Abstract
Background:
Low-density lipoprotein cholesterol (LDL), and especially its oxidized form, renders the atherosclerotic plaque vulnerable to rupture in acute coronary syndromes (ACS). On the other hand, high-density lipoprotein (HDL) is considered an anti-atherogenic molecule. The more re-cent HDL-targeted drugs may prove to be superior to those used before. Indeed, delipidated HDL and HDL mimetics are efficient in increasing HDL levels, while the apoA-I upregulation with RVX-208 appears to offer a clinical benefit which is beyond the HDL related effects. HDL treatment however has not shown a significant improvement in the outcomes of patients with ACS so far, studies have therefore focused again on LDL. In addition to statins and ezetimibe, novel drugs such as PSCK9 in-hibitors and apolipoprotein B inhibitors appear to be both effective and safe for patients with hyper-lipidemia.
Conclusion:
Data suggest these could potentially improve the cardiovascular outcomes of patient with ACS. Yet, there is still research to be done, in order to confirm whether ACS patients would benefit from LDL- or HDL-targeted therapies or a combination of both.
Keywords: High-density lipoprotein, low-density lipoprotein, acute coronary syndromes, outcomes, atherosclerosis, lipid-lowering drugs, statins, ezetimibe
1. INTRODUCTION
Over the last decades, the underlying mechanisms for atherosclerosis have been thoroughly examined. In the effort to improve prognosis in patients with acute coronary syndromes (ACS), a variety of biomarkers have been proposed as possible targets for intervention. It is widely known that (low density lipoprotein) LDL levels are linked to coronary artery disease (CAD), as the oxidative modification of LDL is the initial step that leads to the formation of the nascent atheroma (Fig. 1). Statins have long been considered an indispensable part of the treatment of patients with ACS, owing to their hypolipidemic effect and to their numerous pleiotropic properties that have a positive clinical impact. Indeed, as many studies have reported, statins potently decrease LDL levels and, in tandem, cardiovascular disease (CVD) risk [1].
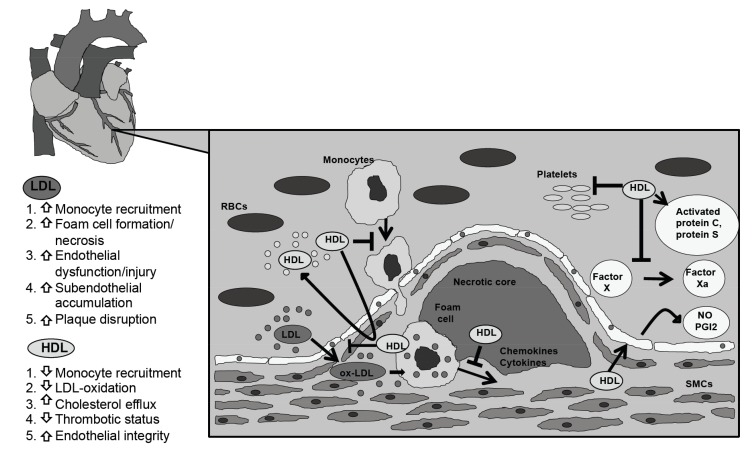
Fig. (1).
The atherogenic role of LDL vs. the atheroprotective properties of HDL. The deposition of the oxidized LDL in the intima media is the first crucial step that leads to the formation of a nascent atheroma. Oxidized LDL particles promote the inflammatory process, as they enhance the expression of adhesion molecules. Monocytes accumulate into the vessel wall, where they mature into tissue macrophages and subsequently into lipid-laden foam cells. These processes cause endothelial dysfunction and exacerbate the thrombotic status. By contrast, HDL mitigates the atherosclerotic process as it presents anti-inflammatory, anti-oxidative and anti-thrombotic properties, which enhance endothelial integrity. HDL also promotes cholesterol efflux from the artery wall and prevents the increase of the necrotic core volume. LDL: low-density lipoprotein; HDL: high-density lipoprotein; ox-LDL: oxidized LDL; NO: nitric oxide; PGI2: prostacyclin; SMCs: smooth muscle cells; RBCs: red blood cells.
Nevertheless, even when the LDL goal is achieved with statin administration residual risk remains, which is mainly attributed to the persistently decreased HDL levels [2]. HDL, as opposed to LDL, has proved to be a potent cardioprotective agent, as HDL levels are inversely linked to CAD incidence [3]. Significant data have revealed that HDL-related drugs may be effective in reducing cardiovascular mortality; however they are not as encouraging or unanimous as expected. HDL is a complex particle (Fig. 2) in terms of size and structure, and the associated molecules present different properties in terms of CVD risk. Additionally, it seems that the atheroprotective role of HDL turns into atherogenic in states of increased inflammatory process [4].
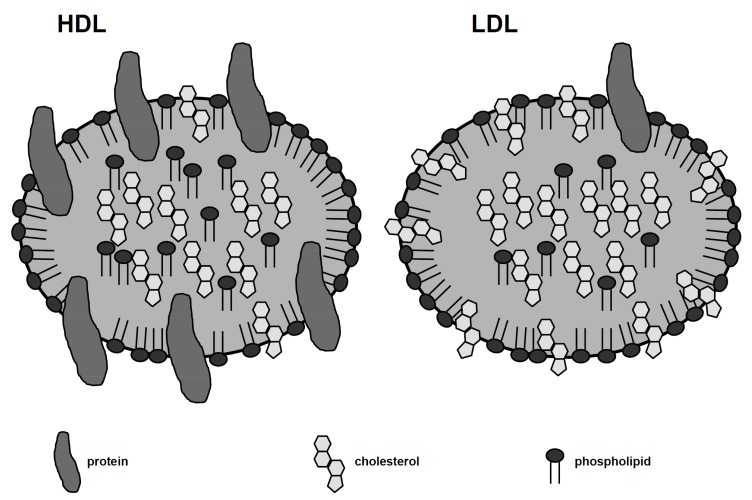
Fig. (2).
Differences in HDL and LDL structure. Lipoprotein particles consist of two basic components: an internal core – an aggregate of cholesteryl esters and triglycerides – and an external phospholipid monolayer, which contains apolipoproteins and unesterified cholesterol. The different lipoproteins are discriminated based on the quantity and specific class of apoliporoteins they contain. 50% of an HDL particle is made up of protein, while the equivalent percentage for an LDL particle is 25%. Apo A-I and apo A-II make up 90% of the HDL’s protein mass, while each LDL particle contains a single apo-B-100 molecule. HDL: high density lipoprotein; LDL: low-density lipoprotein; apo A-I: apolipoprotein A-I.
Several treatment strategies have been tested so far, targeting either the HDL or the LDL metabolism. In the present review article, we aimed to compare the effects of the HDL- vs. the LDL-targeted therapy on the outcomes of patients with ACS.
2. THERAPIES WITH LOW-DENSITY LIPOPROTEIN-LOWERING EFFECT
2.1. Statins
Early studies were in agreement that LDL level reduction substantially decreases CVD risk even in patients with ACS [1, 5, 6]. Nevertheless, the rate of cardiovascular (CV) mortality remained high. Cannon et al. [7] compared the standard therapy of 40 mg pravastatin with 80 mg of atorvastatin daily in patients with recent ACS. At a follow-up of 24 months, the intensive-treatment group demonstrated a decrease in the composite incidence of all-cause mortality, myocardial infarction (MI), unstable angina (UA) requiring hospitalization, revascularization and stroke, and a number of patients needed to treat of 6.25 which was fairly promising as intervention. On further analysis of data, intensive statin treatment also had a protective role against recurrence of primary endpoint events [8]. Similarly, the A to Z trial [9] reported that the intensification of statin treatment in ACS patients (40 mg simvastatin for 1 month and afterwards 80 mg simvastatin daily) decreased the major adverse cardiovascular event (MACE) incidence, compared to the standard treatment (placebo for 4 months, followed by 20 mg simvastatin daily). Moreover, the IDEAL study [10], which enrolled patients with acute MI (AMI), demonstrated that the high-dose atorvastatin regimen was superior to the usual-dose simvastatin one in reducing the risk of recurrent nonfatal MI although with a number needed to treat of 50 to benefit from any coronary event. Nonetheless, no difference was noted in the incidence of coronary death or cardiac arrest with resuscitation. Of particular note, a large meta-analysis of data from almost 170,000 subjects suggested that the more intensive regimens caused an additional 15% reduction in the rate of MACE [11]. This involved separate significant reductions in coronary death or nonfatal MI 13%, coronary revascularization (19%) and ischaemic stroke (16%). The dose-dependent benefit from the LDL lowering effect was demonstrated with both standard and intensive statin regimens with a reduction in all-cause mortality of 10% per 1.0 mmol/L LDL reduction, mostly representing significant reductions in deaths secondary to all cardiac causes without any significant difference in stroke-related deaths. Of note, the adherence to statin treatment is another significant parameter. As a recent prospective cohort of ACS patients concluded, statin non-adherence at a 23-month follow-up significantly increases death rate [12].
Therefore, high-dose of statins has improved CVD outcomes. Nevertheless, some high-risk patients do not reach the target LDL levels, despite receiving intensive statin treatment. At the same time statin intolerance (unable to continue statin, either because of the development of a side effect or because of evidence of abnormal blood markers) might not allow the intensification of statin therapy. Thus, the concomitant use of other regimens that target the LDL metabolism is, in some cases, warranted [13].
Recently, visit-to-visit LDL variability has emerged as an independent predictor of adverse outcomes in patients with CAD [14]. It has been suggested that LDL variability is associated with unpredictable fibrous cap thickness resulting and, hence, plaque vulnerability. Furthermore, erratic LDL levels are more likely to be present in older and frail individuals or might even reflect reduced compliance to medication. It appears that LDL variability is more common with the use of low dose statins compared to high doses.
2.2. Ezetimibe
A number of studies including patients with ACS have suggested that the addition of ezetimibe to background statin treatment increases the percentage of patients who achieve target LDL levels [15]. In a randomized trial including patients with acute MI (AMI), it was noted that 45% of the patients treated with a combination regimen of 10 mg ezetimibe and 40 mg simvastatin exhibited LDL levels below 70 mg/dl by the fourth day of treatment; the equivalent percentage in the group that received monotherapy with 40 mg simvastatin was only 5% [16]. Nevertheless, in patients with a recent MI the statin-ezetimibe co-administration had no effect on the mortality rate, when compared to standard simvastatin monotherapy. In contrast, the intensification of the statin treatment significantly reduced mortality at a 3.2-year follow-up [17].
Importantly, the IMPROVE-IT trial [18] was a double-blind, randomized trial involving 18,144 patients with a recent ACS, that aimed to clarify the impact of ezetimibe-statin co-administration on patients’ outcomes at a 7-year follow-up. It appeared that co-administration of 10 mg ezetimibe and 40 mg simvastatin decreased the rate of the composite cardiovascular end-point (i.e. CV death, nonfatal MI, UA requiring hospitalization, coronary revascularization and nonfatal stroke) when compared to monotherapy with 40 mg simvastatin alone. Approximately 50 patients needed to be treated to meet the primary endpoint. It was also noted that the reduction of LDL cholesterol (LDL-C) to levels below the previous target ones conveyed an additional benefit. In a recent subsequent analysis of the same trial it was shown that the addition of ezetimibe could reduce not only the first adverse cardiovascular events, but also the subsequent ones. Indeed, when the subsequent events were also taken into consideration, it was shown that ezetimibe could reduce total CV events by 9%. The decrease was driven by reductions in total nonfatal MI and total nonfatal stroke [19]. The benefit from ezetimibe addition to statin therapy was stronger among patients with prior coronary artery bypass graft (CABG) who experienced a significantly lower risk of the primary endpoint [20]. Furthermore, a recent meta-analysis showed that ezetimibe is the only add-on therapy that conveys a clinical benefit [21]. In accordance, Savarese et al. [22], in their meta-analysis, concluded that the addition of ezetimibe could reduce the risk of MI and stroke by 13.5% and 16% respectively, even though all-cause mortality and the rates of CV death were unaffected. In complete contrast to the above, Bataggia et al. [23], in their pooled analysis, reported that ezetimibe co-administration, compared to simvastatin monotherapy, increases the rate of all-cause mortality, CV death, non-CV death, MI and stroke.
Despite the favorable results derived from either the intensification of statin therapy or from the statin-ezetimibe co-administration, the CVD risk was still not obliterated. Thus, therapies that aimed to raise HDL seemed a promising alternative.
3. THERAPEUTIC APPROACHES TO RAISE PLASMA HDL
However, over the last decade it has become apparent that the anti-athrosclerotic properties of HDL lie more in its quality rather than absolute HDL levels. HDL appears to lose its protective qualities in certain pathological conditions [24, 25] and with only 5% of the total HDL derived from actual macrophage cholesterol efflux, the amount of HDL seems a poor representative of the beneficial effects of HDL [26, 27]. Over the last few years, several methods have been developed which can assess HDL function [28]. One of these newer methods, macrophage cholesterol efflux appears to more accurately predict cardiovascular events than actual HDL levels [29, 30].
3.1. Niacin
Niacin, a vitamin B, has long been used to increase HDL [31-33]. In the Coronary Drug Project [34], that enrolled men with a history of MI, it was demonstrated that niacin reduced the rate of recurrent non-fatal MI and decreased 15-year total mortality by 11%. The FATS trial [35] investigated the results of intensive lipid-lowering therapy in men with high CVD risk burden and documented CAD. Adverse clinical events, such as death, MI or deteriorating symptoms, were noted only in 2 of 48 patients who were administered niacin and colestipol, as opposed to 10 out of 52 assigned to conventional treatment with placebo or colestipol. Of particular interest are data derived from recent meta-analyses. Phan et al. demonstrated that niacin treatment for 3 years significantly reduced the major CV events risk (8% vs. 21%, p=0.001), interestingly with a number needed to treat of 7.7 and, even though it induced an unfavorable modification of glucose levels, did not cause diabetes mellitus [36].
Most of the studies that investigated the results of niacin administration in statin-naive patients raised questions whether the effects would persist on top statin administration. In this light, the ARBITER 2 trial [37] assessed the effect of extended-release niacin in patients with known CAD that were already on statin therapy. Niacin treatment significantly decreased the progression rate of the common carotid intima-media thickness (IMT) in patients without insulin resistance. However, the reduction in the rate of subsequent coronary events was not found to be significant. Furthermore, the AIM-HIGH trial [38] assigned patients with CVD treated with simvastatin plus ezetimibe to randomly receive either niacin or placebo. Niacin administration induced a favorable alteration in the lipid profile. However, the trial was forced to stop at 3 years, since the rates of death, nonfatal MI, ischemic stroke, hospitalization for ACS and coronary or cerebral revascularization, were not affected. Additionally, all patients included had reached the LDL goal of <80 mg/dl, due to the combination of statin and ezetimibe [39]. The HPS2-THRIVE trial [40] was the most recent trial to address this issue. In this study, 25,673 patients with CVD were randomized to receive either a regimen of 2 g niacin and 40 mg laropiprant or placebo, after the baseline therapy with 40 mg simvastatin with or without 10 mg ezetimibe was standardized so as to achieve the recommended LDL goal. Laropiprant, a selective antagonist of PGD2-receptor subtype-1, was added in order to prevent the prostaglandin-mediated flushing, so that patients’ compliance to niacin would improve [40]. However, the incidence of major adverse vascular events was not affected by the addition of niacin plus laropiprant, which was associated with a higher rate of adverse events [41]. Whether the latter is attributable to niacin per se or laropiprant, cannot be determined but with a 2-by-2 factorial design study [42]. It should be noted that knockout mice lacking the prostaglandin D2 receptor DP1 (inhibited by laropiprant) present increased atherogenesis and thrombogenesis [43].
In a recent meta-regression analysis of data from 35,723 participants which also included the unfavourable results from AIM-HIGH [44] and HPS-THRIVE2 [40] trials, niacin treatment significantly reduced all CV events, major coronary events and any revascularization [45]. However, it appeared that this beneficial effect was related to reductions in total cholesterol, non-HDL and triglyceride levels rather an improvement in HDL.
Overall, niacin has long been used to increase HDL. Controversial data exist with regards to its ability to alter major CV risk. A recent meta-analysis of randomized data showed that niacin treatment significantly reduced all CV events, major coronary events and any revascularization though these effects were likely related to reductions in lipid profile.
3.2. Fibrates
Fibrates are synthetic ligands for peroxisome proliferator activated receptor (PPAR)-α that induce a 5 to 15% increase in HDL levels. Clofibrate was the first fibrate designed and a series of trials have studied its potential benefit on cardiovascular outcomes, but the results were controversial. Goldenberg et al. [46] reported that the mortality rate and the non-fatal MI incidence were significantly reduced among patients with ischemic heart disease treated with clofibrate. However, the reduction was greater for the population with a history of angina rather than ACS. Notably, the impact of clofibrate administration on the mortality rate was independent of its lipid-modifying effect. The combination of clofibrate and niacin seemed to have had more encouraging results. The Stockholm Ischaemic Heart Disease Secondary Prevention Study that involved 555 MI survivors, showed that both total and ischemic heart disease mortality were decreased in the intervention group. Of note, this study noted that the beneficial impact of clofibrate on mortality should be attributed to its lipid-lowering effect [47].
While the results concerning the use of clofibrate continued to be ambiguous, two randomized 5-year trials investigated the impact of gemfibrozil administration on CAD incidence. The Helsinki Heart Study showed that gemfibrozil reduced the incidence of a cardiac end point among 4,081 asymptomatic, dyslipidemic middle-aged men, but did not affect the total death rate [48]. Nonetheless, the analysis of 600 individuals who were previously symptomatic and were excluded from the primary trial, concluded that the incidence of fatal and non-fatal MI and cardiac death were not reduced by gemfibrozil administration [49]. A subsequent trial that concerned men with clinically overt CAD reported a 24% decrease in the combined hazard of cardiovascular mortality, nonfatal MI and stroke with gemfibrozil therapy. Of note, the LDL levels between the intervention and placebo group were similar, while HDL increased by 6% and triglycerides decreased by 31% in the intervention group. This evidence suggested that the rate of MACE was reduced due to the improvement in HDL and triglycerides (TG) levels, regardless of the LDL invariability [50].
The studies assessing the impact of bezafibrate and fenofibrate were more enlightening, even though they mainly addressed diabetic population. The beneficial impact of bezafibrate was confirmed in the Bezafibrate Infarction Prevention study concerning patients with a previous history of MI or angina. Bezafibrate administration tended to decrease the risk of fatal or non-fatal MI and sudden death [51]. In the subsequent 16-year follow-up of the patients enrolled in the study, bezafibrate therapy was shown to reduce long-term mortality by 11%. Of note, the long-term mortality decrease correlated with the degree of the HDL response to treatment [52]. A recent study showed that the administration of bezafibrate on top of statin treatment in diabetic patients with ACS decreased the incidence of 30-day MACE [53]. A similar reduction in the rate of 30-day MACE was also found in smokers with ACS after a combination of bezafibrate and statin [54]. On the contrary, the ACCORD trial [55], which included diabetic patients that received simvastatin on study on admission, showed that the addition of fenofibrate did not add a further clinical benefit to that of statins. Examining a population with diabetes (n=9,795) with less than one third (n=2,131) having established CV disease, the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study showed that fenofibrate did not reduce the primary outcome of major coronary events; however, it reduced total CV events mainly by reducing non-fatal MIs and coronary revascularization, however with a quite high number needed to treat of 100 patients [56]. Despite more favorable changes in lipoprotein profile in women, the effect on CV outcomes was consistent in both males and females [57]. Nevertheless, the interpretation of FIELD findings is limited by factors such as significantly more frequent statin treatment in the placebo group and fenofibrate effect on non-lipid biochemical profile (HbA1c, homocysteine, creatinine). Overall, it appears that patients with triglycerides level above 200 mg/dl benefit most from fibrate treatment with consistent reductions in CV events in the larger studies. Therefore, this subgroup might be the one that should receive fibrate treatment.
In summary, clofibrate was the first fibrate designed and a series of trials have studied its potential benefit on CV outcomes, but the results were controversial. More consistent results were derived from gemfibrozil and bezafibrate in terms of MACE reduction. Also, fibrates have consistently reduced CV events in the subgroup of patients with HDL 200mg/dL and that this subgroup is the one that probably should receive fibrate treatment.
3.3. Glitazones
Thiazolidinediones consist an antidiabetic drug class which is less frequently used nowadays. They exert their effects via activation of peroxisome proliferator-activated receptors (PPAR)-γ. Their use has been associated with numerical increases in HDL levels with the evidence regarding their effect on HDL functional qualities being less clear [58-62]. Overall, there is evidence suggesting that pioglitazone decreases the atheroma burden and MI rate [63, 64]. Nevertheless, rosiglitazone has been mostly associated with unfavorable CV outcomes namely increased the risk of MI and heart failure [65-68].
3.4. Glitazars
Glitazars are agonists with dual action for PPAR α and γ, which were developed with a view to treat different aspects of the metabolic syndrome. The first attempts to show that glitazars might convey a clinical benefit were disappointing, due to the serious adverse effects of muraglitazar and tesaglitazar. Aleglitazar on the other hand, showed advantageous impact on the laboratory profiles in clinical studies [69], but a positive clinical effect was not announced. The AleCardio randomized clinical trial was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial that enrolled 7,226 patients diabetic hospitalized for ACS. Aleglitazar failed to reduce the rate of adverse CV events at a 104-week follow-up and also increased the probability of serious adverse events [70, 71].
3.5. Cholesteryl Ester Transfer Protein Inhibitors
Torcetrapib (Table 1) was the first cholesteryl ester transfer protein (CETP) inhibitor, whose clinical manifestations were assessed by a large-scale randomized, double-blind study that involved subjects with high CVD risk. At a 12-month follow-up, the addition of torcetrapib to atorvastatin treatment increased the rate of adverse CV events, as well as all-cause mortality, even though a 72.1% increase in HDL and a 24.9% decrease in LDL were noted [72]. Thus, the administration of torcetrapib was not further encouraged. The results concerning dalcetrapib were not favorable either. In a large randomized trial, which assigned 15,871 patients with a recent ACS to either dalcetrapib or placebo as add-on therapy, no impact was evident on the cumulative risk of the primary end point (i.e. death from cardiovascular causes, nonfatal MI, ischemic stroke, UA and cardiac arrest with resuscitation) and no significant change was observed in the rates of each separate components [73]. Despite promising initial data [74, 75] evacetrapib did not reduce CV events despite a 130% increase in HDL [76].
Table 1.
Novel therapeutic approaches targeting HDL: evidence from randomized controlled studies.
| Study | Drug | Population | No (Intervention/ Control) | Intervention Group | Control Group | Follow/Up | Impact on Lipid Parameters | Impact on CV Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nissen et al. [124] |
Muraglitazar | Patients with type 2 diabetes | 3,725 (2,374/ 1,351) |
Muraglitazar +/- metformin or glyburide |
Pioglitazone or placebo +/- metformin or glyburide | 24-104 weeks | N/A | ↑ death, MI or nonfatal stroke (RR=2.23, P=0.03) and ↑composite risk of death, MI, nonfatal stroke, CHF or TIA (RR= 2.62, P=0.004) |
||||||
| Rubin et al. [125] | Muraglitazar | Patients with type 2 diabetes | 1,805 | Muraglitazar 2.5 mg or 5 mg | Glimepiride 1 mg | 52 weeks | ↑HDL ↓TG (P<0.0001) |
↓HbA1c with both doses (P<0.0001) ; CV events were similar among the groups (2%); there was a trend towards ↑total mortality |
||||||
| Lincoff et al. [70] | Aleglitazar | Patients with ACS and type 2 diabetes | 7,226 (3,613/3,613) |
Aleglitazar 150 μg | Placebo | 104 weeks | N/A | no effect on CV death, nonfatal MI or nonfatal stroke | ||||||
| Barter et al. [72] | Torcetrapib | Patients at high CV risk | 15,067 (7,533/7,534) |
Torcetrapib+ atorvastatin |
Atorvastatin monotherapy | 12 months | ↑HDL by 72.1% ↓LDL by 24.9% (p<0.001) | ↑risk of MACE (HR=1.25, P=0.001), ↑ death from any cause (HR=1.58, P=0.006) | ||||||
| Schwartz et al. [73] | Dalcetrapib | Patients with recent ACS | 15,871 | 600 mg dalcetrapib + best available evidence-based care | Placebo + best available evidence-based care | 31 months | ↑HDL by 31-40% compared to 4-11% in the placebo group | No effect on the risk of the primary end point; no significant effect on any component of the primary end point or total mortality |
||||||
| Cannon et al. [77] |
Anacetrapib | Patients on statin that had reached LDL goal levels | 1,623 (811/812) |
100 mg anacetrapib | Placebo | 18 months | Intervention group: ↓LDL (from 81 mg/dl to 45 mg/dl); ↑HDL (from 41 mg/dl to 101 mg/dl); placebo group: ↓LDL (from 82 mg/dl to 77 mg/dl); ↑HDL (from 40 mg/dl to 46 mg/dl) (P<0.001) |
CV events occurred in 2% of patients on anacetrapib and 2.6% of patients on placebo (P=0.40) | ||||||
| Nissen et al. [80] | ETC-216 | Patients with ACS | 57 | ETC-216 (5 weekly infusions at 15 mg/kg or 45 mg/kg) |
Placebo | 5 weeks | N/A | ↓atheroma volume in the combined treatment groups by 4.2% (P<0.001) | ||||||
| Tardif et al. [81] | CSL-111 (reconstituted HDL) | Patients with CAD | 138 (123/60 |
CSL-111 (4 weekly infusions of 40 mg/kg or 80 mg/kg) | Placebo | 6 weeks | N/A | no significant effect on the atheroma volume; significant improvement in the plaque characterization index and coronary score on QCA | ||||||
| Study | Drug | Population | No (Intervention/ Control) | Intervention Group | Control Group | Follow/Up | Impact on Lipid Parameters | Impact on CV Outcomes | ||||||
| Tardif et al. [82] | CER-001 (reconstituted HDL) | Patients with ACS | 507 (352/118) |
CER-001 (6 weekly infusions of 3mg/kg, 6 mg/kg or 12 mg/kg) |
Placebo | 6 months | N/A | CER-001 infusions did not reduce coronary atherosclerosis on IVUS and QCA; any MACE occurred in 8,3%, 13,3%, 13,7% and 9,8% of the patients in the placebo, 3mg/kg, 6mg/kg and 12mg/kg group respectively |
||||||
| Chenevard et al. [83] | Reconstituted HDL | Patients with ACS | 29 (19/10) |
Reconstituted HDL | Albumin | 4 hours | ↑plasma HDL (P<0.0001) and ↓plasma LDL (P<0.0001); | no effect on the response to endothelium dependent and in dependent vasodilators; no effect on oxidized LDL and hs CRP levels |
||||||
| Waksman et al. [85] | Delipidated HDL | Patients with ACS | 28 (14/14) |
7 weekly delipidated HDL infusions | Control plasma apheresis/ reinfusions | 14 days | ↑prebeta-like HDL from 5.6% to 79.1%; ↓ alpha-HDL from 92.8% to 20.9% |
There was a trend towards regression in the total atheroma volume assessed by IVUS | ||||||
| Bloedon et al. [87] | Unformulated D-4F (HDL mimetic) | Patients with CHD | 40 (32/8) |
A single dose of 30, 100, 300 or 500 mg of unformulated D-4F |
Placebo | 4 hours | no effect | The HDL anti-inflammatory index significantly improved (P<0.05) | ||||||
| Nicholls et al. [92] | RVX-208 | Statin treated patients with CAD | 299 | RVX-208 at 50, 100 or 150 mg twice daily | Placebo | 12 weeks | ↑apoA-I levels dose-dependently up to 5.6% (P=0.035); ↑ HDL from 3.2% to 8.3% (P=0.02); ↑ large HDL from 11.1% to 21.1% (P=0.003) |
- | ||||||
Abbreviations: HDL: high-density lipoprotein, MI: myocardial infarction, RR: relative ratio, CHF: congestive heart failure, TIA: transient ischemic attack, N/A: not available, Hb1Ac: glycated hemoglobin; TG: triglycerides; ACS: acute coronary syndromes; CV: cardiovascular, MACE: major adverse cardiovascular events; CHD: coronary heart disease; SBP: systolic blood pressure; CRP: C-reactive protein; LDL; low-density lipoprotein; UA: unstable angina; IVUS: intravascular ultrasound; QCA: quantitative coronary angiography; CAD: coronary artery disease; +/-: plus/minus; ↑: increased; ↓:decreased; No: number.
Meanwhile, the DEFINE study [77] concluded that anacetrapib potently increases HDL and reduces LDL levels, with no apparent serious side-effects in patients with CAD or at high risk for CAD. The REVEAL trial, another ongoing phase III clinical study expected by 2017, has been designed to determine whether anacetrapib can reduce the rate of MACE in patients with CVD and low LDL.
3.6. Reconstituted High-density Lipoprotein
Given the positive results of recombinant proapolipoprotein A-I infusion on cholesterol excretion, it was proposed the infusion of recombinant HDL (rHDL) as a direct method to raise HDL levels. Several animal models had already supported this idea, as HDL administration proved able to hinder the atherosclerosis progression and to minimize the established atheromatic lesions in cholesterol-fed rabbits [78].
Franceschini et al. [79] reported 3 cases with hypertriglyceridemia and a marked decrease in HDL levels, but no concomitant CVD. The phenomenon was attributed to a point mutation in the apo-A-I gene, with the resultant lipoprotein molecule known as HDL Milano. In the clinical setting, data concerning the rHDL-Milano is derived mainly from imaging studies that report a regression of coronary atherosclerosis. Still, the impact on outcomes has not been assessed yet [80]. CSL-111, a rHDL that contained ApoA-I derived from human plasma, failed to demonstrate reduction in atheroma plaque by IVUS. Higher doses were accused of raising liver enzymes and thus it was forced to discontinuation [81]. However, the recently designed CER-001 presented a safer profile. Tardif et al., enrolled 507 ACS patients in an attempt to investigate the possible impact of different doses of CER-001 on several imaging parameters, but noticed no significant differences. The major CV events incidence was not significantly affected either [82]. So far, this is the only study to assess the impact of rHDL administrationon the outcomes of ACS patients. Chenevard et al. [83] showed that rHDL fails to improve vascular function in ACS patients, despite having a favorable impact on the lipid profile. Given the production cost and administration limitations, the trials assessing its clinical safety and efficacy remain limited, despite the initial encouraging evidence.
3.7. Delipidated HDL
Selective delipidation of plasma HDL transforms large HDL particles to small, nascent ones that are necessary for the reverse cholesterol transport and, thereby, facilitates this athero-protective process [84]. Waksman et al. investigated the impact of autologous infusions of delipidated HDL in 28 patients with ACS. A favorable alteration of the HDL subgroups’ concentration was noted, as prebeta-like HDL and alpha HDL diverted from 5.6% to 79.1% and from 92.8% to 20.9%, respectively. The atheroma’s volume was decreased and the administration was well-tolerated [85]. However, the efficacy of the above method in decreasing the incidence of clinical coronary events remains to be seen. Nevertheless, given the production cost and administration limitations the clinical data regarding to these regimens are still sparse.
3.8. HDL Mimetics
HDL mimetics are characterized by a number of interesting properties, as several studies using experimental models demonstrated. The 4F peptide hinders the sphingomyelinase-mediated LDL aggregation [86] and exhibits remarkable anti-inflammatory functions [87], while the D-4F peptide significantly reduced atherosclerotic lesions in the aorta of diabetic mice [88], which was also noted with the 5A peptide in another animal model [89]. However, the clinical trials that will address their impact on patients’ outcomes are a distant future, while the emerging evidence is controversial. Bloedon et al. [87] in a study assessing the effect of different single doses of D-4F administration in patients at high CV risk, concluded that the regimen is safe at a single dose and showed potentially improvement of the HDL anti-inflammatory index. However, a 28-day administration of L-4F failed to ameliorate HDL functional biomarkers in patients with CAD [90].
3.9. Stimulation of Apolipoprotein A-I Transcription
The ApoA-I upregulation is another mechanism of enhancing HDL function that generates nascent HDL particles. Nicholls et al. [91] assigned 299 statin-treated patients with stable CAD to receive either placebo or different doses of RVX-208, an oral ApoA-I inducer. The concentrations of HDL and particularly of the large HDL particles increased at 12 weeks. This was declarative of enhanced cholesterol mobilization. The ASSURE and SUSTAIN trials showed that RVX-208 significantly increased apoA-I and HDL levels in patients with low HDL and cardiovascular disease, while it induced a significant decrease in the rate of MACE [92, 93].-Nevertheless, it was only recently shown that the administration of RVX-208 failed to increase the apoA-I or HDL levels or to regress the atherosclerotic process in those patients [92].
Despite the aforementioned promising results, further studies of HDL-targeted therapies have not moved forward as expected probably because of the complexity of production, the formulation currently available (for some no oral formulation) and the production costs [94].
4. FUTURE PERSPECTIVES IN LIPID THERAPY
As the results from studies focused on HDL therapy were not totally encouraging (Table 1), novel therapies that address the LDL-C metabolism were designed.
4.1. Other HDL-targeted Therapies
Several new regimens are under vigorous investigation, but the evidence is, so far, derived mainly from experimental animal models. Liver X receptors (LXR) agonists are thought to facilitate reverse cholesterol transport and increase HDL levels [95], while a novel scavenger receptor class B type I (SR-BI) inhibitor was recently shown to increase HDL concentration by 20% in hypertriglyceridemic patients [96]. Further trials are warranted before any safe clinical conclusions can be drawn.
However, over the last decade it has become apparent that the anti-athrosclerotic properties of HDL lie more in its quality rather than absolute HDL levels. HDL appears to lose its protective qualities in certain pathological conditions [24, 25] and with only 5% of the total HDL derived from actual macrophage cholesterol efflux, the amount of HDL seems a poor representative of the beneficial effects of HDL [26, 27]. Over the last few years, several methods have been developed which can assess HDL function [28]. One of these newer methods, macrophage cholesterol efflux appears to more accurately predict cardiovascular events than actual HDL levels [29, 30] .
4.2. PCSK9 Inhibitors
The proprotein convertase subtilisin/kexin type 9 serine protease gene (PCSK9) mediates the degradation of the hepatic LDL receptor and thus inhibits LDL sequestration and increases LDL levels. Consequently, its loss of function would result in a reduction in LDL levels [97]. Human antibodies have been lately designed to target PCSK9 inhibition and have showed promising results in clinical trials.
Evolocumab (AMG 145) was one of the first anti-PCSK9 monoclonal antibodies that showed efficacy in decreasing the LDL plasma levels. The MENDEL trial, including hypercholesterolemic patients, reported that monotherapy with evolocumab at various doses decreased LDL plasma levels by approximately 50% [98]. This was confirmed in the subsequent phase 3 trial. Evolocumab decreased LDL by 55 to 57% compared to placebo and by 38 to 40% compared to ezetimibe [99]. Evolocumab has been also shown to decrease the levels of Apo B, triglycerides and lipoprotein (a) (LP(a)), while it increases HDL [100]. This is of particular significance for the group of patients that are statin intolerant. The GAUSS-2 trial investigated the impact of evolocumab administration in statin-intolerant patients and concluded that evolocumab was well-tolerated for a 12-month administration [101]. The short-term tolerability of evolocumab in statin-intolerant patients had previously been confirmed by Sullivan et al. [102]. All in all, the OSLER trial [103] was the largest trial to assess the regimen’s efficacy and tolerability and concluded that the beneficial impact of evolocumab continued to exist after 1 year of administration, while the therapy was well-tolerated compared to standard treatment. Indeed, the 24-month follow up of the patients enrolled in the GAUSS-2 trial showed equally promising results. The modifying lipid effect persisted at 24-months [104]. Thus, evolocumab could be an alternative in the case of statin intolerance. Nevertheless, evolocumab also showed efficacy as an add-on therapy. Particularly in high-risk patients that fail to reach the LDL-C goal with intensive statin treatment, the addition of evolocumab has proved to be advantageous. The LAPLACE-TIMI 57 trial included patients who received intensive statin treatment and had plasma LDL levels of >70mg/dl. It was reported that 90% of the patients assigned to evolocumab achieved the LDL-C goal of <70 mg/dl at a 12-week follow-up whereas the rate of high-risk patients that achieve this goal with statin treatment only was 35% [105]. The DESCARTES trial [106] which enrolled patients with increased CV risk, concluded that the addition of evolocumab yielded an additional overall reduction of 57% in the LDL-C levels at a 52-week follow-up. As Raal et al. [107] recently confirmed, the addition of evolocumab is advantageous even in the challenging case of homozygous familial hypercholesterolemia. Evolocumab further reduced LDL-C by 30.9% at a 12 week follow-up when added to stable lipid-lowering background therapy.
The results from alirocumab administration were equally promising. McKenney et al. [108] investigated the effects of alirocumab administration in hypercholesterolemic patients that followed background atorvastatin therapy. Alirocumab as add-on therapy could further decrease LDL-C by 40% to 72%. Roth et al. [109] demonstrated that 90% of dyslipidemic patients treated with alirocumab reach the LDL-C goal of <70 mg/dl. By contrast, only 17% of those treated with intensive atorvastatin monotherapy had LDL-C levels below 70 mg/dl. In fact, as Cannon et al. [110] recently demonstrated, alirocumab is superior to ezetimibe in reducing LDL-C. In patients with high CV risk and persistently high LDL levels, despite optimal statin treatment, the addition of alirocumab decreased LDL-C by 50.6% at a 24-week follow-up. The reduction achieved with the addition of ezetimibe was only 20.7%. The results of the ODYSSEY ALTERNATIVE trial [111] were equally favorable for alirocumab. Among 36 statin-intorelant patients, alirocumab decreased LDL more than ezetimibe and caused fewer muscle adverse events (muscle pain or spasm). Of note, a recent analysis showed that, in patients receiving statins, alirocumab can decrease further triglycerides, apolipoproteins CII and CIII and the cholesterol content of LDL1, LDL2 and LDL3+4 subfractions [112]. Last but not least, bococizumab can significantly decrease LDL as add-on treatment as well and its most efficacious dose of 150 mg every 14 days is under further evaluation in phase 3 clinical trials [113].
Whether the above mentioned favorable lipid-altering effects will be translated in an improvement of the patients’ outcomes are yet to be determined. Robinson et al. [114] recently concluded that the addition of alirocumab to intensive statin treatment can reduce the rate of major adverse CV events in high-risk patients at a follow-up period of 78 weeks. Similarly, the addition of evolocumab reduced the rate of 1-year CV events in high risk patients [115]. Nevertheless, the evidence regarding the vulnerable group of patients with ACS is limited. The ODYSSEY outcomes trial [116] is an ongoing phase 3 study that promised to shed light to this question. It will be investigated whether the addition of alirocumab to intensive statin treatment decreases CV mortality and the rate of nonfatal adverse cardiovascular events in 18.000 patients recently hospitalized for ACS.
There is ongoing debate with regards to the cost-effectiveness of these agents as well as which subgroups of patients would benefit the most. Alirocumab and evolocumab are estimated to cost approximately $14,350 per patient for a one-year in the United States, or approximately $1,200 per month. When used in patients with familial hypercholesterolemia or atherosclerotic CVD who are unable to control LDL‐C levels with maximally tolerated statins and who remain at high risk, addition of evolocumab has been shown to be cost effective [117]. More data are expected from ongoing trials currently being conducted.
4.3. Apolipoprotein B inhibitors: Mipomersen - Lomitapide
Mipomersen is an antisense oligonucleotide inhibitor of apolipoprotein B that has been reported to induce a maximum of 50% decrease in LDL-C levels [118]. As Stein et al. [119] concluded in their trial which involved 124 patients with heterozygous familial hypercholesterolemia and CAD, mipomersen, as add-on therapy to the maximum tolerated statin treatment, can further reduce LDL by 28% in 4 weeks. A 36.9% reduction at the 28th week of treatment was later reported in patients with severe hypercholesterolemia or at high risk for CAD, who were also receiving maximal statin therapy [120]. In high-risk statin-intolerant patients, LDL reduction induced by mipomersen monotherapy reached the rate of 47 ± 18% [121]. Of note, mipomersen has been shown to decrease the small LDL particles to a greater extent than the larger ones [122]. At the same time, lomitapide, an oral microsomal triglyceride transfer protein inhibitor that reduces the apolipoprotein B production has also shown efficacy in decreasing LDL levels by 50.9% in homozygous familial hypercholesterolemia patients [123]. However, the impact of these agents on the CV outcomes in these patients complicated with ACS in particular has not been evaluated yet (Table 2).
Table 2.
Novel therapeutic approaches targeting LDL: evidence from randomized controlled studies.
| Study | Treatment | Population | No | Treatment Arms | Follow/up | Impact on Lipid Parameters | CV Outcomes |
|---|---|---|---|---|---|---|---|
| Raal et al. [107] | Evolocumab | Patients with homozygous FCH | 49 | Evolocumab 420 mg Q4W or placebo | 12 weeks | ↓LDL by 30.9% (P<0.0001) | N/A |
| Sullivan et al. [102] | Evolocumab | Patients with statin intolerance | 160 | Evolocumab at doses of 280 mg, 350 mg or 420 mg; or evolocumab at 420 mg + ezetimibe at 10 mg (SC Q4W); or 10 mg ezetimibe +placebo | 12 weeks | ↓LDL by 15 to 63% (P<0.001) | N/A |
| Stroes et al. [101] |
Evolocumab | Hypercholesterolemic patients with statin intolerance | 307 | Evolocumab 140 mg Q2W or evolocumab 420 mg QM + oral placebo or SC placebo Q2W or QM + daily oral ezetimibe 10 mg |
12 weeks | ↓LDL by 53 to 56% from baseline and by 37 to 39% compared to ezetimibe (P<0.001) | N/A |
| Koren et al. [103] |
Evolocumab | Patients with fasting LDL ≥100 and <190 mg/dl and Framingham risk scores ≤10% | 614 | Oral placebo and SC placebo Q2W; oral placebo and SC placebo QM; ezetimibe and SC placebo Q2W; ezetimibe and SC placebo QM; oral placebo and evolocumab 140 mg Q2W; or oral placebo and evolocumab 420 mg QM |
12 weeks | ↓LDL by 55 to 57% compared to placebo and by 38 to 40% compared to ezetimibe (P<0.001); favorably altered other lipoproteins | N/A |
| Blom et al. [106] | Evolocumab | Patients with a range of CV risks | 901 | Background lipid-lowering therapy with diet al.one or diet plus atorvastatin 10 mg daily, atorvastatin 80 mg daily, or atorvastatin 80 mg daily plus ezetimibe 10 mg daily, evolocumab 420 mg daily or placebo every 4 weeks if LDL ≥75 mg/dl | 52 weeks | ↓LDL by 57% compared to placebo (P<0.001); reductions were 55.7% in the diet-alone group, 61.6% in the 10 mg atorvastatin group, 56.8% in the 80 mg atorvastatin group and 48.5% in the combination of 80 mg atorvastatin and 10 mg ezetimibe group; ↓apo-b, non-HDL, Lp(a) and TG levels |
N/A |
| Mckenney et al. [108] |
Alirocumab | LDL-C ≥100 mg/dl | 183 | SC placebo Q2W; alirocumab 50, 100, or 150 mg Q2W; or alirocumab 200 or 300 mg Q4W, alternating with placebo; patients were on stable-dose atorvastatin 10, 20, or 40 mg for ≥6 weeks |
12 weeks | ↓LDL by 40% to 72% when added to atorvastatin; the reduction was dose- and frequency-dependent | N/A |
| Roth et al. [109] | Alirocumab | LDL ≥100 mg/dl after treatment with 10 mg of atorvastatin for at least 7 weeks | 92 | 80 mg atorvastatin daily plus alirocumab once every 2 weeks, 10 mg of atorvastatin daily plus alirocumab Q2W or 80 mg of atorvastatin daily plus placebo Q2W |
10 weeks | All patients on alirocumab had LDL cholesterol level ≤100 mg/dl and at least 90% of those had LDL < 70 mg/dl; the equivalent percentages for patients on placebo were 52% and 17% |
N/A |
| Study | Treatment | Population | No | Treatment Arms | Follow/up | Impact on Lipid Parameters | CV Outcomes |
| Cannon et al. [110] |
Alirocumab | High CV and elevated LDL-C despite maximal doses of statins | 720 | SC alirocumab 75 mg Q2W +oral placebo or oral ezetimibe 10 mg daily +SC placebo on a background of statin therapy |
104 weeks | ↓ LDL by 50.6% in the alirocumab vs. 20.7% in the ezetimibe group (P<0.0001); 77% of alirocumab and 45.9% of ezetimibe group achieved LDL<1.8 mmol/L (P<0.0001) |
N/A |
| Robinson et al. [114] |
Alirocumab | High CV and LDL ≥70 mg/dl and background treatment despite maximal doses of statins | 2341 | Alirocumab 150 mg or placebo as a 1-ml subcutaneous injection Q2W | 72 weeks | ↓LDL by 62% compared to placebo (p<0.001) | ↓ rate of MACE(HR:0.52, P=0.02) |
| Sabatine et al. [115] |
Evolocumab | Patients who had completed 1 of 12 phase 2 or 3 studies (“parent trials”) of evolocumab | 4465 | Evolocumab (140 mg Q2W or 420 mg monthly) + standard therapy or standard therapy alone. | 11,1 months | ↓LDL by 61% compared to standard therapy alone (p<0.001) | ↓ rate of MACE (HR:0.47%, P=0.003) |
| Stein et al. [119] | Mipomersen | Heterozygous familial hypercholesterolemia | 114 | mipomersen 200 mg SC, QW or placebo |
28 weeks | ↓LDL-C by 28.0% compared with 5.2% [-0.5% to 10.9%] increase with placebo (P<0.001); ↓apo-lipoprotein-B, total cholesterol, lipoprotein(a) (P<0.001) |
N/A |
| Thomas et al. [120] |
Mipomersen | High CV and LDL ≥100 mg/dl and background treatment despite maximal doses of statins | 158 | mipomersen 200 mg SC, QW or placebo for 26 weeks |
24 weeks | ↓ LDL by 36.9% compared with placebo at -4.5% (p < 0.001); target LDL <100 mg/dl in 76% of mipomersen and 38% of placebo patients; ↓apo- B and Lp(a) (P < 0.001) |
N/A |
| Cuchel et al. [122] |
Lomitapide | Homozygous familial hypercholesterolemia | 6 | Lomitapide at 0.03, 0.1, 0.3, and 1.0 mg/kg per day for 4 weeks |
4 weeks |
↓ LDL 50.9% and apo- B levels by 55.6% (P<0.001) | N/A |
CONCLUSION
The observed anti-atherosclerotic properties of HDL have triggered research aiming to improve cardiovascular outcomes of patients with ACS. Nevertheless, the results of these therapies have not been that encouraging so far. The administration of niacin failed to reduce the incidence of MACE, while the data regarding fibrates, glitazones and glitazars were, in some studies, alarming: several of these agents had a negative effect on those patients’ prognosis. The more recent HDL-targeted drugs may prove to be more effective than those used before. Delipidated HDL and HDL mimetics are efficient in increasing HDL levels, while the apoA-I upregulation with RVX-208 has been recently shown to decrease the rate of MACE. Nevertheless, given the production cost and administration limitations, the clinical data regarding to these regimens are still sparse. On the other hand, the newly designed LDL-targeted drugs seem more likely to enter clinical practice. Indeed, LDL reduction, even below the previous target levels, has been shown to convey a clinical benefit. The intensification of statin treatment and the addition of ezetimibe have been proved clinically beneficial. Importantly, the recently designed PSCK9 inhibitors appear to be safe and efficacious in patients with familial hypercholesterolemia, but there is still ongoing debate with regards to possible broader indications and their cost effectiveness.
ACKNOWLEDGEMENTS
Emmanuel Androulakis: edited and revised manuscript, Nikolaos Papageorgiou: wrote and revised manuscript; Effimia Zacharia: wrote manuscript, Eirini Lioudaki: edited and revised the manuscript, Dimitris Bertsias: edited and revised the manuscript, Marietta Charakida: edited and revised the manuscript, Gerasimos Siasos: edited and revised the manuscript, Dimitris Tousoulis: wrote and revised manuscript.
Abbreviations:
- HDL
high-density lipoprotein;
- SC
subcutaneous;
- MI
myocardial infarction,
- HR
hazard ratio,
- CHF
congestive heart failure,
- TIA
transient ischemic attack,
- NR
not reported;
- TG
triglycerides;
- ACS
acute coronary syndromes;
- CV
cardiovascular,
- MACE
major adverse cardiovascular events;
- CHD
coronary heart disease;
- LDL
low-density lipoprotein;
- UA
unstable angina;
- CAD
coronary artery disease;
- FCH
familial hypercholesterolemia;
- +
plus;
- ↓
decrease;
- Q2W
every two weeks;
- QM
once monthly;
- Q4W
every four weeks;
- QW
weekly;
- No
number.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N. Engl. J. Med. 1998;339(19):1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 2.Correia L.C., Rocha M.S., Esteves J.P. HDL-cholesterol level provides additional prognosis in acute coronary syndromes. Int. J. Cardiol. 2009;136(3):307–314. doi: 10.1016/j.ijcard.2008.05.067. [DOI] [PubMed] [Google Scholar]
- 3.Gordon T., Castelli W.P., Hjortland M.C., Kannel W.B., Dawber T.R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 1977;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 4.Rosenson R.S., Brewer H.B., Jr, Ansell B.J., et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016;13(1):48–60. doi: 10.1038/nrcardio.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks F.M., Pfeffer M.A., Moye L.A., et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 6.Stenestrand U., Wallentin L. Early statin treatment following acute myocardial infarction and 1-year survival. JAMA. 2001;285(4):430–436. doi: 10.1001/jama.285.4.430. [DOI] [PubMed] [Google Scholar]
- 7.Cannon C.P., Braunwald E., McCabe C.H., et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 8.Murphy S.A., Cannon C.P., Wiviott S.D., McCabe C.H., Braunwald E. Reduction in recurrent cardiovascular events with intensive lipid-lowering statin therapy compared with moderate lipid-lowering statin therapy after acute coronary syndromes from the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) trial. J. Am. Coll. Cardiol. 2009;54(25):2358–2362. doi: 10.1016/j.jacc.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 9.de Lemos J.A., Blazing M.A., Wiviott S.D., et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292(11):1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen T.R., Faergeman O., Kastelein J.J., et al. High-dose atorvastatin vs. usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294(19):2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 11.Baigent C., Blackwell L., Emberson J., et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet (London, England) 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allonen J., Nieminen M.S., Lokki M., et al. Mortality rate increases steeply with nonadherence to statin therapy in patients with acute coronary syndrome. Clin. Cardiol. 2012;35(11):E22–E27. doi: 10.1002/clc.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ameson K, Amber V, D’Oca K, Mills D, Giles A, Ambegaonkar B. Impact of lipid-lowering therapy on the prevalence of dyslipidaemia in patients at high-risk of cardiovascular events in UK primary care - a retrospective database study. Int. J. Clin. Pract. 2013;67(12):1228–1237. doi: 10.1111/ijcp.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boey E., Gay G.M., Poh K.K., Yeo T.C., Tan H.C., Lee C.H. Visit-to-visit variability in LDL- and HDL-cholesterol is associated with adverse events after ST-segment elevation myocardial infarction: A 5-year follow-up study. Atherosclerosis. 2016;244:86–92. doi: 10.1016/j.atherosclerosis.2015.10.110. [DOI] [PubMed] [Google Scholar]
- 15.Phan B.A., Dayspring T.D., Toth P.P. Ezetimibe therapy: mechanism of action and clinical update. Vasc. Health Risk Manag. 2012;8:415–427. doi: 10.2147/VHRM.S33664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chenot F., Montant P.F., Marcovitch O., Blaimont M., de Meester A., Descamps O.S. Co-administration of ezetimibe and simvastatin in acute myocardial infarction. Eur. J. Clin. Invest. 2007;37(5):357–363. doi: 10.1111/j.1365-2362.2007.01797.x. [DOI] [PubMed] [Google Scholar]
- 17.Pauriah M., Elder D.H., Ogston S., et al. High-potency statin and ezetimibe use and mortality in survivors of an acute myocardial infarction: a population-based study. Heart. 2014;100(11):867–872. doi: 10.1136/heartjnl-2013-304678. [DOI] [PubMed] [Google Scholar]
- 18.Cannon C.P., Blazing M.A., Giugliano R.P., et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 19.Murphy S.A., Cannon C.P., Blazing M.A., et al. Reduction in Total Cardiovascular Events With Ezetimibe/Simvastatin Post-Acute Coronary Syndrome: The IMPROVE-IT Trial. J. Am. Coll. Cardiol. 2016;67(4):353–361. doi: 10.1016/j.jacc.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 20.Eisen A., Cannon C.P., Blazing M.A., et al. The benefit of adding ezetimibe to statin therapy in patients with prior coronary artery bypass graft surgery and acute coronary syndrome in the IMPROVE-IT trial. Eur. Heart J. 2016;37(48):3576–3584. doi: 10.1093/eurheartj/ehw377. [DOI] [PubMed] [Google Scholar]
- 21.Ip C.K., Jin D.M., Gao J.J., et al. Effects of add-on lipid-modifying therapy on top of background statin treatment on major cardiovascular events: A meta-analysis of randomized controlled trials. Int. J. Cardiol. 2015;191:138–148. doi: 10.1016/j.ijcard.2015.04.228. [DOI] [PubMed] [Google Scholar]
- 22.Savarese G., De Ferrari G.M., Rosano G.M., Perrone-Filardi P. Safety and efficacy of ezetimibe: A meta-analysis. Int. J. Cardiol. 2015;201:247–252. doi: 10.1016/j.ijcard.2015.08.103. [DOI] [PubMed] [Google Scholar]
- 23.Battaggia A., Donzelli A., Font M., Molteni D., Galvano A. Clinical efficacy and safety of Ezetimibe on major cardiovascular endpoints: systematic review and meta-analysis of randomized controlled trials. PLoS One. 2015;10(4):e0124587. doi: 10.1371/journal.pone.0124587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Lenten B.J., Wagner A.C., Nayak D.P., Hama S., Navab M., Fogelman A.M. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001;103(18):2283–2288. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 25.Hoang A., Murphy A.J., Coughlan M.T., et al. Advanced glycation of apolipoprotein A-I impairs its anti-atherogenic properties. Diabetologia. 2007;50(8):1770–1779. doi: 10.1007/s00125-007-0718-9. [DOI] [PubMed] [Google Scholar]
- 26.Santos-Gallego C.G., Badimon J.J., Rosenson R.S. Beginning to understand high-density lipoproteins. Endocrinol. Metab. Clin. North Am. 2014;43(4):913–947. doi: 10.1016/j.ecl.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Santos-Gallego C.G. HDL: Quality or quantity? Atherosclerosis. 2015;243(1):121–123. doi: 10.1016/j.atherosclerosis.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Santos-Gallego C.G., Giannarelli C., Badimon J.J. Experimental models for the investigation of high-density lipoprotein-mediated cholesterol efflux. Curr. Atheroscler. Rep. 2011;13(3):266–276. doi: 10.1007/s11883-011-0177-0. [DOI] [PubMed] [Google Scholar]
- 29.Khera A.V., Cuchel M., de la Llera-Moya M., et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011;364(2):127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohatgi A., Khera A., Berry J.D., et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014;371(25):2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blankenhorn D.H., Nessim S.A., Johnson R.L., Sanmarco M.E., Azen S.P., Cashin-Hemphill L. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA. 1987;257(23):3233–3240. [PubMed] [Google Scholar]
- 32.Chai J.T., Digby J.E., Choudhury R.P. GPR109A and vascular inflammation. Curr. Atheroscler. Rep. 2013;15(5):325. doi: 10.1007/s11883-013-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X.Q., Phan B.A., Davis J., et al. Mortality reduction in patients treated with long-term intensive lipid therapy: 25-year follow-up of the Familial Atherosclerosis Treatment Study-Observational Study. J. Clin. Lipidol. 2016;10(5):1091–1097. doi: 10.1016/j.jacl.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canner P.L., Berge K.G., Wenger N.K., et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J. Am. Coll. Cardiol. 1986;8(6):1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 35.Brown G., Albers J.J., Fisher L.D., et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N. Engl. J. Med. 1990;323(19):1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 36.Phan B.A., Munoz L., Shadzi P., et al. Effects of niacin on glucose levels, coronary stenosis progression, and clinical events in subjects with normal baseline glucose levels (<100 mg/dl): a combined analysis of the Familial Atherosclerosis Treatment Study (FATS), HDL-Atherosclerosis Treatment Study (HATS), Armed Forces Regression Study (AFREGS), and Carotid Plaque Composition by MRI during lipid-lowering (CPC) study. Am. J. Cardiol. 2013;111(3):352–355. doi: 10.1016/j.amjcard.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor A.J., Sullenberger L.E., Lee H.J., Lee J.K., Grace K.A. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110(23):3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 38.Albers J.J., Slee A., O’Brien K.D., et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J. Am. Coll. Cardiol. 2013;62(17):1575–1579. doi: 10.1016/j.jacc.2013.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boden W.E., Probstfield J.L., Anderson T., et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 40.Landray M.J., Haynes R., Hopewell J.C., et al. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 2014;371(3):203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 41.Parhofer K.G. Review of extended-release niacin/laropiprant fixed combination in the treatment of mixed dyslipidemia and primary hypercholesterolemia. Vasc. Health Risk Manag. 2009;5:901–908. doi: 10.2147/vhrm.s4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos-Gallego C.G., Badimon J. Niacin for reduction of cardiovascular risk. N. Engl. J. Med. 2014;371(20):1943. doi: 10.1056/NEJMc1411240. [DOI] [PubMed] [Google Scholar]
- 43.Song W.L., Stubbe J., Ricciotti E., et al. Niacin and biosynthesis of PGD(2)by platelet COX-1 in mice and humans. J. Clin. Invest. 2012;122(4):1459–1468. doi: 10.1172/JCI59262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Investigators A-H. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 45.Siniawski D., Santos-Gallego C.G., Badimon J.J., Masson W.M. Niacin is still beneficial. Implications from an updated meta-regression analysis. Acta Cardiol. 2016;71(4):463–472. doi: 10.2143/AC.71.4.3159701. [DOI] [PubMed] [Google Scholar]
- 46.Goldenberg I., Benderly M., Goldbourt U. Update on the use of fibrates: focus on bezafibrate. Vasc. Health Risk Manag. 2008;4(1):131–141. doi: 10.2147/vhrm.2008.04.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlson L.A., Rosenhamer G. Reduction of mortality in the Stockholm Ischaemic Heart Disease Secondary Prevention Study by combined treatment with clofibrate and nicotinic acid. Acta Med. Scand. 1988;223(5):405–418. doi: 10.1111/j.0954-6820.1988.tb15891.x. [DOI] [PubMed] [Google Scholar]
- 48.Frick M.H., Elo O., Haapa K., et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N. Engl. J. Med. 1987;317(20):1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 49.Frick M.H., Heinonen O.P., Huttunen J.K., Koskinen P., Manttari M., Manninen V. Efficacy of gemfibrozil in dyslipidaemic subjects with suspected heart disease. An ancillary study in the Helsinki Heart Study frame population. Ann. Med. 1993;25(1):41–45. doi: 10.3109/07853899309147855. [DOI] [PubMed] [Google Scholar]
- 50.Rubins H.B., Robins S.J., Collins D., et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N. Engl. J. Med. 1999;341(6):410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 51.Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation. 2000;102(1):21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 52.Goldenberg I., Boyko V., Tennenbaum A., Tanne D., Behar S., Guetta V. Long-term benefit of high-density lipoprotein cholesterol-raising therapy with bezafibrate: 16-year mortality follow-up of the bezafibrate infarction prevention trial. Arch. Intern. Med. 2009;169(5):508–514. doi: 10.1001/archinternmed.2008.584. [DOI] [PubMed] [Google Scholar]
- 53.Klempfner R., Goldenberg I., Fisman E.Z., et al. Comparison of statin alone versus bezafibrate and statin combination in patients with diabetes mellitus and acute coronary syndrome. Am. J. Cardiol. 2014;113(1):12–16. doi: 10.1016/j.amjcard.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 54.Klempfner R., Goldenberg I., Fisman E.Z., et al. Bezafibrate treatment is associated with a reduced rate of re-hospitalization in smokers after acute coronary syndrome. Cardiol. J. 2014;21(4):364–369. doi: 10.5603/CJ.a2013.0127. [DOI] [PubMed] [Google Scholar]
- 55.Ginsberg H.N., Elam M.B., Lovato L.C., et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010;362(17):1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keech A., Simes R.J., Barter P., et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet (London, England) 2005;366(9500):1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 57.d’Emden M.C., Jenkins A.J., Li L., et al. Favourable effects of fenofibrate on lipids and cardiovascular disease in women with type 2 diabetes: results from the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2014;57(11):2296–2303. doi: 10.1007/s00125-014-3344-3. [DOI] [PubMed] [Google Scholar]
- 58.Aghamohammadzadeh N., Niafar M., Dalir Abdolahinia E., et al. The effect of pioglitazone on weight, lipid profile and liver enzymes in type 2 diabetic patients. Ther. Adv. Endocrinol. Metab. 2015;6(2):56–60. doi: 10.1177/2042018815574229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Persegol L., Duvillard L., Monier S., et al. No improvement of high-density lipoprotein (HDL) vasorelaxant effect despite increase in HDL cholesterol concentration in type 2 diabetic patients treated with glitazones. J. Clin. Endocrinol. Metab. 2014;99(10):E2015–E2019. doi: 10.1210/jc.2014-2078. [DOI] [PubMed] [Google Scholar]
- 60.Li C., Tu Y., Liu T.R., et al. Rosiglitazone attenuates atherosclerosis and increases high-density lipoprotein function in atherosclerotic rabbits. Int. J. Mol. Med. 2015;35(3):715–723. doi: 10.3892/ijmm.2015.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rizzo M., Vekic J., Koulouris S., et al. Effects of rosiglitazone on fasting and postprandial low- and high-density lipoproteins size and subclasses in type 2 diabetes. Angiol. 2010;61(6):584–590. doi: 10.1177/0003319710366431. [DOI] [PubMed] [Google Scholar]
- 62.Schwing W., Hustak L., Taylor H.C. Paradoxical severe decrease in high-density lipoprotein cholesterol due to rosiglitazone-fenofibrate interaction. Endocr. Pract. 2010;16(3):382–388. doi: 10.4158/EP09307.OR. [DOI] [PubMed] [Google Scholar]
- 63.Dormandy J.A., Charbonnel B., Eckland D.J., et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet (London, England) 2005;366(9493):1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 64.Christoph M., Herold J., Berg-Holldack A., et al. Effects of the PPARgamma agonist pioglitazone on coronary atherosclerotic plaque composition and plaque progression in non-diabetic patients: a double-center, randomized controlled VH-IVUS pilot-trial. Heart Vessels. 2015;30(3):286–295. doi: 10.1007/s00380-014-0480-0. [DOI] [PubMed] [Google Scholar]
- 65.Home P.D., Pocock S.J., Beck-Nielsen H., et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet (London, England) 2009;373(9681):2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 66.Nissen S.E., Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 67.Nissen S.E., Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch. Intern. Med. 2010;170(14):1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 68.Loke Y.K., Kwok C.S., Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ. 2011;342:d1309. doi: 10.1136/bmj.d1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilding J.P. PPAR agonists for the treatment of cardiovascular disease in patients with diabetes. Diabetes Obes. Metab. 2012;14(11):973–982. doi: 10.1111/j.1463-1326.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 70.Lincoff A.M., Tardif J.C., Schwartz G.G., et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA. 2014;311(15):1515–1525. doi: 10.1001/jama.2014.3321. [DOI] [PubMed] [Google Scholar]
- 71.Erdmann E., Califf R., Gerstein H.C., et al. Effects of the dual peroxisome proliferator-activated receptor activator aleglitazar in patients with Type 2 Diabetes mellitus or prediabetes. Am. Heart J. 2015;170(1):117–122. doi: 10.1016/j.ahj.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 72.Barter P.J., Caulfield M., Eriksson M., et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 2007;357(21):2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 73.Schwartz G.G., Olsson A.G., Abt M., et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012;367(22):2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 74.Nicholls S.J., Brewer H.B., Kastelein J.J., et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA. 2011;306(19):2099–2109. doi: 10.1001/jama.2011.1649. [DOI] [PubMed] [Google Scholar]
- 75.Cao G., Beyer T.P., Zhang Y., et al. Evacetrapib is a novel, potent, and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure. J. Lipid Res. 2011;52(12):2169–2176. doi: 10.1194/jlr.M018069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nicholls S.J., Lincoff A.M., Barter P. The ACCELERATE trial: impact of the cholesteryl ester transfer protein inhibitor evacetrapib on cardiovascular outcome.; 65th Annual Scientific Session and Expo of the American College of Cardiology; 2016 April 2-4, 2016; Chicago, IL. ; 2016. [Google Scholar]
- 77.Cannon C.P., Shah S., Dansky H.M., et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 2010;363(25):2406–2415. doi: 10.1056/NEJMoa1009744. [DOI] [PubMed] [Google Scholar]
- 78.Badimon J.J., Badimon L., Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Invest. 1990;85(4):1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franceschini G., Sirtori C.R., Capurso A., II, Weisgraber K.H., Mahley R.W. A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J. Clin. Invest. 1980;66(5):892–900. doi: 10.1172/JCI109956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nissen S.E., Tsunoda T., Tuzcu E.M., et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290(17):2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 81.Tardif J.C., Gregoire J., L’Allier P.L., et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297(15):1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 82.Tardif J.C., Ballantyne C.M., Barter P., et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur. Heart J. 2014;35(46):3277–3286. doi: 10.1093/eurheartj/ehu171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chenevard R., Hurlimann D., Spieker L., et al. Reconstituted HDL in acute coronary syndromes. Cardiovasc. Ther. 2012;30(2):e51–e57. doi: 10.1111/j.1755-5922.2010.00221.x. [DOI] [PubMed] [Google Scholar]
- 84.Sacks F.M., Rudel L.L., Conner A., et al. Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo. J. Lipid Res. 2009;50(5):894–907. doi: 10.1194/jlr.M800622-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waksman R., Torguson R., Kent K.M., et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 2010;55(24):2727–2735. doi: 10.1016/j.jacc.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen S.D., Javanainen M., Rissanen S., et al. Apolipoprotein A-I mimetic peptide 4F blocks sphingomyelinase-induced LDL aggregation. J. Lipid Res. 2015;56(6):1206–1221. doi: 10.1194/jlr.M059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bloedon L.T., Dunbar R., Duffy D., et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 2008;49(6):1344–1352. doi: 10.1194/jlr.P800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morgantini C., Imaizumi S., Grijalva V., Navab M., Fogelman A.M., Reddy S.T. Apolipoprotein A-I mimetic peptides prevent atherosclerosis development and reduce plaque inflammation in a murine model of diabetes. Diabetes. 2010;59(12):3223–3228. doi: 10.2337/db10-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amar M.J., D’Souza W., Turner S., et al. 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J. Pharmacol. Exp. Ther. 2010;334(2):634–641. doi: 10.1124/jpet.110.167890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Watson C.E., Weissbach N., Kjems L., et al. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 2011;52(2):361–373. doi: 10.1194/jlr.M011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nicholls S.J., Gordon A., Johansson J., et al. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J. Am. Coll. Cardiol. 2011;57(9):1111–1119. doi: 10.1016/j.jacc.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 92.Nicholls S.J., Puri R., Wolski K., et al. Effect of the BET Protein Inhibitor, RVX-208, on Progression of Coronary Atherosclerosis: Results of the Phase 2b, Randomized, Double-Blind, Multicenter, ASSURE Trial. Am. J. Cardiovasc. Drugs. 2016;16(1):55–65. doi: 10.1007/s40256-015-0146-z. [DOI] [PubMed] [Google Scholar]
- 93.Gilham D., Wasiak S., Tsujikawa L.M., et al. RVX-208, a BET-inhibitor for treating atherosclerotic cardiovascular disease, raises ApoA-I/HDL and represses pathways that contribute to cardiovascular disease. Atherosclerosis. 2016;247:48–57. doi: 10.1016/j.atherosclerosis.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 94.Kingwell B.A., Chapman M.J., Kontush A., Miller N.E. HDL-targeted therapies: progress, failures and future. Nat. Rev. Drug Discov. 2014;13(6):445–464. doi: 10.1038/nrd4279. [DOI] [PubMed] [Google Scholar]
- 95.Beltowski J. Liver X receptors (LXR) as therapeutic targets in dyslipidemia. Cardiovasc. Ther. 2008;26(4):297–316. doi: 10.1111/j.1755-5922.2008.00062.x. [DOI] [PubMed] [Google Scholar]
- 96.Masson D., Koseki M., Ishibashi M., et al. Increased HDL cholesterol and apoA-I in humans and mice treated with a novel SR-BI inhibitor. Arterioscler. Thromb. Vasc. Biol. 2009;29(12):2054–2060. doi: 10.1161/ATVBAHA.109.191320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horton J.D., Cohen J.C., Hobbs H.H. PCSK9: a convertase that coordinates LDL catabolism. J. Lipid Res. 2009;50(Suppl.):S172–S177. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koren M.J., Scott R., Kim J.B., et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet (London, England) 2012;380(9858):1995–2006. doi: 10.1016/S0140-6736(12)61771-1. [DOI] [PubMed] [Google Scholar]
- 99.Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2531–40. doi: 10.1016/j.jacc.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 100.Stein E.A., Giugliano R.P., Koren M.J., et al. Efficacy and safety of evolocumab (AMG 145), a fully human monoclonal antibody to PCSK9, in hyperlipidaemic patients on various background lipid therapies: pooled analysis of 1359 patients in four phase 2 trials. Eur. Heart J. 2014;35(33):2249–2259. doi: 10.1093/eurheartj/ehu085. [DOI] [PubMed] [Google Scholar]
- 101.Stroes E., Colquhoun D., Sullivan D., et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J. Am. Coll. Cardiol. 2014;63(23):2541–2548. doi: 10.1016/j.jacc.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 102.Sullivan D., Olsson A.G., Scott R., et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308(23):2497–2506. doi: 10.1001/jama.2012.25790. [DOI] [PubMed] [Google Scholar]
- 103.Koren M.J., Giugliano R.P., Raal F.J., et al. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomized trial. Circulation. 2014;129(2):234–243. doi: 10.1161/CIRCULATIONAHA.113.007012. [DOI] [PubMed] [Google Scholar]
- 104.Nissen S.E., Stroes E., Dent-Acosta R.E., et al. Efficacy and Tolerability of Evolocumab vs. Ezetimibe in Patients With Muscle-Related Statin Intolerance: The GAUSS-3 Randomized Clinical Trial. JAMA. 2016;315(15):1580–1590. doi: 10.1001/jama.2016.3608. [DOI] [PubMed] [Google Scholar]
- 105.Desai N.R., Giugliano R.P., Zhou J., et al. AMG 145, a monoclonal antibody against PCSK9, facilitates achievement of national cholesterol education program-adult treatment panel III low-density lipoprotein cholesterol goals among high-risk patients: an analysis from the LAPLACE-TIMI 57 trial (LDL-C assessment with PCSK9 monoclonal antibody inhibition combined with statin thErapy-thrombolysis in myocardial infarction 57). J. Am. Coll. Cardiol. 2014;63(5):430–433. doi: 10.1016/j.jacc.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 106.Blom D.J., Hala T., Bolognese M., et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N. Engl. J. Med. 2014;370(19):1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 107.Raal F.J., Honarpour N., Blom D.J., et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet (London, England) 2015;385(9965):341–350. doi: 10.1016/S0140-6736(14)61374-X. [DOI] [PubMed] [Google Scholar]
- 108.McKenney J.M., Koren M.J., Kereiakes D.J., Hanotin C., Ferrand A.C., Stein E.A. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J. Am. Coll. Cardiol. 2012;59(25):2344–2353. doi: 10.1016/j.jacc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 109.Roth E.M., McKenney J.M., Hanotin C., Asset G., Stein E.A. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N. Engl. J. Med. 2012;367(20):1891–1900. doi: 10.1056/NEJMoa1201832. [DOI] [PubMed] [Google Scholar]
- 110.Cannon C.P., Cariou B., Blom D., et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur. Heart J. 2015;36(19):1186–1194. doi: 10.1093/eurheartj/ehv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moriarty P.M., Thompson P.D., Cannon C.P., et al. Efficacy and safety of alirocumab vs. ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J. Clin. Lipidol. 2015;9(6):758–769. doi: 10.1016/j.jacl.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 112.Toth P.P., Hamon S.C., Jones S.R., et al. Effect of alirocumab on specific lipoprotein non-high-density lipoprotein cholesterol and subfractions as measured by the vertical auto profile method: analysis of 3 randomized trials versus placebo. Lipids Health Dis. 2016;15:28. doi: 10.1186/s12944-016-0197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ballantyne C.M., Neutel J., Cropp A., et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am. J. Cardiol. 2015;115(9):1212–1221. doi: 10.1016/j.amjcard.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 114.Robinson J.G., Farnier M., Krempf M., et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015;372(16):1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 115.Sabatine M.S., Giugliano R.P., Wiviott S.D., et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015;372(16):1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 116.Schwartz G.G., Bessac L., Berdan L.G., et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am. Heart J. 2014;168(5):682–689. doi: 10.1016/j.ahj.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 117.Gandra S.R., Villa G., Fonarow G.C., et al. Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin. Cardiol. 2016;39(6):313–320. doi: 10.1002/clc.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kastelein J.J., Wedel M.K., Baker B.F., et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006;114(16):1729–1735. doi: 10.1161/CIRCULATIONAHA.105.606442. [DOI] [PubMed] [Google Scholar]
- 119.Stein E.A., Dufour R., Gagne C., et al. Apolipoprotein B synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation. 2012;126(19):2283–2292. doi: 10.1161/CIRCULATIONAHA.112.104125. [DOI] [PubMed] [Google Scholar]
- 120.Thomas G.S., Cromwell W.C., Ali S., Chin W., Flaim J.D., Davidson M. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: a randomized, double-blind, placebo-controlled trial. J. Am. Coll. Cardiol. 2013;62(23):2178–2184. doi: 10.1016/j.jacc.2013.07.081. [DOI] [PubMed] [Google Scholar]
- 121.Visser M.E., Wagener G., Baker B.F., et al. Mipomersen, an apolipoprotein B synthesis inhibitor, lowers low-density lipoprotein cholesterol in high-risk statin-intolerant patients: a randomized, double-blind, placebo-controlled trial. Eur. Heart J. 2012;33(9):1142–1149. doi: 10.1093/eurheartj/ehs023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Santos R.D., Raal F.J., Donovan J.M., Cromwell W.C. Mipomersen preferentially reduces small low-density lipoprotein particle number in patients with hypercholesterolemia. J. Clin. Lipidol. 2015;9(2):201–209. doi: 10.1016/j.jacl.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 123.Cuchel M., Bloedon L.T., Szapary P.O., et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N. Engl. J. Med. 2007;356(2):148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 124.Nissen S.E., Wolski K., Topol E.J. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294(20):2581–2586. doi: 10.1001/jama.294.20.joc50147. [DOI] [PubMed] [Google Scholar]
- 125.Rubin C.J., Ledeine J.M., Fiedorek F.T. Improvement of glycaemic and lipid profiles with muraglitazar plus metformin in patients with type 2 diabetes: an active-control trial with glimepiride. Diab. Vasc. Dis. Res. 2008;5(3):168–176. doi: 10.3132/dvdr.2008.028. [DOI] [PubMed] [Google Scholar]