total energy: 7.2J/cm2). |
All statistical analyses were conducted using the Statistical Package for the Social Sciences V.17.0 (SPSS Inc, Chicago, Illinois, USA). Two samples independent T-test and paired T-tests were performed for normally distributed variables, and nonparametric tests were used if variables are not normally distributed. A probability of less than 5% (p<0.05) was considered statistically significant.
3. RESULTS
3.1. Baseline Characteristics (Table 2)
Table 2.
Comparison of baseline characteristics in CXL and KXL group.
| CXL (N=29) | KXL (N=47) | P value | |
|---|---|---|---|
| Age (years) | 29 +/- 7 | 28 +/- 7 | 0.452 |
| Sex (Male: Female) | 21:8 | 37:10 | 0.530 |
| Follow up (months) | 13.10 +/- 3.30 | 12.20 +/- 2.70 | 0.191 |
| UCVA | 0.86 +/- 0.40 | 0.80 +/- 0.30 | 0.400 |
| BCVA | 0.37 +/- 0.30 | 0.40 +/- 0.20 | 0.703 |
| Spherical equivalent (D) | -4.72 +/- 3.60 | -4.30 +/- 3.00 | 0.591 |
| Cylinder (D) | -4.94 +/- 3.40 | -5.50 +/- 2.10 | 0.434 |
| K1 (D) | 47.68 +/- 4.30 | 47.40 +/- 5.00 | 0.808 |
| K2 (D) | 52.29 +/- 5.40 | 52.15 +/- 5.30 | 0.915 |
| Kmean (D) | 49.82 +/- 4.50 | 49.78 +/- 5.00 | 0.969 |
| Central corneal thickness (µm) | 491.52 +/- 46.30 | 492.64 +/- 31.20 | 0.909 |
| Minimal corneal thickness (µm) | 460.10 +/- 44.90 | 467.78 +/- 31.00 | 0.425 |
| Corneal hysteresis (mm Hg) | 7.82 +/- 1.50 | 8.23 +/- 1.60 | 0.273 |
| Corneal resistance factor (mm Hg) | 6.38 +/- 1.40 | 6.28 +/- 1.70 | 0.790 |
| Endothelial cell density (Cells/mm2) | 2860 +/- 368 | 3146 +/- 544 | 0.017 |
Abbreviations and units: UCVA, uncorrected visual acuity; BCVA, best corrected visual acuity; D, Diopter
We included 76 eyes from 76 patients in the studies, of which 29 eyes underwent conventional crosslinking while 47 eyes underwent accelerated crosslinking. The mean age (+/- SD) of the patients was 29.16 +/- 7.3 years and 27.88 +/- 7.1 years in the CXL and KXL group respectively. The majority of patients in each group were male, with 37 patients (78.7%) in the KXL group and 21 patients (72.4%) in the CXL group. The patients were also predominantly Chinese (CXL, N=17, 58.6%; KXL, N=31, 66.0%). The mean follow up period was 13.1 months in the CXL group and 12.2 months in the KXL group. There was no statistical difference between the 2 groups in terms of demographics, preoperative visual acuity, refraction, topography, pachymetry and biomechanical parameters. However, the KXL group had significantly higher preoperative endothelial cell density than the CXL group.
3.2. Visual Acuity and Refractive Outcomes
In the CXL group, there was significant improvement in the UCVA from baseline of 0.13 (P= 0.003) and 0.11 (P=0.017) at 3 months and 6 months respectively. For BCVA, the subjects in the CXL group showed significant improvement from baseline of 0.11 (P=0.037) at 12 months. In the KXL group, there was no statistically significant change in UCVA throughout follow-up, with improvement in BCVA seen at 6 (0.06, P=0.006) and 12 months (0.08, P=0.004). There was no statistically significant difference in the change in both uncorrected and best corrected LogMAR visual acuity from baseline between the 2 groups throughout follow up, except for UCVA at 3 months (CXL: -0.13; KXL: -0.01; P = 0.01).
In terms of refractive outcomes, within the CXL group, there was no change in spherical equivalent (SE) throughout follow up but in the KXL group, the subjects had a significantly more myopic SE compared to baseline at 1 month (-0.72D, P=0.046) and 3 months (-0.65D, P=0.019) only (Table 3). There was no significant change in SE from baseline at 12 months in the KXL group. There was no significant difference in the change in spherical equivalent between the 2 groups up to 12 months, except for the measurement at 1 month (CXL: 1.01D; KXL:-0.72D; P = 0.008).
Table 3.
Refractive changes before and after CXL and KXL.
| Baseline | 1 Month | 3 Months | 6 Months | 12 Months | |
|---|---|---|---|---|---|
| Spherical Equivalent (D) | |||||
| CXL | -4.72 +/- 3.6 | -3.71 +/- 3.6 (P=0.091) | -4.06 +/- 4.5 (P=0.434) | -3.93 +/- 4.03 (P=0.165) | -3.82 +/- 4.4 (P=0.247) |
| KXL | -4.30 +/- 3.1 | -5.25 +/- 4.0 (P=0.046) | -5.08 +/- 4.6 (P=0.019) | -4.79 +/- 3.7 (P=0.379) | -5.11 +/- 4.07 (P=0.131) |
| Cylinder (D) | |||||
| CXL | -4.94 +/- 3.4 | -3.93 +/- 2.7 (P=0.048) | -4.14 +/- 3.1 (P=0.129) | -4.27 +/- 2.8 (P=0.544) | -4.21 +/- 3.5 (P=0.490) |
| KXL | -5.5 +/- 2.1 | -5.7 +/- 2.1 (P=0.013) | -5.77 +/- 2.1 (P=0.009) | -5.73 +/- 2.1 (P=0.026) | -5.82 +/- 2.15 (P=0.002) |
Abbreviations and units: D, Diopter
In the KXL group, there was a statistically significant deterioration in cylinder error from baseline at 1 month (-0.46D, P=0.013), 3 months (-0.46D, P=0.009), 6 months (-0.41D, P=0.026) and 12 months (-0.55D, P=0.002). (Table 3) Subjects in the CXL group had an improvement in cylinder correction at 1 month (1.01D, P=0.048) only. The KXL group also had a significant worsening in cylinder error at 1 month (CXL: 1.01D; KXL: -0.46D; P = 0.008) and 3 months (CXL: 0.80D; KXL: -0.46D; P = 0.025) compared to the CXL group, but this trend was not observed at subsequent follow ups at 6 and 12 months.
3.3. Topographic Outcomes
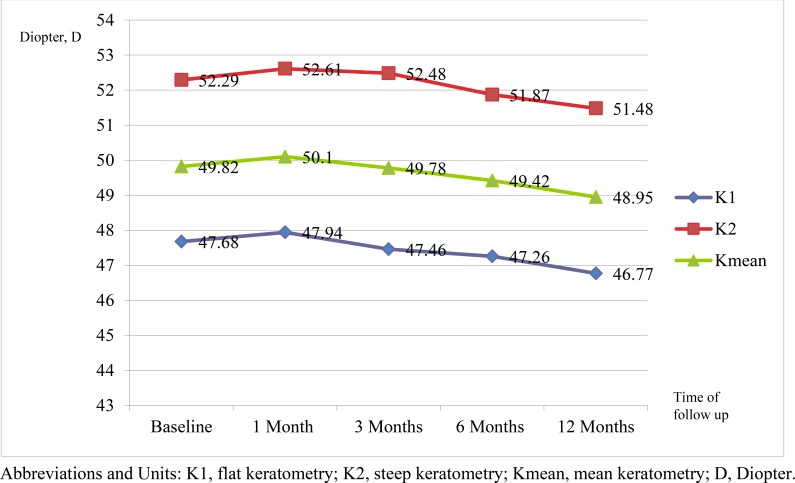
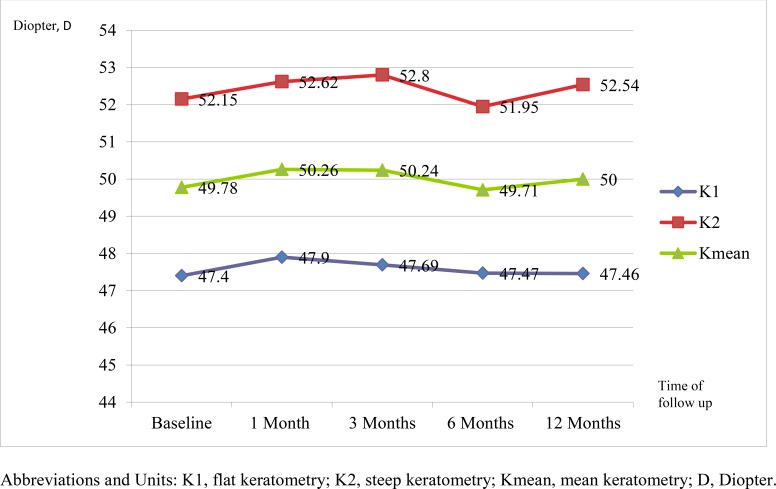
There was no significant increase for K1, K2 and K mean compared to preoperative measurements at 12 months for both CXL (Fig. 1) and KXL (Fig. 2). Similarly, there was no difference between the CXL and KXL group for the change in postoperative K1 (CXL:-0.39D; KXL: -0.13D; P=0.352), K2 (CXL: -0.13D; KXL:-0.21D; P=0.829) and Kmean (CXL:-0.13D; KXL: -0.17D; P=0.626) values at 12 months.
Fig. (1).
Figure showing K1, K2 and Kmean with time in the CXL group.
Fig. (2).
Figure showing K1, K2 and Kmean with time in the KXL group.
3.4. Pachymetric Outcomes
Within the CXL group, there was a significant reduction in central corneal thickness from baseline measurements at 1 month (-8.10 µm, P=0.016), and a significant reduction in minimal corneal thickness was seen at 1 month (-8.97 µm, P=0.041) and 3 months (-9.34 µm, P=0.043). There was no significant change in both measurements from baseline at 12 months. For the KXL group, both the central and minimal corneal thickness measurements were reduced at 1 month (central, -8.03 µm, P=0.044; minimal, -12.08 µm, P=0.005), reaching a maximum at 3 months (central, -19.56 µm, P<0.001; minimal, -21.00 µm, P<0.001) before recovering at 6 months (central, -8.56 µm, P=0.015; minimal -7.97 µm, P=0.026). However, at the 12-month follow up, there was no statistically significant difference in both measurements from baseline.
There was no significant difference in the change in central and minimal corneal thickness with time between the 2 groups, with the exception being the central corneal thickness measurement at 3 months, in which the KXL group showed a greater reduction than the CXL group (CXL: -7.28 µm; KXL -19.56 µm; P=0.042).
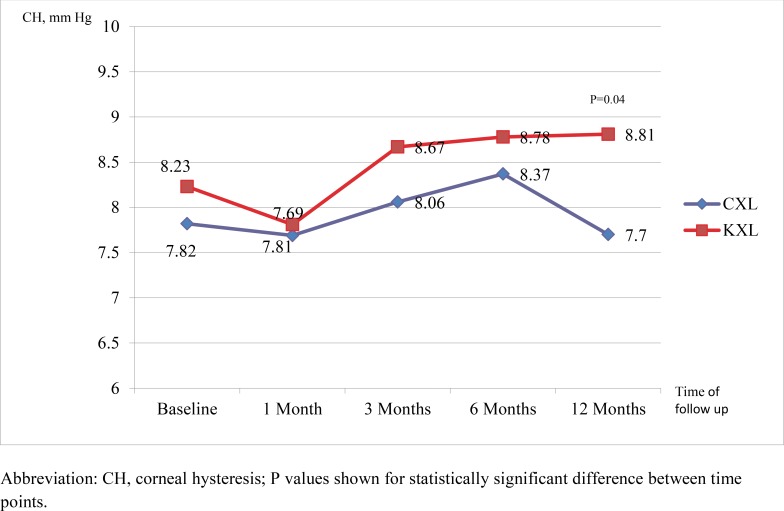
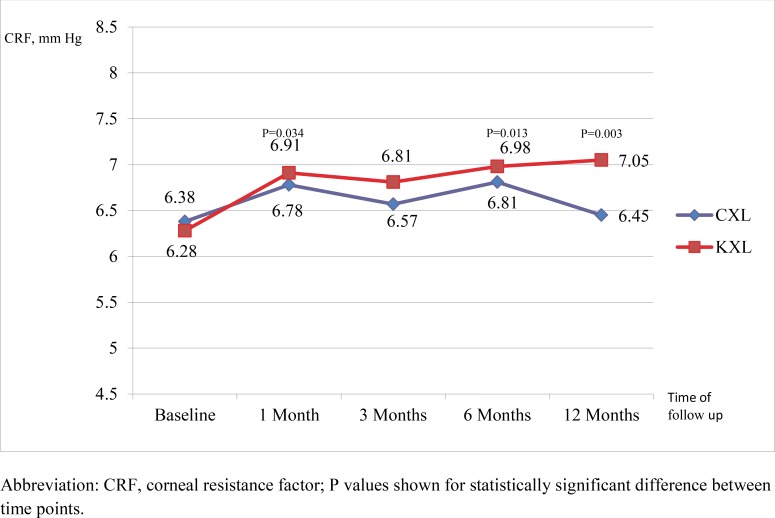
3.5. Biomechanical Outcomes (Figs 3 and 4)
Fig. (3).
Comparison of corneal hysteresis with time between the CXL and KXL group.
Fig. (4).
Comparison of corneal resistance factor with time between the CXL and KXL group.
We found a significant improvement in corneal hysteresis from baseline (0.62 mm Hg, P=0.04) in the KXL group at 12 months. In the KXL group, the corneal hysteresis increased from 8.23 mmHg to 8.81 mmHg at 12 months (P=0.04). In the CXL group, the corneal hysteresis changed from 7.82 mmHg at baseline to 7.7 at 12 months. However, this difference of 0.12 mmHg was not statistically significant (P = 0.621).
There was a statistically significant increase from baseline measurement of corneal resistance factor for those within the KXL group at 1 month (0.61 mm Hg, P=0.034), 6 months (0.78 mm Hg, P=0.013) and 12 months (0.91 mm Hg, P=0.003). For the CXL group, there was no significant change in the corneal resistance factor between baseline and up to 12 months.
3.6. Endothelial Cell Density
There was no statistically significant reduction in endothelial cell density for both the CXL and KXL groups at all time points throughout follow up. In the CXL group, the baseline ECD was 2860 cells/mm2 while that at 12 months was 3002 cells/mm2 (P=0.05). Similarly, in the KXL group, the preoperative ECD was 3146 cells/mm2 while that at 12 months was 2912 cells/mm2 (P=0.06)
3.7. Complications
Two patients in the CXL group developed late onset deep stromal scarring and this has been published elsewhere [20]. Notably, the stromal scar formation occurred away from the visual axis and did not affect the final best corrected visual acuity in both cases. There were no long term complications in the KXL group.
4. DISCUSSION
To date, there is no consensus on the safety and efficacy of accelerated high fluence collagen crosslinking compared to conventional protocol. While some studies have reported similar results in terms of visual acuity, refractive and topographic outcomes, a few have found the effect of accelerated crosslinking on disease stabilization to be limited. (Table 4) Notably, current evidence is based mostly on studies with small sample sizes, which may be underpowered to detect significant differences. Differing irradiance used for accelerated crosslinking protocols (ranging from 7 to 30 mW/cm2), ultraviolet radiation systems, postoperative regimens and follow-up duration further confound any meaningful comparison between studies.
Table 4.
Review of studies comparing conventional and accelerated crosslinking.
| Study | Study design | Conventional crosslinking | Accelerated crosslinking |
Follow up
(months) |
Findings | ||
|---|---|---|---|---|---|---|---|
| N | Protocol/ platform | N | Protocol/ platform | ||||
| Kanellopoulos, 2012 | Prospective randomized comparative case series | 21 | 3mW/cm2 for 30 mins | 21 | 7mW/cm2 for 15 mins | 46 | Comparable changes in visual acuity, refraction, reduction in steepest K, no progression in both groups |
| Cinar et al. 2014 | Prospective comparative case series | 13 | 3mW/cm2 for 30 mins | 13 | 9mW/cm2 for 10 mins | 6 | Comparable visual and refractive results, decrease in Km and Kmax in both groups |
| Brittingham et al. 2014 | Retrospective case series | 81 | 3mW/cm2 for 30 mins UV-X 1000 |
50 | 9mW/cm2 for 10 mins UV-X 2000 |
12 | Increased steepening of anterior cornea in accelerated (+0.72D) compared to conventional group (-0.76D) |
| Ng et al. 2015 | Retrospective comparative case series | 14 | 3mW/cm2 for 30 mins UV-X 1000 |
12 | 9mW/cm2 for 10 mins UV-X 2000 |
12 | Greater reduction in Kmax and Kmean in conventional group compared to accelerated group |
| Hashemi et al. 2015 | Prospective randomized comparative case series | 31 | 3mW/cm2 for 30 mins UV-X |
31 | 18mW/cm2 for 5 mins UV-X |
6 | Comparable visual acuity, refractive, keratometric and biomechanical outcomes |
| Chow et al. 2015 | Prospective comparative case series | 19 | 3mW/cm2 for 30 mins UV-X |
19 | 18mW/cm2 for 5 mins CCL-VARIO |
12 | Comparable visual acuity and refractive outcomes. More topographic flattening in the conventional group compared to accelerated group |
| Hashemian et al. 2014 | Prospective randomized controlled trial | 153 | 3mW/cm2 for 30 mins CCL-VARIO |
77 | 30mW/cm2 for 3 mins CCL-VARIO |
15 | Comparable changes in visual acuity, refraction, endothelial cell density, Kmax, anterior stromal keratocyte density and subbasal nerve density |
| Sherif, 2014 | Prospective randomized comparative study | 11 | 3mW/cm2 for 30 mins UV-X |
14 | 30mW/cm2 for 4 mins and 20s Avedro KXL |
12 | Comparable reduction in Kmax, changes in corneal hysteresis, corneal resistance factor and central corneal thickness |
| Tomita et al. 2014 | Prospective comparative study | 18 | 3mW/cm2 for 30 mins CCL VARIO |
30 | 30mW/cm2 for 3 mins Avedro KXL |
12 | Comparable changes in visual acuity, refractive, keratometric readings, biomechanical responses between the 2 groups |
| Shetty et al. 2015 | Prospective randomized interventional study | 36 | 3mW/cm2 for 30 mins Avedro KXL |
36 | 9mW/cm2 for 10 mins | 12 | Conventional group and accelerated groups with irradiance of 9mW/cm2 and 18mW/cm2 showed better visual, refractive and tomographic improvements. Minimal stabilization of disease in 30mW/cm2 group |
| 33 | 18mW/cm2 for 5 mins | ||||||
| 33 | 30mW/cm2 for 3 mins Avedro KXL for all groups |
||||||
In the present study, the CXL and KXL groups showed improvement in BCVA of 0.11 and 0.08 LogMAR units respectively at 12 months compared to baseline. Shetty et al. in a prospective randomized interventional study of 138 eyes with keratoconus which underwent crosslinking at radiance of 3, 9, 18 or 30mW/cm2, found that while there was an improvement in the corrected distance visual acuity in all groups at 12 months, the change was not significant in the 30mW/cm2 group and the most improvement occurred in the 18mW/cm2 group [12]. However, no such intergroup difference was found in our study. Various authors have reported a reduction in spherical equivalent and cylinder error in both accelerated and conventional crosslinking, but with no significant difference between the 2 groups [10, 17, 18]. Our study showed no difference between the 2 groups at 12 months when the change in spherical equivalent value was considered. There was a significant deterioration in mean cylindrical error in the KXL group at all time points throughout the follow up period. However, for the CXL group, the improvement in cylindrical error was only significant at 1 month. (Table 3) There was no difference between the 2 groups beyond 3 months.
We did not find any differences for K1, K2 and Kmean values after 1 year of follow up. Our results are similar to existing data in which there is generally no significant difference between accelerated and conventional protocols in terms of topographic change [11, 13, 15, 18]. Not all authors agree on that standard and high fluence protocols change corneal topography to the same extent. Shetty et al. noted that the flattening effect of crosslinking was reduced with higher irradiation and shorter treatment duration [12]. A retrospective analysis of 131 eyes with progressive keratoconus by Brittingham et al. [16] even showed a negative effect on topographic outcome at 1 year, with the mean change of -0.76D with standard protocol versus +0.72D in the accelerated group.
Two studies have observed that both central and minimal corneal thickness measurements were reduced to a lesser extent in accelerated high-fluence crosslinking compared to conventional protocol [12, 18]. However, we did not find any difference between the CXL and KXL group in terms of change in central or minimal corneal thickness, which were reduced at 1 month before recovering to near preoperative levels at 12 months. Interestingly, the values for both central and minimal corneal thickness were lowest at 3 months for the KXL group while the cornea was thinnest between 1 and 3 months for the CXL group. Jordan et al. in prospective in vivo confocal microscopy study of corneal microstructural changes after crosslinking for keratoconus, showed the complete absence of stromal keratocyte nuclei in 86% of corneas at 1 month, while anterior stromal edema with hyper-reflective cytoplasm and extracellular lacunae in a honeycomb-like appearance may persist at 3 months postoperatively [21, 22]. These anterior stromal changes were also more pronounced in accelerated crosslinking compared to conventional crosslinking [23]. The reduction in corneal thickness between 1 and 3 months may be a result of progressive re-epithelization and compaction of stromal lamellae after crosslinking. There is also evidence that pachymetry with Scheimpflug imaging system may underestimate corneal thickness in the early postoperative period due to stromal haze and changes in reflectivity [24].
We have demonstrated a positive change in both corneal hysteresis and corneal resistance factor, as measured by the ocular response analyzer, associated with accelerated crosslinking up to 12 months after the procedure (Figs. 3 and 4). This is in contrast to the findings of previous studies on biomechanical properties of the cornea after crosslinking, which reported no change in corneal hysteresis and corneal resistance factor [25-28] To the best of our knowledge, this has not been reported before. Various in vitro animal studies [27, 29] and ex vivo trials on human eyes [30, 33] have provided strong evidence of increased corneal rigidity and resistance to enzymatic digestion with crosslinking [34]. Comparative trials have not shown any difference in biomechanical parameters between conventional and high-fluence, short duration protocols [13, 15, 18]. An ex vivo human corneal study by Kanellopoulos et al. also found the biomechanical effect of CXL studied by resistance to enzymatic digestion in human corneas to be comparable between irradiances of 9, 18 and 30 mW/cm2 [35]. However, before the current study, these effects have never been replicated in clinical studies, likely due to the differences in quantification of corneal rigidity and the high variability in resistance to deformation in irregular keratoconic corneas [27]. We postulate that the improved biomechanical effects demonstrated in accelerated crosslinking may be attributed to possible differences in UV radiation beam profile between the 2 protocols, though this has to be further validated.
This paper may be limited by a lack of sufficient statistical power due to small patient cohorts. There was also no randomization as the 2 groups of patients were treated consecutively. For practical purposes, some of our patients could only stop the use of their RGP contact lens 3 days from the day of evaluation and this may limit the reliability of the keratometric results. Our analysis was limited to the 1 year outcomes even though the CXL group had a longer post-treatment duration. Lastly, we did not have data with regards to the demarcation line which may further substantiate the comparison of treatment efficacy between the 2 protocols.
CONCLUSION
In conclusion, our study has strengthened the evidence on the efficacy and safety of accelerated high-fluence crosslinking compared to conventional crosslinking. Both protocols were effective in stabilizing the keratoconus at 1 year follow up. Our findings also suggested an added biomechanical advantage of accelerated crosslinking at 12 months. Larger prospective randomized controlled trials with longer follow up are necessary to confirm the long term safety and efficacy of accelerated crosslinking.
ACKNOWLEDGEMENTS
This study is supported by the Singapore National Eye Centre Health Research Endowment Fund.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Approved by the Singhealth Centralised Institutional Review Board (CIRB), Informed Consent was Obtained from all Participants.
HUMAN AND ANIMAL RIGHTS
This is a Prospective Interventional Clinical Study.
CONSENT FOR PUBLICATION
Informed consent for the study and publication was obtained from all subjects.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Konstantopoulos A., Mehta J.S. Conventional versus accelerated collagen cross-linking for keratoconus. Eye Contact Lens. 2015;41(2):65–71. doi: 10.1097/ICL.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 2.Mastropasqua L. Collagen cross-linking: When and how? A review of the state of the art of the technique and new perspectives. Eye Vis (Lond) 2015:2: 19. doi: 10.1186/s40662-015-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGhee C.N., Kim B.Z., Wilson P.J. Contemporary treatment paradigms in keratoconus. Cornea. 2015;34(Suppl 10):S16–S23. doi: 10.1097/ICO.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 4.Wollensak G., Spoerl E., Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophthalmol. 2003;135(5):620–627. doi: 10.1016/S0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 5.Sorkin N., Varssano D. Corneal collagen crosslinking: A systematic review. Ophthalmologica. 2014;232(1):10–27. doi: 10.1159/000357979. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher S., Oeftiger L., Mrochen M. Equivalence of biomechanical changes induced by rapid and standard corneal cross-linking, using riboflavin and ultraviolet radiation. Invest. Ophthalmol. Vis. Sci. 2011;52(12):9048–9052. doi: 10.1167/iovs.11-7818. [DOI] [PubMed] [Google Scholar]
- 7.Hammer A., Richoz O., Arba Mosquera S., Tabibian D., Hoogewoud F., Hafezi F. Corneal biomechanical properties at different corneal cross-linking (CXL) irradiances. Invest. Ophthalmol. Vis. Sci. 2014;55(5):2881–2884. doi: 10.1167/iovs.13-13748. [DOI] [PubMed] [Google Scholar]
- 8.Wernli J., Schumacher S., Spoerl E., Mrochen M. The efficacy of corneal cross-linking shows a sudden decrease with very high intensity UV light and short treatment time. Invest. Ophthalmol. Vis. Sci. 2013;54(2):1176–1180. doi: 10.1167/iovs.12-11409. [DOI] [PubMed] [Google Scholar]
- 9.Beshtawi I.M., Akhtar R., Hillarby M.C., O’Donnell C., Zhao X., Brahma A., Carley F., Derby B., Radhakrishnan H. Biomechanical properties of human corneas following low- and high-intensity collagen cross-linking determined with scanning acoustic microscopy. Invest. Ophthalmol. Vis. Sci. 2013;54(8):5273–5280. doi: 10.1167/iovs.13-12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashemian H., Jabbarvand M., Khodaparast M., Ameli K. Evaluation of corneal changes after conventional versus accelerated corneal cross-linking: a randomized controlled trial. J. Refract. Surg. 2014;30(12):837–842. doi: 10.3928/1081597X-20141117-02. [DOI] [PubMed] [Google Scholar]
- 11.Cınar Y., Cingü A.K., Türkcü F.M., Çınar T., Yüksel H., Özkurt Z.G., Çaça I. Comparison of accelerated and conventional corneal collagen cross-linking for progressive keratoconus. Cutan. Ocul. Toxicol. 2014;33(3):218–222. doi: 10.3109/15569527.2013.834497. [DOI] [PubMed] [Google Scholar]
- 12.Shetty R., Pahuja N.K., Nuijts R.M., Ajani A., Jayadev C., Sharma C., Nagaraja H. Current protocols of corneal collagen cross-linking: Visual, refractive, and tomographic outcomes. Am. J. Ophthalmol. 2015;160(2):243–249. doi: 10.1016/j.ajo.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Sherif A.M. Accelerated versus conventional corneal collagen cross-linking in the treatment of mild keratoconus: A comparative study. Clin. Ophthalmol. 2014;8:1435–1440. doi: 10.2147/OPTH.S59840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng A.L., Chan T.C., Cheng A.C. Conventional versus accelerated corneal collagen cross-linking in the treatment of keratoconus. Clin. Experiment. Ophthalmol. 2016;44(1):8–14. doi: 10.1111/ceo.12571. [DOI] [PubMed] [Google Scholar]
- 15.Tomita M., Mita M., Huseynova T. Accelerated versus conventional corneal collagen crosslinking. J. Cataract Refract. Surg. 2014;40(6):1013–1020. doi: 10.1016/j.jcrs.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Brittingham S., Tappeiner C., Frueh B.E. Corneal cross-linking in keratoconus using the standard and rapid treatment protocol: differences in demarcation line and 12-month outcomes. Invest. Ophthalmol. Vis. Sci. 2014;55(12):8371–8376. doi: 10.1167/iovs.14-15444. [DOI] [PubMed] [Google Scholar]
- 17.Kanellopoulos A.J. Long term results of a prospective randomized bilateral eye comparison trial of higher fluence, shorter duration ultraviolet A radiation, and riboflavin collagen cross linking for progressive keratoconus. Clin. Ophthalmol. 2012;6:97–101. doi: 10.2147/OPTH.S27170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashemi H., Fotouhi A., Miraftab M., Bahrmandy H., Seyedian M.A., Amanzadeh K., Heidarian S., Nikbin H., Asgari S. Short-term comparison of accelerated and standard methods of corneal collagen crosslinking. J. Cataract Refract. Surg. 2015;41(3):533–540. doi: 10.1016/j.jcrs.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Chow V.W., Chan T.C., Yu M., Wong V.W., Jhanji V. One-year outcomes of conventional and accelerated collagen crosslinking in progressive keratoconus. Sci. Rep. 2015;5:14425. doi: 10.1038/srep14425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim L.S., Beuerman R., Lim L., Tan D.T. Late-onset deep stromal scarring after riboflavin-UV-A corneal collagen cross-linking for mild keratoconus. Arch. Ophthalmol. 2011;129(3):360–362. doi: 10.1001/archophthalmol.2011.23. [DOI] [PubMed] [Google Scholar]
- 21.Meiri Z., Keren S., Rosenblatt A., Sarig T., Shenhav L., Varssano D. Efficacy of corneal collagen cross-linking for the treatment of keratoconus: A systematic review and meta-analysis. Cornea. 2016;35(3):417–428. doi: 10.1097/ICO.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 22.Jordan C., Patel D.V., Abeysekera N., McGhee C.N. In vivo confocal microscopy analyses of corneal microstructural changes in a prospective study of collagen cross-linking in keratoconus. Ophthalmology. 2014;121(2):469–474. doi: 10.1016/j.ophtha.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Touboul D., Efron N., Smadja D., Praud D., Malet F., Colin J. Corneal confocal microscopy following conventional, transepithelial, and accelerated corneal collagen cross-linking procedures for keratoconus. J. Refract. Surg. 2012;28(11):769–776. doi: 10.3928/1081597X-20121016-01. [DOI] [PubMed] [Google Scholar]
- 24.Antonios R., Fattah M.A., Maalouf F., Abiad B., Awwad S.T. Central corneal thickness after cross-linking using high-definition optical coherence tomography, ultrasound, and dual scheimpflug tomography: A comparative study over one year. Am. J. Ophthalmol. 2016;167:38–47. doi: 10.1016/j.ajo.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Greenstein S.A., Fry K.L., Hersh P.S. In vivo biomechanical changes after corneal collagen cross-linking for keratoconus and corneal ectasia: 1-year analysis of a randomized, controlled, clinical trial. Cornea. 2012;31(1):21–25. doi: 10.1097/ICO.0b013e31821eea66. [DOI] [PubMed] [Google Scholar]
- 26.Gkika M., Labiris G., Giarmoukakis A., Koutsogianni A., Kozobolis V. Evaluation of corneal hysteresis and corneal resistance factor after corneal cross-linking for keratoconus. Graefes Arch. Clin. Exp. Ophthalmol. 2012;250(4):565–573. doi: 10.1007/s00417-011-1897-0. [DOI] [PubMed] [Google Scholar]
- 27.Sedaghat M., Naderi M., Zarei-Ghanavati M. Biomechanical parameters of the cornea after collagen crosslinking measured by waveform analysis. J. Cataract Refract. Surg. 2010;36(10):1728–1731. doi: 10.1016/j.jcrs.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 28.Vinciguerra P., Albè E., Mahmoud A.M., Trazza S., Hafezi F., Roberts C.J. Intra- and postoperative variation in ocular response analyzer parameters in keratoconic eyes after corneal cross-linking. J. Refract. Surg. 2010;26(9):669–676. doi: 10.3928/1081597X-20100331-01. [DOI] [PubMed] [Google Scholar]
- 29.Beckman Rehnman J., Behndig A., Hallberg P., Lindén C. Increased corneal hysteresis after corneal collagen crosslinking: A study based on applanation resonance technology. JAMA Ophthalmol. 2014;132(12):1426–1432. doi: 10.1001/jamaophthalmol.2014.3029. [DOI] [PubMed] [Google Scholar]
- 30.Kling S., Remon L., Pérez-Escudero A., Merayo-Lloves J., Marcos S. Corneal biomechanical changes after collagen cross-linking from porcine eye inflation experiments. Invest. Ophthalmol. Vis. Sci. 2010;51(8):3961–3968. doi: 10.1167/iovs.09-4536. [DOI] [PubMed] [Google Scholar]
- 31.Wollensak G., Iomdina E. Long-term biomechanical properties of rabbit cornea after photodynamic collagen crosslinking. Acta Ophthalmol. 2009;87(1):48–51. doi: 10.1111/j.1755-3768.2008.01190.x. [DOI] [PubMed] [Google Scholar]
- 32.Wollensak G., Spoerl E., Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J. Cataract Refract. Surg. 2003;29(9):1780–1785. doi: 10.1016/S0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 33.Knox Cartwright N.E., Tyrer J.R., Marshall J. in vitro quantification of the stiffening effect of corneal cross-linking in the human cornea using radial shearing speckle pattern interferometry. J. Refract. Surg. 2012;28(7):503–508. doi: 10.3928/1081597X-20120613-01. [DOI] [PubMed] [Google Scholar]
- 34.Spoerl E., Wollensak G., Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr. Eye Res. 2004;29(1):35–40. doi: 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 35.Kanellopoulos A.J., Loukas Y.L., Asimellis G. Cross-linking biomechanical effect in human corneas by same energy, different UV-A fluence: An enzymatic digestion comparative evaluation. Cornea. 2016;35(4):557–561. doi: 10.1097/ICO.0000000000000758. [DOI] [PubMed] [Google Scholar]