Abstract
Background:
The concept of antioxidant therapies assumes high importance as oxidative stress is associated with cardiovascular aging via endothelial dysfunction. This review focuses on exploring the interaction between nrf-2 and ADMA in influencing the nitric oxide pathway and cardiovascular function.
Objective:
A systematic review of literature from 1990 to 2016 was conducted using Pubmed and Google Scholar. The literature suggests a strong influence of nrf-2 activation on up regulation of DDAH I which degrades ADMA, the endogenous inhibitor of nitric oxide synthase. The resulting decrease of ADMA would in turn enhance nitric oxide (NO) production. This would support endothelial function by adequate NO production and homeostasis of endothelial function.
Conclusion:
As NO production has many positive pleiotropic effects in the cardiovascular system, such an interaction could be utilized for designing molecular therapeutics. The targets for therapy need not be limited to activation of nrf-2. Modulation of molecules downstream such as DDAH I can be used to regulate ADMA levels. Most current literature is supported by animal studies. The concept of antioxidant therapies needs to be tested in well-defined randomized control trials. The biochemical basis of nrf-2 activation needs to be substantiated in human studies.
Keywords: NRF-2, ADMA, DDAH, oxidative stress, nitric oxide (NO), homeostasis
1. Introduction
Atherosclerosis, hypertension and consequent cardiomyopathy tend to have a common basis at the level of endothelial dysfunction [1, 2]. The molecular basis of endothelial dysfunction resides largely on oxidative stress and its impact on chemical derangement at the level of transcription, translation and post translational mechanisms. The generation of reactive oxygen species (ROS) in an uncontrolled fashion leads to oxidative stress.
Age-dependent changes in ventricular remodeling and apoptosis in cardiac tissue correlate with oxidative stress [3-5]. Nrf2, a transcription factor is involved in regulation of genes that contain specific sequences called Antioxidant Response Elements (AREs) [6, 7]. ADMA is an endogenous competitive inhibitor of nitric oxide synthase [8-13]. Nrf2 decreases oxidative stress by regulating the expression of antoxidant genes while ADMA enhances oxidative stress by inhibiting the nitric oxide pathway via competitive inhibition of nitric oxide synthase pathway. This review will therefore focus on the interaction of two molecules Nrf2 (Nuclear erythroid-2-p45-related factor-2) and ADMA (Asymmetric DiMethyl Arginine). Therapeutic strategies to target these two molecules could probably help restore homeostasis during the aging process.
2. Nrf2 IN CARDIOVASCULAR AGING
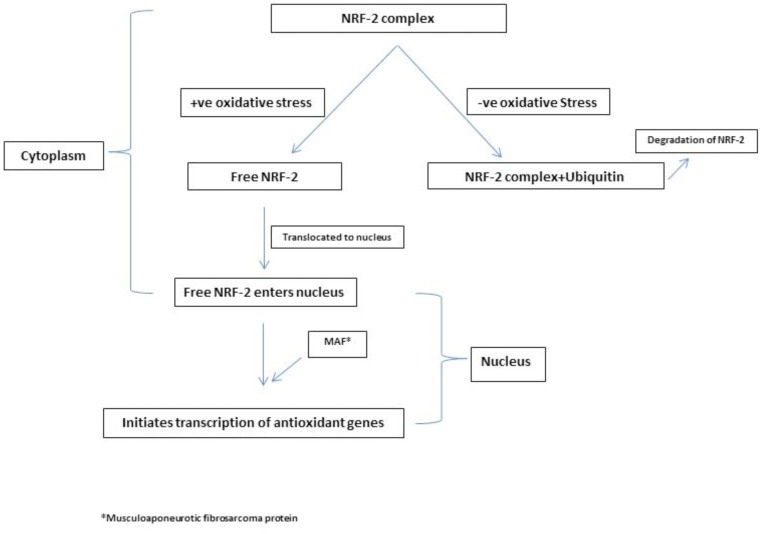
ROS which comprises free radicals such as superoxide anion (∙O2−) and other small molecules (hydrogen peroxide (H2O2), hydroxyl radical (∙OH), and hypochlorite (OCl∙−)) are produced as a result of redox reactions in the system. The synthesis and degradation of these molecules need to be in a homeostatic equilibrium to maintain health. ROS function as important regulators of health and disease processes at the biochemical level. Increased ROS is the molecular basis of cardiovascular diseases as it leads to necrosis and apoptosis of the cardiomyocytes. Other important players in this process are the antioxidant pathways that operate to regulate the ROS metabolism. The pathways of antioxidant mechanisms are the free radical scavengers such as vitamins and other antioxidant molecules, hepatic detoxification and conjugation systems as well as proteins which contain AREs. One of the important modulators of the AREs is Nrf2. Nrf2 in its turn regulates a number of proteins/ enzymes involved in pathogenesis and consequent structural remodeling underlying cardiomyopathy. The addition and removal of ubiiquitin to nrf-2 alters the half- life and location of this molecule and dictates its role in antioxidation. A simplified version of complex molecular interactions involved in this process is depicted in Fig. (1) [6, 14-18].
Fig. (1).
Modulation of NRF-2 by oxidative stress.
Nrf-2 studies in rodent models have suggested a protective role in cardiac remodeling secondary to hypertension [17]. The antioxidant gene superoxide dismutase (SOD) that is regulated by nrf-2 has been shown to be upregulated in a mouse model system of extracellular SOD deficiency under hemodynamic stress [18]. In rat cultured cardiomyocytes overexpression of heme oygenase -1 (HO-1) appears to attenuate cardiac hypertrophy induced by angiotensin II. This is relevant as HO-1 is one of the antioxidant genes that is upregulated by nrf-2 [19]. In adult spontaneously hypertensive rats, up regulation of the HO-1 appears to suppress the hypertensive stress induced hypertrophy [20]. Additionally synthetic activators of nrf-2 have been shown to protect mice subjected to hemodynamic stress from pathological response to stress suggesting a role for nrf-2 up regulation of antioxidant genes [21, 22].
3. ROLE OF ANTIOXIDANT TARGETS OF nrf-2 IN CARDIOMYOPATHY
In ischemia-reperfusion injury ROS generated can damage cell membranes and affect the subcellular proteins as well as activate pro apoptotic pathways [23]. Additionally such changes at the cellular and subcellular levels can lead to ventricular remodeling, progressive chamber dilation, wall thinning, and eventually heart failure secondary to ischemic cardiomyopathy [24]. In adult male rats who were subjected to thirty minutes of ischemia followed by reperfusion treatment with alpha lipoic acid appeared to attenuate cardio toxic changes and remodeling by decreasing myocyte necrosis, apoptosis and inflammation status post ischemia-reperfusion injury. This effect was found to be mediated via up regulation of the PI3 kinase/ Akt pathway and consequent nuclear translocation of nrf-2 which in turn led to induction of HO-1 an antioxidant enzyme [25]. The cardio protective role of fumarate has also been shown in knockout mice lacking the fumarate hydratase enyme to be mediated via up regulation of nrf-2 and the subsequent antioxidant targets [26]. The HO-1 protein has been shown to be up regulated in failing hearts and the effect reversed when these patients were supported on left ventricular assist devices [27].
In a diabetic mouse model overexpression of HO-1 was noted to reverse cardiac dysfunction [28]. Up regulation of other antioxidant genes such as Mn SOD and glutathione peroxidase have been noted in mouse models to protect stress induced cardiac dysfunction [29, 30]. These studies suggest a role for nrf-2 and downstream regulation of the antioxidant genes in diabetic cardiomyopathy.
In anthracycline cardiotoxicity it is still debated whether nrf-2 plays a role as animal model systems do not show consistency in up regulation of nrf-2 and its target antioxidant genes [31, 32]. This area needs further investigations.
Additionally nrf-2 has been implicated in cardiovascular aging because of its down regulation in aging [33-36]. In the drosophila intestinal stem cells loss of nrf-2 function is associated with age dependent cell degeneration suggesting an important role in redox homeostasis [37]. Further investigations in clinical heart failure and the human system are warranted in this area.
4. Effect of ADMA and NO on cardiovascular aging
Increase in oxidative stress and decreased nitric oxide (NO) responsiveness is also noted in aging. Platelet NO response appears to be affected with aging and elevated in post-menopausal women. NO resistance can be explained at the biochemical level as a result of depletion of NO by superoxide anion, resulting in oxidative stress. Inactivation of sGC has been suggested to contribute to this. The major antioxidant protein Thioredoxin (TRX) is inhibited by Thioredoxin (TRX) -interacting protein (TXNIP) which also deranges NO signaling. TXNIP increases with age. Other molecules like thrombospondin (TSP) also increase with age and activate NADPH oxidase resulting in more ROS production and inactivation of soluble guanylate cyclase [38-43].
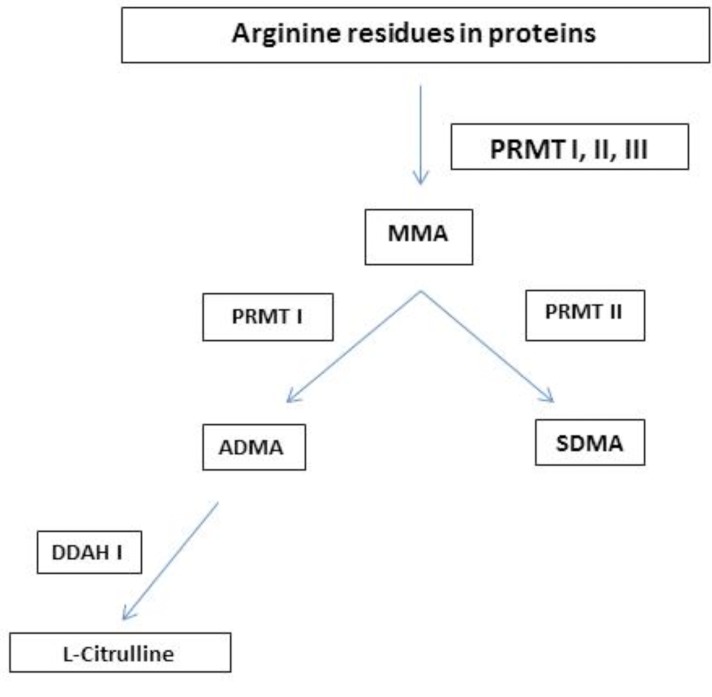
ADMA has been shown to increase with age [44, 45]. Increased ADP-induced platelet aggregation, plasma ADMA concentrations, as well as reductions in platelet NO responsiveness have been associated with aging [46, 47]. Deterioration in platelet NO responsiveness and increases in ADMA concentrations correlate with aging. However the finding that ADMA is elevated in older women versus men is still debated [47-49]. The levels of ADMA are influenced by renal function as well as its enzymatic metabolism [50-54]. The ADMA metabolic pathway is briefly summarized in Fig. (2).
Fig. (2).
Metabolism of ADMA.
5. REGULATION OF ADMA BY nrf-2
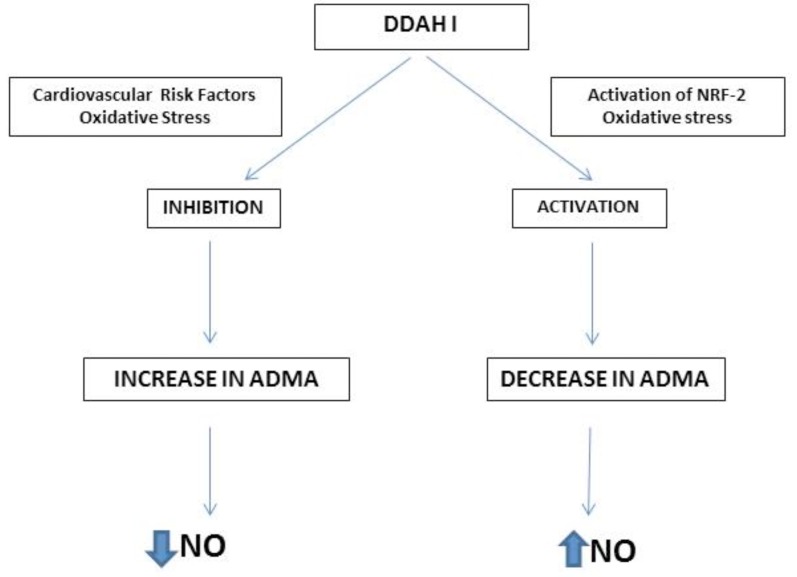
The interaction of Nrf with ARE results in transcriptional activation of antioxidant genes that afford cytoprotection from oxidative stress. An interesting aspect of nrf-2 regulation of antioxidant mechanisms is its role in activating dimethyl arginine dimethyl aminohydrolase (DDAH-1) which metabolizes ADMA. Such an interaction would regulate ADMA levels in the cell which would enhance the NO pathway as ADMA is an endogenous competitive inhibitor of NO synthase. It has been shown that activation of nrf-2 in a human renal glomerular endothelial cell system by tert-butylhydroquinone significantly increased NO and the activities of NOS and DDAH and decreased ADMA [55]. The mechanism of action involved activation of nrf-2 and downstream up regulation of the antioxidant genes such as hemoxygenase-1 as well as eNOS, DDAH-1 and -2 and PPAR-γ. One of the notable effects was the translocation of Nrf2 into the nucleus. As NO production has many positive pleiotropic effects in the cardiovascular system, such an interaction could be utilized for designing molecular therapeutics. Upregulation of the antioxidant genes cause reduction of reactive oxygen species, while activation of DDAH-1 and -2 reduces ADMA. Activation of PPAR-γ by nrf-2 would increase eNOS and its phosphorylation thus integrating all pathways that enhance endothelial NO generation. Fig. (3) illustrates the interaction of nrf-2, ADMA and the NO pathway.
Fig. (3).
Interaction between NRF-2, ADMA and NO pathway.
6. INTERACTION BETWEEN ADMA, HOMOCYSTEINE AND nrf-2
The interactions between ADMA, homocysteine and nrf-2 are rather complex. Investigations on the role of ADMA in aging of endothelial cells in vitro showed significant increase in senescence and accelerated telomere shortening with concomitant decrease in telomerase activity [56]. Decreases in DDAH accompanied these changes [56]. Antioxidants such as pyrrolidine dithiocarbamate decreased DDAH activity and ablated the effect of ADMA in vitro. S-adenosylhomocysteine (SAM), an inhibitor of s-adenosyl methytransferase has been found to nullify the effects of ADMA on endothelial senescence. SAM loses its methyl group to a methyl acceptor to form s-adenosylhomocysteine (SAH) which is in turn converted to homocysteine. Hyperhomocysteinemia is regarded as an important cardiovascular risk factor. Association between increased levels of homocysteine and cardiovascular disease may be explained by low SAM levels or a low SAM/SAH ratio. Homocysteine increases endothelial senescence via DNA hypomethylation mechanisms involving the human telomerase reverse transcriptase in vitro [57]. Biochemical pathways link homocysteine and ADMA. Both molecules have been correlated with arterial stiffness positively and may influence vascular aging in patients with systemic lupus erythematosis who have accelerated atherosclerotic disease [58].
7. Therapeutic targets
Molecular therapeutics such as flavonoid and phenolic antioxidants including resveratrol have been implicated in inducing the AREs [59]. Animal studies show benefits of resveratrol in decreasing deleterious effects of oxidative stress via activation of nrf-2 in animal models [60, 61]. In clinical trials high doses of resveratrol have been shown to be beneficial in hypertension management [62, 63].
8. Balance between ROS and antioxidants
Oxidative stress occurs when the balance between production and degradation of ROS is lost. Hence it is imperative to evaluate the physiological roles of ROS when deciding on treatments that decrease ROS. Oxidative stress increases with aging associated with mitochondrial abnormalities [64]. It has been postulated that oxidative stress induced dysfunction of the endothelium is the link to cardiovascular aging [65]. Vascular oxidative stress has been linked to nrf-2 dysfunction [36]. Despite all evidence of the bad effects of ROS, existing antioxidant therapies are yet to be proven as beneficial. This is possibly due to lack of existing knowledge on the right balance between ROS species and antioxidant molecules so that normal physiological processes are not hampered. Increase in antioxidants causes increased mortality [66] suggests that defining the fine balance is crucial to discovering efficient therapies against oxidative stress [67].
ROS production has been said to be the cause and effect of the aging processes. Present day knowledge about ROS signaling shows that not only do they participate in dysregulation of cell signaling resulting in cardiovascular pathology but they also positively modulate signaling in normal cell physiology. ROS is involved in physiological cell signaling and helps homeostatic regulation.
Conclusion
The targets for therapy need not be limited to activation of nrf-2 but modulation of molecules downstream such as DDAH I would in turn decrease ADMA levels and support endothelial function by adequate NO production and homeostasis of endothelial function [68-70]. Many compounds found in nature such as cucurmin, catechins, allicins, sulfurophanes and lycopenes have been known to activate nrf-2 and its antioxidant actions [71]. Modulation of the antioxidant pathways via nrf-2 activation appears to be attractive as a therapeutic strategy as this effect can be transduced to a number of downstream molecules [72] but needs to be demonstrated in human studies. The most crucial aspect of antioxidant therapy is defining the right balance to allow enough ROS function for normal physiological processes. Such balance can most likely be achieved by identifying specific molecular therapies to which antioxidant therapies can be targeted.
Future Directions
The concept of antioxidant therapies needs to be tested in well-defined randomized control trials. The biochemical basis of nrf-2 activation needs to be substantiated in human studies as most of the current literature is based on animal models. The molecules tested as therapeutic agents need further investigation as to their efficacy and toxicity in the human system. It is unclear if nrf-2 pathway modulation is more effective when used in prophylaxis versus therapy. Additionally the anti-inflammatory component of this pathway needs to be explored further in the aging process. As oxidative stress and aging go hand in hand, it is imperative to investigate new therapeutic regimens to attenuate the aging process and its detrimental effects. The fact that ROS and antioxidants are compartmentalized opens up future avenues of targeting antioxidant therapies that are more efficient. Directing antioxidant therapies to particular compartments at the subcellular level to disrupt pathological reactions would likely be more specific than the systemic prophylactic therapeutics reported in literature. This would most probably eliminate undesirable side effects noted in a systemic approach which indiscriminately targets all pathway including physiological cell signaling required for the normal metabolic processes.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Seals D.R., Jablonski K.L., Donato A.J. Aging and vascular endothelial function in humans. Clin. Sci. (Lond.) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higashi Y., Kihara Y., Noma K. Endothelial dysfunction and hypertension in aging. Hypertens. Res. 2012;35:1039–1047. doi: 10.1038/hr.2012.138. [DOI] [PubMed] [Google Scholar]
- 3.Sheydina A., Riordon D.R., Boheler K.R. Molecular mechanisms of cardiomyocyte aging. Clin. Sci. (Lond.) 2011;121:315–329. doi: 10.1042/CS20110115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai D.F., Chen T., Johnson S.C., Szeto H., Rabinovitch P.S. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid. Redox Signal. 2012;16:1492–1526. doi: 10.1089/ars.2011.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capell B.C., Collins F.S., Nabel E.G. Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ. Res. 2007;101:13–26. doi: 10.1161/CIRCRESAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- 6.Zhou S, Sun W, Zhang Z, Zheng Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid Med Cell Longev. 2014 doi: 10.1155/2014/260429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen B., Lu Y., Chen Y., Cheng J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015;225:R83–R99. doi: 10.1530/JOE-14-0662. [DOI] [PubMed] [Google Scholar]
- 8.Santhanam L., Lim H.K., Lim H.K., et al. iNOS-dependent S-nitrosylation and activation of arginase 1 contributes to age related endothelial dysfunction. Circ. Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- 9.Cooke J.P., Ghebremariam Y.T. DDAH says NO to ADMA. Arterioscler. Thromb. Vasc. Biol. 2011;31:1462–1464. doi: 10.1161/ATVBAHA.111.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Böger R.H., Vallance P., Cooke J.P. Asymmetric dimethylarginine (ADMA): a key regulator of nitric oxide synthase. Atheroscler. Suppl. 2003;4:1–3. doi: 10.1016/s1567-5688(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 11.Cooke J.P. The pivotal role of nitric oxide for vascular health. Can. J. Cardiol. 2004;20(Suppl. B):7B–15B. [PubMed] [Google Scholar]
- 12.Böger R.H., Cooke J.P., Vallance P. ADMA: an emerging cardiovascular risk factor. Vasc. Med. 2005;10(Suppl. 1):S1–S2. doi: 10.1177/1358836X0501000101. [DOI] [PubMed] [Google Scholar]
- 13.Cooke J.P. ADMA: its role in vascular disease. Vasc. Med. 2005;10(Suppl. 1):S11–S17. doi: 10.1177/1358836X0501000103. [DOI] [PubMed] [Google Scholar]
- 14.Circu M.L., Aw T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afanas'ev I. ROS and RNS signaling in heart disorders: could antioxidant treatment be successful? Oxid Med Cell Longev. 2011 doi: 10.1155/2011/293769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hybertson B.M., Gao B., Bose S.K., McCord J.M. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Ichikawa T., Villacorta L., et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler. Thromb. Vasc. Biol. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 18.Lu Z., Xu X., Hu X., et al. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;51:1925. doi: 10.1161/HYPERTENSIONAHA.107.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu C.M., Chen Y.H., Chiang M.T., Chau L.Y. Heme oxygenase-1 inhibits angiotensin II-induced cardiac hypertrophy in vitro and in vivo. Circulation. 2004;110:309–316. doi: 10.1161/01.CIR.0000135475.35758.23. [DOI] [PubMed] [Google Scholar]
- 20.Ndisang J.F., Jadhav A. Upregulating the heme oxygenase system suppresses left ventricular hypertrophy in adult spontaneously hypertensive rats for 3 months. J. Card. Fail. 2009;15:616–628. doi: 10.1016/j.cardfail.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Xing Y., Niu T., Wang W., et al. Triterpenoid dihydro-CDDO-trifluoroethyl amide protects against maladaptive cardiac remodeling and dysfunction in mice: a critical role of Nrf2. PLoS One. 2012;7:e44899. doi: 10.1371/journal.pone.0044899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin H., Li H.F., Chen H.H., et al. Activating transcription factor 3 protects against pressure-overload heart failure via the autophagy molecule Beclin-1 pathway. Mol. Pharmacol. 2014;85:682–691. doi: 10.1124/mol.113.090092. [DOI] [PubMed] [Google Scholar]
- 23.Braunersreuther V., Jaquet V. Reactive oxygen species in myocardial reperfusion injury: from physiopathology to therapeutic approaches. Curr. Pharm. Biotechnol. 2012;13:97–114. doi: 10.2174/138920112798868782. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y. Myocardial repair/remodelling following infarction: Roles of local factors. Cardiovasc. Res. 2009;81:482–490. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng C., Sun Z., Tong G., et al. α-Lipoic acid reduces infarct size and preserves cardiac function in rat myocardial ischemia/reperfusion injury through activation of PI3K/Akt/Nrf2 pathway. PLoS One. 2013;8:e58371. doi: 10.1371/journal.pone.0058371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashrafian H., Czibik G., Bellahcene M., et al. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15:361–371. doi: 10.1016/j.cmet.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabellus F., Schmid C., Levkau B., et al. Reduction of hypoxia-inducible heme oxygenase-1 in the myocardium after left ventricular mechanical support. J. Pathol. 2002;197:230–237. doi: 10.1002/path.1106. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y., Zhang L., Qiao Y., et al. Heme oxygenase-1 prevents cardiac dysfunction in streptozotocin-diabetic mice by reducing inflammation, oxidative stress, apoptosis and enhancing autophagy. PLoS One. 2013;8:e75927. doi: 10.1371/journal.pone.0075927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen X., Zheng S., Metreveli N.S., Epstein P.N. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 30.Iwata K., Nishinaka T., Matsuno K., Yabe-Nishimura C. Increased gene expression of glutathione peroxidase-3 in diabetic mouse heart. Biol. Pharm. Bull. 2006;29:1042–1045. doi: 10.1248/bpb.29.1042. [DOI] [PubMed] [Google Scholar]
- 31.Nordgren K.K., Wallace K.B. Keap1 redox-dependent regulation of doxorubicin-induced oxidative stress response in cardiac myoblasts. Toxicol. Appl. Pharmacol. 2014;274:107–116. doi: 10.1016/j.taap.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Jirkovsky E., Popelová O., Kriváková-Stanková P., et al. Chronic anthracycline cardiotoxicity: molecular and functional analysis with focus on nuclear factor erythroid 2-related factor 2 and mitochondrial biogenesis pathways. J. Pharmacol. Exp. Ther. 2012;343:468–478. doi: 10.1124/jpet.112.198358. [DOI] [PubMed] [Google Scholar]
- 33.Ungvari Z., Bailey-Downs L., Gautam T., et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Safdar A., deBeer J., Tarnopolsky M.A. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic. Biol. Med. 2011;49:1487–1493. doi: 10.1016/j.freeradbiomed.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Suh J.H., Shenvi S.V., Dixon B.M., et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungvari Z., Bailey-Downs L., Sosnowska D., et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hochmuth C.E., Biteau B., Bohmann D., Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chirkov Y.Y., Horowitz J.D. Impaired tissue responsiveness to organic nitrates and nitric oxide: a new therapeutic frontier? Pharmacol. Ther. 2007;116:287–305. doi: 10.1016/j.pharmthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Sverdlov A.L., Chan W.P., Procter N.E., Chirkov Y.Y., Ngo D.T., Horowitz J.D. Reciprocal regulation of NO signaling and TXNIP expression in humans: impact of aging and ramipril therapy. Int. J. Cardiol. 2013;168:4624–4630. doi: 10.1016/j.ijcard.2013.07.159. [DOI] [PubMed] [Google Scholar]
- 40.Lee S., Kim S.M., Lee R.T. Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance. Antioxid. Redox Signal. 2013;18:1165–1207. doi: 10.1089/ars.2011.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts D.D., Miller T.W., Rogers N.M., Yao M., Isenberg J.S. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers N.M., Roberts D.D., Isenberg J.S. Age-associated induction of cell membrane CD47 limits basal and temperature-induced changes in cutaneous blood flow. Ann. Surg. 2013;258:184–191. doi: 10.1097/SLA.0b013e31827e52e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao M., Rogers N.M., Csanyi G., et al. Thrombospondin-1 activation of signal-regulatory protein-alpha stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J. Am. Soc. Nephrol. 2014;25:1171–1186. doi: 10.1681/ASN.2013040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marliss E.B., Chevalier S., Gougeon R., et al. Elevations of plasma methylarginines in obesity and ageing are related to insulin sensitivity and rates of protein turnover. Diabetologia. 2006;49:351–359. doi: 10.1007/s00125-005-0066-6. [DOI] [PubMed] [Google Scholar]
- 45.Chan W.P., Ngo D.T., Sverdlov A.L., et al. Premature aging of cardiovascular/platelet function in polycystic ovarian syndrome. Am. J. Med. 2012;126:640.e1–640.e7. doi: 10.1016/j.amjmed.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Procter N.E., Chong C.R., Sverdlov A.L., Chan W.P., Chirkov Y.Y., Horowitz J.D. Aging of platelet nitric oxide signaling: pathogenesis, clinical implications, and therapeutics. Semin. Thromb. Hemost. 2014;40:660–668. doi: 10.1055/s-0034-1389082. [DOI] [PubMed] [Google Scholar]
- 47.Sverdlov A.L., Ngo D.T., Chan W.P., Chirkov Y.Y., Horowitz J.D. Aging of the nitric oxide system: are we as old as our NO? J. Am. Heart Assoc. 2014;3(4):e000973. doi: 10.1161/JAHA.114.000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kielstein J.T., Bode-Boger S.M., Frolich J.C., Ritz E., Haller H., Fliser D. Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation. 2003;107:1891–1895. doi: 10.1161/01.CIR.0000060496.23144.A7. [DOI] [PubMed] [Google Scholar]
- 49.Hov G.G., Sagen E., Bigonah A., Asberg A. Health-associated reference values for arginine, asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) measured with high-performance liquid chromatography. Scand. J. Clin. Lab. Invest. 2007;67:868–876. doi: 10.1080/00365510701429836. [DOI] [PubMed] [Google Scholar]
- 50.Achan V., Broadhead M., Malaki M., et al. Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler. Thromb. Vasc. Biol. 2003;23:1455–1459. doi: 10.1161/01.ATV.0000081742.92006.59. [DOI] [PubMed] [Google Scholar]
- 51.Vallance P., Leone A., Calver A., Collier J., Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 52.Abbasi F., Asagmi T., Cooke J.P., et al. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am. J. Cardiol. 2001;88:1201–1203. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 53.Boger R.H., Bode-Boger S.M., Szuba A., et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: Its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 54.Blackwell S. The biochemistry, measurement and current clinical significance of asymmetric dimethylarginine. Ann. Clin. Biochem. 2010;47:17–28. doi: 10.1258/acb.2009.009196. [DOI] [PubMed] [Google Scholar]
- 55.Luo Z., Aslam S., Welch W.J., Wilcox C.S. Activation of nuclear factor erythroid 2-related factor 2 coordinates dimethylarginine dimethylaminohydrolase/PPAR-γ/endothelial nitric oxide synthase pathways that enhance nitric oxide generation in human glomerular endothelial cells. Hypertension. 2015;65:896–902. doi: 10.1161/HYPERTENSIONAHA.114.04760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bode-Böger S.M., Scalera F., Martens-Lobenhoffer J. Asymmetric dimethylarginine (ADMA) accelerates cell senescence. Vasc. Med. 2005;10(Suppl. 1):S65–S71. doi: 10.1177/1358836X0501000110. [DOI] [PubMed] [Google Scholar]
- 57.Zhang D., Sun X., Liu J., Xie X., Cui W., Zhu Y. Homocysteine accelerates senescence of endothelial cells via DNA hypomethylation of human telomerase reverse transcriptase. Arterioscler. Thromb. Vasc. Biol. 2015;35:71–78. doi: 10.1161/ATVBAHA.114.303899. [DOI] [PubMed] [Google Scholar]
- 58.Perna M., Roman M.J., Alpert D.R., et al. Relationship of asymmetric dimethylarginine and homocysteine to vascular aging in systemic lupus erythematosus patients. Arthritis Rheum. 2010;62:1718–1722. doi: 10.1002/art.27392. [DOI] [PubMed] [Google Scholar]
- 59.Rubiolo J.A., Mithieux G., Vega F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 60.Haskó G., Pacher P. Endothelial Nrf2 activation: A new target for resveratrol? Am. J. Physiol. Heart Circ. Physiol. 2010;299:H10–H12. doi: 10.1152/ajpheart.00436.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hao E., Lang F., Chen Y., et al. Resveratrol alleviates endotoxin-induced myocardial toxicity via the Nrf2 transcription factor. PLoS One. 2013;8:250. doi: 10.1371/journal.pone.0069452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y., Ma W., Zhang P., He S., Huang D. Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials. Clin. Nutr. 2015;34:27–34. doi: 10.1016/j.clnu.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 63.Carrizzo A., Puca A., Damato A., et al. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension. 2013;62:359–366. doi: 10.1161/HYPERTENSIONAHA.111.01009. [DOI] [PubMed] [Google Scholar]
- 64.Ungvari Z., Sonntag W.E., Csiszar A. Mitochondria and aging in the vascular system. Int. J. Mol. Med. 2010;88:1021–1027. doi: 10.1007/s00109-010-0667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brandes R.P., Fleming I., Busse R. Endothelial aging. Cardiovasc. Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 66.Yoshihara D., Fujiwara N., Suzuki K. Antioxidants: benefits and risks for long-term health. Maturitas. 2010;67:103–107. doi: 10.1016/j.maturitas.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Puca A.A., Carrizzo A., Villa F., et al. Vascular ageing: the role of oxidative stress. Int. J. Biochem. Cell Biol. 2013;45:556–559. doi: 10.1016/j.biocel.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 68.Wadham C., Mangoni A.A. Dimethylarginine dimethylaminohydro-lase regulation: a novel therapeutic target in cardiovascular disease. Expert Opin. Drug Metab. Toxicol. 2009;5:303–319. doi: 10.1517/17425250902785172. [DOI] [PubMed] [Google Scholar]
- 69.Cooke J.P., Ghebremariam Y.T. DDAH says NO to ADMA. Arterioscler. Thromb. Vasc. Biol. 2011;31:1462–1464. doi: 10.1161/ATVBAHA.111.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghebremariam Y.T., Erlanson D.A., Cooke J.P. A novel and potent inhibitor of dimethylarginine dimethylaminohydrolase: a modulator of cardiovascular nitric oxide. J. Pharmacol. Exp. Ther. 2014;348:69–76. doi: 10.1124/jpet.113.206847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cardozo L.F., Pedruzzi L.M., Stenvinkel P., et al. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie. 2013;95:1525–1533. doi: 10.1016/j.biochi.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 72.Xu Z., Wang S., Ji H., et al. Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Sci. Rep. 2016;6:30252. doi: 10.1038/srep30252. [DOI] [PMC free article] [PubMed] [Google Scholar]